Partial Hydrogenation of Anthracene with In Situ Hydrogen Produced from Water-Gas Shift Reaction over Fe-Based Catalysts
Abstract
1. Introduction
2. Results and Discussion
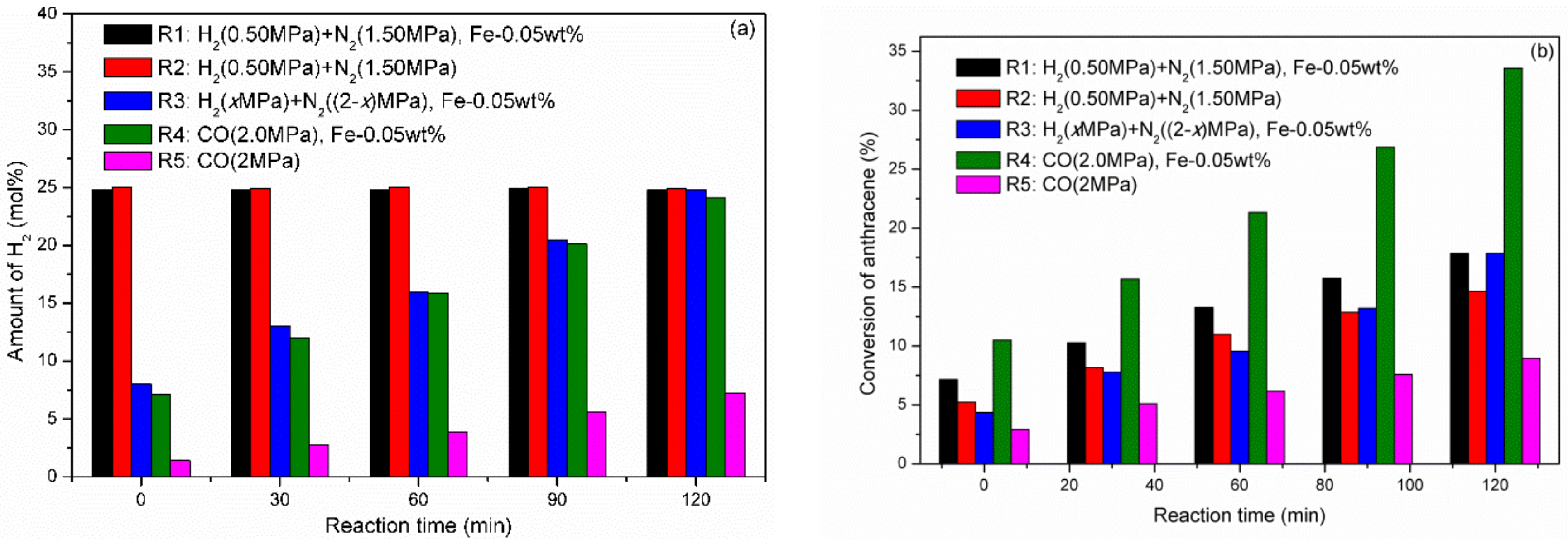
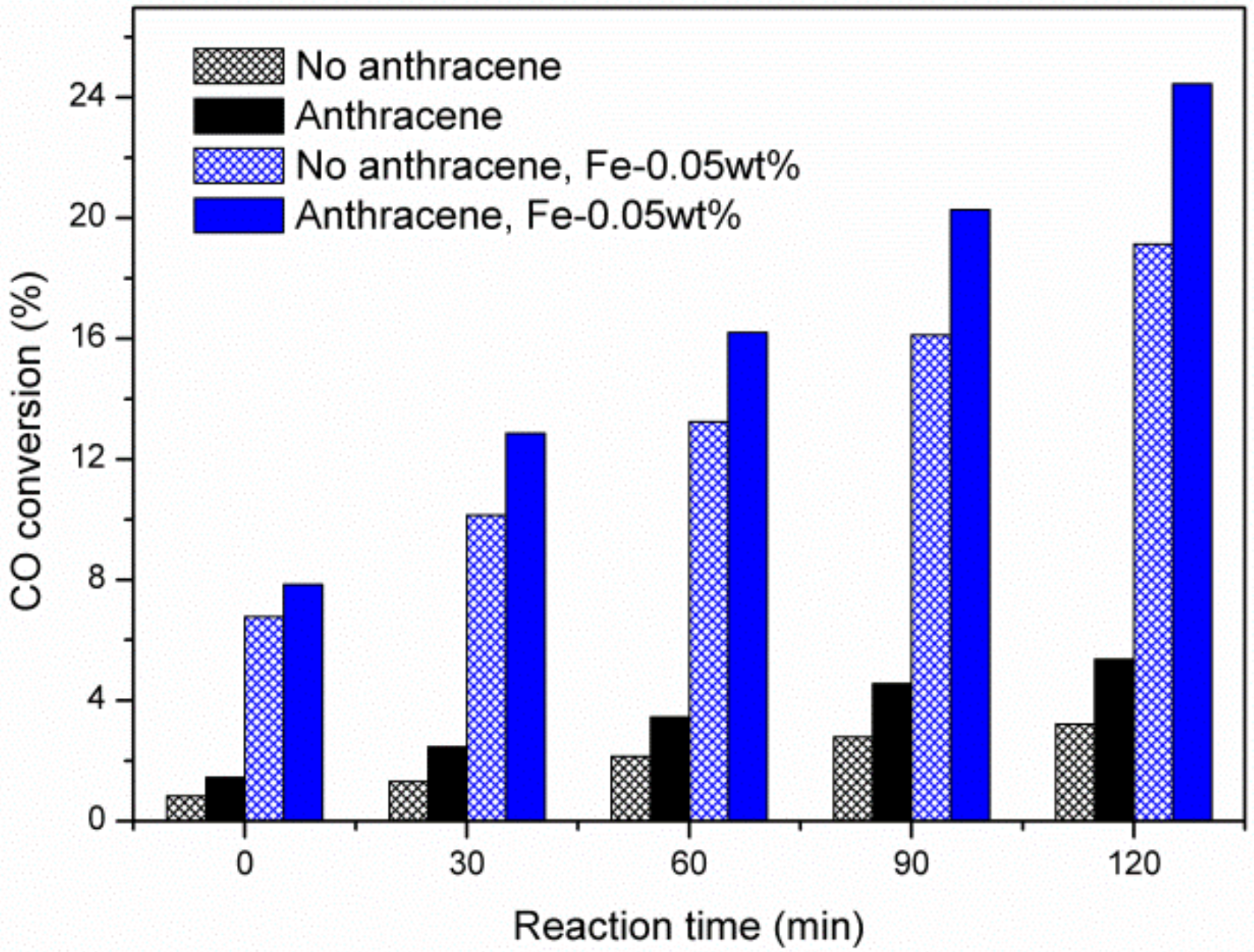
2.1. Partial Hydrogenation of Anthracene under CO-H2O, N2-H2O, and H2-H2O over Fe-Based Catalysts
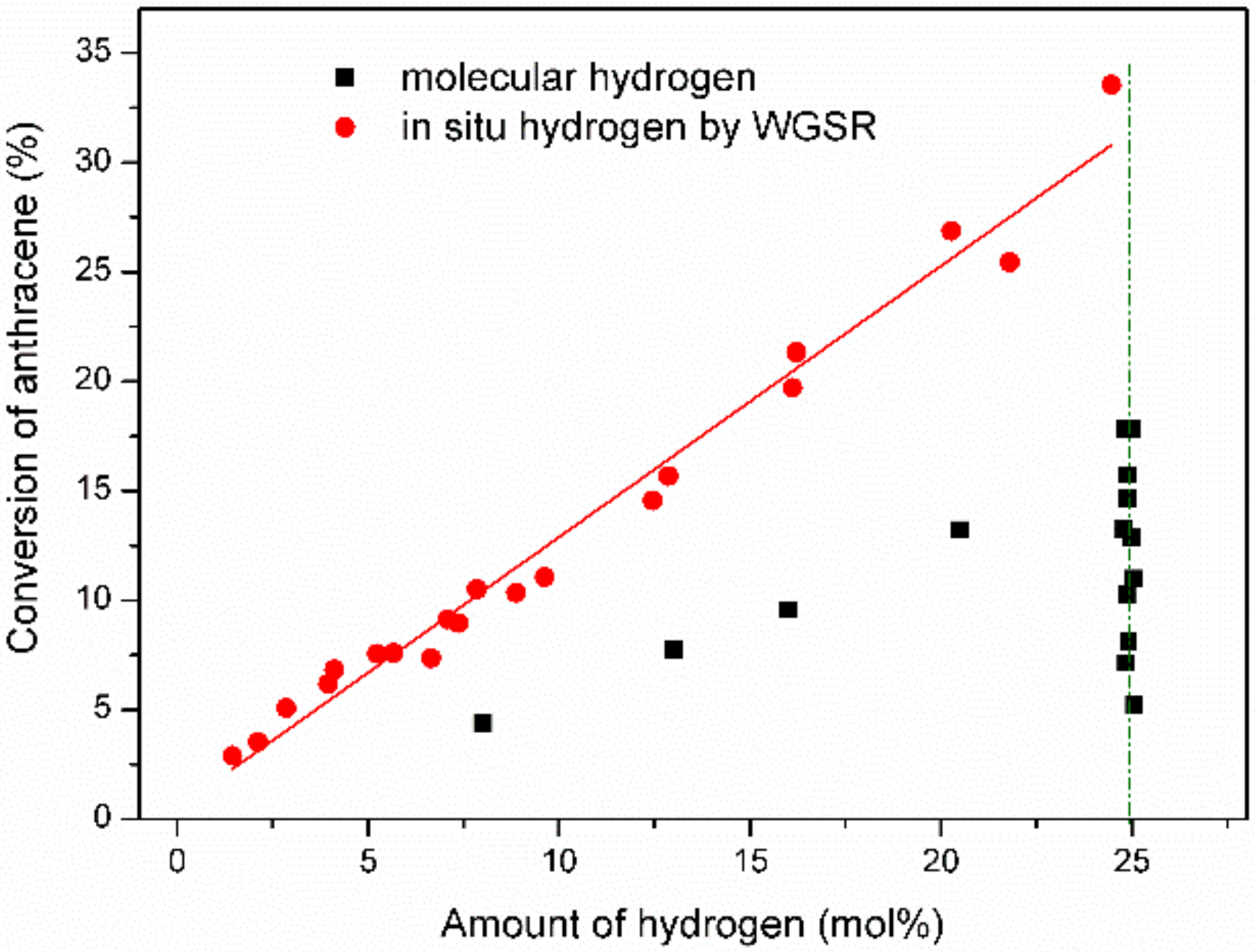
2.2. Comparison of In Situ Hydrogen Produced by WGSR and Molecular Hydrogen
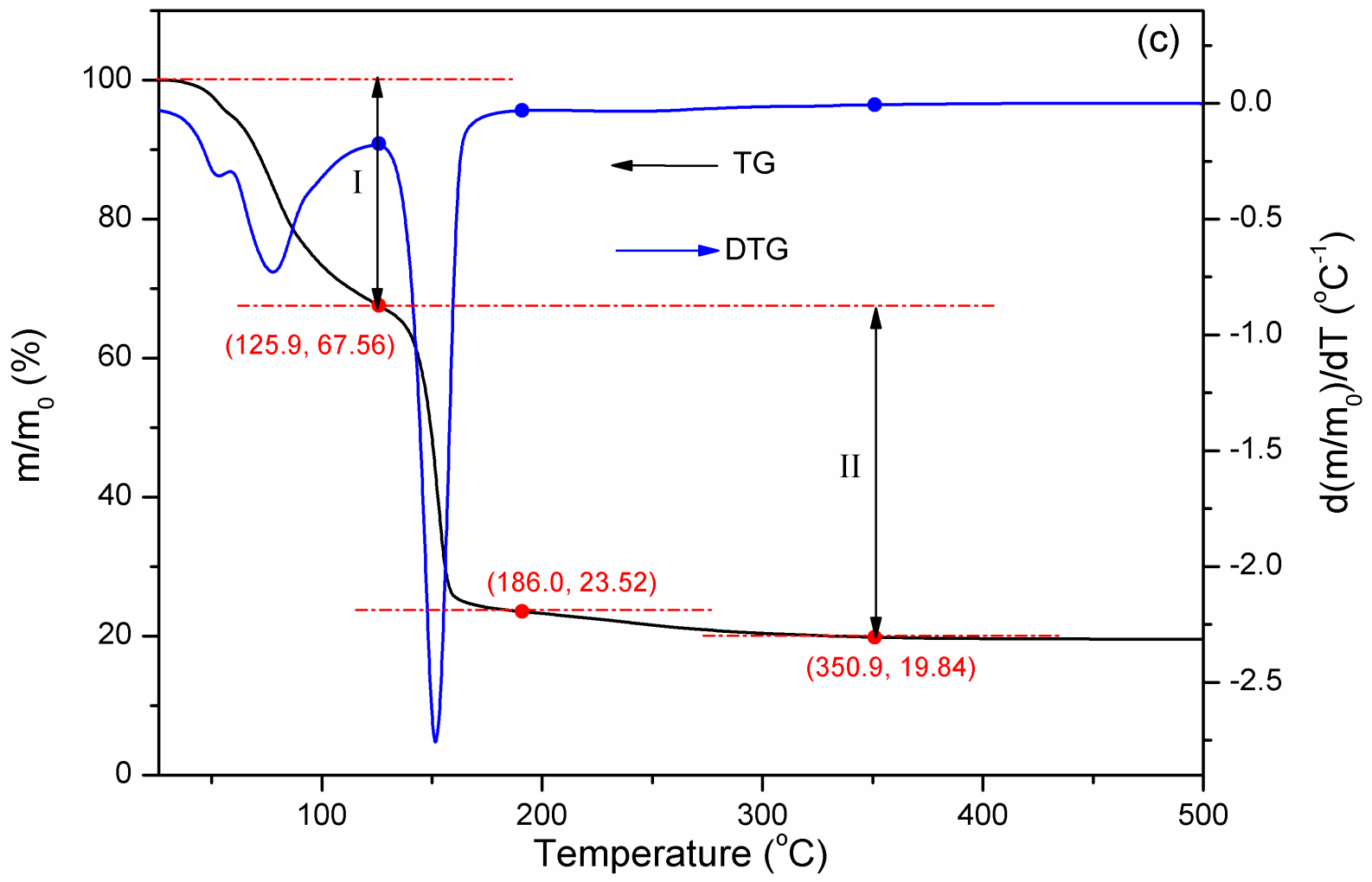
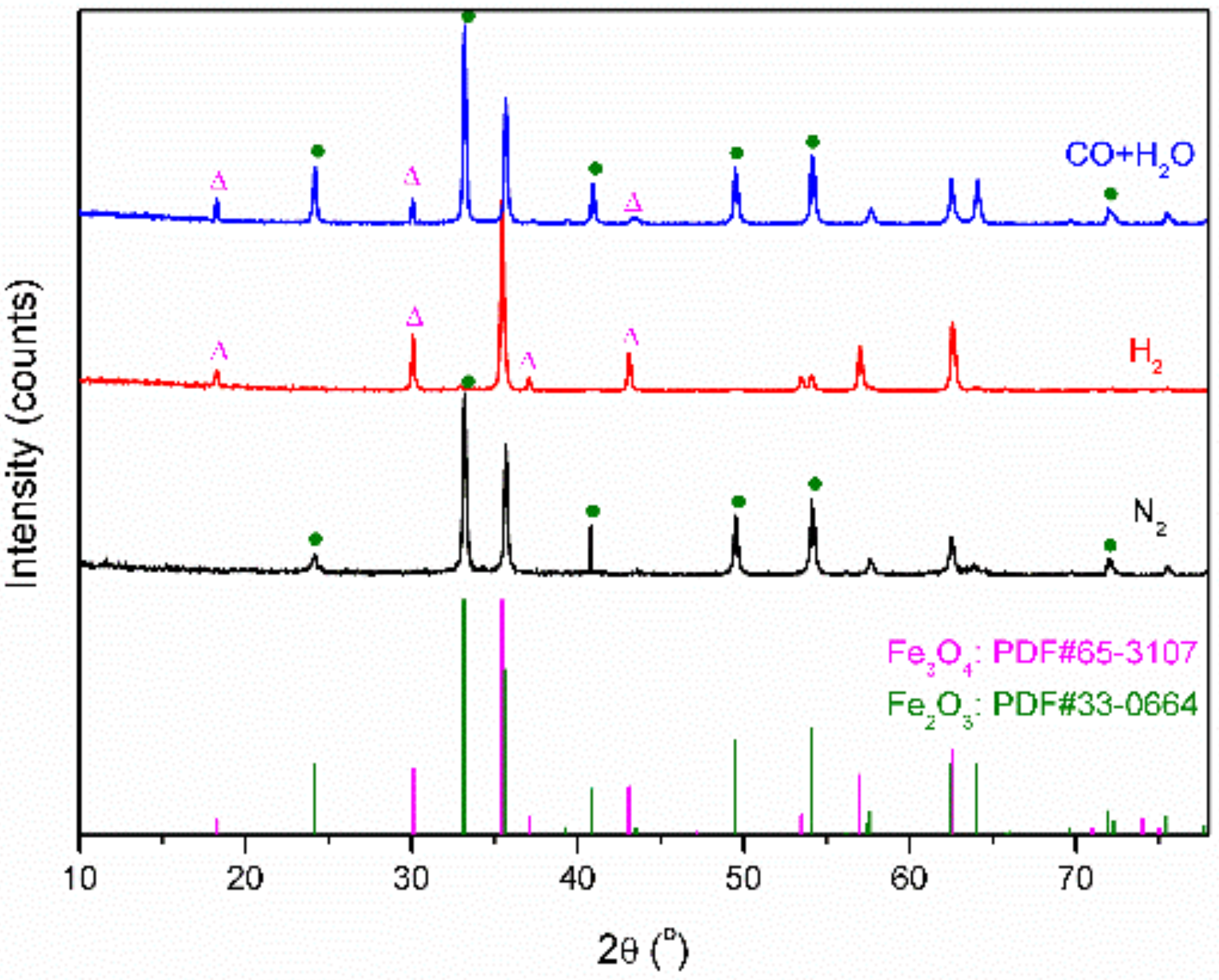
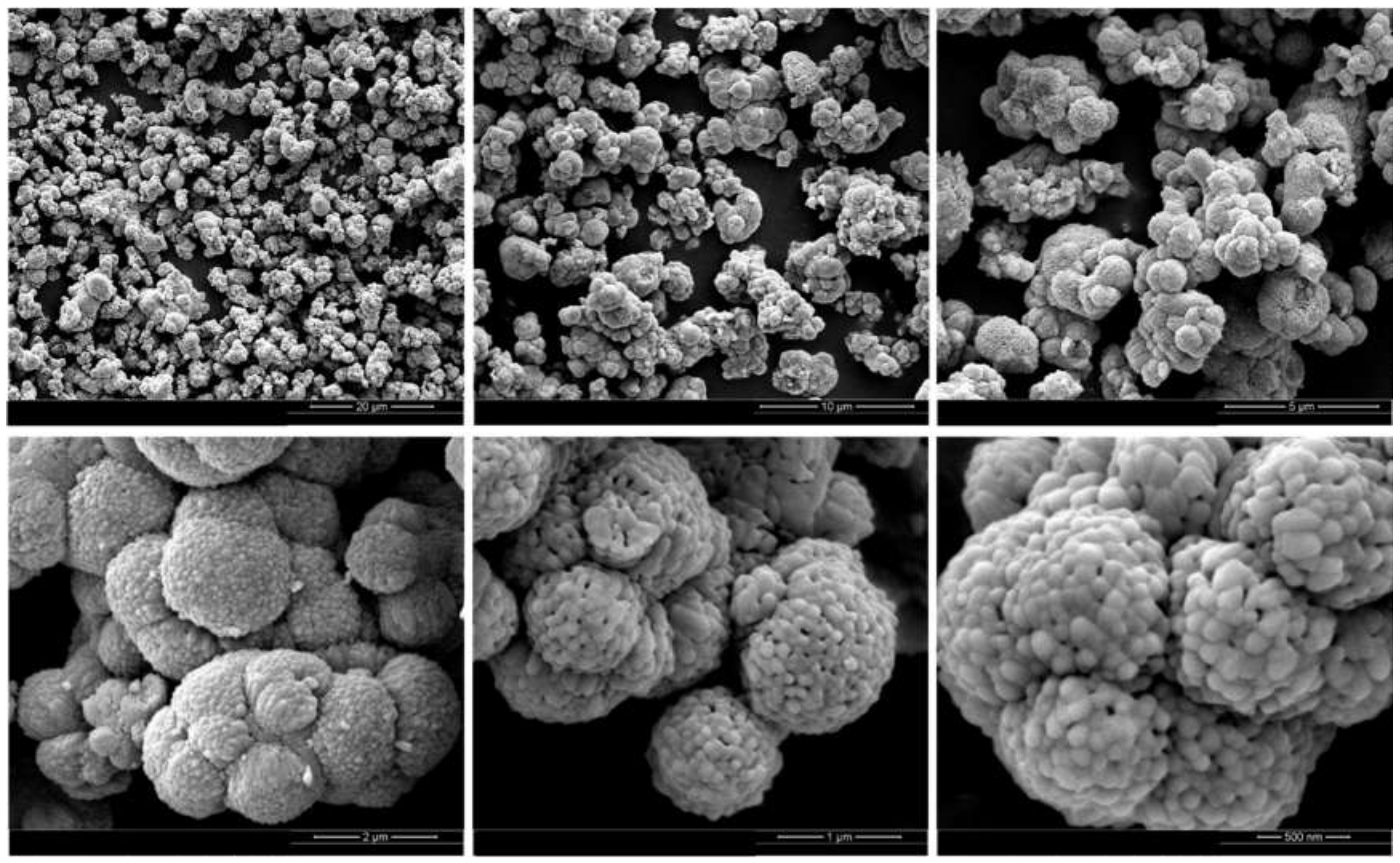
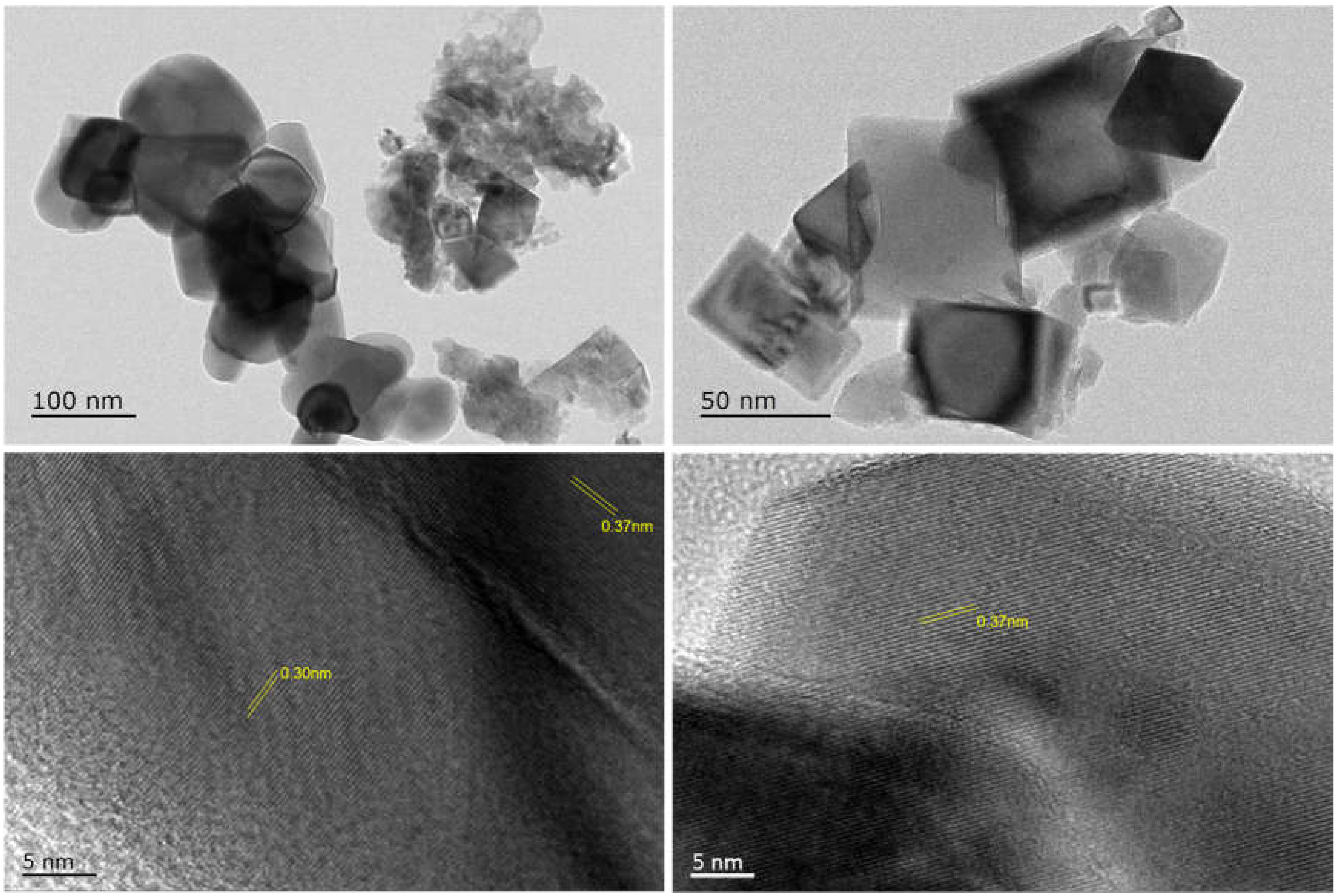
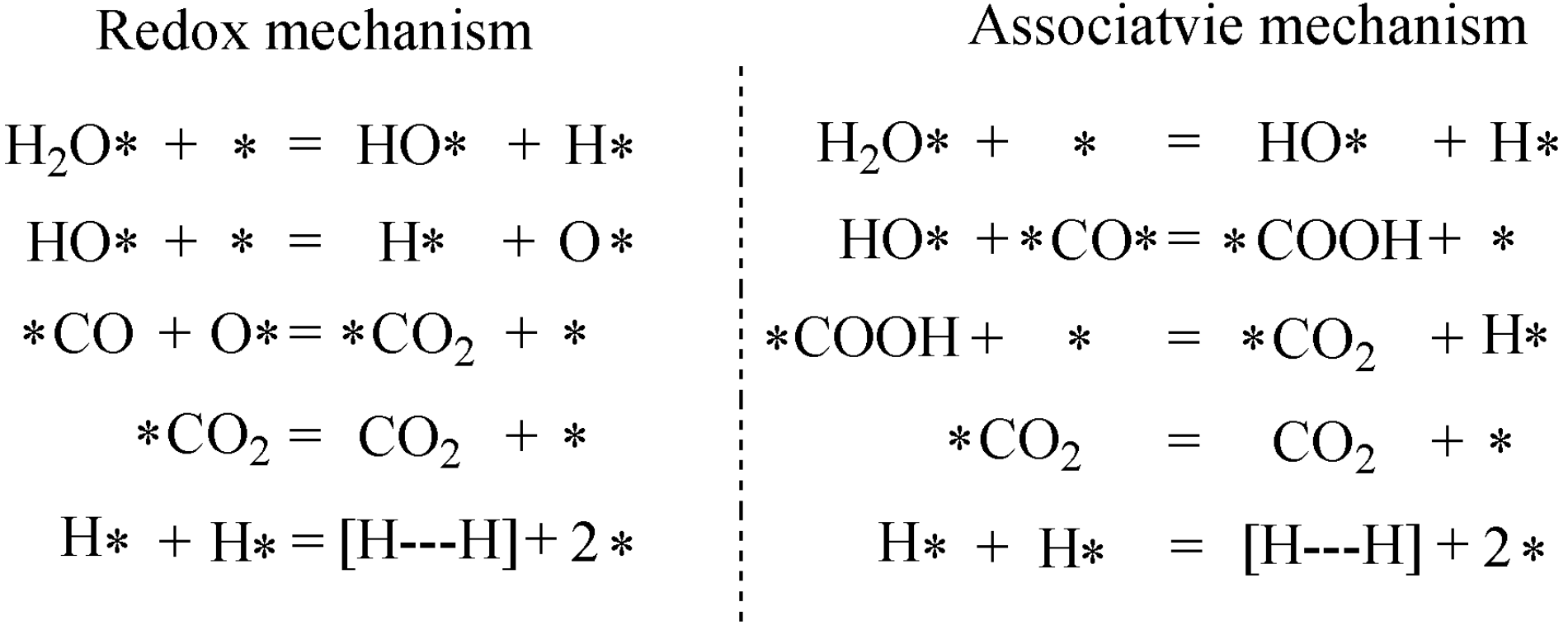
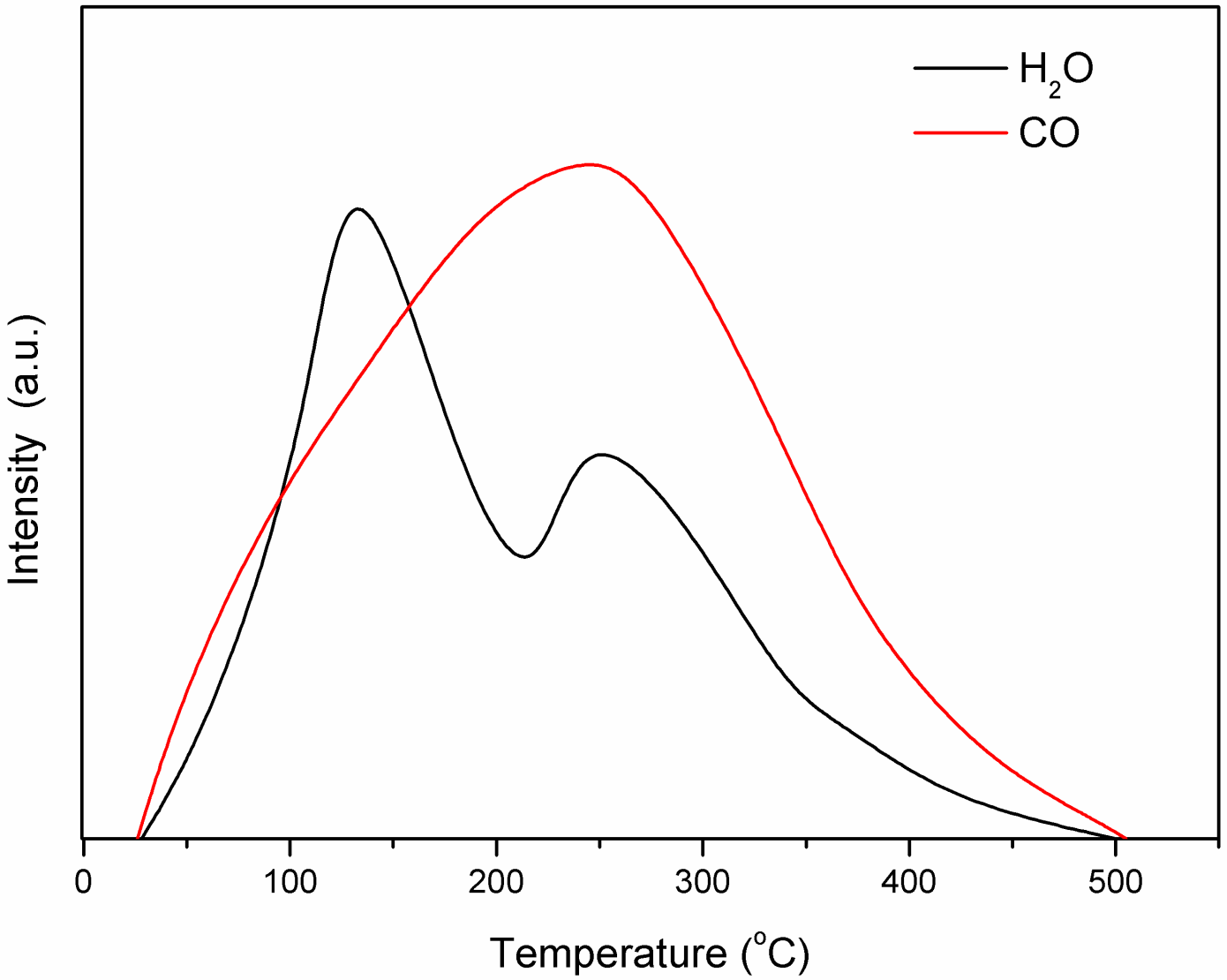
2.3. Characterization of Fe Catalytic Species during Hydrogenation of Anthracene under CO-H2O
3. Materials and Methods
3.1. Materials
3.2. Hydrogenation Experiment
3.3. Characterization of Catalytic Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borden, K. The challenges of processing and transporting heavy crude. Oil Gas Facil. 2015, 2, 22–26. [Google Scholar] [CrossRef]
- Muñoz, J.A.D.; Ancheyta, J.; Castañeda, L.C. Required viscosity values to assure proper transportation of crude oil by pipeline. Energy Fuels 2016, 30, 8850–8854. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, S.; Sun, X.; Xu, Z.; Xu, C. Storage stability of the visbreaking product from Venezuela heavy oil. Energy Fuels 2010, 24, 3970–3976. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Zhuang, S.; Li, F.; Guo, L.; Guo, A. Thermal upgrading of inferior residue without or with hydrogen donor. Acta Petrol. Sin. (Pet. Process. Sect.) 2014, 30, 439–445. [Google Scholar]
- Joshi, J.B.; Pandit, A.B.; Kataria, K.L.; Kulkarni, R.P.; Sawarkar, A.N.; Tandon, D.; Ram, Y.; Kumar, M.M. Petroleum residue upgradation via visbreaking: A review. Ind. Eng. Chem. Res. 2008, 47, 8960–8988. [Google Scholar] [CrossRef]
- Gray, M.R.G.; Mccaffrey, W.C. Role of chain reactions and olefin formation in cracking, hydroconversion, and coking of petroleum and bitumen fractions. Energy Fuels 2002, 16, 756–766. [Google Scholar] [CrossRef]
- Fischer, F.; Schrader, H. The production of oils by hydrogenation of coal. I. The hydrogenation of coal and other solid fuels by means of sodium formate. Brennst. Chem. 1921, 2, 161–173. [Google Scholar]
- Ng, F.T.T.; Tsakiri, S.K. Activation of water in emulsion for catalytic desulphurization of benzothiophene. Fuel 1992, 71, 1309–1314. [Google Scholar] [CrossRef]
- Ng, F.T.T. Upgrading heavy oil/bitumen emulsions via in situ hydrogen generation. Prepr. Symp. Am. Chem. Soc. Div. Fuel Chem. 1998, 43, 456–460. [Google Scholar]
- Ng, F.T.T.; Moll, J. Hydrogen production from bitumen emulsion for desulfurization and upgrading. Prepr. Symp. Am. Chem. Soc. Div. Fuel Chem. 2012, 57, 853. [Google Scholar]
- Jia, L. Oil Sands bitumen emulsion upgrading by using in situ hydrogen generated through the water gas shift reaction. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, December 2014. [Google Scholar]
- Liu, H.; Wang, Z.; Zhao, X.; Li, Y.; Chen, K.; Guo, A. Partial upgrading of vacuum residue from Canadian oil sand bitumen under CO/H2-H2O. J. Fuel Chem. Technol. 2018, 46, 45–53. [Google Scholar]
- Ng, F.T.T.; Milad, I.K. Catalytic desulphurization of benzothiophene in an emulsion via in situ generated H2. Appl. Catal. A 2000, 200, 243–254. [Google Scholar] [CrossRef]
- Liu, K. Hydrodesulfurization and hydrodenitrogenation of model compounds using in-situ hydrogen over nano-dispersed Mo sulfide based catalysts. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, October 2010. [Google Scholar]
- Liu, K.; Ng, F.T.T. Effect of the nitrogen heterocyclic compounds on hydrodesulfurization using in situ hydrogen and a dispersed Mo catalyst. Catal. Today 2010, 149, 28–34. [Google Scholar] [CrossRef]
- Yue, X.M.; Wei, X.Y.; Zhang, S.Q.; Liu, F.J.; Zong, Z.M.; Yang, X.Q. Hydrogenation of polycyclic aromatic hydrocarbons over a solid superacid. Fuel Process. Technol. 2017, 161, 283–288. [Google Scholar] [CrossRef]
- Schachtl, E.; Zhong, L.; Kondratieva, E.; Hein, J.; Gutiérrez, O.Y.; Jentys, A.; Lercher, J.A. Understanding Ni promotion of MoS2/γ-Al2O3 and its implications for the hydrogenation of phenanthrene. ChemCatChem 2016, 7, 4118–4130. [Google Scholar] [CrossRef]
- Fan, H.; Wu, T.; Yang, G.; Zhang, P.; Ding, G.; Wang, W.; Jiang, T.; Han, B. Effect of CO2/water mixture on the selective hydrocracking of anthracene. Catal. Commun. 2013, 33, 42–46. [Google Scholar] [CrossRef]
- Cai, X.; Long, J.; Wang, N.; Zhu, X.; Liu, Z.; Liu, Y.; Tian, S. Molecular characterization and transformation of PAHs in the hydrotreating process of light cycle oil. Acta Petrol. Sin. (Pet. Process. Sect.) 2017, 33, 403–410. [Google Scholar]
- Liu, H.; Ji, S.; Wang, Z.; Guo, A.; Chen, K. The catalytic performance of metalloporphyrins during hydrogenation of anthracene. Energy Technol. 2015, 3, 145–154. [Google Scholar] [CrossRef]
- Alemán-Vázquez, L.O.; Torres-Mancera, P.; Ancheyta, J.; Ramírez-Salgado, J. Use of hydrogen donors for partial upgrading of heavy petroleum. Energy Fuels 2016, 30, 9050–9060. [Google Scholar] [CrossRef]
- Yang, Z.; Zong, S.; Long, J. Quantum mechanical studies on the micro-structure of tetrahydro-naphthalene. Comput. Appl. Chem. 2012, 29, 465–468. [Google Scholar]
- Ye, W. The primary exploration on the chemical structure and refining properties of different types of petroleum molecules. Ph.D. Thesis, Research Institute of Petroleum Processing, Beijing, China, June, 2016. [Google Scholar]
- Luo, Y. Handbook of Chemical Bond Energies; Science Press: Beijing, China, 2005. [Google Scholar]
- Jiang, X.; Zong, S.; Li, X.; Zhao, Y. Molecular simulation of the hydrogen donating ability of hydrocarbon molecule. Acta Petrol. Sin. (Pet. Process. Sect.) 2012, 28, 254–259. [Google Scholar]
- Alemán-Vázquez, L.O.; Cano-Domínguez, J.L.; García-Gutiérrez, J.L. Effect of tetralin, decalin and naphthalene as hydrogen donors in the upgrading of heavy oils. Procedia Eng. 2012, 42, 532–539. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.D.; Lee, H.; Lee, Y.K. Beneficial roles of H-donors as diluent and H-shuttle for asphaltenes in catalytic upgrading of vacuum residue. Chem. Eng. J. 2017, 314, 1–10. [Google Scholar] [CrossRef]
- Kubo, J.; Higashi, H.; Yoshiharu Ohmoto, A.; Arao, H. Heavy oil hydroprocessing with the addition of hydrogen-donating hydrocarbons derived from petroleum. Energy Fuels 1996, 10, 474–481. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, S.; Liu, H.; Chen, K.; Guo, A. Hydrogen transfer of petroleum residue subfractions during thermal processing under hydrogen. Energy Technol. 2015, 3, 259–264. [Google Scholar] [CrossRef]
- Guo, A.; Wang, Z.; Zhang, H.; Zhang, X.; Wang, Z. Hydrogen transfer and coking propensity of petroleum residues under thermal processing. Energy Fuels 2010, 24, 3093–3100. [Google Scholar] [CrossRef]
- Sheng, Q.; Wang, G.; Duan, M.; Ren, A.; Yao, L.; Hu, M.; Gao, J. Determination of hydrogen-donating ability of industrial distillate narrow fractions. Energy Fuels 2016, 30, 10314–10321. [Google Scholar] [CrossRef]
- Gould, K.A.; Wiehe, I.A. Natural hydrogen donors in petroleum resids. Energy Fuels 2007, 21, 1199–1204. [Google Scholar] [CrossRef]
- Li, N.; Yan, B.; Xiao, X. A review of laboratory-scale research on upgrading heavy oil in supercritical water. Energies 2015, 8, 8962–8989. [Google Scholar] [CrossRef]
- Arai, K.; Tadafumi Adschiri, A.; Watanabe, M. Hydrogenation of hydrocarbons through partial oxidation in supercritical water. Ind. Eng. Chem. Res. 2010, 7, 273–281. [Google Scholar] [CrossRef]
- Sato, T.; Sumita, T.; Itoh, N. Effect of CO addition on upgrading bitumen in supercritical water. J. Supercrit. Fluids 2015, 104, 171–176. [Google Scholar] [CrossRef]
- Maity, S.K.; Ancheyta, J.; Marroquín, G. Catalytic aquathermolysis used for viscosity reduction of heavy crude oils: A review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Jia, D. Direct coal liquefaction with Fe3O4 nanocatalysts prepared by a simple solid-state method. Energies 2017, 10, 886. [Google Scholar] [CrossRef]
- Weng, S.; Gao, J.; Wu, Y.; Zhao, C.; Wu, Z. Mossbauer spectroscopic study of iron catalyst in coal hydroliquefaction II. On the transformation and the hydrogenation activity of ferric oxide under various reaction conditions. J. Fuel Chem. Technol. 1990, 18, 103–108. [Google Scholar]
- Siewe, C.N.S.; Ng, F.T.T. Hydrodesulfurization of cold lake diesel fraction using dispersed catalysts: Influence of hydroprocessing medium and sources of H2. Energy Fuels 1998, 12, 598–606. [Google Scholar] [CrossRef]
- Takemura, Y.; Itoh, H.; Ouchi, K. Catalytic hydrodesulfurization of residual oil by a mixture of carbon monoxide and water. Sekiyu Gakkaishi 2008, 24, 357–362. [Google Scholar] [CrossRef][Green Version]
- Liu, C.; Ng, F.T.T. HDS of DBT using in situ generated hydrogen in the presence of dispersed Mo catalysts II. Comparison between in situ hydrogen and molecular H2. Chin. J. Catal. 1999, 20, 505–510. [Google Scholar]
- Hook, B.D.; Akgerman, A. Desulfurization of dibenzothiophene by in-situ hydrogen generation through a water gas shift reaction. Ind. Eng. Chem. Process Des. Dev. 1986, 25, 278–284. [Google Scholar] [CrossRef]
- Lee, R.Z.; Ng, F.T.T. Effect of water on HDS of DBT over a dispersed Mo catalyst using in situ generated hydrogen. Catal. Today 2006, 116, 505–511. [Google Scholar] [CrossRef]
- Patlolla, A.; Carino, E.V.; Ehrlich, S.N.; Stavitski, E.; Frenkel, A.I. Application of operando XAS, XRD, and Raman spectroscopy for phase speciation in water gas shift reaction catalysts. ACS Catal. 2013, 2, 2216–2223. [Google Scholar] [CrossRef]
- Huang, L.; Han, B.; Zhang, Q.; Fan, M.; Cheng, H. Mechanistic study on water gas shift reaction on the Fe3O4 (111) reconstructed surface. J. Phys. Chem. C 2015, 119, 28934–28945. [Google Scholar] [CrossRef]
- Chen, L.; Ni, G.; Han, B.; Zhou, C.; Wu, J. Mechanism of water gas shift reaction on Fe3O4 (111) surface. Acta Chim. Sin. 2011, 69, 393–398. [Google Scholar]
- Adschiri, T.; Shibata, R.; Sato, T.; Masaru Watanabe, A.; Arai, K. Catalytic hydrodesulfurization of dibenzothiophene through partial oxidation and a water-gas shift reaction in supercritical water. Ind. Eng. Chem. Res. 1998, 37, 2634–2638. [Google Scholar] [CrossRef]


| Run. No. | Temperature/°C | Atmosphere (Initial Pressure/MPa) | Fe(NO3)3·9H2O/Fe wt% |
|---|---|---|---|
| R1 | 400 | H2(0.50) + N2(1.50) | 0.05 |
| R2 | 400 | H2(0.50) + N2(1.50) | 0 |
| R3 | 400 | H2(x) + N2(2 − x) | 0.05 |
| R3(x = 0.16) | 400 | H2(0.16) + N2(1.84) | 0.05 |
| R3(x = 0.26) | 400 | H2(0.26) + N2(1.74) | 0.05 |
| R3(x = 0.32) | 400 | H2(0.32) + N2(1.68) | 0.05 |
| R3(x = 0.41) | 400 | H2(0.41) + N2(1.59) | 0.05 |
| R4 | 400 | CO(2) | 0.05 |
| R5 | 400 | CO(2) | 0 |
| R6 | 400 | N2(2) | 0 |
| R7 | 400 | N2(2) | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Fan, S.; Gong, X.; Wang, J.; Guo, A.; Chen, K.; Wang, Z. Partial Hydrogenation of Anthracene with In Situ Hydrogen Produced from Water-Gas Shift Reaction over Fe-Based Catalysts. Catalysts 2020, 10, 1379. https://doi.org/10.3390/catal10121379
Liu H, Fan S, Gong X, Wang J, Guo A, Chen K, Wang Z. Partial Hydrogenation of Anthracene with In Situ Hydrogen Produced from Water-Gas Shift Reaction over Fe-Based Catalysts. Catalysts. 2020; 10(12):1379. https://doi.org/10.3390/catal10121379
Chicago/Turabian StyleLiu, He, Shiguang Fan, Xu Gong, Jian Wang, Aijun Guo, Kun Chen, and Zongxian Wang. 2020. "Partial Hydrogenation of Anthracene with In Situ Hydrogen Produced from Water-Gas Shift Reaction over Fe-Based Catalysts" Catalysts 10, no. 12: 1379. https://doi.org/10.3390/catal10121379
APA StyleLiu, H., Fan, S., Gong, X., Wang, J., Guo, A., Chen, K., & Wang, Z. (2020). Partial Hydrogenation of Anthracene with In Situ Hydrogen Produced from Water-Gas Shift Reaction over Fe-Based Catalysts. Catalysts, 10(12), 1379. https://doi.org/10.3390/catal10121379








