2.1. MoS2 Electrodeposition
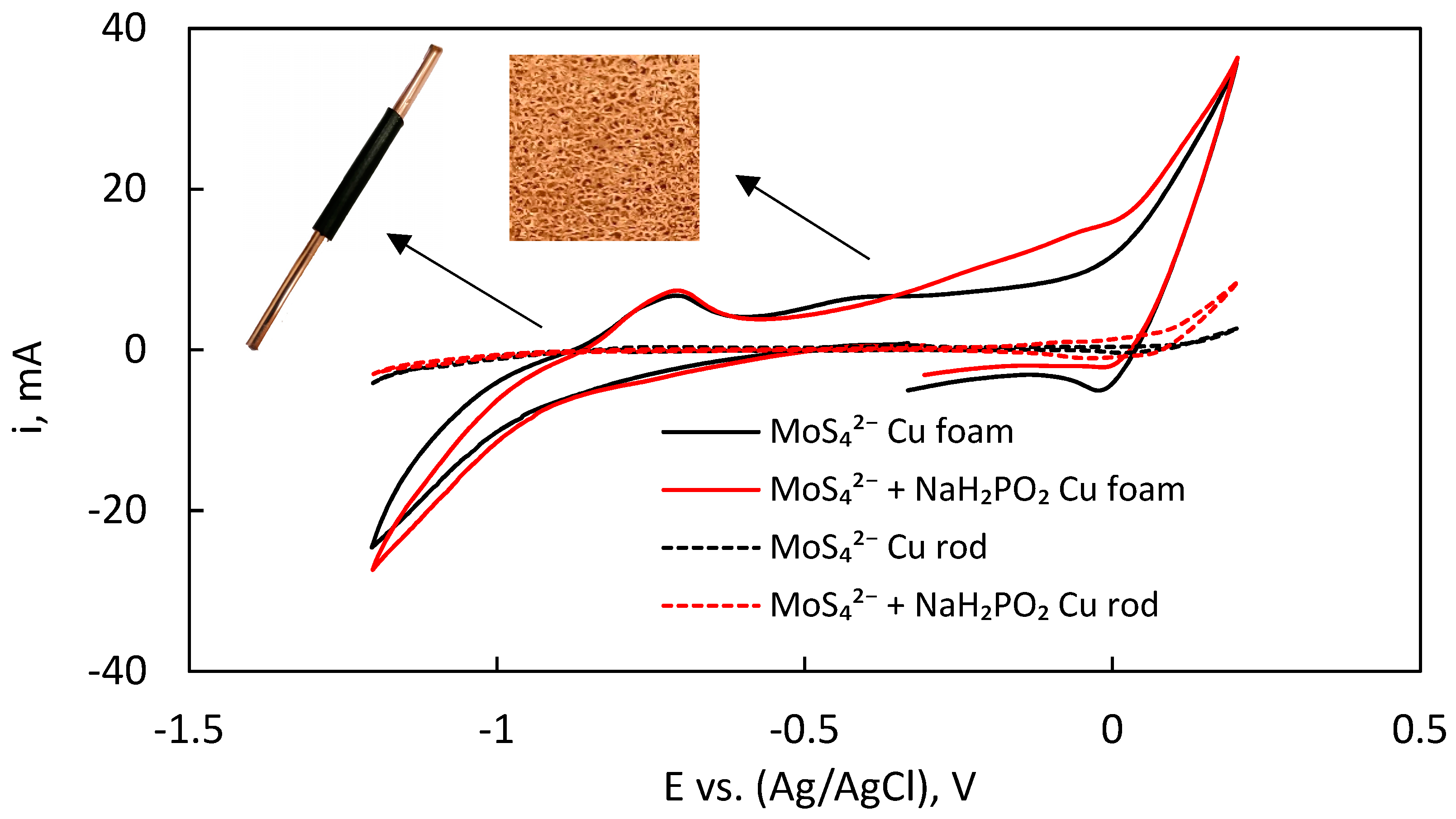
The electrodeposition of MoS
2 films in both solutions (without and with hypophosphite) was investigated by cyclic voltammetry to establish a working potential range, where the dominating cathodic reaction is MoS
42− reduction to MoS
2. Firstly, CV scans were taken on a 1 cm
2 surface area copper rod electrode, at potentials from −1.2 to 0.2 V vs. Ag/AgCl (
Figure 1). No prominent cathodic peaks are present in these cycles due to overlaying of MoS
42− reduction peaks with hydrogen evolution, which occurs across the entire potential range. At about −1 V, the cathodic current begins to increase more rapidly, which reveals that MoS
42− reduction and hydrogen evolution are occurring. The addition of NaH
2PO
2 does not change the profile of the cycles, nor do any peaks become apparent. There is a small difference in the anodic part of the cycles, but in this system the anodic current is entirely dominated by the corrosion of the copper substrate, and the oxidation of electrodeposited MoS
2. CVs performed on the Cu-foam electrodes have a slight difference in the cathodic profile of the curves. A minor increase in the current is observed with the addition of hypophosphite, but no distinguishable reductive peaks appear. A small anodic peak at −0.68 V is seen in both cases and is likely caused by rapid hydrogen desorption from the large surface area and catalyst-covered electrode. The sharp increase in oxidative current at higher anodic potentials again occurs due to above-discussed reasons.
All further research was conducted on the Cu-foam electrodes, prepared as described in
Section 3.2. Galvanostatic conditions were chosen for the electrodeposition, because reductive peaks were not visible in CV, and the geometrical surface area of the substrates is uncertain. Therefore, a cathodic current of 10 mA corresponding to a potential, where intense hydrogen evolution does not yet occur, was selected. At this current, the potential settles at ~1.05 V, which is almost identical to the one we used for potentiostatic deposition in the precluding research [
34]. The films were electrodeposited at increasing charges passed through the cell (from 10 to 40 C). This range was selected to encompass a wider array of film thicknesses: thinner MoS
2 films (10–20 C) were expected to have better adhesion to the substrate, more resistance to delamination and more favourable electron transfer kinetics from the catalyst/solution interface to the substrate. In contrast, thicker films may exhibit poorer physical stability due to internal stresses and weaker chemical bonding to the substrate. However, the increased catalyst loading (higher charged passed) should result in better HER catalytic activity.
To relate electrodeposition parameters to catalyst loading, the films, electrodeposited for discrete amounts of charge passed, were weighed, thus measuring the mass of the MoS
2 deposits. The reductive deposition of MoS
2 conforms to Faraday’s law—there is a linear correlation between charge passed and mass of the electrodeposited film. Up to 0.25 mg of material is deposited per 1 C. In terms of current efficiency, this corresponds to ~32%, which is a known limit for MoS
42− electroreduction [
24]. However, a decrease in current efficiency was observed with increasing charge up to 40 C. This can be caused by the formation of an active electrocatalytic film, which catalyses simultaneous hydrogen evolution, and in turn inhibits MoS
2 electrodeposition. The current efficiency then drops to ~23%. It must be noted that the amount of material deposited without and with NaH
2PO
2 is almost identical. The calculated efficiencies are slightly lower for the electrolyte with hypophosphite, but not by any significant degree. Therefore, the addition of NaH
2PO
2 has no effect on the kinetics or mechanism of reductive MoS
2 deposition. The actual catalyst loading for films, electrodeposited for a similar amount of charge without or with hypophosphite, can be assumed to be identical. For that reason, the changes in the films’ structure, morphology and catalytic activity discussed below have to be attributed to changes in the intrinsic properties of the MoS
2 films and not extrinsic parameters, e.g., catalyst loading.
2.2. Composition and Surface Morphology
The MoS
2 films, electrodeposited without and with NaH
2PO
2 were examined by SEM, and their chemical composition was determined by EDX. A visible effect of hypophosphite is observed on the morphology of the electrodeposited MoS
2 films. The deposits grow more evenly and with less apparent roughness (
Figure 2). In the base electrolyte, MoS
2 appears to grow in clusters of nodules, protruding from the surface and away from the substrate, but often not connecting amongst each other. This leaves empty gaps in the film and creates disorder in the structure. In contrast, the addition of NaH
2PO
2 causes the formation of more compact films. There are fewer disconnected nodules, and MoS
2 growth occurs homogenously over a larger surface area. Overall, this change in morphology would certainly cause a decrease in the micro-level roughness of the deposits. Some cracks can be seen in the thicker MoS
2 films (e.g., deposited at 30 C), which have also been observed in our previous studies, and are directly related to the thickness and consequent tensile stress of the electrodeposited films. Fewer cracks are seen for the films, electrodeposited with hypophosphite, feasibly due to the lower stress of the deposits.
Interestingly, sodium hypophosphite provides a similar effect on the electrodeposition of various transition metal alloys. For example, when depositing a Fe-W-P film, a certain concentration of NaH
2PO
2 in the deposition solution resulted in lower stress of the film and smaller crystallite sizes; the grain sizes decreased when the NaH
2PO
2 concentration was increased [
38]. Similarly, for Co-Ni-P electrodeposits, the amount of NaH
2PO
2 in the electrodeposition solution was observed to affect the surface morphology—the surface appeared smooth and less nodular [
39].
The elemental analysis showed that deposited MoS
2 films are rather thin; therefore, a strong signal from the substrate material (Cu) is present in the EDX spectra (
Table 1). A significant amount of oxygen is also detected in all samples, which may be due to surface oxidation that has been documented to occur, as MoS
2 active sites readily oxidise when the film is left to dry in the open air.
There is no direct correlation between electrodeposition conditions and composition of MoS
2 films. The deposits are sulphur-deficient, and, for those obtained from the base solution, the MoS
2−x stoichiometry ranges from MoS
1.72 to MoS
1.87. Some difference in composition is observed for the films, electrodeposited with hypophosphite. The amount of Mo and S in atomic per cent increases with passed charge, and the background signal of the copper substrate consequently decreases. In MoS
2 films, electrodeposited with hypophosphite, the Mo:S ratios are closer to the stoichiometric. The film deposited for 10 C even exhibits a sulphur surplus, which may be caused by a chemical reaction, for example the formation of copper sulphide. A small amount of phosphorus is present in the EDX spectra, but, in some cases, it was smaller than the reliable detection limit deviation. It must be concluded that there exists a possibility of phosphide incorporation into the MoS
2 structure, but, if so, its amount is near the detection limit. Because the active sites of MoS
2 for HER catalysis are sulphur vacancies, it can be suspected that the stoichiometry difference will have an effect on the electrocatalytic activity of these deposited films. Indeed, the research has shown that there is an optimal amount of point defects (active sites) that results in best HER catalytic activity [
40].
2.3. X-ray Photoelectron Spectroscopy
X-ray photoelectron spectra were obtained for the MoS
2 films, electrodeposited with and without NaH
2PO
2, to investigate the possible differences in their structure (
Figure 3). The sulphur 2p region is determined by a single doublet corresponding to S 2p
1/2 and S 2p
3/2 lines that is characteristic for the presence of the 2H-MoS
2 phase. For the MoS
2 films, electrodeposited without NaH
2PO
2 (
Figure 3b), the 2p
3/2 maximum is seen at a binding energy of 161.9 eV, whereas deposition with hypophosphite leads to the shift of the peak position towards slightly higher energies at 162.1 eV. Another S 2p doublet was added in the model for better fitting of the spectra. A second doublet in the S 2p region has been attributed to the existence of different S-S bonds in the material (terminal and bridging bonds) [
19,
20]. However, in our case, the second S 2p doublet appears more similar to those that have been reported to be caused by sulphur residuals from polysulphide that may have formed in the deposition solution. Thus, it can be inferred from the spectra that the near-surface sulphur atoms exist almost entirely in one oxidation state.
Deconvolution of the Mo 3d region revealed that Mo exists in multiple oxidation states, and is coordinated by different elements (
Figure 3a,c). The films electrodeposited without hypophosphite (
Figure 3a) show a spectrum that is almost entirely decided by two peaks at 231.7 eV and 234.8 eV. Judging by these binding energies, these peaks likely belong to Mo
6+ oxides (3d
5/2 232.3–232.5 eV; 3d
3/2 235.4–235.7 eV [
41,
42,
43]). These oxides may have been electrodeposited from residual MoO
42− in the deposition solution, or appeared through oxidation of uncoordinated Mo sites on the surface. The peaks deconvolute into two doublets marked Mo
6+-O and Mo
6+-iO in
Figure 3, which also suggests that the oxide exists in different states.
The peaks at binding energies of 229.35 and 232.3 eV have been reported to correspond to Mo
4+ 3d
5/2 and 3d
3/2 components of 2H-MoS
2 (marked Mo
4+-S) [
44]. This, along with the previously discussed characteristic S 2p peaks, confirms the presence of the 2H-MoS
2 phase. The origin of the doublet (3d
5/2 230.2 eV; 3d
3/2 233.2 eV) is ambiguous. Such binding energies correspond well to Mo
4+ oxides [
41,
42], which may exist in the electrodeposited film due to reasons discussed above related to Mo
6+ oxides. However, a very similar binding energy (230.76 eV) may also be attributed to an intermediate Mo oxidation state (e.g., 5+) that is only partially coordinated by sulphur (with a sulphur vacancy—active site) [
20,
45]. The depressed, wide peaks at a very high binding energies (234.5–237.8 eV) could not be attributed to any state of Mo and are assumed to be caused by some residual compound from the solution.
XPS analysis showed that within films, electrodeposited without NaH2PO2, molybdenum is widely coordinated by oxygen, i.e., the film contains a lot of molybdenum oxide. This is also supported by EDX analysis, where the amount of oxygen in these films was relatively large. When electrodeposition is carried out with hypophosphite, a much stronger Mo4+-S bond signal is observed. Because the S 2p signal does not change in any major way, it is assumed that the material retains the same 2H-MoS2 structure, and that the effect of NaH2PO2 on the electrochemical deposition of MoS2 is mainly targeting the suppression of Mo-O bond formation, or, conversely, the assistance of Mo-S bond formation.
2.4. Electrocatalytic Activity for HER
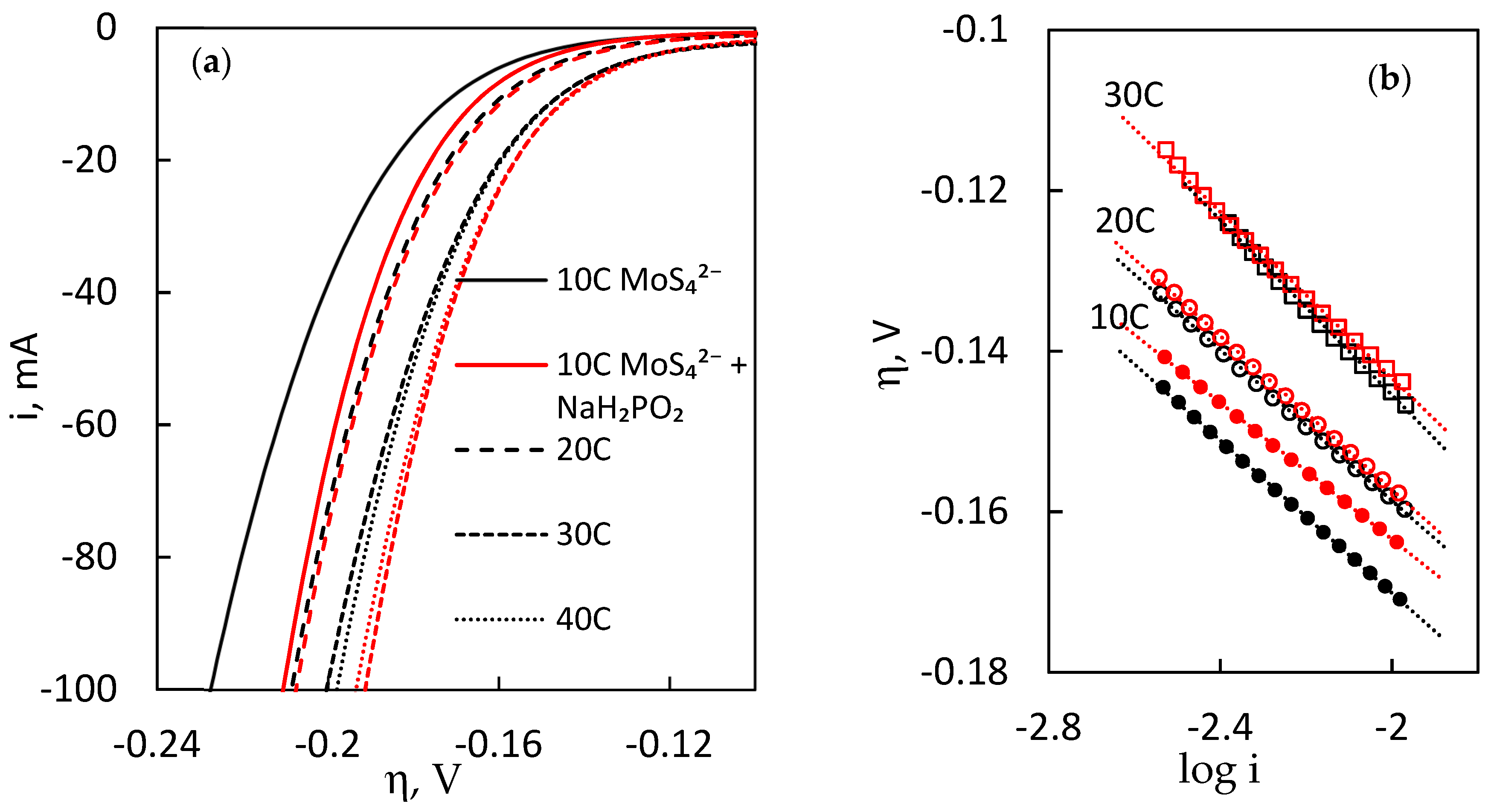
The most common and conventionally informative way to characterise the electrocatalytic activity of an electrode is linear sweep voltammetry (LSV) in a wide overpotential range. As MoS
2 is a catalyst in acidic media, the electrodeposited films were studied by LSV in 0.5 M H
2SO
4. The initial potential was set close to thermodynamic equilibrium (0 V vs. SHE), and a scan rate of 2 mV s
−1 was applied. A cut-off condition of 100 mA of cathodic current was set to end the scan. In this way, three scans were recorded, and the third scan was selected for analysis (
Figure 4a). Note that the cathodic current on the ordinate axis has not been normalised into geometrical surface area, because it would be incorrect for such an electrode, where current density distribution does not guarantee equal HER turnover over the entire electrochemically active surface area. More discussion on this issue follows in the
Section 2.5. It can be seen that MoS
2 films, electrodeposited for increased passed charge (higher catalyst loading) predictably have better total activity. The onset overpotential (arbitrary, where the HER current would begin to sharply rise) is also lower for higher-loading electrodes. Thus, a MoS
2 film electrodeposited from the base MoS
42− solution for 10 C could reach a HER cathodic current of 100 mA at an overpotential of −0.227 V. In comparison, a film electrodeposited for 10 C in the presence of NaH
2PO
2 could reach −100 mA at −0.21 V, representing a 17-mV improvement. The effect of adding sodium hypophosphite into the electrodeposition solution has a prominent effect on the electrocatalytic activity of the films—their total activity generally increases. On LSV curves, this results in lower overpotentials, required to reach the same HER current. A smaller, but similar difference is observed in all cases. Because the mass of the Cu-foam MoS
2 electrodes (catalyst loading) is alike, this must be caused by changes of the intrinsic activity of the film.
Two more important parameters characterising the electrocatalytic HER activity of the MoS
2 films are the Tafel slope and exchange current. The Tafel slopes are shown in
Figure 4b, and the corresponding values are presented in
Table 2. The determined values for slopes (40–50 mV dec
−1) are commonly reported for MoS
2-based electrodes and materials, where HER undergoes via mixed Volmer–Heyrovsky kinetics. MoS
2 films, electrodeposited with hypophosphite, have slightly lower Tafel slopes. For exchange current, only a small change of this parameter is seen upon addition of NaH
2PO
2 as
I0 decreases in most cases. However, due to the potential variance in the geometrical surface area of Cu-foam electrodes, these values are not necessarily correct representations of the intrinsic activity of the electrodeposited films.
The electrochemical stability of electrodeposited MoS
2 films was tested by a galvanostatic step technique: the cathodic current was set at incrementally increasing values (from −10 to −100 mA), and the potential response was measured for 500 s in order to approach a steady state value (
Figure 5). In terms of stability, all films are stable at lower applied currents (up to −40 mA), and a slight decrease in overpotential is observed over each 500-s step. Furthermore, MoS
2 films electrodeposited from the solution with hypophosphite possess worse catalytic activity at lower currents than in the absence of it, as they reach the same current density at higher overpotentials. However, at cathodic currents higher than −50 mA, the curves overlap, and the films electrodeposited with hypophosphite exhibit improved catalytic activity. These results are in line with the Tafel analysis discussed above, because these films were found to have lower Tafel slopes values, and, as a result, their catalytic activity reaches its full ability only at high applied currents or overpotentials. However, at higher cathodic currents (over −50 mA), a rapid drop in measured overpotential is observed, which indicates a loss in catalytic activity. This is likely due to the cathodic corrosion, i.e., shortening of the polymeric S-Mo-S chains by cleavage of Mo-S bonds, as described in [
20]. Experimental observations support this theory, because the colourless sulphuric acid solution attained a faint yellow hue, likely due to sulphide ions dissolving into the solution.
2.5. HER Study by EIS
EIS can be an informative and non-destructive method to characterise a heterogeneous catalyst-solution interface. This method is especially useful to characterise electrodes that have an uncertain geometric surface area (such as metallic foams) based on the parameters of double-layer (
Cdl) and polarisation (
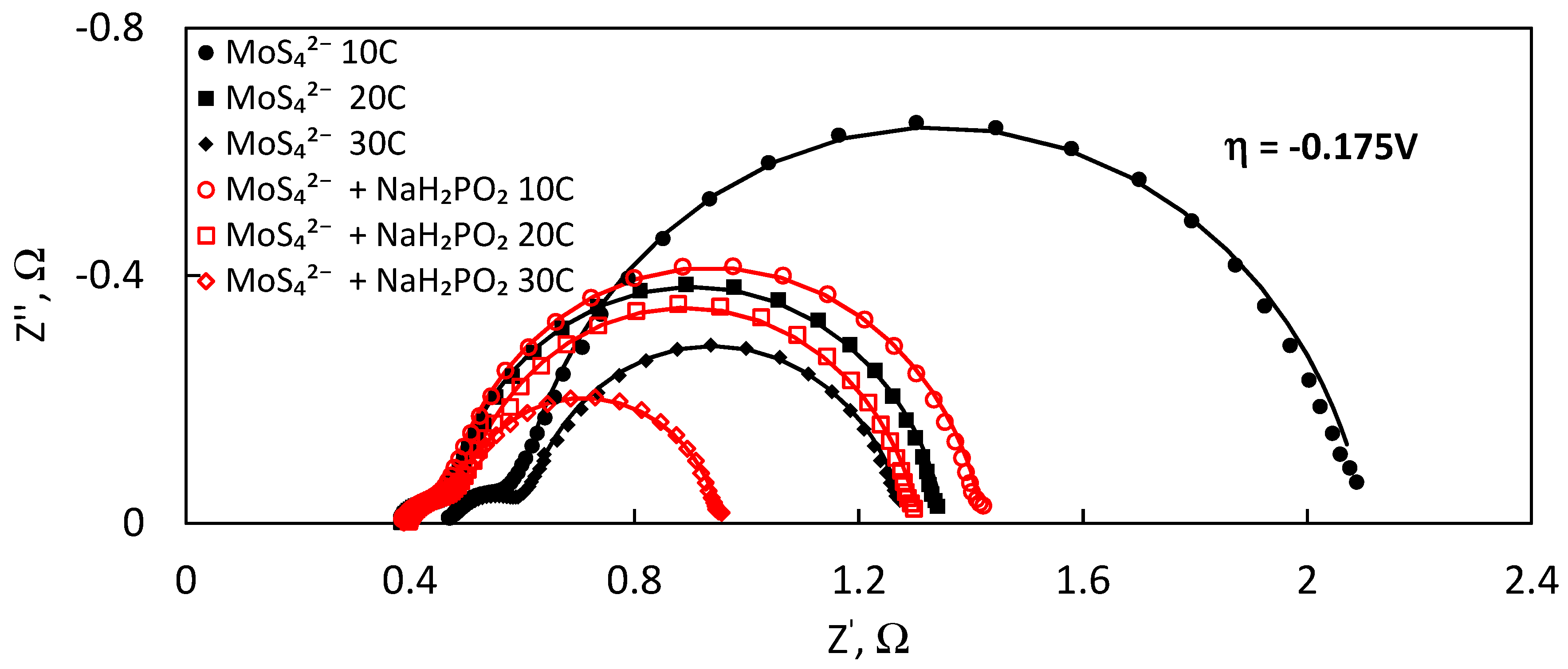
Cp) capacitance that are directly proportional to the electrochemically and electrocatalytically active surface area. The spectra were registered at increasingly cathodic overpotentials (0, −0.1, −0.15, −0.175, −0.2, −0.25 and −0.3 V) to obtain more data (in addition to Tafel analysis) on the kinetics of HER catalysis. Based on the results of Kramers–Kronig procedure, the system was found to be linear and stable, and spectra obtained even at high overpotentials were of good quality. The impedance of the system decreases dramatically with the increase of the overpotential (
Figure 6), owing to the intensity of HER. Two semicircles become especially pronounced from −0.15 V, when hydrogen evolution begins to occur at an increasing rate (
Figure 6a). At higher overpotentials, the semicircles become similar in magnitude (
Figure 6b). If the spectra were to be registered at even higher overpotentials, only the high-frequency semicircle could be distinguished. The Bode plots (
Figure 6c) also show two distinguishable peaks, with the low-frequency peak shrinking in magnitude when the overpotential is increased. Such behaviour of the system can be described by equivalent electric circuit (EEC) containing two time constants in the system: τ
HF and τ
LF shown in
Figure 7. This EEC is commonly used to interpret EIS data for electrode processes containing adsorbed intermediate compounds, and it is often applied to model HER in both acidic and alkaline media [
46]. Here, the double layer capacitance
Cdl and polarisation capacitance
Ca are represented by constant phase elements that account the inhomogeneity of the surface. In agreement with the previously discussed spectra, this model contains two time constants:
τHF =
CdlRct and
τLF =
CaRa. Values of CPE were recalculated into capacitance by Brug et al.’s Equation [
47]:
Here,
Tdl and
Ta are values of the CPE
dl and CPE
a elements, respectively.
Although at lower overpotentials the system behaves in a moderately blocking manner (
Figure 6d), the rate of HER increases rapidly, reactive resistance decreases and the system becomes almost conductive.
EIS spectra vary with amount of charge used for MoS
2 film electrodeposition from the solutions with and without hypophosphite ions (
Figure 8). At the same overpotential, the shape of the spectra remains identical, and two semicircles can be distinguished for all films. It means that the mechanism of HER does not change. The magnitude of the impedance spectra, however, decreases with increasing charge used for electrodeposition of MoS
2. The most significant difference is obtained for the low-frequency semicircle, which, as discussed below, is correlated to the polarisation resistance and capacitance. The spectra also show that the MoS
2 films which were electrodeposited with hypophosphite have lower impedance magnitudes and are thus more catalytically active.
All of the parameters of the EEC are correlated to the material’s electrocatalytic activity towards HER, and therefore their relation to the applied overpotential reveals important information about kinetics. The inverse of charge transfer resistance (1/
Rct) is directly proportional to the reaction rate, assuming a simplified Volmer–Heyrovsky mechanism. The 1/
Rct−ŋ plots for all studied MoS
2 films (
Figure 9) show that the rate of HER is generally higher when the films were electrodeposited at more charges passed for MoS
2 deposition. Furthermore, addition of hypophosphite to the electrodeposition solution increases the catalytic activity of the films. The tendencies in terms of HER activity as a function of deposition charge correspond to the polarisation curves seen in
Figure 4.
The measured double layer capacitance
Cdl is directly proportional to the electrochemically active surface area of the electrode, and it is especially important to approximate when the geometrical surface area is uncertain. The values of
Cdl also vary as a function of overpotential (
Figure 10) and reach a peak at ~−0.2 V. Overall, the
Cdl values of the MoS
2 films, electrodeposited with hypophosphite, are larger throughout the entire overpotential range, meaning that the observed smoother surface morphology in fact results in a larger electrochemically active surface area in contact with the electrolyte. The apparent values of
Cdl are on the order of 10–100 mF, which is a conceivably large value, given the vast surface area of the Cu-foam electrode and the affinity of MoS
2 to adsorb H
+ under these experimental conditions.
As discussed above, the low-frequency RC-element in the equivalent circuit used to fit the impedance data causes the formation of a large depressed semicircle that is strongly correlated to the applied overpotential (
Figure 6 and
Figure 8). It is therefore expected that the values of
Ca and
Ra should also change significantly.
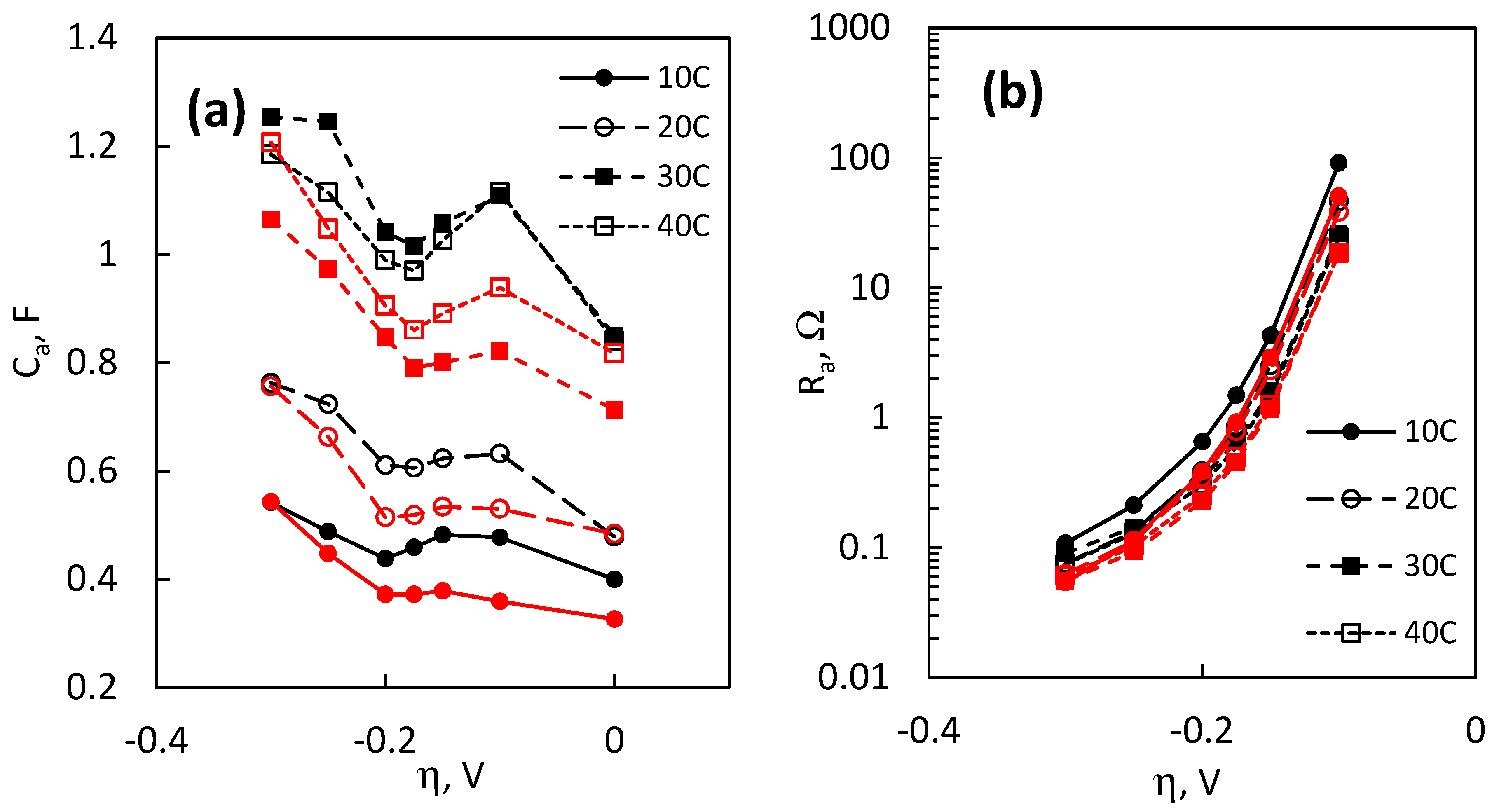
Ca, which can also be imagined as a pseudocapacitance that arises from the modulating surface coverage by adsorbed hydrogen H
ads, is relatively constant until −0.2 V, after which it increases linearly with applied overpotential (
Figure 11a). As it is generally assumed that with increasingly negative overpotential the surface coverage Θ
H approaches to 1 [
6],
Ca could be expected to plateau at even larger overpotentials, but that was not observed in this experimental series. When considering the effect of hypophosphite on the electrodeposited MoS
2 films, it can be seen that the values of
Ca are lower than for the respective films from the base solution. This suggests that there is a change in the surface coverage by H
ads when the films act electrocatalytically, but the precise kinetic reason is difficult to establish from these data alone. In addition, as the difference seems to exist only in the intermediate overpotential range and at −0.3 V, the
Ca values for the respective compared MoS
2 films overlap. The other component in the low-frequency RC-element—an adsorption resistance,
Ra—decreases exponentially with applied overpotential, and it is the most significantly affected parameter of the system (
Figure 11b). Note that here the data from 0 V are not presented, but
Ra can be imagined to trend towards infinity.
Ra values decrease by several magnitudes over the measured overpotential range (for example, for a MoS
2 film, deposited for 30 C, a decrease from 26.1 Ω at −0.1 V to 0.09 Ω at −0.3 V is seen).
Ra as a function of η begins to plateau at overpotentials larger than −0.2 V and could conceivably reach a constant value. Here, it can also be seen that the MoS
2 films, electrodeposited with NaH
2PO
2, exhibit lower
Ra values than those electrodeposited in the base solution.
All the data from the EIS fitting and calculations can be summed up in terms of time constants and their dependence on the HER overpotential. Because the time constant is a measure of the system response rate to an applied overpotential perturbation, the τ
HF and τ
LF values will be correlated to HER kinetics. The high frequency time constant τ
HF =
Rct⋅Cdl rises until it reaches a peak value, after which it begins to decrease (
Figure 12a). This tendency was also seen for the double layer capacitance (
Figure 10) and is likely related to the surface coverage by H
ads. At a certain overpotential, the two capacitances diverge:
Ca increases, while
Cdl begins to drop, and this point corresponds to the highest value of τ
HF. It is interesting to note that the high frequency response at low and high overpotentials is similar; the peak only occurs at ~−0.2 V, at which point hydrogen evolution is already intense with a current of up to −100 mA, as seen from polarisation experiments. The low-frequency time constant is a product of
Ca and
Ra, and, because of the small changes of
Ca, it is completely determined by the
Ra component. Like
Ra, τ
LF decreases exponentially with overpotential by several magnitudes over the measured range (
Figure 12b). At high overpotentials of −0.3 V, both τ
HF and τ
LF become comparable for all samples. For example, for the MoS
2 film, electrodeposited for 10 C with NaH
2PO
2, τ
LF is 0.029 s
−1, τ
HF is 0.0078 s
−1 and τ
LF/τ
HF = 3.7. It is also apparent that films with less catalyst loading (i.e., less electrodeposition charge used for MoS
2 deposition) have a faster response to perturbation (lower τ
LF values). In fact, these results correlate directly to the Tafel slopes presented in
Table 2: the film with the lowest Tafel slope also has the lowest τ
LF values at the entire overpotential range. The results provide an insightful observation: although higher catalyst loading does result in increased total electrode activity, the intrinsic activity is degraded.
2.6. Turnover Frequency and Active Site Calculations
In a previous attempt to apply EIS for the approximation of the number of active sites on a MoS
2 electrocatalyst, it has been shown that with certain assumptions the values of
Ca obtained from impedance fitting could be used in calculations [
28]. However, there the number was likely underestimated due to the low applied overpotential (−0.1 V). In theory, the surface coverage by adsorbed hydrogen approaches Θ
H = 1 at highly negative overpotentials [
6]. Therefore, the same analysis was done in this study in order to approximate the number of active sites on the MoS
2 deposited on the Cu-foam electrodes, and to calculate the turnover frequencies.
It must be noted that both physically and mathematically the number of active sites will depend on the applied overpotential, so the following calculations were performed using the most cathodic overpotential for which experimental data were obtained in this study: −0.3 V. The following assumptions were made: hydrogen adsorption is a one-electron process, one active site adsorbs one H
+ and Θ
H ~≈ 1. Then, the charge needed to attain a monolayer of H
ads is:
where
Ca is the adsorption capacitance, η is the applied overpotential (−0.3 V).
The number of active sites is calculated from Equation (4):
It may then be inferred, from the results in
Figure 11a, that at sufficiently negative overpotentials the compared MoS
2 films that were electrodeposited with and without hypophosphite will have the same number of active sites. Indeed, no significant difference is seen in the values, as presented in
Table 3.
Na increases with electrodeposition charge as more material is deposited. The values in total for the whole electrode are on the order of 10
18, which, accounting for the large geometrical surface area of the substrate, are within range of commonly reported values. In comparison, the previous application of this method resulted in
Na of up to 2.8 × 10
16 sites cm
−2 for MoS
2, electrodeposited on a Cu rod electrode [
28].
The turnover frequencies (number of hydrogen molecules evolved per site per second) were then calculated using the obtained number of active sites (
Table 3). For comparison, TOFs at overpotential of −0.19 V (where experimental data were available for all measurements) are also given. Even though hydrogen evolution is vigorous and large cathodic currents are observed at this overpotential (see
Figure 4a), the actual TOFs are fairly small owing to the large number of active sites. However, the turnover frequencies of MoS
2 films electrodeposited with NaH
2PO
2 are marginally higher than those of MoS
2 electrodeposited from the base solution. This again suggests that incorporation of hypophosphite into the electrodeposition solution results in catalytic MoS
2 films that exhibit higher per-site activity, as the enhanced HER performance can no longer be attributed to changes in surface morphology alone.
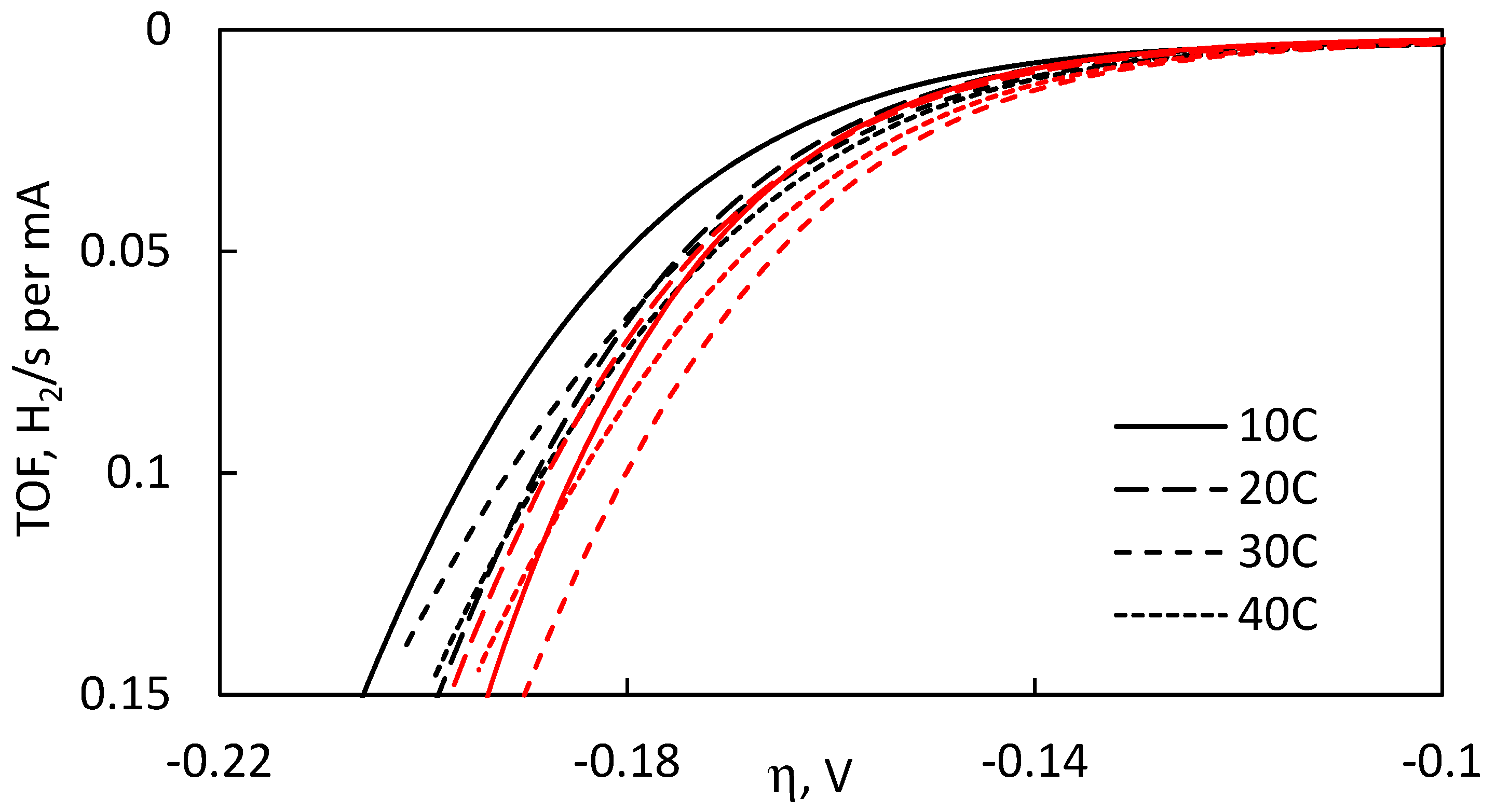
It has been suggested that the ideal way to compare catalysts regardless of geometrical surface area is plotting the turnover frequency as a function of overpotential [
48]. Therefore,
Figure 13 presents these dependencies for all catalytic films examined in this study. It can be seen that the MoS
2 films, electrodeposited with NaH
2PO
2, exhibit distinctly better electrocatalytic activity. They reach higher TOF values at lower overpotentials, which signals a more rapid hydrogen evolution performance of their active sites. The TOF values may be susceptible to error due to the method used in approximation of the number of active sites. However, regardless of possible errors in calculations, the general tendency is that using hypophosphite in the electrodeposition bath did result in enhanced catalytic performances of the deposited MoS
2 films.