Phthalocyanine-Grafted Titania Nanoparticles for Photodegradation of Ibuprofen
Abstract
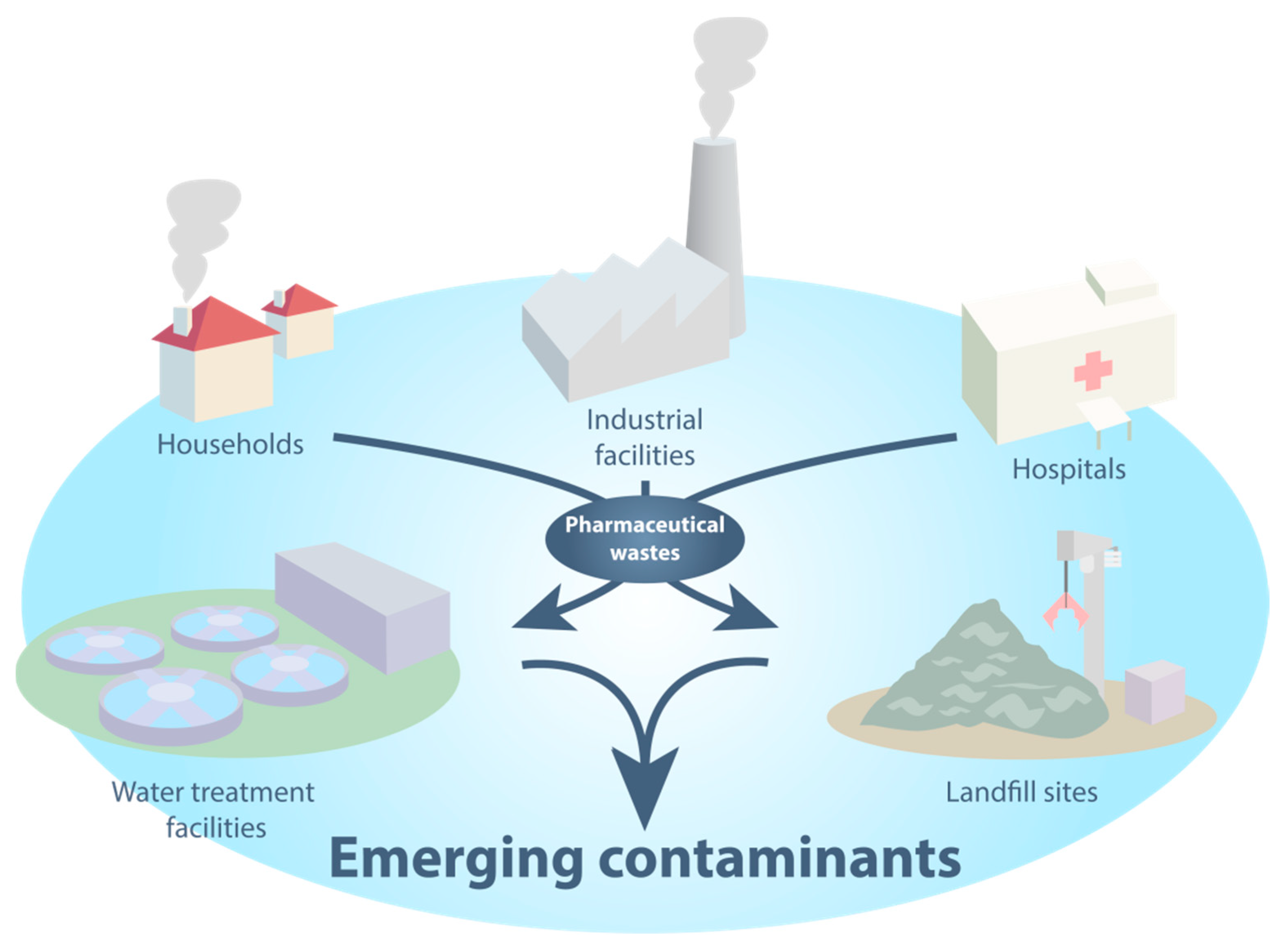
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of the Nanoparticles
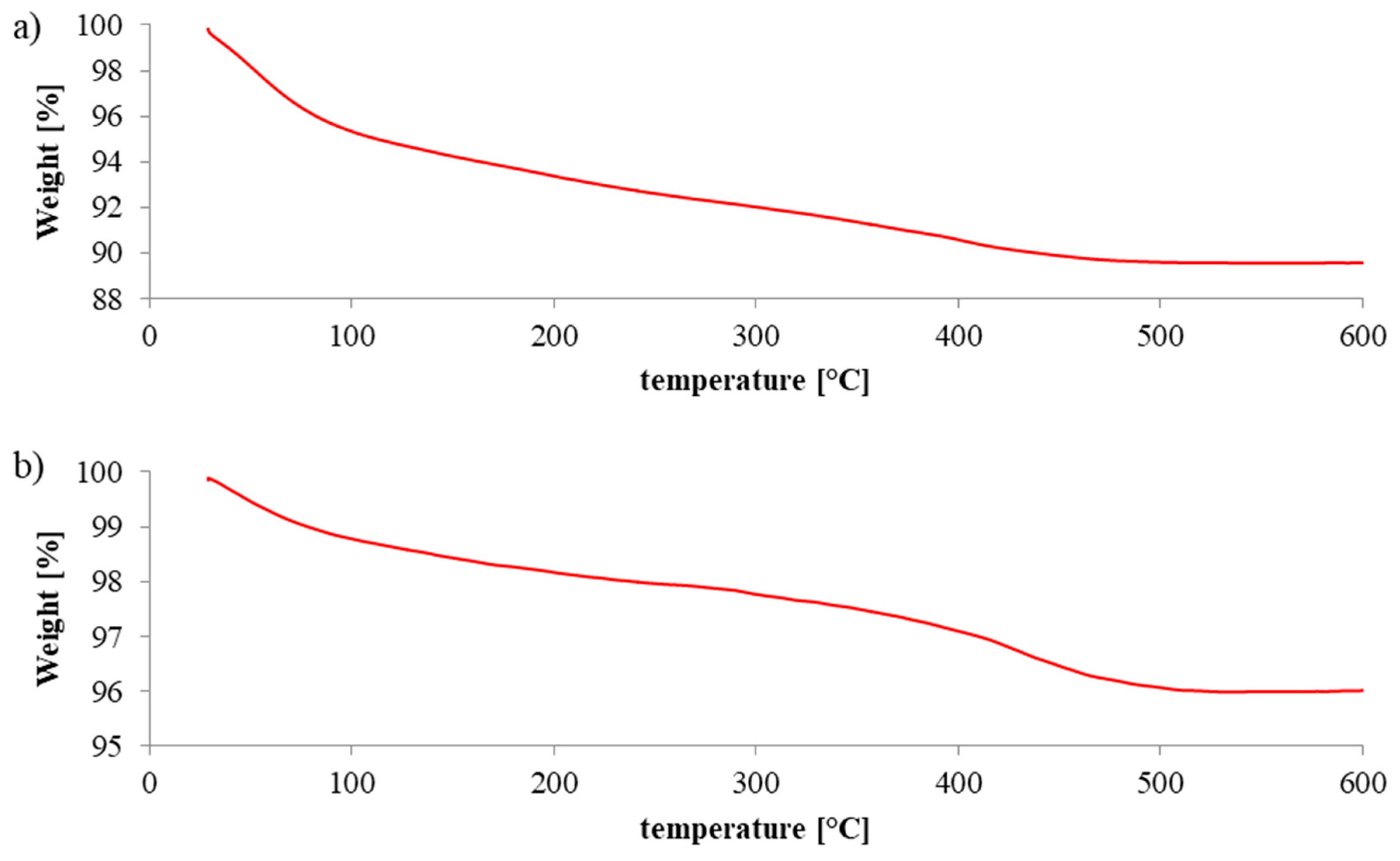
2.2. Thermoanalysis
2.3. X-ray Powder Diffraction Analysis
2.4. XPS
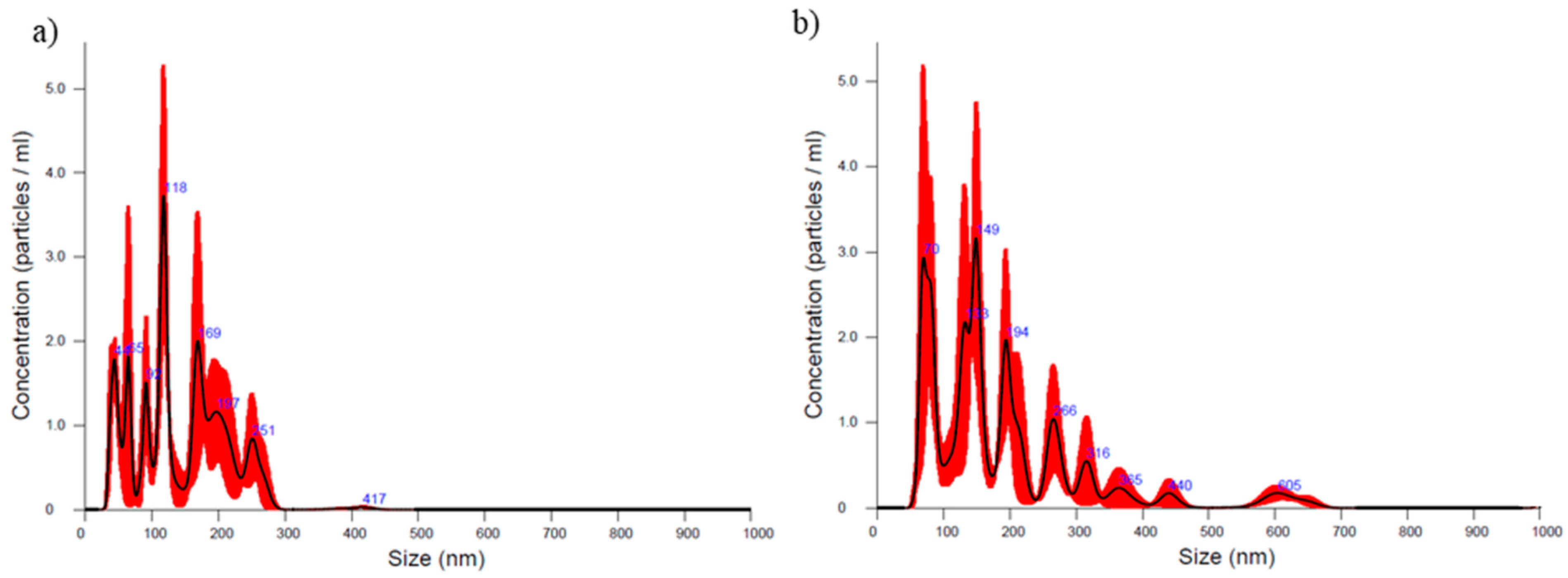
2.5. Particle Size
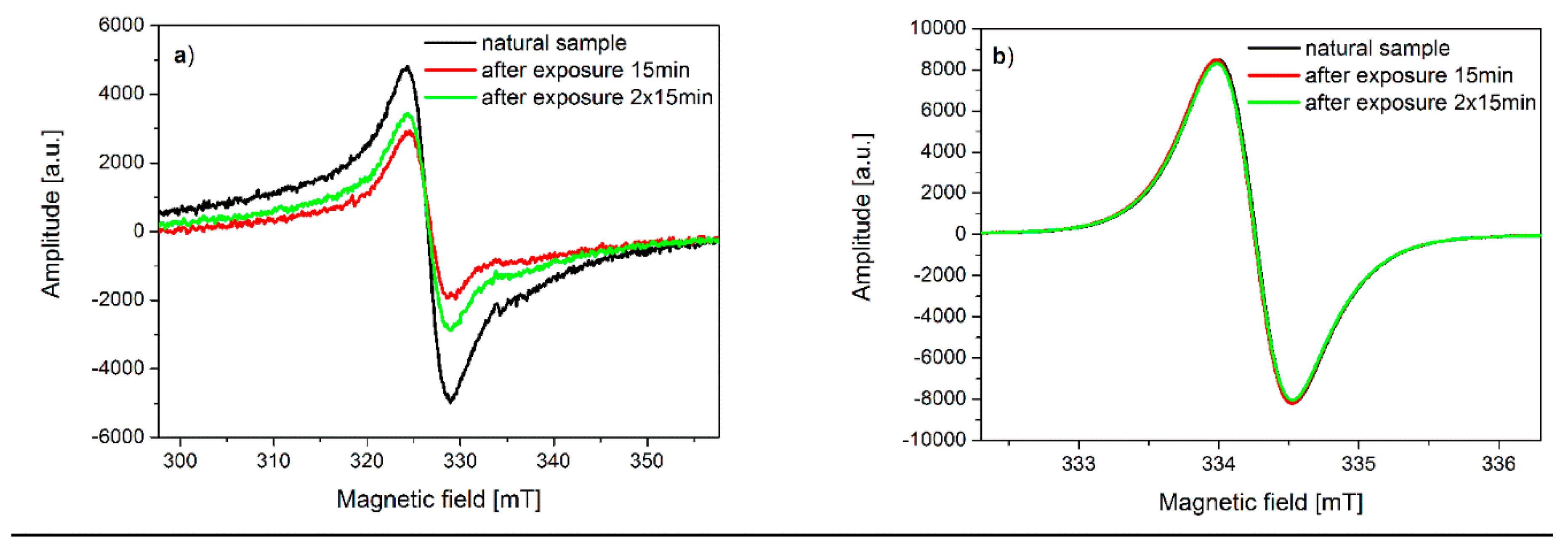
2.6. Electron Spin Resonance Spectroscopy
2.7. BET Surface Area Analysis
2.8. Photochemical Studies
2.8.1. Photodegradation of Ibuprofen
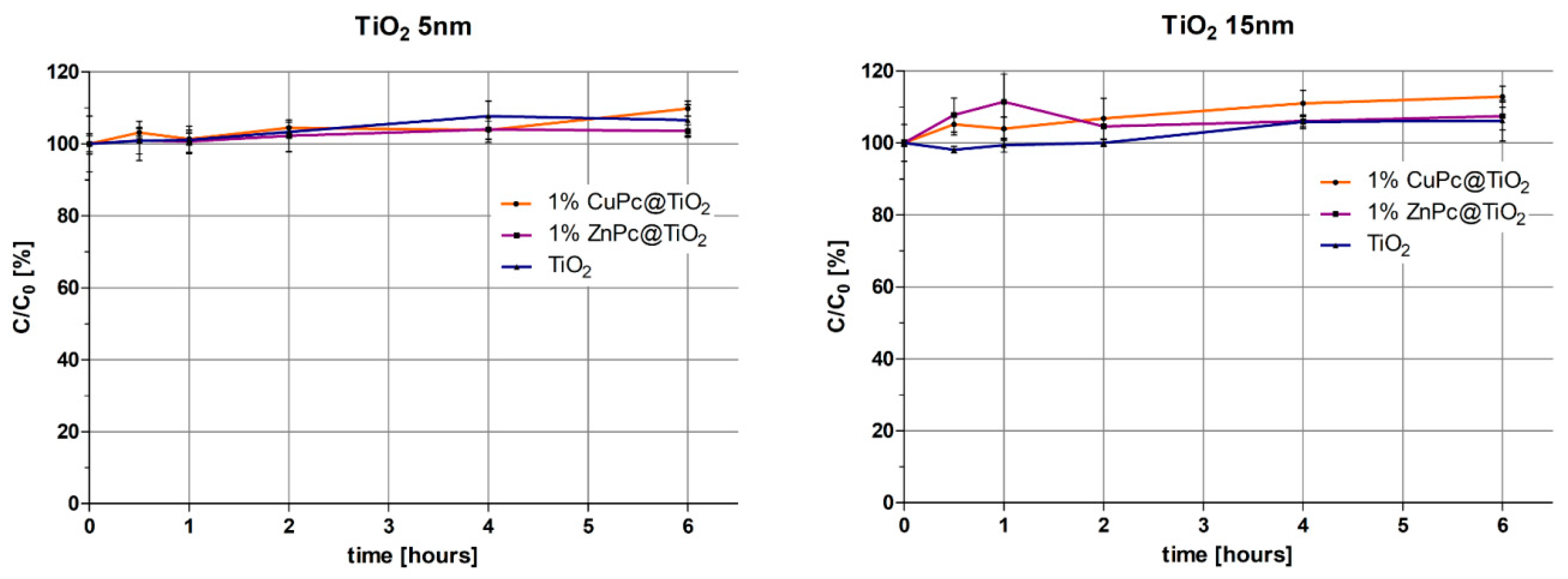
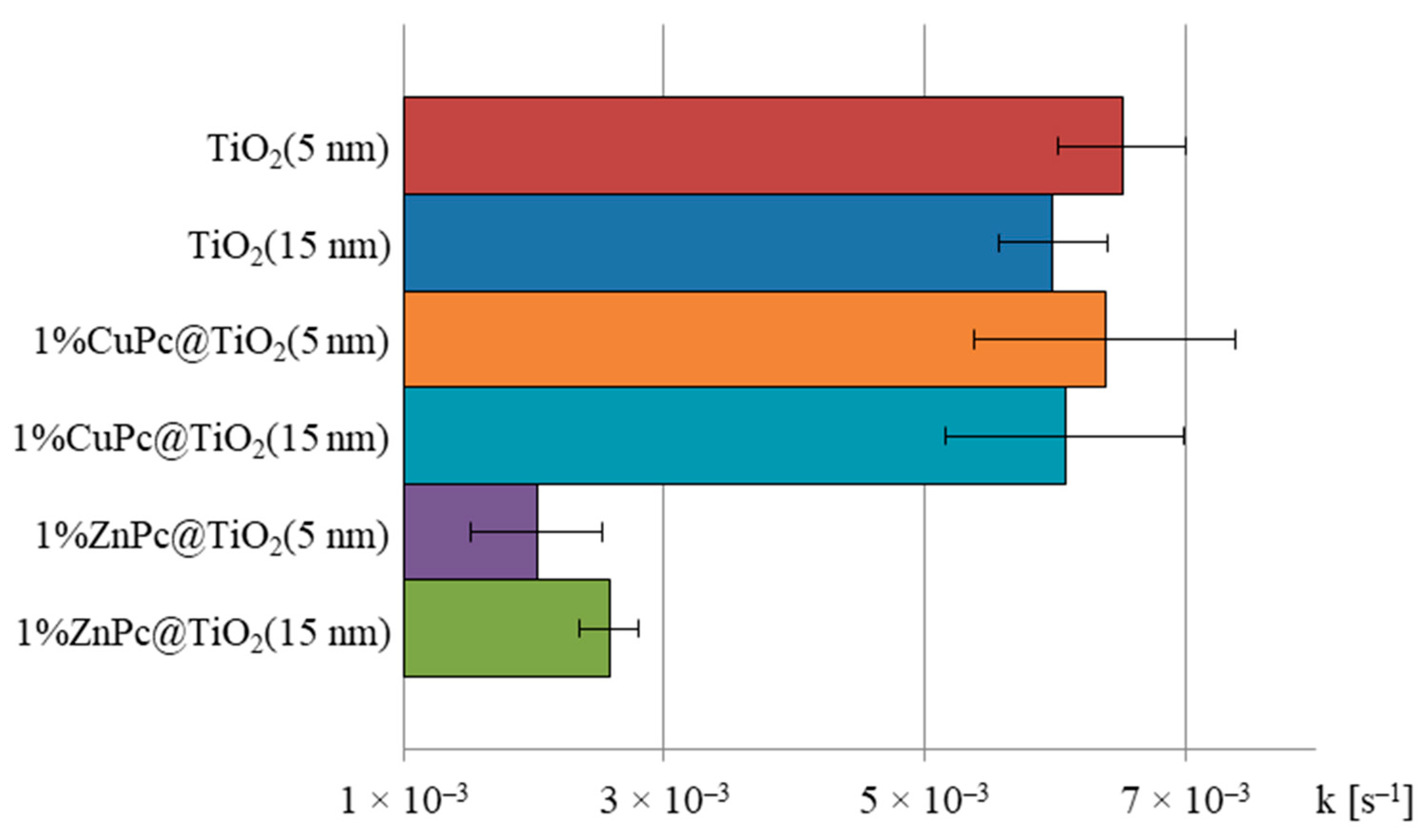
2.8.2. Calculation of Photodegradation Constants for Various Conditions Applied
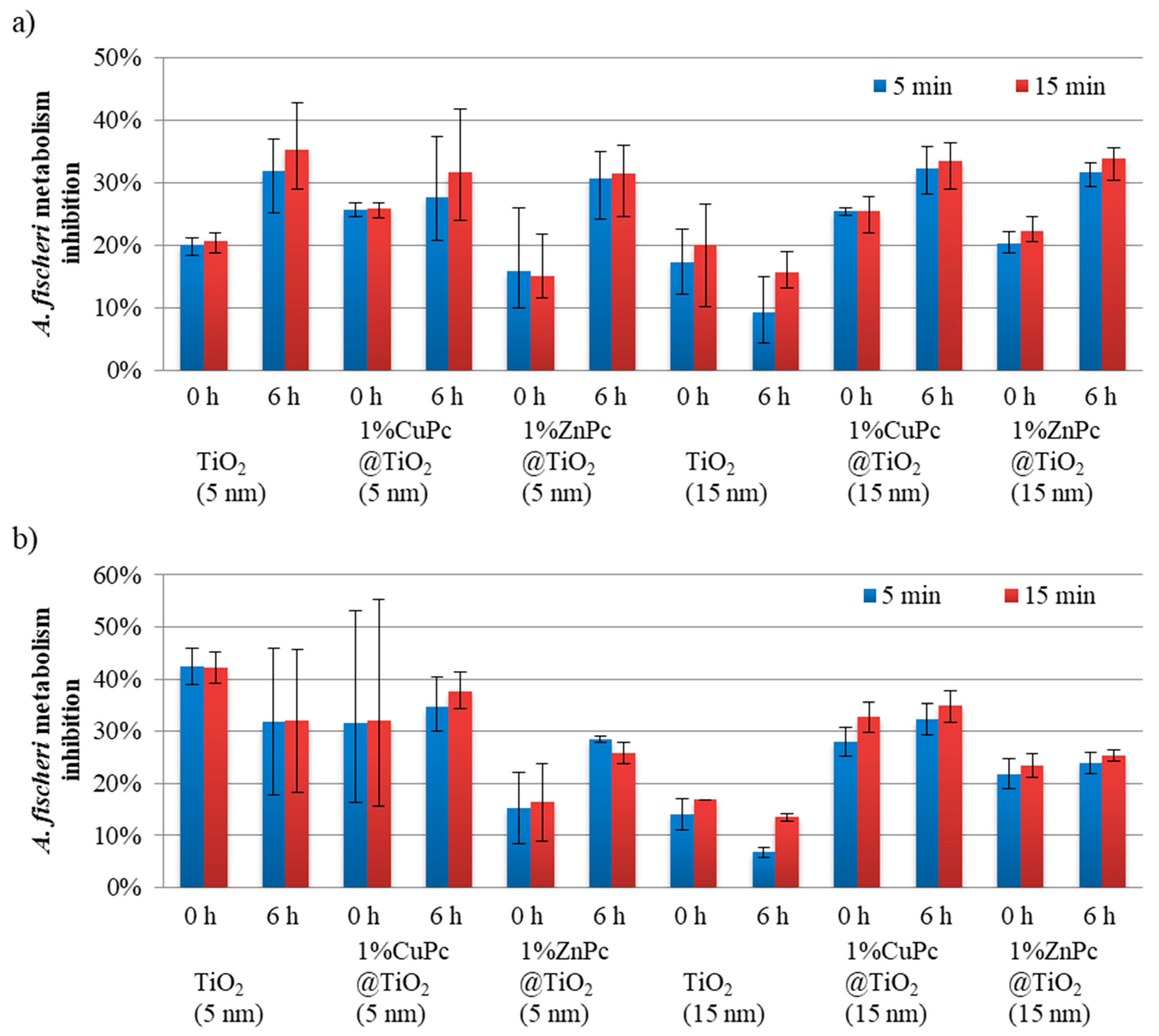
2.9. Acute Toxicity Assessment
3. Materials and Methods
3.1. Materials and Instruments
3.2. Preparation of Nanoparticles
3.3. Nanoparticle Characterization
3.3.1. Thermogravimetric Analysis
3.3.2. X-ray Powder Diffraction
3.3.3. X-ray Photoelectron Spectroscopy
3.3.4. Particle Size
3.3.5. Electron Spin Resonance Spectroscopy
3.3.6. BET Surface Area Analysis
3.4. Photochemical Studies
3.4.1. Set-Up of the Photocatalytic Experiment
3.4.2. LC-MS/MS Analysis of the Samples
3.5. Microtox® Acute Toxicity Assessment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ling, S.D.; Davey, A.; Reeves, S.E.; Gaylard, S.; Davies, P.L.; Stuart-Smith, R.D.; Edgar, G.J. Pollution signature for temperate reef biodiversity is short and simple. Mar. Pollut. Bull. 2018, 130, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Z. Industrial water pollution, water environment treatment and health risks in China. Environ. Pollut. 2016, 218, 358–365. [Google Scholar] [CrossRef] [PubMed]
- La Farré, M.; Pérez, S.; Kantiani, L.; Barceló, D. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal. Chem. 2008, 27, 991–1007. [Google Scholar] [CrossRef]
- Candido, J.P.; Andrade, S.J.; Fonseca, A.L.; Silva, F.S.; Silva, M.R.A.; Kondo, M.M. Ibuprofen removal by heterogeneous photocatalysis and ecotoxicological evaluation of the treated solutions. Environ. Sci. Pollut. Res. 2016, 23, 19911–19920. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Mestre, A.; Andrade, M.; Ania, C. Ibuprofen in the Aquatic Environment: Occurrence, Ecotoxicity and Water Remediation Technologies. In Ibuprofen: Clinical Pharmacology, Medical Uses and Adverse Effects; Nova Science Pub Inc.: Hauppauge, NY, USA, 2013; pp. 1–84. ISBN 978-1-62618-659-0. [Google Scholar]
- Kostich, M.S.; Batt, A.L.; Lazorchak, J.M. Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation. Environ. Pollut. 2014, 184, 354–359. [Google Scholar] [CrossRef]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically active compounds in aqueous environment: A status, toxicity and insights of remediation. Environ. Res. 2019, 176, 108542. [Google Scholar] [CrossRef]
- Godoy, A.A.; Kummrow, F.; Pamplin, P.A.Z. Occurrence, ecotoxicological effects and risk assessment of antihypertensive pharmaceutical residues in the aquatic environment - A review. Chemosphere 2015, 138, 281–291. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Wei, Q.; Wei, D. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef]
- Sim, W.-J.; Lee, J.-W.; Lee, E.-S.; Shin, S.-K.; Hwang, S.-R.; Oh, J.-E. Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere 2011, 82, 179–186. [Google Scholar] [CrossRef]
- Santos, L.H.; Gros, M.; Rodriguez-Mozaz, S.; Delerue-Matos, C.; Pena, A.; Barceló, D.; Montenegro, M.C.B.S.M. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: Identification of ecologically relevant pharmaceuticals. Sci. Total Environ. 2013, 461–462, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Zioris, I.; Danis, T.; Bikiaris, D.; Lambropoulou, D. Comprehensive investigation of a wide range of pharmaceuticals and personal care products in urban and hospital wastewaters in Greece. Sci. Total Environ. 2019, 694, 133565. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Kim, H.W.; Oh, J.-E.; Park, H.-S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Pharmaceuticals in Drinking-Water; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- IQVIA Institute for Human Data Sciences. The Global Use of Medicine in 2019 and Outlook to 2023-Forecasts and Areas to Watch; IQVIA: Durham, NC, USA, 2019. [Google Scholar]
- Méndez-Arriaga, F.; Torres-Palma, R.A.; Pétrier, C.; Esplugas, S.; Gimenez, J.; Pulgarin, C. Ultrasonic treatment of water contaminated with ibuprofen. Water Res. 2008, 42, 4243–4248. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Choina, J.; Kosslick, H.; Fischer, C.; Flechsig, G.-U.; Frunza, L.; Schulz, A. Photocatalytic decomposition of pharmaceutical ibuprofen pollutions in water over titania catalyst. Appl. Catal. B Environ. 2013, 129, 589–598. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Bechelany, M.; Nasr, M.; Jarvis, J.; Schaub, T.; Sapkota, R.R.; Miele, P.; Wang, H.; Xu, P. Adsorption and photocatalytic oxidation of ibuprofen using nanocomposites of TiO2 nanofibers combined with BN nanosheets: Degradation products and mechanisms. Chemosphere 2019, 220, 921–929. [Google Scholar] [CrossRef]
- Jallouli, N.; Pastrana-Martínez, L.M.; Ribeiro, A.R.; Moreira, N.F.F.; Faria, J.L.; Hentati, O.; Silva, A.M.T.; Ksibi, M. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Moro, P.; Donzello, M.P.; Ercolani, C.; Monacelli, F.; Moretti, G. Tetrakis-2,3-[5,6-di-(2-pyridyl)-pyrazino]porphyrazine and its Cu(II) complex as sensitizers in the TiO2-based photo-degradation of 4-nitrophenol. J. Photochem. Photobiol. Chem. 2011, 220, 77–83. [Google Scholar] [CrossRef]
- Simmler, W. Air, 6. Photochemical Degradation. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; Volume 50, pp. 464–488. ISBN 0007-4977. [Google Scholar]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Mozia, S.; Morawski, A.W. The performance of a hybrid photocatalysis–MD system for the treatment of tap water contaminated with ibuprofen. Catal. Today 2012, 193, 213–220. [Google Scholar] [CrossRef]
- Hiroto, S.; Miyake, Y.; Shinokubo, H. Synthesis and Functionalization of Porphyrins through Organometallic Methodologies. Chem. Rev. 2017, 117, 2910–3043. [Google Scholar] [CrossRef] [PubMed]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Banfield, J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?-Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mele, G.; del Sole, R.; Vasapollo, G.; García-López, E.; Palmisano, L.; Jun, L.; Słota, R.; Dyrda, G. TiO2-based photocatalysts impregnated with metallo-porphyrins employed for degradation of 4-nitrophenol in aqueous solutions: Role of metal and macrocycle. Res. Chem. Intermed. 2007, 33, 433–448. [Google Scholar] [CrossRef]
- Vignesh, K.; Rajarajan, M.; Suganthi, A. Photocatalytic degradation of erythromycin under visible light by zinc phthalocyanine-modified titania nanoparticles. Mater. Sci. Semicond. Process. 2014, 23, 98–103. [Google Scholar] [CrossRef]
- Fadel, M.; Kassab, K.; Abdel Fadeel, D. Zinc phthalocyanine-loaded PLGA biodegradable nanoparticles for photodynamic therapy in tumor-bearing mice. Lasers Med. Sci. 2010, 25, 283–292. [Google Scholar] [CrossRef]
- Cabir, B.; Yurderi, M.; Caner, N.; Agirtas, M.S.; Zahmakiran, M.; Kaya, M. Methylene blue photocatalytic degradation under visible light irradiation on copper phthalocyanine-sensitized TiO2 nanopowders. Mater. Sci. Eng. B 2017, 224, 9–17. [Google Scholar] [CrossRef]
- Altın, İ.; Sökmen, M.; Bıyıklıoğlu, Z. Quaternized zinc(II) phthalocyanine-sensitized TiO2: Surfactant-modified sol–gel synthesis, characterization and photocatalytic applications. Desalin. Water Treat. 2016, 57, 16196–16207. [Google Scholar] [CrossRef]
- Flak, D.; Yate, L.; Nowaczyk, G.; Jurga, S. Hybrid ZnPc@TiO2 nanostructures for targeted photodynamic therapy, bioimaging and doxorubicin delivery. Mater. Sci. Eng. C 2017, 78, 1072–1085. [Google Scholar] [CrossRef] [PubMed]
- Theivasanthi, T.; Alagar, M. Titanium dioxide (TiO2) Nanoparticles XRD Analyses: An Insight. arXiv 2013, arXiv:13071091. [Google Scholar]
- Duan, M.; Li, J.; Li, M.; Zhang, Z.; Wang, C. Pt(II) porphyrin modified TiO2 composites as photocatalysts for efficient 4-NP degradation. Appl. Surf. Sci. 2012, 258, 5499–5504. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, B.; Zhu, S.; Yao, Y.; Ye, Y.; Lu, W.; Chen, W. Photocatalytic activity of phthalocyanine-sensitized TiO2–SiO2 microparticles irradiated by visible light. Mater. Sci. Semicond. Process. 2014, 25, 148–152. [Google Scholar] [CrossRef]
- Mali, S.S.; Kim, H.; Kim, J.H.; Patil, P.S.; Kook Hong, C. Synthesis and characterization of planar heterojunction hybrid polymer solar cells based on copper pthalocyanine (CuPc) and titanium dioxide. Ceram. Int. 2014, 40, 643–649. [Google Scholar] [CrossRef]
- Soema, P.C.; Willems, G.-J.; Jiskoot, W.; Amorij, J.-P.; Kersten, G.F. Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach. Eur. J. Pharm. Biopharm. 2015, 94, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, N.K.; Natashina, U.A.; Tamarov, K.P.; Gongalsky, M.B.; Solovyev, V.V.; Kudryavtsev, A.A.; Sivakov, V.; Osminkina, L.A. Recycling of silicon: From industrial waste to biocompatible nanoparticles for nanomedicine. Mater. Res. Express 2017, 4, 95026. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Model List of Essential Medicines, 21st ed.; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Kanabar, D.J. A clinical and safety review of paracetamol and ibuprofen in children. Inflammopharmacology 2017, 25, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, D.W.; Kelly, J.P.; Battista, D.R.; Malone, M.K.; Weinstein, R.B.; Shiffman, S. Exceeding the daily dosing limit of nonsteroidal anti-inflammatory drugs among ibuprofen users. Pharmacoepidemiol. Drug Saf. 2018, 27, 322–331. [Google Scholar] [CrossRef]
- Tixier, C.; Singer, H.P.; Oellers, S.; Müller, S.R. Occurrence and Fate of Carbamazepine, Clofibric Acid, Diclofenac, Ibuprofen, Ketoprofen and Naproxen in Surface Waters. Environ. Sci. Technol. 2003, 37, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Miège, C.; Choubert, J.M.; Ribeiro, L.; Eusèbe, M.; Coquery, M. Fate of pharmaceuticals and personal care products in wastewater treatment plants–Conception of a database and first results. Environ. Pollut. 2009, 157, 1721–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.; Silva, L.; Laranjeiro, C.; Lino, C.; Pena, A. Selected Pharmaceuticals in Different Aquatic Compartments: Part I—Source, Fate and Occurrence. Molecules 2020, 25, 1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simazaki, D.; Kubota, R.; Suzuki, T.; Akiba, M.; Nishimura, T.; Kunikane, S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015, 76, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Peñas-Garzón, M.; Gómez-Avilés, A.; Belver, C.; Rodriguez, J.J.; Bedia, J. Degradation pathways of emerging contaminants using TiO2-activated carbon heterostructures in aqueous solution under simulated solar light. Chem. Eng. J. 2020, 392, 124867. [Google Scholar] [CrossRef]
- Achilleos, A.; Hapeshi, E.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Fatta-Kassinos, D. UV-A and Solar Photodegradation of Ibuprofen and Carbamazepine Catalyzed by TiO2. Sep. Sci. Technol. 2010, 45, 1564–1570. [Google Scholar] [CrossRef]
- Da Silva, J.C.C.; Teodoro, J.A.R.; de Cássia Franco Afonso, R.J.; Aquino, S.F.; Augusti, R. Photolysis and photocatalysis of ibuprofen in aqueous medium: Characterization of by-products via liquid chromatography coupled to high-resolution mass spectrometry and assessment of their toxicities against Artemia Salina. J. Mass Spectrom. 2014, 49, 145–153. [Google Scholar] [CrossRef]
- Romeiro, A.; Azenha, M.E.; Canle, M.; Rodrigues, V.H.N.; Da Silva, J.P.; Burrows, H.D. Titanium Dioxide Nanoparticle Photocatalysed Degradation of Ibuprofen and Naproxen in Water: Competing Hydroxyl Radical Attack and Oxidative Decarboxylation by Semiconductor Holes. ChemistrySelect 2018, 3, 10915–10924. [Google Scholar] [CrossRef]
- Jiménez-Salcedo, M.; Monge, M.; Tena, M.T. Photocatalytic degradation of ibuprofen in water using TiO2/UV and g-C3N4/visible light: Study of intermediate degradation products by liquid chromatography coupled to high-resolution mass spectrometry. Chemosphere 2019, 215, 605–618. [Google Scholar] [CrossRef]
- Patterson, K.; Howlett, K.; Patterson, K.; Wang, B.; Jiang, L. Photodegradation of ibuprofen and four other pharmaceutical pollutants on natural pigments sensitized TiO2 nanoparticles. Water Environ. Res. 2020. [Google Scholar] [CrossRef]
- Méndez-Arriaga, F.; Esplugas, S.; Giménez, J. Photocatalytic degradation of non-steroidal anti-inflammatory drugs with TiO2 and simulated solar irradiation. Water Res. 2008, 42, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Dhir, A. Transition metal doped TiO2 mediated photocatalytic degradation of anti-inflammatory drug under solar irradiations. J. Environ. Chem. Eng. 2016, 4, 1267–1273. [Google Scholar] [CrossRef]
- Agustina, T.E.; Ang, H.M.; Vareek, V.K. A review of synergistic effect of photocatalysis and ozonation on wastewater treatment. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 264–273. [Google Scholar] [CrossRef]
- Ogunsipe, A.; Chen, J.-Y.; Nyokong, T. Photophysical and photochemical studies of zinc(II) phthalocyanine derivatives—Effects of substituents and solvents. New J. Chem. 2004, 28, 822–827. [Google Scholar] [CrossRef]
- Li, G.; Lv, L.; Fan, H.; Ma, J.; Li, Y.; Wan, Y.; Zhao, X.S. Effect of the agglomeration of TiO2 nanoparticles on their photocatalytic performance in the aqueous phase. J. Colloid Interface Sci. 2010, 348, 342–347. [Google Scholar] [CrossRef]
- Johnson, B.T. Microtox® Acute Toxicity Test. In Small-scale Freshwater Toxicity Investigations; Blaise, C., Férard, J.-F., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 69–105. ISBN 978-1-4020-3119-9. [Google Scholar]
- Czech, B.; Jośko, I.; Oleszczuk, P. Ecotoxicological evaluation of selected pharmaceuticals to Vibrio fischeri and Daphnia magna before and after photooxidation process. Ecotoxicol. Environ. Saf. 2014, 104, 247–253. [Google Scholar] [CrossRef]
- Shemer, H.; Linden, K.G. Photolysis, oxidation and subsequent toxicity of a mixture of polycyclic aromatic hydrocarbons in natural waters. J. Photochem. Photobiol. Chem. 2007, 187, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Lü, X.; Li, J.; Wang, C.; Duan, M.; Luo, Y.; Yao, G.; Wang, J.-L. Enhanced photoactivity of CuPp-TiO2 photocatalysts under visible light irradiation. Appl. Surf. Sci. 2010, 257, 795–801. [Google Scholar] [CrossRef]
- Recillas, S.; García, A.; González, E.; Casals, E.; Puntes, V.; Sánchez, A.; Font, X. Use of CeO2, TiO2 and Fe3O4 nanoparticles for the removal of lead from water. Desalination 2011, 277, 213–220. [Google Scholar] [CrossRef] [Green Version]





| TiO2 Nanoparticle Size | Pc, Pc : TiO2 Ratio (m/m) | Symbol of the Material |
|---|---|---|
| 5 nm | CuPc, 1 : 100 | 1%CuPc@TiO2(5 nm) |
| 5 nm | ZnPc, 1 : 100 | 1%ZnPc@TiO2(5 nm) |
| 15 nm | CuPc, 1 : 100 | 1%CuPc@TiO2(15 nm) |
| 15 nm | ZnPc, 1 : 100 | 1%ZnPc@TiO2(15 nm) |
| Material | Particle Size | Polydispersity Index a |
|---|---|---|
| TiO2 (5 nm) | 145.7 ± 68.4 nm | 0.22 |
| 1%CuPc@TiO2 (5 nm) | 103.5 ± 87.8 nm | 0.72 |
| 1%ZnPc@TiO2 (5 nm) | 158.7 ± 74.4 nm | 0.22 |
| TiO2 (15 nm) | 201.6 ± 139.8 nm | 0.48 |
| 1%CuPc@TiO2 (15 nm) | 144.5 ± 129.1 nm | 0.80 |
| 1%ZnPc@TiO2 (15 nm) | 236.2 ± 128.1 nm | 0.29 |
| Material | BET Surface Area [m2/g] |
|---|---|
| TiO2 (5 nm) | 264.8 ± 1.2 |
| 1%CuPc@TiO2 (5 nm) | 249.7 ± 1.5 |
| 1%ZnPc@TiO2 (5 nm) | 244.3 ± 1.5 |
| TiO2 (15 nm) | 104.6 ± 0.3 |
| 1%CuPc@TiO2 (15 nm) | 96.8 ± 0.3 |
| 1%ZnPc@TiO2 (15 nm) | 100.4 ± 0.3 |
| Samples | Regression Parameters a | Kinetic Parameters [k (s−1)] b | |||
|---|---|---|---|---|---|
| 1%ZnPc@TiO2(5 nm) | a | −0.1214 | k | 2.024 × 10−3 | s−1 |
| Δα | 0.0303 | Δκ | 5.051 × 10−4 | s−1 | |
| b = y(0) | 1 504 745.2 | ||||
| Δβ | 1.098 | ||||
| sa | 0.0117 | ||||
| sb | 0.0364 | ||||
| sy | 0.0611 | ||||
| r | −0.982 | ||||
| 1%ZnPc@TiO2(15 nm) | a | −0.1548 | k | 2.580 × 10−3 | s−1 |
| Δα | 0.0136 | Δκ | 2.279 × 10−4 | s−1 | |
| b = y(0) | 912 073.1 | ||||
| Δβ | 1.043 | ||||
| sa | 0.00531 | ||||
| sb | 0.0164 | ||||
| sy | 0.02757 | ||||
| r | −0.998 | ||||
| 1%CuPc@TiO2(5 nm) | a | −0.3832 | k | 6.387 × 10−3 | s−1 |
| Δα | 0.0601 | Δκ | 1.002 × 10−3 | s−1 | |
| b = y(0) | 1 160 993.9 | ||||
| Δβ | 1.204 | ||||
| sa | 0.0233 | ||||
| sb | 0.0722 | ||||
| sy | 0.121 | ||||
| r | −0.993 | ||||
| 1%CuPc@TiO2(15 nm) | a | −0.3649 | k | 6.083 × 10−3 | s−1 |
| Δα | 0.0548 | Δκ | 9.149 × 10−4 | s−1 | |
| b =y(0) | 1 242 276.5 | ||||
| Δβ | 1.184 | ||||
| sa | 0.0213 | ||||
| sb | 0.0659 | ||||
| sy | 0.11 | ||||
| r | −0.993 | ||||
| TiO2(5 nm) | a | −0.391 | k | 6.517 × 10−3 | s−1 |
| Δα | 0.0292 | Δκ | 4.876 × 10−4 | s−1 | |
| b = y(0) | 1 158 957.5 | ||||
| Δβ | 1.0945 | ||||
| sa | 0.011381 | ||||
| sb | 0.0351 | ||||
| sy | 0.0589 | ||||
| r | −0.998 | ||||
| TiO2(15 nm) | a | −0.359 | k | 5.988 × 10−3 | s−1 |
| Δα | 0.0252 | Δκ | 4.202 × 10−4 | s−1 | |
| b = y(0) | 1 111 784.1 | ||||
| Δβ | 1.08 | ||||
| sa | 0.0098 | ||||
| sb | 0.0302 | ||||
| sy | 0.0508 | ||||
| r | −0.999 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krakowiak, R.; Musial, J.; Frankowski, R.; Spychala, M.; Mielcarek, J.; Dobosz, B.; Krzyminiewski, R.; Sikorski, M.; Bendzinska-Berus, W.; Tykarska, E.; et al. Phthalocyanine-Grafted Titania Nanoparticles for Photodegradation of Ibuprofen. Catalysts 2020, 10, 1328. https://doi.org/10.3390/catal10111328
Krakowiak R, Musial J, Frankowski R, Spychala M, Mielcarek J, Dobosz B, Krzyminiewski R, Sikorski M, Bendzinska-Berus W, Tykarska E, et al. Phthalocyanine-Grafted Titania Nanoparticles for Photodegradation of Ibuprofen. Catalysts. 2020; 10(11):1328. https://doi.org/10.3390/catal10111328
Chicago/Turabian StyleKrakowiak, Rafal, Joanna Musial, Robert Frankowski, Marcin Spychala, Jadwiga Mielcarek, Bernadeta Dobosz, Ryszard Krzyminiewski, Marek Sikorski, Wioletta Bendzinska-Berus, Ewa Tykarska, and et al. 2020. "Phthalocyanine-Grafted Titania Nanoparticles for Photodegradation of Ibuprofen" Catalysts 10, no. 11: 1328. https://doi.org/10.3390/catal10111328






