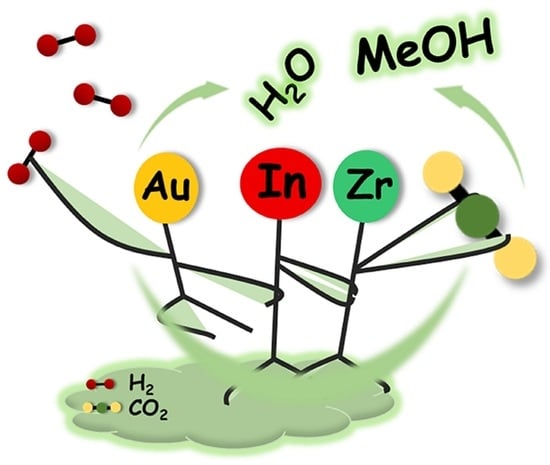
A Highly Active Au/In2O3-ZrO2 Catalyst for Selective Hydrogenation of CO2 to Methanol
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.3. Catalyst Activity Test
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar]
- Su, X.; Xu, J.; Liang, B.; Duan, H.; Hou, B.; Huang, Y. Catalytic carbon dioxide hydrogenation to methane: A review of recent studies. J. Energy Chem. 2016, 25, 553–565. [Google Scholar]
- Wang, J.; Li, G.; Li, Z.; Tang, C.; Feng, Z.; An, H.; Liu, H.; Liu, T.; Li, C. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 2017, 3, e1701290. [Google Scholar]
- Studt, F.; Sharafutdinov, I.; Abild-Pedersen, F.; Elkjær, C.F.; Hummelshøj, J.S.; Dahl, S.; Chorkendorff, I.; Nørskov, J.K. Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat. Chem. 2014, 6, 320–324. [Google Scholar]
- Liao, F.; Wu, X.-P.; Zheng, J.; Li, M.M.-J.; Kroner, A.; Zeng, Z.; Hong, X.; Yuan, Y.; Gong, X.-Q.; Tsang, S.C.E. A promising low pressure methanol synthesis route from CO2 hydrogenation over Pd@Zn core–shell catalysts. Green Chem. 2017, 19, 270–280. [Google Scholar]
- Xiang, W.; Zhang, Y.; Chen, Y.; Liu, C.J.; Tu, X. Synthesis, characterization and application of defective metal–organic frameworks: Current status and perspectives. J. Mater. Chem. A 2020, 8, 21526–21546. [Google Scholar]
- Gutterød, E.S.; Lazzarini, A.; Fjermestad, T.; Kaur, G.; Manzoli, M.; Bordiga, S.; Svelle, S.; Lillerud, K.P.; Skúlason, E.; Øien-Ødegaard, S.; et al. Hydrogenation of CO2 to methanol by Pt nanoparticles encapsulated in UiO-67: Deciphering the role of the metal-organic framework. J. Am. Chem. Soc. 2020, 142, 999–1009. [Google Scholar] [PubMed]
- Ye, J.; Liu, C.; Ge, Q. DFT study of CO2 adsorption and hydrogenation on the In2O3 surface. J. Phys. Chem. C 2012, 116, 7817–7825. [Google Scholar]
- Ye, J.; Liu, C.; Mei, D.; Ge, Q. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3(110): A DFT Study. ACS Catal. 2013, 3, 1296–1306. [Google Scholar]
- Sun, K.; Fan, Z.; Ye, J.; Yan, J.; Ge, Q.; Li, Y.; He, W.; Yang, W.; Liu, C.-J. Hydrogenation of CO2 to methanol over In2O3 catalyst. J. CO2 Util. 2015, 12, 1–6. [Google Scholar]
- Tsoukalou, A.; Abdala, P.M.; Stoian, D.; Huang, X.; Willinger, M.-G.; Fedorov, A.; Müller, C.R. Structural evolution and dynamics of an In2O3 catalyst for CO2 hydrogenation to methanol: An operando XAS-XRD and in situ TEM study. J. Am. Chem. Soc. 2019, 141, 13497–13505. [Google Scholar] [PubMed]
- Chen, T.-Y.; Cao, C.; Chen, T.-B.; Ding, X.; Huang, H.; Shen, L.; Cao, X.; Zhu, M.; Xu, J.; Gao, J.; et al. Unraveling highly tunable selectivity in CO2 hydrogenation over bimetallic In-Zr oxide catalysts. ACS Catal. 2019, 9, 8785–8797. [Google Scholar]
- Dou, M.; Zhang, M.; Chen, Y.; Yu, Y. Mechanistic insight into the modification of the surface stability of In2O3 catalyst through metal oxide doping. Catal. Lett. 2018, 148, 3723–3731. [Google Scholar]
- Tsoukalou, A.; Abdala, P.M.; Armutlulu, A.; Willinger, E.; Fedorov, A.; Müller, C.R. Operando X-ray absorption spectroscopy identifies a monoclinic ZrO2: In solid solution as the active phase for the hydrogenation of CO2 to methanol. ACS Catal. 2020, 10, 10060–10067. [Google Scholar]
- Frei, M.S.; Mondelli, C.; Cesarini, A.; Krumeich, F.; Hauert, R.; Stewart, J.A.; Curulla Ferré, D.; Pérez-Ramírez, J. Role of zirconia in indium oxide-catalyzed CO2 hydrogenation to methanol. ACS Catal. 2020, 10, 1133–1145. [Google Scholar]
- Martin, O.; Martín, A.J.; Mondelli, C.; Mitchell, S.; Segawa, T.F.; Hauert, R.; Drouilly, C.; Curulla-Ferré, D.; Pérez-Ramírez, J. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation. Angew. Chem. Int. Ed. 2016, 55, 6261–6265. [Google Scholar]
- Yang, C.; Pei, C.; Luo, R.; Liu, S.; Wang, Y.; Wang, Z.; Zhao, Z.-J.; Gong, J. Strong electronic oxide–support interaction over In2O3 /ZrO2 for highly selective CO2 hydrogenation to methanol. J. Am. Chem. Soc. 2020. [Google Scholar] [CrossRef]
- Rui, N.; Zhang, F.; Sun, K.; Liu, Z.; Xu, W.; Stavitski, E.; Senanayake, S.D.; Rodriguez, J.A.; Liu, C.-J. Hydrogenation of CO2 to methanol on a Auδ+–In2O3–x catalyst. ACS Catal. 2020, 10, 11307–11317. [Google Scholar]
- Rui, N.; Sun, K.; Shen, C.; Liu, C.-J. Density functional theoretical study of Au4/In2O3 catalyst for CO2 hydrogenation to methanol: The strong metal-support interaction and its effect. J. CO2 Util. 2020, 42, 101313. [Google Scholar]
- Shi, Z.; Tan, Q.; Wu, D. A novel core–shell structured CuIn@SiO2 catalyst for CO2 hydrogenation to methanol. AIChE J. 2019, 65, 1047–1058. [Google Scholar]
- Shi, Z.; Tan, Q.; Tian, C.; Pan, Y.; Sun, X.; Zhang, J.; Wu, D. CO2 hydrogenation to methanol over Cu-In intermetallic catalysts: Effect of reduction temperature. J. Catal. 2019, 379, 78–89. [Google Scholar]
- Bavykina, A.; Yarulina, I.; Al Abdulghani, A.J.; Gevers, L.; Hedhili, M.N.; Miao, X.; Galilea, A.R.; Pustovarenko, A.; Dikhtiarenko, A.; Cadiau, A.; et al. Turning a methanation Co catalyst into an In–Co methanol producer. ACS Catal. 2019, 9, 6910–6918. [Google Scholar]
- Ye, J.; Liu, C.-J.; Mei, D.; Ge, Q. Methanol synthesis from CO2 hydrogenation over a Pd4/In2O3 model catalyst: A combined DFT and kinetic study. J. Catal. 2014, 317, 44–53. [Google Scholar]
- Rui, N.; Wang, Z.; Sun, K.; Ye, J.; Ge, Q.; Liu, C.-J. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy. Appl. Catal. B 2017, 218, 488–497. [Google Scholar]
- Frei, M.S.; Mondelli, C.; García-Muelas, R.; Kley, K.S.; Puértolas, B.; López, N.; Safonova, O.V.; Stewart, J.A.; Curulla Ferré, D.; Pérez-Ramírez, J. Atomic-scale engineering of indium oxide promotion by palladium for methanol production via CO2 hydrogenation. Nat. Commun. 2019, 10, 3377. [Google Scholar]
- Cai, Z.; Dai, J.; Li, W.; Tan, K.B.; Huang, Z.; Zhan, G.; Huang, J.; Li, Q. Pd supported on MIL-68(In)-derived In2O3 nanotubes as superior catalysts to boost CO2 hydrogenation to methanol. ACS Catal. 2020, 10, 13275–13289. [Google Scholar]
- Wang, J.; Sun, K.; Jia, X.; Liu, C.-J. CO2 hydrogenation to methanol over Rh/In2O3 catalyst. Catal. Today 2020. [Google Scholar] [CrossRef]
- Jia, X.; Sun, K.; Wang, J.; Shen, C.; Liu, C.-J. Selective hydrogenation of CO2 to methanol over Ni/In2O3 catalyst. J. Energy Chem. 2020, 50, 409–415. [Google Scholar]
- Sun, K.; Rui, N.; Zhang, Z.; Sun, Z.; Ge, Q.; Liu, C.-J. A highly active Pt/In2O3 catalyst for CO2 hydrogenation to methanol with enhanced stability. Green Chem. 2020, 22, 5059–5066. [Google Scholar]
- Han, Z.; Tang, C.; Wang, J.; Li, L.; Li, C. Atomically dispersed Ptn+ species as highly active sites in Pt/In2O3 catalysts for methanol synthesis from CO2 hydrogenation. J. Catal. 2020. [Google Scholar] [CrossRef]
- Su, J.; Wang, D.; Wang, Y.; Zhou, H.; Liu, C.; Liu, S.; Wang, C.; Yang, W.; Xie, Z.; He, M. Direct conversion of syngas into light olefins over zirconium-doped indium(III) oxide and SAPO-34 bifunctional catalysts: Design of oxide component and construction of reaction network. ChemCatChem 2018, 10, 1536–1541. [Google Scholar]
- Zhu, H.; Wang, X.; Yang, F.; Yang, X. Template-free, surfactantless route to fabricate In(OH)3 monocrystalline nanoarchitectures and their conversion to In2O3. Cryst. Growth Des. 2008, 8, 950–956. [Google Scholar]
- Luo, M.-F.; Yan, Z.-L.; Jin, L.-Y.; He, M. Raman spectroscopic study on the structure in the surface and the bulk shell of CexPr1-xO2-δ mixed oxides. J. Phys. Chem. B 2006, 110, 13068–13071. [Google Scholar] [PubMed]
- Jiang, X.; Nie, X.; Gong, Y.; Moran, C.M.; Wang, J.; Zhu, J.; Chang, H.; Guo, X.; Walton, K.S.; Song, C. A combined experimental and DFT study of H2O effect on In2O3/ZrO2 catalyst for CO2 hydrogenation to methanol. J. Catal. 2020, 383, 283–296. [Google Scholar]
- Cui, Y.; Xu, L.; Chen, M.; Lian, X.; Wu, C.-e.; Yang, B.; Miao, Z.; Wang, F.; Hu, X. Facilely fabricating mesoporous nanocrystalline Ce–Zr solid solution supported CuO-based catalysts with advanced low-temperature activity toward CO oxidation. Catal. Sci. Technol. 2019, 9, 5605–5625. [Google Scholar]
- Gonçalves, A.; Puna, J.F.; Guerra, L.; Campos Rodrigues, J.; Gomes, J.F.; Santos, M.T.; Alves, D. Towards the development of syngas/biomethane electrolytic production, using liquefied biomass and heterogeneous catalyst. Energies 2019, 12, 3787. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Dang, S.; Li, S.; Bu, X.; Liu, Z.; Qiu, M.; Yang, C.; Wang, H.; Zhong, L.; Sun, Y. Direct production of lower olefins from CO2 conversion via bifunctional catalysis. ACS Catal. 2018, 8, 571–578. [Google Scholar]
- Frei, M.; Mondelli, C.; Short, M.; Pérez-Ramírez, J. Methanol as a hydrogen carrier: Kinetic and thermodynamic drivers for its CO2-based synthesis and reforming over heterogeneous catalysts. ChemSusChem 2020, 13, 1–9. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Sun, K.; Wang, J.; Zhang, Z.; Liu, C. A Highly Active Au/In2O3-ZrO2 Catalyst for Selective Hydrogenation of CO2 to Methanol. Catalysts 2020, 10, 1360. https://doi.org/10.3390/catal10111360
Lu Z, Sun K, Wang J, Zhang Z, Liu C. A Highly Active Au/In2O3-ZrO2 Catalyst for Selective Hydrogenation of CO2 to Methanol. Catalysts. 2020; 10(11):1360. https://doi.org/10.3390/catal10111360
Chicago/Turabian StyleLu, Zhe, Kaihang Sun, Jing Wang, Zhitao Zhang, and Changjun Liu. 2020. "A Highly Active Au/In2O3-ZrO2 Catalyst for Selective Hydrogenation of CO2 to Methanol" Catalysts 10, no. 11: 1360. https://doi.org/10.3390/catal10111360
APA StyleLu, Z., Sun, K., Wang, J., Zhang, Z., & Liu, C. (2020). A Highly Active Au/In2O3-ZrO2 Catalyst for Selective Hydrogenation of CO2 to Methanol. Catalysts, 10(11), 1360. https://doi.org/10.3390/catal10111360