Promoting Effect of Mn on In Situ Synthesized Cu-SSZ-13 for NH3-SCR
Abstract
:1. Introduction
2. Results and Discussion
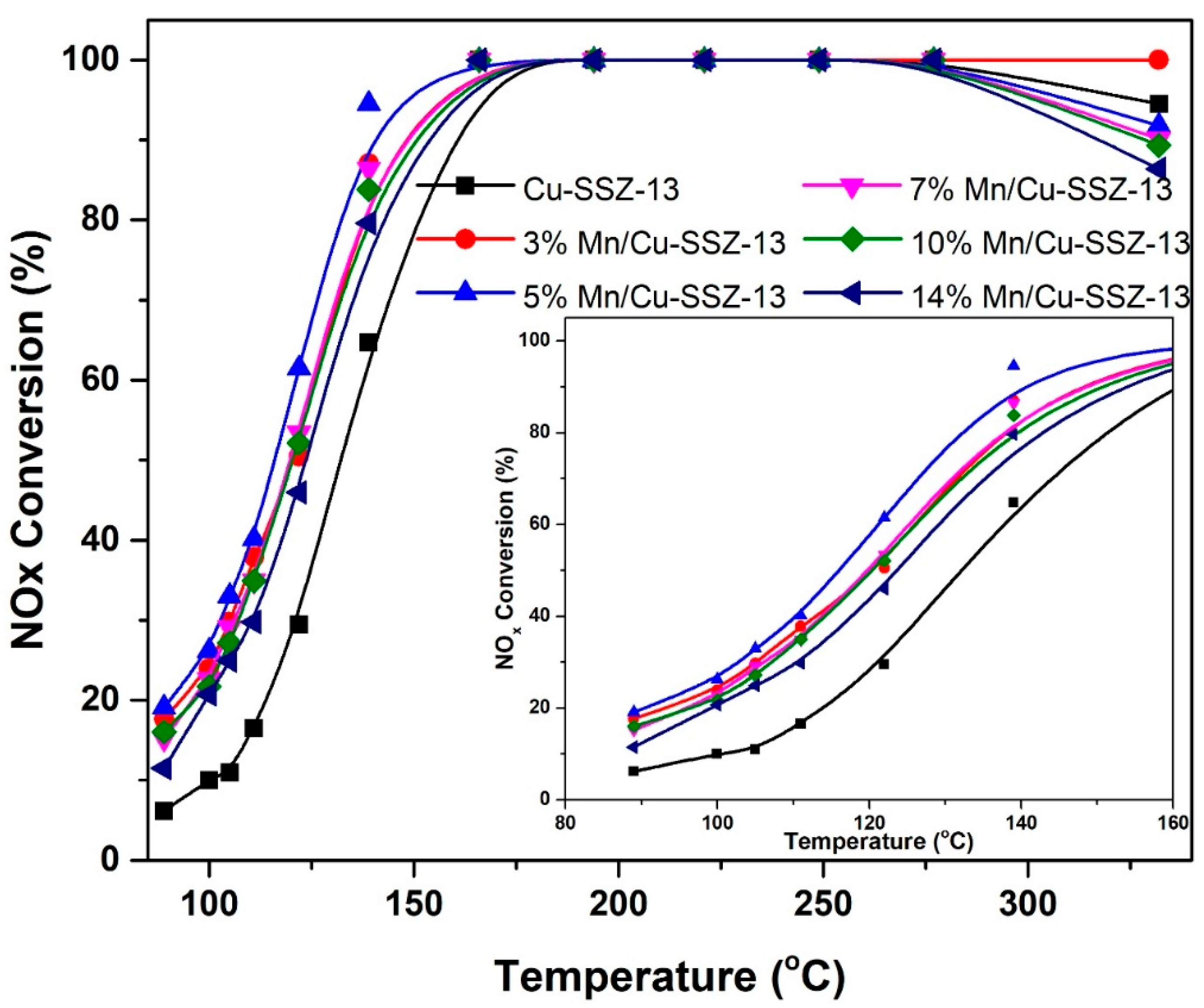
2.1. NH3-SCR Performance
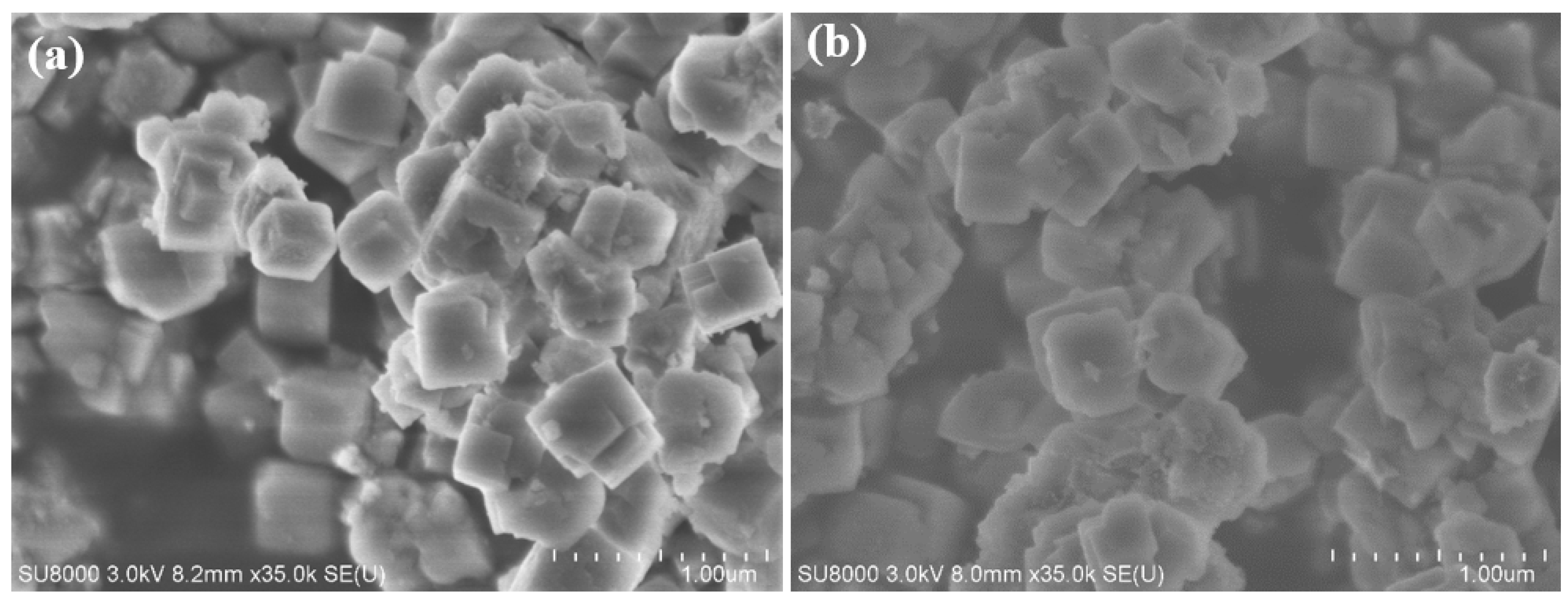
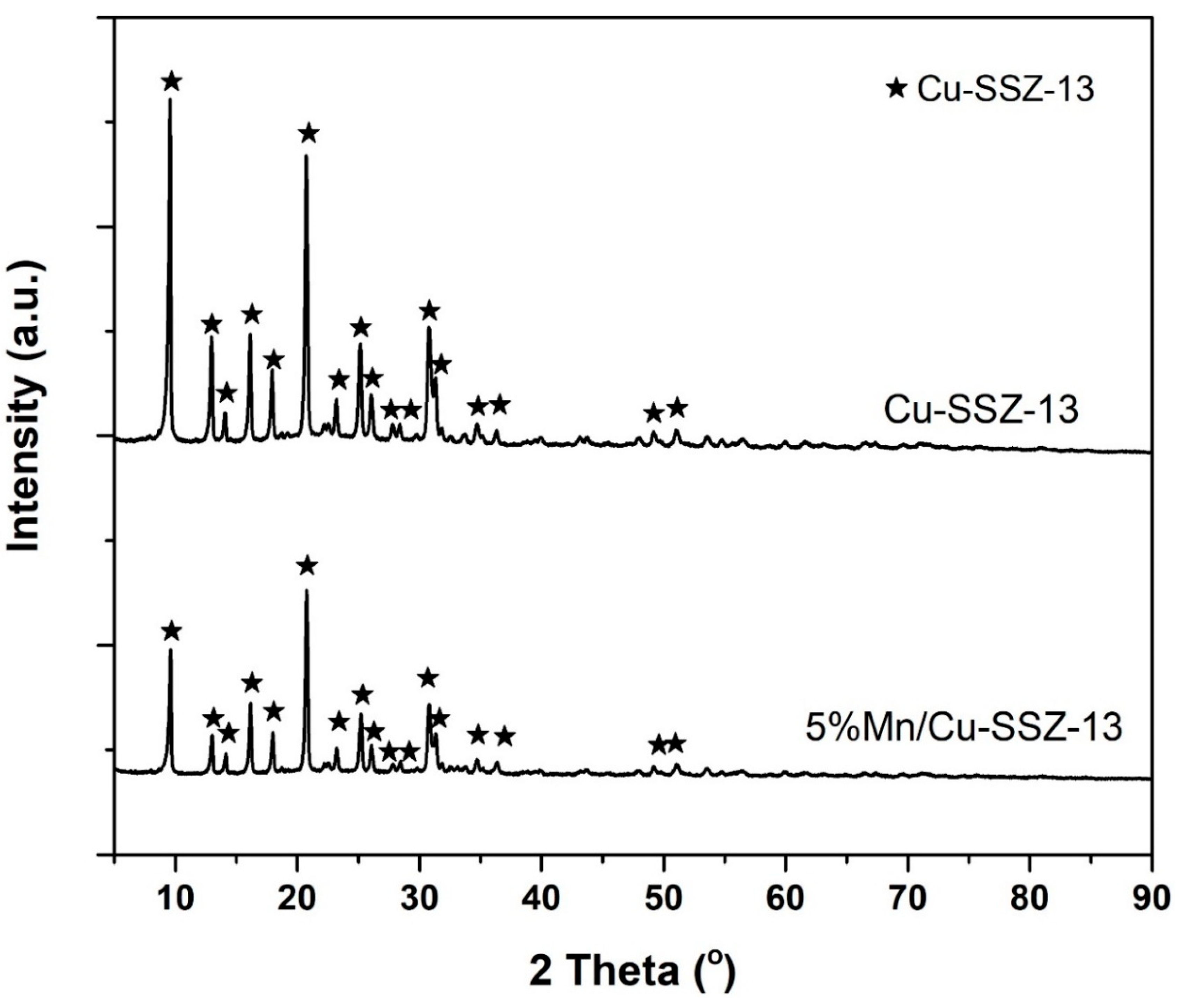
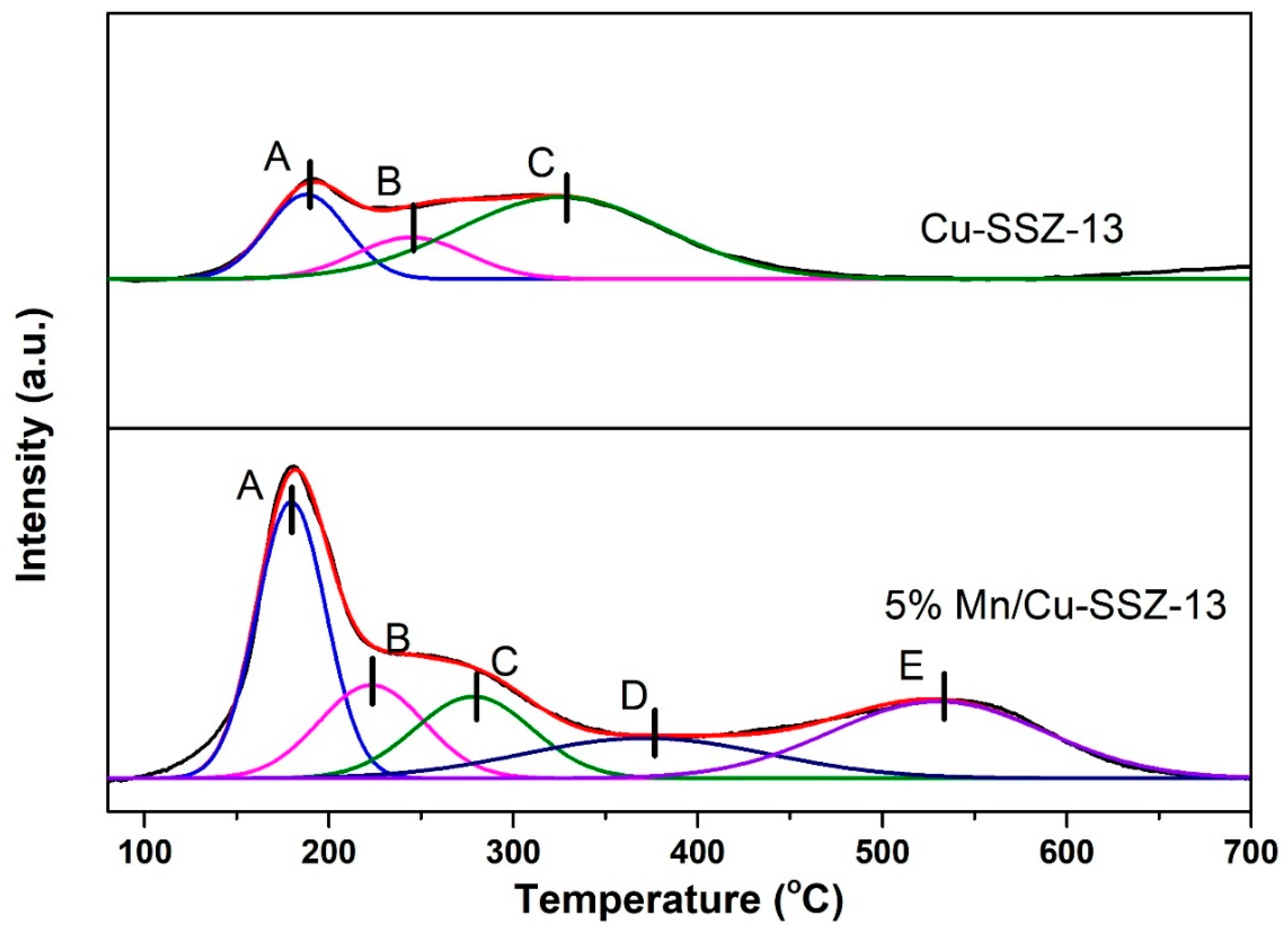
2.2. Characterization of Catalysts
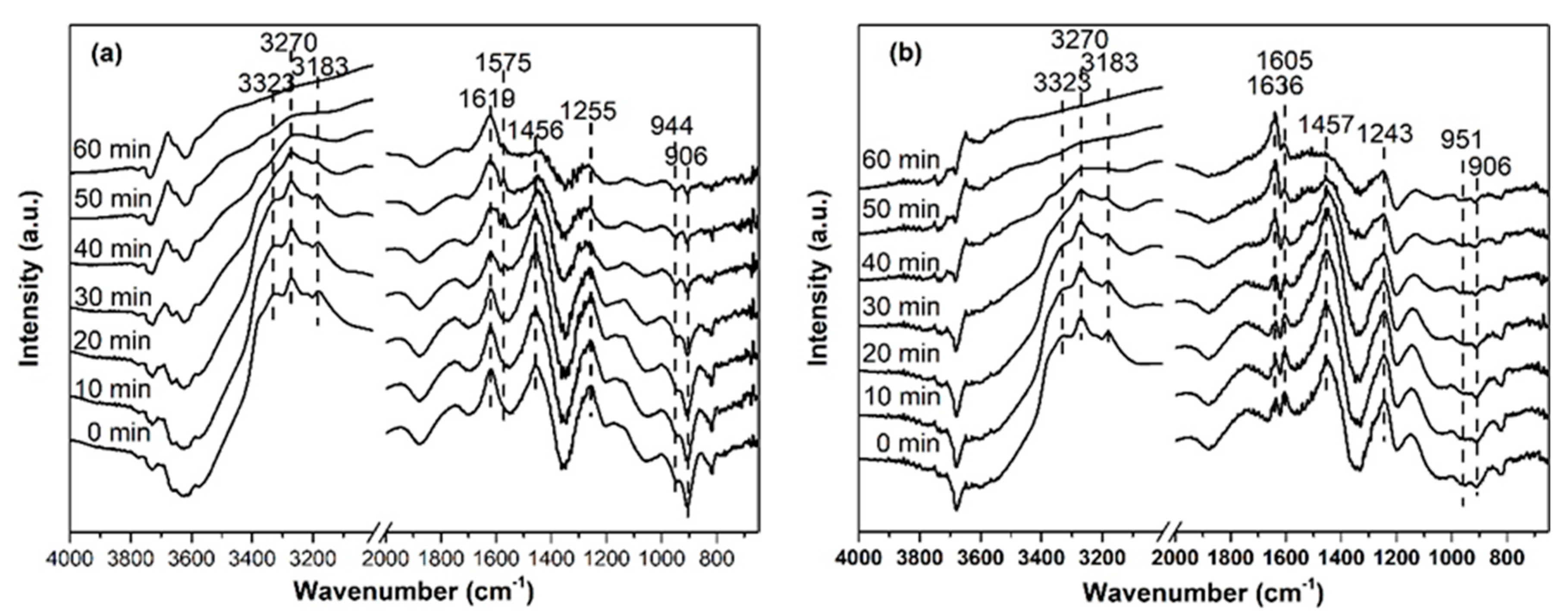
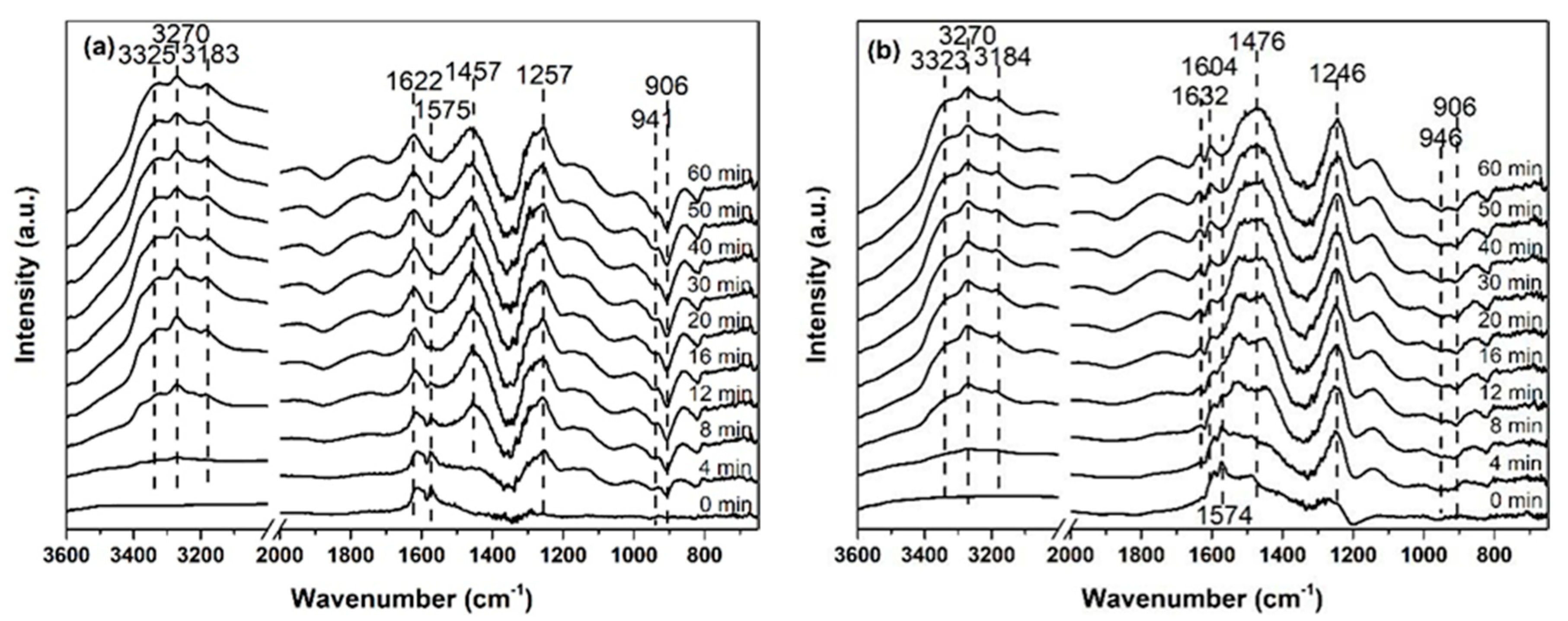
2.3. Investigation of Reaction Process
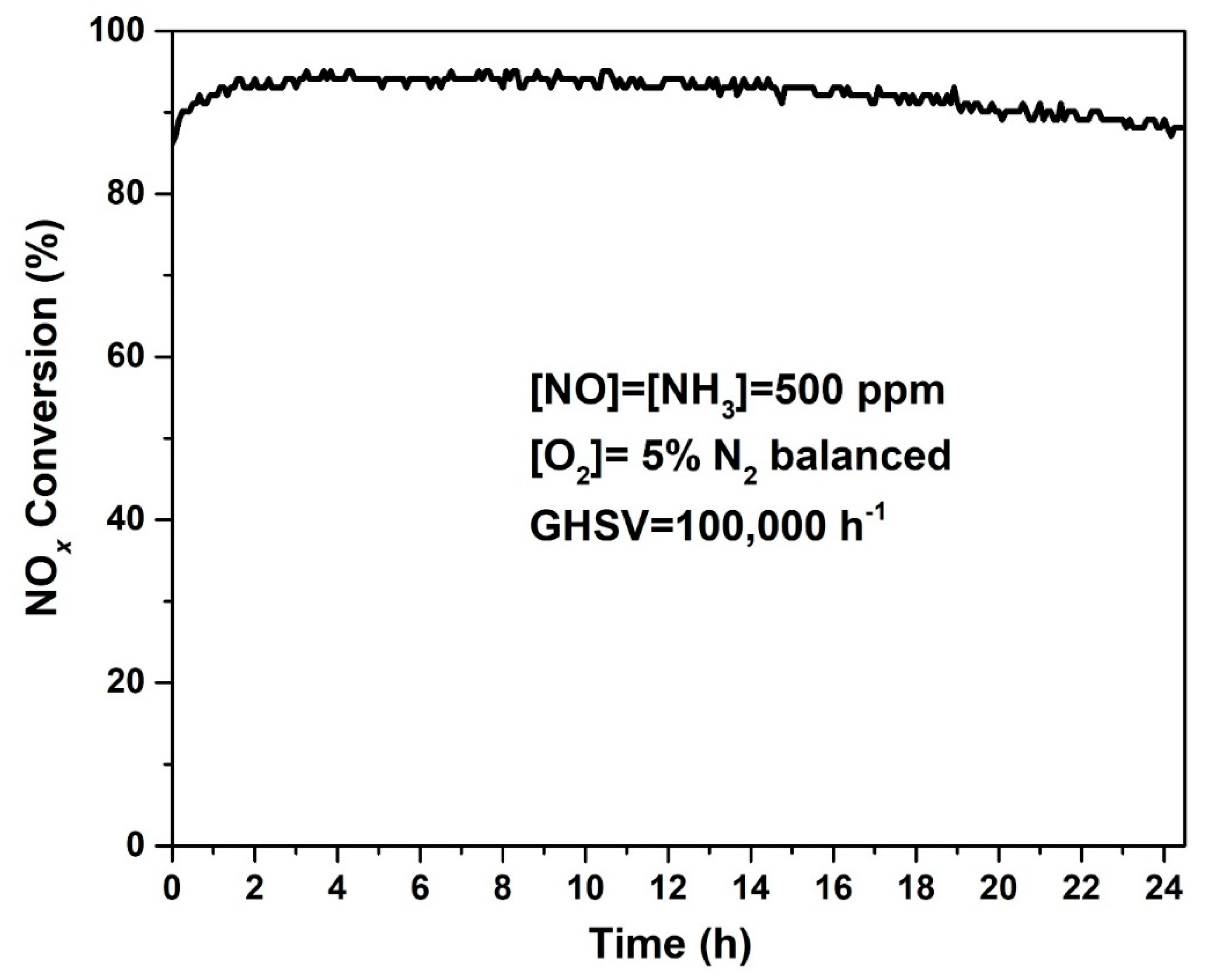
2.4. The Stability of 5% Mn/Cu-SSZ-13
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tan, Y.; Henderick, P.; Yoon, S.; Herner, J.; Montes, T.; Boriboonsomsin, K.; Johnson, K.; Scora, G.; Sandez, D.; Durbin, T.D. On-Board Sensor-Based NOx Emissions from Heavy-Duty Diesel Vehicles. Environ. Sci. Technol. 2019, 53, 5504–5511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, W.W.; Goodwin, S.R.; Michalski, G. Nitrogen stable isotope composition (delta15N) of vehicle-emitted NOx. Environ. Sci. Technol. 2015, 49, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Franco, V.; Mock, P.; Kolke, R.; Zhang, S.; Wu, Y.; German, J. Experimental Assessment of NOx Emissions from 73 Euro 6 Diesel Passenger Cars. Environ. Sci. Technol. 2015, 49, 14409–14415. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, S.; Gao, S.; Zhang, J.; Wang, H.; Wu, Z. Niobium oxide confined by ceria nanotubes as a novel SCR catalyst with excellent resistance to potassium, phosphorus, and lead. Appl. Catal. B Environ. 2018, 231, 299–309. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, D.; Zhang, L.; Huang, L.; Li, H.; Gao, R.; Shi, L.; Zhang, J. Comparative study of 3D ordered macroporous Ce0.75Zr0.2M0.05O2−δ(M = Fe, Cu, Mn, Co) for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2014, 4, 93–101. [Google Scholar] [CrossRef]
- Li, P.; Xin, Y.; Li, Q.; Wang, Z.; Zhang, Z.; Zheng, L. Ce-Ti amorphous oxides for selective catalytic reduction of NO with NH3: Confirmation of Ce-O-Ti active sites. Environ. Sci. Technol. 2012, 46, 9600–9605. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Chen, J.; Tang, X. Self-Prevention of Well-Defined-Facet Fe2O3/MoO3 against Deposition of Ammonium Bisulfate in Low-Temperature NH3-SCR. Environ. Sci. Technol. 2018, 52, 11796–11802. [Google Scholar] [CrossRef]
- Han, S.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Effect of the hydrothermal aging temperature and Cu/Al ratio on the hydrothermal stability of CuSSZ-13 catalysts for NH3-SCR. Catal. Sci. Technol. 2017, 7, 703–717. [Google Scholar] [CrossRef]
- Gao, F.; Peden, C.H. Recent Progress in Atomic-Level Understanding of Cu/SSZ-13 Selective Catalytic Reduction Catalysts. Catalysts 2018, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Kwak, J.H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tran, D.; Burton, S.D.; Szanyi, J.; Lee, J.H.; Peden, C.H.F. Effects of hydrothermal aging on NH3-SCR reaction over Cu/zeolites. J. Catal. 2012, 287, 203–209. [Google Scholar] [CrossRef]
- Paolucci, C.; Khurana, I.; Parekh, A.A.; Li, S.; Shih, A.J.; Li, H.; Di Iorio, J.R.; Albarracin-Caballero, J.D.; Yezerets, A.; Miller, J.T.; et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 2017, 357, 898–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Kwak, J.; Szanyi, J.; Peden, C.F. Current Understanding of Cu-Exchanged Chabazite Molecular Sieves for Use as Commercial Diesel Engine DeNOx Catalysts. Top. Catal. 2013, 56, 1441–1459. [Google Scholar] [CrossRef]
- Ren, L.; Zhu, L.; Yang, C.; Chen, Y.; Sun, Q.; Zhang, H.; Li, C.; Nawaz, F.; Meng, X.; Xiao, F.S. Designed copper-amine complex as an efficient template for one-pot synthesis of Cu-SSZ-13 zeolite with excellent activity for selective catalytic reduction of NOx by NH3. Chem. Commun. 2011, 47, 9789–9791. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, Y.; Zeng, S.; Zhu, L.; Sun, Q.; Zhang, H.; Yang, C.; Meng, X.; Yang, X.; Xiao, F.-S. Design and Synthesis of a Catalytically Active Cu-SSZ-13 Zeolite from a Copper-Amine Complex Template. Chin. J. Catal. 2012, 33, 92–105. [Google Scholar] [CrossRef]
- Shan, Y.; Du, J.; Yu, Y.; Shan, W.; Shi, X.; He, H. Precise control of post-treatment significantly increases hydrothermal stability of in-situ synthesized cu-zeolites for NH3-SCR reaction. Appl. Catal. B Environ. 2020, 266. [Google Scholar] [CrossRef]
- Xie, L.; Liu, F.; Shi, X.; Xiao, F.-S.; He, H. Effects of post-treatment method and Na co-cation on the hydrothermal stability of Cu–SSZ-13 catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2015, 179, 206–212. [Google Scholar] [CrossRef]
- Wang, D.; Gao, F.; Peden, C.H.F.; Li, J.; Kamasamudram, K.; Epling, W.S. Selective Catalytic Reduction of NOx with NH3 over a Cu-SSZ-13 Catalyst Prepared by a Solid-State Ion-Exchange Method. ChemCatChem 2014, 6, 1579–1583. [Google Scholar] [CrossRef]
- Kim, J.; Cho, S.J.; Kim, D.H. Facile Synthesis of KFI-type Zeolite and Its Application to Selective Catalytic Reduction of NOx with NH3. ACS Catal. 2017, 7, 6070–6081. [Google Scholar] [CrossRef]
- Hammershøi, P.S.; Negri, C.; Berlier, G.; Bordiga, S.; Beato, P.; Janssens, T.V.W. Temperature-programmed reduction with NO as a characterization of active Cu in Cu-CHA catalysts for NH3-SCR. Catal. Sci. Technol. 2019, 9, 2608–2619. [Google Scholar] [CrossRef]
- Liu, Z.; Yi, Y.; Zhang, S.; Zhu, T.; Zhu, J.; Wang, J. Selective catalytic reduction of NOx with NH3 over Mn-Ce mixed oxide catalyst at low temperatures. Catal. Today 2013, 216, 76–81. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G. A Highly Effective Catalyst of Sm-MnOx for the NH3-SCR of NOx at Low Temperature: Promotional Role of Sm and Its Catalytic Performance. ACS Catal. 2015, 5, 5973–5983. [Google Scholar] [CrossRef]
- Kang, M.; Park, E.D.; Kim, J.M.; Yie, J.E. Cu–Mn mixed oxides for low temperature NO reduction with NH3. Catal. Today 2006, 111, 236–241. [Google Scholar] [CrossRef]
- Zuo, J.; Chen, Z.; Wang, F.; Yu, Y.; Wang, L.; Li, X. Low-Temperature Selective Catalytic Reduction of NOx with NH3 over Novel Mn–Zr Mixed Oxide Catalysts. Ind. Eng. Chem. Res. 2014, 53, 2647–2655. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, L.; Wang, X.; Zou, W.; Cao, Y.; Sun, J.; Tang, C.; Gao, F.; Deng, Y.; Dong, L. Synthesis, characterization and catalytic performance of FeMnTiOx mixed oxides catalyst prepared by a CTAB-assisted process for mid-low temperature NH3-SCR. Appl. Catal. A Gen. 2015, 505, 235–242. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, S.; Zhang, C.; Wang, Q.; Louis, B. Synthesis and catalytic performance of Cu1Mn0.5Ti0.5Ox mixed oxide as low-temperature NH3 -SCR catalyst with enhanced SO2 resistance. Appl. Catal. B Environ. 2018, 238, 236–247. [Google Scholar] [CrossRef]
- Tang, X.; Wang, C.; Gao, F.; Ma, Y.; Yi, H.; Zhao, S.; Zhou, Y. Effect of hierarchical element doping on the low-temperature activity of manganese-based catalysts for NH3-SCR. J. Environ. Chem. Eng. 2020, 8, 104399–104408. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Ye, Y.; Shen, F.; Wang, H.; Chen, R. SSZ-13-supported manganese oxide catalysts for low temperature selective catalytic reduction of NOx by NH3. J. Chem. Sci. 2017, 129, 765–774. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwon, H.J.; Heo, I.; Nam, I.-S.; Cho, B.K.; Choung, J.W.; Cha, M.-S.; Yeo, G.K. Mn–Fe/ZSM5 as a low-temperature SCR catalyst to remove NOx from diesel engine exhaust. Appl. Catal. B Environ. 2012, 126, 9–21. [Google Scholar] [CrossRef]
- Yao, W.; Gang-gang, L.; Shao-qing, Z.; Xin-yan, Z.; Xin, Z.; Zheng-ping, H. Promoting effect of Ce and Mn addition on Cu-SSZ-39 zeolites for NH3-SCR reaction: Activity, hydrothermal stability, and mechanism study. Chem. Eng. J. 2020, 393. [Google Scholar] [CrossRef]
- Song, C.; Zhang, L.; Li, Z.; Luac, Y.; Li, K. Co-Exchange of Mn: A Simple Method to Improve Both the Hydrothermal Stability and Activity of Cu–SSZ-13 NH3–SCR Catalysts. Catalysts 2019, 9, 455. [Google Scholar] [CrossRef] [Green Version]
- Shan, Y.; Shan, W.; Shi, X.; Du, J.; Yu, Y.; He, H. A comparative study of the activity and hydrothermal stability of Al-rich Cu-SSZ-39 and Cu-SSZ-13. Appl. Catal. B Environ. 2020, 264, 118511. [Google Scholar] [CrossRef]
- Shan, Y.; Sun, Y.; Du, J.; Zhang, Y.; He, H.; Yu, Y.; Shan, W.; He, H. Hydrothermal aging alleviates the inhibition effects of NO2 on Cu-SSZ-13 for NH3-SCR. Appl. Catal. B Environ. 2020, 275, 119105. [Google Scholar] [CrossRef]
- Kwak, J.H.; Zhu, H.; Lee, J.H.; Peden, C.H.F.; Szanyi, J. Two different cationic positions in Cu-SSZ-13? Chem. Commun. 2012, 48, 4758–4760. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.K.; Min, K.M.; Hong, S.B.; Nam, I.-S.; Cho, B.K. Hydrothermal stability of CuSSZ13 for reducing NOx by NH3. J. Catal. 2014, 311, 447–457. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Shan, Y.; Du, J.; Liu, K.; He, H. Hydrothermal Stability Enhancement of Al-Rich Cu-SSZ-13 for NH3 Selective Catalytic Reduction Reaction by Ion Exchange with Cerium and Samarium. Ind. Eng. Chem. Res. 2020, 59, 6416–6423. [Google Scholar] [CrossRef]
- Du, J.; Qu, Z.; Dong, C.; Song, L.; Qin, Y.; Huang, N. Low-temperature abatement of toluene over Mn-Ce oxides catalysts synthesized by a modified hydrothermal approach. Appl. Surf. Sci. 2018, 433, 1025–1035. [Google Scholar] [CrossRef]
- Sun, P.; Guo, R.-T.; Liu, S.-M.; Wang, S.-X.; Pan, W.-G.; Li, M.-Y. The enhanced performance of MnOx catalyst for NH3-SCR reaction by the modification with Eu. Appl. Catal. A Gen. 2017, 531, 129–138. [Google Scholar] [CrossRef]
- Deng, H.; Kang, S.; Ma, J.; Wang, L.; Zhang, C.; He, H. Role of Structural Defects in MnOx Promoted by Ag Doping in the Catalytic Combustion of Volatile Organic Compounds and Ambient Decomposition of O3. Environ. Sci. Technol. 2019, 53, 10871–10879. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; He, H. Tuning the Chemical State of Silver on Ag–Mn Catalysts to Enhance the Ozone Decomposition Performance. Environ. Sci. Technol. 2020, 54, 11566–11575. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Mei, D.; Wang, Y.; Szanyi, J.; Peden, C.H.F. Selective Catalytic Reduction over Cu/SSZ-13: Linking Homo- and Heterogeneous Catalysis. J. Am. Chem. Soc. 2017, 139, 4935–4942. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Xu, M.; Huang, Y.; Wang, J.; Wang, J.; Lv, L.; Qi, G.; Li, W.; Shen, M. Insight of platinum poisoning Cu/SAPO-34 during NH3-SCR and its promotion on catalysts regeneration after hydrothermal treatment. Appl. Catal. B Environ. 2017, 204, 525–536. [Google Scholar] [CrossRef]
- Wang, J.; Yu, T.; Wang, X.; Qi, G.; Xue, J.; Shen, M.; Li, W. The influence of silicon on the catalytic properties of Cu/SAPO-34 for NOx reduction by ammonia-SCR. Appl. Catal. B Environ. 2012, 127, 137–147. [Google Scholar] [CrossRef]
- Liu, X.; Xiaodong, W.; Weng, D.; Si, Z.; Ran, R. Evolution of copper species on Cu/SAPO-34 SCR catalysts upon hydrothermal aging. Catal. Today 2017, 281, 596–604. [Google Scholar] [CrossRef]
- Chen, X.; Wang, P.; Fang, P.; Ren, T.; Liu, Y.; Cen, C.; Wang, H.; Wu, Z. Tuning the property of Mn-Ce composite oxides by titanate nanotubes to improve the activity, selectivity and SO2/H2O tolerance in middle temperature NH3-SCR reaction. Fuel Process. Technol. 2017, 167, 221–228. [Google Scholar] [CrossRef]
- Tian, W.; Yang, H.; Fan, X.; Zhang, X. Catalytic reduction of NOx with NH3 over different-shaped MnO2 at low temperature. J. Hazard. Mater. 2011, 188, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhao, H.; Luo, X.; Shi, K.; Wang, W.; Chen, Q.; Fan, H.; Wu, T. Promotion effect and mechanism of the addition of Mo on the enhanced low temperature SCR of NOx by NH3 over MnOx/γ-Al2O3 catalysts. Appl. Catal. B Environ. 2019, 245, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Liu, F.; Liu, K.; Shi, X.; He, H. Inhibitory effect of NO2 on the selective catalytic reduction of NOx with NH3 over one-pot-synthesized Cu–SSZ-13 catalyst. Catal. Sci. Technol. 2014, 4, 1104–1110. [Google Scholar] [CrossRef]
- Shi, X.; Liu, F.; Xie, L.; Shan, W.; He, H. NH3-SCR Performance of Fresh and Hydrothermally Aged Fe-ZSM-5 in Standard and Fast Selective Catalytic Reduction Reactions. Environ. Sci. Technol. 2013, 47, 3293–3298. [Google Scholar] [CrossRef] [PubMed]
- Lezcano-Gonzalez, I.; Deka, U.; Arstad, B.; Deyne, A.V.Y.-D.; Hemelsoet, K.; Waroquier, M.; Van Speybroeck, V.; Weckhuysen, B.M.; Beale, A.M. Determining the storage, availability and reactivity of NH3within Cu-Chabazite-based Ammonia Selective Catalytic Reduction systems. Phys. Chem. Chem. Phys. 2014, 16, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Liu, C.; Toyao, T.; Maeno, Z.; Ogura, M.; Nakazawa, N.; Inagaki, S.; Kubota, Y.; Shimizu, K.-I. Formation and Reactions of NH4NO3 during Transient and Steady-State NH3-SCR of NOx over H-AFX Zeolites: Spectroscopic and Theoretical Studies. ACS Catal. 2020, 10, 2334–2344. [Google Scholar] [CrossRef]
- Ruggeri, M.P.; Nova, I.; Tronconi, E.; Pihl, J.A.; Toops, T.J.; Partridge, W.P. In-situ DRIFTS measurements for the mechanistic study of NO oxidation over a commercial Cu-CHA catalyst. Appl. Catal. B Environ. 2015, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Shi, X.; Shan, Y.; Xu, G.; Sun, Y.; Wang, Y.; Yu, Y.; Shan, W.; He, H. Effects of SO2 on Cu-SSZ-39 catalyst for the selective catalytic reduction of NOx with NH3. Catal. Sci. Technol. 2020, 10, 1256–1263. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Li, K.; Liu, S.; Chen, J.; Li, J.; Gao, F.; Peden, C.H.F. Using Transient FTIR Spectroscopy to Probe Active Sites and Reaction Intermediates for Selective Catalytic Reduction of NO on Cu/SSZ-13 Catalysts. ACS Catal. 2019, 9, 6137–6145. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Wei, Z.; Kollar, M.; Gao, F.; Wang, Y.; Szanyi, J.; Peden, C.H.F. A comparative study of N2O formation during the selective catalytic reduction of NOx with NH3 on zeolite supported Cu catalysts. J. Catal. 2015, 329, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Hadjiivanov, K.I. Identification of Neutral and Charged NxOySurface Species by IR Spectroscopy. Catal. Rev. 2000, 42, 71–144. [Google Scholar] [CrossRef]
- Xie, L.; Liu, F.; Ren, L.; Shi, X.; Xiao, F.-S.; He, H. Excellent Performance of One-Pot Synthesized Cu-SSZ-13 Catalyst for the Selective Catalytic Reduction of NOx with NH3. Environ. Sci. Technol. 2013, 48, 566–572. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Wang, J.; Shi, X.; Shan, Y.; Zhang, Y.; He, H. Promoting Effect of Mn on In Situ Synthesized Cu-SSZ-13 for NH3-SCR. Catalysts 2020, 10, 1375. https://doi.org/10.3390/catal10121375
Du J, Wang J, Shi X, Shan Y, Zhang Y, He H. Promoting Effect of Mn on In Situ Synthesized Cu-SSZ-13 for NH3-SCR. Catalysts. 2020; 10(12):1375. https://doi.org/10.3390/catal10121375
Chicago/Turabian StyleDu, Jinpeng, Jingyi Wang, Xiaoyan Shi, Yulong Shan, Yan Zhang, and Hong He. 2020. "Promoting Effect of Mn on In Situ Synthesized Cu-SSZ-13 for NH3-SCR" Catalysts 10, no. 12: 1375. https://doi.org/10.3390/catal10121375



