Self-Regeneration Effect of Three-Way Catalysts during Thermal Aging Procedure
Abstract
1. Introduction
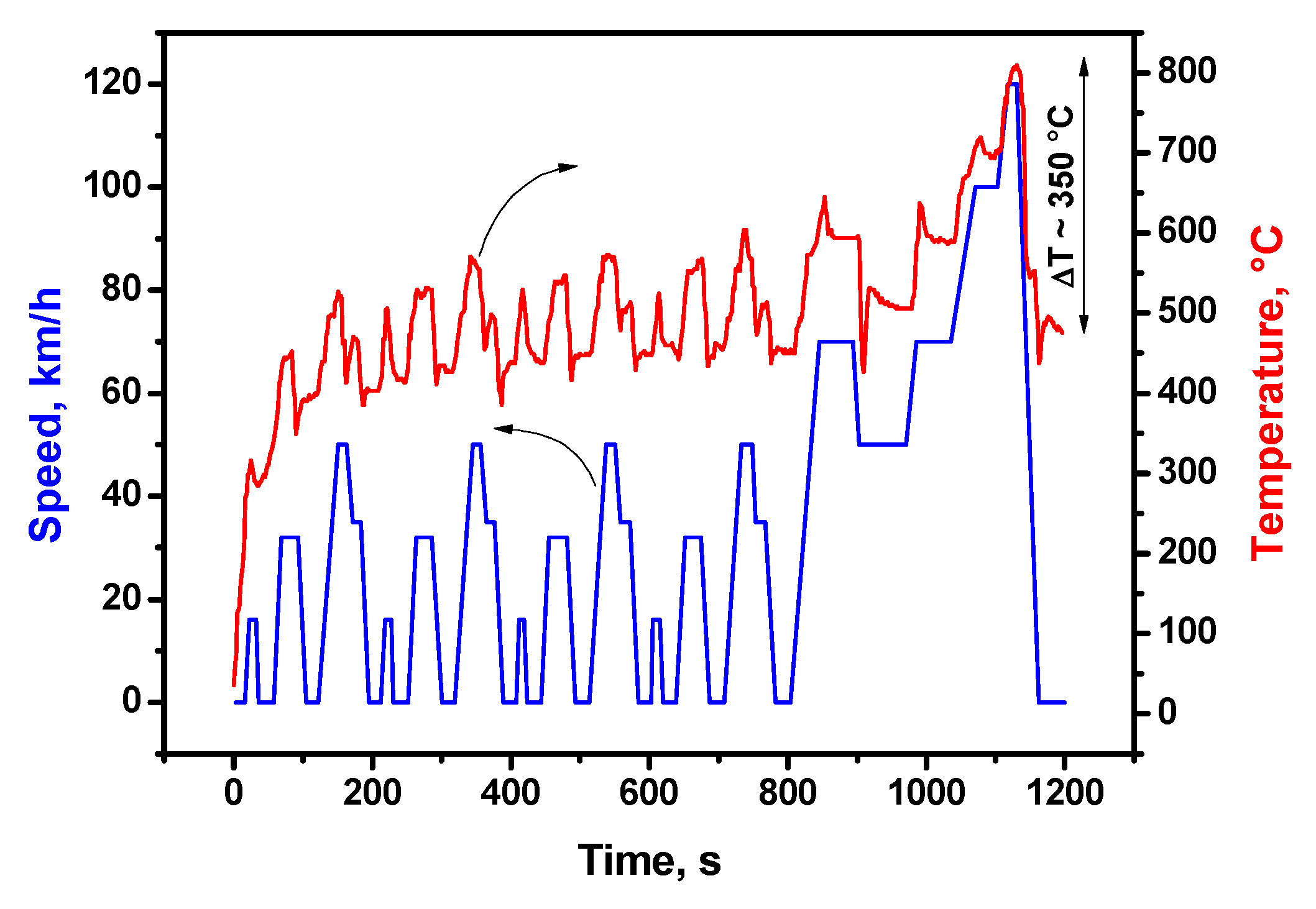
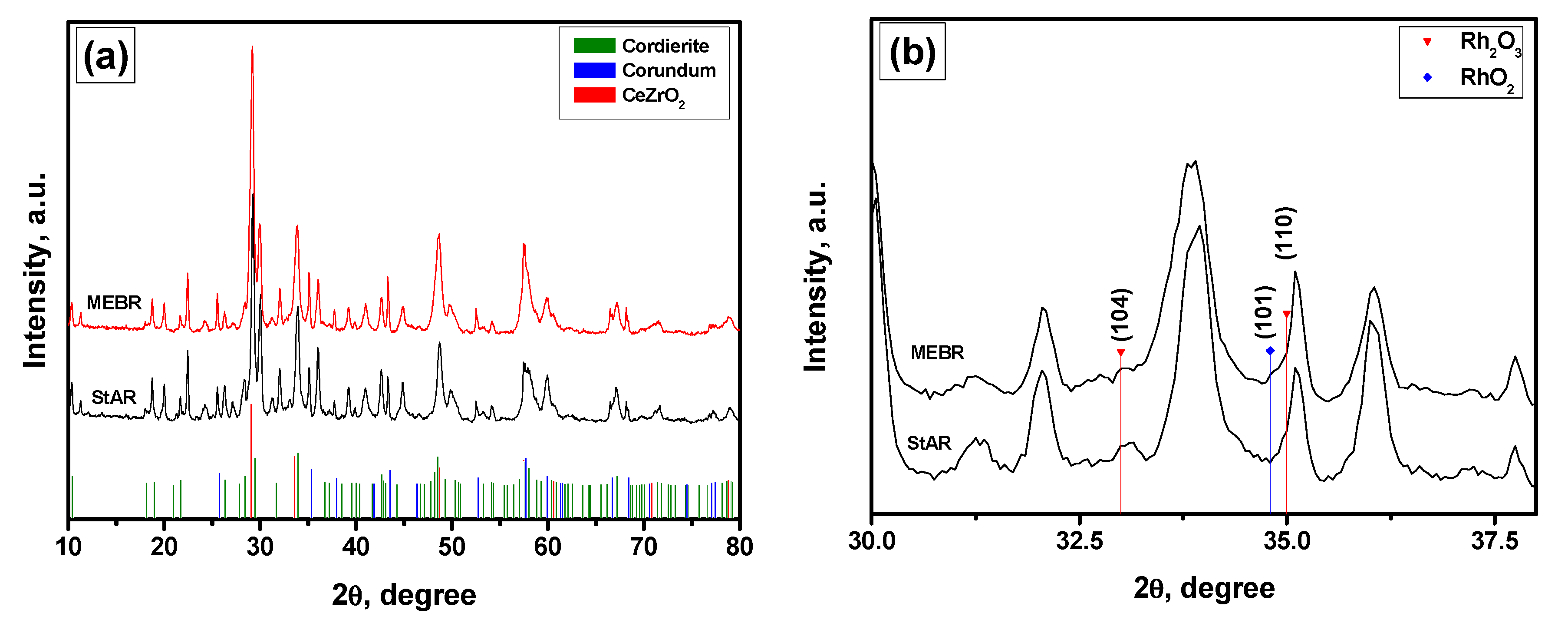
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of the Catalysts
3.2. Characterization of the Catalysts
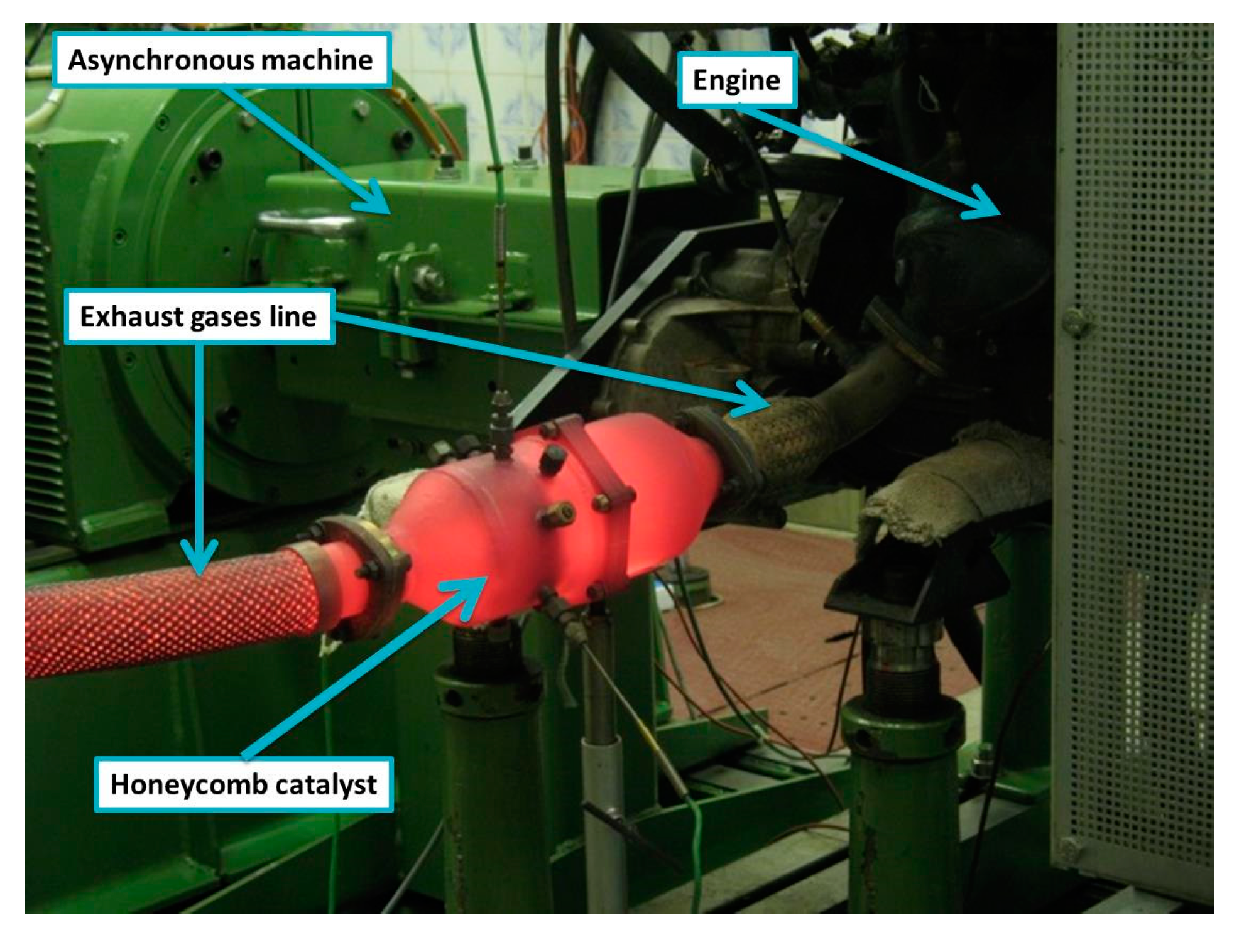
3.3. Testing and Aging of the Catalysts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heck, R.M.; Farrauto, R.J.; Gulati, S. Catalytic Air Pollution Control; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Twigg, M.V. Catalytic control of emissions from cars. Catal. Today 2011, 163, 33–41. [Google Scholar] [CrossRef]
- Shelef, M.; Graham, G.W. Why Rhodium in Automotive Three-Way Catalysts? Catal. Rev. 1994, 36, 433–457. [Google Scholar] [CrossRef]
- González-Velasco, J.R.; Botas, J.; Ferret, R.; González-Marcos, M.P.; Marc, J.-L.; Gutiérrez-Ortiz, J.I. Thermal aging of Pd/Pt/Rh automotive catalysts under a cycled oxidizing–reducing environment. Catal. Today 2000, 59, 395–402. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Neto, A.A.; Cardoso, M.J.B.; Zotin, F.M.Z. Commercial automotive catalysts: Chemical, structural and catalytic evaluation, before and after aging. Catal. Today 2008, 574–581. [Google Scholar] [CrossRef]
- Fathali, A.; Olsson, L.; Ekström, F.; Laurell, M.; Andersson, B. Hydrothermal Aging-Induced Changes in Washcoats of Commercial Three-Way Catalysts. Top. Catal. 2013, 56, 323–328. [Google Scholar] [CrossRef]
- Kang, S.B.; Han, S.J.; Nam, S.B.; Nam, I.-S.; Cho, B.K.; Kim, C.H.; Oh, S.H. Effect of Aging Atmosphere on Thermal Sintering of Modern Commercial TWCs. Top. Catal. 2013, 56, 298–305. [Google Scholar] [CrossRef]
- Ramanathan, K.; Oh, S.H. Modeling and analysis of rapid catalyst aging cycles. Chem. Eng. Res. Des. 2014, 92, 350–361. [Google Scholar] [CrossRef]
- Lupescu, J.A.; Schwank, J.W.; Dahlberg, K.A.; Seo, C.Y.; Fisher, G.B.; Peczonczyk, S.L.; Rhodes, K.; Jagner, M.J.; Haack, L.P. Pd model catalysts: Effect of aging environment and lean redispersion. Appl. Catal. B Environ. 2016, 183, 343–360. [Google Scholar] [CrossRef]
- Lupescu, J.A.; Schwank, J.W.; Fisher, G.B.; Chen, X.; Peczonczyk, S.L.; Drews, A.R. Pd model catalysts: Effect of aging duration on lean redispersion. Appl. Catal. B Environ. 2016, 185, 189–202. [Google Scholar] [CrossRef]
- Burch, R.; Loader, P.; Cruise, N. An investigation of the deactivation of Rh/alumina catalysts under strong oxidising conditions. Appl. Catal. A Gen. 1996, 147, 375–394. [Google Scholar] [CrossRef]
- Angelidis, T.; Koutlemani, M.; Sklavounos, S.; Lioutas, C.; Voulgaropoulos, A.; Papadakis, V.; Emons, H. Causes of deactivation and an effort to regenerate a commercial spent three-way catalyst. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 155–164. [Google Scholar]
- Birgersson, H.; Boutonnet, M.; Järås, S.; Eriksson, L. Deactivation and Regeneration of Spent Three-Way Automotive Exhaust Gas Catalysts (TWC). Top. Catal. 2004, 433–437. [Google Scholar] [CrossRef]
- Lassi, U.; Hietikko, M.; Rahkamaa-Tolonen, K.; Kallinen, K.; Savimäki, A.; Härkönen, M.; Laitinen, R.; Keiski, R.L. Deactivation Correlations over Pd/Rh Monoliths: The Role of Gas Phase Composition. Top. Catal. 2004, 30, 457–462. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Scofield, C.F.; Neto, A.A.; Cardoso, M.J.B.; Zotin, F.M.Z. Thermal deactivation of Pt/Rh commercial automotive catalysts. Chem. Eng. J. 2010, 160, 85–92. [Google Scholar] [CrossRef]
- He, B.J.-J.; Wang, C.-X.; Zheng, T.-T.; Zhao, Y.-K. Thermally Induced Deactivation and the Corresponding Strategies for Improving Durability in Automotive Three-Way Catalysts. Johns. Matthey Technol. Rev. 2016, 60, 196–203. [Google Scholar] [CrossRef]
- Zheng, T.; He, J.; Zhao, Y.; Xia, W.; He, J. Precious metal-support interaction in automotive exhaust catalysts. J. Rare Earths 2014, 32, 97–107. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Lampert, J.K.; Hobson, M.C.; Waterman, E.M. Thermal decomposition and reformation of PdO catalysts; support effects. Appl. Catal. B Environ. 1995, 6, 263–270. [Google Scholar] [CrossRef]
- Vedyagin, A.A.; Volodin, A.M.; Stoyanovskii, V.O.; Mishakov, I.V.; Medvedev, D.A.; Noskov, A.S. Characterization of active sites of Pd/Al2O3 model catalysts with low Pd content by luminescence, EPR and ethane hydrogenolysis. Appl. Catal. B Environ. 2011, 103, 397–403. [Google Scholar] [CrossRef]
- Lieske, H.; Voelter, J. Palladium redispersion by spreading of palladium(II) oxide in oxygen treated palladium/alumina. J. Phys. Chem. 1985, 89, 1841–1842. [Google Scholar] [CrossRef]
- Morgan, K.; Goguet, A.; Hardacre, C. Metal Redispersion Strategies for Recycling of Supported Metal Catalysts: A Perspective. ACS Catal. 2015, 5, 3430–3445. [Google Scholar] [CrossRef]
- Lupescu, J.A.; Schwank, J.W.; Fisher, G.B.; Hangas, J.; Peczonczyk, S.L.; Paxton, W.A. Pd model catalysts: Effect of air pulse length during redox aging on Pd redispersion. Appl. Catal. B Environ. 2018, 223, 76–90. [Google Scholar] [CrossRef]
- Seo, C.Y.; Chena, X.; Sunb, K.; Allard, L.F.; Fisher, G.B.; Schwank, J.W. Palladium redispersion at high temperature within the Pd@SiO2 core@shell structure. Catal. Commun. 2018, 108, 73–76. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Pulvermacher, B. Kinetics of crystallite sintering during heat treatment of supported metal catalysts. AIChE J. 1973, 19, 356–364. [Google Scholar] [CrossRef]
- Bett, J. Crystallite growth of platinum dispersed on graphitized carbon black. J. Catal. 1974, 35, 307–316. [Google Scholar] [CrossRef]
- Flynn, P. A model of supported metal catalyst sintering I. Development of model. J. Catal. 1974, 34, 390–399. [Google Scholar] [CrossRef]
- Wanke, S.E.; Flynn, P.C. The Sintering of Supported Metal Catalysts. Catal. Rev. 1975, 12, 93–135. [Google Scholar] [CrossRef]
- Li, L.; Plessow, P.N.; Rieger, M.; Sauer, S.; Sánchez-Carrera, R.S.; Schaefer, A.; Abild-Pedersen, F. Modeling the Migration of Platinum Nanoparticles on Surfaces Using a Kinetic Monte Carlo Approach. J. Phys. Chem. C 2017, 121, 4261–4269. [Google Scholar] [CrossRef]
- Bourdon, J. (Ed.) Growth and Properties of Metal Clusters: Applications to Catalysis and the Photographic Process—International Conference Proceedings; Elsevier: Amsterdam, The Netherlands, 1980; Volume 4, p. 15. [Google Scholar]
- Zhou, Y.; Nakashima, M.; White, J.M. Interaction of platinum with ceria and silica. J. Phys. Chem. 1988, 92, 812–818. [Google Scholar] [CrossRef]
- Stoyanovskii, V.O.; Vedyagin, A.A.; Aleshina, G.I.; Volodin, A.M.; Noskov, A.S. Characterization of Rh/Al2O3 catalysts after calcination at high temperatures under oxidizing conditions by luminescence spectroscopy and catalytic hydrogenolysis. Appl. Catal. B Environ. 2009, 90, 141–146. [Google Scholar] [CrossRef]
- Stoyanovskii, V.O.; Vedyagin, A.A.; Volodin, A.M.; Kenzhin, R.M.; Shubin, Y.V.; Plusnin, P.E.; Mishakov, I.V. Peculiarity of Rh bulk diffusion in La-doped alumina and its impact on CO oxidation over Rh/Al2O3. Catal. Commun. 2017, 97, 18–22. [Google Scholar] [CrossRef]
- Stoyanovskii, V.O.; Vedyagin, A.A.; Volodin, A.M.; Kenzhin, R.M.; Bespalko, Y.N.; Plusnin, P.E.; Shubin, Y.V. Optical spectroscopy of Rh3+ ions in the lanthanum-aluminum oxide systems. J. Lumin. 2018, 204, 609–617. [Google Scholar] [CrossRef]
- Alikin, E.A.; Vedyagin, A.A. High Temperature Interaction of Rhodium with Oxygen Storage Component in Three-Way Catalysts. Top. Catal. 2016, 59, 1033–1038. [Google Scholar] [CrossRef]
- Kenzhin, R.M.; Alikin, E.A.; Denisov, S.P.; Vedyagin, A.A. Study on Thermal Stability of Ceria-Supported Rhodium Catalysts. Mater. Sci. Forum 2019, 950, 190–194. [Google Scholar] [CrossRef]
- Alikin, E.A.; Denisov, S.P.; Vedyagin, A.A. Partial Regeneration of Model TWC after High-Temperature Aging on Engine Bench. Top. Catal. 2018, 62, 324–330. [Google Scholar] [CrossRef]
- Zou, R.; Wen, S.; Zhang, L.; Liu, L.; Yue, D. Preparation of Rh–SiO2 fiber catalyst with superior activity and reusability by electrospinning. RSC Adv. 2015, 5, 99884–99891. [Google Scholar] [CrossRef]
- Kato, K.; Abe, Y.; Kawamura, M.; Sasaki, K. Preparation of RhO2Thin Films by Reactive Sputtering and Their Characterizations. Jpn. J. Appl. Phys. 2001, 40, 2399–2402. [Google Scholar] [CrossRef]
- Satsuma, A.; Osaki, K.; Yanagihara, M.; Ohyama, J.; Shimizu, K. Low temperature combustion over supported Pd catalysts—Strategy for catalyst design. Catal. Today 2015, 258, 83–89. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Q.; Li, G.; Zhou, R. Effect of rare earth (La, Nd, Pr, Sm and Y) on the performance of Pd/Ce0.67Zr0.33MO2−δ three-way catalysts. J. Environ. Chem. Eng. 2013, 1, 534–543. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, M.; Huang, X.; Hou, Y.; Yue, M.; Zhong, Q.; Zhang, Y. The effect of hydrogen peroxide on properties of Ce0.35Zr0.55La0.055Pr0.045O2 oxides and the catalytic performance used on Pd supported three-way catalyst. J. Rare Earths 2017, 35, 1092–1101. [Google Scholar] [CrossRef]
- Zheng, Q.; Farrauto, R.J.; Deeba, M.; Valsamakis, I. Part I: A Comparative Thermal Aging Study on the Regenerability of Rh/Al2O3 and Rh/CexOy-ZrO2 as Model Catalysts for Automotive Three Way Catalysts. Catalysts 2015, 5, 1770–1796. [Google Scholar] [CrossRef]
- Porsin, A.V.; Denisov, S.P.; Danchenko, N.M.; Alikin, E.A.; Smirnov, M.Y.; Bukhtiyarov, V.I.; Bol’shakov, A.M. Migration of supported platinum metals in catalysts for automotive exhaust purification. In Proceedings of the III International Conference “Catalysis: Fundamentals and Application”, Novosibirsk, Russia, 4–8 July 2007; pp. 557–559. [Google Scholar]
- Vedyagin, A.A.; Kenzhin, R.M.; Tashlanov, M.Y.; Alikin, E.A.; Stoyanovskii, V.O.; Plyusnin, P.E.; Shubin, Y.V.; Mishakov, I.V.; Smirnov, M.Y.; Kalinkin, A.V.; et al. Effect of La Addition on the Performance of Three-Way Catalysts Containing Palladium and Rhodium. Top. Catal. 2020, 63, 152–165. [Google Scholar] [CrossRef]
- Nefedov, V.I. X-Ray Photoelectron Spectroscopy of Chemical Compounds: Handbook; Khimiya: Moscow, Russia, 1984. [Google Scholar]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]



| Aging Conditions | T50, °C | Conversion at 400 °C, % | ||||
|---|---|---|---|---|---|---|
| THC | CO | NOx | THC | CO | NOx | |
| Standard aging regime (StAR) | 336 | 290 | 280 | 66 | 99 | 89 |
| Model engine braking regime (MEBR) | 313 | 285 | 276 | 97 | 98 | 100 |
| Aging Conditions | Rh3d5/2 | [Rh]/[Al] | [C]/[Al] | |
|---|---|---|---|---|
| Rh2O3 | RhO2 | |||
| Standard aging regime (StAR) | 308.3 | - | 0.0050 | 0.927 |
| Model engine braking regime (MEBR) | 308.4 | 310.5 | 0.0072 | 0.742 |
| Sample | Aging Conditions | T50, °C | Conversion at 400 °C, % | ||||
|---|---|---|---|---|---|---|---|
| THC | CO | NOx | THC | CO | NOx | ||
| Rh/Al2O3 | StAR | 291 | 280 | 272 | 89 | 95 | 88 |
| Rh/Al2O3 | MEBR | 361 | 326 | 312 | 69 | 93 | 90 |
| Rh/CeZrO2 | StAR | 356 | 326 | 306 | 68 | 90 | 96 |
| Rh/CeZrO2 | MEBR | 305 | 288 | 278 | 80 | 80 | 97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alikin, E.A.; Denisov, S.P.; Bubnov, K.V.; Vedyagin, A.A. Self-Regeneration Effect of Three-Way Catalysts during Thermal Aging Procedure. Catalysts 2020, 10, 1257. https://doi.org/10.3390/catal10111257
Alikin EA, Denisov SP, Bubnov KV, Vedyagin AA. Self-Regeneration Effect of Three-Way Catalysts during Thermal Aging Procedure. Catalysts. 2020; 10(11):1257. https://doi.org/10.3390/catal10111257
Chicago/Turabian StyleAlikin, Evgeny A., Sergey P. Denisov, Konstantin V. Bubnov, and Aleksey A. Vedyagin. 2020. "Self-Regeneration Effect of Three-Way Catalysts during Thermal Aging Procedure" Catalysts 10, no. 11: 1257. https://doi.org/10.3390/catal10111257
APA StyleAlikin, E. A., Denisov, S. P., Bubnov, K. V., & Vedyagin, A. A. (2020). Self-Regeneration Effect of Three-Way Catalysts during Thermal Aging Procedure. Catalysts, 10(11), 1257. https://doi.org/10.3390/catal10111257







