Copper- and Nitrogen-Codoped Graphene with Versatile Catalytic Performances for Fenton-Like Reactions and Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Results and Discussion
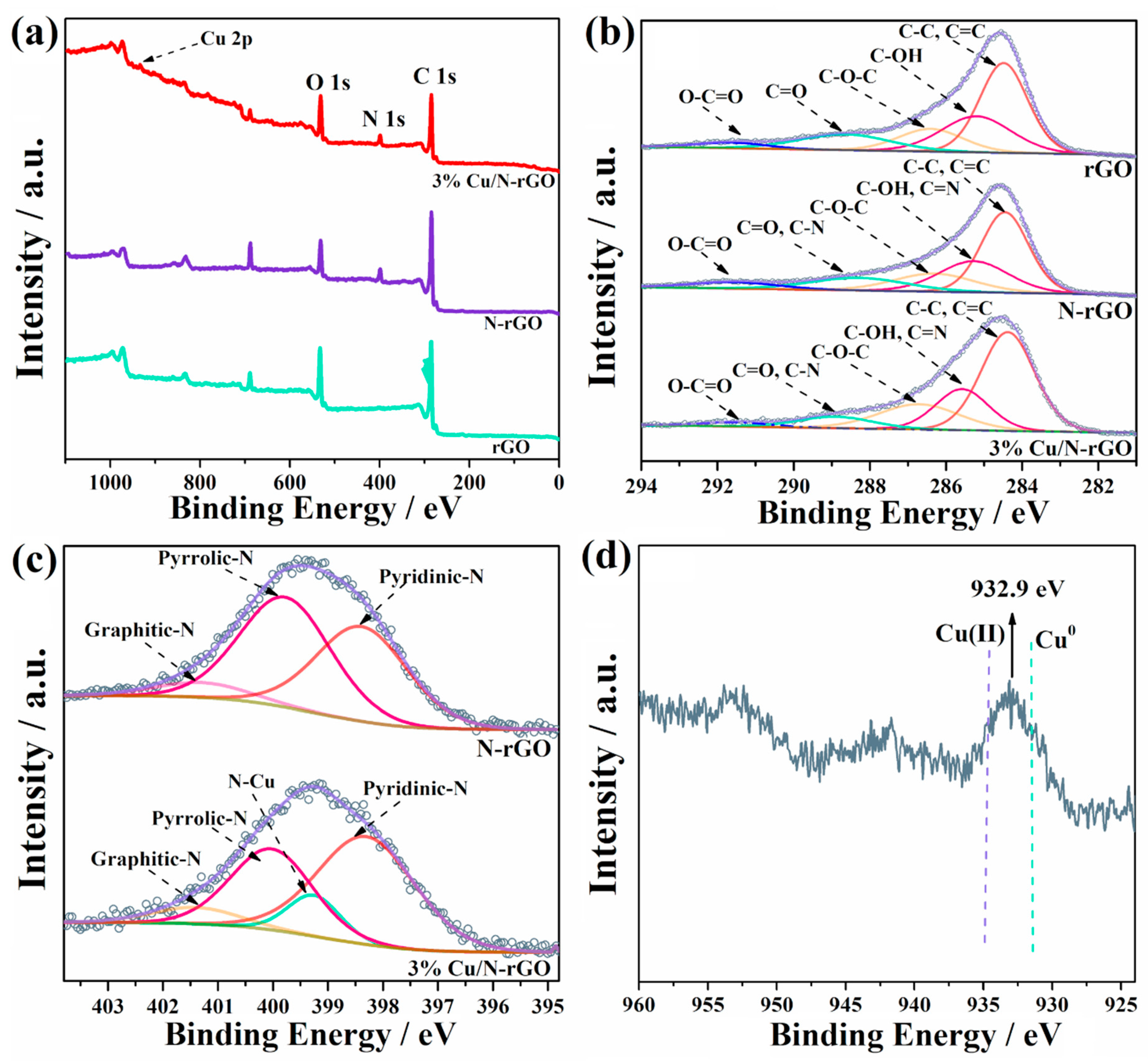
2.1. Characterizations of Cu/N-rGO
2.2. H2O2 Activation Catalyzed by Cu/N-rGO for the Oxidative Degradation of Rhodamine B (RhB)
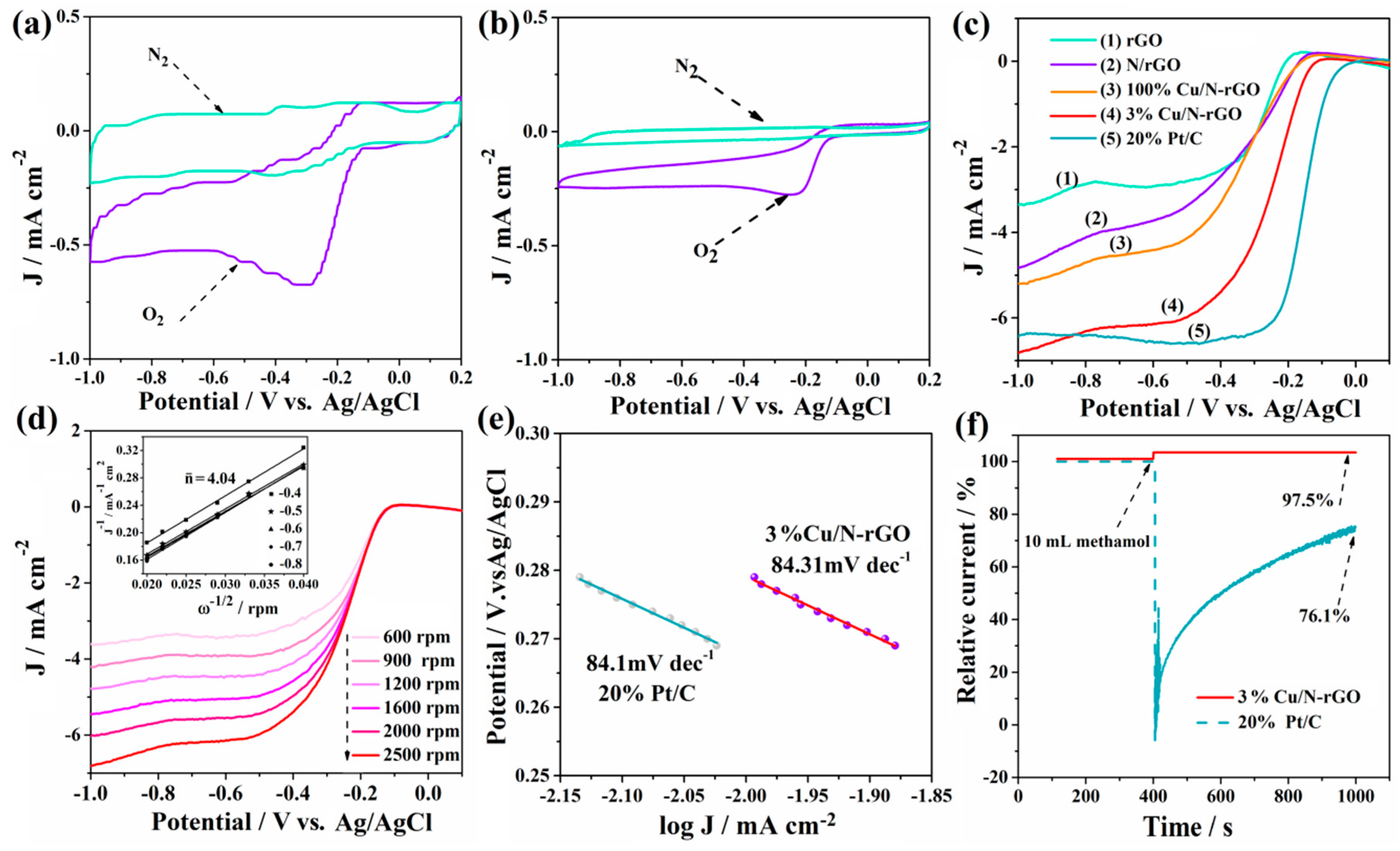
2.3. Electrocatalytic Performance of Cu/N-rGO
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of the Cu/N-rGO Catalyst
3.3. Characterizations
3.4. Adsorption and Degradation Experiments
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peng, Z.; Qiu, X.; Yu, Y.; Jiang, D.; Wang, H.; Cai, G.; Zhang, X.; Dong, Z. Polydopamine coated prussian blue analogue derived hollow carbon nanoboxes with FeP encapsulated for hydrogen evolution. Carbon 2019, 152, 16–23. [Google Scholar] [CrossRef]
- Tan, H.; Tang, J.; Kim, J.; Kaneti, Y.V.; Kang, Y.M.; Sugahara, Y.; Yamauchi, Y. Rational design and construction of nanoporous iron- and nitrogen-doped carbon electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2019, 7, 1380–1393. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Xu, J. Separation of hydrogen sulfide from gas phase using Ce3+/Mn2+-enhanced fenton-like oxidation system. Chem. Eng. J. 2019, 359, 1486–1492. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Z.; Zhu, L.; Wang, N.; Tang, H. Bisulfite-induced drastic enhancement of bisphenol A degradation in Fe3+-H2O2 Fenton system. Chem. Eng. J. 2019, 361, 1190–1197. [Google Scholar] [CrossRef]
- Liu, P.; Hu, Y.; Liu, X.; Wang, T.; Xi, P.; Xi, S.; Gao, D.; Wang, J. Cu and Co nanoparticle-Co-decorated N-doped graphene nanosheets: A high efficiencybifunctional electrocatalyst for rechargeable Zn–air batteries. J. Mater. Chem. A 2019, 7, 12851–12858. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Z.; Meng, S.; Wang, Y.; Li, D. Regulating charge transfer over 3D Au/ZnO hybrid inverse opal toward efficiently photocatalytic degradation of bisphenol A and photoelectrochemical water splitting. Chem. Eng. J. 2020, 393, 124676. [Google Scholar] [CrossRef]
- Su, J.; Yang, Y.; Xia, G.; Chen, J.; Jiang, P.; Chen, Q. Ruthenium-cobalt nanoalloys encapsulated in nitrogen-doped graphene as active electrocatalysts for producing hydrogen in alkaline media. Nat. Commun. 2017, 8, 14969. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ye, X.; Chen, G.; Li, D.; Meng, S.; Chen, S. Synthesis of BiPO4 by crystallization and hydroxylation with boosted photocatalytic removal of organic pollutants in air and water. J. Hazard. Mater. 2020, 399, 122999. [Google Scholar] [CrossRef]
- Dias, J.A.; Arantes, V.L.; Ramos, A.S.; Giraldi, T.R.; Minucci, M.Z.; Maestrelli, S.C. Characterization and photocatalytic evaluation of ZnO–Co3O4 particles obtained by high energy milling. Part II: Photocatalytic properties. Ceram. Int. 2016, 42, 3485–3490. [Google Scholar] [CrossRef]
- Wang, X.R.; Liu, J.Y.; Liu, Z.W.; Wang, W.C.; Luo, J.; Han, X.P.; Du, X.W.; Qiao, S.Z.; Yang, J. Identifying the Key Role of Pyridinic-N-Co Bonding in Synergistic Electrocatalysis for Reversible ORR/OER. Adv. Mater. 2018, 30, e1800005. [Google Scholar] [CrossRef]
- Peng, J.; Xue, J.; Li, J.; Du, Z.; Wang, Z.; Gao, S. Catalytic effect of low concentration carboxylated multi-walled carbon nanotubes on the oxidation of disinfectants with Cl-substituted structure by a Fenton-like system. Chem. Eng. J. 2017, 321, 325–334. [Google Scholar] [CrossRef]
- Waki, K.; Wong, R.A.; Oktaviano, H.S.; Fujio, T.; Nagai, T.; Kimoto, K.; Yamada, K. Non-nitrogen doped and non-metal oxygen reduction electrocatalysts basedon carbon nanotubes: Mechanism and origin of ORR activity. Energy Environ. Sci. 2014, 7, 1950–1958. [Google Scholar] [CrossRef]
- Kannan, M.V.; Kumar, G.G. Current status, key challenges and its solutions in the design and development of graphene based ORR catalysts for themicrobial fuel cell applications. Biosens. Bioelectron. 2016, 77, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhang, Y.; Zhang, C.; Miao, D.; Li, J.; Liu, H.; Wang, L.; Gao, S. Removal of triclosan in a Fenton-like system mediated by graphene oxide: Reaction kinetics and ecotoxicity evaluation. Sci. Total Environ. 2019, 673, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ding, Z.; Liu, P.; Antonietti, M.; Fu, X.; Wang, X. Metal-free activation of H2O2 by g-C3N4 under visible light irradiation for the degradation of organic pollutants. Phys. Chem. Chem. Phys. 2012, 14, 1455–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Xie, M.; Meng, S.; Fu, X.; Chen, S. Coupled systems for selective oxidation of aromatic alcohols to aldehydes and reduction of nitrobenzene into aniline using CdS/g-C3N4 photocatalyst under visible light irradiation. Appl. Catal. B Environ. 2014, 158–159, 382–390. [Google Scholar] [CrossRef]
- Qin, Y.; Ding, Y.; Tang, H. Highly efficient visible-light photocatalytic activity of graphitic carbon nitride prepared from melamine-thiourea molecular composite. J. Environ. Chem. Eng. 2016, 4, 4374–4384. [Google Scholar] [CrossRef]
- Mahajan, M.; Singla, G.; Singh, K.; Pandey, O.P. Synthesis of grape-like carbon nanospheres and their application as photocatalyst and electrocatalyst. J. Solid State Chem. 2015, 232, 108–117. [Google Scholar] [CrossRef]
- Shen, M.; Ruan, C.; Chen, Y.; Jiang, C.; Ai, K.; Lu, L. Covalent entrapment of cobalt-iron sulfides in N-doped mesoporous carbon: Extraordinary bifunctional electrocatalysts for oxygen reduction and evolution reactions. ACS Appl. Mater. Interfaces 2015, 7, 1207–1218. [Google Scholar] [CrossRef]
- Wu, H.; Li, H.; Zhao, X.; Liu, Q.; Wang, J.; Xiao, J.; Xie, S.; Si, R.; Yang, F.; Miao, S.; et al. Highly doped and exposed Cu(I)-N active sites within graphene towards efficient oxygen reduction for zinc–air batteries. Energy Environ. Sci. 2016, 9, 3736–3745. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Xi, S.; Miao, S.; Ding, J.; Cai, W.; Liu, S.; Yang, X.; Yang, H.; Gao, J.; et al. Single Cobalt Atoms Anchored on Porous N-Doped Graphene with Dual Reaction Sites for Efficient Fenton-like Catalysis. J. Am. Chem. Soc. 2018, 140, 12469–12475. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, H.; Lian, C.; Wei, F.; Zhang, D.; Wu, G.; Chen, B.; Wang, S. Fe, Co, Ni nanocrystals encapsulated in nitrogen-doped carbon nanotubes as Fenton-like catalysts for organic pollutant removal. J. Hazard. Mater. 2016, 314, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, Y.; Zhu, L.; Tang, H.Q. Nitrogen-Doped Reduced Graphene Oxide as a Bifunctional Material for Removing Bisphenols: Synergistic Effect between Adsorption and Catalysis. Environ. Sci. Technol. 2015, 49, 6855–6864. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, Q.; Liu, J.; Ma, X.; Rao, Y.; Shao, X.; Li, Z.; Wu, W.; Ning, H.; Wu, M. Synergistically enhanced activity of nitrogen-doped carbon dots/graphene composites for oxygen reduction reaction. Appl. Surf. Sci. 2017, 423, 909–916. [Google Scholar] [CrossRef]
- Qu, L.T.; Liu, Y.; Baek, J.B.; Dai, L.M. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.T.; Wang, J.L. Fe3O4-MWCNT Magnetic Nanocomposites as Efficient Fenton-Like Catalysts for Degradation of Sulfamethazine in Aqueous Solution. Chem. Select. 2017, 2, 10727–10735. [Google Scholar]
- Tang, J.T.; Wang, J.L. Fenton-like degradation of sulfamethoxazole using Fe-based magnetic nanoparticles embedded into mesoporous carbon hybrid as an efficient catalyst. Chem. Eng. J. 2018, 351, 1085–1094. [Google Scholar] [CrossRef]
- Lei, M.; Guo, S.; Wang, Z.; Zhu, L.; Tang, H. Ultrarapid and Deep Debromination of Tetrabromodiphenyl Ether over Noble-Metal-Free Cu/TiO2 Nanocomposites under Mild Conditions. Environ. Sci. Technol. 2018, 52, 11743–11751. [Google Scholar] [CrossRef]
- Lei, M.; Wang, N.; Zhu, L.; Zhou, Q.; Nie, G.; Tang, H. Photocatalytic reductive degradation of polybrominated diphenyl ethers on CuO/TiO2 nanocomposites: A mechanism based on the switching of photocatalytic reduction potential being controlled by the valence state of copper. Appl. Catal. B Environ. 2016, 182, 414–423. [Google Scholar] [CrossRef]
- Ding, Y.; Tang, H.; Zhang, S.; Wang, S.; Tang, H. Efficient degradation of carbamazepine by easily recyclable microscaled CuFeO2 mediated heterogeneous activation of peroxymonosulfate. J. Hazard. Mater. 2016, 317, 686–694. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, J.; Wang, Z.; Xu, Y.; Xing, Z.; Zhang, X.; Guan, Y.; Liao, G.; Li, X. High-loaded single Cu atoms decorated on N doped graphene for boosting Fenton-like catalysis under neutral pH. J. Mater. Chem. A 2020, 8, 13685–13693. [Google Scholar] [CrossRef]
- Lin, L.; Yang, Z.K.; Jiang, Y.F.; Xu, A.W. Nonprecious Bimetallic (Fe,Mo)−N/C Catalyst for Efficient Oxygen Reduction Reaction. ACS Catal. 2016, 6, 4449–4454. [Google Scholar] [CrossRef]
- Jiang, K.; Siahrostami, S.; Akey, A.J.; Li, Y.; Lu, Z.; Lattimer, J.; Hu, Y.; Stokes, C.; Gangishetty, M.; Chen, G.; et al. Transition-Metal Single Atoms in a Graphene Shell as Active Centers for Highly Efficient Artificial Photosynthesis. Chem 2017, 3, 950–960. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Waller, G.; Liu, Y.; Liu, M.; Wong, C.P. Facile Synthesis of Nitrogen-Doped Graphene via Pyrolysis of Graphene Oxide and Urea, and its Electrocatalytic Activity toward the Oxygen-Reduction Reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, L.; Zhang, Y.; Zou, J.; Tang, H. Cobalt particles encapsulated and nitrogen-doped bamboo-like carbon nanotubes as a catalytic and adsorptive bifunctional material for efficient removal of organic pollutants from wastewater. J. Environ. Chem. Eng. 2017, 5, 5322–5330. [Google Scholar] [CrossRef]
- Ju, W.; Bagger, A.; Hao, G.P.; Varela, A.S.; Sinev, I.; Bon, V.; Cuenya, B.R.; Kaskel, S.; Rossmeisl, J.; Strasser, P. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2. Nat. Commun. 2017, 8, 944. [Google Scholar] [CrossRef]
- Qu, Y.; Li, Z.; Chen, W.; Lin, Y.; Yuan, T.; Yang, Z.; Zhao, C.; Wang, J.; Zhao, C.; Wang, X.; et al. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms. Nat. Catal. 2018, 1, 781–786. [Google Scholar] [CrossRef]
- Galtayries, A.; Bonnelle, J.P. XPS and ISS studies on the interaction of H2S with polycrystalline Cu, Cu2O and CuO surfaces. Surf. Interface Anal. 1995, 23, 171–179. [Google Scholar] [CrossRef]
- Wang, Y.; Biradar, A.V.; Wang, G.; Sharma, K.K.; Duncan, C.T.; Rangan, S.; Asefa, T. Controlled Synthesis of Water-Dispersible Faceted Crystalline Copper Nanoparticles and Their Catalytic Properties. Chem. Eur. J. 2010, 16, 10735–10743. [Google Scholar] [CrossRef]
- Li, L.; Huang, J.; Hu, X.; Zhang, S.; Dai, Q.; Chai, H.; Gu, L. Activation of sodium percarbonate by vanadium for the degradation of aniline in water: Mechanism and identification of reactive species. Chemosphere 2019, 215, 647–656. [Google Scholar] [CrossRef]
- Zhou, L.; Song, W.; Chen, Z.; Yin, G. Degradation of Organic Pollutants in Wastewater by Bicarbonate-Activated Hydrogen Peroxide with a Supported Cobalt Catalyst. Environ. Sci. Technol. 2013, 47, 3833–3839. [Google Scholar] [CrossRef] [PubMed]
- Bagal, M.V.; Gogate, P.R. Sonochemical degradation of alachlor in the presence of process intensifying additives. Sep. Purif. Technol. 2012, 90, 92–100. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, H.; Yang, W.; Zhou, H.; Gao, N.; Fu, C.; Li, S.; Li, H.; Kuang, Y. In Situ Self-Template Synthesis of Fe-N-Doped Double-Shelled Hollow Carbon Microspheres for Oxygen Reduction Reaction. ACS Nano 2018, 12, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Yao, T.; Zheng, L.; Lin, Y.; Ju, H.; Zhu, J.; Hong, X.; Deng, Z.; Zhou, G.; Wei, S.; et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. Int. Ed. 2016, 55, 10800–10805. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Chen, Z.; Kong, F.; Qiao, Y.; Kong, A.; Shan, Y. Space-confined synthesis of multilayer Cu–N doped graphene nanosheets for efficient oxygen electroreduction. Dalton Trans. 2017, 46, 8586–8592. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Sui, Q.; Lyu, S.; Hao, H.; Schröder, H.F.; Gebhardt, W. Elucidation of theoxidation mechanisms and pathways of sulfamethoxazole degradation under Fe(II) activated percarbonate treatment. Sci. Total Environ. 2018, 640, 973–980. [Google Scholar] [CrossRef]
- Ge, L.; Moor, K.; Zhang, B.; He, Y.; Kim, J.H. Electron Transfer Mediation by Aqueous C60 Aggregatesin H2O2/UV Advanced Oxidation of Indigo Carmine. Nanoscale 2014, 6, 13579–13585. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Fu, X.; Lu, C.; HoonKim, S. Mesoporous sulfur-modified iron oxide as an effective Fenton-like catalyst for degradation of bisphenol A. Appl. Catal. B Environ. 2016, 184, 132–141. [Google Scholar] [CrossRef]
- Hummer, W.; Offeman, R. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339–1340. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, X.; Wang, X.; Huang, C.; Zhu, L. Copper- and Nitrogen-Codoped Graphene with Versatile Catalytic Performances for Fenton-Like Reactions and Oxygen Reduction Reaction. Catalysts 2020, 10, 1326. https://doi.org/10.3390/catal10111326
Liao X, Wang X, Huang C, Zhu L. Copper- and Nitrogen-Codoped Graphene with Versatile Catalytic Performances for Fenton-Like Reactions and Oxygen Reduction Reaction. Catalysts. 2020; 10(11):1326. https://doi.org/10.3390/catal10111326
Chicago/Turabian StyleLiao, Xiuxiang, Xiaobo Wang, Cuiyu Huang, and Lihua Zhu. 2020. "Copper- and Nitrogen-Codoped Graphene with Versatile Catalytic Performances for Fenton-Like Reactions and Oxygen Reduction Reaction" Catalysts 10, no. 11: 1326. https://doi.org/10.3390/catal10111326




