Impact of Coordination Features of Co(II)-Glycine Complex on the Surface Sites of Co/SiO2 for Fischer–Tropsch Synthesis
Abstract
:1. Introduction
2. Results and Discussion
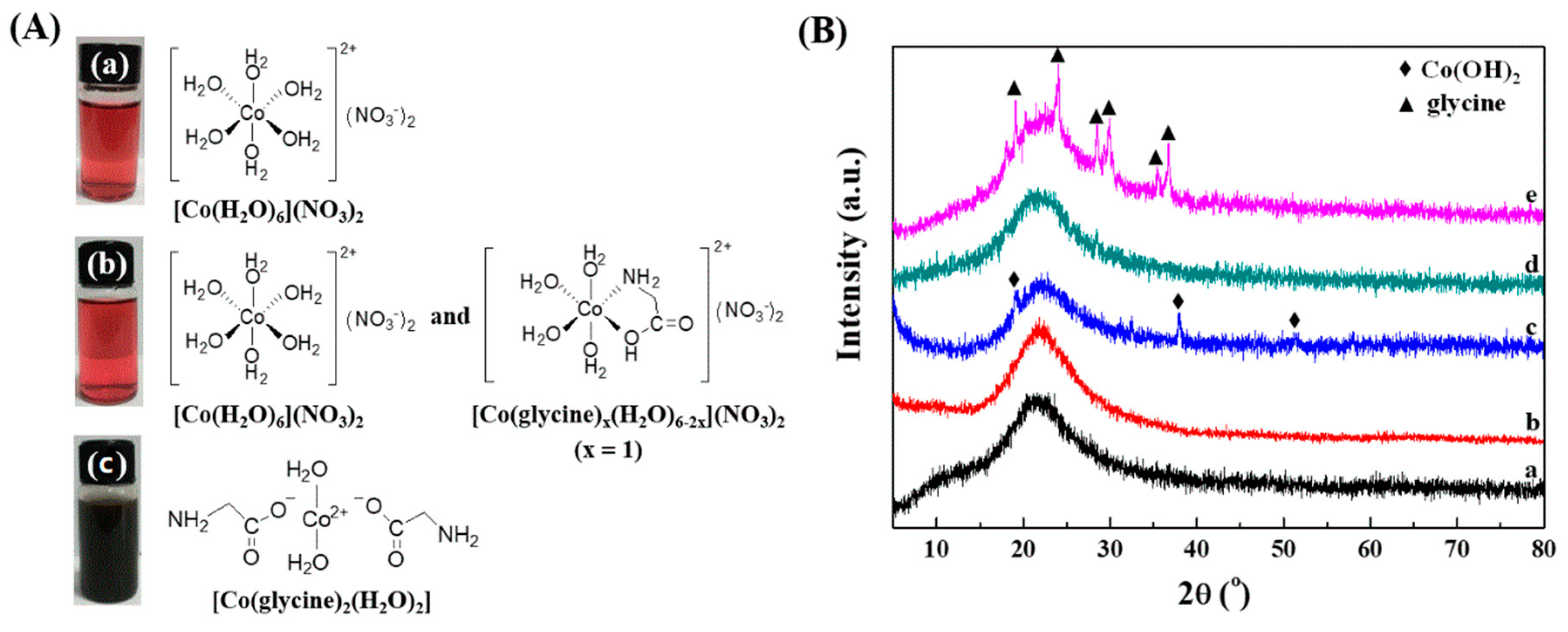
2.1. Preparation of SiO2-Supported Catalysts with Different Co(II)-Glycine Complexes
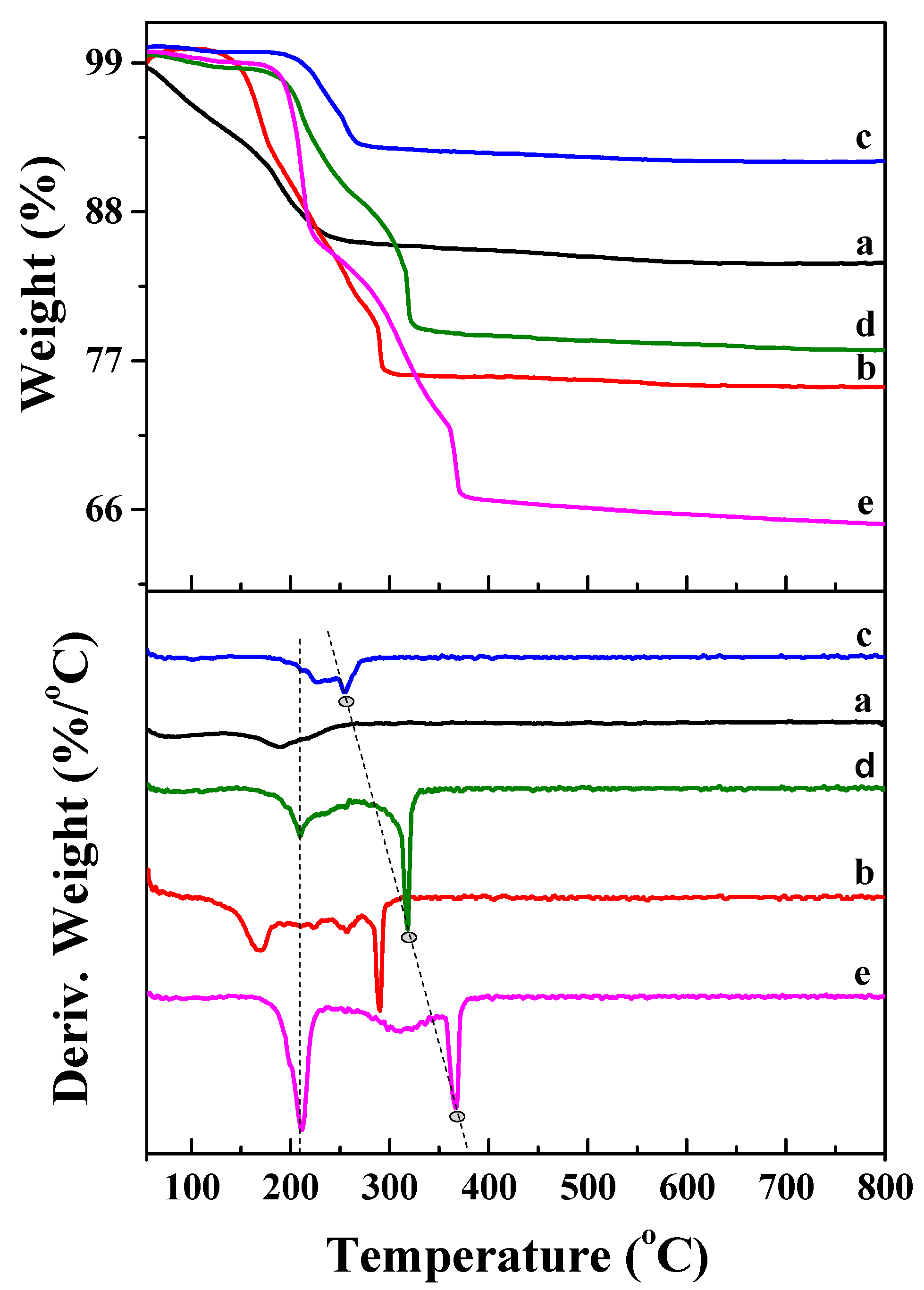
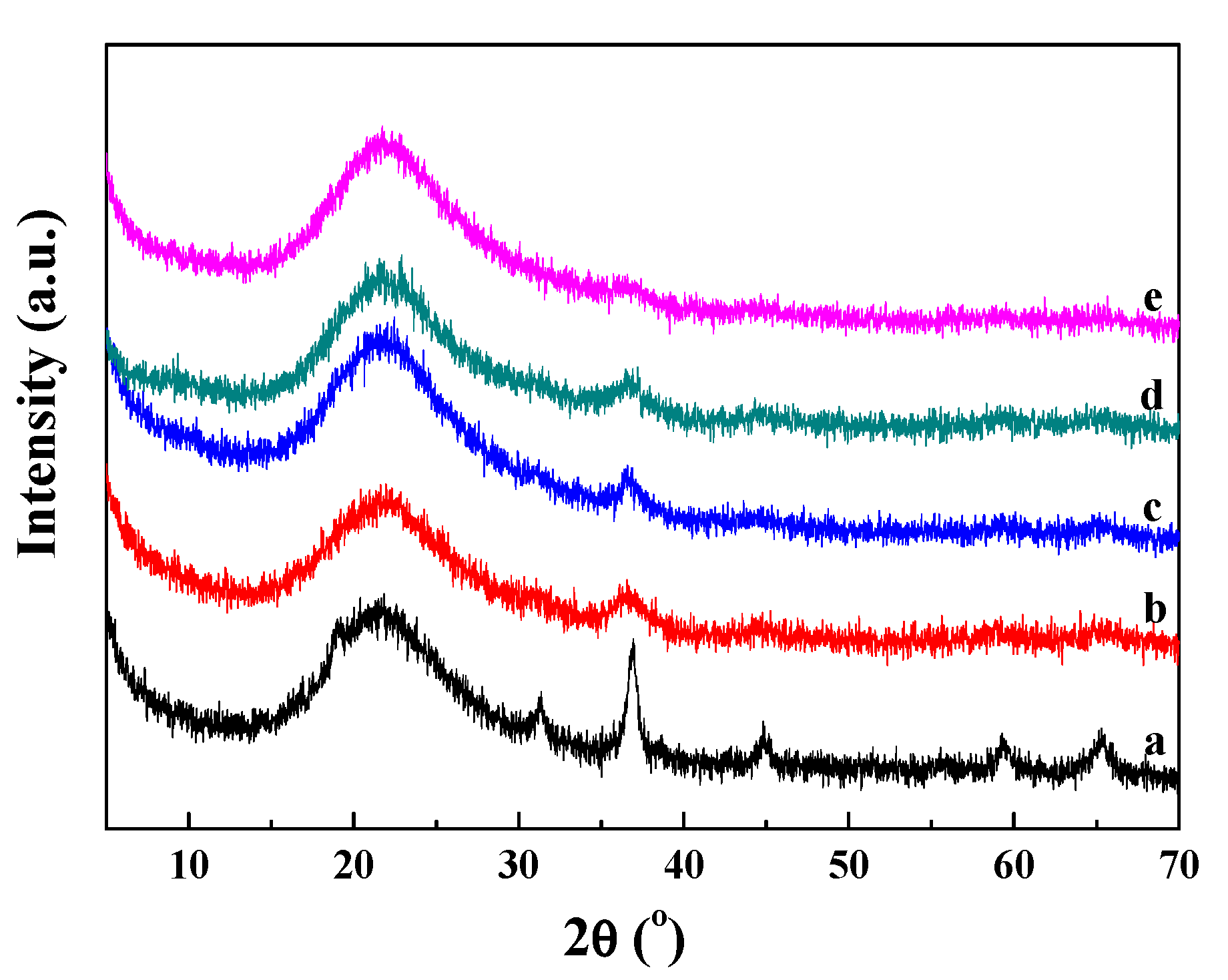
2.2. Textural and Structural Properties of Catalysts
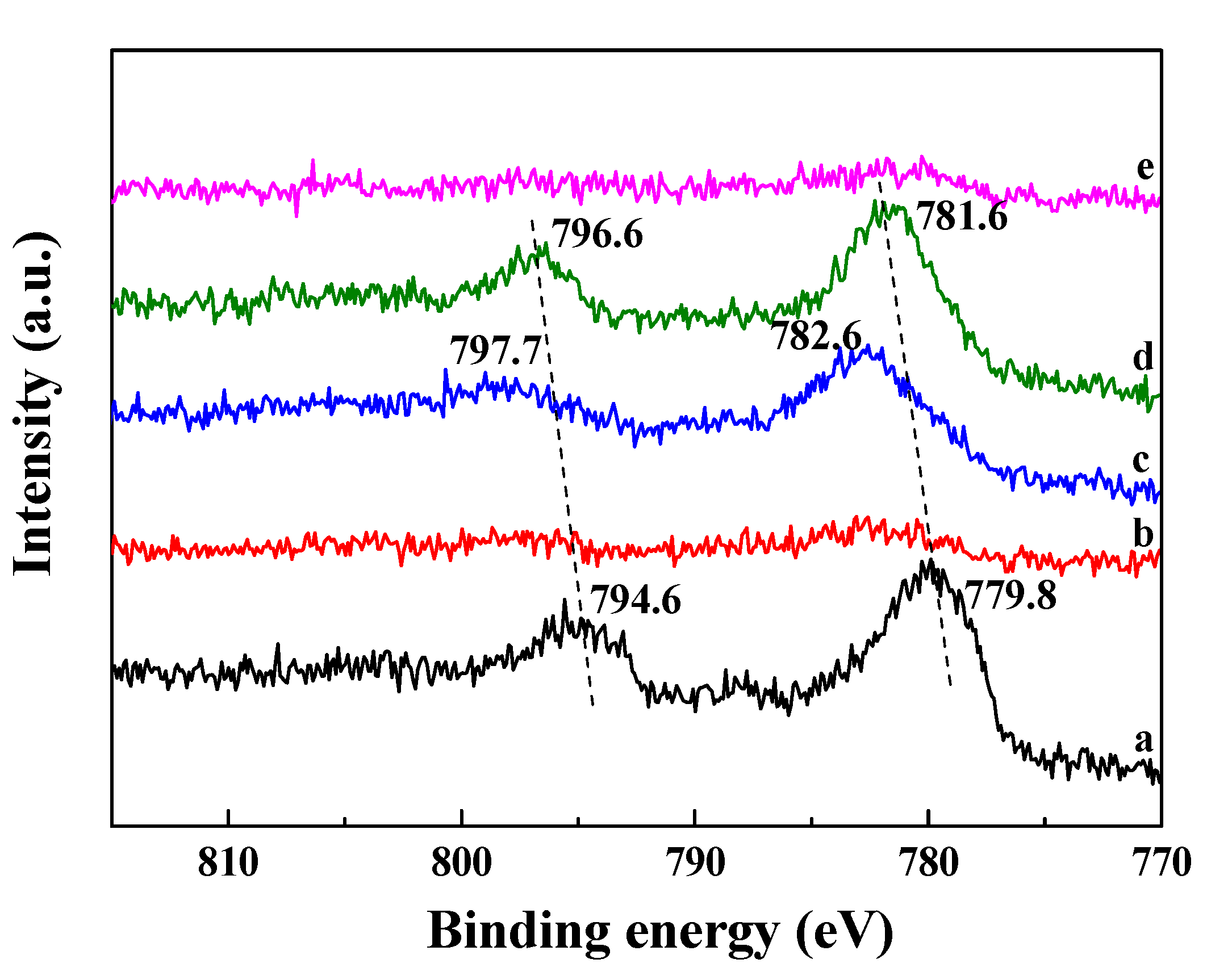
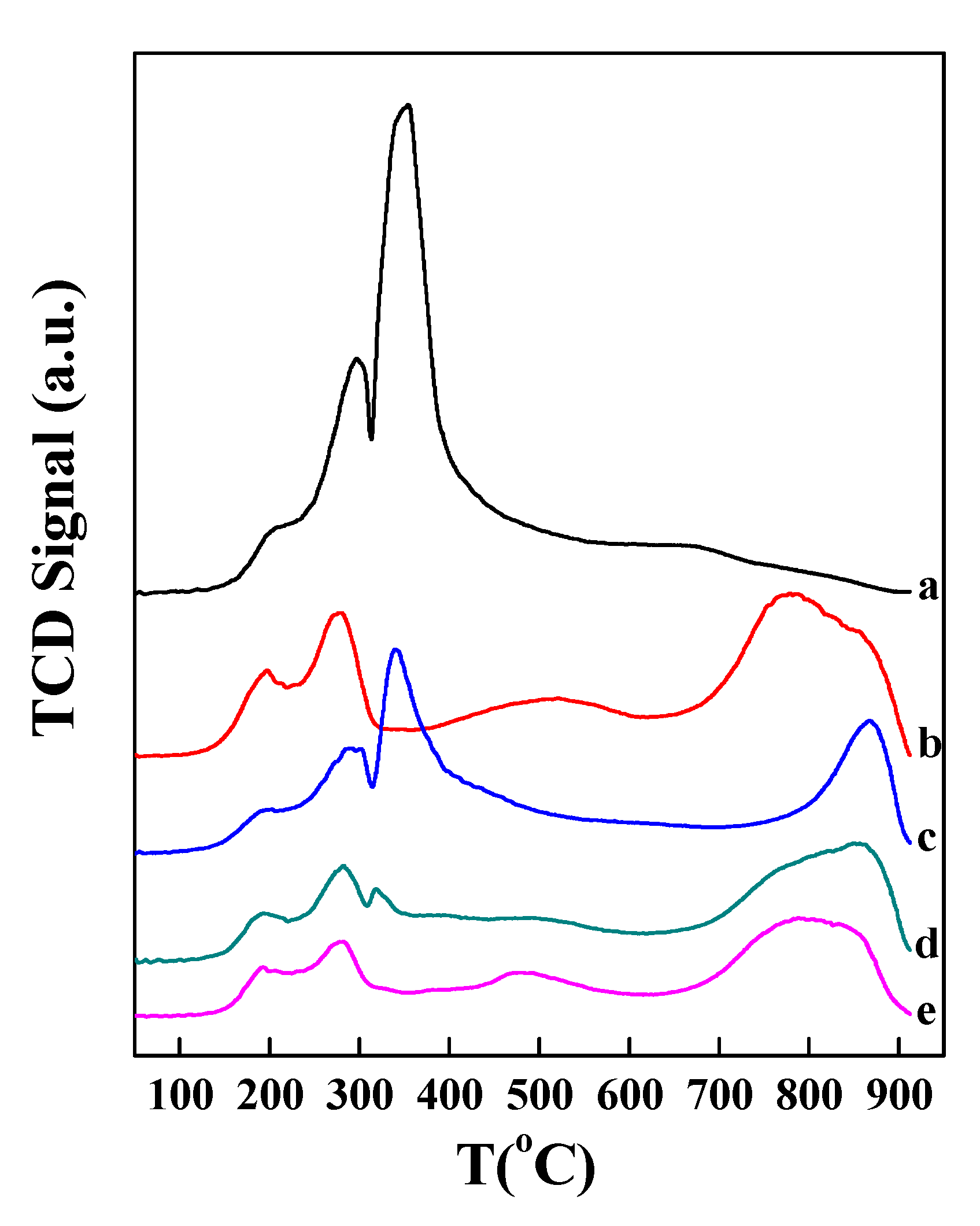
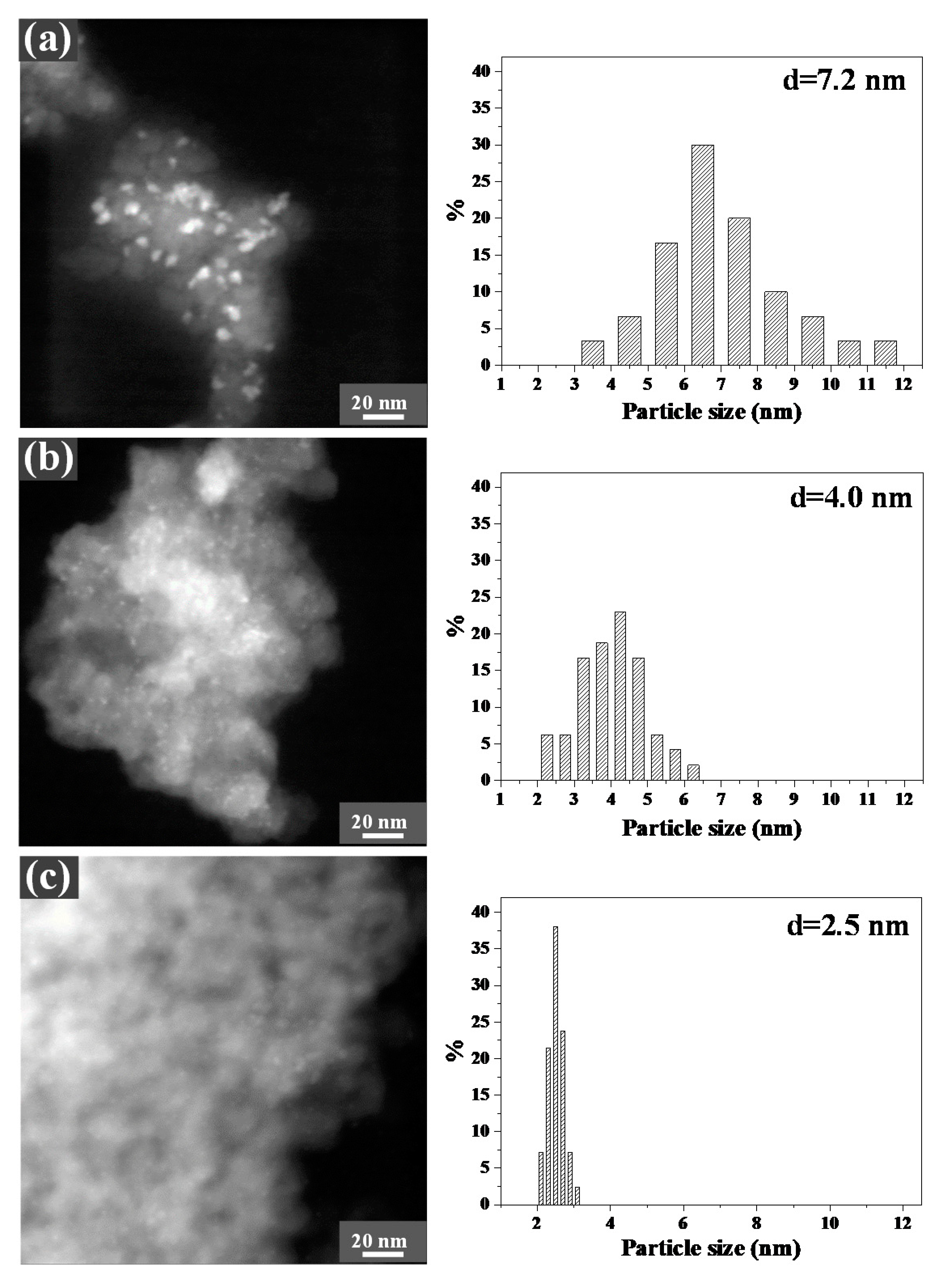
2.3. Co Particle Size and Reduction Behavior of the Catalysts
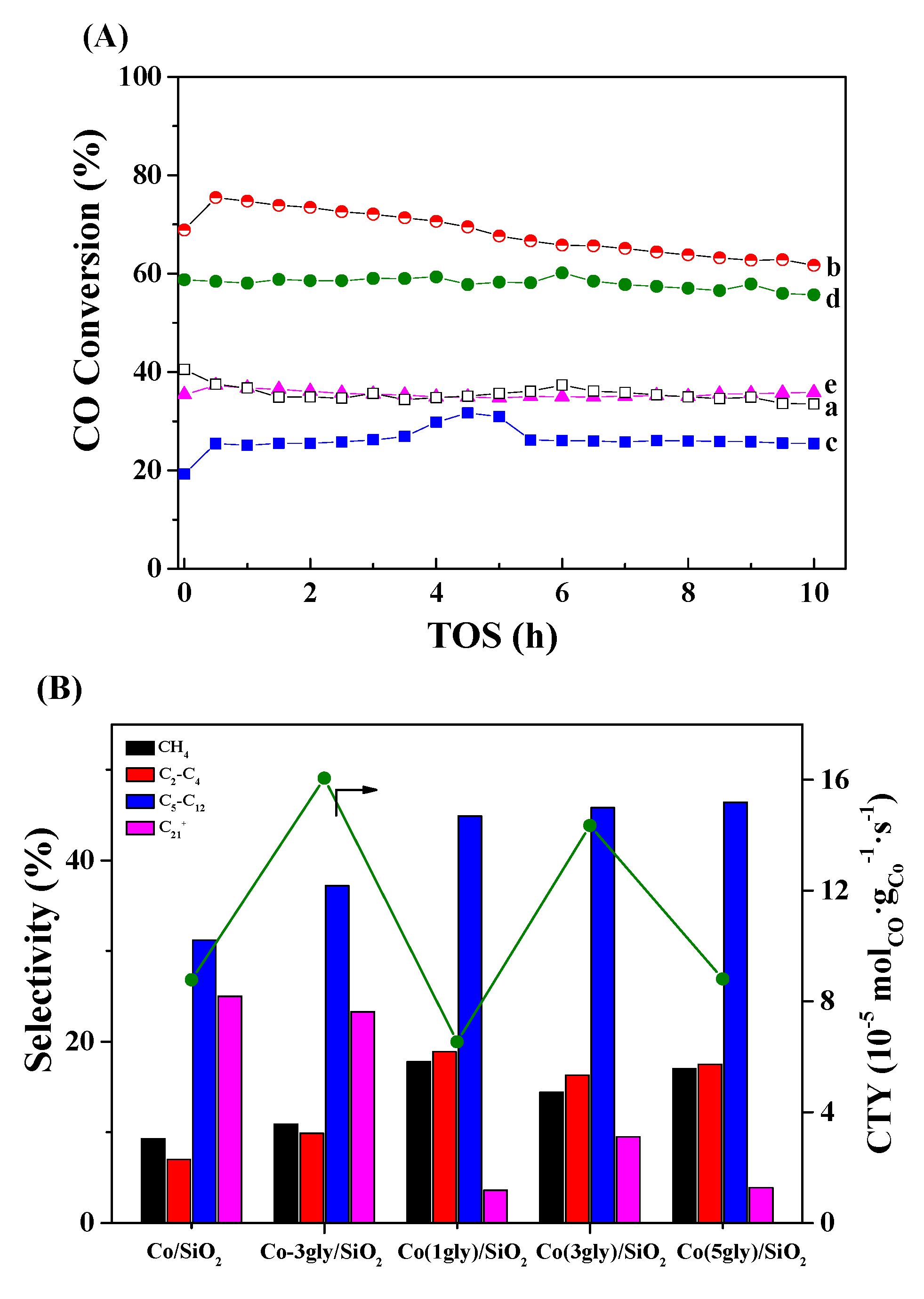
2.4. FT Performance
3. Materials and Methods
3.1. Materials
3.2. Preparation of Catalyst
3.3. Characterizations
3.4. Catalytic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Q.; Kang, J.; Wang, Y. Development of novel catalysts for Fischer-Tropsch synthesis: Tuning the product selectivity. ChemCatChem 2010, 2, 1030–1058. [Google Scholar] [CrossRef]
- Chen, Q.P.; Tian, Y.; Lyu, S.S.; Zhao, N.; Ma, K.; Ding, T.; Jiang, Z.; Wang, L.H.; Zhang, J.; Zheng, L.R.; et al. Confined small-sized cobalt catalysts stimulate carbon-chain growth reversely by modifying ASF law of Fischer-Tropsch synthesis. Nat. Commun. 2018, 9, 3250–3258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.Y.; Chen, L.N.; Zhang, S.C.; Su, J.; Somorjai, G.A. A Mini review of cobalt-based nanocatalyst in Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2020, 602, 117701. [Google Scholar] [CrossRef]
- Van de Water, L.G.A.; Bezemer, G.L.; Bergwerff, J.A.; Versluijs-Helder, M.; Weckhuysen, B.M.; De Jong, K.P. Spatially resolved UV-vis microspectroscopy on the preparation of alumina-supported Co Fischer-Tropsch catalysts: Linking activity to Co distribution and speciation. J. Catal. 2016, 242, 287–298. [Google Scholar] [CrossRef]
- Girardon, J.S.; Quinet, E.; Griboval-Constant, A.; Chernavskii, P.A.; Gengembre, L.; Khodakov, A.Y. Cobalt dispersion, reducibility, and surface sites in promoted silica-supported Fischer-Tropsch catalysts. J. Catal. 2007, 248, 143–157. [Google Scholar] [CrossRef]
- Shi, L.; Zeng, C.Y.; Lin, Q.H.; Lu, P.; Niu, W.Q.; Tsubaki, N. Citric acid assisted one-step synthesis of highly dispersed metallic Co/SiO2 without further reduction: As-prepared Co/SiO2 catalysts for Fischer–Tropsch synthesis. Catal. Today 2014, 228, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Hao, Q.-Q.; Liu, Z.-W.; Zhang, B.; Wang, G.-W.; Ma, C.; Frandsen, W.; Li, J.; Liu, Z.-T.; Hao, Z.; Su, D. Porous montmorillonite heterostructures directed by a single alkyl ammonium template for controlling the product distribution of Fischer-Tropsch synthesis over cobalt. Chem. Mater. 2012, 24, 972–974. [Google Scholar] [CrossRef]
- Sun, S.L.; Tsubaki, N.; Fujimoto, K. The reaction performances and characterization of Fischer-Tropsch synthesis Co/SiO2 catalysts prepared from mixed cobalt salts. Appl. Catal. A Gen. 2000, 202, 121–131. [Google Scholar] [CrossRef]
- Mochizuki, T.; Hara, T.; Koizumi, N.; Yamada, M. Surface structure and Fischer-Tropsch synthesis activity of highly active Co/SiO2 catalysts prepared from the impregnating solution modified with some chelating agents. Appl. Catal. A Gen. 2007, 317, 97–104. [Google Scholar] [CrossRef]
- Girardon, J.S.; Lermontov, A.S.; Gengembre, L.; Chernavskii, P.A.; Griboval-Constant, A.; Khodakov, A.Y. Effect of Cobalt Precursor and Pretreatment conditions on the structure and Patalytic Performance of cobalt Silica-Supported Fischer-Tropsch catalyst. J. Catal. 2005, 230, 339–352. [Google Scholar] [CrossRef]
- Mochizuki, T.; Hara, T.; Koizumi, N.; Yamada, M. Novel preparation method of highly active Co/SiO2 catalyst for Fischer-Tropsch synthesis with chelating agents. Catal. Lett. 2007, 113, 165–169. [Google Scholar] [CrossRef]
- Jos van Dillen, A.; Terörde, R.J.A.M.; Lensveld, D.J.; Geus, J.W.; de Jong, K.P. Synthesis of supported catalysts by impregnation and drying using aqueous chelated metal complexes. J. Catal. 2003, 216, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, N.; Ibi, Y.; Hongo, D.; Hamabe, Y.; Suzuki, S.; Hayasaka, Y.; Shindo, T.; Yamada, M. Mechanistic aspects of the role of chelating agents in enhancing Fischer-Tropsch synthesis activity of Co/SiO2 catalyst: Importance of specific interaction of Co with chelate complex during calcination. J. Catal. 2012, 289, 151–163. [Google Scholar] [CrossRef]
- Hong, J.P.; Marceau, E.; Khodakov, A.Y.; Griboval-Constant, A.; Fontaine, C.L.; Villain, F.; Briois, V.; Chernavskii, P.A. Impact of Sorbitol addition on the structure and performance of silica-supported cobalt catalysts for Fischer-Tropsch synthesis. Catal. Today 2011, 175, 528–533. [Google Scholar] [CrossRef]
- Bambal, A.S.; Guggilla, V.S.; Kugler, E.L.; Gardner, T.H.; Dadyburjor, D.B. Poisoning of a silica-supported cobalt catalyst due to presence of sulfur impurities in syngas during Fischer-Tropsch synthesis: Effects of chelating agent. Ind. Eng. Chem. Res. 2014, 53, 5846–5857. [Google Scholar] [CrossRef]
- Shi, L.; Tao, K.; Kawabata, T.; Shimamura, T.; Zhang, X.J.; Tsubaki, N. Surface impregnation combustion method to prepare nanostructured metallic catalysts without further reduction: As-burnt Co/SiO2 catalysts for Fischer-Tropsch synthesis. ACS Catal. 2011, 1, 1225–1233. [Google Scholar] [CrossRef]
- Munnik, P.; Krans, N.A.; de Jongh, P.E.; de Jong, K.P. Effects of drying conditions on the synthesis of Co/SiO2 and Co/Al2O3 Fischer-Tropsch catalysts. ACS Catal. 2014, 4, 3219–3226. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Control and impact of the nanoscale distribution of supported cobalt particles used in Fischer-Tropsch catalysis. J. Am. Chem. Soc. 2014, 136, 7333–7340. [Google Scholar] [CrossRef] [PubMed]
- den Breejen, J.P.; Sietsma, J.R.A.; Friedrich, H.; Bitter, J.H.; de Jong, K.P. Design of supported cobalt catalysts with maximum activity for the Fischer-Tropsch synthesis. J. Catal. 2010, 270, 146–152. [Google Scholar] [CrossRef]
- Gorboletova, G.G.; Metlin, A.A. Standard Thermodynamic functions of Co2+ complexation with glycine and L-histidine in aqueous solution. J. Phys. Chem. 2016, 90, 334–338. [Google Scholar] [CrossRef]
- Sun, K.Q.; Marceau, E.; Che, M. Evolution of nickel speciation during preparation of Ni-SiO2 catalysts: Effect of the number of chelating ligands in [Ni(en)x(H2O)6–2x]2+ precursor complexes. Phys. Chem. Chem. Phys. 2006, 8, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, R.H.; Liu, J.X.; Li, W.X. Atomistic theory of ostwald ripening and disintegration of supported metal particles under reaction conditions. J. Am. Chem. Soc. 2013, 135, 1760–1771. [Google Scholar] [CrossRef] [PubMed]
- Kistamurthy, D.; Saib, A.M.; Moodley, D.J.; Niemantsverdriet, J.W.; Weststrate, C.J. Ostwald ripening on a planar Co/SiO2 catalyst exposed to model Fischer-Tropsch synthesis conditions. J. Catal. 2015, 328, 123–129. [Google Scholar] [CrossRef]
- Hong, J.P.; Wang, B.; Xiao, G.Q.; Wang, N.; Zhang, Y.H.; Khodakov, A.Y.; Li, J.L. Tuning the metal-support interaction and enhancing the stability of titania-supported cobalt Fischer-Tropsch catalysts via carbon nitride coating. ACS Catal. 2020, 10, 5554–5566. [Google Scholar] [CrossRef]
- Eschemann, T.O.; Jong, K.P.D. Deactivation behavior of Co/TiO2 catalysts during Fischer-Tropsch synthesis. ACS Catal. 2015, 5, 3181–3188. [Google Scholar] [CrossRef]
- Loosdrecht, J.V.D.; Ciobîcâ, I.M.; Gibson, P.; Govender, N.S.; Moodley, D.J.; Saib, A.M.; Weststrate, C.J.; Niemantsverdriet, J.W. Providing fundamental and applied insights into Fischer-Tropsch catalysis: Sasol-eindhoven university of technology collaboration. ACS Catal. 2016, 6, 3840–3855. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Marceau, E.; Che, M.; Pepe, C. Influence of the morphology and impurities of Ni(OH)2 on the synthesis of neutral Ni(II)-amino acid complexes. J. Solid State Chem. 2007, 180, 3469–3478. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Marceau, E.; Beaunier, P.; Che, M.; Train, C. Stabilization of hexagonal close-packed metallic nickel for alumina-supported systems prepared from Ni(II) glycinate. J. Solid State Chem. 2007, 180, 22–30. [Google Scholar] [CrossRef]
- Koizumi, N.; Mochizuki, T.; Yamada, M. Preparation of highly active catalysts for ultra-clean fuels. Catal. Today 2009, 141, 34–42. [Google Scholar] [CrossRef]
- Reuel, R.C.; Bartholomew, C.H. The stoichiometries of H2 and CO adsorptions on cobalt: Effects of support and preparation. J. Catal. 1984, 85, 63–77. [Google Scholar] [CrossRef]
- Puskas, I.; Fleisch, T.H.; Kaduk, J.A.; Marshall, C.L.; Meyers, B.L.; Castagnola, M.J.; Indacochea, J.E. Novel aspects of the physical chemistry of Co/SiO2 Fischer-Tropsch catalyst preparations. Cobalt oxide-induced silica migration during calcination of cobalt nitrate-impregnated high surface area silica. Appl. Catal. A Gen. 2007, 316, 197–206. [Google Scholar] [CrossRef]
- Lü, B.Z.; Qi, W.J.; Luo, M.S.; Liu, Q.L.; Guo, L. Fischer-Tropsch synthesis: ZIF-8@ZIF-67-derived cobalt nanoparticle-embedded nanocage catalysts. Ind. Eng. Chem. Res. 2020, 59, 12352–12359. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.M.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.K.; Sun, B.; Qiao, M.H.; Wei, J.; Yue, Q.; Wang, C.; Deng, Y.H.; Kaliaguine, S.; Zhao, D.Y. A General chelate-assisted co-assembly to metallic nanoparticles incorporated ordered mesoporous carbon catalysts for Fischer-Tropsch synthesis. J. Am. Chem. Soc. 2012, 134, 17653–17660. [Google Scholar] [CrossRef]
- Bezemer, G.L.; Bitter, J.H.; Kuipers, H.P.C.E.; Oosterbeek, H.; Holewijn, J.E.; Xu, X.; Kapteijn, F.; van Dillen, A.J.; de Jong, K.P. Cobalt particle size effects in the Fischer-Tropsch reaction studied with carbon nanofiber supported catalysts. J. Am. Chem. Soc. 2006, 128, 3956–3964. [Google Scholar] [CrossRef] [Green Version]
- Breejen, J.P.D.; Radstake, P.B.; Bezemer, G.L.; Bitter, J.H.; Frøseth, V.; Holmen, A.; Jong, K.P.D. On the origin of the cobalt particle size effects in Fischer-Tropsch catalysis. J. Am. Chem. Soc. 2009, 131, 7197–7203. [Google Scholar] [CrossRef]
| Catalysts | BET Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) |
|---|---|---|---|
| SiO2 | 354.2 | 1.22 | 13.77 |
| Co/SiO2 | 250.9 | 0.56 | 9.00 |
| Co-3gly/SiO2 | 296.9 | 0.72 | 9.69 |
| Co(1gly)/SiO2 | 207.5 | 0.47 | 7.97 |
| Co(3gly)/SiO2 | 239.0 | 0.50 | 8.33 |
| Co(5gly)/SiO2 | 210.7 | 0.60 | 7.97 |
| Catalysts | Co Size (nm) | Reduction Degree a (%) | Co Dispersion b (%) | Surface Co Density (10−2 mmol/gcat) | |
|---|---|---|---|---|---|
| d(Co)TEM | d(Co)Hc | ||||
| Co/SiO2 | 7.2 | 9.4 | 52.9 | 5.4 | 6.4 |
| Co-3gly/SiO2 | 4.0 | 3.4 | 38.3 | 10.9 | 13.0 |
| Co(1gly)/SiO2 | --- | 7.9 | 28.7 | 3.5 | 4.2 |
| Co(3gly)/SiO2 | 2.5 | 2.4 | 21.3 | 8.5 | 10.1 |
| Co(5gly)/SiO2 | --- | 2.1 | 16.7 | 7.8 | 9.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Q.-Q.; Hu, M.; Xie, Z.-X.; Ma, X.; Wang, W.; Ren, H.-P. Impact of Coordination Features of Co(II)-Glycine Complex on the Surface Sites of Co/SiO2 for Fischer–Tropsch Synthesis. Catalysts 2020, 10, 1295. https://doi.org/10.3390/catal10111295
Hao Q-Q, Hu M, Xie Z-X, Ma X, Wang W, Ren H-P. Impact of Coordination Features of Co(II)-Glycine Complex on the Surface Sites of Co/SiO2 for Fischer–Tropsch Synthesis. Catalysts. 2020; 10(11):1295. https://doi.org/10.3390/catal10111295
Chicago/Turabian StyleHao, Qing-Qing, Min Hu, Zhi-Xia Xie, Xiaoxun Ma, Wei Wang, and Hua-Ping Ren. 2020. "Impact of Coordination Features of Co(II)-Glycine Complex on the Surface Sites of Co/SiO2 for Fischer–Tropsch Synthesis" Catalysts 10, no. 11: 1295. https://doi.org/10.3390/catal10111295





