Surface Modification of Catalysts via Atomic Layer Deposition for Pollutants Elimination
Abstract
:1. Introduction
2. Conformal Coating
2.1. Applications in Photocatalytic Degradation of Organic Pollutants
2.2. Applications in Removal of Air Pollutants
3. Uniform Particle Deposition
4. Area-Selective Deposition
5. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal-metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Ye, B.; Kim, S.-I.; Lee, M.; Ezazi, M.; Kim, H.-D.; Kwon, G.; Lee, D.H. Synthesis of oxygen functionalized carbon nanotubes and their application for selective catalytic reduction of NOx with NH3. RSC Adv. 2020, 10, 16700–16708. [Google Scholar] [CrossRef]
- Caruso, R.A.; Antonietti, M. Sol−Gel Nanocoating: An Approach to the Preparation of Structured Materials. Chem. Mater. 2001, 13, 3272–3282. [Google Scholar] [CrossRef]
- Yu, J.; Pan, Y.; Wang, C.; Lai, Z. ZIF-8 membranes with improved reproducibility fabricated from sputter-coated ZnO/alumina supports. Chem. Eng. Sci. 2016, 141, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.-M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Weimer, A.W. Particle atomic layer deposition. J. Nanopart. Res. 2019, 21, 1–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Liu, W.; Luo, Q.; Yin, R.; Wang, B.; Weissenrieder, J.; Soldemo, M.; Yan, H.; Lin, Y.; Sun, Z.; et al. Atomically dispersed iron hydroxide anchored on Pt for preferential oxidation of CO in H2. Nat. Cell Biol. 2019, 565, 631–635. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Jin, Y.; Wu, T.; Ma, L.; Liang, X. Supported Single Fe Atoms Prepared via Atomic Layer Deposition for Catalytic Reactions. ACS Appl. Nano Mater. 2020, 3, 2867–2874. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Zuo, W.; Kong, L.; Cui, H. Z-scheme 2D/3D g-C3N4@ZnO with enhanced photocatalytic activity for cephalexin oxidation under solar light. Chem. Eng. J. 2018, 352, 412–422. [Google Scholar] [CrossRef]
- Duan, H.; You, R.; Xu, S.; Li, Z.; Qian, K.; Cao, T.; Huang, W.; Bao, X. Pentacoordinated Al3+—Stabilized Active Pd Structures on Al2O3—Coated Palladium Catalysts for Methane Combustion. Angew. Chem. Int. Ed. 2019, 58, 12043–12048. [Google Scholar] [CrossRef]
- Jackson, D.H.K.; Schwartz, M.M.; Ngo, C.; Facteau, D.; Pylypenko, S.; Marshall, C.L.; Dameron, A.A. Hydrocarbon catalyzed-selective catalytic reduction catalysts using core-shell atomic layer deposited CeO2 and ZrO2. J. Vac. Sci. Technol. A 2019, 37, 020919. [Google Scholar] [CrossRef]
- Richey, N.E.; De Paula, C.; Bent, S.F. Understanding chemical and physical mechanisms in atomic layer deposition. J. Chem. Phys. 2020, 152, 040902. [Google Scholar] [CrossRef]
- Hagen, D.; Pemble, M.E.; Karppinen, M. Atomic layer deposition of metals: Precursors and film growth. Appl. Phys. Rev. 2019, 6, 041309. [Google Scholar] [CrossRef]
- Cao, K.; Cai, J.; Shan, B.; Chen, R. Surface functionalization on nanoparticles via atomic layer deposition. Sci. Bull. 2020, 65, 678–688. [Google Scholar] [CrossRef] [Green Version]
- Sobel, N.; Hess, C. Nanoscale Structuring of Surfaces by Using Atomic Layer Deposition. Angew. Chem. Int. Ed. 2015, 54, 15014–15021. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, G.; Prakash, J.; Zheng, Y.; Sun, S. Rational Design of Novel Catalysts with Atomic Layer Deposition for the Reduction of Carbon Dioxide. Adv. Energy Mater. 2019, 9, 1900889. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Qin, Y. Application of atomic layer deposition in fabricating high-efficiency electrocatalysts. Chin. J. Catal. 2020, 41, 227–241. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, Y. Interface Tailoring of Heterogeneous Catalysts by Atomic Layer Deposition. ACS Catal. 2018, 8, 10064–10081. [Google Scholar] [CrossRef]
- O’Neill, B.J.; Jackson, D.H.K.; Lee, J.; Canlas, C.; Stair, P.C.; Marshall, C.L.; Elam, J.W.; Kuech, T.F.; Dumesic, J.A.; Huber, G.W. Catalyst Design with Atomic Layer Deposition. ACS Catal. 2015, 5, 1804–1825. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Elam, J.W.; Stair, P.C. Atomic layer deposition—Sequential self-limiting surface reactions for advanced catalyst “bottom-up” synthesis. Surf. Sci. Rep. 2016, 71, 410–472. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, K.O.; Gahlmann, T.; Riedl, T. Atomic Layer Deposition of Functional Layers in Planar Perovskite Solar Cells. Sol. RRL 2019, 4, 1900332. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zheng, K.; Sun, X. Addressing Interfacial Issues in Liquid-Based and Solid-State Batteries by Atomic and Molecular Layer Deposition. Joule 2018, 2, 2583–2604. [Google Scholar] [CrossRef] [Green Version]
- Azizpour, H.; Talebi, M.; Tichelaar, F.; Sotudeh-Gharebagh, R.; Guo, J.; Van Ommen, J.R.; Mostoufi, N. Effective coating of titania nanoparticles with alumina via atomic layer deposition. Appl. Surf. Sci. 2017, 426, 480–496. [Google Scholar] [CrossRef]
- Jang, E.; Kim, D.W.; Hong, S.H.; Park, Y.M.; Park, T.J. Visible light-driven g-C3N4@ZnO heterojunction photocatalyst synthesized via atomic layer deposition with a specially designed rotary reactor. Appl. Surf. Sci. 2019, 487, 206–210. [Google Scholar] [CrossRef]
- Cao, M.; Wang, P.; Ao, Y.; Wang, C.; Hou, J.; Qian, J. Visible light activated photocatalytic degradation of tetracycline by a magnetically separable composite photocatalyst: Graphene oxide/magnetite/cerium-doped titania. J. Colloid Interface Sci. 2016, 467, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.J.; Ko, W.C.; Kang, S.; Kim, S.M.; Jeong, Y.K. Surface passivation of highly stable TiO2/V2O5 photocatalyst by atomic layer deposited-Al2O3. Appl. Surf. Sci. 2020, 507, 145128. [Google Scholar] [CrossRef]
- Shenouda, S.; Hussien, M.S.; Parditka, B.; Csík, A.; Takats, V.; Erdelyi, Z. Novel amorphous Al-rich Al2O3 ultra-thin films as active photocatalysts for water treatment from some textile dyes. Ceram. Int. 2020, 46, 7922–7929. [Google Scholar] [CrossRef]
- Jang, E.; Kim, W.J.; Kim, D.W.; Hong, S.H.; Ali, I.; Park, Y.M.; Park, T.J. Atomic layer deposition with rotary reactor for uniform hetero-junction photocatalyst, g-C3N4@TiO2 core–shell structures. RSC Adv. 2019, 9, 33180–33186. [Google Scholar] [CrossRef]
- Trochowski, M.; Kobielusz, M.; Mróz, K.; Surówka, M.; Hämäläinen, J.; Iivonen, T.; Leskelä, M.; Macyk, W. How insignificant modifications of photocatalysts can significantly change their photocatalytic activity. J. Mater. Chem. A 2019, 7, 25142–25154. [Google Scholar] [CrossRef]
- Cheng, H.-E.; Lin, C.-Y.; Hsu, C.-M. Fabrication of SnO2-TiO2 core-shell nanopillar-array films for enhanced photocatalytic activity. Appl. Surf. Sci. 2017, 396, 393–399. [Google Scholar] [CrossRef]
- Yang, H.; Zhai, L.; Li, K.; Liu, X.; Deng, B.; Xu, W. A highly efficient nano-graphite-doped TiO2 photocatalyst with a unique sea-island structure for visible-light degradation. Catal. Sci. Technol. 2020, 10, 1161–1170. [Google Scholar] [CrossRef]
- Birnal, P.; De Lucas, M.C.M.; Pochard, I.; Domenichini, B.; Imhoff, L. Photocatalytic properties of atomic layer deposited TiO2 inverse opals and planar films for the degradation of dyes. Appl. Surf. Sci. 2020, 512, 145693. [Google Scholar] [CrossRef]
- Wang, H.; Ma, F.; Sun, Y.-S.; Zhou, L.; Zeng, D.-J.; Qin, Y.; Xu, Y.-K.; Chen, Y.; Xu, K.-W.; Ma, D.-Y. Band bending and valence band shifting of sub-monolayer TiO2 functionalized SnO2 nanowires. J. Mater. Sci. Mater. Electron. 2019, 31, 637–643. [Google Scholar] [CrossRef]
- Wan, G.; Peng, X.; Zeng, M.; Yu, L.; Wang, K.; Li, X.; Wang, G. The Preparation of Au@TiO2 Yolk–Shell Nanostructure and its Applications for Degradation and Detection of Methylene Blue. Nanoscale Res. Lett. 2017, 12, 535. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-H.; Wang, C.-C.; Liao, S.-Y.; Gan, J.-Y.; Perng, T.-P. CNT/TiO2 core-shell structures prepared by atomic layer deposition and characterization of their photocatalytic properties. Thin Solid Films 2016, 616, 151–159. [Google Scholar] [CrossRef]
- Marchetti, F.; Laidani, N.B.; Scarpa, M.; Gottardi, G.; Moser, E. Graphene films decorated with TiO2 grown by atomic layer deposition: Characterization and photocatalytic activity study under UV–visible light. Appl. Surf. Sci. 2019, 470, 484–495. [Google Scholar] [CrossRef]
- Xu, H.; Han, F.; Xia, C.; Wang, S.; Ramachandran, R.M.; Detavernier, C.; Wei, M.; Lin, L.; Zhuiykov, S. Wafer-Scale Fabrication of Sub-10 nm TiO2-Ga2O3 n-p Heterojunctions with Efficient Photocatalytic Activity by Atomic Layer Deposition. Nanoscale Res. Lett. 2019, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-Q.; Zhao, X.-R.; Chen, J.; Zhang, W.; Li, M.; Zhu, L.; Zhang, X.-J.; Wu, D.; Li, A.-D. TiOxNy Modified TiO2 Powders Prepared by Plasma Enhanced Atomic Layer Deposition for Highly Visible Light Photocatalysis. Sci. Rep. 2018, 8, 12131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.; Ahn, C.; Park, J.; Jeon, S. 3D nanostructured N-doped TiO2 photocatalysts with enhanced visible absorption. Nanoscale 2018, 10, 9747–9751. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Han, C.; Shao, C.; Yang, S.; Li, X.; Li, B.; Li, X.; Ma, J.; Liu, Y. ZnO/ZnFe2O4 Janus Hollow Nanofibers with Magnetic Separability for Photocatalytic Degradation of Water-Soluble Organic Dyes. ACS Appl. Nano Mater. 2019, 2, 4879–4890. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, C.; Li, X.; Han, C.; Yang, S.; Ma, J.; Li, X.; Liu, Y. Immobilization of ZnO/polyaniline heterojunction on electrospun polyacrylonitrile nanofibers and enhanced photocatalytic activity. Mater. Chem. Phys. 2018, 214, 507–515. [Google Scholar] [CrossRef]
- Park, K.-H.; Han, G.D.; Kim, B.J.; Kang, E.H.; Park, J.S.; Shim, J.H.; Park, H.-D. Effects of atomic layer deposition conditions on the formation of thin ZnO films and their photocatalytic characteristics. Ceram. Int. 2019, 45, 18823–18830. [Google Scholar] [CrossRef]
- Gu, C.; Xiong, S.; Zhong, Z.; Wang, Y.; Xing, W. A promising carbon fiber-based photocatalyst with hierarchical structure for dye degradation. RSC Adv. 2017, 7, 22234–22242. [Google Scholar] [CrossRef] [Green Version]
- Bakos, L.P.; Justh, N.; Costa, U.C.M.D.S.B.D.; László, K.; Lábár, J.L.; Igricz, T.; Varga-Josepovits, K.; Pasierb, P.; Faärm, E.; Ritala, M.; et al. Photocatalytic and Gas Sensitive Multiwalled Carbon Nanotube/TiO2-ZnO and ZnO-TiO2 Composites Prepared by Atomic Layer Deposition. Nanomaterials 2020, 10, 252. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Xiong, S.; Wang, Y. Atomic layer deposition of hybrid metal oxides on carbon nanotube membranes for photodegradation of dyes. Compos. Commun. 2019, 12, 39–46. [Google Scholar] [CrossRef]
- Wang, X.; Jin, Y.; Liang, X. Significant photocatalytic performance enhancement of TiO2 by CeO2 atomic layer deposition. Nanotechnology 2017, 28, 505709. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Patel, R.L.; Liang, X. Significant improvement in TiO2 photocatalytic activity through controllable ZrO2 deposition. RSC Adv. 2018, 8, 25829–25834. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.K.; Kim, Y.-K.; Choi, B.J.; Chung, T.-M.; Han, J.H. SnO-decorated TiO2 nanoparticle with enhanced photocatalytic performance for methylene blue degradation. Appl. Surf. Sci. 2019, 480, 1089–1092. [Google Scholar] [CrossRef]
- Shin, S.C.; Park, B.K.; Chung, T.-M.; Han, J.H. Highly efficient photocatalytic methylene blue degradation over Sn(O,S)/TiO2 photocatalyst fabricated via powder atomic layer deposition of SnO and subsequent sulfurization. Mater. Lett. 2020, 272, 127868. [Google Scholar] [CrossRef]
- You, J.; Guo, Y. Atomic layer deposition of fcc-FePt nanoparticles on g-C3N4 for magnetically recyclable photocatalysts with enhanced photocatalytic performance. Ceram. Int. 2019, 45, 2451–2456. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, W.; Zhao, L. BaZrO3/Au and BaZrO3/Au-Pd hetero-structures: The characteristics and mechanism for their photocatalytic performance. Ceram. Int. 2019, 45, 23808–23814. [Google Scholar] [CrossRef]
- Scott, T.; Zhao, H.; Deng, W.; Feng, X.; Li, Y. Photocatalytic degradation of phenol in water under simulated sunlight by an ultrathin MgO coated Ag/TiO2 nanocomposite. Chemosphere 2019, 216, 1–8. [Google Scholar] [CrossRef]
- Merenda, A.; Weber, M.; Bechelany, M.; Allioux, F.-M.; Hyde, L.; Kong, L.; Dumée, L.F. Fabrication of Pd-TiO2 nanotube photoactive junctions via Atomic Layer Deposition for persistent pesticide pollutants degradation. Appl. Surf. Sci. 2019, 483, 219–230. [Google Scholar] [CrossRef]
- Liang, X.; Weimer, A.W. Photoactivity passivation of TiO2 nanoparticles using molecular layer deposited (MLD) polymer films. J. Nanopart. Res. 2009, 12, 135–142. [Google Scholar] [CrossRef]
- Ye, L.; Kropp, J.A.; Gougousi, T. In situ infrared spectroscopy study of the surface reactions during the atomic layer deposition of TiO2 on GaAs (100) surfaces. Appl. Surf. Sci. 2017, 422, 666–674. [Google Scholar] [CrossRef]
- Wang, X.; Donovan, A.R.; Patel, R.L.; Shi, H.; Liang, X. Adsorption of metal and metalloid ions onto nanoporous microparticles functionalized by atomic layer deposition. J. Environ. Chem. Eng. 2016, 4, 3767–3774. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.; Regmi, C.; Schäfer, A.; Richards, B. Photocatalytic degradation of organic dye via atomic layer deposited TiO2 on ceramic membranes in single-pass flow-through operation. J. Membr. Sci. 2020, 604, 118015. [Google Scholar] [CrossRef]
- Li, D.; Yan, X.; Lin, C.; Huang, S.; Tian, Z.R.; He, B.; Yang, Q.; Yu, B.; He, X.; Li, J.; et al. Synthesis of ZnO/Si Hierarchical Nanowire Arrays for Photocatalyst Application. Nanoscale Res. Lett. 2017, 12, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markovic, M.K.; Peter, R.; Badovinac, I.J.; Šarić, I.; Perčić, M.; Radičić, R.; Marković, D.; Knez, M.; Ambrožić, G. ‘Sandwich’-like hybrid ZnO thin films produced by a combination of atomic layer deposition and wet-chemistry using a mercapto silane as single organic precursor. Nanotechnology 2020, 31, 185603. [Google Scholar] [CrossRef]
- Di Mauro, A.; Cantarella, M.; Nicotra, G.; Privitera, V.; Impellizzeri, G. Low temperature atomic layer deposition of ZnO: Applications in photocatalysis. Appl. Catal. B Environ. 2016, 196, 68–76. [Google Scholar] [CrossRef]
- Liu, R.; Peng, M.; Zhang, H.; Wan, X.; Shen, M. Atomic layer deposition of ZnO on graphene for thin film transistor. Mater. Sci. Semicond. Process. 2016, 56, 324–328. [Google Scholar] [CrossRef]
- Walter, T.N.; Lee, S.; Zhang, X.; Chubarov, M.; Redwing, J.M.; Jackson, T.N.; Mohney, S.E. Atomic layer deposition of ZnO on MoS2 and WSe2. Appl. Surf. Sci. 2019, 480, 43–51. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Yu, L.; Kovarik, L.; Zhang, X.; Hoffman, A.S.; Gallo, A.; Bare, S.R.; Sokaras, D.; Kroll, T.; et al. Identification of the active complex for CO oxidation over single-atom Ir-on-MgAl2O4 catalysts. Nat. Catal. 2019, 2, 149–156. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Mao, X.; Foucher, A.; Stach, E.A.; Gorte, R.J. A Study of Support Effects for CH4 and CO Oxidation over Pd Catalysts on ALD-Modified Al2O3. Catal. Lett. 2019, 149, 905–915. [Google Scholar] [CrossRef]
- Monai, M.; Montini, T.; Melchionna, M.; Duchoň, T.; Kúš, P.; Chen, C.; Tsud, N.; Nasi, L.; Prince, K.C.; Veltruská, K.; et al. The effect of sulfur dioxide on the activity of hierarchical Pd-based catalysts in methane combustion. Appl. Catal. B Environ. 2017, 202, 72–83. [Google Scholar] [CrossRef]
- Onn, T.M.; Monai, M.; Dai, S.; Fonda, E.; Montini, T.; Pan, X.; Graham, G.W.; Fornasiero, P.; Gorte, R.J. Smart Pd Catalyst with Improved Thermal Stability Supported on High-Surface-Area LaFeO3Prepared by Atomic Layer Deposition. J. Am. Chem. Soc. 2018, 140, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- Onn, T.M.; Dai, S.; Chen, J.; Pan, X.; Graham, G.W.; Gorte, R.J. High-Surface Area Ceria-Zirconia Films Prepared by Atomic Layer Deposition. Catal. Lett. 2017, 147, 1464–1470. [Google Scholar] [CrossRef]
- Cao, K.; Liu, X.; Zhu, Q.; Shan, B.; Chen, R. Atomically Controllable Pd@Pt Core-Shell Nanoparticles towards Preferential Oxidation of CO in Hydrogen Reactions Modulated by Platinum Shell Thickness. ChemCatChem 2015, 8, 326–330. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts for soot combustion: Investigations on the surface sensitivity. Appl. Catal. B Environ. 2015, 165, 742–751. [Google Scholar] [CrossRef]
- Machida, M.; Murata, Y.; Kishikawa, K.; Zhang, D.; Ikeue, K. On the Reasons for High Activity of CeO2 Catalyst for Soot Oxidation. Chem. Mater. 2008, 20, 4489–4494. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Toivonen, J.; Maydannik, P.; Kääriäinen, T.; Sillanpää, M.; Homola, T.; Cameron, D.C. Atomic layer deposition of cerium oxide for potential use in diesel soot combustion. J. Vac. Sci. Technol. A 2016, 34, 031506. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Homola, T.; Bryukvin, A.; Cameron, D.C. Catalytic Performance of Ag2O and Ag Doped CeO2 Prepared by Atomic Layer Deposition for Diesel Soot Oxidation. Coatings 2018, 8, 237. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, B.J.; Jackson, D.H.K.; Crisci, A.J.; Farberow, C.A.; Shi, F.; Alba-Rubio, A.C.; Lu, J.; Dietrich, P.J.; Gu, X.; Marshall, C.L.; et al. Stabilization of Copper Catalysts for Liquid-Phase Reactions by Atomic Layer Deposition. Angew. Chem. Int. Ed. 2013, 52, 13808–13812. [Google Scholar] [CrossRef]
- Wang, X.; Hu, W.; Deng, B.; Liang, X. Selective hydrogenation of citral over supported Pt catalysts: Insight into support effects. J. Nanopart. Res. 2017, 19, 56. [Google Scholar] [CrossRef]
- Ye, X.; Wang, H.; Lin, Y.; Liu, X.; Cao, L.; Gu, J.; Lu, J. Insight of the stability and activity of platinum single atoms on ceria. Nano Res. 2019, 12, 1401–1409. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single-atom Catalysis Using Pt/Graphene Achieved through Atomic Layer Deposition. Sci. Rep. 2013, 3, 1775. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.-R.; Crosby, L.; George, C.; Kennedy, R.M.; Schweitzer, N.M.; Wen, J.; Van Duyne, R.P.; Stair, P.C.; Poeppelmeier, K.R.; Marks, L.D.; et al. Morphology and CO Oxidation Activity of Pd Nanoparticles on SrTiO3Nanopolyhedra. ACS Catal. 2018, 8, 4751–4760. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; He, X.; White, T.A.; Liang, X. Highly Active and Stable Fe/SiO2 Catalyst Synthesized by Atomic Layer Deposition for CO Oxidation. Catal. Lett. 2020, 150, 3296–3303. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Wu, T.; Liu, Y.; Liang, X. Synthesis of Highly Dispersed and Highly Stable Supported Au–Pt Bimetallic Catalysts by a Two-Step Method. Catal. Lett. 2016, 146, 2606–2613. [Google Scholar] [CrossRef]
- Shang, Z.; Liang, X. “Core–Shell” Nanostructured Supported Size-Selective Catalysts with High Catalytic Activity. Nano Lett. 2016, 17, 104–109. [Google Scholar] [CrossRef]
- Tang, W.; Lu, X.; Liu, F.; Du, S.; Weng, J.; Hoang, S.; Wang, S.; Nam, C.-Y.; Gao, P.-X. Ceria-based nanoflake arrays integrated on 3D cordierite honeycombs for efficient low-temperature diesel oxidation catalyst. Appl. Catal. B Environ. 2019, 245, 623–634. [Google Scholar] [CrossRef]
- Hoang, S.; Lu, X.; Tang, W.; Wang, S.; Du, S.; Nam, C.-Y.; Ding, Y.; Vinluan, R.D.; Zheng, J.; Gao, P.-X. High performance diesel oxidation catalysts using ultra-low Pt loading on titania nanowire array integrated cordierite honeycombs. Catal. Today 2019, 320, 2–10. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, Y.; Li, J.; Weimer, A.W. Reaction mechanism studies for platinum nanoparticle growth by atomic layer deposition. J. Nanopart. Res. 2011, 13, 3781–3788. [Google Scholar] [CrossRef]
- Han, S.W.; Kim, D.H.; Jeong, M.-G.; Park, K.J.; Kim, Y.D. CO oxidation catalyzed by NiO supported on mesoporous Al2O3 at room temperature. Chem. Eng. J. 2016, 283, 992–998. [Google Scholar] [CrossRef]
- Jeong, M.-G.; Park, E.J.; Jeong, B.; Kim, D.H.; Kim, Y.D. Toluene combustion over NiO nanoparticles on mesoporous SiO2 prepared by atomic layer deposition. Chem. Eng. J. 2014, 237, 62–69. [Google Scholar] [CrossRef]
- Fang, M.; Ho, J.C. Area-Selective Atomic Layer Deposition: Conformal Coating, Subnanometer Thickness Control, and Smart Positioning. ACS Nano 2015, 9, 8651–8654. [Google Scholar] [CrossRef] [PubMed]
- Canlas, C.P.; Lu, J.; Ray, N.A.; Grosso-Giordano, N.A.; Lee, S.; Elam, J.W.; Winans, R.E.; Van Duyne, R.P.; Stair, P.C.; Notestein, J.M. Shape-selective sieving layers on an oxide catalyst surface. Nat. Chem. 2012, 4, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gu, X.-K.; Zheng, X.; Pan, H.; Zhu, J.; Chen, S.; Cao, L.; Li, W.-X.; Lu, J. Disentangling the size-dependent geometric and electronic effects of palladium nanocatalysts beyond selectivity. Sci. Adv. 2019, 5, eaat6413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liu, Y.; Liang, X. Hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran over supported Pt–Co bimetallic catalysts under mild conditions. Green Chem. 2018, 20, 2894–2902. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Liu, Y.; Park, J.; Liang, X. Atomic layer deposited Pt-Co bimetallic catalysts for selective hydrogenation of α, β-unsaturated aldehydes to unsaturated alcohols. J. Catal. 2018, 366, 61–69. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Q.Q.; Liu, W.; Lin, Y.; Guan, Q.; Zheng, X.; Pan, H.; Zhu, J.; Sun, Z.; Wei, S.; et al. Quasi Pd1Ni single-atom surface alloy catalyst enables hydrogenation of nitriles to secondary amines. Nat. Commun. 2019, 10, 4998–4999. [Google Scholar] [CrossRef]
- Lu, J.; Low, K.-B.; Lei, Y.; Libera, J.A.; Nicholls, A.; Stair, P.C.; Elam, J.W. Toward atomically-precise synthesis of supported bimetallic nanoparticles using atomic layer deposition. Nat. Commun. 2014, 5, 3264. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Yao, Q.; Yan, H.; Li, J.; Lu, J. Precisely Applying TiO2 Overcoat on Supported Au Catalysts Using Atomic Layer Deposition for Understanding the Reaction Mechanism and Improved Activity in CO Oxidation. J. Phys. Chem. C 2015, 120, 478–486. [Google Scholar] [CrossRef]
- Wang, C.; Yao, Q.; Cao, L.; Li, J.; Chen, S.; Lu, J. Precise Tailoring of Ir-FeOx Interfaces for Improved Catalytic Performance in Preferential Oxidation of Carbon Monoxide in Hydrogen. J. Phys. Chem. C 2019, 123, 29262–29270. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, C.; Wang, H.; Yan, H.; Lu, J. Revisiting the Au Particle Size Effect on TiO2-Coated Au/TiO2 Catalysts in CO Oxidation Reaction. J. Phys. Chem. C 2016, 120, 9174–9183. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Q.; Lang, Y.; Cao, K.; Chu, S.; Shan, B.; Chen, R. Oxide-Nanotrap-Anchored Platinum Nanoparticles with High Activity and Sintering Resistance by Area-Selective Atomic Layer Deposition. Angew. Chem. Int. Ed. 2017, 56, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, K.; Zhang, Z.; Hu, X.; Tian, H.; Zhang, Y.; Yang, X. MnO2–Graphene-oxide-scroll–TiO2 composite catalyst for low-temperature NH3-SCR of NO with good steam and SO2 resistance obtained by low-temperature carbon-coating and selective atomic layer deposition. Catal. Sci. Technol. 2019, 9, 1602–1608. [Google Scholar] [CrossRef]
- Lv, P.; Zhao, C.; Lee, W.J.; Huo, S.; Kwon, S.-H.; Fang, J.; Yang, Y. Less is more: Enhancement of photocatalytic activity of g-C3N4 nanosheets by site-selective atomic layer deposition of TiO2. Appl. Surf. Sci. 2019, 494, 508–518. [Google Scholar] [CrossRef]
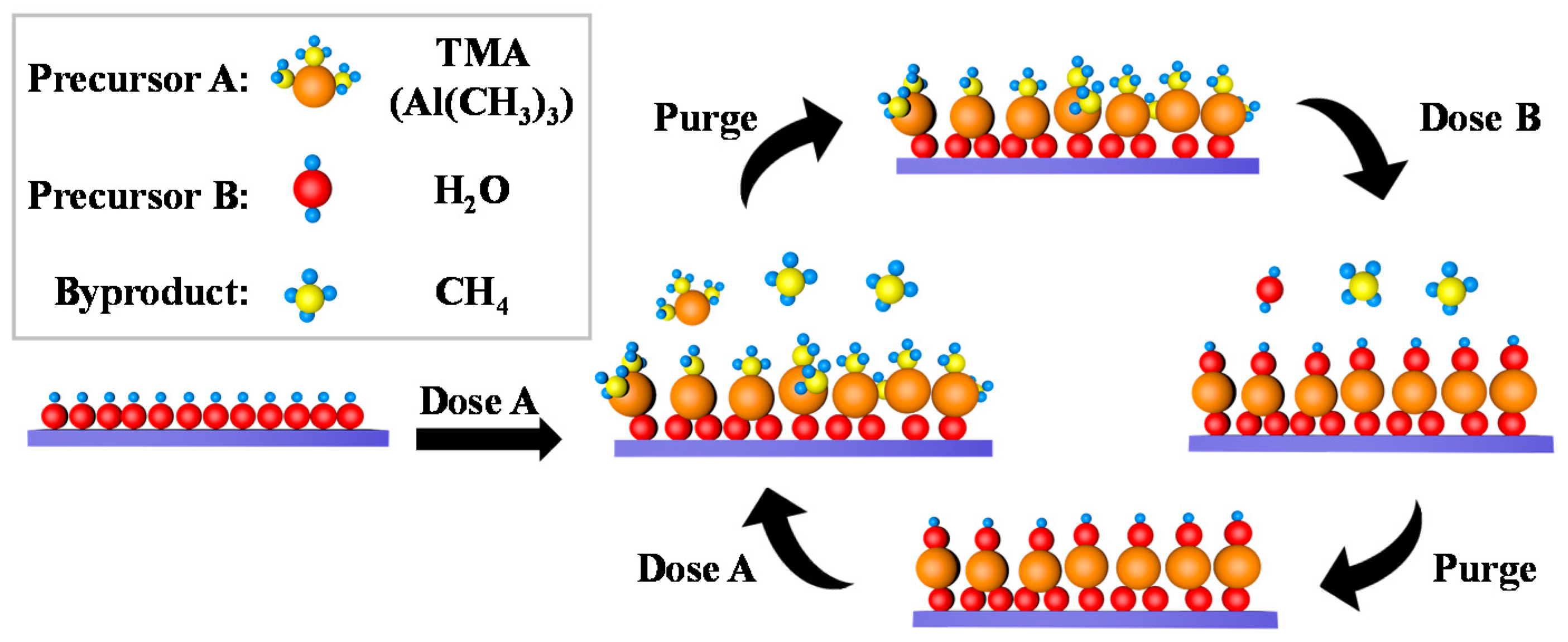

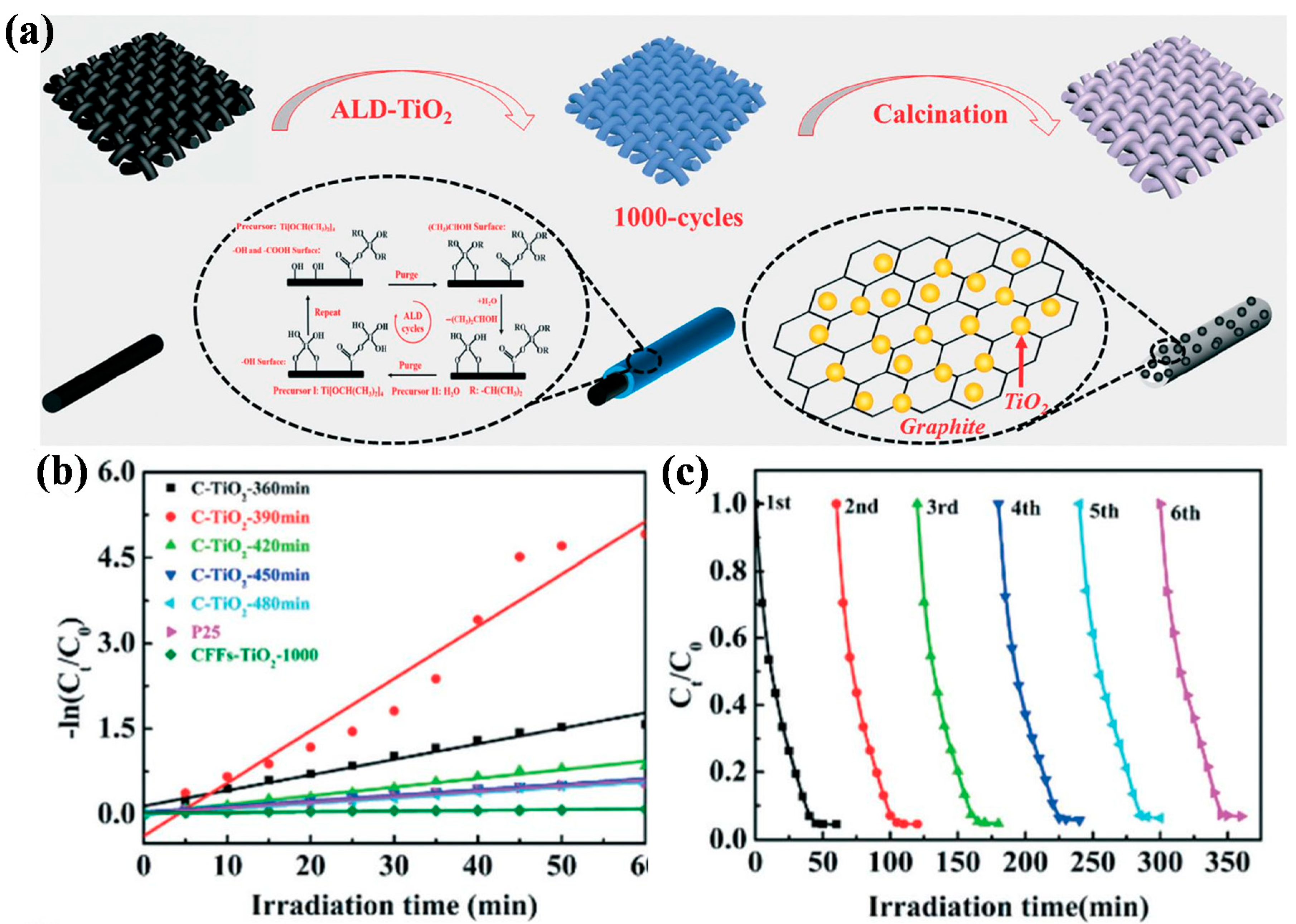
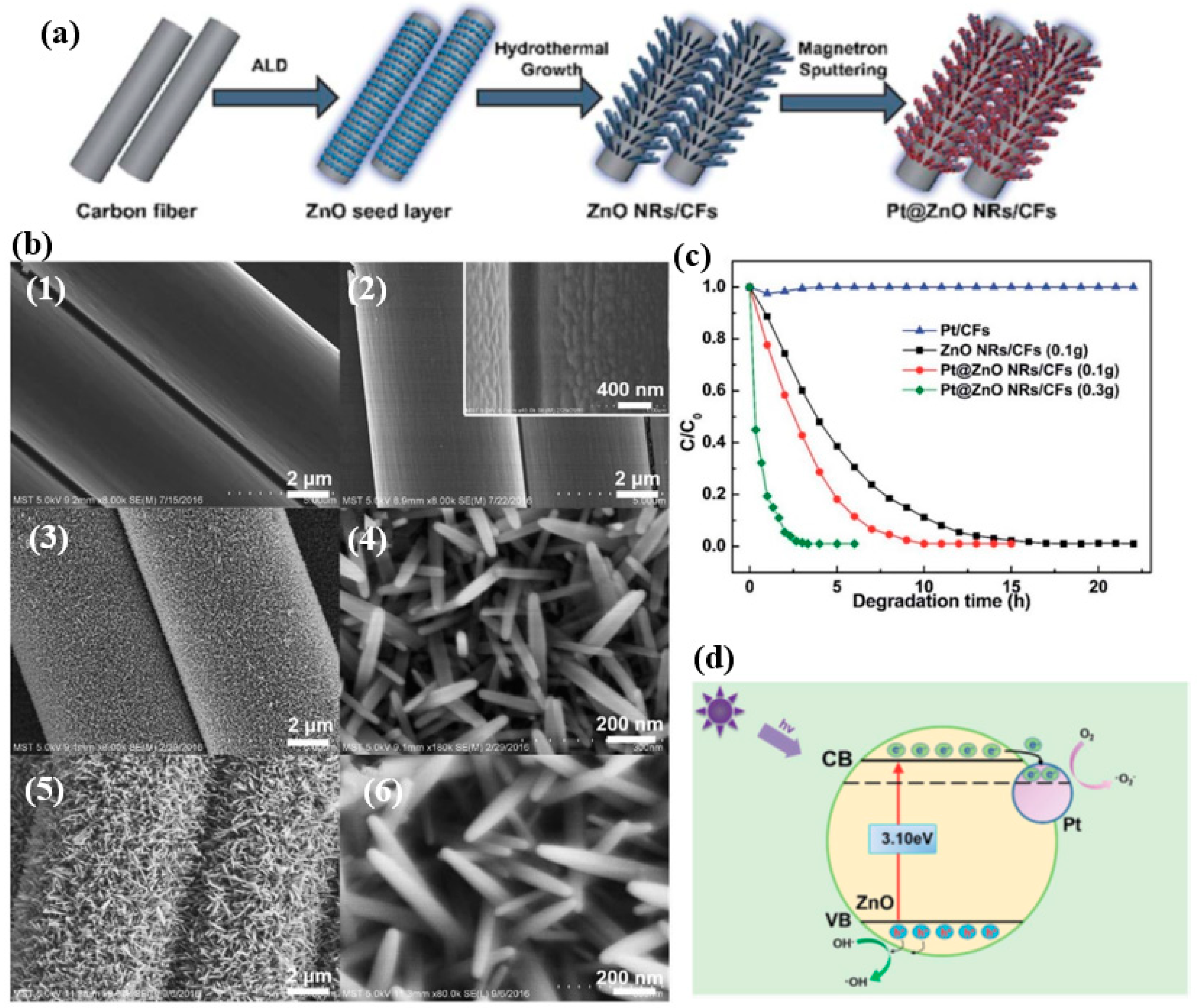
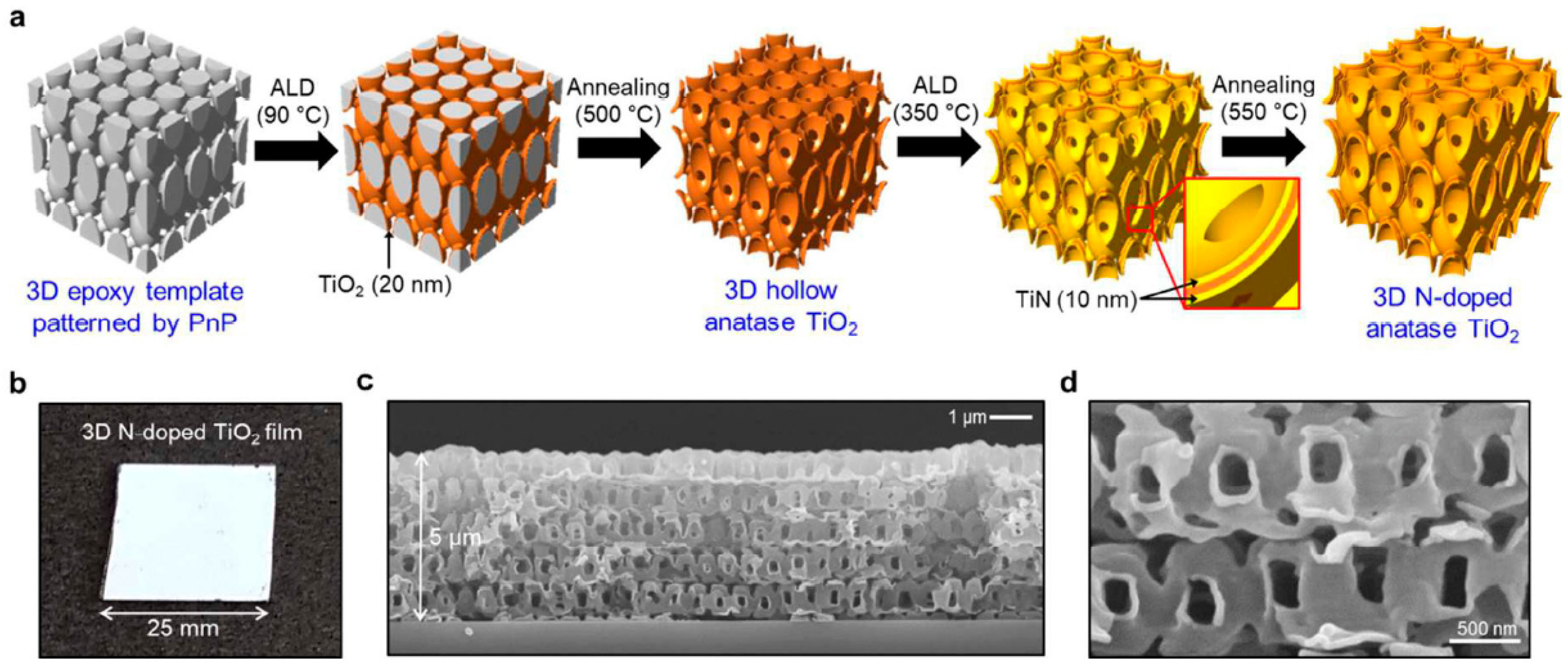
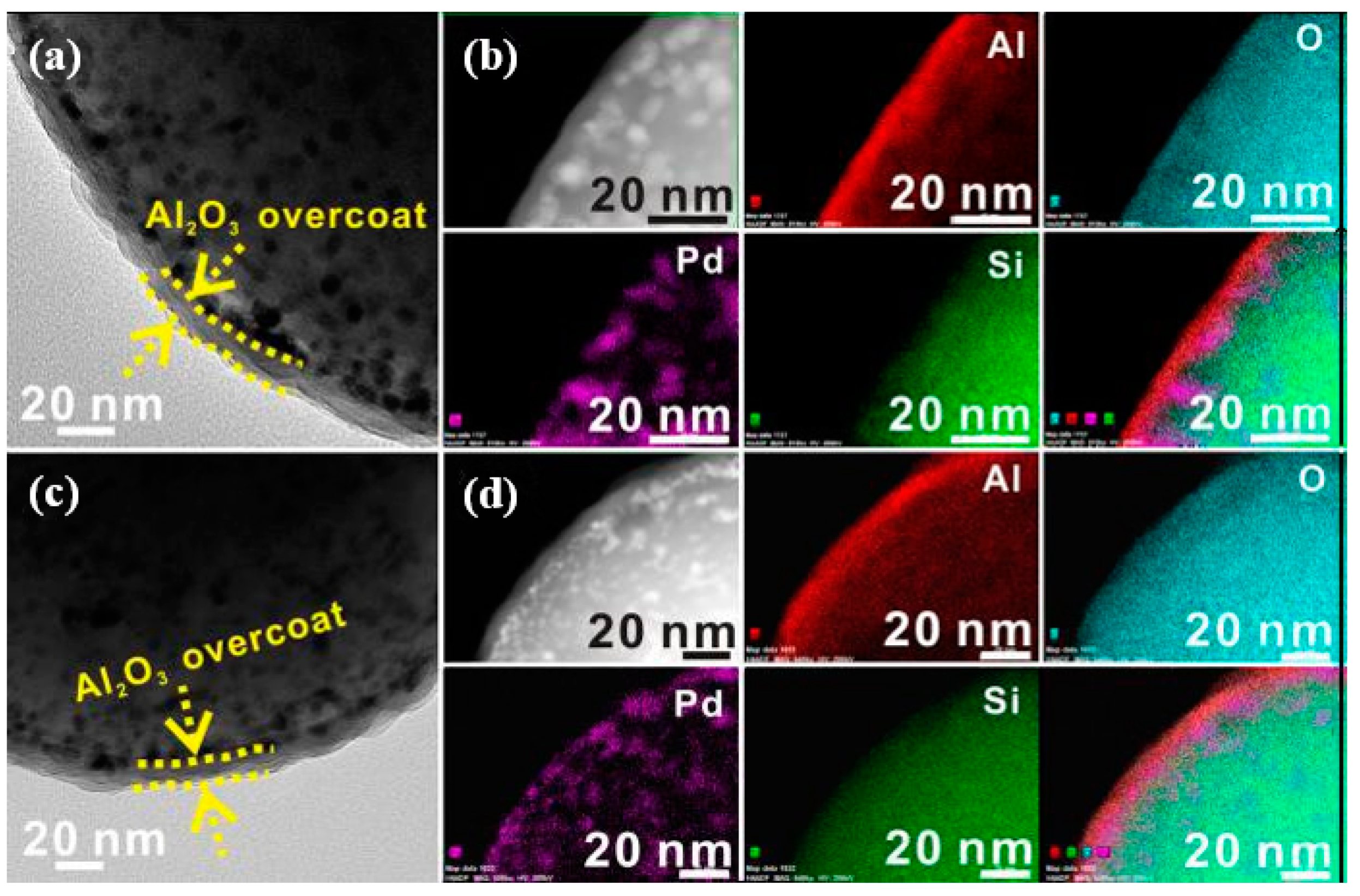
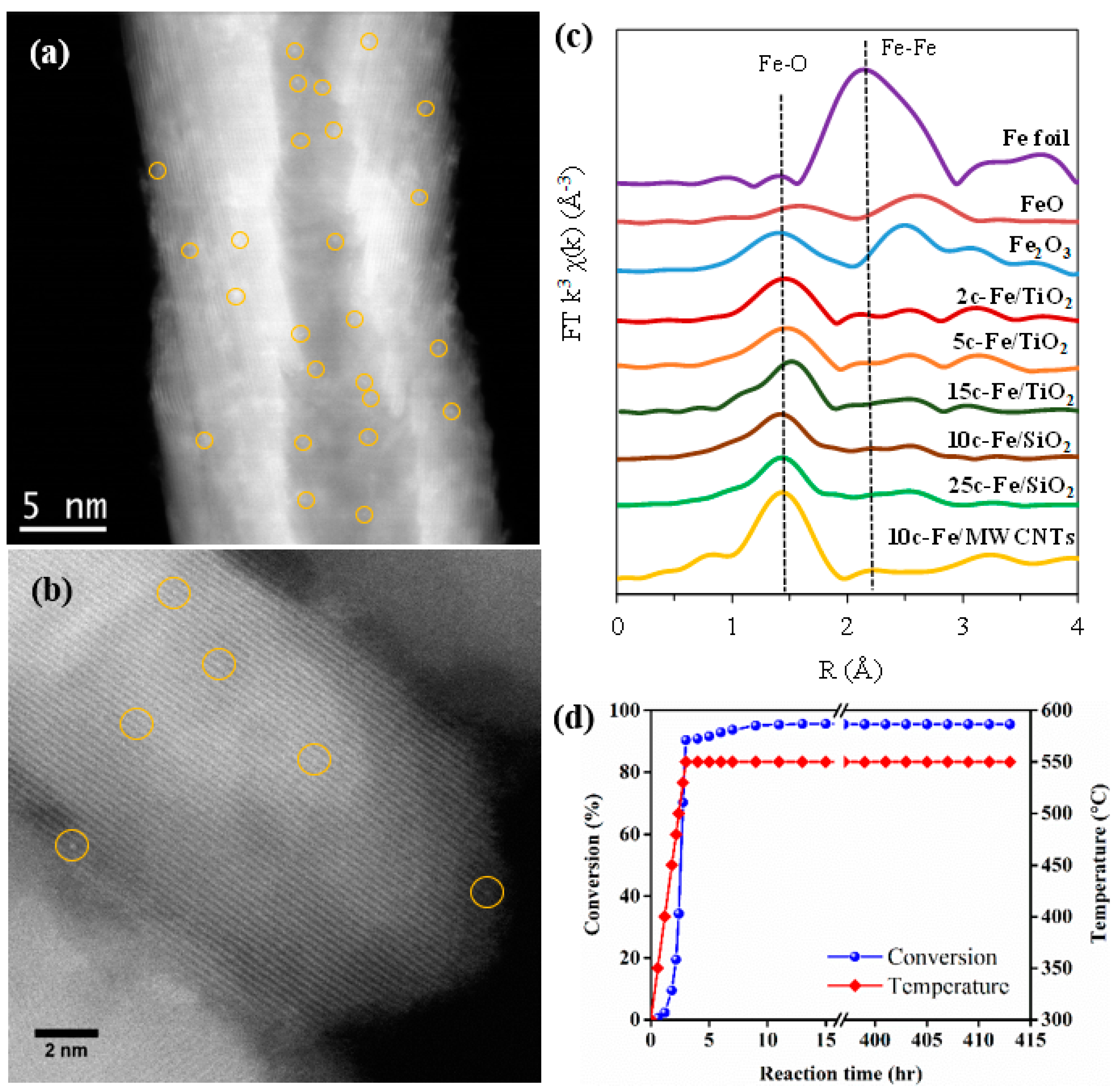
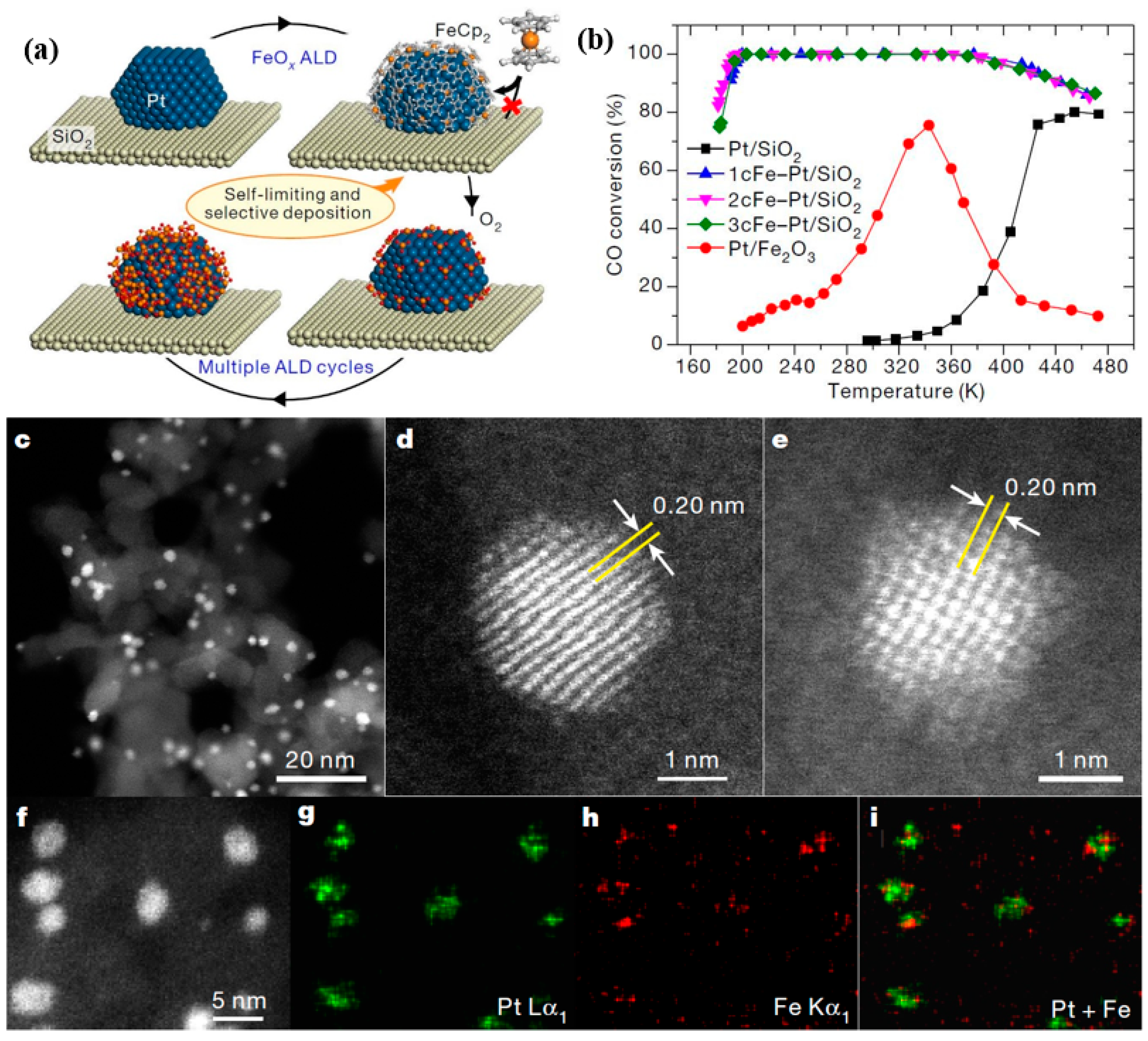
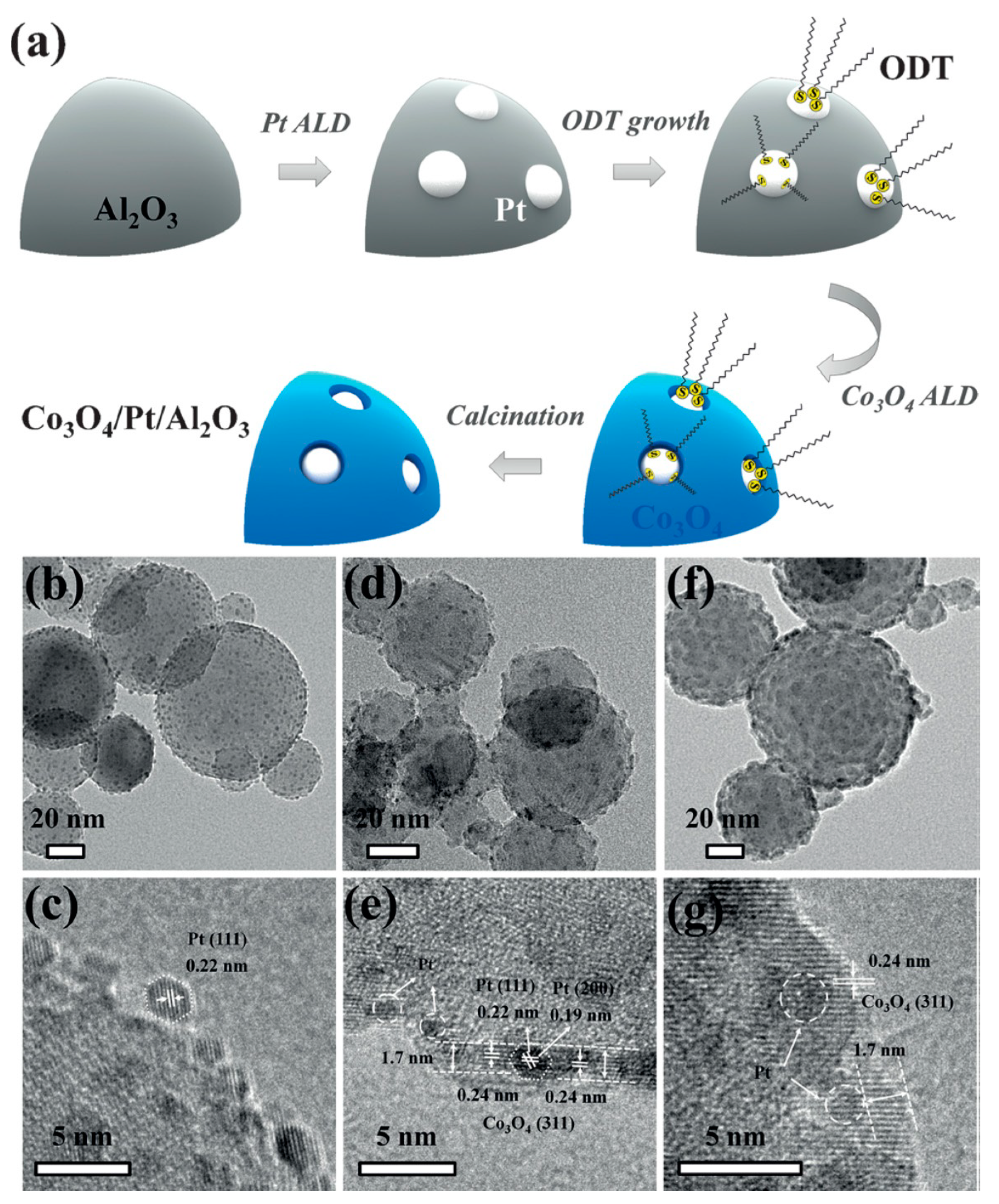

| Entry | ALD | Number of Cycles | Catalyst | Pollutant | Light | kapp (min−1) 1 | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Al2O3 | 10 | TiO2/V2O5/Al2O3 | RhB | UV light | - | [31] |
| 2 | Al2O3 | (−8 nm) | Al-rich Al2O3 ultra-thin film | RB5 | UV light | 0.163 | [32] |
| 3 | TiO2 | 5 | g-C3N4@TiO2 | RhB | Visible light | 0.00324 | [33] |
| 4 | TiO2 | 30 | 30TiO2@P25 | 2,4-D | λ >320 nm | 5.0 | [34] |
| 5 | SnO2, TiO2 | 1000, 200 | SnO2@TiO2 nanopillar-array | MB | UV light | - | [35] |
| 6 | TiO2 | 1000 | C-TiO2-390 | MB | Visible light | 0.0917 | [36] |
| 7 | TiO2 | 250 | TiO2 inverse opals | MB | UV light | 0.00415 | [37] |
| 8 | TiO2 | 10 | SnO2/TiO2 core shell nanowires | MO | UV light | 0.00147 | [38] |
| 9 | TiO2 | 80 | Au-80@TiO2 | MB | UV light | - | [39] |
| 10 | TiO2 | 400 | CNT/TiO2 | MB | UV light | 0.0101 | [40] |
| 11 | TiO2 | 1125 | TiO2/Graphene | Methyl red | UV-vis light | 0.0064 | [41] |
| 12 | TiO2, Ga2O3 | −(6.5 nm, 8.0 nm) | TiO2-Ga2O3 | MO | UV light | - | [42] |
| 13 | TiN | 100 | TiO2@100TiN | MO | Visible light | 0.027 | [43] |
| 14 | TiN | (−10 nm) | 3D N-doped TiO2 | MB | Solar light | - | [44] |
| 15 | ZnO | 100 | g-C3N4@ZnO | cephalexin | Solar light | 0.0735 | [14] |
| 16 | ZnO | 300 | ZnO/ZFO-2 JHNFs | MB | Visible light | 0.0187 | [45] |
| 17 | ZnO | 5 | g-C3N4@ZnO | MB | Visible light | 0.0263 | [29] |
| 18 | ZnO | 400 | PAN@PANI@ZnO nanofibers | MB | UV light | 0.040 | [46] |
| 19 | ZnO | 400 | ZnO/glass | MB | UV light | 0.0037 | [47] |
| 20 | ZnO | 300 | Pt@ZnO NRs/CFs | MO | UV light | - | [48] |
| 21 | ZnO | 120 | CNT-ZnO | MO | UV light | 0.0026 | [49] |
| 22 | ZnO, TiO2 | 30, 30 | ZnO-TiO2/CNT membrane | MB | UV light | 0.0203 | [50] |
| 23 | CeO2 | 40 | CeO2/TiO2 | MB | UV light | 0.079 | [51] |
| 24 | ZrO2 | 45 | ZrO2/TiO2 | MB | UV light | 0.127 | [52] |
| 25 | SnO | 236 | SnO/TiO2 | MB | UV-vis light | 0.010 | [53] |
| 26 | SnO | (−3 nm) | Sn (O,S)/TiO2 | MB | Visible light | 0.114 | [54] |
| 27 | Fe, Pt | 30, 30 | g-C3N4/FePt-2 | RhB | Visible light | 0.0891 | [55] |
| 28 | Pd | 10 | BaZrO3/Au-Pd | RhB | UV light | 0.0408 | [56] |
| 29 | Fe | 2 | Fe/TiO2 | MB | UV light | 0.155 | [13] |
| 30 | MgO | 5 | 5_MgO@Ag_TiO2 | phenol | Solar light | - | [57] |
| 31 | Pd | 50 | Pd-TiO2 | 2,4-D | UV-vis light | - | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhao, Z.; Zhang, C.; Li, Q.; Liang, X. Surface Modification of Catalysts via Atomic Layer Deposition for Pollutants Elimination. Catalysts 2020, 10, 1298. https://doi.org/10.3390/catal10111298
Wang X, Zhao Z, Zhang C, Li Q, Liang X. Surface Modification of Catalysts via Atomic Layer Deposition for Pollutants Elimination. Catalysts. 2020; 10(11):1298. https://doi.org/10.3390/catal10111298
Chicago/Turabian StyleWang, Xiaofeng, Zhe Zhao, Chengcheng Zhang, Qingbo Li, and Xinhua Liang. 2020. "Surface Modification of Catalysts via Atomic Layer Deposition for Pollutants Elimination" Catalysts 10, no. 11: 1298. https://doi.org/10.3390/catal10111298





