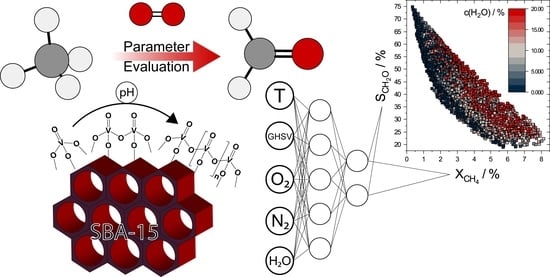
Increasing the Efficiency of Optimized V-SBA-15 Catalysts in the Selective Oxidation of Methane to Formaldehyde by Artificial Neural Network Modelling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
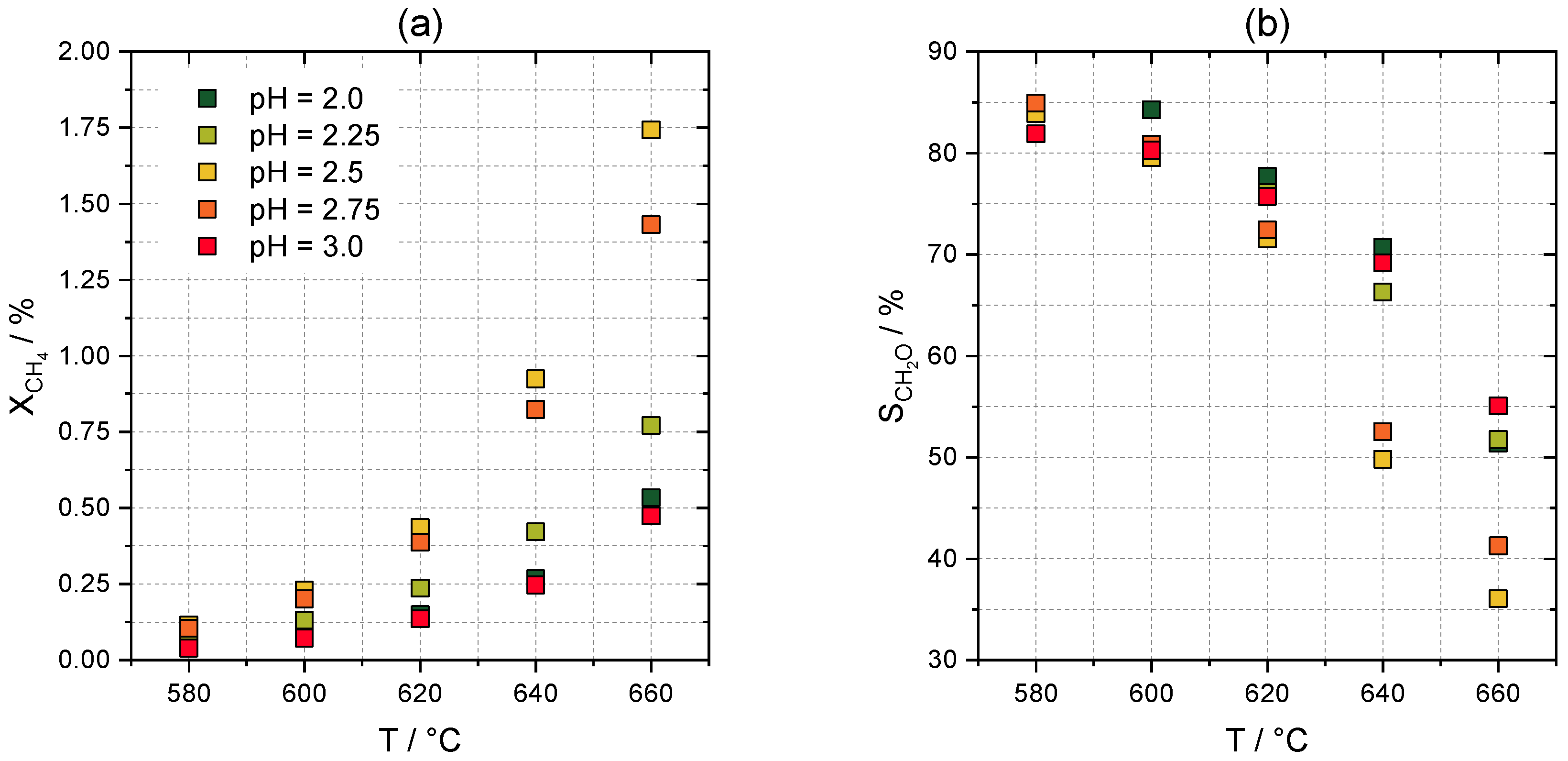
2.2. Catalytic Tests
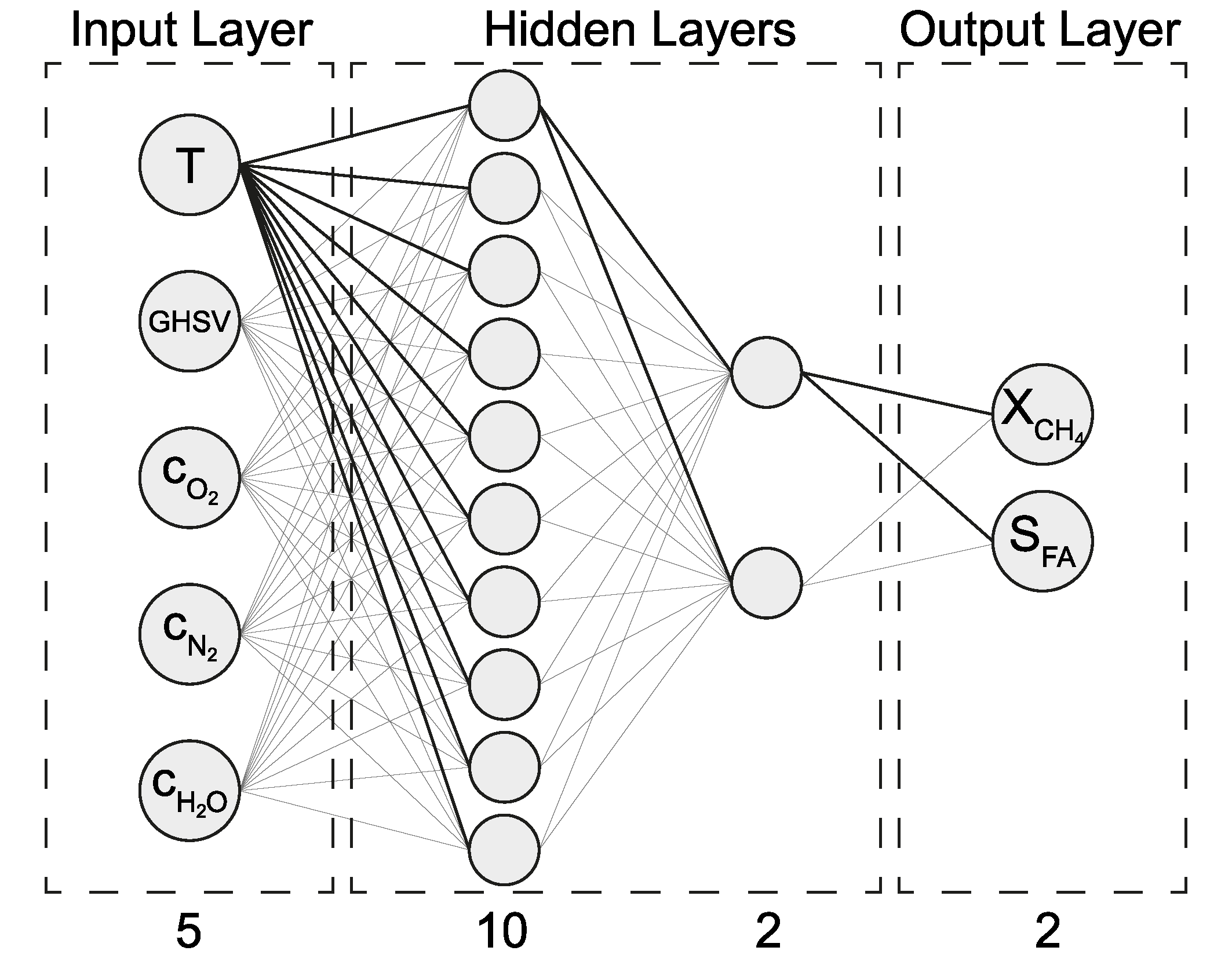
2.3. ANN Modelling
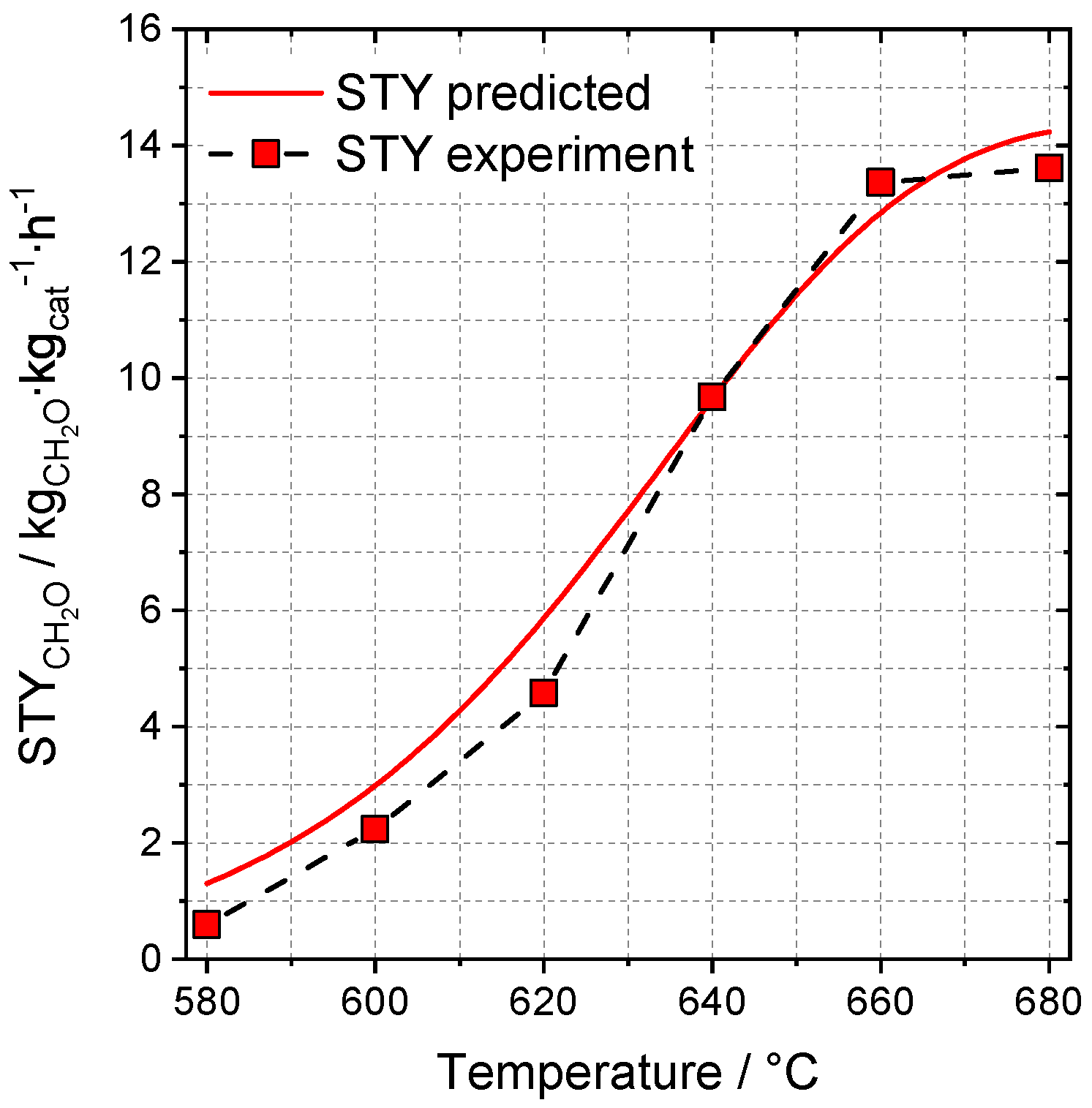
2.3.1. Model Selection and Training Process
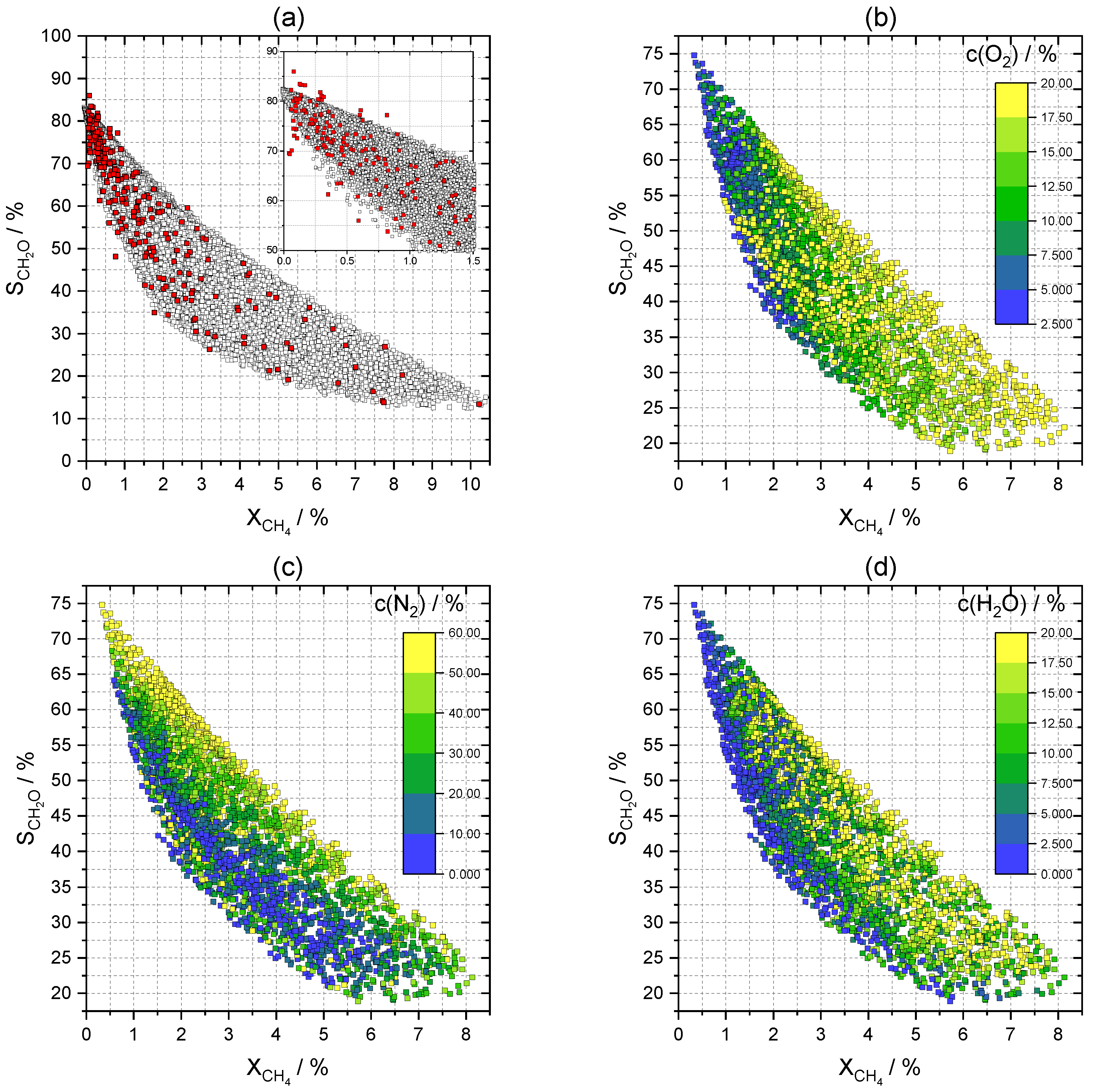
2.3.2. Effects on Selectivity and Conversion
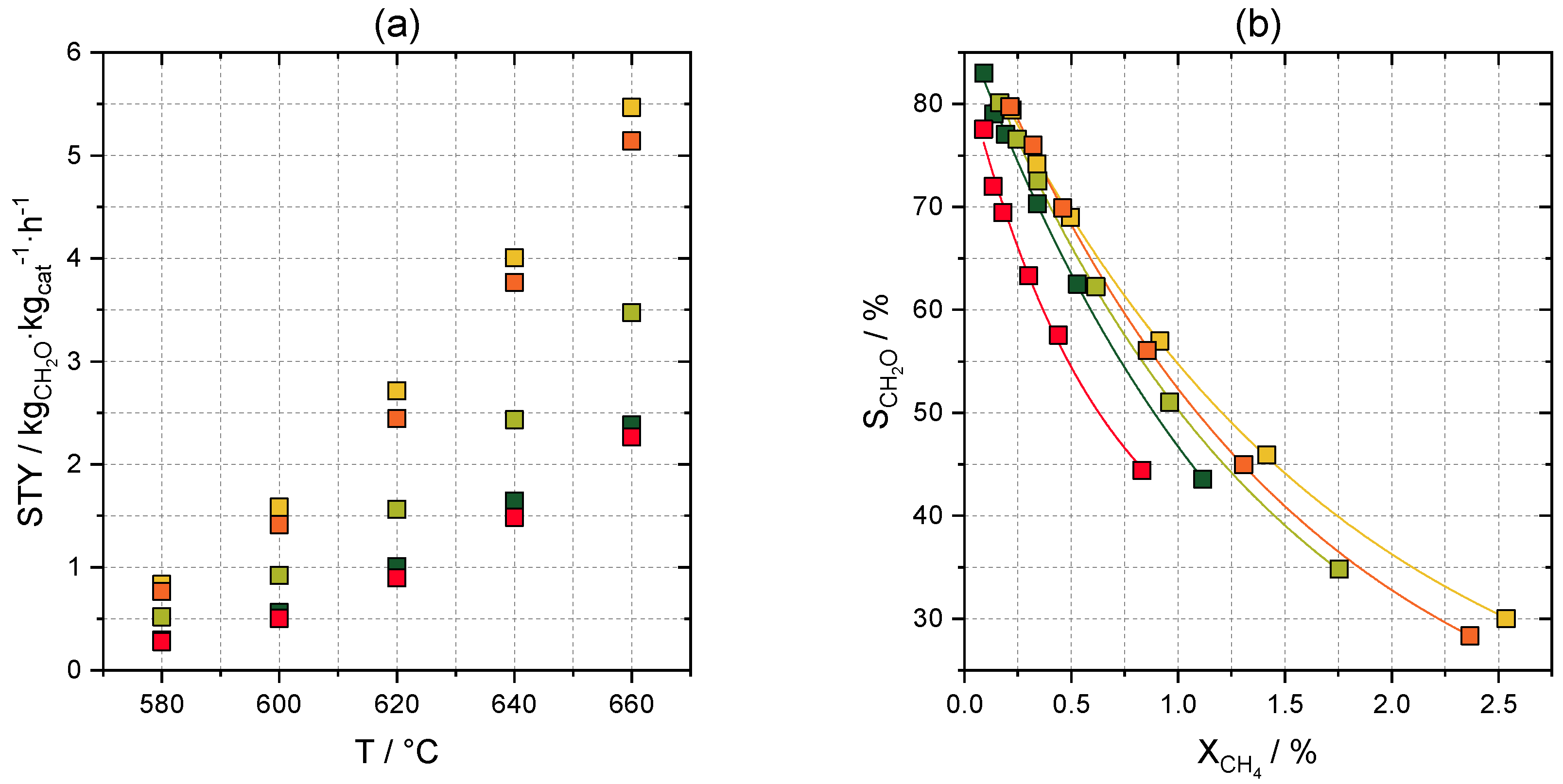
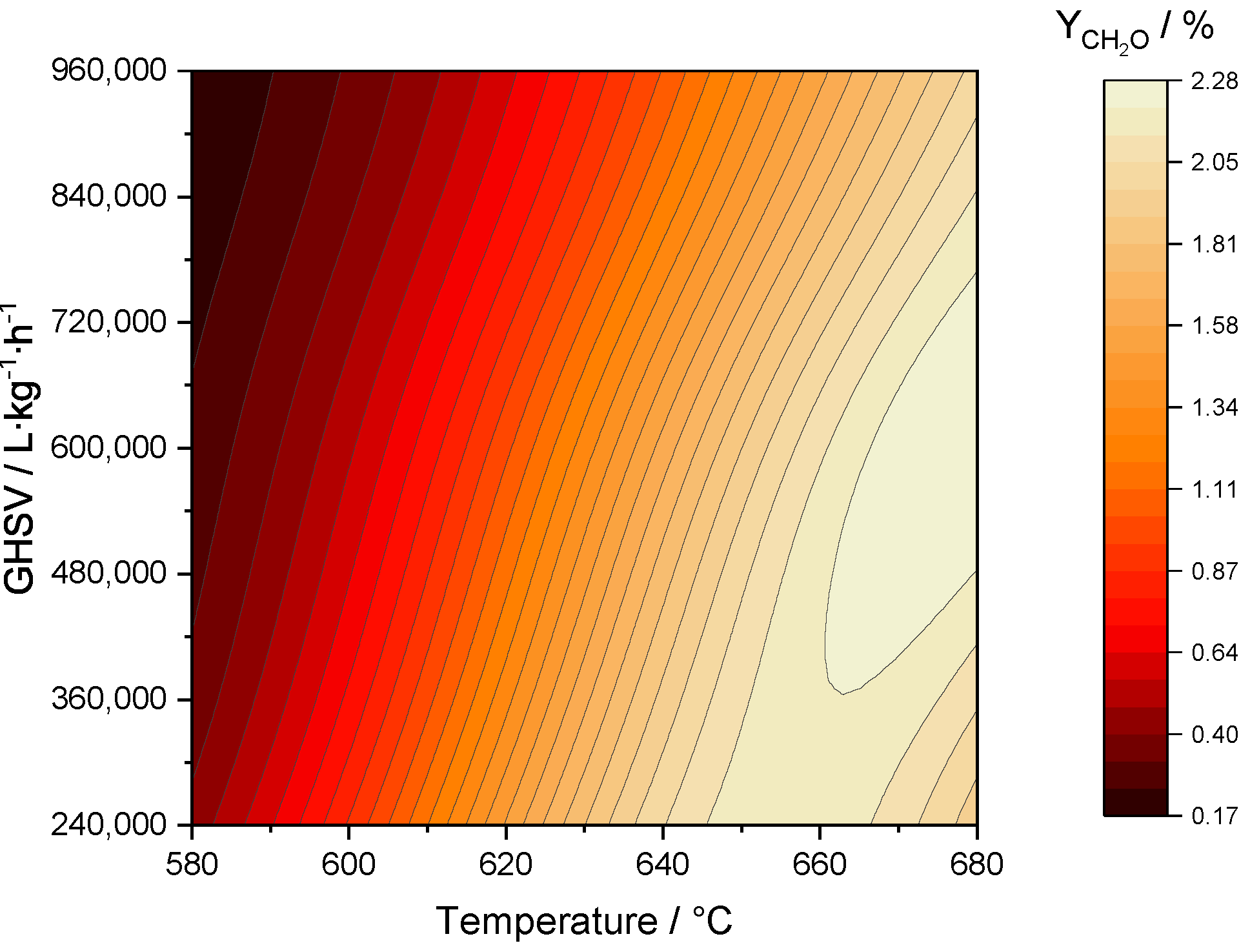
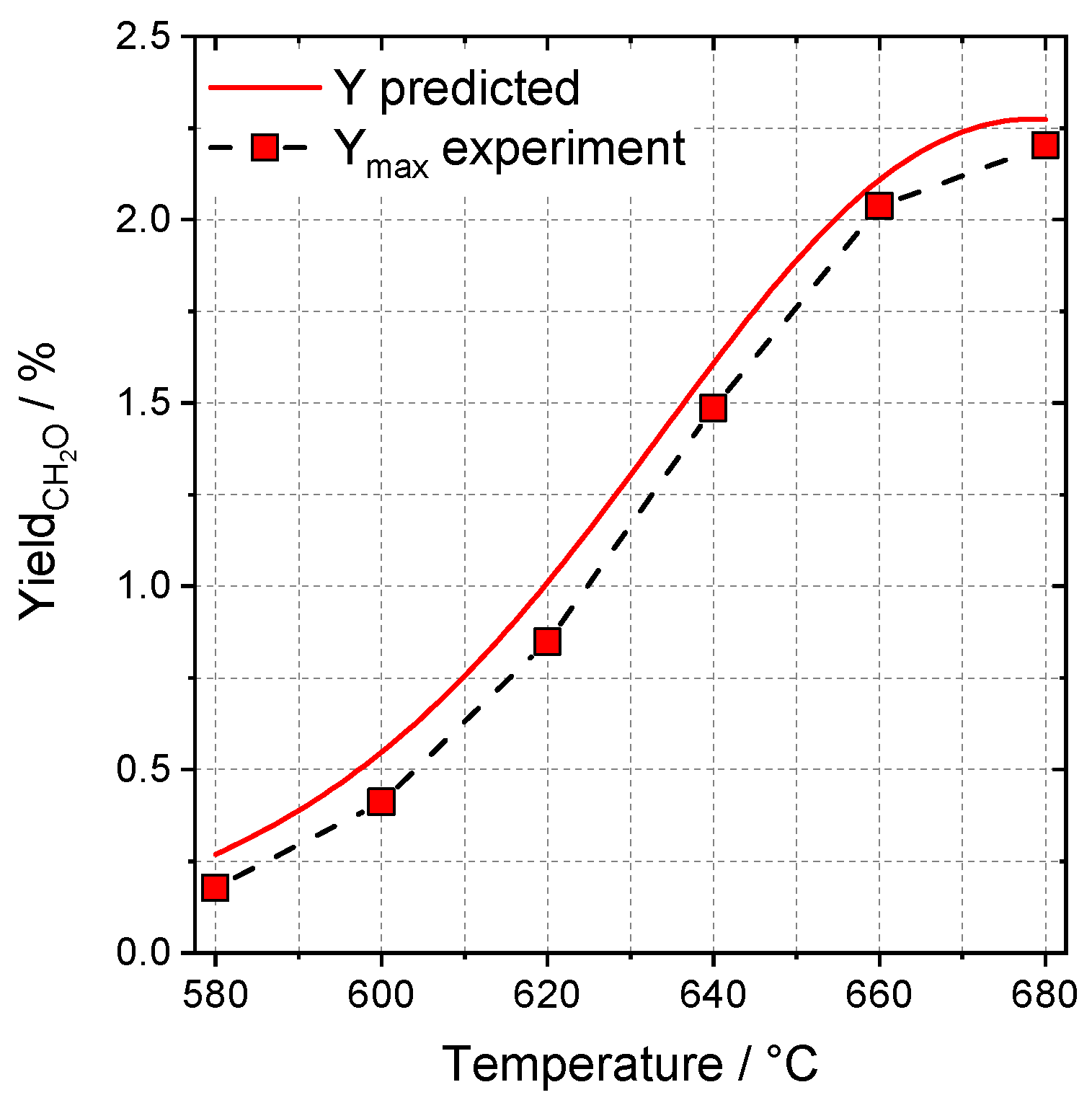
2.3.3. Optimization of Formaldehyde Yield
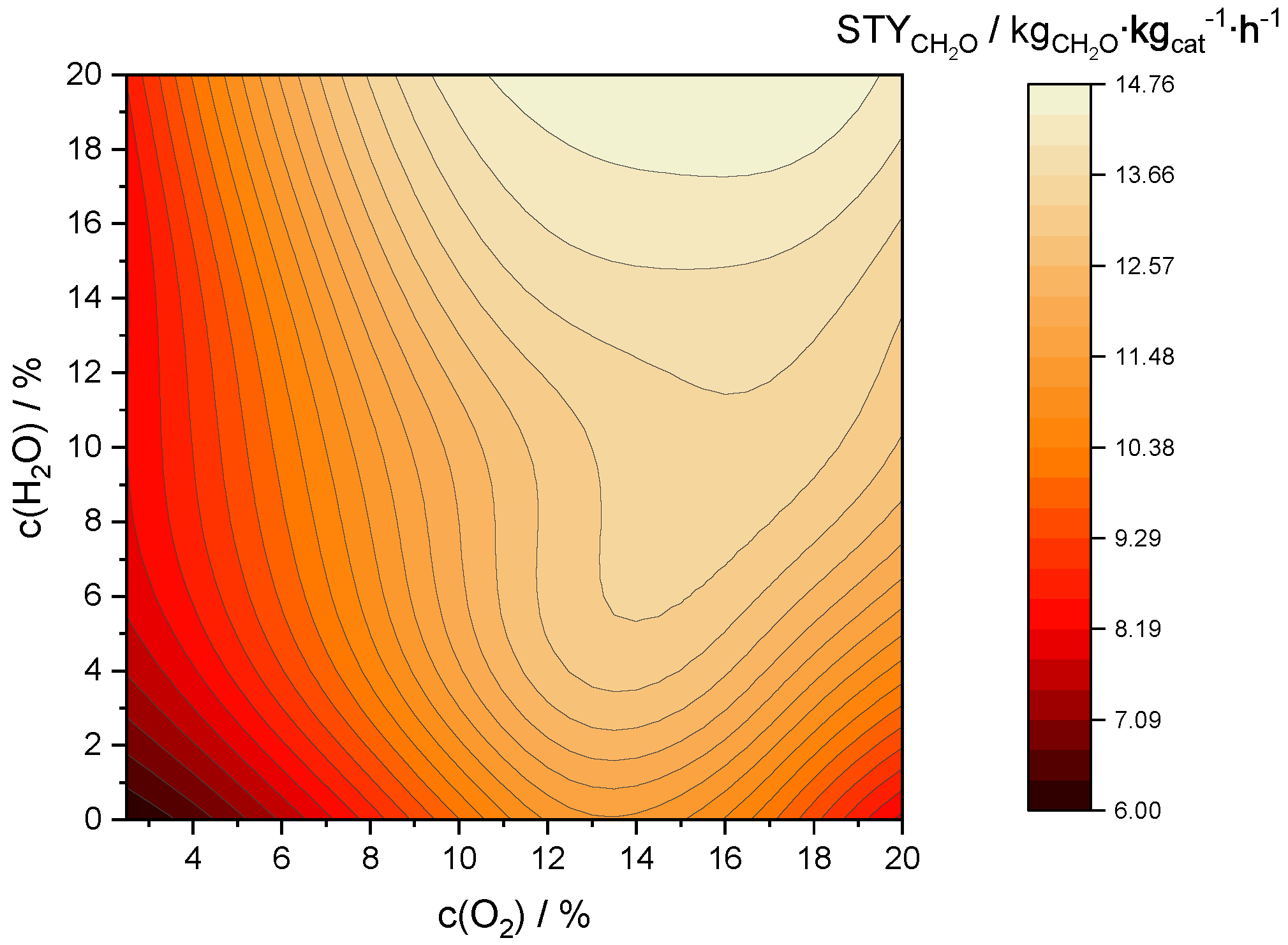
2.3.4. Optimization of Space-Time Yield
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
3.4. ANN Modelling
3.5. Collection of Training Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Company BP. BP Statistical Review of World Energy; British Petroleum Co.: London, UK, 2020. [Google Scholar]
- Horn, R.; Schlogl, R. Methane Activation by Heterogeneous Catalysis. Catal. Lett. 2015, 145, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Baba, T.; Miyaji, A. Catalysis and the Mechanism of Methane Conversion to Chemicals; Springer Nature Singapore Pte Ltd.: Singapore, 2020. [Google Scholar]
- Kondratenko, E.V.; Peppel, T.; Seeburg, D.; Kondratenko, V.A.; Kalevaru, N.; Martin, A.; Wohlrab, S. Methane conversion into different hydrocarbons or oxygenates: Current status and future perspectives in catalyst development and reactor operation. Catal. Sci. Technol. 2017, 7, 366–381. [Google Scholar] [CrossRef]
- Seeburg, D.; Bentrup, U.; Kunkel, B.; Vu, T.T.H.; Dang, T.T.H.; Wohlrab, S. Influence of hydrothermal ageing time on the performance of in situ prepared VMCM-41 catalysts in the selective oxidation of methane to formaldehyde. Microporous Mesoporous Mater. 2019, 288, 109581. [Google Scholar] [CrossRef]
- Nguyen, L.D.; Loridant, S.; Launay, H.; Pigamo, A.; Dubois, J.L.; Millet, J.M.M. Study of new catalysts based on vanadium oxide supported on mesoporous silica for the partial oxidation of methane to formaldehyde: Catalytic properties and reaction mechanism. J. Catal. 2006, 237, 38–48. [Google Scholar] [CrossRef]
- Ruddy, D.A.; Ohler, N.L.; Bell, A.T.; Tilley, T.D. Thermolytic molecular precursor route to site-isolated vanadia-silica materials and their catalytic performance in methane selective oxidation. J. Catal. 2006, 238, 277–285. [Google Scholar] [CrossRef]
- Pirovano, C.; Schonborn, E.; Wohlrab, S.; Kalevaru, V.N.; Martin, A. On the performance of porous silica supported VOx catalysts in the partial oxidation of methane. Catal. Today 2012, 192, 20–27. [Google Scholar] [CrossRef]
- Liu, Y.M.; Feng, W.L.; Li, T.C.; He, H.Y.; Dai, W.L.; Huang, W.; Cao, Y.; Fan, K.N. Structure and catalytic properties of vanadium oxide supported on mesocellulous silica foams (MCF) for the oxidative dehydrogenation of propane to propylene. J. Catal. 2006, 239, 125–136. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.H.; Ohishi, Y.; Shishido, T.; Takehira, K. Synthesis of V-MCM-41 by template-ion exchange method and its catalytic properties in propane oxidative dehydrogenation. Catal. Lett. 2001, 72, 215–219. [Google Scholar] [CrossRef]
- Hess, C.; Hoefelmeyer, J.D.; Tilley, T.D. Spectroscopic characterization of highly dispersed vanadia supported on SBA-15. J. Phys. Chem. B 2004, 108, 9703–9709. [Google Scholar] [CrossRef]
- Baltes, M.; Cassiers, K.; Van Der Voort, P.; Weckhuysen, B.M.; Schoonheydt, R.A.; Vansant, E.F. MCM-48-supported vanadium oxide catalysts, prepared by the molecular designed dispersion of VO(acac)(2): A detailed study of the highly reactive MCM-48 surface and the structure and activity of the deposited VOx. J. Catal. 2001, 197, 160–171. [Google Scholar] [CrossRef]
- Zhu, H.B.; Ould-Chikh, S.; Dong, H.L.; Llorens, I.; Saih, Y.; Anjum, D.H.; Hazemann, J.L.; Basset, J.M. VOx/SiO2 Catalyst Prepared by Grafting VOCl3 on Silica for Oxidative Dehydrogenation of Propane. Chemcatchem 2015, 7, 3332–3339. [Google Scholar] [CrossRef]
- Högerl, M.P.; Serena Goh, L.M.; Abou-Hamad, E.; Barman, S.; Dachwald, O.; Pasha, F.A.; Pelletier, J.; Köhler, K.; D’Elia, V.; Cavallo, L.; et al. SOMC grafting of vanadium oxytriisopropoxide (VO(OiPr)3) on dehydroxylated silica; analysis of surface complexes and thermal restructuring mechanism. RSC Adv. 2018, 8, 20801–20808. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, M.; Park, D.W. Highly selective synthesis of cyclododecanone over mesostructured VSBA-15 catalysts. Appl. Catal. A Gen. 2010, 388, 22–30. [Google Scholar] [CrossRef]
- Jurado, M.J.; Gracia, M.D.; Campelo, J.M.; Luque, R.; Marinas, J.M.; Romero, A.A. Selective epoxidation of alkenes using highly active V-SBA-15 materials: Microwave vs. conventional heating. J. Mater. Chem. 2009, 19, 8603–8609. [Google Scholar] [CrossRef]
- Ying, F.; Li, J.H.; Huang, C.J.; Weng, W.Z.; Wan, H.L. Direct synthesis and superior catalytic performance of V-containing SBA-15 mesoporous materials for oxidative dehydrogenation of propane. Catal. Lett. 2007, 115, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Janiszewska, E.; Held, A.; Nowinska, K.; Kowalak, S. One-pot synthesis of vanadium-containing silica SBA-3 materials and their catalytic activity for propene oxidation. RSC Adv. 2019, 9, 4671–4681. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Haller, G.L. Preparation of Highly Ordered Vanadium-Substituted MCM-41: Stability and Acidic Properties†. J. Phys. Chem. B 2002, 106, 8437–8448. [Google Scholar] [CrossRef]
- Dang, T.T.H.; Seeburg, D.; Radnik, J.; Kreyenschulte, C.; Atia, H.; Vu, T.T.H.; Wohlrab, S. Influence of V-sources on the catalytic performance of VMCM-41 in the selective oxidation of methane to formaldehyde. Catal. Commun. 2018, 103, 56–59. [Google Scholar] [CrossRef]
- Wang, H.Q.; Qian, W.; Chen, J.; Wu, Y.; Xu, X.Y.; Wang, J.; Kong, Y. Spherical V-MCM-48: The synthesis, characterization and catalytic performance in styrene oxidation. RSC Adv. 2014, 4, 50832–50839. [Google Scholar] [CrossRef]
- Piumetti, M.; Bonelli, B.; Massiani, P.; Millot, Y.; Dzwigaj, S.; Gaberova, L.; Armandi, M.; Garrone, E. Novel vanadium-containing mesocellular foams (V-MCF) obtained by direct synthesis. Microporous Mesoporous Mater. 2011, 142, 45–54. [Google Scholar] [CrossRef]
- Aktas, O.; Yasyerli, S.; Dogu, G.; Dogu, T. Structural variations of MCF and SBA-15-like mesoporous materials as a result of differences in synthesis solution pH. Mater. Chem. Phys. 2011, 131, 151–159. [Google Scholar] [CrossRef]
- Post, K.; Robins, R.G. Thermodynamic diagrams for the vanadium-water system at 298·15K. Electrochim. Acta 1976, 21, 401–405. [Google Scholar] [CrossRef]
- Larson, J.W. Thermochemistry of Vanadium(5+) in Aqueous-Solutions. J. Chem. Eng. Data 1995, 40, 1276–1280. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.H.; Wan, H.Q.; Kong, Y.; Wu, X.C.; Dong, L.; Li, B.Q.; Chen, Y. The states of vanadium species in V-SBA-15 synthesized under different pH values. Microporous Mesoporous Mater. 2008, 110, 508–516. [Google Scholar] [CrossRef]
- Zhao, L.N.; Dong, Y.L.; Zhan, X.L.; Cheng, Y.; Zhu, Y.J.; Yuan, F.L.; Fu, H.G. One-pot Hydrothermal Synthesis of Mesoporous V-SBA-16 with a Function of the pH of the Initial Gel and its Improved Catalytic Performance for Benzene Hydroxylation. Catal. Lett. 2012, 142, 619–626. [Google Scholar] [CrossRef]
- Zhang, J.; Burkle-Vitzthum, V.; Marquaire, P.M. An Investigation on the Role of NO2 in the Oxidation of Methane to Formaldehyde. Combust. Sci. Technol. 2015, 187, 1139–1156. [Google Scholar] [CrossRef]
- Bañares, M.A.; Cardoso, J.H.; Hutchings, G.J.; Correa Bueno, J.M.; Fierro, J.L.G. Selective oxidation of methane to methanol and formaldehyde over V2O5/SiO2 catalysts. Role of NO in the gas phase. Catal. Lett. 1998, 56, 149–153. [Google Scholar] [CrossRef]
- Khan, M.M.; Somorjai, G.A. A Kinetic-Study of Partial Oxidation of Methane with Nitrous-Oxide on a Molybdena Silica Catalyst. J. Catal. 1985, 91, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Berndt, H.; Martin, A.; Bruckner, A.; Schreier, E.; Muller, D.; Kosslick, H.; Wolf, G.U.; Lucke, B. Structure and catalytic properties of VOx/MCM materials for the partial oxidation of methane to formaldehyde. J. Catal. 2000, 191, 384–400. [Google Scholar] [CrossRef]
- Meltser, L.Z.; Garibyan, T.A.; Grigoryan, R.R.; Muradyan, A.A.; Kurina, L.N. Effect of Homogeneous Initiator on Heterogeneous-Homogeneous Oxidation of Methane. React. Kinet. Catal. Lett. 1989, 38, 229–236. [Google Scholar] [CrossRef]
- Tsang, W.; Hampson, R.F. Chemical Kinetic Data Base for Combustion Chemistry. Part I. Methane and Related Compounds. J. Phys. Chem. Ref. Data 1986, 15, 1087–1279. [Google Scholar] [CrossRef]
- Takemoto, T.; Tabata, K.; Teng, Y.; Yao, S.; Nakayama, A.; Suzuki, E. Optimization of C1-Oxygenates for the Selective Oxidation of Methane in a Gas-Phase Reaction of CH4−O2−NO at Atmospheric Pressure. Energy Fuels 2001, 15, 44–51. [Google Scholar] [CrossRef]
- Mackie, J.C. Partial Oxidation of Methane-the Role of the Gas-Phase Reactions. Catal. Rev. 1991, 33, 169–240. [Google Scholar] [CrossRef]
- Wachs, I.E. Recent conceptual advances in the catalysis science of mixed metal oxide catalytic materials. Catal. Today 2005, 100, 79–94. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C. Response Surface Methodology: Process and Product in Optimization Using Designed Experiments; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1995. [Google Scholar]
- Toyao, T.; Maeno, Z.; Takakusagi, S.; Kamachi, T.; Takigawa, I.; Shimizu, K. Machine Learning for Catalysis Informatics: Recent Applications and Prospects. ACS Catal. 2020, 10, 2260–2297. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Liu, Z.J. Application of Artificial Neural Networks for Catalysis: A Review. Catalysts 2017, 7, 306. [Google Scholar] [CrossRef]
- Abiodun, O.I.; Jantan, A.; Omolara, A.E.; Dada, K.V.; Mohamed, N.A.; Arshad, H. State-of-the-art in artificial neural network applications: A survey. Heliyon 2018, 4, e00938. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, W.S.; Pitts, W. A logical calculus of the ideas immanent in nervous activity. Bull. Math. Biophys. 1943, 5, 115–133. [Google Scholar] [CrossRef]
- Cybenko, G. Approximation by superpositions of a sigmoidal function. Math. Control Signals Syst. 1989, 2, 303–314. [Google Scholar] [CrossRef]
- Jain, A.K.; Mao, J.C.; Mohiuddin, K.M. Artificial neural networks: A tutorial. Computer 1996, 29, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, J.; Zupan, J. Neural Networks in Chemistry. Angew. Chem. Int. Ed. 1993, 32, 503–527. [Google Scholar] [CrossRef]
- Goldsmith, B.R.; Esterhuizen, J.; Liu, J.-X.; Bartel, C.J.; Sutton, C. Machine learning for heterogeneous catalyst design and discovery. AlChE J. 2018, 64, 2311–2323. [Google Scholar] [CrossRef]
- Panerati, J.; Schnellmann, M.A.; Patience, C.; Beltrame, G.; Patience, G.S. Experimental methods in chemical engineering: Artificial neural networks–ANNs. Can. J. Chem. Eng. 2019, 97, 2372–2382. [Google Scholar] [CrossRef]
- Timoshenko, J.; Frenkel, A.I. “Inverting” X-ray Absorption Spectra of Catalysts by Machine Learning in Search for Activity Descriptors. ACS Catal. 2019, 9, 10192–10211. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Kiyohara, S. Machine learning approaches for ELNES/XANES. Microscopy 2020, 69, 92–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darabi Mahboub, M.J.; Rostamizadeh, M.; Dubois, J.-L.; Patience, G.S. Partial oxidation of 2-methyl-1,3-propanediol to methacrylic acid: Experimental and neural network modeling. RSC Adv. 2016, 6, 114123–114134. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Gao, X.T.; Bare, S.R.; Weckhuysen, B.M.; Wachs, I.E. In situ spectroscopic investigation of molecular structures of highly dispersed vanadium oxide on silica under various conditions. J. Phys. Chem. B 1998, 102, 10842–10852. [Google Scholar] [CrossRef] [Green Version]
- Schraml-Marth, M.; Wokaun, A.; Pohl, M.; Krauss, H.-L. Spectroscopic investigation of the structure of silica-supported vanadium oxide catalysts at submonolayer coverages. J. Chem. Soc. Faraday Trans. 1991, 87, 2635–2646. [Google Scholar] [CrossRef]
- Bulánek, R.; Čapek, L.; Setnička, M.; Čičmanec, P. DR UV–vis Study of the Supported Vanadium Oxide Catalysts. J. Phys. Chem. C 2011, 115, 12430–12438. [Google Scholar] [CrossRef]
- Humbert, B.; Burneau, A.; Gallas, J.P.; Lavalley, J.C. Origin of the Raman Bands, D1 and D2, in High Surface-Area and Vitreous Silicas. J. Non-Cryst. Solids 1992, 143, 75–83. [Google Scholar] [CrossRef]
- Galeener, F.L.; Mikkelsen, J.C. Vibrational dynamics in 18O-substituted vitreous SiO2. Phys. Rev. B 1981, 23, 5527–5530. [Google Scholar] [CrossRef]
- Borodko, Y.; Ager Iii, J.W.; Marti, G.E.; Song, H.; Niesz, K.; Somorjai, G.A. Structure sensitivity of vibrational spectra of mesoporous silica SBA-15 and Pt/SBA-15. J. Phys. Chem. B 2005, 109, 17386–17390. [Google Scholar] [CrossRef] [PubMed]
- Döbler, J.; Pritzsche, M.; Sauer, J. Vibrations of Silica Supported Vanadia: Variation with Particle Size and Local Surface Structure. J. Phys. Chem. C 2009, 113, 12454–12464. [Google Scholar] [CrossRef]
- Strunk, J.; Banares, M.A.; Wachs, I.E. Vibrational Spectroscopy of Oxide Overlayers. Top. Catal. 2017, 60, 1577–1617. [Google Scholar] [CrossRef]
- Das, N.; Eckert, H.; Hu, H.; Wachs, I.E.; Walzer, J.F.; Feher, F.J. Bonding states of surface vanadium(V) oxide phases on silica: Structural characterization by vanadium-51 NMR and Raman spectroscopy. J. Phys. Chem. 1993, 97, 8240–8243. [Google Scholar] [CrossRef]
- Izumi, Y.; Konishi, K.; Obaid, D.M.; Miyajima, T.; Yoshitake, H. X-ray absorption fine structure combined with X-ray fluorescence spectroscopy. Monitoring of vanadium sites in mesoporous titania, excited under visible light by selective detection of vanadium Kbeta5,2 fluorescence. Anal. Chem. 2007, 79, 6933–6940. [Google Scholar] [CrossRef]
- Hobson, A.J.; Stewart, D.I.; Bray, A.W.; Mortimer, R.J.G.; Mayes, W.M.; Riley, A.L.; Rogerson, M.; Burke, I.T. Behaviour and fate of vanadium during the aerobic neutralisation of hyperalkaline slag leachate. Sci. Total Environ. 2018, 643, 1191–1199. [Google Scholar] [CrossRef]
- Kepert, D.L. The Early Transition Metals; Academic Press: London, UK, 1972. [Google Scholar]
- Lincoln, W.P.; Skrzypek, J. Synergy of Clustering Multiple Back Propagation Networks. Proceedings of Advances in Neural Information Processing Systems, Denver, CO, USA, 27–30 November 1989; pp. 650–657. [Google Scholar]
- Horn, D.; Naftaly, U.; Intrator, N. Large Ensemble Averaging. In Neural Networks: Tricks of the Trade; Orr, G.B., Müller, K.-R., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 133–139. [Google Scholar] [CrossRef]
- Hansen, L.K.; Salamon, P. Neural Network Ensembles. IEEE Trans. Pattern Anal. Mach. Intell. 1990, 12, 993–1001. [Google Scholar] [CrossRef] [Green Version]
- Spencer, N.D.; Pereira, C.J. V2O5-SiO2-Catalyzed Methane Partial Oxidation with Molecular-Oxygen. J. Catal. 1989, 116, 399–406. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press LLC Taylor & Francis Group: Boca Raton, FL, USA; Florence, Italy, 2016. [Google Scholar]
- Launay, H.; Loridant, S.; Pigamo, A.; Dubois, J.L.; Millet, J.M.M. Vanadium species in new catalysts for the selective oxidation of methane to formaldehyde: Specificity and molecular structure dynamics with water. J. Catal. 2007, 246, 390–398. [Google Scholar] [CrossRef]
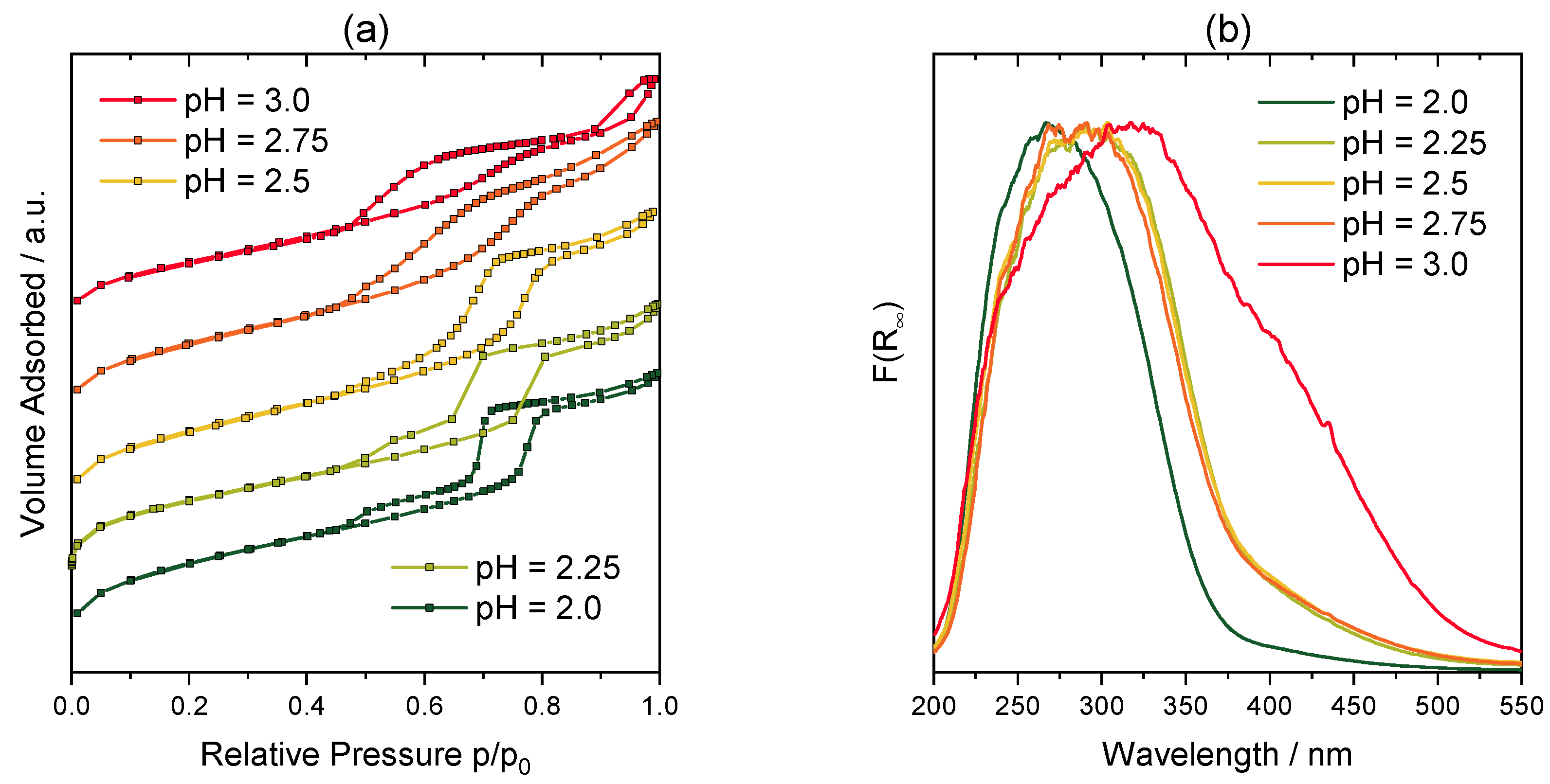


| Catalyst | V%/wt% | SSA/m2∙g−1 | Pore Volume/cm−3∙g−1 |
|---|---|---|---|
| V-SBA-15(2.0) | 1.1 | 771 | 0.94 |
| V-SBA-15(2.25) | 1.4 | 757 | 0.92 |
| V-SBA-15(2.5) | 1.9 | 725 | 1.00 |
| V-SBA-15(2.75) | 1.7 | 692 | 0.97 |
| V-SBA-15(2.0) | 1.9 | 574 | 0.82 |
| Input | Output |
|---|---|
| Temperature T | Conversion of methane X(CH4) |
| Gas hourly space velocity (GHSV) | Selectivity towards formaldehyde S(CH2O) |
| Concentration of oxygen c(O2) | |
| Concentration of nitrogen c(N2) | |
| Concentration of water c(H2O) |
| GHSV a/L∙kg−1∙h−1 | c(O2) b/% | c(N2) c/% | c(H2O) c/% |
|---|---|---|---|
| 960,000 | 2.5 | 0 | 0 |
| 720,000 | 5 | 20 | 2.5 |
| 480,000 | 10 | 40 | 5 |
| 240,000 | 15 | 60 | 10 |
| 20 | 15 | ||
| 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunkel, B.; Kabelitz, A.; Buzanich, A.G.; Wohlrab, S. Increasing the Efficiency of Optimized V-SBA-15 Catalysts in the Selective Oxidation of Methane to Formaldehyde by Artificial Neural Network Modelling. Catalysts 2020, 10, 1411. https://doi.org/10.3390/catal10121411
Kunkel B, Kabelitz A, Buzanich AG, Wohlrab S. Increasing the Efficiency of Optimized V-SBA-15 Catalysts in the Selective Oxidation of Methane to Formaldehyde by Artificial Neural Network Modelling. Catalysts. 2020; 10(12):1411. https://doi.org/10.3390/catal10121411
Chicago/Turabian StyleKunkel, Benny, Anke Kabelitz, Ana Guilherme Buzanich, and Sebastian Wohlrab. 2020. "Increasing the Efficiency of Optimized V-SBA-15 Catalysts in the Selective Oxidation of Methane to Formaldehyde by Artificial Neural Network Modelling" Catalysts 10, no. 12: 1411. https://doi.org/10.3390/catal10121411
APA StyleKunkel, B., Kabelitz, A., Buzanich, A. G., & Wohlrab, S. (2020). Increasing the Efficiency of Optimized V-SBA-15 Catalysts in the Selective Oxidation of Methane to Formaldehyde by Artificial Neural Network Modelling. Catalysts, 10(12), 1411. https://doi.org/10.3390/catal10121411