In Situ Synthesis of Sn-Beta Zeolite Nanocrystals for Glucose to Hydroxymethylfurfural (HMF)
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Synthesized Sn-Beta Nanocrystals
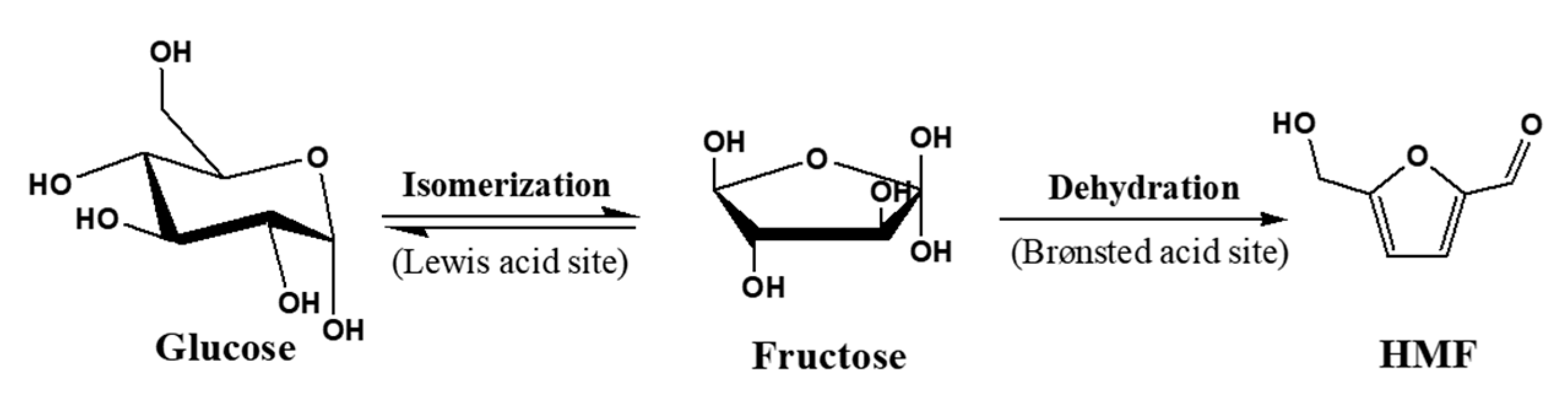
2.2. Catalytic Test in the Glucose Conversion to 5-hydroxymethylfurfural (5-HMF)
3. Experimental Section
3.1. Chemicals and Materials
3.2. In Situ Synthesis of Sn-Beta Zeolite Nanocrystals
3.3. Characterization of Catalysts
3.4. Catalytic Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kohli, K.; Prajapati, R.; Sharma, B. Bio-based chemicals from renewable biomass for integrated biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Trapp, A.M. The chemical conversion of biomass-derived saccharides: An overview. J. Braz. Chem. Soc. 2017, 28, 1586607. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Romashov, L.V.; Galkin, K.I.; Ananikov, V.P. Chemical transformations of biomass-derived C6-furanic platform chemicals for sustainable energy research, materials science, and synthetic building blocks. ACS Sustain. Chem. Eng. 2018, 6, 8064–8092. [Google Scholar] [CrossRef]
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-hydroxymethylfurfural (HMF) production from real biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Lu, Y.; Jameel, H.; Chang, H.M.; Ma, L. High conversion of glucose to 5-hydroxymethylfurfural using hydrochloric acid as a catalyst and sodium chloride as a promoter in a water/γ-valerolactone system. RSC Adv. 2017, 7, 14330–14336. [Google Scholar] [CrossRef]
- Torres-Olea, B.; Mérida-Morales, S.; García-Sancho, C.; Cecilia, J.A.; Maireles-Torres, P. Catalytic activity of mixed Al2O3-ZrO2 oxides for glucose conversion into 5-hydroxymethylfurfural. Catalysts 2020, 10, 878. [Google Scholar] [CrossRef]
- Tempelman, C.; Jacobs, U.; Hut, T.; Pereira de Pina, E.; Van Munster, M.; Cherkasov, N.; Degirmenci, V. Sn exchanged acidic ion exchange resin for the stable and continuous production of 5-HMF from glucose at low temperature. Appl. Catal. A 2019, 588, 117267. [Google Scholar] [CrossRef]
- Zhang, M.; Su, K.; Song, H.; Li, Z.; Cheng, B. The excellent performance of amorphous Cr2O3, SnO2, SrO and graphene oxide-ferric oxide in glucose conversion into 5-HMF. Catal. Commun. 2015, 69, 76–80. [Google Scholar] [CrossRef]
- Silahua-Pavón, A.A.; Espinosa-González, C.G.; Ortiz-Chi, F.; Pacheco-Sosa, J.G.; Pérez-Vidal, H.; Arévalo-Pérez, J.C.; Godavarthi, S.; Torres-Torres, J.G. Production of 5-HMF from glucose using TiO2-ZrO2 catalysts: Effect of the sol-gel synthesis additive. Catal. Commun. 2019, 129, 105723. [Google Scholar] [CrossRef]
- Oozeerally, R.; Ramkhelawan, S.D.K.; Burnett, D.L.; Tempelman, C.H.L.; Degirmenci, V. ZIF-8 metal organic framework for the conversion of glucose to fructose and 5-hydroxymethyl furfural. Catalysts 2019, 9, 812. [Google Scholar] [CrossRef]
- Dapsens, P.Y.; Mondelli, C.; Jagielski, J.; Hauert, R.; Pérez-Ramírez, J. Hierarchical Sn-MFI zeolites prepared by facile top-down methods for sugar isomerisation. Catal. Sci. Technol. 2014, 4, 2302–2311. [Google Scholar] [CrossRef]
- Gardner, D.W.; Huo, J.; Hoff, T.C.; Johnson, R.L.; Shanks, B.H.; Tessonnier, J.-P. Insights into the hydrothermal stability of ZSM-5 under relevant biomass conversion reaction conditions. ACS Catal. 2015, 5, 4418–4422. [Google Scholar] [CrossRef]
- Xue, Z.; Ma, M.-G.; Li, Z.; Mu, T. Advances in the conversion of glucose and cellulose to 5-hydroxymethylfurfural over heterogeneous catalysts. RSC Adv. 2016, 6, 98874–98892. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, Y. Chapter 5—Porous materials for catalysis: Toward sustainable synthesis and applications of zeolites. Sustain. Nanoscale Eng. 2020, 115–137. [Google Scholar] [CrossRef]
- Khan, W.; Jia, X.; Wu, Z.; Choi, J.; Yip, A. Incorporating hierarchy into conventional zeolites for catalytic biomass conversions: A review. Catalysts 2019, 9, 127. [Google Scholar] [CrossRef]
- Abildstrøm, J.O.; Ali, Z.N.; Mentzel, U.V.; Mielby, J.; Kegnæs, S.; Kegnæs, M. Mesoporous MEL, BEA, and FAU zeolite crystals obtained by in situ formation of carbon template over metal nanoparticles. New J. Chem. 2016, 40, 4223–4227. [Google Scholar] [CrossRef]
- Siqueira, B.G.; Silva, M.A.P.; Moraes, C. Synthesis of HMF from glucose in aqueous medium using niobium and titanium oxides. Braz. J. Petroleum Gas 2013, 7, 71–82. [Google Scholar] [CrossRef]
- Tamura, M.; Chaikittisilp, W.; Yokoi, T.; Okubo, T. Incorporation process of Ti species into the framework of MFI type zeolite. Micropor. Mesopor. Mater. 2008, 112, 202–210. [Google Scholar] [CrossRef]
- Bayu, A.; Karnjanakom, S.; Kusakabe, K.; Abudula, A.; Guan, G. Preparation of Sn-β-zeolite via immobilization of Sn/choline chloride complex for glucose-fructose isomerization reaction. Chin. J. Catal. 2017, 38, 426–433. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, L.; Wang, H.; Miao, G.; Kong, L.; Li, S.; Sun, Y. Efficient production of lactic acid from sugars over Sn-Beta zeolite in water: Catalytic performance and mechanistic insights. Sustain. Ener. Fuels 2019, 3, 1163–1171. [Google Scholar] [CrossRef]
- Yang, G.; Wang, C.; Lyu, G.; Lucia, L.A.; Chen, J. Catalysis of glucose to 5-hydroxymethylfurfural using Sn-Beta zeolites and a Brønsted acid in biphasic systems. BioResources 2015, 10, 5863–5875. [Google Scholar] [CrossRef]
- Moliner, M.; Román-Leshkov, Y.; Davis, M.E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc. Natl. Acad. Sci. USA 2010, 107, 6164–6168. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ren, L.; Alhassan, S.M.; Tsapatsis, M. Glucose isomerization in dioxane/water with Sn-β catalyst: Improved catalyst stability and use for HMF production. Chem. Commun. 2019, 55, 14942–14945. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ding, J.; Jiang, J.-G.; Zhu, Z.; Wu, P. One-pot synthesis of 5-hydroxymethylfurfural from glucose using bifunctional [Sn,Al]-Beta catalysts. Chin. J. Catal. 2015, 36, 820–828. [Google Scholar] [CrossRef]
- Li, Y.-P.; Head-Gordon, M.; Bell, A.T. Analysis of the reaction mechanism and catalytic activity of metal-substituted Beta zeolite for the isomerization of glucose to fructose. ACS Catal. 2014, 4, 1537–1545. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Xie, Q.; Wang, J.; Hou, B.; Jia, L.; Cui, J.; Li, D. Conversion of fructose into furfural or 5-hydroxymethylfurfural over HY zeolites selectively in γ-butyrolactone. Appl. Catal. A 2019, 572, 51–60. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, J.; Xiong, G.; Li, X.; Liu, L.; Guo, H. The synthesis of hierarchical Sn-Beta zeolite via aerosol-assisted hydrothermal method combined with a mild base treatment. Micropor. Mesopor. Mater. 2019, 287, 85–92. [Google Scholar] [CrossRef]
- Corma, A.; Nemeth, L.T.; Renz, M.; Valencia, S. Sn-zeolite beta as a heterogeneous chemoselective catalyst for Baeyer-Villiger oxidations. Nature 2001, 412, 423–425. [Google Scholar] [CrossRef]
- Dijkmans, J.; Schutyser, W.; Dusselier, M.; Sels, B.F. Snβ-zeolite catalyzed oxido-reduction cascade chemistry with biomass-derived molecules. Chem. Commun. 2016, 52, 6712–6715. [Google Scholar] [CrossRef]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C6 carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Takagaki, A.; Ohara, M.; Nishimura, S.; Ebitani, K. A one-pot reaction for biorefinery: Combination of solid acid and base catalysts for direct production of 5-hydroxymethylfurfural from saccharides. Chem. Commun. 2009, 41, 6276–6278. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Takagaki, A.; Nishimura, S.; Ebitani, K. Syntheses of 5-hydroxymethylfurfural and levoglucosan by selective dehydration of glucose using solid acid and base catalysts. Appl. Catal. A 2010, 383, 149–155. [Google Scholar] [CrossRef]
- Nikolla, E.; Román-Leshkov, Y.; Moliner, M.; Davis, M.E. “One-Pot” synthesis of 5-(hydroxymethyl)furfural from carbohydrates using tin-Beta zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Dumesic, J.A. Solvent effects on fructose dehydration to 5-hydroxymethylfurfural in biphasic systems saturated with inorganic salts. Top Catal. 2009, 52, 297–303. [Google Scholar] [CrossRef]
- Patil, S.K.R.; Lund, C.R.F. Formation and growth of humins via aldol addition and condensation during acid-catalyzed conversion of 5-hydroxymethylfurfural. Energy Fuels 2011, 25, 4745–4755. [Google Scholar] [CrossRef]
- Candu, N.; El Fergani, M.; Verziu, M.; Cojocaru, B.; Jurca, B.; Apostol, N.; Teodorescu, C.; Parvulescu, V.I.; Coman, S.M. Efficient glucose dehydration to HMF onto Nb-BEA catalysts. Catal. Today 2018, 325, 109–116. [Google Scholar] [CrossRef]
- Na, K.; Choi, M.; Ryoo, R. Cyclic diquaternary ammoniums for nanocrystalline BEA, MTW and MFI zeolites with intercrystalline mesoporosity. J. Mater. Chem. 2009, 19, 6713–6719. [Google Scholar] [CrossRef]
- Rodaum, C.; Thivasasith, A.; Suttipat, D.; Witoon, T.; Pengpanich, S.; Wattanakit, C. Modified acid-base ZSM-5 derived from core-shell ZSM-5@aqueous miscible organic-layered double hydroxides for catalytic cracking of n-pentane to light olefins. ChemCatChem 2020, 12, 4288–4296. [Google Scholar] [CrossRef]
- Dijkmans, J.; Gabriëls, D.; Dusselier, M.; de Clippel, F.; Vanelderen, P.; Houthoofd, K.; Malfliet, A.; Pontikes, Y.; Sels, B.F. Productive sugar isomerization with highly active Sn in dealuminated β zeolites. Green Chem. 2013, 15, 2777–2785. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Li, X.; Ren, J.; Zhou, L.; Lu, T.; Su, Y. Synthesis of Sn-containing nanosized Beta zeolite as efficient catalyst for transformation of glucose to methyl lactate. ACS Sustain. Chem. Eng. 2018, 6, 8256–8265. [Google Scholar] [CrossRef]
- Van der Graaff, W.N.P.; Tempelman, C.H.L.; Pidko, E.A.; Hensen, E.J.M. Influence of pore topology on synthesis and reactivity of Sn-modified zeolite catalysts for carbohydrate conversions. Catal. Sci. Tech. 2017, 7, 3151–3162. [Google Scholar] [CrossRef]
- Jiao, L.; Sun, S.; Meng, X.; Ji, P. Sn-based porous coordination polymer synthesized with two ligands for tandem catalysis producing 5-hydroxymethylfurfural. Catalysts 2019, 739, 1–13. [Google Scholar] [CrossRef]
- Dai, W.; Wang, C.; Tang, B.; Wu, G.; Guan, N.; Xie, Z.; Hunger, M.; Li, L. Lewis acid catalysis confined in zeolite cages as a strategy for sustainable heterogeneous hydration of epoxides. ACS Catal. 2016, 6, 2955–2964. [Google Scholar] [CrossRef]
- Liu, M.; Jia, S.; Li, C.; Zhang, A.; Song, C.; Guo, X. Facile preparation of Sn-β zeolites by post-synthesis (isomorphous substitution) method for isomerization of glucose to fructose. Chin. J. Catal. 2014, 35, 723–732. [Google Scholar] [CrossRef]
- Van der Graaff, W.N.P.; Li, G.; Mezari, B.; Pidko, E.A.; Hensen, E.J.M. Synthesis of Sn-Beta with exclusive and high framework Sn content. ChemCatChem 2015, 7, 1152–1160. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Ryoo, R. The synthesis of a hierarchically porous BEA zeolitevia pseudomorphic crystallization. ChemComm. 2009, 20, 2845–2847. [Google Scholar]
- Saenluang, K.; Imyen, T.; Wannapakdee, W.; Suttipat, D.; Dugkhuntod, P.; Ketkaew, M.; Thivasasith, A.; Wattanakit, C. Hierarchical nanospherical ZSM-5 nanosheets with uniform Al distribution for alkylation of benzene with ethanol. ACS Appl. Nano Mater. 2020, 3, 3252–3263. [Google Scholar] [CrossRef]

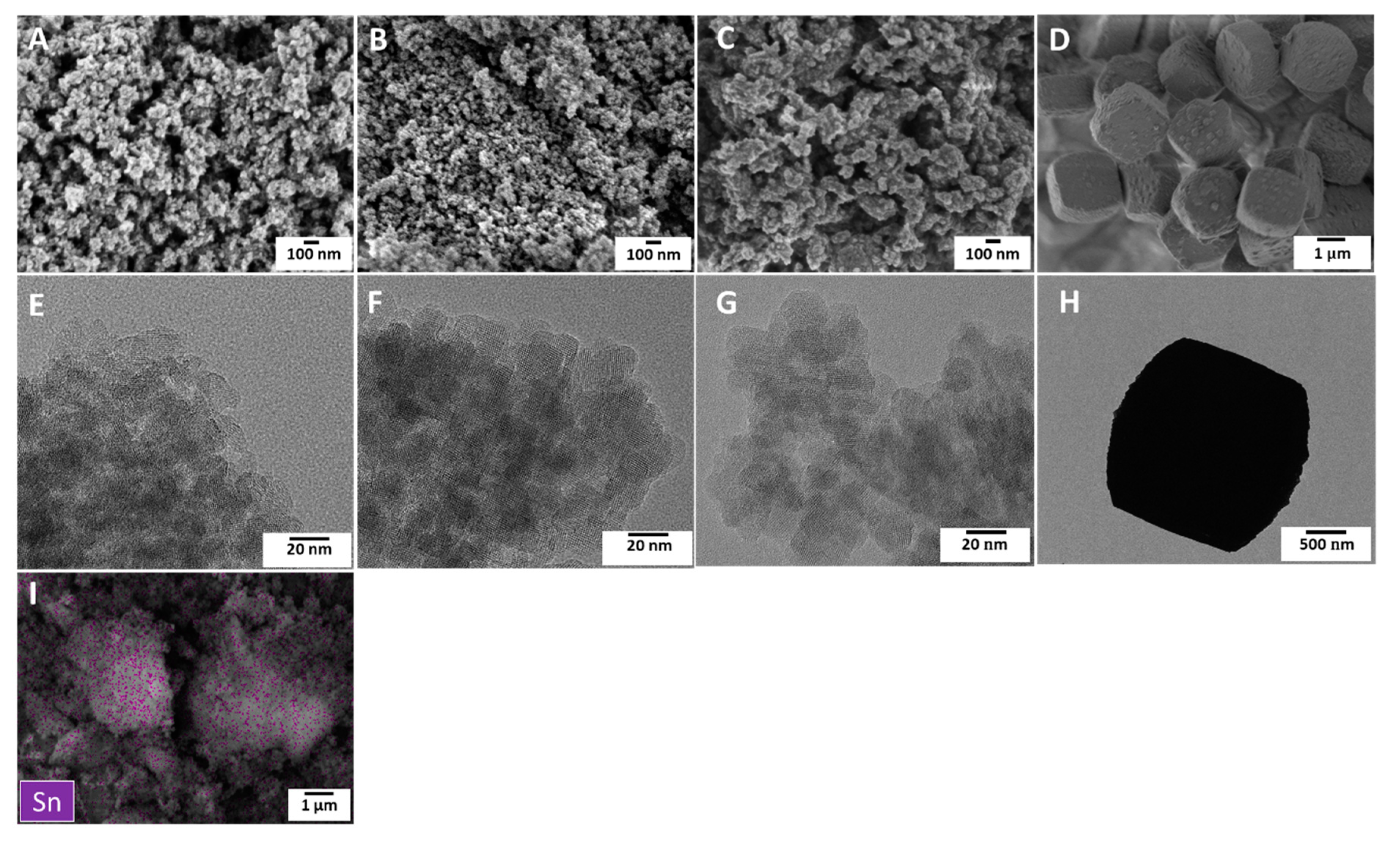
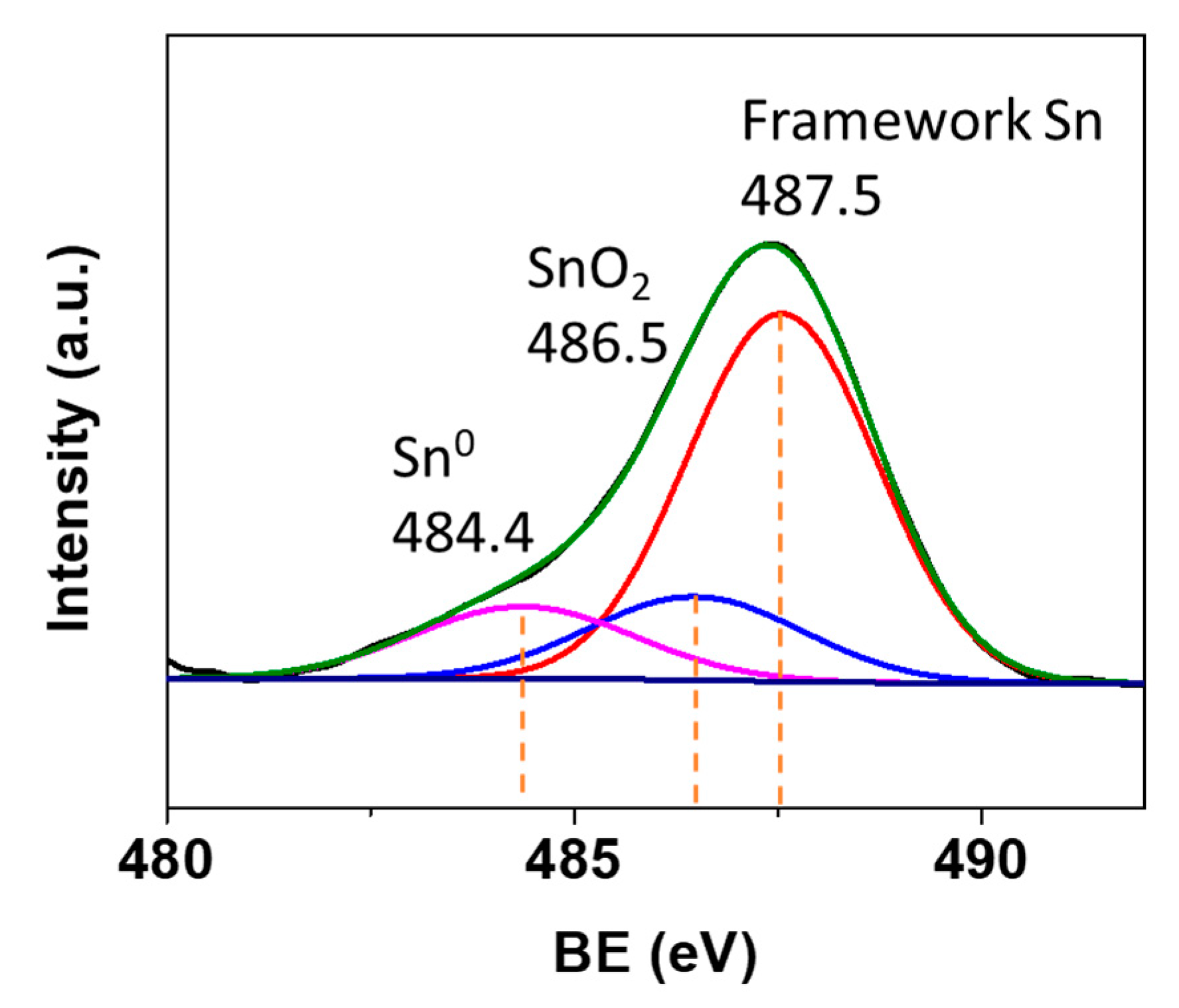
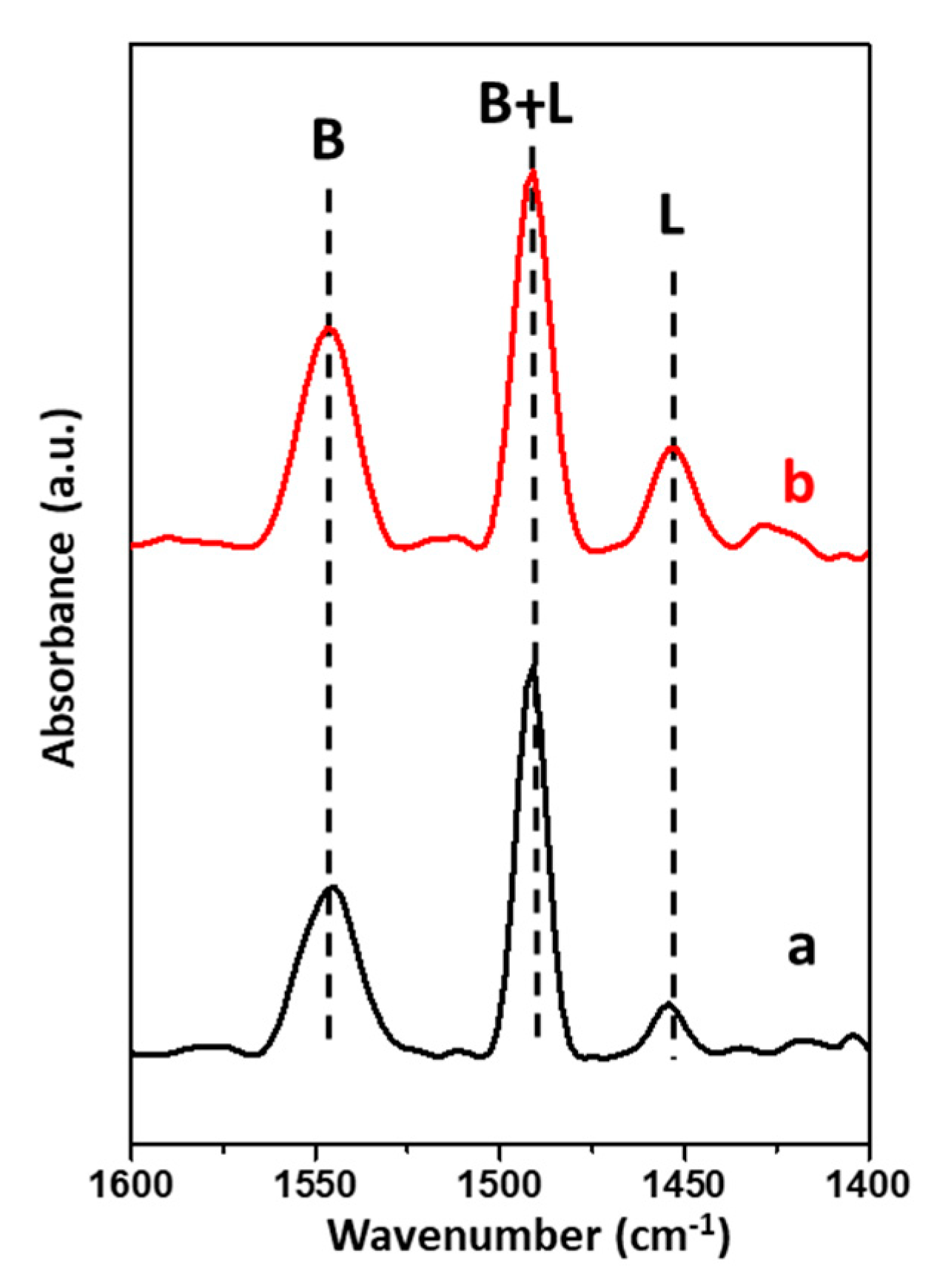
| Sample | SBET a (m2/g) | Smicro b (m2/g) | Sext c (m2/g) | Vtotal d (cm3/g) | Vmicro e (cm3/g) | Vext f (cm3/g) | Vext/Vtotal g |
|---|---|---|---|---|---|---|---|
| Bare Beta | 711 | 670 | 41 | 1.49 | 0.16 | 1.33 | 0.89 |
| 0.4 wt% Sn-Beta | 492 | 470 | 22 | 2.05 | 0.10 | 1.95 | 0.95 |
| Samples/Tmax (°C) | Acid Site Density (mmol g−1) a | ||
|---|---|---|---|
| Weak (180 °C) | Strong (300–550 °C) | Total | |
| Bare Beta | 0.318 | 0.441 | 0.759 |
| 0.4 wt% Sn-Beta | 0.237 | 0.424 | 0.661 |
| Commercial Beta (Beta-COM) | 0.340 | 0.449 | 0.789 |
| Conventional ZSM-5 (ZSM-5-CON) | 0.440 | 0.423 | 0.863 |
| Samples | Time (h) | T (°C) | Conversion (%) | Product Selectivity (%) | Yield (%) g | MB (%) h | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glu a | Fru b | Lac c | FA d | LA e | HMF f | |||||
| Bare Beta | 48 | 120 | 61.1 | 36.1 | 16.4 | 13.9 | 12.0 | 21.6 | 13.2 | 86 |
| 0.7 wt% Sn-Beta | 48 | 120 | 73.8 | 33.5 | 6.2 | 5.8 | 25.4 | 29.1 | 21.5 | 68 |
| 0.4 wt% Sn-Beta | 48 | 120 | 72.0 | 37.9 | 5.1 | 5.6 | 24.9 | 26.5 | 19.1 | 74 |
| 0.4 wt% Sn-Beta | 24 | 120 | 57.5 | 38.8 | 4.8 | 13.7 | 16.5 | 26.2 | 15.1 | 82 |
| 0.4 wt% Sn-Beta | 24 | 140 | 94.1 | 0.7 | 16.4 | 42.9 | 40 | 0.0 | 0.0 | 67 |
| Samples | Phase a | Time (h) | T (°C) | Conversion (%) | Product Selectivity (%) | Yield (%) h | MB (%) i | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glu b | Fru c | Lac d | FA e | LA f | HMF g | ||||||
| 0.4 wt% Sn-Beta | aq. | 24 | 120 | 57.5 | 38.8 | 4.8 | 13.7 | 16.5 | 26.2 | 15.1 | 82 |
| 0.4 wt% Sn-Beta | bi | 24 | 120 | 98.4 | 5.9 | 42.0 | 8.8 | 0.0 | 42.7 | 42.0 | 61 |
| 0.4 wt% Sn-Beta | bi. | 4 | 120 | 77.3 | 24.3 | 36.3 | 6.6 | 0.5 | 32.3 | 25.0 | 81 |
| 0.4 wt% Sn-Beta | bi. | 24 | 100 | 80.3 | 22.5 | 31.2 | 9.8 | 0.4 | 36.1 | 29.0 | 68 |
| 0.4 wt% Sn-Beta | bi. | 4 | 100 | 30.1 | 75.3 | 8.2 | 3.1 | 0 | 13.4 | 4.1 | 93 |
| Beta-COM | bi. | 24 | 100 | 44.9 | 45.8 | 19.3 | 4.6 | 0 | 30.3 | 13.6 | 89 |
| ZSM-5-CON | bi. | 24 | 100 | 42.7 | 45.9 | 16.2 | 4.3 | 0 | 33.6 | 14.4 | 91 |
| ZSM-5-COM | bi | 24 | 100 | 59.4 | 40.5 | 21.4 | 9.7 | 0.3 | 28.1 | 16.7 | 81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saenluang, K.; Thivasasith, A.; Dugkhuntod, P.; Pornsetmetakul, P.; Salakhum, S.; Namuangruk, S.; Wattanakit, C. In Situ Synthesis of Sn-Beta Zeolite Nanocrystals for Glucose to Hydroxymethylfurfural (HMF). Catalysts 2020, 10, 1249. https://doi.org/10.3390/catal10111249
Saenluang K, Thivasasith A, Dugkhuntod P, Pornsetmetakul P, Salakhum S, Namuangruk S, Wattanakit C. In Situ Synthesis of Sn-Beta Zeolite Nanocrystals for Glucose to Hydroxymethylfurfural (HMF). Catalysts. 2020; 10(11):1249. https://doi.org/10.3390/catal10111249
Chicago/Turabian StyleSaenluang, Kachaporn, Anawat Thivasasith, Pannida Dugkhuntod, Peerapol Pornsetmetakul, Saros Salakhum, Supawadee Namuangruk, and Chularat Wattanakit. 2020. "In Situ Synthesis of Sn-Beta Zeolite Nanocrystals for Glucose to Hydroxymethylfurfural (HMF)" Catalysts 10, no. 11: 1249. https://doi.org/10.3390/catal10111249
APA StyleSaenluang, K., Thivasasith, A., Dugkhuntod, P., Pornsetmetakul, P., Salakhum, S., Namuangruk, S., & Wattanakit, C. (2020). In Situ Synthesis of Sn-Beta Zeolite Nanocrystals for Glucose to Hydroxymethylfurfural (HMF). Catalysts, 10(11), 1249. https://doi.org/10.3390/catal10111249





