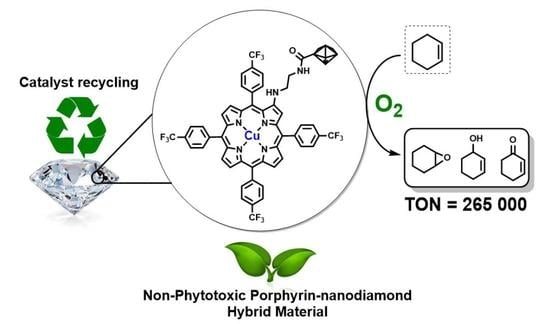
Porphyrin–Nanodiamond Hybrid Materials—Active, Stable and Reusable Cyclohexene Oxidation Catalysts
Abstract
1. Introduction
2. Results
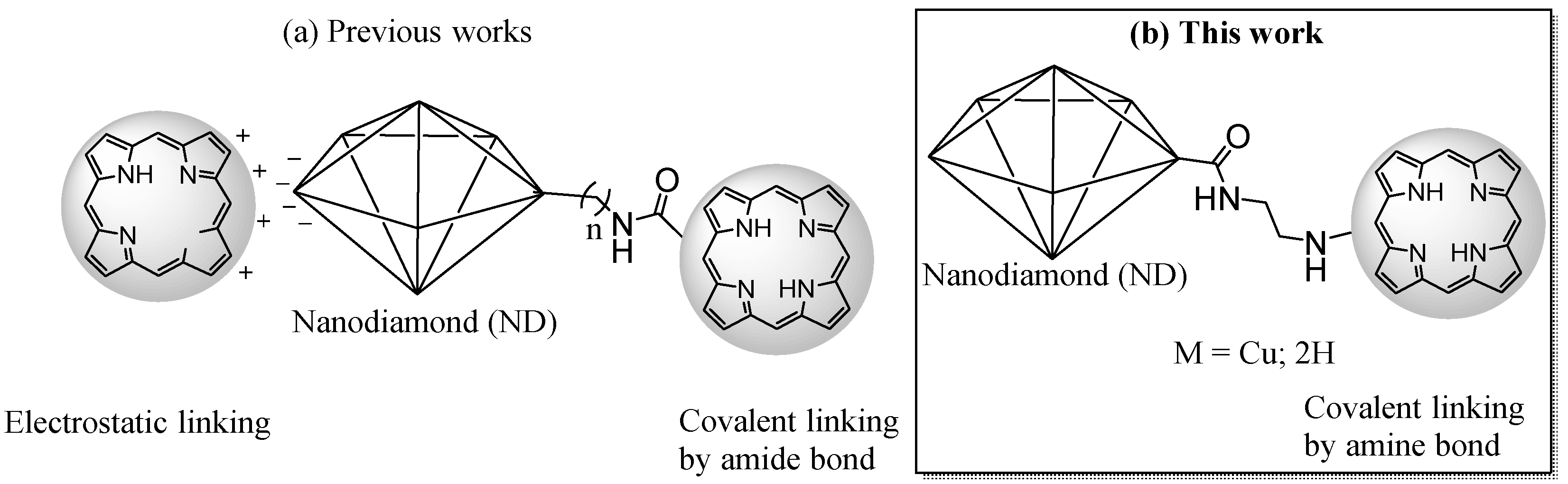
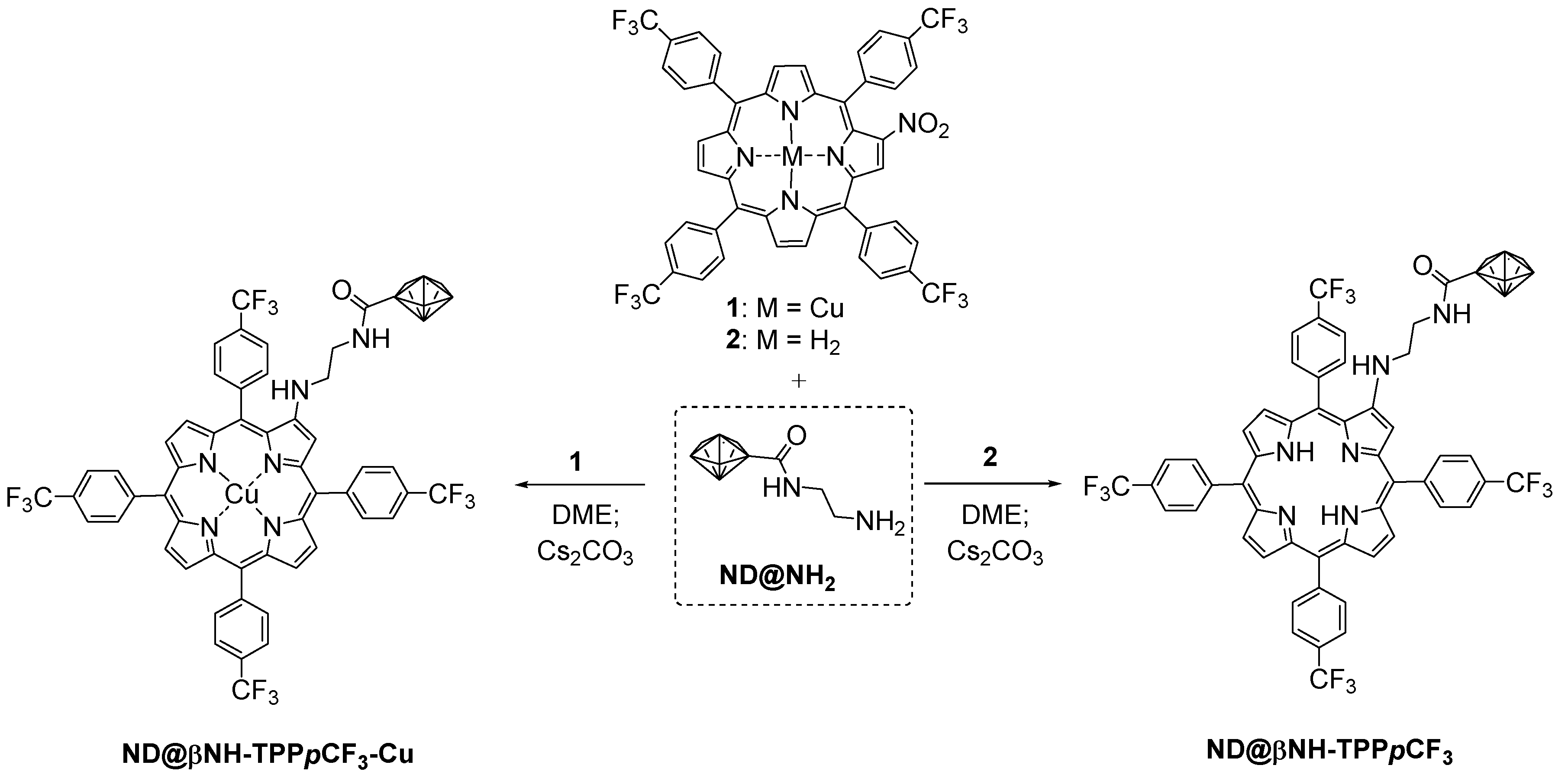
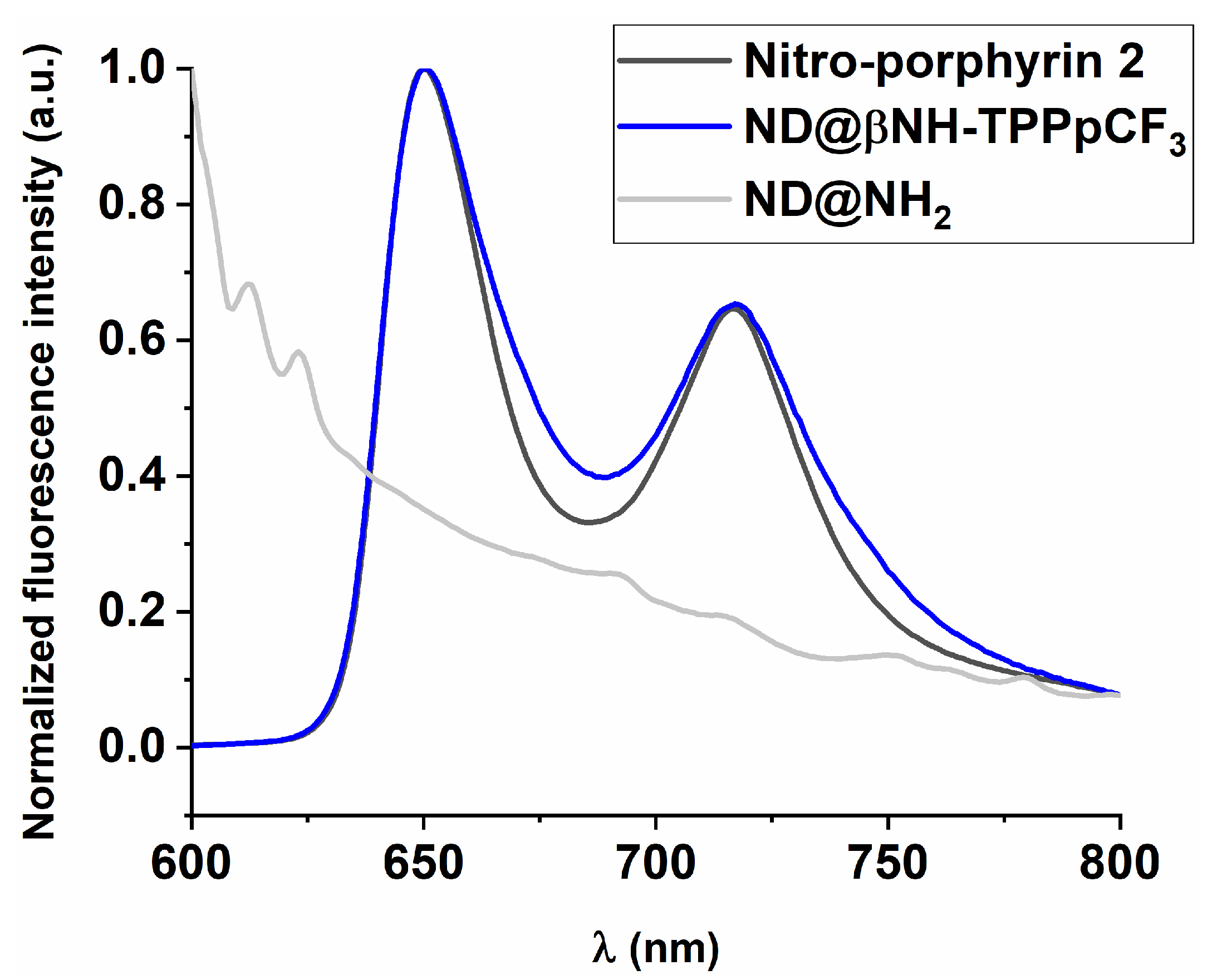
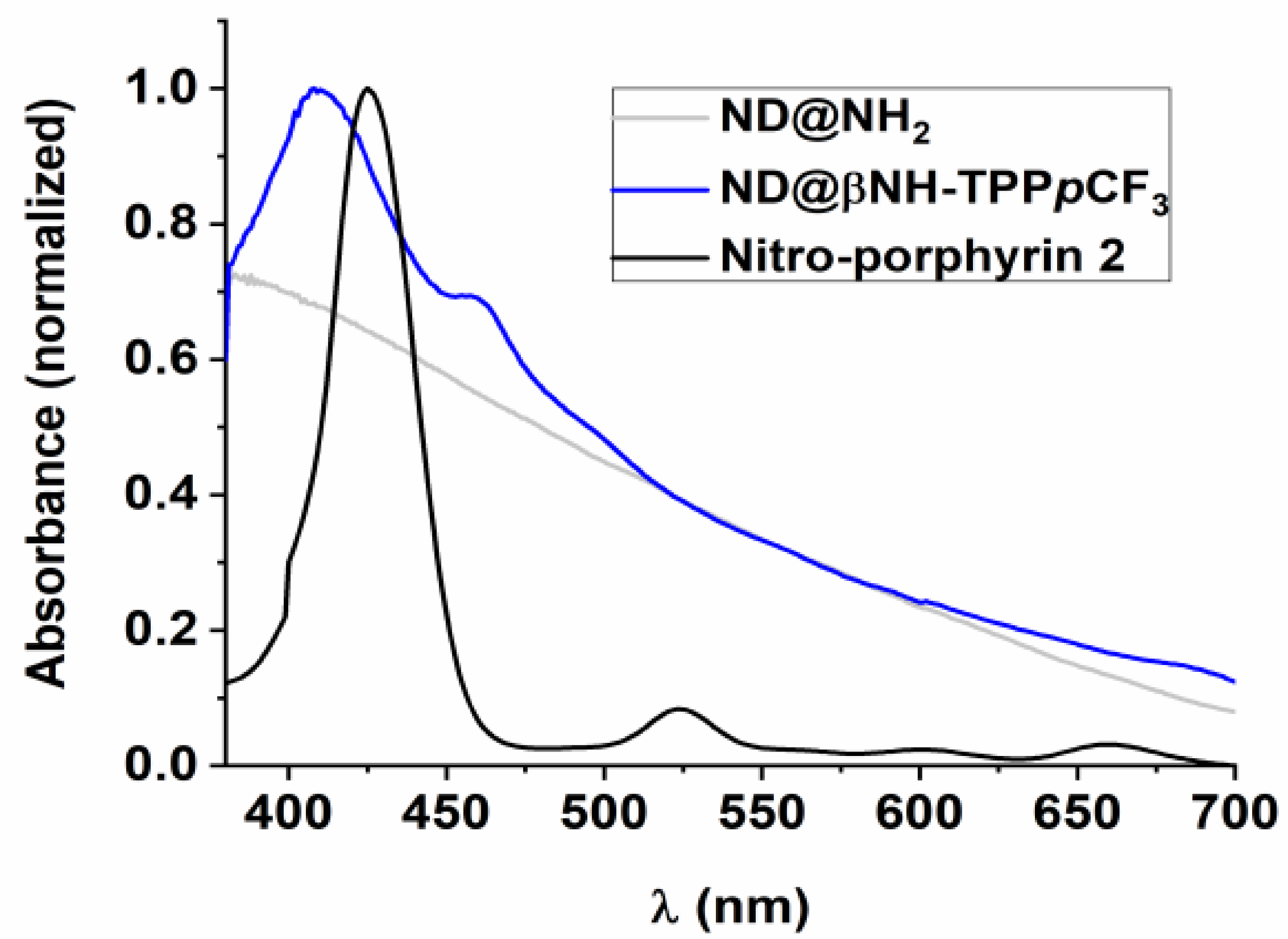
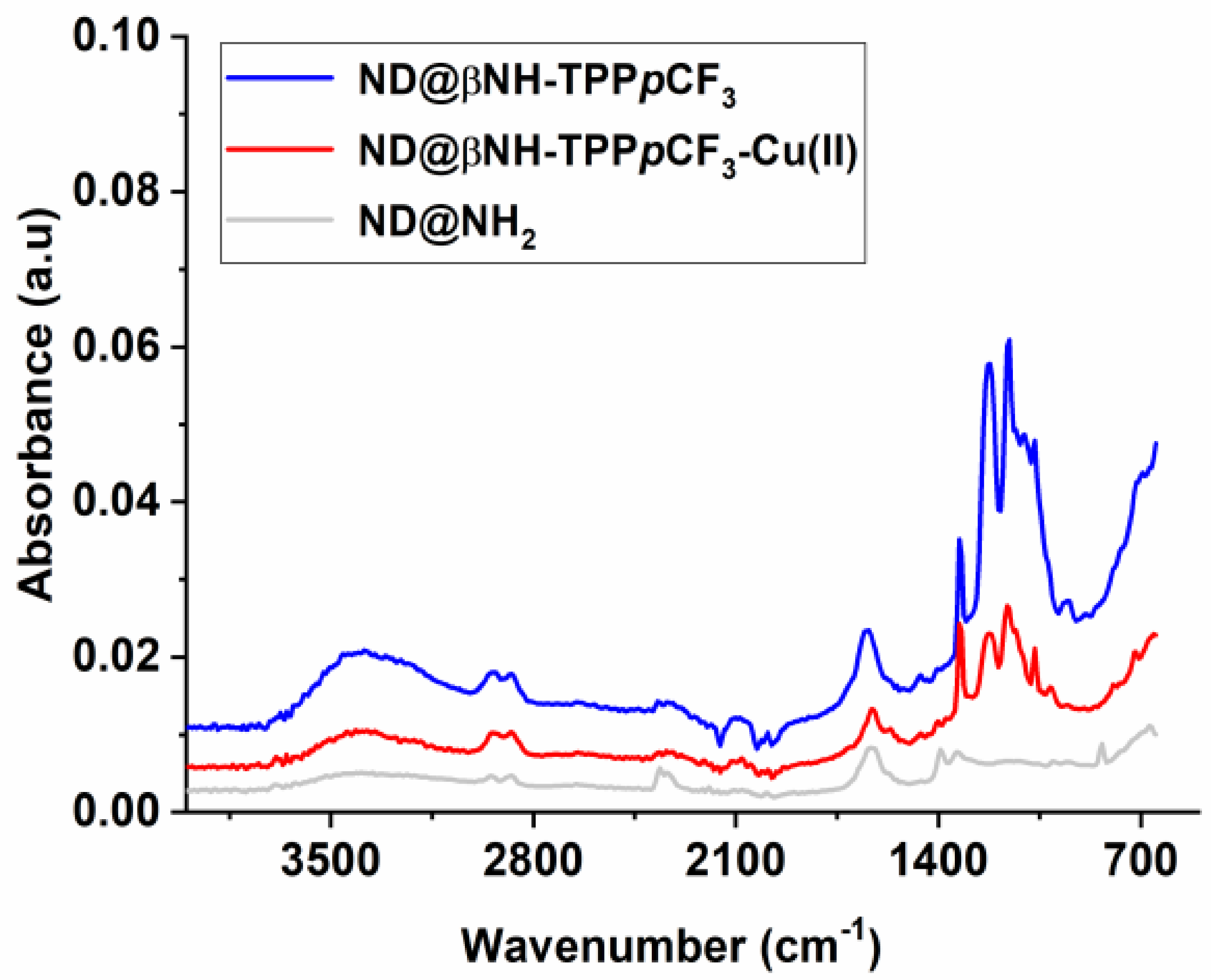
2.1. Synthesis and Characterization of Porphyrin-Functionalized Nanodiamonds (ND@βNH-TPPpCF3-Cu(II) and ND@βNH-TPPpCF3)
2.2. Cytotoxic and Genotoxic Evaluation of Porphyrin-Functionalized Nanodiamonds in Allium Cepa Assay
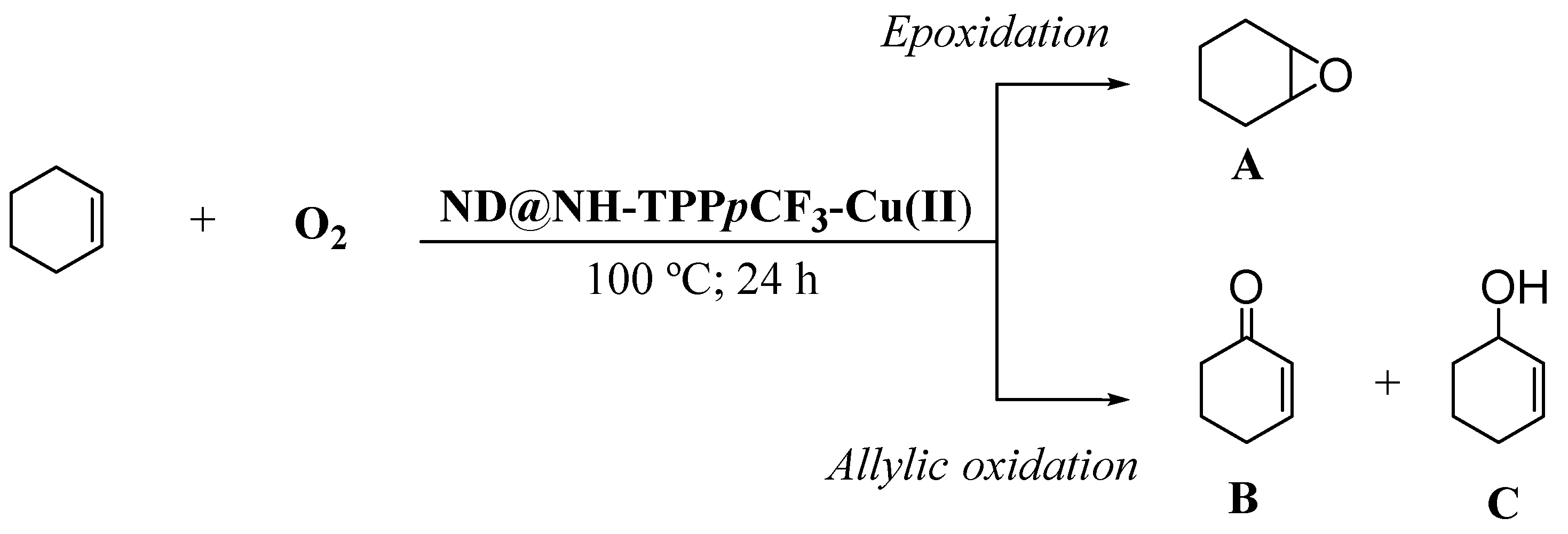
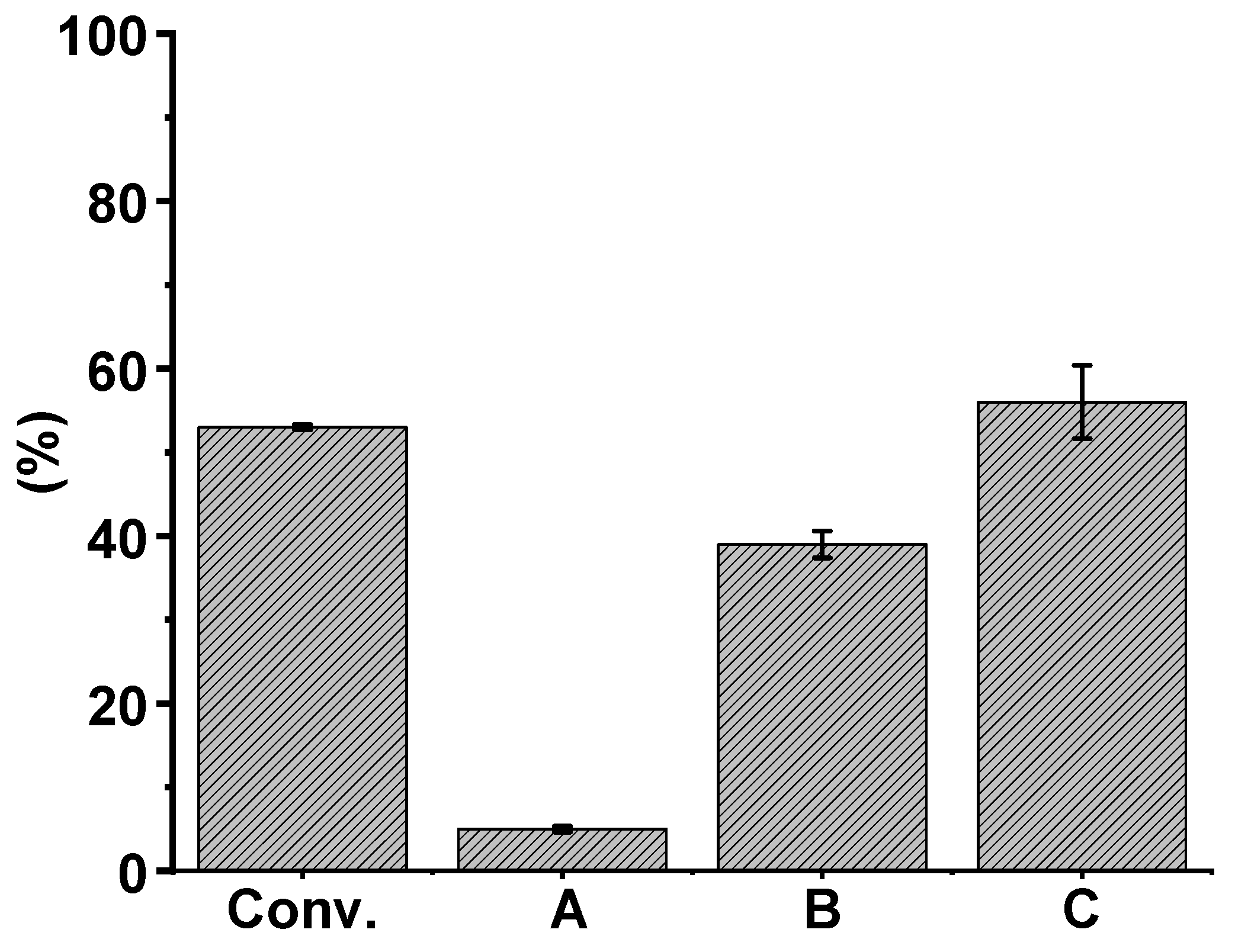
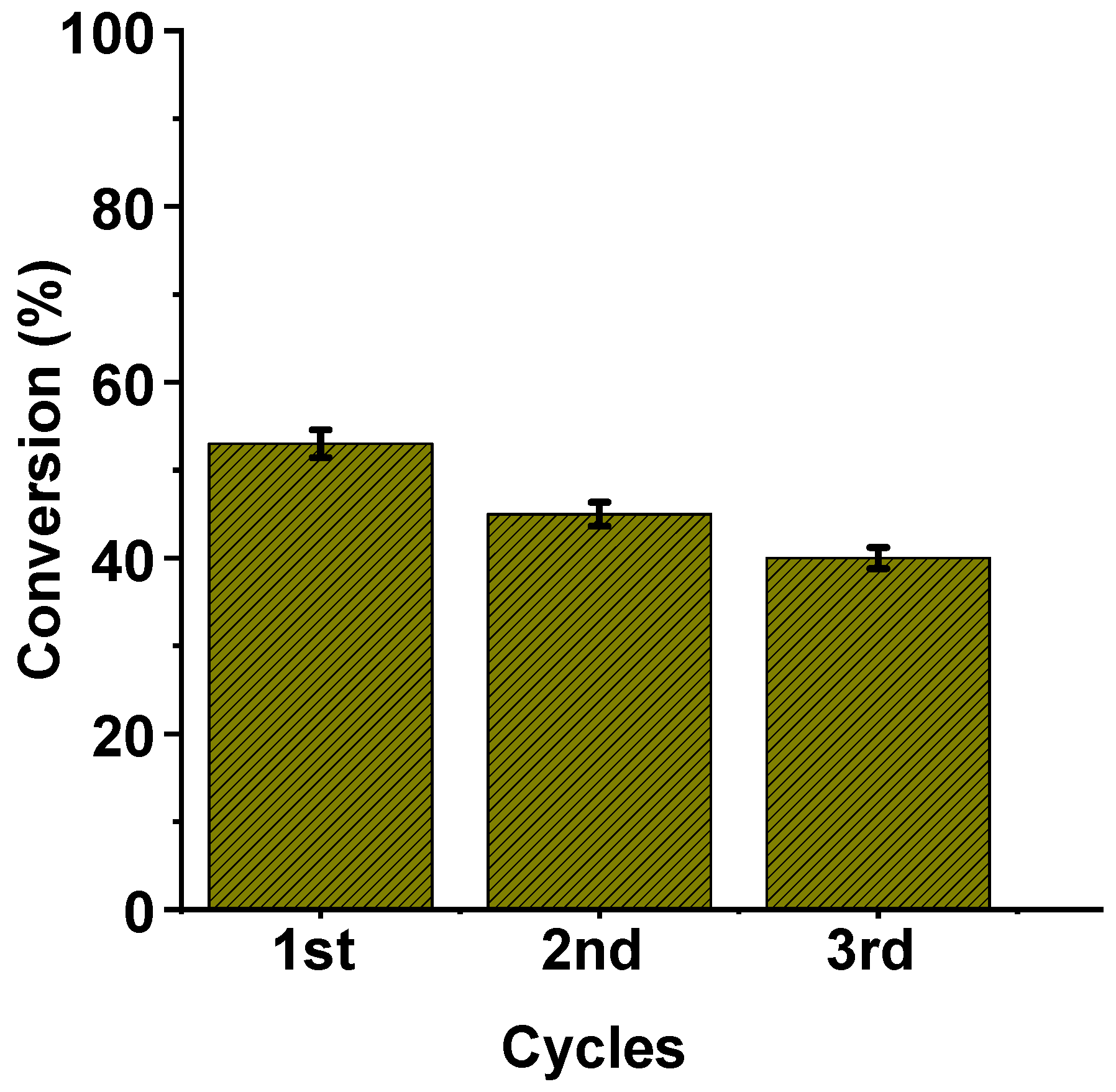
2.3. Catalytic Oxidation Experiments
3. Materials and Methods
3.1. Characterization of Chemicals and Materials
3.2. Synthesis of Porphyrin Precursors and Metalloporphyrins
3.3. Immobilization of Nitro-Functionalized Porphyrins 1 and 2 onto ND@NH2
3.4. Cytotoxic and Genotoxic Evaluation of Porphyrin-Functionalized Nanodiamonds in Allium Cepa Assays
3.5. Procedure for Oxidation of Cyclohexene Using ND@βNH-TPPpCF3-Cu(II) as Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Danilenko, V.V. On the history of the discovery of nanodiamond synthesis. Phys. Solid State 2004, 46, 595–599. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, X.; Su, D.S.; Centi, G.; Perathoner, S. Catalysis by hybrid sp2/sp3nanodiamonds and their role in the design of advanced nanocarbon materials. Chem. Soc. Rev. 2018, 47, 8438–8473. [Google Scholar] [CrossRef]
- Lai, H.; Chen, F.; Lu, M.; Stenzel, M.H.; Xiao, P. Polypeptide-Grafted Nanodiamonds for Controlled Release of Melittin to Treat Breast Cancer. ACS Macro Lett. 2017, 6, 796–801. [Google Scholar] [CrossRef]
- Ishchenko, A.A.; Mchedlov-Petrossyan, N.O.; Kriklya, N.N.; Kryshtal, A.P.; Ōsawa, E.; Kulinich, A.V. Interaction of Polymethine Dyes with Detonation Nanodiamonds. ChemPhysChem 2019, 20, 1028–1035. [Google Scholar] [CrossRef]
- Sinolits, A.V.; Chernysheva, M.G.; Matveeva, O.D.; Popov, A.G.; Badun, G.A. Chitosan adsorption on nanodiamonds: Stability and mechanism. Fuller Nanotub. Carbon Nanostruct. 2019, 28, 299–303. [Google Scholar] [CrossRef]
- Guo, H.; Hu, H.; Yu, X.; Naito, K.; Zhang, Q. Covalent Functionalization of Nanodiamonds with Natural Amino Acids and Ascorbic Acids. J. Nanosci. Nanotechnol. 2019, 19, 7574–7583. [Google Scholar] [CrossRef]
- Patoary, N.H.; Rai, A.; Patel, K.P.; Rebecca, A.; Zhang, W.; Ulrich, A.J.; Galib, M.; Desai, T.; Zivanovic, S.; Yousufuddin, M.; et al. Directed covalent assembly of nanodiamonds into thin films. Diam. Relat. Mater. 2020, 101, 9. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Carabineiro, S.A.C.; Buijnsters, J.G.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M. Nanodiamond-TiO2Composites for Heterogeneous Photocatalysis. ChemPlusChem 2013, 78, 750. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Silva, A.R.; Ribeiro, C.; Carabineiro, S.A.C.; Buijnsters, J.G.; Lanceros-Méndez, S. Nanodiamonds/poly(vinylidene fluoride) composites for tissue engineering applications. Compos. Part B Eng. 2017, 111, 37–44. [Google Scholar] [CrossRef]
- Whitlow, J.; Pacelli, S.; Paul, A. Multifunctional nanodiamonds in regenerative medicine: Recent advances and future directions. J. Control. Release 2017, 261, 62–86. [Google Scholar] [CrossRef]
- Day, A.H.; Adams, S.J.; Gines, L.; Williams, O.A.; Johnson, B.R.; Fallis, I.A.; Loveridge, E.J.; Bahra, G.S.; Oyston, P.C.; Herrera, J.M.; et al. Synthetic routes, characterization and photophysical properties of luminescent, surface functionalized nanodiamonds. Carbon 2019, 152, 335–343. [Google Scholar] [CrossRef]
- Solarska-Ściuk, K.; Kleszczyńska, H. Possibilities of nanodiamonds application—Biological and medical aspects. Acta Pol. Pharm. Drug Res. 2019, 76, 779–796. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Nanodiamonds: Emerging face of future nanotechnology. Carbon 2019, 143, 678–699. [Google Scholar] [CrossRef]
- Sangiao, E.T.; Holban, A.M.; Gestal, M.C. Applications of Nanodiamonds in the Detection and Therapy of Infectious Diseases. Materials 2019, 12, 1639. [Google Scholar] [CrossRef]
- Matshitse, R.; Ngoy, B.P.; Managa, M.; Mack, J.; Nyokong, T. Photophysical properties and photodynamic therapy activities of detonated nanodiamonds-BODIPY-phthalocyanines nanoassemblies. Photodiagnosis Photodyn. Ther. 2019, 26, 101–110. [Google Scholar] [CrossRef]
- Matshitse, R.; Managa, M.; Nyokong, T. The modulation of the photophysical and photodynamic therapy activities of a phthalocyanine by detonation nanodiamonds: Comparison with graphene quantum dots and carbon nanodots. Diam. Relat. Mater. 2020, 101, 107617. [Google Scholar] [CrossRef]
- Lai, H.; Stenzel, M.H.; Xiao, P. Surface engineering and applications of nanodiamonds in cancer treatment and imaging. Int. Mater. Rev. 2020, 65, 189–225. [Google Scholar] [CrossRef]
- Muller, O.; Pichot, V.; Merlat, L.; Schmidlin, L.; Spitzer, D. Nonlinear optical behavior of porphyrin functionalized nanodiamonds: An efficient material for optical power limiting. Appl. Opt. 2016, 55, 3801–3808. [Google Scholar] [CrossRef]
- Muller, O.; Pichot, V.; Merlat, L.; Spitzer, D. Optical limiting properties of surface functionalized nanodiamonds probed by the Z-scan method. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Afandi, A.; Howkins, A.; Boyd, I.W.; Jackman, R.B. Nanodiamonds for device applications: An investigation of the properties of boron-doped detonation nanodiamonds. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Radulaski, M.; Zhang, J.L.; Tzeng, Y.; Lagoudakis, K.G.; Ishiwata, H.; Dory, C.; Fischer, K.A.; Kelaita, Y.A.; Sun, S.; Maurer, P.C.; et al. Nanodiamond Integration with Photonic Devices. Laser Photon Rev. 2019, 13, 14. [Google Scholar] [CrossRef]
- Shenderova, O.; Vargas, A.; Turner, S.; Ivanov, D.M.; Ivanov, M.G. Nanodiamond-Based Nanolubricants: Investigation of Friction Surfaces. Tribol. Trans. 2014, 57, 1051–1057. [Google Scholar] [CrossRef]
- Marko, M.; Kyle, J.P.; Branson, B.; Terrell, E.J. Tribological Improvements of Dispersed Nanodiamond Additives in Lubricating Mineral Oil. J. Tribol. Trans. ASME 2015, 137, 7. [Google Scholar] [CrossRef]
- Picollo, F.; Mino, L.; Battiato, A.; Tchernij, S.D.; Forneris, J.; Martina, K.; Sacco, M.; Tagliapietra, S.; Vittone, E.; Olivero, P.; et al. Synthesis and characterization of porphyrin functionalized nanodiamonds. Diam. Relat. Mater. 2019, 91, 22–28. [Google Scholar] [CrossRef]
- Arnaut, L.G.; Pereira, M.M.; Dąbrowski, J.M.; Silva, E.F.F.; Schaberle, F.A.; Abreu, A.R.; Rocha, L.B.; Barsan, M.M.; Urbańska, K.; Stochel, G.; et al. Photodynamic Therapy Efficacy Enhanced by Dynamics: The Role of Charge Transfer and Photostability in the Selection of Photosensitizers. Chem. A Eur. J. 2014, 20, 5346–5357. [Google Scholar] [CrossRef]
- Gonçalves, N.P.F.; Simões, A.V.C.; Abreu, A.R.; Abrunhosa, A.J.; Dąbrowski, J.M.; Pereira, M.M. Synthesis and biological distribution study of a new carbon-11 labeled porphyrin for PET imaging. Photochemical and biological characterization of the non-labeled porphyrin. J. Porphyr. Phthalocyanines 2015, 19, 946–955. [Google Scholar] [CrossRef]
- Simões, A.V.C.; Pinto, S.M.A.; Calvete, M.J.F.; Gomes, C.M.F.; Ferreira, N.C.; Castelo-Branco, M.; Llop, J.; Pereira, M.M.; Abrunhosa, A.J. Synthesis of a new 18F labeled porphyrin for potential application in positron emission tomography. In vivo imaging and cellular uptake. RSC Adv. 2015, 5, 99540–99546. [Google Scholar] [CrossRef]
- Pucelik, B.; Paczyński, R.; Dubin, G.; Pereira, M.M.; Arnaut, L.G.; Dąbrowski, J.M. Properties of halogenated and sulfonated porphyrins relevant for the selection of photosensitizers in anticancer and antimicrobial therapies. PLoS ONE 2017, 12, e0185984. [Google Scholar] [CrossRef]
- Dias, L.D.; Carrilho, R.M.B.; Henriques, C.A.; Calvete, M.J.F.; Masdeu-Bultó, A.M.; Claver, C.; Rossi, L.M.; Pereira, M.M. Hybrid Metalloporphyrin Magnetic Nanoparticles as Catalysts for Sequential Transformation of Alkenes and CO2 into Cyclic Carbonates. ChemCatChem 2018, 10, 2792–2803. [Google Scholar] [CrossRef]
- Dias, L.D.; De Carvalho, A.L.M.B.; Pinto, S.M.A.; Aquino, G.L.B.; Calvete, M.J.F.; Rossi, L.M.; Marques, M.P.M.; Pereira, M.M. Bioinspired-Metalloporphyrin Magnetic Nanocomposite as a Reusable Catalyst for Synthesis of Diastereomeric (−)-Isopulegol Epoxide: Anticancer Activity Against Human Osteosarcoma Cells (MG-63). Molecules 2019, 24, 52. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, E.; Scorsone, E.; Girard, H.; Pichot, V.; Spitzer, D.; Bergonzo, P. Metalloporphyrin-functionalised diamond nano-particles as sensitive layer for nitroaromatic vapours detection at room-temperature. Sens. Actuators B Chem. 2010, 151, 191–197. [Google Scholar] [CrossRef]
- Sato, K.; Aoki, M.; Noyori, R. A “Green” Route to Adipic Acid: Direct Oxidation of Cyclohexenes with 30 Percent Hydrogen Peroxide. Science 1998, 281, 1646–1647. [Google Scholar] [CrossRef] [PubMed]
- Nowak, I.; Ziolek, M. Effect of texture and structure on the catalytic activity of mesoporous niobosilicates for the oxidation of cyclohexene. Microporous Mesoporous Mater. 2005, 78, 281–288. [Google Scholar] [CrossRef]
- Cao, H.; Zhu, B.; Yang, Y.; Xu, L.; Yu, L.; Xu, Q. Recent advances on controllable and selective catalytic oxidation of cyclohexene. Chin. J. Catal. 2018, 39, 899–907. [Google Scholar] [CrossRef]
- Xing, Y.; Dai, L. Nanodiamonds for nanomedicine. Nanomedicine 2009, 4, 207–218. [Google Scholar] [CrossRef]
- Rocha, R.P.; Sousa, J.P.; Silva, A.M.; Pereira, M.F.; Figueiredo, J. Catalytic activity and stability of multiwalled carbon nanotubes in catalytic wet air oxidation of oxalic acid: The role of the basic nature induced by the surface chemistry. Appl. Catal. B Environ. 2011, 104, 330–336. [Google Scholar] [CrossRef]
- Sundar, L.S.; Anjum, N.A.; Ferro, M.; Pereira, E.; Singh, M.K.; Sousa, A.C.M. Biocompatibility and biotoxicity of in-situ synthesized carboxylated nanodiamond-cobalt oxide nanocomposite. J. Mater. Sci. Technol. 2017, 33, 879–888. [Google Scholar] [CrossRef]
- Chernysheva, M.G.; Myasnikov, I.Y.; Badun, G.A.; Matorin, D.N.; Gabbasova, D.T.; Konstantinov, A.I.; Korobkov, V.I.; Kulikova, N.A. Humic substances alter the uptake and toxicity of nanodiamonds in wheat seedlings. J. Soils Sediments 2016, 18, 1335–1346. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Figueiredo, J.L.; Carabineiro, S.A.; Buijnsters, J.G.; Figueiredo, J.L.; Silva, A.M.; Faria, J.L. Photocatalytic activity of functionalized nanodiamond-TiO2 composites towards water pollutants degradation under UV/Vis irradiation. Appl. Surf. Sci. 2018, 458, 839–848. [Google Scholar] [CrossRef]
- Basiuk, V.A.; Terrazas, T.; Luna-Martínez, N.; Basiuk, E.V. Phytotoxicity of carbon nanotubes and nanodiamond in long-term assays with Cactaceae plant seedlings. Fuller. Nanotub. Carbon Nanostruct. 2018, 27, 141–149. [Google Scholar] [CrossRef]
- Leme, D.M.; Marin-Morales, M.A. Allium cepa test in environmental monitoring: A review on its application. Mutat. Res. Rev. Mutat. Res. 2009, 682, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, A.M.D.R.; Varejão, J.M.T.B.; Pereira, M.M. Some new aspects related to the synthesis ofmeso-substituted porphyrins. J. Heterocycl. Chem. 1991, 28, 635–640. [Google Scholar] [CrossRef]
- Crossley, M.J.; King, L.G.; Pyke, S.M. A new and highly efficient synthesis of hydroxyporphyrins. Tetrahedron 1987, 43, 4569–4577. [Google Scholar] [CrossRef]
- Crossley, M.J.; King, L.G.; Pyke, S.M.; Tansey, C.W. Reaction of 5-nitro-octaethylporphyrins with nucleophiles. J. Porphyr. Phthalocyanines 2002, 6, 685–694. [Google Scholar] [CrossRef]
- Rebelo, S.L.H.; Gonçalves, A.R.; Pereira, M.M.; Simões, M.M.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Epoxidation reactions with hydrogen peroxide activated by a novel heterogeneous metalloporphyrin catalyst. J. Mol. Catal. A Chem. 2006, 256, 321–323. [Google Scholar] [CrossRef]
- Vicente, M.G.H.; Smith, K.M. Syntheses and Functionalizations of Porphyrin Macrocycles. Curr. Org. Synth. 2014, 11, 3–28. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Li, J.; Tao, L.; Wei, Y. A comparative study of cellular uptake and cytotoxicity of multi-walled carbon nanotubes, graphene oxide, and nanodiamond. Toxicol. Res. 2012, 1, 62–68. [Google Scholar] [CrossRef]
- Maas, M. Carbon Nanomaterials as Antibacterial Colloids. Materials 2016, 9, 617. [Google Scholar] [CrossRef]
- Marcon, L.; Riquet, F.; Vicogne, D.; Szunerits, S.; Bodart, J.-F.; Boukherroub, R. Cellular and in vivo toxicity of functionalized nanodiamond in Xenopus embryos. J. Mater. Chem. 2010, 20, 8064–8069. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Fedutik, Y.; Mao, Z.; Gao, C. Nanodiamonds of Different Surface Chemistry Influence the Toxicity and Differentiation of Rat Bone Mesenchymal Stem Cells In Vitro. J. Nanosci. Nanotechnol. 2019, 19, 5426–5434. [Google Scholar] [CrossRef]
- Fusco, L.; Avitabile, E.; Armuzza, V.; Orecchioni, M.; Istif, A.; Bedognetti, D.; Da Ros, T.; Delogu, L.G. Impact of the surface functionalization on nanodiamond biocompatibility: A comprehensive view on human blood immune cells. Carbon 2020, 160, 390–404. [Google Scholar] [CrossRef]
- Cohen, S.; Kozuch, S.; Hazan, C.; Shaik, S. Does Substrate Oxidation Determine the Regioselectivity of Cyclohexene and Propene Oxidation by Cytochrome P450? J. Am. Chem. Soc. 2006, 128, 11028–11029. [Google Scholar] [CrossRef]
- Bohström, Z.; Rico-Lattes, I.; Holmberg, K. Oxidation of cyclohexene into adipic acid in aqueous dispersions of mesoporous oxides with built-in catalytical sites. Green Chem. 2010, 12, 1861–1869. [Google Scholar] [CrossRef]
- Henriques, C.A.; Fernandes, A.; Rossi, L.M.; Ribeiro, M.F.; Calvete, M.J.F.; Pereira, M.M. Biologically Inspired and Magnetically Recoverable Copper Porphyrinic Catalysts: A Greener Approach for Oxidation of Hydrocarbons with Molecular Oxygen. Adv. Funct. Mater. 2016, 26, 3359–3368. [Google Scholar] [CrossRef]
- Denekamp, I.M.; Antens, M.; Slot, T.K.; Rothenberg, G. Selective Catalytic Oxidation of Cyclohexene with Molecular Oxygen: Radical Versus Nonradical Pathways. ChemCatChem 2018, 10, 1035–1041. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Zhu, T.; Li, Y.; Ruan, J.; Li, G. Effective solvent-free oxidation of cyclohexene to allylic products with oxygen by mesoporous etched halloysite nanotube supported Co2+. RSC Adv. 2018, 8, 14870–14878. [Google Scholar] [CrossRef]
- Yang, G.; Du, H.; Liu, J.; Zhou, Z.; Hu, X.; Zhang, Z. Oxidation of olefins using molecular oxygen catalyzed by a part per million level of recyclable copper catalyst under mild conditions. Green Chem. 2017, 19, 675–681. [Google Scholar] [CrossRef]
- Hill, A.G.; Angell, F.G.; Macdonald, A.M.G. Book reviews. Analyst 1967, 92, 537–538. [Google Scholar] [CrossRef]
- Scherer, M.D.; Sposito, J.C.; Falco, W.F.; Grisolia, A.B.; Andrade, L.H.; Lima, S.; Machado, G.; Nascimento, V.A.; Gonçalves, D.A.; Wender, H.; et al. Cytotoxic and genotoxic effects of silver nanoparticles on meristematic cells of Allium cepa roots: A close analysis of particle size dependence. Sci. Total. Environ. 2019, 660, 459–467. [Google Scholar] [CrossRef]
| Sample | C 1s | N 1s | O 1s | F 1s | Cu 2p |
|---|---|---|---|---|---|
| Pristine NDs 1 | 91.6 | 2.1 | 6.3 | - | - |
| ND@NH2 1 | 96.0 | 2.1 | 1.9 | - | - |
| ND@βNH-TPPpCF3-Cu(II) | 94.7 | 2.0 | 1.4 | 1.8 | 0.06 |
| ND@βNH-TPPpCF3 | 91.2 | 2.2 | 3.4 | 3.2 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, L.D.; Rodrigues, F.M.S.; Calvete, M.J.F.; Carabineiro, S.A.C.; Scherer, M.D.; Caires, A.R.L.; Buijnsters, J.G.; Figueiredo, J.L.; Bagnato, V.S.; Pereira, M.M. Porphyrin–Nanodiamond Hybrid Materials—Active, Stable and Reusable Cyclohexene Oxidation Catalysts. Catalysts 2020, 10, 1402. https://doi.org/10.3390/catal10121402
Dias LD, Rodrigues FMS, Calvete MJF, Carabineiro SAC, Scherer MD, Caires ARL, Buijnsters JG, Figueiredo JL, Bagnato VS, Pereira MM. Porphyrin–Nanodiamond Hybrid Materials—Active, Stable and Reusable Cyclohexene Oxidation Catalysts. Catalysts. 2020; 10(12):1402. https://doi.org/10.3390/catal10121402
Chicago/Turabian StyleDias, Lucas D., Fábio M. S. Rodrigues, Mário J. F. Calvete, Sónia A. C. Carabineiro, Marisa D. Scherer, Anderson R. L. Caires, Josephus G. Buijnsters, José L. Figueiredo, Vanderlei S. Bagnato, and Mariette M. Pereira. 2020. "Porphyrin–Nanodiamond Hybrid Materials—Active, Stable and Reusable Cyclohexene Oxidation Catalysts" Catalysts 10, no. 12: 1402. https://doi.org/10.3390/catal10121402
APA StyleDias, L. D., Rodrigues, F. M. S., Calvete, M. J. F., Carabineiro, S. A. C., Scherer, M. D., Caires, A. R. L., Buijnsters, J. G., Figueiredo, J. L., Bagnato, V. S., & Pereira, M. M. (2020). Porphyrin–Nanodiamond Hybrid Materials—Active, Stable and Reusable Cyclohexene Oxidation Catalysts. Catalysts, 10(12), 1402. https://doi.org/10.3390/catal10121402