Two Scenarios of Dechlorination of the Chlorinated Hydrocarbons over Nickel-Alumina Catalyst
Abstract
1. Introduction
2. Results
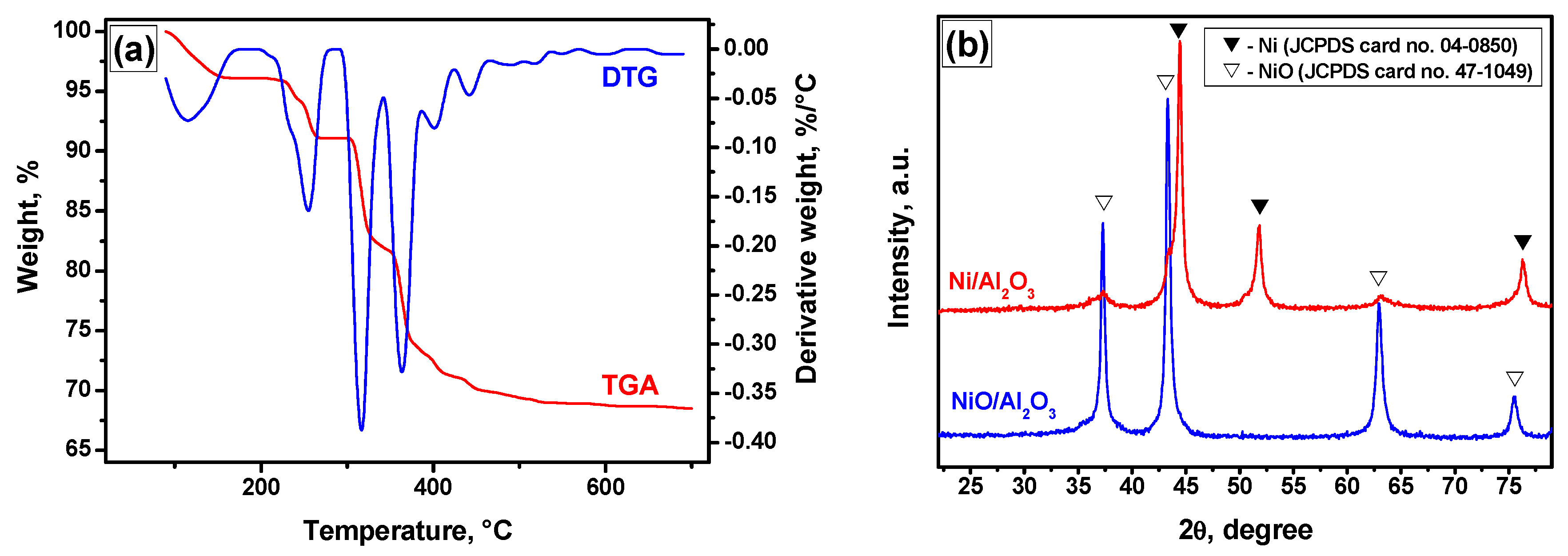
2.1. Reduction Behavior of the NiO/Al2O3 Precursor
2.2. The Features of Nickel Chloride Interaction with Hydrogen
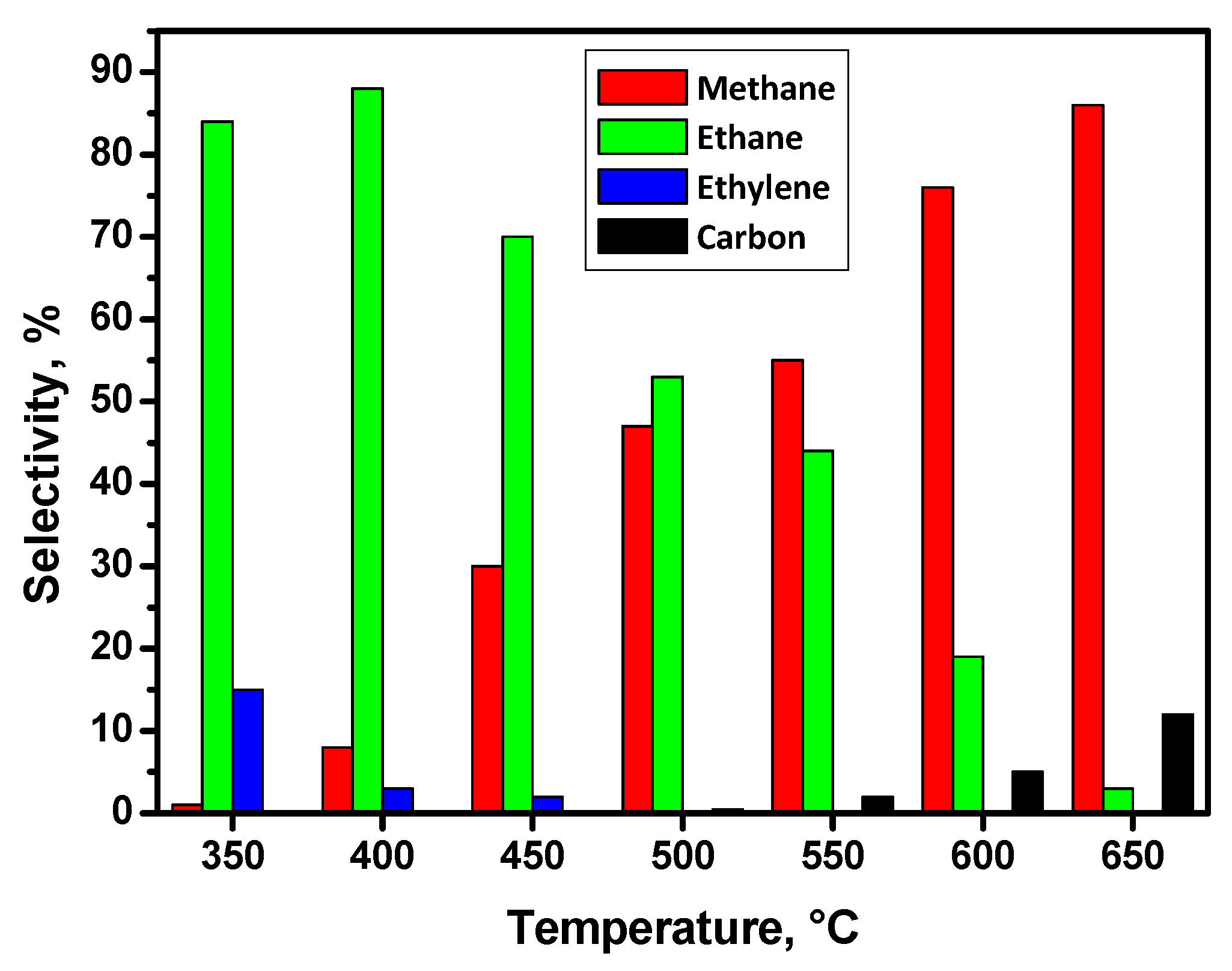
2.3. (Hydro)Dechlorination of C2H4Cl2
2.4. Hydrodechlorination of CHCl3
2.5. (Hydro)Dechlorination of C6H5Cl
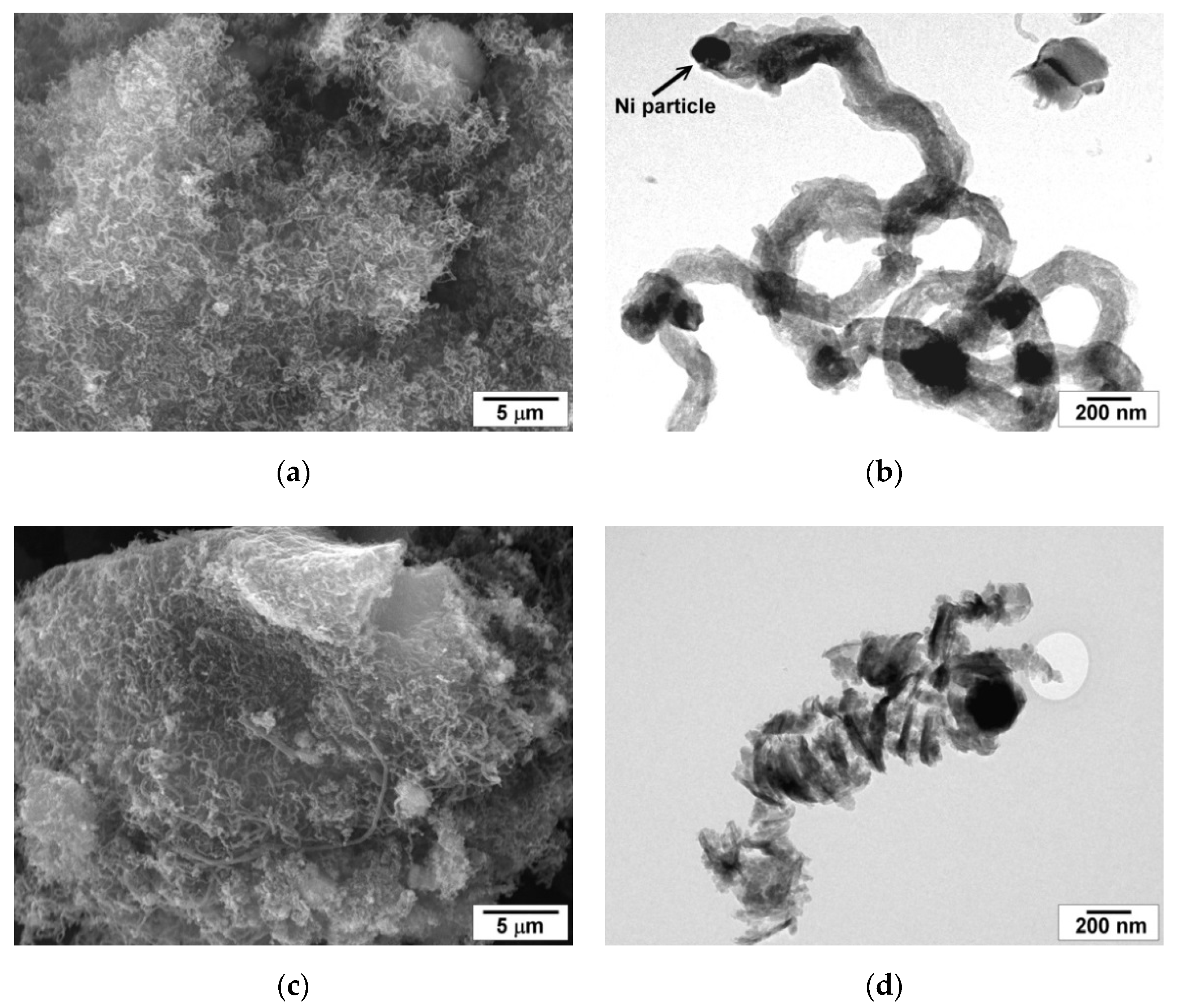
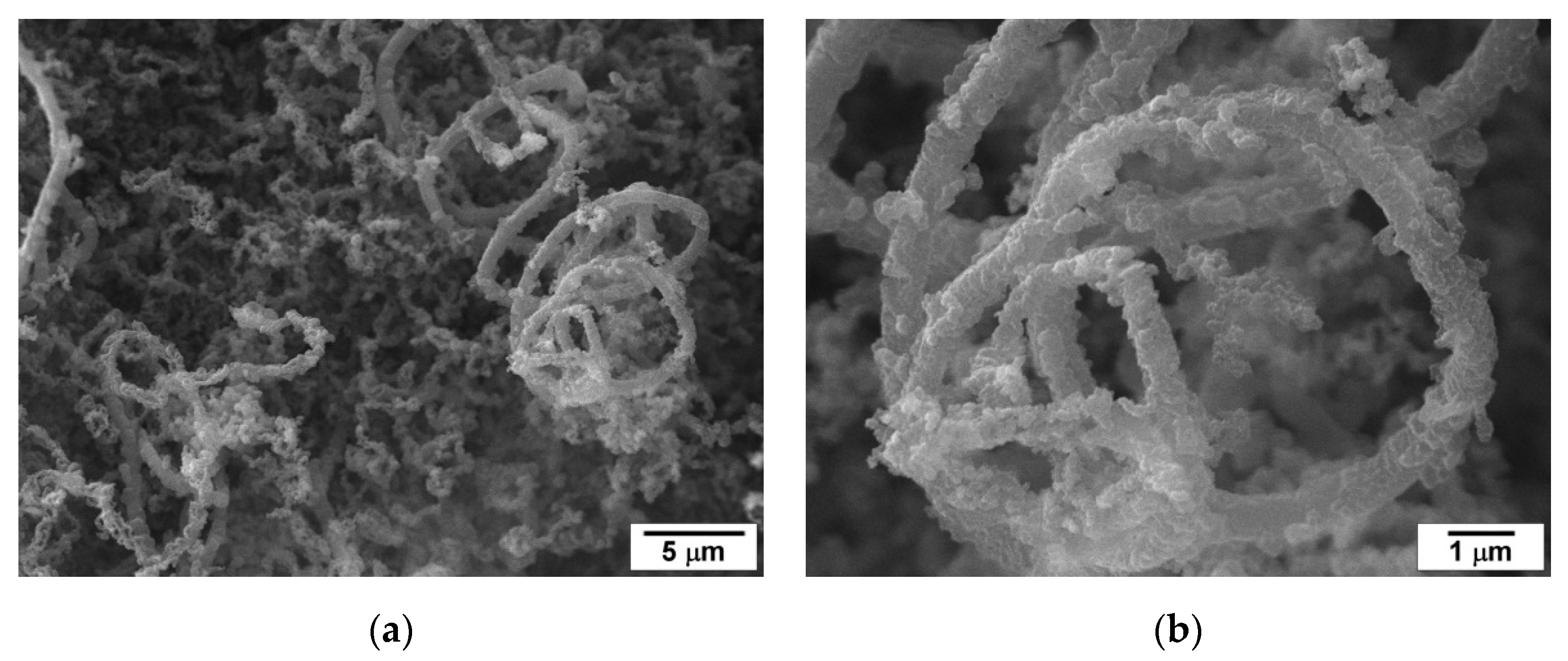
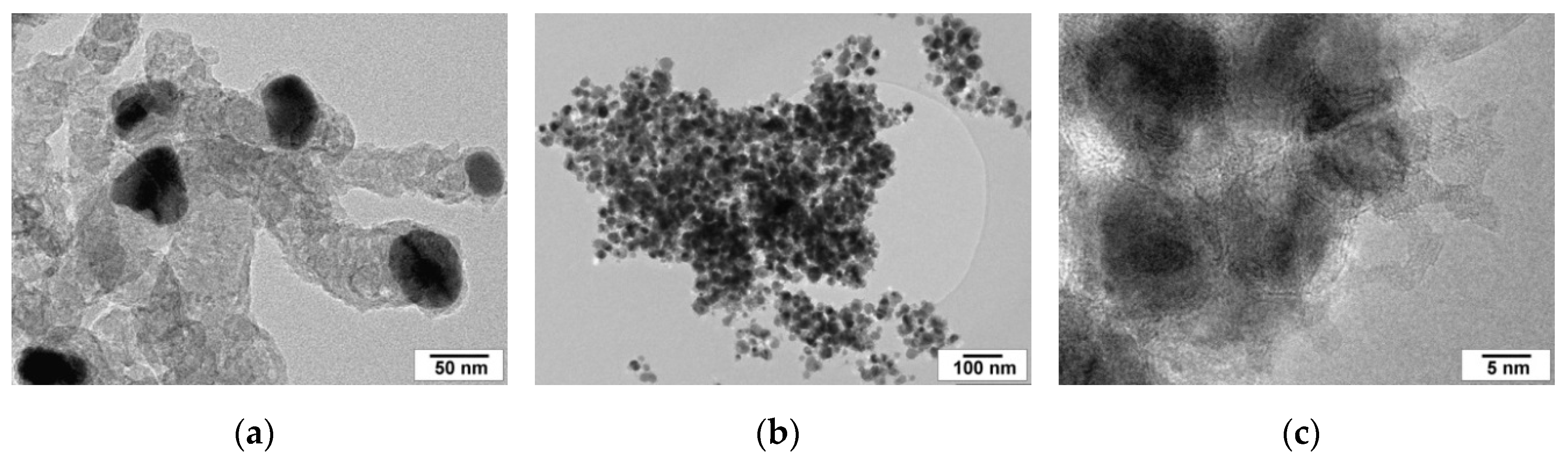
2.6. Structure and Morphology of the Carbonaceous Deposits
3. Discussion
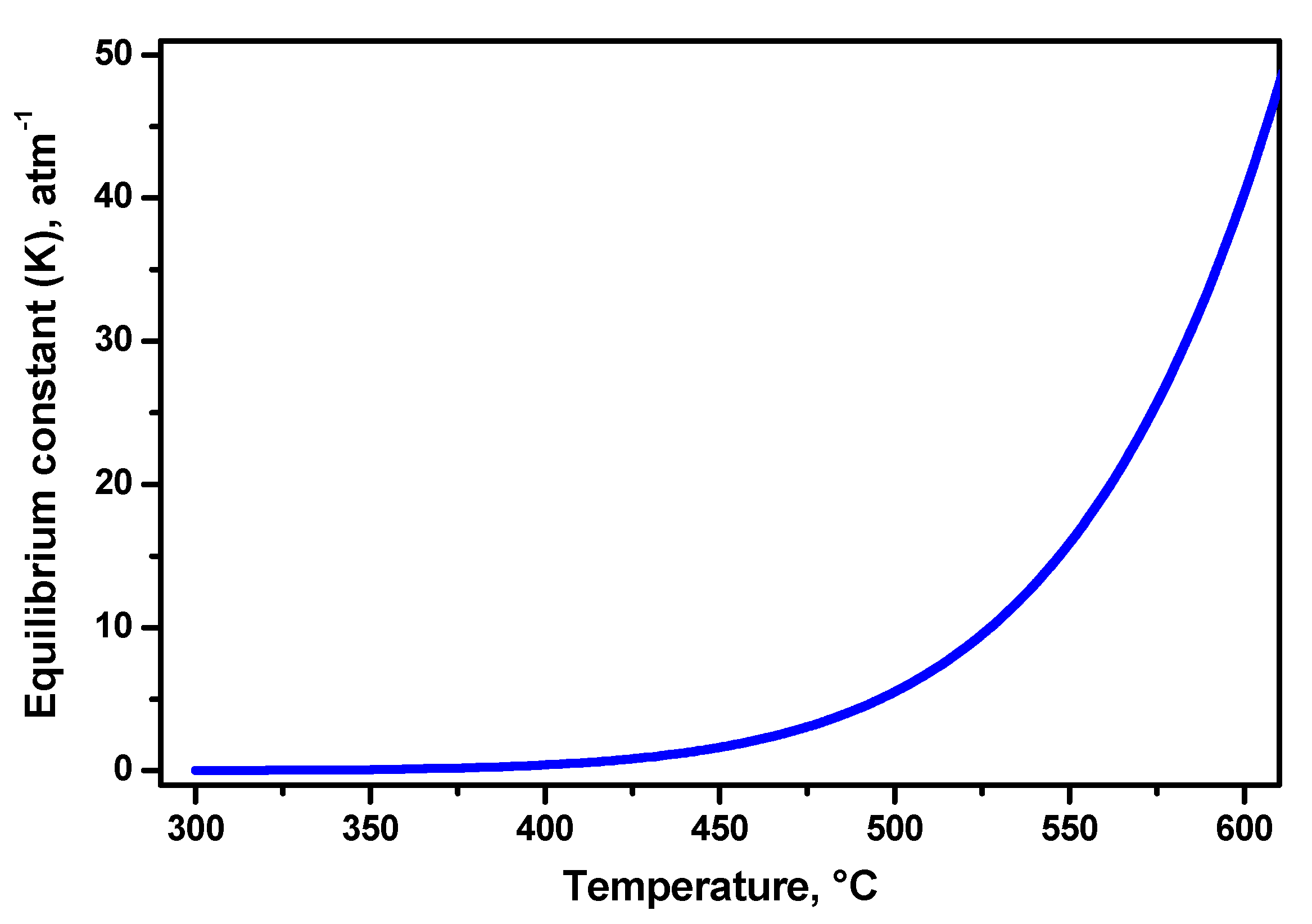
3.1. Path (i) Catalyst Deactivation via the Formation of the Nickel Chloride
3.2. Path (ii) Catalytic Hydrodechlorination
3.3. Path (iii) Catalytic Pyrolysis via the “Carbide Cycle” Mechanism
4. Materials and Methods
4.1. Preparation of Ni/Al2O3 Catalyst
4.2. Processing of DCE, CLF and CB on Ni/Al2O3 Catalyst
4.3. Characterization of Catalyst and Carbonaceous Products
5. Conclusions
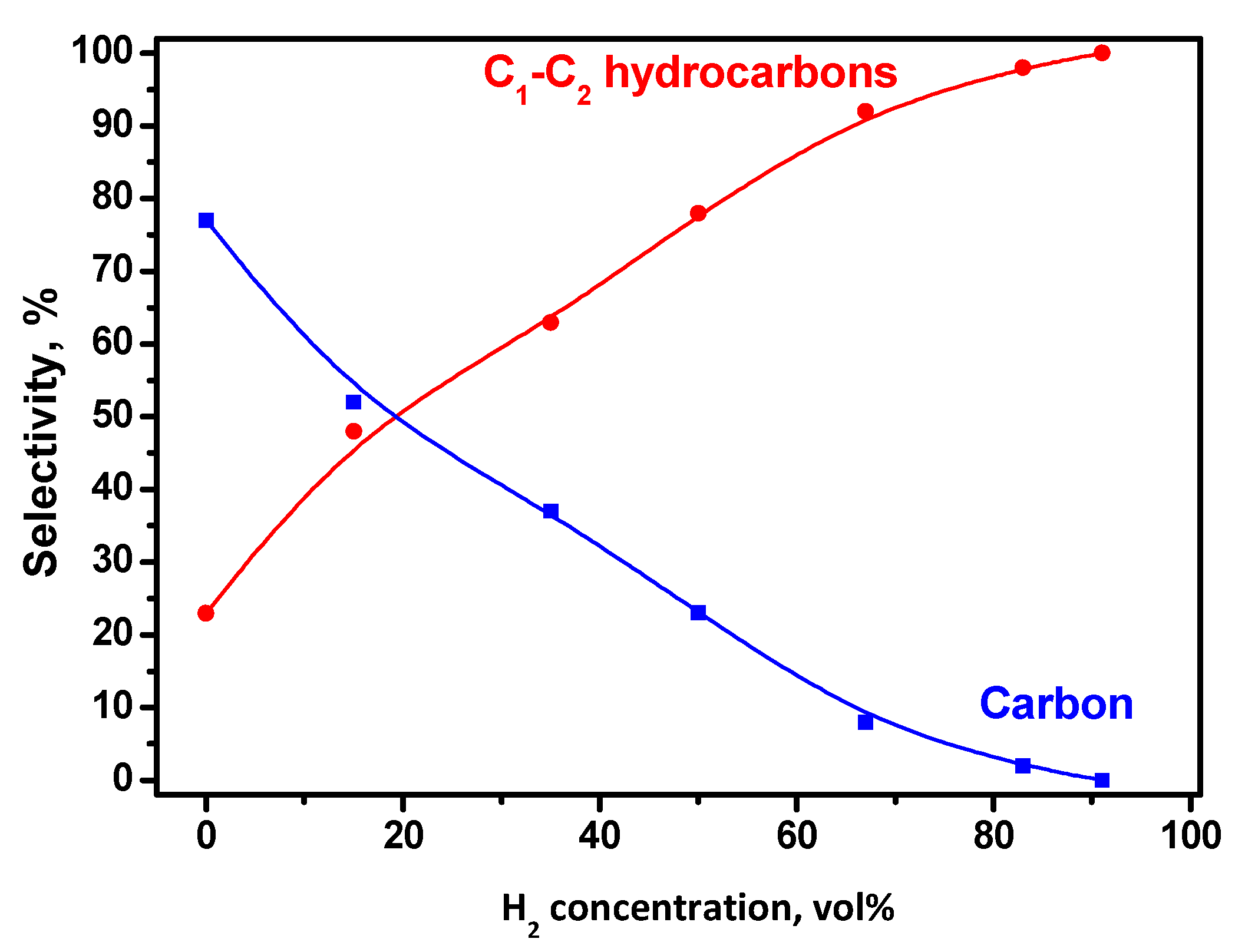
- Catalytic hydrodechlorination regime. It implies the replacement of C-Cl bonds with C-H or C-C bonds in the ClHC molecule. This route is characterized by a high selectivity for hydrocarbons (including olefins). It prevails in a region of low and moderate temperatures at a significant excess of hydrogen in the reaction mixture.
- Catalytic pyrolysis regime. It proceeds via the “carbide cycle” mechanism. The complete destruction of all bonds in a ClHC molecule leads to the formation of nanostructured carbon as the main product. This route is effective at high temperatures (above 450 °C) without hydrogen addition (for ClHCs with an intramolecular ratio [H]:[Cl] > 1). The appearance of hydrogen in the reaction mixture (or reaction products) leads to partial gasification of the deposited carbon with the formation of methane.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Rivas, B.; López-Fonseca, R.; González-Velasco, J.R.; Gutiérrez-Ortiz, M.A. On the mechanism of the catalytic destruction of 1,2-dichloroethane over Ce/Zr mixed oxide catalysts. J. Mol. Catal. A Chem. 2007, 278, 181–188. [Google Scholar] [CrossRef]
- Barrabés, N.; Föttinger, K.; Dafinov, A.; Medina, F.; Rupprechter, G.; Llorca, J.; Sueiras, J. Study of Pt–CeO2 interaction and the effect in the selective hydrodechlorination of trichloroethylene. Appl. Catal. B Environ. 2009, 87, 84–91. [Google Scholar] [CrossRef]
- Garetto, T.F.; Vignatti, C.; Borgna, A.; Monzón, A. Deactivation and regeneration of Pt/Al2O3 catalysts during the hydrodechlorination of carbon tetrachloride. Appl. Catal. B Environ. 2009, 87, 211–219. [Google Scholar] [CrossRef]
- Meshesha, B.; Chimentao, R.J.; Medina, F.; Sueiras, J.; Cesteros, Y.; Salagre, P.; Figueras, F. Catalytic hydrodechlorination of 1,2,4-trichlorobenzene over Pd/Mg(Al)O catalysts. Appl. Catal. B Environ. 2009, 87, 70–77. [Google Scholar] [CrossRef]
- Huang, Q.; Xue, X.; Zhou, R. Decomposition of 1,2-dichloroethane over CeO2 modified USY zeolite catalysts: Effect of acidity and redox property on the catalytic behavior. J. Hazard. Mater. 2010, 183, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Sotowa, C.; Watanabe, Y.; Yatsunami, S.; Korai, Y.; Mochida, I. Catalytic dehydrochlorination of 1,2-dichloroethane into vinyl chloride over polyacrylonitrile-based active carbon fiber. Appl. Catal. A Gen. 1999, 180, 317–323. [Google Scholar] [CrossRef]
- Gwinn, M.R.; Johns, D.O.; Bateson, T.F.; Guyton, K.Z. A review of the genotoxicity of 1,2-dichloroethane (EDC). Mutat. Res. Mutat. Res. 2011, 727, 42–53. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, J.; Wang, W.; Wan, H.; Xu, Z.; Zheng, S.; Zhu, D. Enhanced selective hydrodechlorination of 1,2-dichloroethane to ethylene on Pt–Ag/TiO2 catalysts prepared by sequential photodeposition. Appl. Catal. B Environ. 2012, 125, 172–179. [Google Scholar] [CrossRef]
- Flid, M.; Kartashov, L.; Treger, Y. Theoretical and Applied Aspects of Hydrodechlorination Processes—Catalysts and Technologies. Catalysts 2020, 10, 216. [Google Scholar] [CrossRef]
- Meyer, R.J.; Kim, D.I.; Allen, D.T.; Jo, J.H. Catalytic hydrodechlorination of 1,3-dichloropropene. Chem. Eng. Sci. 1999, 54, 3627–3634. [Google Scholar] [CrossRef]
- Ordóñez, S.; Sastre, H.; Díez, F.V. Hydrodechlorination of aliphatic organochlorinated compounds over commercial hydrogenation catalysts. Appl. Catal. B Environ. 2000, 25, 49–58. [Google Scholar] [CrossRef]
- Heinrichs, B. Palladium–silver sol–gel catalysts for selective hydrodechlorination of 1,2-dichloroethane into ethylene IV. Deactivation mechanism and regeneration. J. Catal. 2003, 220, 215–225. [Google Scholar] [CrossRef]
- Heinrichs, B.; Schoebrechts, J.-P.; Pirard, J.-P. Palladium–Silver Sol–Gel Catalysts for Selective Hydrodechlorination of 1,2-Dichloroethane into Ethylene. J. Catal. 2001, 200, 309–320. [Google Scholar] [CrossRef]
- Śrębowata, A.; Juszczyk, W.; Kaszkur, Z.; Karpiński, Z. Hydrodechlorination of 1,2-dichloroethane on active carbon supported palladium–nickel catalysts. Catal. Today 2007, 124, 28–35. [Google Scholar] [CrossRef]
- Arevalo-Bastante, A.; Martin-Martinez, M.; Álvarez-Montero, M.A.; Rodríguez, J.J.; Gómez-Sainero, L. Properties of Carbon-supported Precious Metals Catalysts under Reductive Treatment and Their Influence in the Hydrodechlorination of Dichloromethane. Catalysts 2018, 8, 664. [Google Scholar] [CrossRef]
- Liu, S.; Martin-Martinez, M.; Álvarez-Montero, M.A.; Arevalo-Bastante, A.; Rodríguez, J.J.; Gómez-Sainero, L. Recycling of Gas Phase Residual Dichloromethane by Hydrodechlorination: Regeneration of Deactivated Pd/C Catalysts. Catalysts 2019, 9, 733. [Google Scholar] [CrossRef]
- Choi, Y.H.; Lee, W.Y. Effect of Ni loading and calcination temperature on catalyst performance and catalyst deactivation of Ni/SiO2 in the hydrodechlorination of 1,2-dichloropropane into propylene. Catal. Lett. 2000, 67, 155–161. [Google Scholar] [CrossRef]
- Kim, P.; Kim, H.; Joo, J.B.; Kim, W.; Song, I.K.; Yi, J. Effect of nickel precursor on the catalytic performance of Ni/Al2O3 catalysts in the hydrodechlorination of 1,1,2-trichloroethane. J. Mol. Catal. A Chem. 2006, 256, 178–183. [Google Scholar] [CrossRef]
- Chary, K.V.; Rao, P.V.R.; Vishwanathan, V. Synthesis and high performance of ceria supported nickel catalysts for hydrodechlorination reaction. Catal. Commun. 2006, 7, 974–978. [Google Scholar] [CrossRef]
- Morato, A. Conversion under hydrogen of dichlorodifluoromethane and chlorodifluoromethane over nickel catalysts. Appl. Catal. B Environ. 1999, 23, 175–185. [Google Scholar] [CrossRef]
- Śrębowata, A.; Juszczyk, W.; Kaszkur, Z.; Sobczak, J.W.; Kępiński, L.; Karpiński, Z. Hydrodechlorination of 1,2-dichloroethane and dichlorodifluoromethane over Ni/C catalysts: The effect of catalyst carbiding. Appl. Catal. A Gen. 2007, 319, 181–192. [Google Scholar] [CrossRef]
- Liu, L.; Sun, X.; Li, W.; An, Y.; Li, H. Electrochemical hydrodechlorination of perchloroethylene in groundwater on a Ni-doped graphene composite cathode driven by a microbial fuel cell. RSC Adv. 2018, 8, 36142–36149. [Google Scholar] [CrossRef]
- Kamińska, I.I.; Śrębowata, A. Active carbon-supported nickel–palladium catalysts for hydrodechlorination of 1,2-dichloroethane and 1,1,2-trichloroethene. Res. Chem. Intermed. 2015, 41, 9267–9280. [Google Scholar] [CrossRef]
- Golubina, E.V.; Rostovshchikova, T.N.; Lokteva, E.S.; Maslakov, K.I.; Nikolaev, S.; Egorova, T.B.; Gurevich, S.A.; Kozhevin, V.M.; Yavsin, D.A.; Yermakov, A.Y. Chlorobenzene hydrodechlorination on bimetallic catalysts prepared by laser electrodispersion of NiPd alloy. Pure Appl. Chem. 2018, 90, 1685–1701. [Google Scholar] [CrossRef]
- Huang, B.; Qian, W.; Yu, C.; Wang, T.; Zeng, G.; Lei, C. Effective catalytic hydrodechlorination of o-, p- and m-chloronitrobenzene over Ni/Fe nanoparticles: Effects of experimental parameter and molecule structure on the reduction kinetics and mechanisms. Chem. Eng. J. 2016, 306, 607–618. [Google Scholar] [CrossRef]
- Han, Y.; Liu, C.; Horita, J.; Yan, W. Trichloroethene (TCE) hydrodechlorination by Ni Fe nanoparticles: Influence of aqueous anions on catalytic pathways. Chemosphere 2018, 205, 404–413. [Google Scholar] [CrossRef]
- Sahu, R.S.; Li, D.-L.; Andoongc, R. Unveiling the hydrodechlorination of trichloroethylene by reduced graphene oxide supported bimetallic Fe/Ni nanoparticles. Chem. Eng. J. 2018, 334, 30–40. [Google Scholar] [CrossRef]
- Kim, D.I.; Allen, D.T. Catalytic Hydroprocessing of Chlorinated Olefins. Ind. Eng. Chem. Res. 1997, 36, 3019–3026. [Google Scholar] [CrossRef]
- Park, Y.; Kang, T.; Cho, Y.-S.; Kim, P.; Park, J.-C.; Yi, J. Finely-dispersed Ni/Cu catalysts supported on mesoporous silica for the hydrodechlorination of chlorinated hydrocarbons. Adv. Pharmacol. 2003, 146, 637–640. [Google Scholar] [CrossRef]
- Juszczyk, W.; Colmenares, J.C.; Śrębowata, A.; Karpiński, Z. The effect of copper and gold on the catalytic behavior of nickel/alumina catalysts in hydrogen-assisted dechlorination of 1,2-dichloroethane. Catal. Today 2011, 169, 186–191. [Google Scholar] [CrossRef]
- Popov, M.; Bannov, A. Growth of carbon nanofibers by the catalytic decomposition of methane over Ni-Cu/Al2O3 catalyst. Mater. Today Proc. 2020, 31, 489–491. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Chesnokov, V.V.; Buyanov, R.A.; Pakhomov, N.A. Decomposition of Chlorinated Hydrocarbons on Iron-Group Metals. Kinet. Catal. 2001, 42, 543–548. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Chesnokov, V.V.; Buyanov, R.A.; Chuvilin, A. Conditions of Preparation and Structure of Disordered Graphite Filaments Formed upon Decomposition of Chlorine Derivatives of Hydrocarbons on Ni- and Co-Containing Catalysts. Dokl. Phys. Chem. 2002, 386, 207–210. [Google Scholar] [CrossRef]
- Nieto-Márquez, A.; Valverde, J.; Keane, M.A. Catalytic growth of structured carbon from chloro-hydrocarbons. Appl. Catal. A Gen. 2007, 332, 237–246. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Vedyagin, A.A.; Bauman, Y.I.; Shubin, Y.V.; Buyanov, R.A. Synthesis of Carbon Nanofibers via Catalytic Chemical Vapor Deposition of Halogenated Hydrocarbons. In Carbon Nanofibers: Synthesis, Applications and Performance; Chang-Seop, L., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2018; pp. 77–181. [Google Scholar]
- Bauman, Y.I.; Mishakov, I.V.; Vedyagin, A.A.; Dmitriev, S.V.; Mel’Gunov, M.S.; Buyanov, R.A. Processing of organochlorine waste components on bulk metal catalysts. Catal. Ind. 2012, 4, 261–266. [Google Scholar] [CrossRef]
- Lin, W.-H.; Lee, T.-T.; Li, Y.-Y. Chlorine effect on formation of turbostratic carbon nanofibers by a mixture of 1,2-dichloroethane and ethanol. J. Taiwan Inst. Chem. Eng. 2014, 45, 1883–1891. [Google Scholar] [CrossRef]
- Maboya, W.K.; Coville, N.J.; Mhlanga, S.D. The synthesis of carbon nanomaterials using chlorinated hydrocarbons over a Fe-Co/CaCO3 catalyst. South Afr. J. Chem. 2016, 69, 15–26. [Google Scholar] [CrossRef]
- Chesnokov, V.; Buyanov, R.; Zaikovskii, V.I. Stages of Filamentary Carbon Growth from Hydrocarbons on Nickelcontaining Catalysts and Causes of their Deactivation. Eurasian Chem. J. 2007, 5, 253. [Google Scholar] [CrossRef]
- Mishakov, I.; Chesnokov, V.; Buyanov, R.; Chuvilin, A. Morphology and structure of carbon resulting from decomposition of chlorohydrocarbons on nickel and cobalt containing catalysts. React. Kinet. Catal. Lett. 2002, 76, 361–367. [Google Scholar] [CrossRef]
- Estelle, J.; Salagre, P.; Cesteros, Y.; Serra, M.; Medina, F.; Sueiras, J.E. Comparative study of the morphology and surface properties of nickel oxide prepared from different precursors. Solid State Ionics 2003, 156, 233–243. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.-W. Temperature-programmed-reduction studies of nickel oxide/alumina catalysts: Effects of the preparation method. Thermochim. Acta 1995, 256, 457–465. [Google Scholar] [CrossRef]
- Janković, B.; Adnađević, B.; Mentus, S. The kinetic study of temperature-programmed reduction of nickel oxide in hydrogen atmosphere. Chem. Eng. Sci. 2008, 63, 567–575. [Google Scholar] [CrossRef]
- Baran, R.; Kaminska, I.; Śrębowata, A.; Dzwigaj, S. Selective hydrodechlorination of 1,2-dichloroethane on NiSiBEA zeolite catalyst: Influence of the preparation procedure on a high dispersion of Ni centers. Microporous Mesoporous Mater. 2013, 169, 120–127. [Google Scholar] [CrossRef]
- Egawa, C.; Osawa, S.; Oki, S. Ethylene hydrogenation on Ni(100) surface. Surf. Sci. 2003, 529, 349–358. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Buyanov, R.A. The formation of carbon filaments upon decomposition of hydrocarbons catalysed by iron subgroup metals and their alloys. Russ. Chem. Rev. 2000, 69, 623–638. [Google Scholar] [CrossRef]
- Dasaeva, G.S.; Flid, M.R.; Dmitriev, Y.K.; Kartashov, L.M.; Treger, Y.A. Catalytic hydrodechlorination of 1,2-dichloroethane. Russ. Chem. Ind. 2000, 3, 49–54. [Google Scholar]
- Dasaeva, G.S.; Flid, M.R.; Dmitriev, Y.K.; Kartashov, L.M.; Treger, Y.A. Catalytic hydrodechlorination of 1,1,2-trichloroethane. Russ. Chem. Ind. 2000, 4, 44–46. [Google Scholar]
- Chambers, A.; Baker, R.T.K. Influence of Chlorine on the Decomposition of Ethylene over Iron and Cobalt Particles. J. Phys. Chem. B 1997, 101, 1621–1630. [Google Scholar] [CrossRef]
- Bauman, Y.I.; Mishakov, I.V.; Rudneva, Y.V.; Popov, A.A.; Rieder, D.; Korneev, D.V.; Serkova, A.N.; Shubin, Y.V.; Vedyagin, A.A. Catalytic synthesis of segmented carbon filaments via decomposition of chlorinated hydrocarbons on Ni-Pt alloys. Catal. Today 2020, 348, 102–110. [Google Scholar] [CrossRef]
- Kovalchuk, V.I.; D’Itri, J.L. Catalytic chemistry of chloro- and chlorofluorocarbon dehalogenation: From macroscopic observations to molecular level understanding. Appl. Catal. A Gen. 2004, 271, 13–25. [Google Scholar] [CrossRef]
- Pirard, S.L.; Pirard, J.-P.; Heyen, G.; Schoebrechts, J.-P.; Heinrichs, B. Experimental procedure and statistical data treatment for the kinetic study of selective hydrodechlorination of 1,2-dichloroethane into ethylene over a Pd-Ag sol–gel catalyst. Chem. Eng. J. 2011, 173, 801–812. [Google Scholar] [CrossRef]
- Bauman, Y.I.; Shorstkaya, Y.V.; Mishakov, I.V.; Plyusnin, P.E.; Shubin, Y.V.; Korneev, D.V.; Stoyanovskii, V.O.; Vedyagin, A.A. Catalytic conversion of 1,2-dichloroethane over Ni-Pd system into filamentous carbon material. Catal. Today 2017, 293–294, 23–32. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Bauman, Y.I.; Streltsov, I.A.; Korneev, D.V.; Vinokurova, O.B.; Vedyagin, A.A. The regularities of the formation of carbon nanostructures from hydrocarbons based on the composition of the reaction mixture. Resour. Technol. 2016, 2, 61–67. [Google Scholar] [CrossRef]
- Zhou, K. Carbon Nanomaterials; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781351123587. [Google Scholar] [CrossRef]

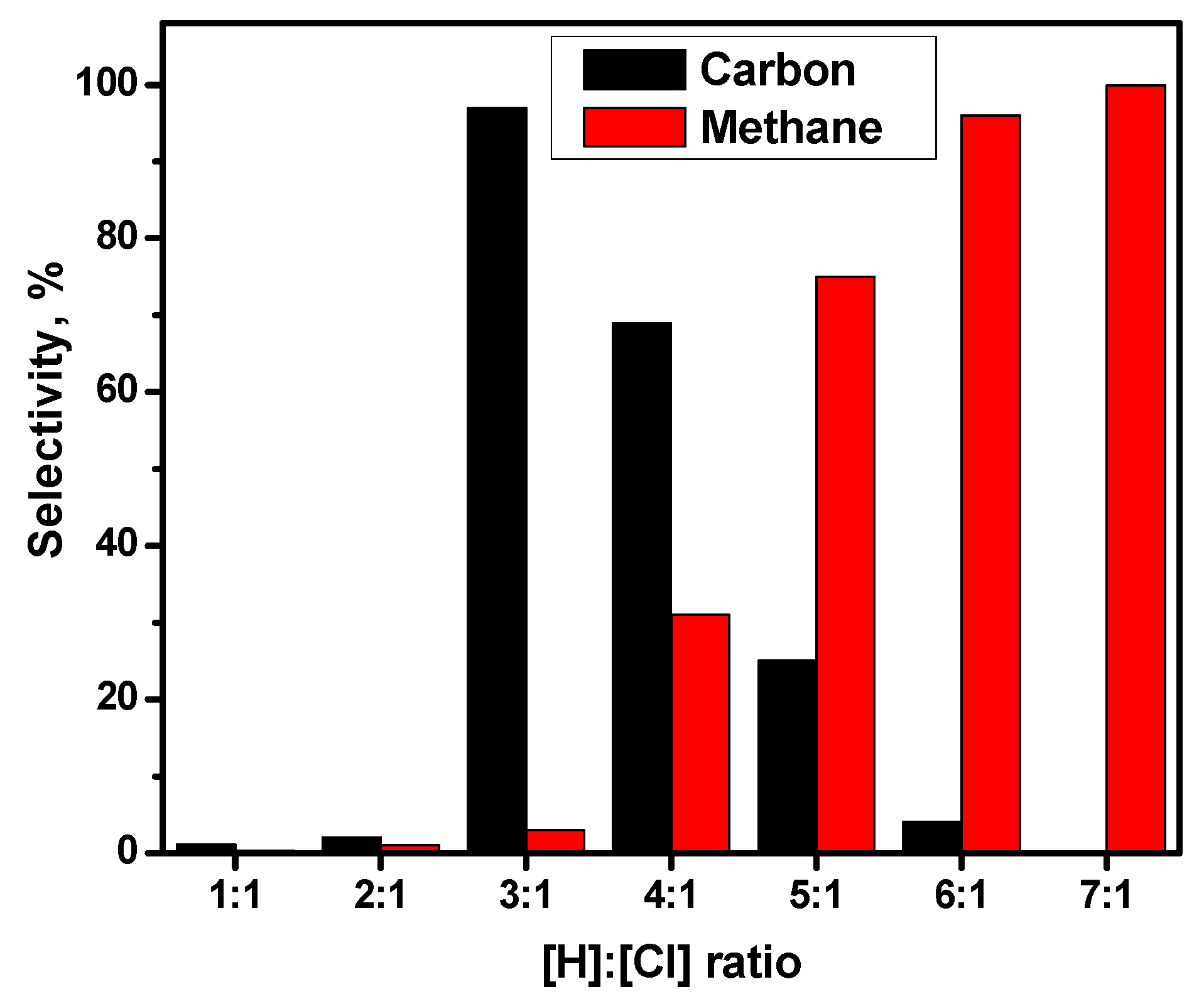

| Temperature, °C | 1,2-DCE Conversion, % | Selectivity, % | ||
|---|---|---|---|---|
| C | CH4 | C2H4 + C2H6 | ||
| 400 | 98.0 | 0 | 8.1 | 91.9 |
| 500 | 99.6 | 0.5 | 47.3 | 52.2 |
| 600 | 99.4 | 5.0 | 76.1 | 18.9 |
| Composition of the Reaction Mixture | Temperature, °C | CB Conversion, % | Selectivity, % | ||||
|---|---|---|---|---|---|---|---|
| Ar | H2 | C6H5Cl | C | CH4 | C6H6 | ||
| 8 | 1 | 1 | 500 | 96 | 88.5 | 8.5 | 3.0 |
| 8 | 1 | 1 | 350 | 88 | 0 | traces | ~100 |
| - | 9 | 1 | 350 | 100 | 0 | 0.2 | 99.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishakov, I.V.; Vedyagin, A.A.; Bauman, Y.I.; Potylitsyna, A.R.; Kadtsyna, A.S.; Chesnokov, V.V.; Nalivaiko, A.Y.; Gromov, A.A.; Buyanov, R.A. Two Scenarios of Dechlorination of the Chlorinated Hydrocarbons over Nickel-Alumina Catalyst. Catalysts 2020, 10, 1446. https://doi.org/10.3390/catal10121446
Mishakov IV, Vedyagin AA, Bauman YI, Potylitsyna AR, Kadtsyna AS, Chesnokov VV, Nalivaiko AY, Gromov AA, Buyanov RA. Two Scenarios of Dechlorination of the Chlorinated Hydrocarbons over Nickel-Alumina Catalyst. Catalysts. 2020; 10(12):1446. https://doi.org/10.3390/catal10121446
Chicago/Turabian StyleMishakov, Ilya V., Aleksey A. Vedyagin, Yury I. Bauman, Arina R. Potylitsyna, Anastasiya S. Kadtsyna, Vladimir V. Chesnokov, Anton Yu. Nalivaiko, Alexander A. Gromov, and Roman A. Buyanov. 2020. "Two Scenarios of Dechlorination of the Chlorinated Hydrocarbons over Nickel-Alumina Catalyst" Catalysts 10, no. 12: 1446. https://doi.org/10.3390/catal10121446
APA StyleMishakov, I. V., Vedyagin, A. A., Bauman, Y. I., Potylitsyna, A. R., Kadtsyna, A. S., Chesnokov, V. V., Nalivaiko, A. Y., Gromov, A. A., & Buyanov, R. A. (2020). Two Scenarios of Dechlorination of the Chlorinated Hydrocarbons over Nickel-Alumina Catalyst. Catalysts, 10(12), 1446. https://doi.org/10.3390/catal10121446









