Oxidative Hydroxylation of Aryl Boronic Acid Catalyzed by Co-porphyrin Complexes via Blue-Light Irradiation
Abstract
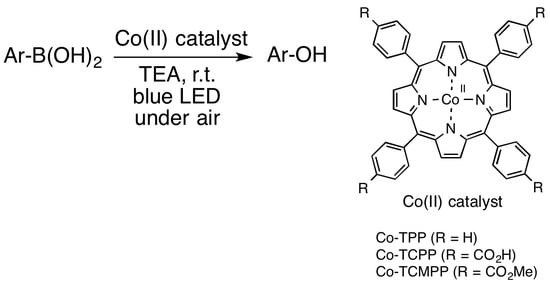
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Meso-Tetraphenylporphyrin H2(TPP)
3.2. Preparation of 5,10,15,20-Tetrakis[4-(methoxycarbonyl)phenyl]porphyrin H2(TMCPP)
3.3. Preparation of 5,10,15,20-Tetrakis(4-carboxyphenyl)porphyrin H2(TCPP)
3.4. Metallation of Porphyrins
3.5. Preparation of Co(II)TCPP Immobilized on Chitosan (CTS)
3.6. Preparation of Polyaniline Emeraldine Hydrochloride
3.7. General Method for Hydroxylation Reaction
3.7.1. Hydroxylation Reaction Catalyzed by Co(II)TPP, Co(II)TMCPP, Co(II)TCPP
3.7.2. Hydroxylation Reaction Catalysed by Co(II)TCPP Supported on Polyaniline
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeyakumar, K.; Chand, D.K. Selective oxidation of sulfides to sulfoxides and sulfones at room temperature using H2O2 and a Mo (VI) salt as catalyst. Tetrahedron Lett. 2006, 47, 4573–4576. [Google Scholar] [CrossRef]
- Molloy, J.J.; Clohessy, T.A.; Irving, C.; Anderson, N.A.; Lloyd-Jones, G.C.; Watson, A.J.B. Chemoselective oxidation of aryl organoboron systems enabled by boronic acid-selective phase transfer. Chem. Sci. 2017, 8, 1551–1559. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.; Pan, X.; Leng, W.; Chen, J.; He, H. The highly efficient air oxidation of aryl and alkyl boronic acids by a microwave-assisted protocol under transition metal-free conditions. Green Chem. 2019, 21, 4614–4618. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, R.; Falck, J.R. Mild and rapid hydroxylation of aryl/heteroaryl boronic acids and boronate esters with N-oxides. Org. Lett. 2012, 14, 3494–3497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goswami, A.; Chatterjee, N. Organic hypervalent iodine (III) catalyzed ipso-hydroxylation of aryl-and alkylboronic acids/esters. Tetrahedron Lett. 2015, 56, 1524–1527. [Google Scholar]
- Romero, N.A.; Nicewicz, D.A. Organic photoredox catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef]
- Huang, Z.; Gu, Y.; Liu, X.; Zhang, L.; Cheng, Z.; Zhu, X. Metal-Free Atom Transfer Radical Polymerization of Methyl Methacrylate with ppm Level of Organic Photocatalyst. Macromol. Rapid Commun. 2017, 38, 1600461. [Google Scholar] [CrossRef] [PubMed]
- Pitre, S.P.; McTiernan, C.D.; Ismaili, H.; Scaiano, J.C. Mechanistic insights and kinetic analysis for the oxidative hydroxylation of arylboronic acids by visible light photoredox catalysis: A metal-free alternative. J. Am. Chem. Soc. 2013, 135, 13286–13289. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Ishii, C.; Kimura, M. Pd-Catalyzed Dehydrogenative Oxidation of Alcohols to Functionalized Molecules. Org. Process Res. Dev. 2019, 23, 1709–1717. [Google Scholar] [CrossRef]
- Xie, H.-Y.; Han, L.-S.; Huang, S.; Lei, X.; Cheng, Y.; Zhao, W.; Sun, H.; Wen, X.; Xu, Q.-L. N-Substituted 3 (10 H)-Acridones as Visible-Light, Water-Soluble Photocatalysts: Aerobic Oxidative Hydroxylation of Arylboronic Acids. J. Org. Chem. 2017, 82, 5236–5241. [Google Scholar] [CrossRef]
- Gualandi, A.; Savoini, A.; Saporetti, R.; Franchi, P.; Lucarini, M.; Cozzi, P.G. A facile hydroxylation of arylboronic acids mediated by sodium ascorbate. Org. Chem. Front. 2018, 5, 1573–1578. [Google Scholar] [CrossRef]
- Silveira-Dorta, G.; Monzón, D.M.; Crisóstomo, F.P.; Martín, T.; Martín, V.S.; Carrillo, R. Oxidation with air by ascorbate-driven quinone redox cycling. Chem. Commun. 2015, 51, 7027–7030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotoučová, H.; Strnadová, I.; Kovandová, M.; Chudoba, J.; Dvořáková, H.; Cibulka, R. Biomimetic aerobic oxidative hydroxylation of arylboronic acids to phenols catalysed by a flavin derivative. Org. Biomol. Chem. 2014, 12, 2137–2142. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Chen, J.; Liu, X.; Lu, L.; Davis, R.L.; Jørgensen, K.A.; Xiao, W. Highly efficient aerobic oxidative hydroxylation of arylboronic acids: Photoredox catalysis using visible light. Angew. Chem. Int. Ed. 2012, 51, 784–788. [Google Scholar] [CrossRef]
- Castro-Godoy, W.D.; Schmidt, L.C.; Argüello, J.E. A Green Alternative for the Conversion of Arylboronic Acids/Esters into Phenols Promoted by a Reducing Agent, Sodium Sulfite. Eur. J. Org. Chem. 2019, 2019, 3035–3039. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K.; Sritularak, B.; Benchanak, K.; Lipipun, V.; Mathew, J.; Schinazi, R.F. Phenolics with antiviral activity from Millettia erythrocalyx and Artocarpus lakoocha. Nat. Prod. Res. 2005, 19, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- Mulakayala, N.; Kumar, K.M.; Rapolu, R.K.; Kandagatla, B.; Rao, P.; Oruganti, S.; Pal, M. Catalysis by Amberlite IR-120 resin: A rapid and green method for the synthesis of phenols from arylboronic acids under metal, ligand, and base-free conditions. Tetrahedron Lett. 2012, 53, 6004–6007. [Google Scholar] [CrossRef]
- Kim, H.-S.; Joo, S.-R.; Shin, U.S.; Kim, S.-H. Recyclable CNT-chitosan nanohybrid film utilized in copper-catalyzed aerobic ipso-hydroxylation of arylboronic acids in aqueous media. Tetrahedron Lett. 2018, 59, 4597–4601. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Shao, C.; Su, D.; Cheng, G.; Hu, Y. Highly efficient synthesis of phenols by copper-catalyzed oxidative hydroxylation of arylboronic acids at room temperature in water. Org. Lett. 2010, 12, 1964–1967. [Google Scholar] [CrossRef]
- Schwarz, F.P.; Gouterman, M.; Muljiani, Z.; Dolphin, D.H. Energy transfer between covalently linked metal porphyrins. Bioinorg. Chem. 1972, 2, 1–32. [Google Scholar] [CrossRef]
- Hong, Y.H.; Han, J.W.; Jung, J.; Nakagawa, T.; Lee, Y.-M.; Nam, W.; Fukuzumi, S. Photocatalytic Oxygenation Reactions with a Cobalt Porphyrin Complex Using Water as an Oxygen Source and Dioxygen as an Oxidant. J. Am. Chem. Soc. 2019, 141, 9155–9159. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gao, B.; Jiao, W. A novel method for immobilization of Co tetraphenylporphyrins on P (4VP-co-St)/SiO2: Efficient catalysts for aerobic oxidation of ethylbenzenes. Appl. Surf. Sci. 2009, 255, 4109–4113. [Google Scholar] [CrossRef]
- Shen, D.-H.; Ji, L.-T.; Liu, Z.-G.; Sheng, W.-B.; Guo, C.-C. Ethylbenzene oxidation over hybrid metalloporphyrin@ silica nanocomposite microspheres. J. Mol. Catal. A-Chem. 2013, 379, 15–20. [Google Scholar]
- e Silva, R.C.; da Silva, L.O.; de Andrade Bartolomeu, A.; Brocksom, T.J.; de Oliveira, K.T. Recent applications of porphyrins as photocatalysts in organic synthesis: Batch and continuous flow approaches. Beilstein J. Org. Chem. 2020, 16, 917–955. [Google Scholar] [CrossRef]
- Guo, C.-C.; Liu, Q.; Wang, X.-T.; Hu, H.-Y. Selective liquid phase oxidation of toluene with air. Appl. Catal. A Gen. 2005, 282, 55–59. [Google Scholar] [CrossRef]
- Kumar, S.; Silva, J.D.A.E.; Wani, M.Y.; Gil, J.C.; Sobral, A.J. Carbon dioxide capture and conversion by an environmentally friendly chitosan based meso-tetrakis (4-sulfonatophenyl) porphyrin. Carbohydr. Polym. 2017, 175, 575–583. [Google Scholar] [CrossRef]
- Haber, J.; Kłosowski, M.; Połtowicz, J. Co-oxidation of styrene and iso-butyraldehyde in the presence of polyaniline-supported metalloporphyrins. J. Mol. Catal. A Chem. 2003, 201, 167–178. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.; Shi, J.; Zou, X.; Huang, X.; Tahir, H.E. Preparation of conducting polyaniline/protoporphyrin composites and their application for sensing VOCs. Food Chem. 2019, 276, 291–297. [Google Scholar] [CrossRef]
- Gottam, R.; Srinivasan, P.; La, D.D.; Bhosale, S.V. Improving the photocatalytic activity of polyaniline and a porphyrin via oxidation to obtain a salt and a charge-transfer complex. New J. Chem. 2017, 41, 14595–14601. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, C.M.; Li, J.; Lu, J. Electrocatalysis of template-electrosynthesized cobalt− porphyrin/polyaniline nanocomposite for oxygen reduction. J. Phys. Chem. C 2008, 112, 18578–18583. [Google Scholar] [CrossRef]
- Huang, G.; Yuan, R.X.; Peng, Y.; Chen, X.F.; Zhao, S.K.; Wei, S.J.; Guo, W.X.; Chen, X. Oxygen oxidation of ethylbenzene over manganese porphyrin is promoted by the axial nitrogen coordination in powdered chitosan. RSC Adv. 2016, 6, 48571–48579. [Google Scholar] [CrossRef]
- Mo, L.-Q.; Huang, X.-F.; Wang, G.-C.; Huang, G.; Liu, P. Full use of factors promoting catalytic performance of chitosan supported manganese porphyrin. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, G.; Pielichowski, J.; Grzesik, M. Characteristics of polyaniline cobalt supported catalysts for epoxidation reactions. Sci. World J. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Elkhalafy, S.H.; Hassanein, M.T.; Abd-Elal, M.F.; Atia, A.A. Oxidation of azo dye Orange II with hydrogen peroxide catalyzed by 5, 10, 15, 20-tetrakis [4-(diethylmethylammonio) phenyl] porphyrinato-cobalt (II) tetraiodide in aqueous solution. J. Saudi Chem. Soc. 2020, 24, 520–526. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritleng, V.; Sirlin, C.; Pfeffer, M. Ru-, Rh-, and Pd-Catalyzed C−C Bond Formation Involving C−H Activation and Addition on Unsaturated Substrates: Reactions and Mechanistic Aspects. Chem. Rev. 2002, 102, 1731–1770. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Atom efficiency and catalysis in organic synthesis. Pure Appl. Chem. 2000, 72, 1233–1246. [Google Scholar] [CrossRef]
- Serra, V.V.; Andrade, S.M.; Neves, M.G.; Cavaleiro, J.A.S.; Costa, S.M.B. J-aggregate formation in bis-(4-carboxyphenyl) porphyrins in water: pH and counterion dependence. New J. Chem. 2010, 34, 2757–2765. [Google Scholar] [CrossRef]
- Ajit, S.; Palaniappan, S.; Kumar, P.U.; Madhusudhanachary, P. One-pot direct synthesis of fluorescent polyaniline-porphyrin macrospheres from porphyrin. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 884–889. [Google Scholar] [CrossRef]
- Toyao, T.; Ueno, N.; Miyahara, K.; Matsui, Y.; Kim, T.-H.; Horiuchi, Y.; Ikeda, H.; Matsuoka, M. Visible-light, photoredox catalyzed, oxidative hydroxylation of arylboronic acids using a metal–organic framework containing tetrakis (carboxyphenyl) porphyrin groups. Chem. Commun. 2015, 51, 16103–16106. [Google Scholar] [CrossRef]
- Maiti, N.C.; Mazumdar, S.; Periasamy, N. J-and H-aggregates of porphyrin− surfactant complexes: Time-resolved fluorescence and other spectroscopic studies. J. Phys. Chem. B 1998, 102, 1528–1538. [Google Scholar] [CrossRef]
- Abraham, B.; Nieto-Pescador, J.; Gundlach, L. Ultrafast relaxation dynamics of photoexcited zinc-porphyrin: Electronic-vibrational coupling. J. Phys. Chem. Lett. 2016, 7, 3151–3156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, S.; Xu, J.; Boyer, C. Exploiting metalloporphyrins for selective living radical polymerization tunable over visible wavelengths. J. Am. Chem. Soc. 2015, 137, 9174–9185. [Google Scholar] [CrossRef] [Green Version]
- Ishida, Y.; Shimada, T.; Masui, D.; Tachibana, H.; Inoue, H.; Takagi, S. Efficient Excited Energy Transfer Reaction in Clay/Porphyrin Complex toward an Artificial Light-Harvesting System. J. Am. Chem. Soc. 2011, 133, 14280–14286. [Google Scholar] [CrossRef] [PubMed]
- Akins, D.L.; Zhu, H.-R.; Guo, C. Aggregation of Tetraaryl-Substituted Porphyrins in Homogeneous Solution. J. Phys. Chem. 1996, 100, 5420–5425. [Google Scholar] [CrossRef]
- Luo, D.; Huang, Y.; Hong, X.; Chen, D.; Li, G.; Huang, X.; Gao, W.; Liu, M.; Zhou, Y.; Wu, H. Phthalocyanine Zinc-catalyzed Hydroxylation of Aryl Boronic Acids under Visible Light. Adv. Synth. Catal. 2019, 361, 961–964. [Google Scholar] [CrossRef]
- Shin, E.-J.; Joo, S.-R.; Kim, S.-H. Cooperation of biopolymer chitosan with hydrogen peroxide for ipso-hydroxylation of arylboronic acids under green conditions. Tetrahedron Lett. 2019, 60, 1509–1513. [Google Scholar] [CrossRef]
- Jiang, H.; Lykke, L.; Pedersen, S.U.; Xiao, W.-J.; Jørgensen, K.A. A practical electromediated ipso-hydroxylation of aryl and alkyl boronic acids under an air atmosphere. Chem. Commun. 2012, 48, 7203–7205. [Google Scholar] [CrossRef]
- Stejskal, J.; Gilbert, R.G. Polyaniline. Preparation of a conducting polymer (IUPAC technical report). Pure Appl. Chem. 2002, 74, 857–867. [Google Scholar] [CrossRef] [Green Version]
- MacDiarmid, A.G.; Epstein, A.J. Polyaniline: Synthesis, Chemistry and Processing; Pennsylvania Univ. Philadelphia Dept. of Chemistry: Philadelphia, PA, USA, 1992. [Google Scholar]
- Abu-Thabit, N.Y. Chemical oxidative polymerization of polyaniline: A practical approach for preparation of smart conductive textiles. J. Chem. Educ. 2016, 93, 1606–1611. [Google Scholar] [CrossRef]




| Entry | Catalyst | Additives | Solvent | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | none | none | H2O | 12 | 0 |
| 2 | none | H2O2 | CH3CN/H2O | 6 | 18 |
| 3 | Pd(OAc)2 | TEA | CH3CN/H2O | 12 | 0 |
| 4 | H2TPP | TEA | CH3CN/H2O | 12 | 0 |
| 5 | Co-TPP | TEA | CH3CN/H2O | 12 | 40 |
| 6 | Co-TCMPP | TEA | CH3CN/H2O | 12 | 60 |
| 7 | Co-TCPP | TEA | CH3CN/H2O | 12 | 70 |
| 8 | Co-TPP/PANI | TEA | pyridine | 6 | 0 |
| 9 | Co-TPP/PANI | TEA | DMF | 6 | 0 |
| 10 | Co-TPP/PANI | TEA | CH3CN | 6 | 0 |
| 11 | Co-TPP/PANI | TEA | H2O | 6 | 42 |
| 12 | Co-TCPP/PANI | TEA | CH3CN/H2O | 6 | 90 |
| 13 | Co-TCPP/Cts | TEA | CH3CN/H2O | 6 | 88 |
| 14 2 | Co-TCPP/PANI | TEA | CH3CN/H2O | 6 | 0 |
| 15 3 | Co-TCPP/PANI | TEA | CH3CN/H2O | 6 | 0 |
| Entry | Boronic Acid (R1, R2, R3) | Time (h) | Yield (%) |
|---|---|---|---|
| 1 | Phenyl boronic acid (R1, R2, R3 = H) | 6 | 90 |
| 2 | p-Methylphenyl boronic acid (R2 = Me) | 4 | 96 |
| 3 | 2,4,6-Trimethylphenyl boronic acid (R1, R2, R3 = Me) | 6 | 92 |
| 4 | p-Bromophenyl boronic acid (R2 = Br) | 12 | 80 |
| 5 | 1-Naphthylboronic acid | 4 | 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atia, A.A.; Kimura, M. Oxidative Hydroxylation of Aryl Boronic Acid Catalyzed by Co-porphyrin Complexes via Blue-Light Irradiation. Catalysts 2020, 10, 1262. https://doi.org/10.3390/catal10111262
Atia AA, Kimura M. Oxidative Hydroxylation of Aryl Boronic Acid Catalyzed by Co-porphyrin Complexes via Blue-Light Irradiation. Catalysts. 2020; 10(11):1262. https://doi.org/10.3390/catal10111262
Chicago/Turabian StyleAtia, Alaa A., and Masanari Kimura. 2020. "Oxidative Hydroxylation of Aryl Boronic Acid Catalyzed by Co-porphyrin Complexes via Blue-Light Irradiation" Catalysts 10, no. 11: 1262. https://doi.org/10.3390/catal10111262