Rapid Ammonia Carriers for SCR Systems Using MOFs [M2(adc)2(dabco)] (M = Co, Ni, Cu, Zn)
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Characterization of the MOFs
2.2. Ammonia Uptake Capacity
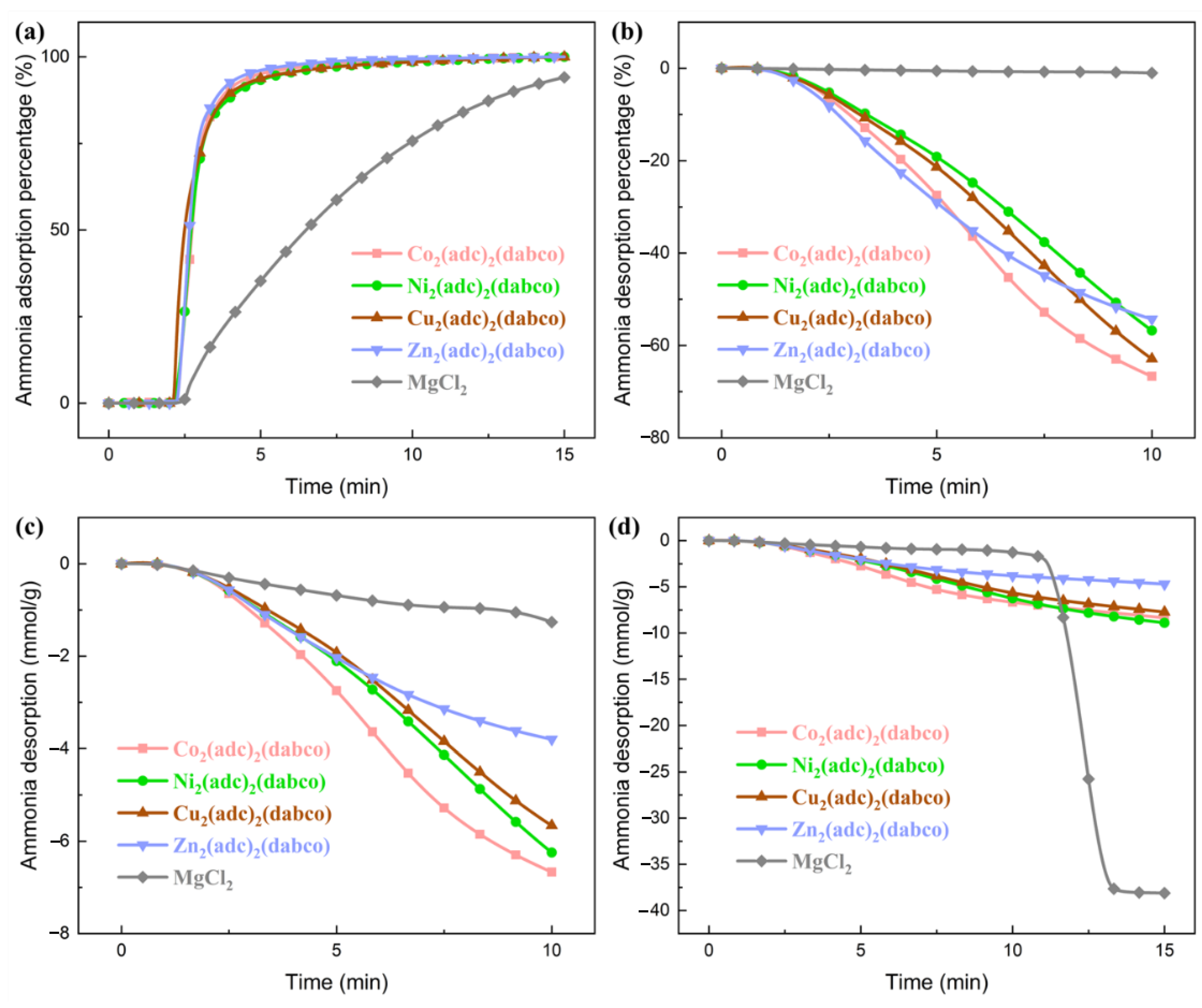
2.3. Kinetics of the Ammonia Adsorption and Desorption
3. Materials and Methods
3.1. MOFs Synthesis
3.2. Structure Characterization
3.3. Ammonia Adsorption and Desorption Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Damma, D.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. A Review of Low Temperature NH3-SCR for Removal of NOx. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Anenberg, S.C.; Miller, J.; Minjares, R.; Du, L.; Henze, D.K.; Lacey, F.; Malley, C.S.; Emberson, L.; Franco, V.; Klimont, Z.; et al. Impacts and mitigation of excess diesel-related NOx emissions in 11 major vehicle markets. Nature 2017, 545, 467–471. [Google Scholar] [CrossRef]
- Jonson, J.E.; Borken-Kleefeld, J.; Simpson, D.; Nyíri, A.; Posch, M.; Heyes, C. Impact of excess NOx emissions from diesel cars on air quality, public health and eutrophication in Europe. Environ. Res. Lett. 2017, 12, 094017. [Google Scholar] [CrossRef]
- Ko, S.; Park, J.; Kim, H.; Kang, G.; Lee, J.; Kim, J.; Lee, J. NOx Emissions from Euro 5 and Euro 6 Heavy-Duty Diesel Vehicles under Real Driving Conditions. Energies 2020, 13, 218. [Google Scholar] [CrossRef]
- Vignesh, R.; Ashok, B. Critical interpretative review on current outlook and prospects of selective catalytic reduction system for De-NOx strategy in compression ignition engine. Fuel 2020, 276, 117996. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Marti, T. NOx-Reduction in Diesel Exhaust Gas with Urea and Selective Catalytic Reduction. Combust. Sci. Technol. 1996, 121, 85–102. [Google Scholar] [CrossRef]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Chi, J.N. Control Challenges for Optimal NOx Conversion Efficiency from SCR Aftertreatment Systems; SAE Technical Paper 2009-01-0905; SAE International: Warrendale, PA, USA, 2009. [Google Scholar]
- Szymaszek, A.; Samojeden, B.; Motak, M. The Deactivation of Industrial SCR Catalysts—A Short Review. Energies 2020, 13, 3870. [Google Scholar] [CrossRef]
- Fu, M.; Li, C.; Lu, P.; Qu, L.; Zhang, M.; Zhou, Y.; Yu, M.; Fang, Y. A review on selective catalytic reduction of NOx by supported catalysts at 100–300 °C—Catalysts, mechanism, kinetics. Catal. Sci. Technol. 2013, 4, 14–25. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Tsuzuki, M.; Satsuma, A. Effects of hydrogen and oxygenated hydrocarbons on the activity and SO2-tolerance of Ag/Al2O3 for selective reduction of NO. Appl. Catal. B Environ. 2007, 71, 80–84. [Google Scholar] [CrossRef]
- Renard, J.J.; Calidonna, S.E.; Henley, M.V. Fate of ammonia in the atmosphere—A review for applicability to hazardous releases. J. Hazard. Mater. 2004, 108, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsson, P.L.T. Urea-SCR in Automotive Applications. Top. Catal. 2004, 28, 177–184. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Kleemann, M. Urea-SCR: A promising technique to reduce NOx emissions from automotive diesel engines. Catal. Today 2000, 59, 335–345. [Google Scholar] [CrossRef]
- Johannessen, T.; Schmidt, H.; Svagin, J.; Johansen, J.; Oechsle, J.; Bradley, R. Ammonia Storage and Delivery Systems for Automotive NOx Aftertreatment; SAE Technical Paper 2008-01-1027; SAE International: Warrendale, PA, USA, 2008. [Google Scholar]
- Choi, B.-C.; Kim, Y.K.; Jhung, W.-N.; Lee, C.-H.; Hwang, C.-Y. Experimental investigation on melting characteristics of frozen urea–water-solutions for a diesel SCR de-NOx-system. Appl. Therm. Eng. 2013, 50, 1235–1245. [Google Scholar] [CrossRef]
- Liu, C.Y.; Aika, K. Ammonia Absorption into Alkaline Earth Metal Halide Mixtures as an Ammonia Storage Material. Ind. Eng. Chem. Res. 2004, 43, 7484–7491. [Google Scholar] [CrossRef]
- Wang, Z.X.; Wang, L.W.; Gao, P.; Yu, Y.; Wang, R.Z. Analysis of composite sorbents for ammonia storage to eliminate NOx emission at low temperatures. Appl. Therm. Eng. 2018, 128, 1382–1390. [Google Scholar] [CrossRef]
- Zhu, H.; Gu, X.; Yao, K.; Gao, L.; Chen, J. Large-Scale Synthesis of MgCl2·6NH3 as an Ammonia Storage Material. Ind. Eng. Chem. Res. 2009, 48, 5317–5320. [Google Scholar] [CrossRef]
- Tekin, A.; Hummelshøj, J.S.; Jacobsen, H.S.; Sveinbjörnsson, D.; Blanchard, D.; Nørskov, J.K.; Vegge, T. Ammonia dynamics in magnesium ammine from DFT and neutron scattering. Energy Environ. Sci. 2010, 3, 448–456. [Google Scholar] [CrossRef]
- Reardon, H.; Hanlon, J.M.; Grant, M.; Fullbrook, I.; Gregory, D.H. Ammonia Uptake and Release in the MnX2–NH3 (X = Cl, Br) Systems and Structure of the Mn(NH3)nX2 (n = 6, 2) Ammines. Crystals 2012, 2, 193–212. [Google Scholar] [CrossRef]
- Yim, S.D.; Kim, S.J.; Baik, J.H.; Nam, I.; Mok, Y.S.; Lee, J.-H.; Cho, B.K.; Oh, S.H. Decomposition of Urea into NH3 for the SCR Process. Ind. Eng. Chem. Res. 2004, 43, 4856–4863. [Google Scholar] [CrossRef]
- Johannessen, T.; Schmidt, H.; Frey, A.M.; Christensen, C.H. Improved Automotive NOx Aftertreatment System: Metal Ammine Complexes as NH3 Source for SCR Using Fe-Containing Zeolite Catalysts. Catal. Lett. 2009, 128, 94–100. [Google Scholar] [CrossRef]
- Johannessen, T. 3rd Generation SCR System Using Solid Ammonia Storage and Direct Gas Dosing: Expanding the SCR window for RDE. In Proceedings of the Directions in Engine-Efficiency and Emissions Research (DEER) Conference, Dearborn, MI, USA, 16–19 October 2012. [Google Scholar]
- Christensen, C.H.; Sørensen, R.Z.; Johannessen, T.; Quaade, U.J.; Honkala, K.; Elmøe, T.D.; Køhlera, R.; Nørskov, J.K. Metal ammine complexes for hydrogen storage. J. Mater. Chem. 2005, 15, 4106–4108. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Sabo, M.; Henschel, A.; Fröde, H.; Klemm, E.; Kaskel, S. Solution infiltration of palladium into MOF-5: Synthesis, physisorption and catalytic properties. J. Mater. Chem. 2007, 17, 3827–3832. [Google Scholar] [CrossRef]
- Johnsen, R.E.; Jensen, P.B.; Norby, P.; Vegge, T. Temperature- and Pressure-Induced Changes in the Crystal Structure of Sr(NH3)8Cl2. J. Phys. Chem. C 2014, 118, 24349–24356. [Google Scholar] [CrossRef]
- Jacobsen, H.S.; Hansen, H.A.; Andreasen, J.W.; Shi, Q.; Andreasen, A.; Feidenhans’l, R.; Nielsen, M.M.; Ståhl, K.; Vegge, T. Nanoscale structural characterization of Mg(NH3)6Cl2 during NH3 desorption: An in situ small angle X-ray scattering study. Chem. Phys. Lett. 2007, 441, 255–260. [Google Scholar] [CrossRef]
- Helminen, J.; Helenius, J.; Paatero, E.; Turunen, I. Adsorption Equilibria of Ammonia Gas on Inorganic and Organic Sorbents at 298.15 K. J. Chem. Eng. Data 2001, 46, 391–399. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. MOF–graphite oxide nanocomposites: Surface characterization and evaluation as adsorbents of ammonia. J. Mater. Chem. 2009, 19, 6521–6528. [Google Scholar] [CrossRef]
- Doonan, C.J.; Tranchemontagne, D.J.; Glover, T.G.; Hunt, J.R.; Yaghi, O.M. Exceptional ammonia uptake by a covalent organic framework. Nat. Chem. 2010, 2, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Mendoza, B.; Bandosz, T.J. Reactive Adsorption of Ammonia on Cu-Based MOF/Graphene Composites. Langmuir 2010, 26, 15302–15309. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Deng, S. Ammonia adsorption and its effects on framework stability of MOF-5 and MOF-177. J. Colloid Interface Sci. 2010, 348, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.; Doonan, C.J.; Yaghi, O.M. Postsynthetic Modification of a Metal–Organic Framework for Stabilization of a Hemiaminal and Ammonia Uptake. Inorg. Chem. 2011, 50, 6853–6855. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, H.G.W.; da Silva, I.; Briggs, L.; Carter, J.H.; Morris, C.G.; Savage, M.; Easun, T.L.; Manuel, P.; Murray, C.A.; Tang, C.C.; et al. Ammonia Storage by Reversible Host–Guest Site Exchange in a Robust Metal–Organic Framework. Angew. Chem. Int. Ed. 2018, 57, 14778–14781. [Google Scholar] [CrossRef]
- Rieth, A.J.; Dincă, M. Controlled Gas Uptake in Metal–Organic Frameworks with Record Ammonia Sorption. J. Am. Chem. Soc. 2018, 140, 3461–3466. [Google Scholar] [CrossRef]
- Rieth, A.J.; Tulchinsky, Y.; Dincă, M. High and Reversible Ammonia Uptake in Mesoporous Azolate Metal–Organic Frameworks with Open Mn, Co, and Ni Sites. J. Am. Chem. Soc. 2016, 138, 9401–9404. [Google Scholar] [CrossRef]
- Van Humbeck, J.F.; McDonald, T.M.; Jing, X.; Wiers, B.M.; Zhu, G.; Long, J.R. Ammonia Capture in Porous Organic Polymers Densely Functionalized with Brønsted Acid Groups. J. Am. Chem. Soc. 2014, 136, 2432–2440. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Yang, C.; Wang, S.; Yang, J.; Li, J. Antenna-Protected Metal–Organic Squares for Water/Ammonia Uptake with Excellent Stability and Regenerability. ACS Sustain. Chem. Eng. 2017, 5, 5082–5089. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Wang, H.; Chen, Y.; Zhang, M. MOF-74 as an Efficient Catalyst for the Low-Temperature Selective Catalytic Reduction of NOx with NH3. ACS Appl. Mater. Interfaces 2016, 8, 26817–26826. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Z.; Lin, F.; Wang, H.; Wang, Y. Single-atom Automobile Exhaust Catalysts. ChemNanoMat 2020. [Google Scholar] [CrossRef]
- Katz, M.J.; Howarth, A.J.; Moghadam, P.Z.; DeCoste, J.B.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. High volumetric uptake of ammonia using Cu-MOF-74/Cu-CPO-27. Dalton Trans. 2016, 45, 4150–4153. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Friedel, J.; Terfort, A. The oriented and patterned growth of fluorescent metal–organic frameworks onto functionalized surfaces. Beilstein J. Nanotechnol. 2012, 3, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Furukawa, S.; Kondo, M.; Uehara, H.; Sakata, O.; Kitagawa, S. Sequential Functionalization of Porous Coordination Polymer Crystals. Angew. Chem. Int. Ed. 2011, 50, 8057–8061. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Zhou, K.; Kabir, N.A.; Chen, Y.; Ke, X.; Van Tendeloo, G.; Verpoort, F. Tuning metal sites of DABCO MOF for gas purification at ambient conditions. Microporous Mesoporous Mater. 2015, 201, 277–285. [Google Scholar] [CrossRef]
- Peterson, G.W.; Wagner, G.W.; Balboa, A.; Mahle, J.; Sewell, T.; Karwacki, C.J. Ammonia Vapor Removal by Cu3(BTC)2 and Its Characterization by MAS NMR. J. Phys. Chem. C 2009, 113, 13906–13917. [Google Scholar] [CrossRef]
- Eigen, M. Fast elementary steps in chemical reaction mechanisms. Pure Appl. Chem. 1963, 6, 97–116. [Google Scholar] [CrossRef]
- Cao, Z.; Grimaldos Osorio, N.; Cai, X.; Feng, P.; Akhtar, F. Carbon-reinforced MgCl2 composites with high structural stability as robust ammonia carriers for selective catalytic reduction system. J. Environ. Chem. Eng. 2020, 8, 103584. [Google Scholar] [CrossRef]

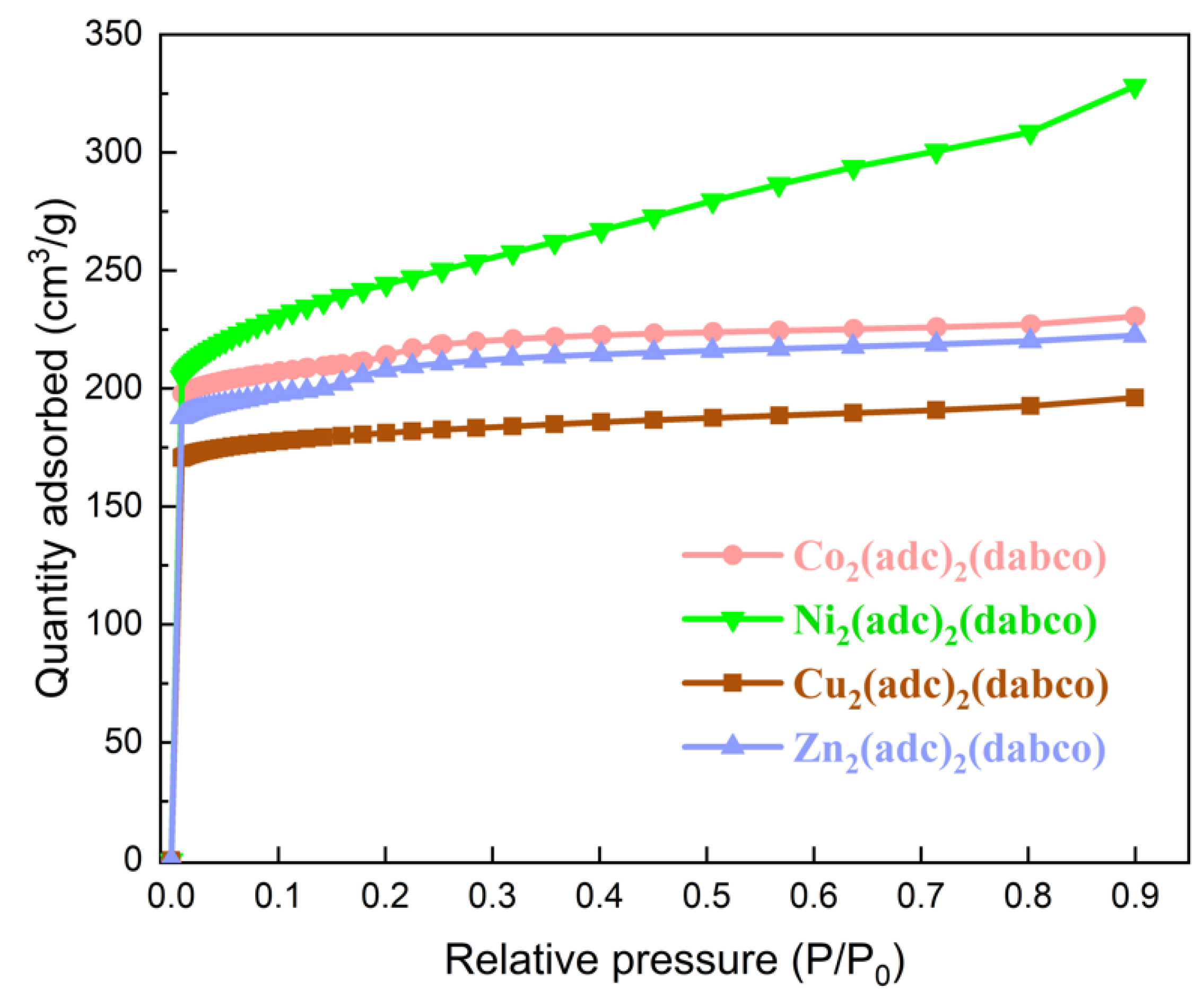
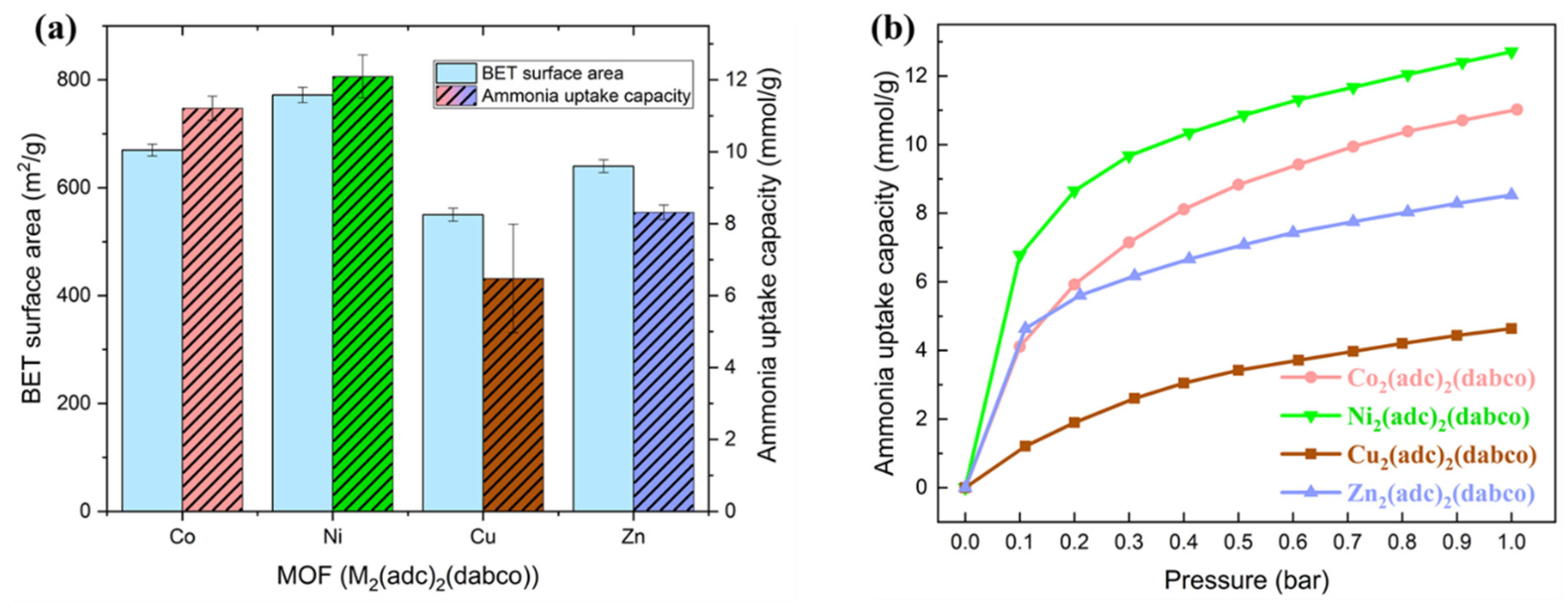
| BET Surface Area (m2/g) | Micropore Area (m2/g) | External Surface Area (m2/g) | Micropore Volume (cm3/g) | |
|---|---|---|---|---|
| Co2(adc)2(dabco) | 670 | 501 | 169 | 0.26 |
| Ni2(adc)2(dabco) | 772 | 472 | 299 | 0.25 |
| Cu2(adc)2(dabco) | 550 | 475 | 74 | 0.25 |
| Zn2(adc)2(dabco) | 640 | 444 | 195 | 0.23 |
| MgCl2 | 3 | 1 | 2 | 0.00 |
| Net Adsorption Time to Reach 90% Ammonia Uptake (min) | Ammonia Desorption Percentage within 10 min [1 bar, 125 °C] | Ammonia Desorption within 10 min [1 bar, 125 °C] (mmol/g) | |
|---|---|---|---|
| Co2(adc)2(dabco) | 2 | 67% | 7 |
| Ni2(adc)2(dabco) | 2 | 57% | 6 |
| Cu2(adc)2(dabco) | 2 | 63% | 6 |
| Zn2(adc)2(dabco) | 2 | 54% | 4 |
| MgCl2 | 11 | 1% | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Landström, K.N.; Akhtar, F. Rapid Ammonia Carriers for SCR Systems Using MOFs [M2(adc)2(dabco)] (M = Co, Ni, Cu, Zn). Catalysts 2020, 10, 1444. https://doi.org/10.3390/catal10121444
Cao Z, Landström KN, Akhtar F. Rapid Ammonia Carriers for SCR Systems Using MOFs [M2(adc)2(dabco)] (M = Co, Ni, Cu, Zn). Catalysts. 2020; 10(12):1444. https://doi.org/10.3390/catal10121444
Chicago/Turabian StyleCao, Zhejian, Kritika Narang Landström, and Farid Akhtar. 2020. "Rapid Ammonia Carriers for SCR Systems Using MOFs [M2(adc)2(dabco)] (M = Co, Ni, Cu, Zn)" Catalysts 10, no. 12: 1444. https://doi.org/10.3390/catal10121444
APA StyleCao, Z., Landström, K. N., & Akhtar, F. (2020). Rapid Ammonia Carriers for SCR Systems Using MOFs [M2(adc)2(dabco)] (M = Co, Ni, Cu, Zn). Catalysts, 10(12), 1444. https://doi.org/10.3390/catal10121444





