The Enhanced Performance of N-Modified Activated Carbon Promoted with Ce in Selective Catalytic Reduction of NOx with NH3
Abstract
:1. Introduction
2. Results and Discussion
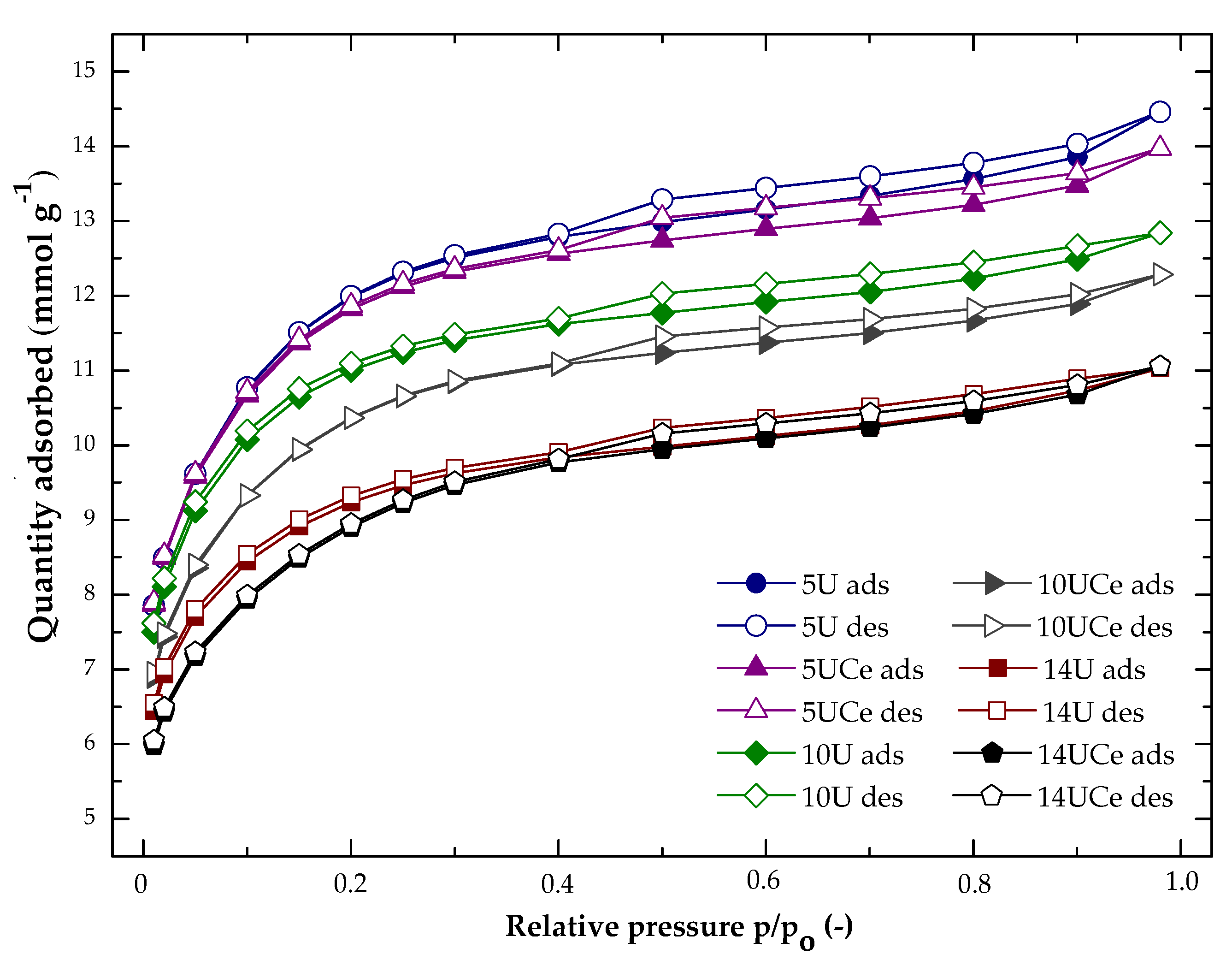
2.1. Nitrogen Adsorption at −196 °C
2.2. X-ray Diffraction Analysis
2.3. Fourier Transform Infrared Spectroscopy Analysis
2.4. SEM and EDX Analysis
2.4.1. SEM Analysis
2.4.2. EDX Analysis
2.5. Thermogravimetric Analysis
2.6. Catalytic Performance Tests
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.; Liu, Y.; Wu, Z. The poisoning mechanisms of different zinc species on a ceria-based NH3-SCR catalyst and the co-effects of zinc and gas-phase sulfur/chlorine species. J. Colloid Interface Sci. 2020, 566, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Szymaszek, A.; Samojeden, B.; Motak, M. The Deactivation of Industrial SCR Catalysts—A Short Review. Energies 2020, 13, 3870. [Google Scholar] [CrossRef]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Samojeden, B.; Motak, M.; Grzybek, T. The influence of the modification of carbonaceous materials on their catalytic properties in SCR-NH3. A short review. C. R. Chim. 2015, 18, 1049–1073. [Google Scholar] [CrossRef]
- Ferella, F. A review on management and recycling of spent selective catalytic reduction catalysts. J. Clean. Prod. 2020, 246, 118990. [Google Scholar] [CrossRef]
- Gonçalves, A.A.S.; Ciesielczyk, F.; Samojeden, B.; Jaroniec, M. Toward development of single-atom ceramic catalysts for selective catalytic reduction of NO with NH3. J. Hazard. Mater. 2020, 401, 123413. [Google Scholar] [CrossRef]
- Motak, M. Montmorillonites modified with polymer and promoted with copper as DeNOx catalysts. Catal. Today 2008, 137, 247–252. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, J.; Lin, Y.; Guo, J.; Zhu, T. Distribution of SO2 Oxidation Products in the SCR of NO over V2O5/TiO2 Catalysts at Different Temperatures. Ind. Eng. Chem. Res. 2020, 59, 5177–5185. [Google Scholar] [CrossRef]
- Liu, Z.G.; Ottinger, N.A.; Cremeens, C.M. Vanadium and tungsten release from V-based selective catalytic reduction diesel aftertreatment. Atmos. Environ. 2015, 104, 154–161. [Google Scholar] [CrossRef]
- Grzybek, T. Layered clays as SCR deNOx catalysts. Catal. Today 2007, 119, 125–132. [Google Scholar] [CrossRef]
- Che, Y.; Zhou, J.; Wang, Z. Plasma modification of activated carbon fibers for adsorption of SO2. Plasma Sci. Technol. 2013, 15, 1047–1052. [Google Scholar] [CrossRef]
- Fang, Z.; Yu, X.; Tu, S.T. Catalytic oxidation of NO on activated carbons. Energy Procedia 2019, 158, 2366–2371. [Google Scholar] [CrossRef]
- Liu, X.; Tian, J.; Li, Y.; Sun, N.; Mi, S.; Xie, Y.; Chen, Z. Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J. Hazard. Mater. 2019, 373, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Grzybek, T.; Klinik, J.; Samojeden, B.; Suprun, V.; Papp, H. Nitrogen-promoted active carbons as DeNOx catalysts. 1. The influence of modification parameters on the structure and catalytic properties. Catal. Today 2008, 137, 228–234. [Google Scholar] [CrossRef]
- Barrabas, S.B.; Baini, R.; Sutan, N.M.; Yakub, I.; Anwar, K.; Zauzi, N.S.A.; Wahab, N.A.; Sulaiman, H. HC-SCR: NOx Reduction using Mn and Cu Catalysts Impregnated in Coconut and Palm Kernel Shell Activated Carbon. MATEC Web Conf. 2016, 87, 03004. [Google Scholar] [CrossRef] [Green Version]
- Klinik, J.; Samojeden, B.; Grzybek, T.; Suprun, W.; Papp, H.; Gläser, R. Nitrogen promoted activated carbons as DeNOx catalysts. 2. the influence of water on the catalytic performance. Catal. Today 2011, 176, 303–308. [Google Scholar] [CrossRef]
- Samojeden, B.; Grzybek, T. The influence of the promotion of N-modified activated carbon with iron on NO removal by NH3-SCR. Energy 2016, 116, 1484–1491. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Y.; Cheng, C.; Hao, J.; Zhu, T. On the Nature of Nitrogen-Containing Groups in the SCR of NO Over Functionalized Activated Coke. Waste Biomass Valoriz. 2020, 11, 1691–1699. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Xing, Z.; Bao, C.; Liang, G. Preparation and characterization of CeO2-MoO3/TiO2 catalysts for selective catalytic reduction of NO with NH3. Aerosol Air Qual. Res. 2017, 17, 2726–2734. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Zhang, H.; Dong, L. Ceria-based catalysts for low-temperature selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2016, 6, 1248–1264. [Google Scholar] [CrossRef]
- Shen, B.; Yao, Y.; Ma, H.; Liu, T. Ceria modified MnOx/TiO2-pillared clays catalysts for the selective catalytic reduction of NO with NH3 at low temperature. Chin. J. Catal. 2011, 32, 1803–1811. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, S.; Qiu, T.; Shen, S. A novel catalyst of CeO2/Al2O3 for selective catalytic reduction of NO by NH3. Catal. Commun. 2009, 11, 20–23. [Google Scholar] [CrossRef]
- Song, Z.; Ning, P.; Zhang, Q.; Li, H.; Zhang, J.; Wang, Y.; Liu, X.; Huang, Z. Activity and hydrothermal stability of CeO2-ZrO2-WO3 for the selective catalytic reduction of NOx with NH3. J. Environ. Sci. (China) 2016, 42, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Woo, T.K.; Hermansson, K. Adsorption of NO on unreduced and reduced CeO2 surfaces: A plane-wave DFT study. Surf. Sci. 2006, 600, 4953–4960. [Google Scholar] [CrossRef]
- Nolan, M. Molecular adsorption on the doped (110) ceria surface. J. Phys. Chem. C 2009, 113, 2425–2432. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Li, J.; Zhu, J.; Ma, L. Novel V2O5-CeO2/TiO2 catalyst with low vanadium loading for the selective catalytic reduction of NOx by NH3. Appl. Catal. B Environ. 2014, 158–159, 11–19. [Google Scholar] [CrossRef]
- Xu, C.; Liu, J.; Zhao, Z.; Yu, F.; Cheng, K.; Wei, Y.; Duan, A.; Jiang, G. NH3-SCR denitration catalyst performance over vanadium-titanium with the addition of Ce and Sb. J. Environ. Sci. (China) 2015, 31, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tan, S.; Wang, X.; Li, M.; Li, S.; Li, W. Regeneration of sulfur-poisoned CeO2 catalyst for NH3-SCR of NOx. Catal. Commun. 2016, 86, 67–71. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A General Treatment and Classification of the Solute Adsorption Isotherm I. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Velasco, L.F.; Guillet-Nicolas, R.; Dobos, G.; Thommes, M.; Lodewyckx, P. Towards a better understanding of water adsorption hysteresis in activated carbons by scanning isotherms. Carbon N. Y. 2016, 96, 753–758. [Google Scholar] [CrossRef]
- Vivo-Vilches, J.F.; Bailón-García, E.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Maldonado-Hódar, F.J. Tailoring the surface chemistry and porosity of activated carbons: Evidence of reorganization and mobility of oxygenated surface groups. Carbon N. Y. 2014, 68, 520–530. [Google Scholar] [CrossRef]
- Huang, B.; Liu, G.; Wang, P.; Zhao, X.; Xu, H. Effect of nitric acid modification on characteristics and adsorption properties of lignite. Processes 2019, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Li, C.; Zeng, G.; He, L.; Peng, D.; Cui, H.; Li, S.; Zhai, Y. Low temperature selective catalytic reduction of NO by activated carbon fiber loading lanthanum oxide and ceria. Appl. Catal. B Environ. 2010, 96, 157–161. [Google Scholar] [CrossRef]
- Dai, H.; Qiu, Y.P.; Dai, H.B.; Wang, P. Ni-Pt/CeO2 Loaded on Granular Activated Carbon: An Efficient Monolithic Catalyst for Controlled Hydrogen Generation from Hydrous Hydrazine. ACS Sustain. Chem. Eng. 2018, 6, 9876–9882. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.; Zhang, J.; Du, X.; Li, S.; Zeng, J.; Yi, Y.; Zeng, G. Simultaneous removal of NO and HgO from simulated flue gas over CoOx-CeO2 loaded biomass activated carbon derived from maize straw at low temperatures. Chem. Eng. J. 2018, 342, 339–349. [Google Scholar] [CrossRef]
- Zou, J.; Oladipo, J.; Fu, S.; Al-Rahbi, A.; Yang, H.; Wu, C.; Cai, N.; Williams, P.; Chen, H. Hydrogen production from cellulose catalytic gasification on CeO2/Fe2O3 catalyst. Energy Convers. Manag. 2018, 171, 241–248. [Google Scholar] [CrossRef]
- Bueno-López, A. Diesel soot combustion ceria catalysts. Appl. Catal. B Environ. 2014, 146, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Atribak, I.; López-Suárez, F.E.; Bueno-López, A.; García-García, A. New insights into the performance of ceria-zirconia mixed oxides as soot combustion catalysts. Identification of the role of “active oxygen” production. Catal. Today 2011, 176, 404–408. [Google Scholar] [CrossRef]
- Guillén-Hurtado, N.; Bueno-López, A.; García-García, A. Catalytic performances of ceria and ceria-zirconia materials for the combustion of diesel soot under NOx/O2 and O2. Importance of the cerium precursor salt. Appl. Catal. A Gen. 2012, 437–438, 166–172. [Google Scholar] [CrossRef]
- Vratny, F.; Kern, S.; Gugliotta, F. The thermal decomposition of cerium (III) nitrate hydrate. J. Inorg. Nucl. Chem. 1961, 17, 281–285. [Google Scholar] [CrossRef]
- Halepoto, A.; Kashif, M.; Su, Y.; Cheng, J.; Deng, W.; Zhao, B. Preparations and Characterization on Fe Based Catalyst Supported on Coconut Shell Activated Carbon CS(AC) and SCR of NOx-HC. Catal. Surv. Asia 2020, 24, 123–133. [Google Scholar] [CrossRef]
- Liu, K.; Yu, Q.; Wang, B.; Qin, Q.; Wei, M.; Fu, Q. Low temperature selective catalytic reduction of nitric oxide with urea over activated carbon supported metal oxide catalysts. Environ. Technol. 2020, 41, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V. Preparation of Activated Carbon-SnO2, TiO2, and WO3 Catalysts. Study by FT-IR Spectroscopy. Ind. Eng. Chem. Res. 2016, 55, 5200–5206. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Ania, C.O. Chapter 4 Surface chemistry of activated carbons and its characterization. Interface Sci. Technol. 2006, 7, 159–229. [Google Scholar]
- Song, X.; Jiang, N.; Li, Y.; Xu, D.; Qiu, G. Synthesis of CeO2-coated SiO2 nanoparticle and dispersion stability of its suspension. Mater. Chem. Phys. 2008, 110, 128–135. [Google Scholar] [CrossRef]
- Setiabudi, A.; Makkee, M.; Moulijn, J.A. The role of NO2 and O2 in the accelerated combustion of soot in diesel exhaust gases. Appl. Catal. B Environ. 2004, 50, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Din, I.U.; Shaharun, M.S.; Subbarao, D.; Naeem, A. Surface modification of carbon nanofibers by HNO3 treatment. Ceram. Int. 2016, 42, 966–970. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Polyaniline-cerium oxide (PAni-CeO2) coated graphene oxide for enhancement of epoxy coating corrosion protection performance on mild steel. Corros. Sci. 2018, 137, 111–126. [Google Scholar] [CrossRef]
- Müller, J.O.; Su, D.S.; Jentoft, R.E.; Kröhnert, J.; Jentoft, F.C.; Schlögl, R. Morphology-controlled reactivity of carbonaceous materials towards oxidation. Catal. Today 2005, 102–103, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Le, Q. Synthesis and performance of cerium oxide as anode materials for lithium ion batteries by a chemical precipitation method. J. Alloys Compd. 2016, 669, 1–7. [Google Scholar] [CrossRef]
- Ikuma, Y.; Oosawa, H.; Shimada, E.; Kamiya, M. Effect of microwave radiation on the formation of Ce2O(CO3)2·H2O in aqueous solution. Solid State Ionics 2002, 151, 347–352. [Google Scholar] [CrossRef]
- Nejar, N.; Makkee, M.; Illán-Gómez, M.J. Catalytic removal of NOx and soot from diesel exhaust: Oxidation behaviour of carbon materials used as model soot. Appl. Catal. B Environ. 2007, 75, 11–16. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, X.; Feng, Y.; Si, Z.; Weng, D.; Shi, L. Low-temperature SCR activity and SO2 deactivation mechanism of Ce-modified V2O5-WO3/TiO2 catalyst. Prog. Nat. Sci. Mater. Int. 2015, 25, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Li, Y.; Xu, Z.; Xiong, J.; Zhu, T. Transformation of functional groups in the reduction of NO with NH3 over nitrogen-enriched activated carbons. Fuel 2018, 223, 312–323. [Google Scholar] [CrossRef]
- Yao, L.; Liu, Q.; Mossin, S.; Nielsen, D.; Kong, M.; Jiang, L.; Yang, J.; Ren, S.; Wen, J. Promotional effects of nitrogen doping on catalytic performance over manganese-containing semi-coke catalysts for the NH3-SCR at low temperatures. J. Hazard. Mater. 2020, 387. [Google Scholar] [CrossRef] [PubMed]
- Białas, A.; Szlendak, J.; Czosnek, C.; Motak, M. Activated Carbon as a Support of Catalysts for the Removal of Nitrogen Oxides. Mineral. Polonica 2020, 16, 9–16. [Google Scholar] [CrossRef]
- Amanpour, J.; Salari, D.; Niaei, A.; Mousavi, S.M.; Panahi, P.N. Optimization of Cu/activated carbon catalyst in low temperature selective catalytic reduction of NO process using response surface methodology. J. Environ. Sci. Heal Part A Toxic/Hazard. Subst. Environ. Eng. 2013, 48, 879–886. [Google Scholar] [CrossRef]
- Marberger, A.; Ferri, D.; Elsener, M.; Kröcher, O. The Significance of Lewis Acid Sites for the Selective Catalytic Reduction of Nitric Oxide on Vanadium-Based Catalysts. Angew. Chem. 2016, 128, 12168–12173. [Google Scholar] [CrossRef]
- Zeng, Z.; Lu, P.; Li, C.; Zeng, G.; Jiang, X.; Zhai, Y.; Fan, X. Selective catalytic reduction (SCR) of NO by urea loaded on activated carbon fibre (ACF) and CeO2/ACF at 30 °C: The SCR mechanism. Environ. Technol. 2012, 33, 1331–1337. [Google Scholar] [CrossRef]
- You, X.; Sheng, Z.; Yu, D.; Yang, L.; Xiao, X.; Wang, S. Influence of Mn/Ce ratio on the physicochemical properties and catalytic performance of graphene supported MnOx-CeO2 oxides for NH3 -SCR at low temperature. Appl. Surf. Sci. 2017, 423, 845–854. [Google Scholar] [CrossRef]
- Dastgheib, S.A.; Salih, H.; Ilangovan, T.; Mock, J. NO Oxidation by Activated Carbon Catalysts: Impact of Carbon Characteristics, Pressure, and the Presence of Water. ACS Omega 2020. [Google Scholar] [CrossRef] [PubMed]
- Adapa, S.; Gaur, V.; Verma, N. Catalytic oxidation of NO by activated carbon fiber (ACF). Chem. Eng. J. 2006, 116, 25–37. [Google Scholar] [CrossRef]
- Klinik, J. Tekstura Porowatych Ciał Stałych; Akademia Górniczo-Hutnicza im. St. Staszica w Krakowie Ośrodek Edukacji Niestacjonarnej: Kraków, Poland, 2000; ISBN 83-913223-2-7. [Google Scholar]










| No. | Sample Code | SBET (m2·g−1) | Volume of Micropores (cm3·g−1) | Average Pore Diameter (nm) | Total Pore Volume (cm3·g−1) | Micropore Contribution (%) |
|---|---|---|---|---|---|---|
| 1 | AC | 722 | 0.36 | 2.00 | 0.45 | 80.00% |
| 2 | 5U | 813 | 0.47 | 2.36 | 0.50 | 94.00% |
| 3 | 5UCe | 880 | 0.49 | 2.38 | 0.52 | 94.20% |
| 4 | 10U | 829 | 0.44 | 2.22 | 0.48 | 91.60% |
| 5 | 10UCe | 888 | 0.48 | 2.12 | 0.51 | 94.11% |
| 6 | 14U | 812 | 0.42 | 1.98 | 0.46 | 91.30% |
| 7 | 14UCe | 873 | 0.45 | 1.99 | 0.48 | 93.70% |
| No. | Sample | Approximate Weight (wt.%) | ||||
|---|---|---|---|---|---|---|
| C | N | O | Ce | Si | ||
| 1 | 5U | 45.00 | 41.00 | 11.45 | 0 | 2.55 |
| 2 | 5UCe | 41.14 | 36.09 | 20.09 | 2.68 | 0 |
| 3 | 10U | 43.02 | 41.31 | 12.44 | 0 | 3.24 |
| 4 | 10UCe | 40.15 | 35.72 | 21.17 | 2.95 | 0 |
| 5 | 14U | 39.89 | 42.35 | 14.16 | 0 | 3.70 |
| 6 | 14UCe | 38.12 | 40.28 | 21.36 | 0.23 | 0 |
| Sample | Weight Loss in the Particular Temperature Range (%) | |
|---|---|---|
| 80–380 °C | 140–300 °C | |
| 5U | 3.03 | 1.06 |
| 10U | 3.27 | 1.06 |
| 14U | 3.48 | 1.12 |
| 80–300 °C | 140–300 °C | |
| 5UCe | 1.87 | 1.50 |
| 10UCe | 3.42 | 1.73 |
| 14UCe | 4.26 | 3.04 |
| No. | Sample Code | Concentration of HNO3 Used for The Synthesis (mol·dm−3) | Modification with Ce |
|---|---|---|---|
| 1 | AC | non-modified sample | |
| 2 | 5U | 5 | - |
| 3 | 5UCe | 5 | + |
| 4 | 10U | 10 | - |
| 5 | 10UCe | 10 | + |
| 6 | 14U | 14 | - |
| 7 | 14UCe | 14 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saad, M.; Szymaszek, A.; Białas, A.; Samojeden, B.; Motak, M. The Enhanced Performance of N-Modified Activated Carbon Promoted with Ce in Selective Catalytic Reduction of NOx with NH3. Catalysts 2020, 10, 1423. https://doi.org/10.3390/catal10121423
Saad M, Szymaszek A, Białas A, Samojeden B, Motak M. The Enhanced Performance of N-Modified Activated Carbon Promoted with Ce in Selective Catalytic Reduction of NOx with NH3. Catalysts. 2020; 10(12):1423. https://doi.org/10.3390/catal10121423
Chicago/Turabian StyleSaad, Marwa, Agnieszka Szymaszek, Anna Białas, Bogdan Samojeden, and Monika Motak. 2020. "The Enhanced Performance of N-Modified Activated Carbon Promoted with Ce in Selective Catalytic Reduction of NOx with NH3" Catalysts 10, no. 12: 1423. https://doi.org/10.3390/catal10121423
APA StyleSaad, M., Szymaszek, A., Białas, A., Samojeden, B., & Motak, M. (2020). The Enhanced Performance of N-Modified Activated Carbon Promoted with Ce in Selective Catalytic Reduction of NOx with NH3. Catalysts, 10(12), 1423. https://doi.org/10.3390/catal10121423







