N-Hydroxyphthalimide on a Polystyrene Support Coated with Co(II)-Containing Ionic Liquid as a New Catalytic System for Solvent-Free Ethylbenzene Oxidation
Abstract
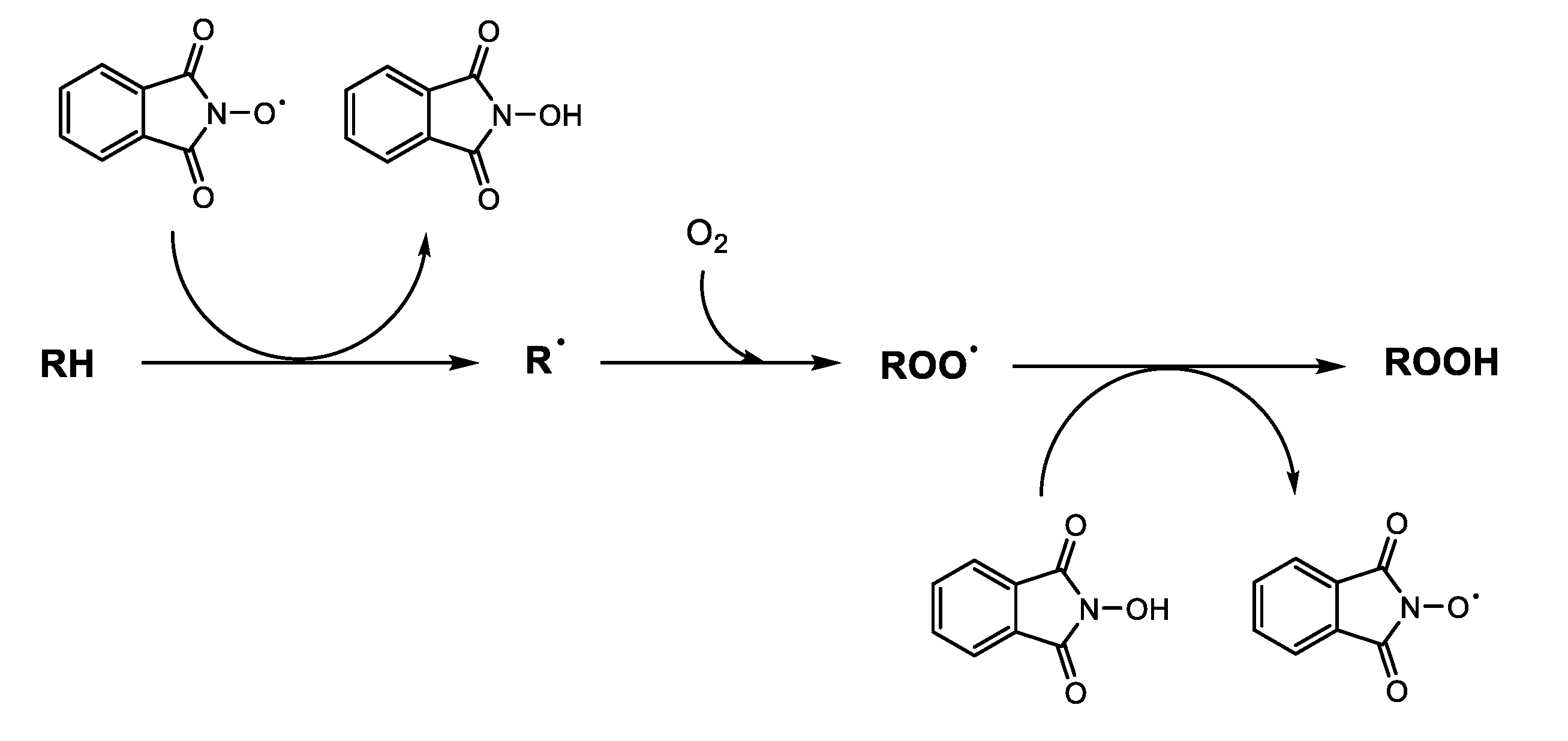
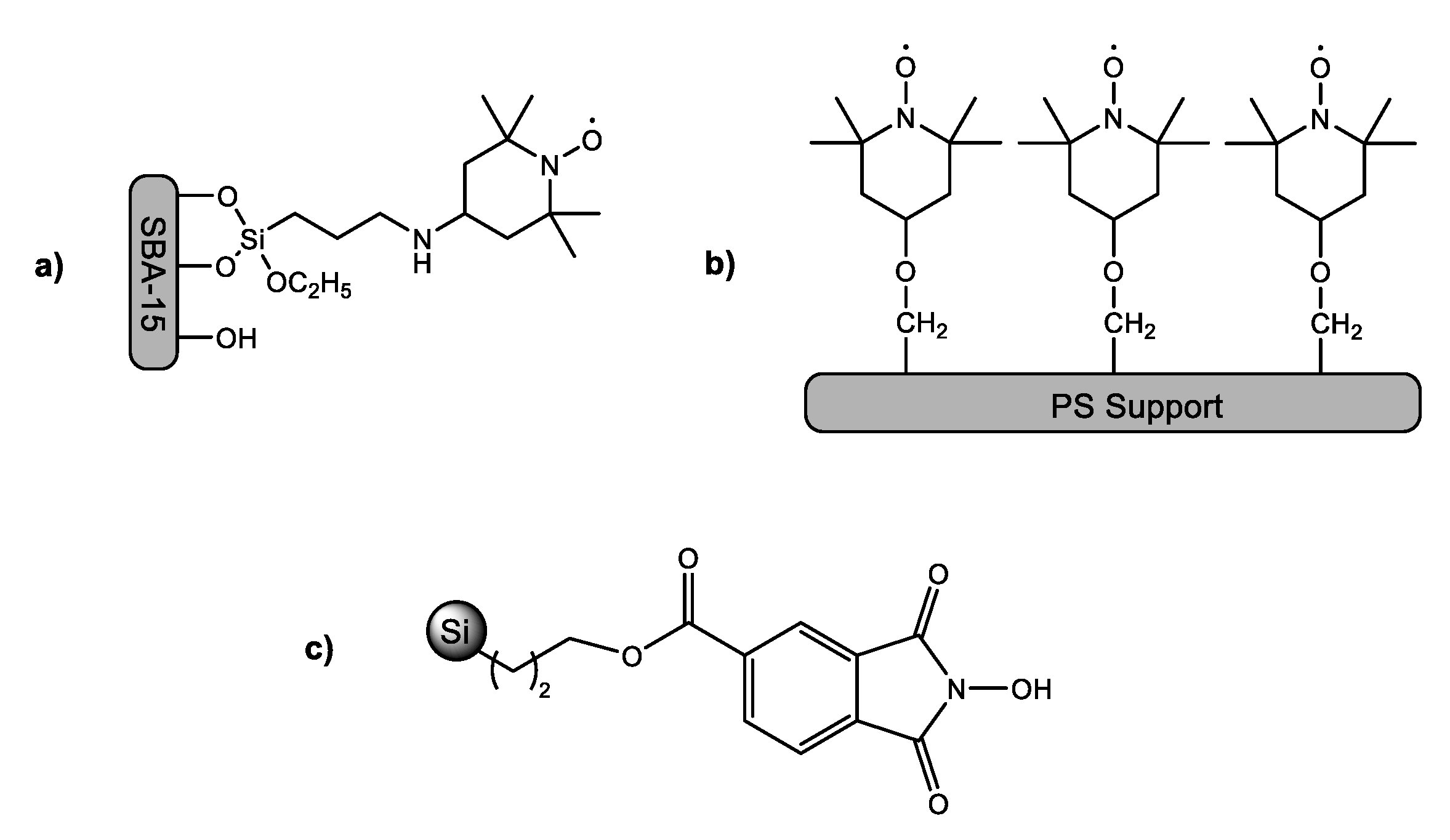
:1. Introduction
2. Results and Discussion
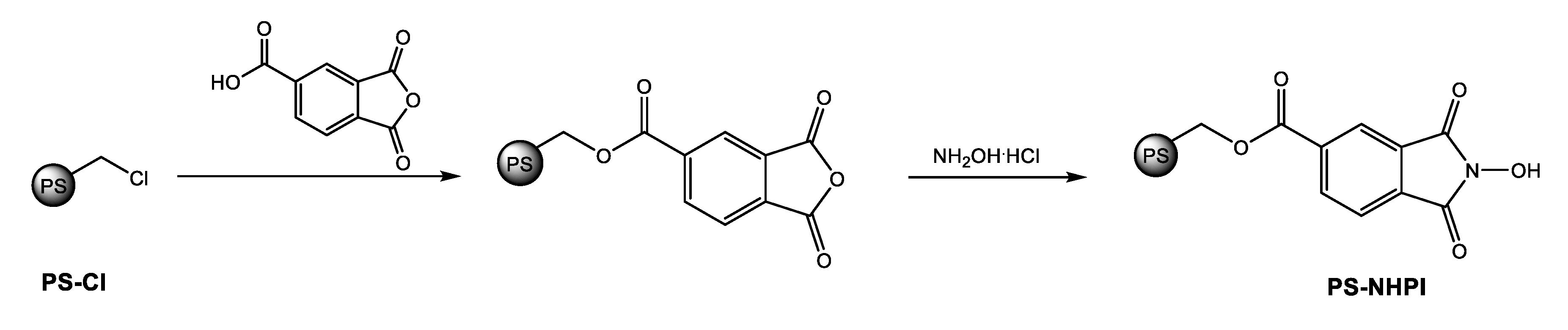
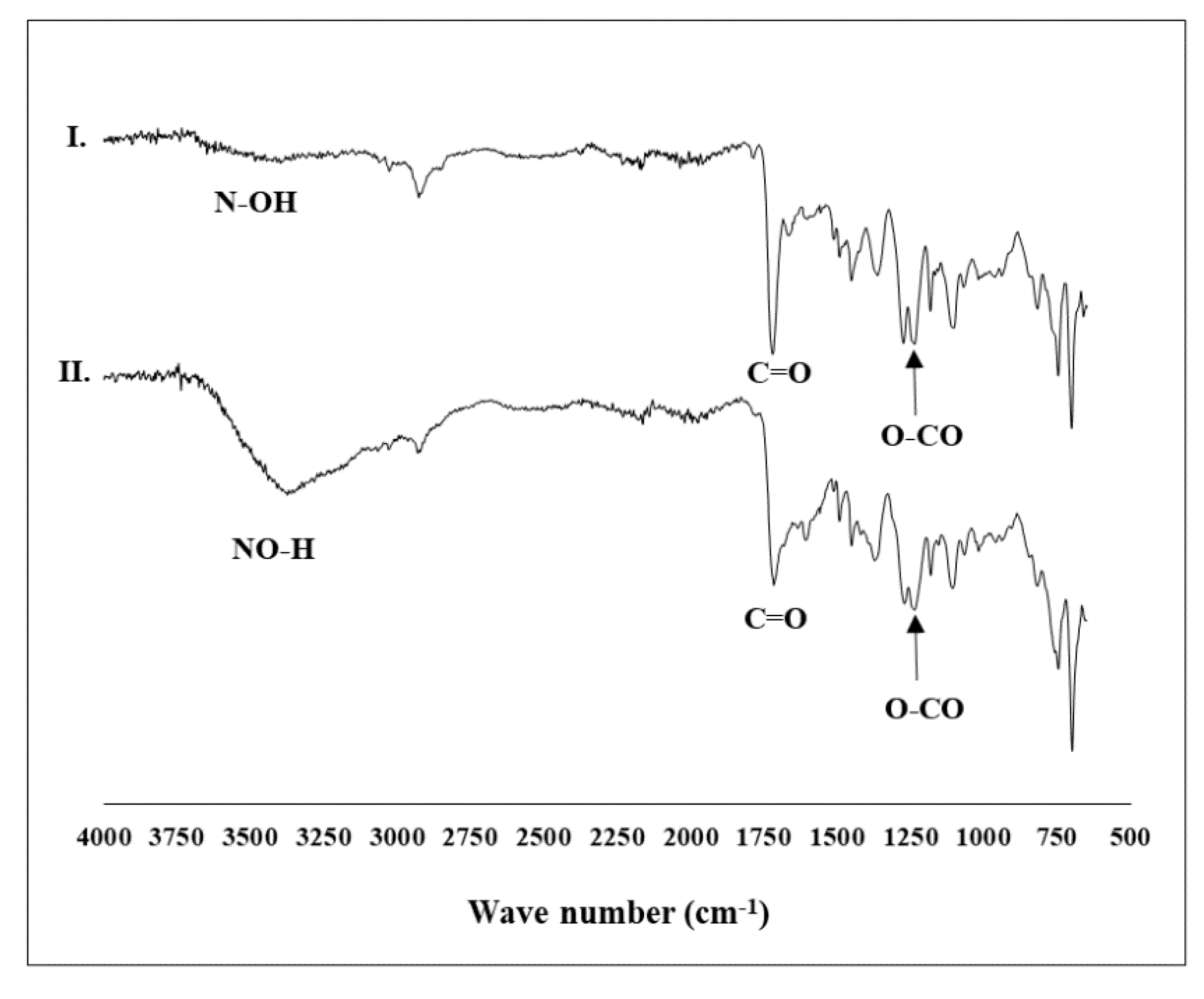
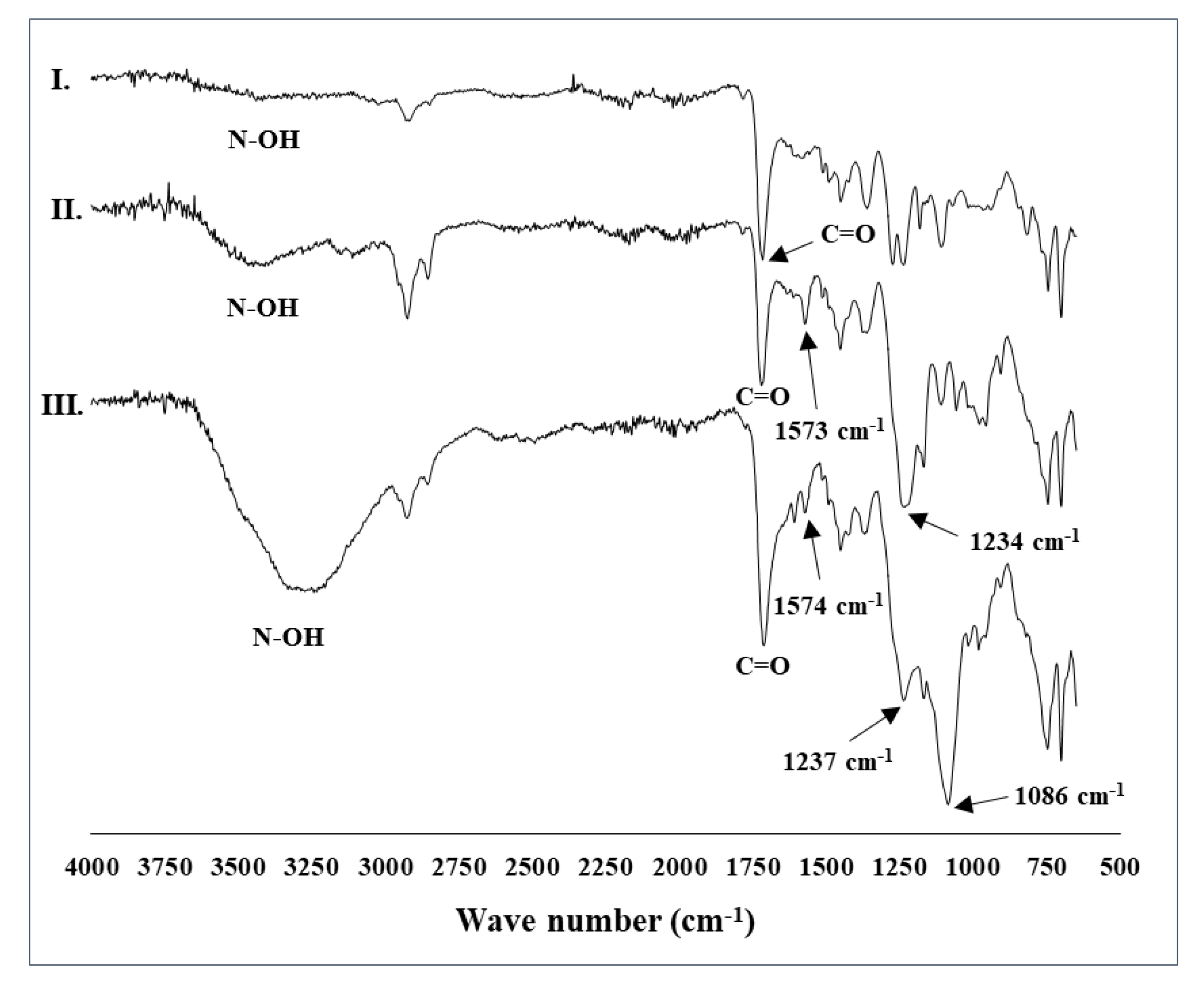
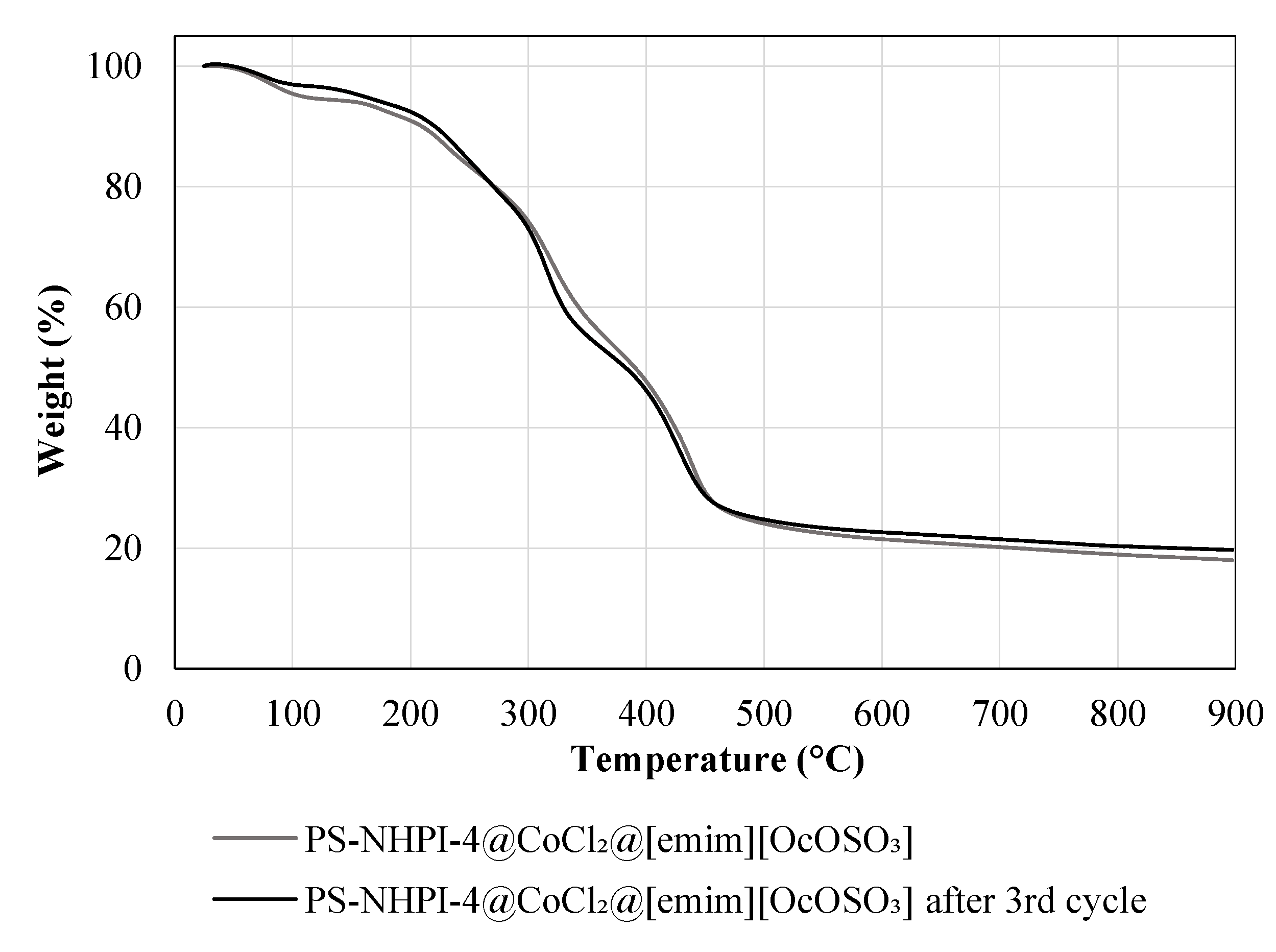
2.1. Preparation of the Catalytic System
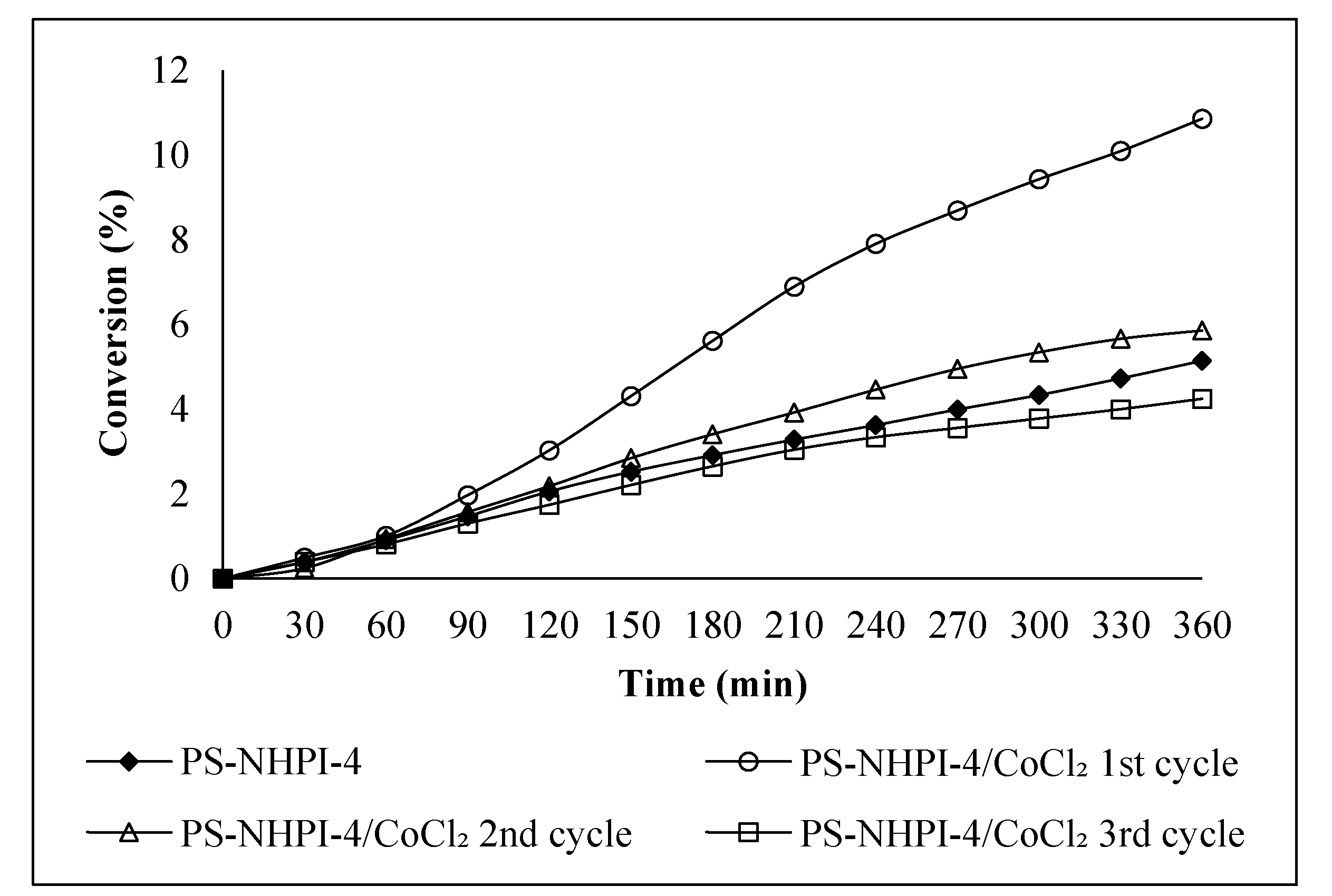
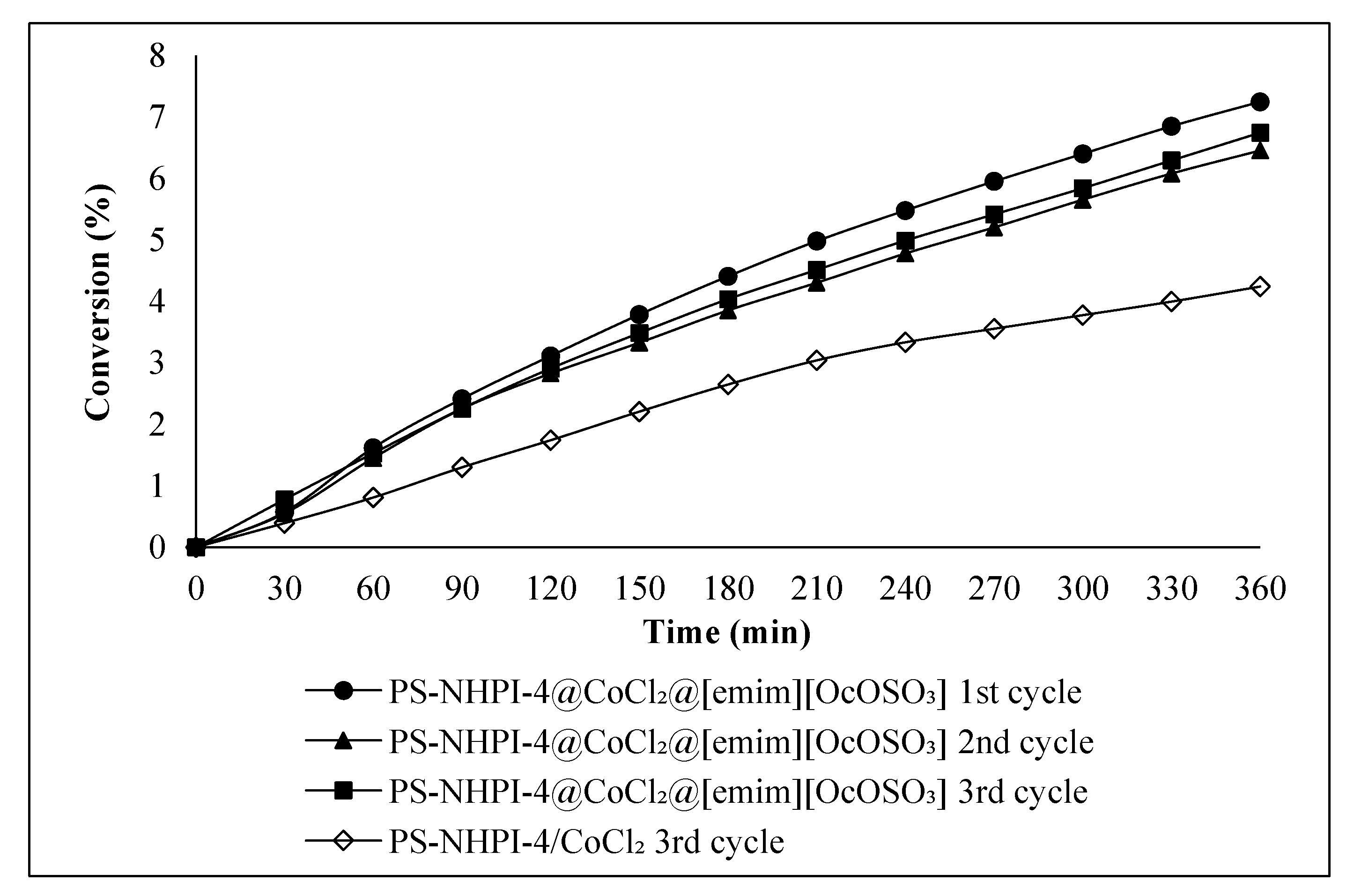
2.2. Ethylbenzene Oxidation
3. Materials and Methods
3.1. Materials
3.2. PS-NHPI Synthesis
3.3. PS-NHPI@CoCl2@[emim][OcOSO3] Preparation
3.4. General Procedure for Ethylbenzene Oxidation
3.5. Analytic Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Teles, H.J.; Hermans, I.F.; Gerhard, S.R.A. Oxidation. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Weissermel, K.; Arpe, H.-J. Industrial Organic Chemistry; VCH: Weinheim, Germany, 1997. [Google Scholar]
- Recupero, F.; Punta, C. Free radical functionalization of organic compounds catalyzed by N-hydroxyphthalimide. Chem. Rev. 2007, 107, 3800–3842. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, P.; Wang, Y.; Li, H. Metal-free allylic/benzylic oxidation strategies with molecular oxygen: Recent advances and future prospects. Green Chem. 2014, 16, 2344–2374. [Google Scholar] [CrossRef]
- Coseri, S. Phthalimide-N-Oxyl (PINO) radical, a powerful catalytic agent: Its generation and versatility towards various organic substrates. Catal. Rev. Sci. Eng. 2009, 51, 218–292. [Google Scholar] [CrossRef]
- Melone, L.; Punta, C. Metal-free aerobic oxidations mediated by N-hydroxyphthalimide. A concise review. Beilstein J. Org. Chem. 2013, 9, 1296–1310. [Google Scholar] [CrossRef] [Green Version]
- Orlińska, B.; Zawadiak, J. Aerobic oxidation of isopropylaromatic hydrocarbons to hydroperoxides catalyzed by N-hydroxyphthalimide. React. Kinet. Mech. Catal. 2013, 110, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Orlińska, B. N-Hydroxyphthalimide in combination with Cu(II), Co(II) or Mn(II) salts as catalytic systems for the oxidation of isopropyl-aromatic hydrocarbons with oxygen. Tetrahedron Lett. 2010, 51, 4100–4102. [Google Scholar] [CrossRef]
- Ishii, Y.; Nakayama, K.; Takeno, M.; Sakaguchi, S.; Iwahama, T.; Nishiyama, Y. A novel catalysis of N-hydroxyphthalimide in the oxidation of organic substrates by molecular oxygen. J. Org. Chem. 1995, 60, 3934–3935. [Google Scholar] [CrossRef]
- Tashiro, Y.; Iwahama, T.; Sakaguchi, S.; Ishii, Y. A new strategy for the preparation of terephthalic acid by the aerobic oxidation of p-xylene using N-hydroxyphthalimide as a catalyst. Adv. Synth. Catal. 2001, 343, 220–225. [Google Scholar] [CrossRef]
- Dobras, G.; Lisicki, D.; Pyszny, D.; Orlińska, B. Badania reakcji utleniającego rozszczepienia α-metylostyrenu tlenem wobec N-hydroksyftalimidu w alternatywnych rozpuszczalnikach. Przemysł Chem. 2019, 1, 124–129. [Google Scholar] [CrossRef]
- Lisicki, D.; Orlińska, B. Oxidation of cycloalkanes catalysed by N-hydroxyimides in supercritical carbon dioxide. Chem. Pap. 2020, 74, 711–716. [Google Scholar] [CrossRef] [Green Version]
- Yavari, I.; Karimi, E. N-hydroxyphthalimide-catalyzed oxidative production of phthalic acids from xylenes using O2/HNO3 in an ionic liquid. Synth. Commun. 2009, 39, 3420–3427. [Google Scholar] [CrossRef]
- Wang, J.R.; Liu, L.; Wang, Y.F.; Zhang, Y.; Deng, W.; Guo, Q.X. Aerobic oxidation with N-hydroxyphthalimide catalysts in ionic liquid. Tetrahedron Lett. 2005, 46, 4647–4651. [Google Scholar] [CrossRef]
- Koguchi, S.; Kitazume, T. Synthetic utilities of ionic liquid-supported NHPI complex. Tetrahedron Lett. 2006, 47, 2797–2801. [Google Scholar] [CrossRef]
- Lu, T.; Lu, M.; Yu, W.; Liu, Z. Remarkable effect of PEG-1000-based dicationic ionic liquid for N-hydroxyphthalimide-catalyzed aerobic selective oxidation of alkylaromatics. Croat. Chem. Acta 2012, 85, 277–282. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, T. PEG1000-DAIL enhanced catalysis activity: Oxidation of ethylbenzene and its derivatives by N-hydroxyphthalimide and oxime in 1000-based dicationic acidic ionic liquid. Chiang Mai J. Sci. 2014, 41, 138–147. [Google Scholar]
- Dobras, G.; Orlińska, B. Aerobic oxidation of alkylaromatic hydrocarbons to hydroperoxides catalysed by N-hydroxyimides in ionic liquids as solvents. Appl. Catal. A Gen. 2018, 561, 59–67. [Google Scholar] [CrossRef]
- Amorati, R.; Lucarini, M.; Mugnaini, V.; Pedulli, G.F.; Minisci, F.; Recupero, F.; Fontana, F.; Astolfi, P.; Greci, L. Hydroxylamines as oxidation catalysts: Thermochemical and kinetic studies. J. Org. Chem. 2003, 68, 1747–1754. [Google Scholar] [CrossRef]
- Melone, L.; Prosperini, S.; Ercole, G.; Pastori, N.; Punta, C. Is it possible to implement N-hydroxyphthalimide homogeneous catalysis for industrial applications? A case study of cumene aerobic oxidation. J. Chem. Technol. Biotechnol. 2014, 89, 1370–1378. [Google Scholar] [CrossRef]
- Rajabi, F.; Clark, J.H.; Karimi, B.; Macquarrie, D.J. The selective aerobic oxidation of methylaromatics to benzaldehydes using a unique combination of two heterogeneous catalysts. Org. Biomol. Chem. 2005, 3, 725–726. [Google Scholar] [CrossRef]
- Hermans, I.; Van Deun, J.; Houthoofd, K.; Peeters, J.; Jacobs, P.A. Silica-immobilized N-hydroxyphthalimide: An efficient heterogeneous autoxidation catalyst. J. Catal. 2007, 251, 204–212. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Mavvaji, M.; Tajbakhsh, M.; Lasemi, Z. Synthesis and characterization of N-hydroxyphthalimide immobilized on SiO2-coated Fe3O4 nanoparticles as magnetic catalyst for oxidation of benzyl alcohols and hydrocarbons. J. Iran. Chem. Soc. 2018, 15, 893–904. [Google Scholar] [CrossRef]
- Kasperczyk, K.; Orlińska, B.; Witek, E.; Łątka, P.; Zawadiak, J.; Proniewicz, L. Polymer-supported N-hydroxyphthalimide as catalyst for toluene and p-methoxytoluene aerobic oxidation. Catal. Lett. 2015, 145, 1856–1867. [Google Scholar] [CrossRef]
- Gao, B.; Meng, S.; Yang, X. Synchronously synthesizing and immobilizing N-hydroxyphthalimide on polymer microspheres and catalytic performance of solid catalyst in oxidation of ethylbenzene by molecular oxygen. Org. Process Res. Dev. 2015, 19, 1374–1382. [Google Scholar] [CrossRef]
- Łątka, P.; Kasperczyk, K.; Orlińska, B.; Drozdek, M.; Skorupska, B.; Witek, E. N-hydroxyphthalimide Immobilized on poly(HEA-Co-DVB) as catalyst for aerobic oxidation processes. Catal. Lett. 2016, 146, 1991–2000. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Bi, C. Some catalytic characteristics of compositional catalysts of immobilized N-hydroxyphthalimide and metal salts in aerobic oxidation of 1-phenylethanol. Catal. Commun. 2018, 115, 6–11. [Google Scholar] [CrossRef]
- Łątka, P.; Berniak, T.; Drozdek, M.; Witek, E.; Kuśtrowski, P. Formation of N-hydroxyphthalimide species in poly(vinyl-diisopropyl-phtalate ester-co-styrene-co-divinylbenzene) and its application in aerobic oxidation of p-methoxytoluene. Catal. Commun. 2018, 115, 73–77. [Google Scholar] [CrossRef]
- Culica, M.E.; Kasperczyk, K.; Baron, R.I.; Biliuta, G.; Macsim, A.M.; Lazea-Stoyanova, A.; Orlinska, B.; Coseri, S. Recyclable polymer-supported N-hydroxyphthalimide catalysts for selective oxidation of pullulan. Materials 2019, 12, 3585. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, R.; Mavvaji, M.; Tajbakhsh, M.; Lasemi, Z. Synthesis and characterization of N-hydroxyphthalimide immobilized on NaY nano-zeolite as a novel and efficient catalyst for the selective oxidation of hydrocarbons and benzyl alcohols. React. Kinet. Mech. Catal. 2018, 124, 839–855. [Google Scholar] [CrossRef]
- Kernchen, U.; Etzold, B.; Korth, W.; Jess, A. Solid catalyst ionic liquid layer (SCILL)—A new concept to improve selectivity illustrated by hydrogenation of cyclooctadiene. Chem. Eng. Technol. 2007, 30, 985–994. [Google Scholar] [CrossRef]
- Steinrück, H.P.; Wasserscheid, P. Ionic liquids in catalysis. Catal. Lett. 2015, 145, 380–397. [Google Scholar] [CrossRef]
- Arras, J.; Steffan, M.; Shayeghi, Y.; Claus, P. The promoting effect of a dicyanamide based ionic liquid in the selective hydrogenation of citral. Chem. Commun. 2008, 34, 4058–4060. [Google Scholar] [CrossRef] [PubMed]
- Arras, J.; Steffan, M.; Shayeghi, Y.; Ruppert, D.; Claus, P. Regioselective catalytic hydrogenation of citral with ionic liquids as reaction modifiers. Green Chem. 2009, 11, 716–723. [Google Scholar] [CrossRef]
- Antonels, N.C.; Benjamin Williams, M.; Meijboom, R.; Haumann, M. Well-defined dendrimer encapsulated ruthenium SCILL catalysts for partial hydrogenation of toluene in liquid-phase. J. Mol. Catal. A Chem. 2016, 421, 156–160. [Google Scholar] [CrossRef]
- Friedrich, M.F.; Lucas, M.; Claus, P. Selective hydrogenation of propyne on a solid Pd/Al2O3 catalyst modified with ionic liquid layer (SCILL). Catal. Commun. 2017, 88, 73–76. [Google Scholar] [CrossRef]
- Karimi, B.; Badreh, E. SBA-15-functionalized TEMPO confined ionic liquid: An efficient catalyst system for transition-metal-free aerobic oxidation of alcohols with improved selectivity. Org. Biomol. Chem. 2011, 9, 4194–4198. [Google Scholar] [CrossRef]
- Podolean, I.; Pavel, O.D.; Manyar, H.G.; Taylor, S.F.R.; Ralphs, K.; Goodrich, P.; Pârvulescu, V.I.; Hardacre, C. SCILLs as selective catalysts for the oxidation of aromatic alcohols. Catal. Today 2019, 333, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Pavel, O.D.; Podolean, I.; Parvulescu, V.I.; Taylor, S.F.R.; Manyar, H.G.; Ralphs, K.; Goodrich, P.; Hardacre, C. Impact of SCILL catalysts for the S-S coupling of thiols to disulfides. Faraday Discuss. 2018, 206, 535–547. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Liu, D.; Xia, Z.; Gao, J.; Zhang, T.; Ma, J.; Zhang, D.; Tong, Z. Supported ionic-liquid layer on polystyrene-TEMPO resin: A highly efficient catalyst for selective oxidation of activated alcohols with molecular oxygen. Monatsh. Chem. 2013, 144, 251–254. [Google Scholar] [CrossRef]
- Dobras, G.; Kasperczyk, K.; Jurczyk, S.; Orlińska, B. N-hydroxyphthalimide supported on silica coated with ionic liquids containing CoCl2 (SCILLs) as new catalytic system for solvent-free ethylbenzene oxidation. Catalysts 2020, 10, 252. [Google Scholar] [CrossRef] [Green Version]
- Dobras, G.; Sitko, M.; Petroselli, M.; Caruso, M.; Cametti, M.; Punta, C.; Orlińska, B. Solvent-free aerobic oxidation of ethylbenzene promoted by NHPI/Co(II) catalytic system: The key role of ionic liquids. ChemCatChem 2020, 12, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; Taylor & Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Das, S.K.; Sarkar, M. Rotational dynamics of coumarin-153 and 4-aminophthalimide in 1-ethyl-3-methylimidazolium alkylsulfate Ionic liquids: Effect of alkyl chain length on the rotational dynamics. J. Phys. Chem. B 2012, 116, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Kasperczyk, K.; Orlińska, B.; Zawadiak, J. Aerobic oxidation of cumene catalysed by 4-alkyloxycarbonyl-N-hydroxyphthalimide. Cent. Eur. J. Chem. 2014, 12, 1176–1182. [Google Scholar] [CrossRef]
- Zawadiak, J.; Gilner, D.; Kulicki, Z.; Baj, S. Concurrent iodimetric determination of cumene hydroperoxide and dicumenyl peroxide used for reaction control in dicumenyl peroxide synthesis. Analyst 1993, 118, 1081–1083. [Google Scholar] [CrossRef]
- Denney, D.B.; Goodyear, W.F.; Goldstein, B. Concerning the mechanism of the reduction of hydroperoxides by trisubstituted phosphines and trisubstituted phosphites. J. Am. Chem. Soc. 1960, 82, 1393–1395. [Google Scholar] [CrossRef]

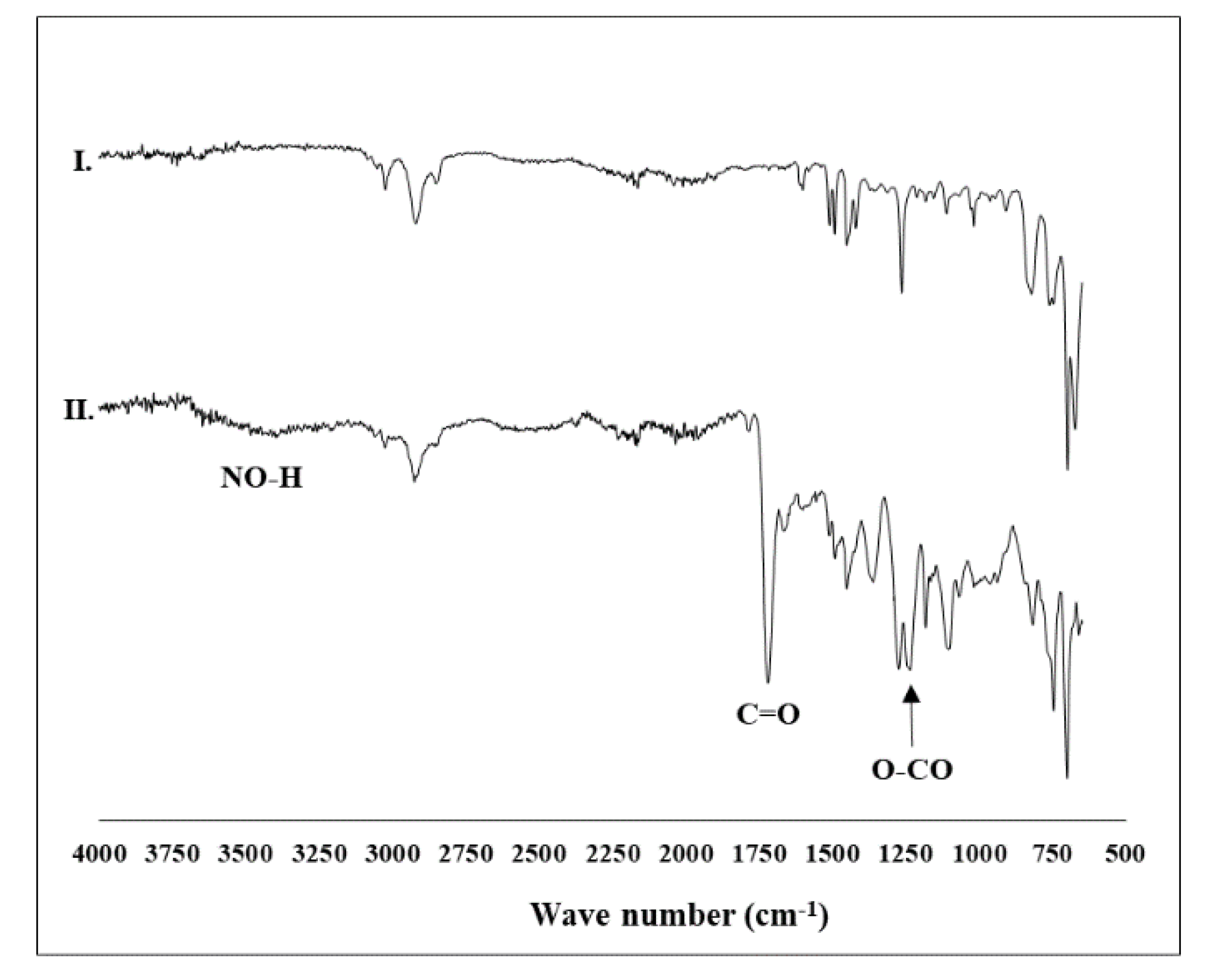


| Resin | Loading (mmol Cl/g) | DVB (%) | Practical Size | FT-IR (ATR; cm−1) | ||
|---|---|---|---|---|---|---|
| a | b | (mesh) | (μm) | |||
| PS-Cl-1 | ~5.5 | 5.5 | 5.5 | 16–50 | 297–1190 | 3023, 2921, 1611, 1511, 1443, 1421, 1264, 1111, 1019, 913, 823, 744, 672 |
| PS-Cl-2 | 2.5–4.0 | 4.24 | 1 | 50–100 | 149–297 | 3025, 2920, 1602, 1510, 1493, 1451, 1421, 1265, 1111, 1019, 908, 823, 760, 699, 674 |
| PS-Cl-3 | 3.5–4.5 | 3.94 | 1 | 100–200 | 74–149 | 3025, 2920, 1602, 1510, 1493, 1452, 1421, 1265, 1111, 1019, 909, 822, 760, 699, 673 |
| PS-Cl-4 | 3.5–4.5 | 4.39 | 1 | 200–400 | 37–74 | 3024, 2919, 1602, 1510, 1493, 1451, 1421, 1264, 1111, 1019, 909, 823, 746, 700, 673 |
| PS-Cl-5 | 2.0–2.5 | 2.78 | 2 | 200–400 | 37–74 | 3025, 2919, 1601, 1510, 1493, 1452, 1420, 1265, 1112, 1028, 907, 823, 757, 697, 676 |
| Immobilized NHPI | Loading (mmol NHPI/g) a | Size b (μm) | Shape b | FT-IR (ATR; cm−1) |
|---|---|---|---|---|
| PS-NHPI-1 | 2.60 | 2–15 | irregular | 3397br, 2925, 1786, 1717, 1449, 1362, 1275, 1245, 1183, 1103, 1070, 962, 817, 770, 746, 709 |
| PS-NHPI-2 | 2.63 | 149–297 20–170 | spherical irregular | 3398br, 2925, 1784, 1720, 1668, 1452, 1363, 1274, 1236, 1182, 1103, 1069, 817, 747, 701 |
| PS-NHPI-3 | 2.13 | 74–149 | spherical | 3398br, 2925, 1788, 1721, 1601, 1452, 1361, 1274, 1235, 1182, 1110, 1069, 1018, 817, 747, 701 |
| PS-NHPI-4 | 2.17 | 37–74 | spherical | 3439br, 2918, 1783, 1717, 1451, 1361, 1274, 1237, 1182, 1109, 818, 746, 701 |
| PS-NHPI-5 | 1.70 | 37–74 | spherical | 3439br, 3025, 2917, 1785, 1723, 1601, 1492, 1452, 1373, 1278, 1246, 1182, 1108, 819, 748, 699 |
| Entry | Catalyst | Loading (mmol NHPI/g) | DVB in Resin (%) | PS-NHPI h | nO2 i (mmol) | α (%) | SEBOOH (%) | SAP (%) | SPEOH (%) | TON j | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (μm) | Shape | ||||||||||
| 1 | − | − | − | − | − | 0.49 | 3.0 | 57.8 | 12.9 | 29.2 | − |
| 2 a | PS-NHPI-4 | 2.17 | 1 | 37–74 | spherical | 0.84 | 5.1 | 29.0 | 12.7 | 58.3 | 3.9 |
| 3 b | PS-NHPI-4@[emim][OcOSO3] | 2.17 | 1 | 37–74 | spherical | 0.90 | 5.5 | 19.1 | 20.4 | 60.5 | 4.1 |
| 4 c | CoCl2 | − | − | − | 0.73 | 4.5 | 46.3 | 22.1 | 31.4 | − | |
| 5 d | PS-NHPI-1/CoCl2 | 2.60 | 5.5 | 2–15 | irregular | 1.71 | 10.5 | 4.7 | 78.1 | 17.2 | 6.6 |
| 6 d | PS-NHPI-2/CoCl2 | 2.63 | 1 | 149–297 20–170 | spherical irregular | 1.55 | 9.5 | 18.7 | 50.4 | 30.9 | 5.9 |
| 7 d | PS-NHPI-3/CoCl2 | 2.13 | 1 | 74–149 | spherical | 1.18 | 7.2 | 23.2 | 45.1 | 31.7 | 5.5 |
| 8 d | PS-NHPI-4/CoCl2 | 2.17 | 1 | 37–74 | spherical | 1.78 | 10.9 | 1.8 | 64.8 | 33.4 | 8.2 |
| 9 d | PS-NHPI-5/CoCl2 | 1.70 | 2 | 37–74 | spherical | 1.31 | 8.0 | 14.4 | 53.3 | 32.3 | 7.7 |
| 10 e | PS-NHPI-1@CoCl2@[emim][OcOSO3] | 2.60 | 5.5 | 2–15 | irregular | 1.08 | 6.6 | 12.7 | 73.7 | 11.6 | 4.2 |
| 11 e | PS-NHPI-2@CoCl2@[emim][OcOSO3] | 2.63 | 1 | 149–297 20–170 | spherical irregular | 0.99 | 6.1 | 14.6 | 55.5 | 28.3 | 3.8 |
| 12 e | PS-NHPI-3@CoCl2@[emim][OcOSO3] | 2.13 | 1 | 74–149 | spherical | 0.96 | 5.9 | 10.6 | 67.9 | 20.3 | 4.5 |
| 13 e | PS-NHPI-4@CoCl2@[emim][OcOSO3] | 2.17 | 1 | 37–74 | spherical | 1.18 | 7.2 | 5.6 | 76.5 | 16.5 | 5.4 |
| 14 e | PS-NHPI-5@CoCl2@[emim][OcOSO3] | 1.70 | 2 | 37–74 | spherical | 1.08 | 6.6 | 10.3 | 63.9 | 25.8 | 6.3 |
| 15 f | CoCl2/[emim][OcOSO3] | − | − | − | − | 0.56 | 3.4 | 34.3 | 23.5 | 39.5 | − |
| 16 g | PS-NHPI-4/CoCl2/[emim][OcOSO3] | 2.17 | 1 | 37–74 | spherical | 1.13 | 6.9 | 23.3 | 35.7 | 40.0 | 5.2 |
| Entry | Cycle | Catalyst | Loading (mmol NHPI/g) | DVB in resin (%) | PS-NHPI | α (%) | SEBOOH (%) | SAP (%) | SPEOH (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Size e (μm) | Shape e | |||||||||
| 1 a | 1 | PS-NHPI-1/CoCl2 | 2.60 | 5.5 | 2–15 | irregular | 10.5 | 4.7 | 78.1 | 17.2 |
| 2 | 2 | 7.8 | 13.6 | 54.3 | 32.0 | |||||
| 3 | 3 | 6.5 | 26.8 | 40.5 | 32.7 | |||||
| 4 a | 1 | PS-NHPI-2/CoCl2 | 2.63 | 1 | 149–297 20–170 | spherical irregular | 9.5 | 18.7 | 50.4 | 30.9 |
| 5 | 2 | 6.2 | 37.8 | 36.1 | 26.1 | |||||
| 6 | 3 | 4.6 | 40.5 | 35.4 | 24.1 | |||||
| 7 a | 1 | PS-NHPI-3/CoCl2 | 2.13 | 1 | 74–149 | spherical | 7.2 | 23.2 | 45.1 | 31.7 |
| 8 | 2 | 7.1 | 28.7 | 36.7 | 34.6 | |||||
| 9 | 3 | 4.3 | 35.3 | 38.5 | 26.2 | |||||
| 10 a | 1 | PS-NHPI-4/CoCl2 | 2.17 | 1 | 37–74 | spherical | 10.9 | 1.8 | 64.8 | 33.4 |
| 11 | 2 | 5.9 | 31.1 | 39.2 | 29.7 | |||||
| 12 | 3 | 4.2 | 45.7 | 30.3 | 24.0 | |||||
| 13 a | 1 | PS-NHPI-5/CoCl2 | 1.70 | 2 | 37–74 | spherical | 8.0 | 14.4 | 53.3 | 32.3 |
| 14 | 2 | 6.1 | 15.6 | 50.6 | 32.5 | |||||
| 15 | 3 | 7.2 | 13.4 | 49.8 | 36.8 | |||||
| 16 | 4 | 5.8 | 19.1 | 45.3 | 35.6 | |||||
| 17 b | 1 | PS-NHPI-1@CoCl2@[emim][OcOSO3] | 2.60 | 5.5 | 2–15 | irregular | 6.6 | 12.7 | 73.7 | 11.6 |
| 18 | 2 | 6.1 | 13.2 | 71.2 | 15.6 | |||||
| 19 | 3 | 5.2 | 18.5 | 69.4 | 10.3 | |||||
| 20 b | 1 | PS-NHPI-2@CoCl2@[emim][OcOSO3] | 2.63 | 1 | 149–297 20–170 | spherical irregular | 6.1 | 14.6 | 55.5 | 28.3 |
| 21 | 2 | 5.8 | 18.9 | 49.5 | 30.7 | |||||
| 22 | 3 | 5.4 | 19.3 | 50.6 | 30.1 | |||||
| 23 b | 1 | PS-NHPI-3@CoCl2@[emim][OcOSO3] | 2.13 | 1 | 74–149 | spherical | 5.9 | 10.6 | 67.9 | 20.3 |
| 24 | 2 | 5.7 | 9.0 | 68.0 | 22.5 | |||||
| 25 | 3 | 5.4 | 10.9 | 66.2 | 22.4 | |||||
| 26 b | 1 | PS-NHPI-4@CoCl2@[emim][OcOSO3] | 2.17 | 1 | 37–74 | spherical | 7.2 | 5.6 | 76.5 | 16.5 |
| 27 | 2 | 6.5 | 7.6 | 70.5 | 21.1 | |||||
| 28 | 3 | 6.7 | 7.1 | 63.9 | 28.4 | |||||
| 29 b | 1 | PS-NHPI-5@CoCl2@[emim][OcOSO3] | 1.70 | 2 | 37–74 | spherical | 6.6 | 10.3 | 64.1 | 25.6 |
| 30 | 2 | 6.1 | 11.9 | 57.7 | 30.4 | |||||
| 31 | 3 | 5.6 | 13.5 | 54.5 | 32.0 | |||||
| 32 c | 1 | CoCl2 | − | − | − | − | 4.5 | 46.3 | 22.1 | 31.4 |
| 33 d | 1 | PS-NHPI-4 | 2.17 | 1 | 37–74 | spherical | 5.1 | 29.0 | 12.7 | 58.3 |
| Resin | Loading (mmol Cl/g) | Amount of resin (g) | TA (mmol) | Et3N (mmol) | Dioxane (mL) | NH2OH·HCl (mmol) | Pyridine: C2H4Cl2 (mL) | Immobilized NHPI | Amount of immobilized NHPI (g) |
|---|---|---|---|---|---|---|---|---|---|
| PS-Cl-1 | 5.5 | 2 | 44 | 110 | 60 | 110 | 220 | PS-NHPI-1 | 3.34 |
| PS-Cl-2 | 2.5–4.0 | 2 | 32 | 80 | 60 | 80 | 160 | PS-NHPI-2 | 3.42 |
| PS-Cl-3 | 3.5–4.5 | 2 | 36 | 90 | 60 | 135 | 270 | PS-NHPI-3 | 4.68 |
| 1 | 18 | 45 | 30 | ||||||
| PS-Cl-4 | 3.5–4.5 | 2 | 36 | 90 | 60 | 90 | 180 | PS-NHPI-4 | 3.10 |
| PS-Cl-5 | 2.0–2.5 | 2 | 20 | 50 | 60 | 50 | 100 | PS-NHPI-5 | 1.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talik, G.; Osial, A.; Grymel, M.; Orlińska, B. N-Hydroxyphthalimide on a Polystyrene Support Coated with Co(II)-Containing Ionic Liquid as a New Catalytic System for Solvent-Free Ethylbenzene Oxidation. Catalysts 2020, 10, 1367. https://doi.org/10.3390/catal10121367
Talik G, Osial A, Grymel M, Orlińska B. N-Hydroxyphthalimide on a Polystyrene Support Coated with Co(II)-Containing Ionic Liquid as a New Catalytic System for Solvent-Free Ethylbenzene Oxidation. Catalysts. 2020; 10(12):1367. https://doi.org/10.3390/catal10121367
Chicago/Turabian StyleTalik, Gabriela, Anna Osial, Mirosława Grymel, and Beata Orlińska. 2020. "N-Hydroxyphthalimide on a Polystyrene Support Coated with Co(II)-Containing Ionic Liquid as a New Catalytic System for Solvent-Free Ethylbenzene Oxidation" Catalysts 10, no. 12: 1367. https://doi.org/10.3390/catal10121367





