The Fatty-Acid Hydratase Activity of the Most Common Probiotic Microorganisms
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials and General Methods
3.2. Analytical Methods and Characterization of the Products Deriving from the Biotransformation Experiments
3.2.1. Instruments and Analytic Condition
3.2.2. GC-MS Analyses
3.2.3. Determination of the Absolute Configuration and of the Optical Purity of the HFAs
3.3. Microorganisms and Biotransformation Experiments
3.3.1. Microorganisms and Media
3.3.2. General Procedure for the Biotransformation Experiments Using Anaerobic Flasks
3.3.3. General Procedure for the Biotransformation Experiments Using Aerobic Flasks
3.3.4. General Procedure for Preparative Biotransformations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- George Kerry, R.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatti-Kaul, R.; Chen, L.; Dishisha, T.; Enshasy, H.E. Lactic acid bacteria: From starter cultures to producers of chemicals. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Huang, J.; Sun, L.; Xu, F.; Zhang, W.; Zhan, J. An efficient process for co-production of γ-aminobutyric acid and probiotic Bacillus subtilis cells. Food Sci. Biotechnol. 2019, 28, 155–163. [Google Scholar] [CrossRef]
- Carević, M.; Vukašinović-Sekulić, M.; Ćorović, M.; Rogniaux, H.; Ropartz, D.; Veličković, D.; Bezbradica, D. Evaluation of β-galactosidase from Lactobacillus acidophilus as biocatalyst for galacto-oligosaccharides synthesis: Product structural characterization and enzyme immobilization. J. Biosci. Bioeng. 2018, 126, 697–704. [Google Scholar] [CrossRef]
- Gaya, P.; Peirotén, Á.; Landete, J.M. Transformation of plant isoflavones into bioactive isoflavones by lactic acid bacteria and bifidobacteria. J. Funct. Foods 2017, 39, 198–205. [Google Scholar] [CrossRef]
- Cheng, J.-R.; Liu, X.-M.; Chen, Z.-Y.; Zhang, Y.-S.; Zhang, Y.-H. Mulberry anthocyanin biotransformation by intestinal probiotics. Food Chem. 2016, 213, 721–727. [Google Scholar] [CrossRef]
- Basholli-Salihu, M.; Schuster, R.; Mulla, D.; Praznik, W.; Viernstein, H.; Mueller, M. Bioconversion of piceid to resveratrol by selected probiotic cell extracts. Bioprocess. Biosyst. Eng. 2016, 39, 1879–1885. [Google Scholar] [CrossRef] [Green Version]
- Gobinath, D.; Prapulla, S.G. Permeabilized probiotic Lactobacillus plantarum as a source of β-galactosidase for the synthesis of prebiotic galactooligosaccharides. Biotechnol. Lett. 2014, 36, 153–157. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shafei, K.; Sharaf, O.M.; Effat, B.A.; Asem, F.M.; El-Aasar, M. Screening of some potentially probiotic lactic acid bacteria for their ability to synthesis conjugated linoleic acid. Int. J. Dairy Technol. 2010, 63, 62–69. [Google Scholar] [CrossRef]
- Serra, S.; De Simeis, D. Use of Lactobacillus rhamnosus (ATCC 53103) as Whole-Cell Biocatalyst for the Regio- and Stereoselective Hydration of Oleic, Linoleic, and Linolenic Acid. Catalysts 2018, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Wallen, L.L.; Benedict, R.G.; Jackson, R.W. The microbiological production of 10-hydroxystearic acid from oleic acid. Arch. Biochem. Biophys. 1962, 99, 249–253. [Google Scholar] [CrossRef]
- Bevers, L.E.; Pinkse, M.W.H.; Verhaert, P.D.E.M.; Hagen, W.R. Oleate hydratase catalyzes the hydration of a nonactivated carbon-carbon bond. J. Bacteriol. 2009, 191, 5010–5012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engleder, M.; Pichler, H. On the current role of hydratases in biocatalysis. Appl. Microbiol. Biotechnol. 2018, 102, 5841–5858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demming, R.M.; Fischer, M.-P.; Schmid, J.; Hauer, B. (de)hydratases—recent developments and future perspectives. Curr. Opin. Chem. Biol. 2018, 43, 43–50. [Google Scholar] [CrossRef]
- Hiseni, A.; Arends, I.W.C.E.; Otten, L.G. New cofactor-independent hydration biocatalysts: Structural, biochemical, and biocatalytic characteristics of carotenoid and oleate hydratases. ChemCatChem 2015, 7, 29–37. [Google Scholar] [CrossRef]
- Kim, K.-R.; Oh, D.-K. Production of hydroxy fatty acids by microbial fatty acid-hydroxylation enzymes. Biotechnol. Adv. 2013, 31, 1473–1485. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, X. Production of long-chain hydroxy fatty acids by microbial conversion. Appl. Microbiol. Biotechnol. 2013, 97, 3323–3331. [Google Scholar] [CrossRef]
- Lu, W.; Ness, J.E.; Xie, W.; Zhang, X.; Minshull, J.; Gross, R.A. Biosynthesis of monomers for plastics from renewable oils. J. Am. Chem. Soc. 2010, 132, 15451–15455. [Google Scholar] [CrossRef]
- Serra, S.; Fuganti, C.; Brenna, E. Biocatalytic preparation of natural flavours and fragrances. Trends Biotechnol. 2005, 23, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, J.; Kishino, S.; Yonejima, Y. Intestinal tract-protecting agent containing hydroxylated fatty acid. U.S. Patent 2016/0000739 A1, 2017. [Google Scholar]
- Schütz, R.; Rawlings, A.V.; Wandeler, E.; Jackson, E.; Trevisan, S.; Monneuse, J.-M.; Bendik, I.; Massironi, M.; Imfeld, D. Bio-derived hydroxystearic acid ameliorates skin age spots and conspicuous pores. Int. J. Cosmetic Sci. 2019, 41, 240–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolar, M.J.; Konduri, S.; Chang, T.; Wang, H.; McNerlin, C.; Ohlsson, L.; Härröd, M.; Siegel, D.; Saghatelian, A. Linoleic acid esters of hydroxy linoleic acids are anti-inflammatory lipids found in plants and mammals. J. Biol. Chem. 2019, 294, 10698–10707. [Google Scholar] [CrossRef] [PubMed]
- Calonghi, N.; Boga, C.; Telese, D.; Bordoni, S.; Sartor, G.; Torsello, C.; Micheletti, G. Synthesis of 9-hydroxystearic acid derivatives and their antiproliferative activity on HT 29 cancer cells. Molecules 2019, 24, 3714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, N.; Cai, P.; Wu, D.; Pan, Y.; Curtis, J.M.; Gänzle, M.G. High-speed counter-current chromatography (hsccc) purification of antifungal hydroxy unsaturated fatty acids from plant-seed oil and Lactobacillus cultures. J. Agr. Food Chem. 2017, 65, 11229–11236. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Takahashi, T.; Murayama, S.Y.; Kawaguchi, M.; Matsuo, K.; Nakamura, M. Protective efficacy of a hydroxy fatty acid against gastric Helicobacter infections. Helicobacter 2017, 22, e12430. [Google Scholar] [CrossRef]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. BioEssays 2016, 38, 1167–1176. [Google Scholar] [CrossRef]
- Kishino, S.; Takeuchi, M.; Park, S.-B.; Hirata, A.; Kitamura, N.; Kunisawa, J.; Kiyono, H.; Iwamoto, R.; Isobe, Y.; Arita, M.; et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc. Natl. Acad. Sci. USA 2013, 110, 17808–17813. [Google Scholar] [CrossRef] [Green Version]
- Kang, W.-R.; Park, C.-S.; Shin, K.-C.; Kim, K.-R.; Oh, D.-K. 13-hydroxy-9Z,15Z-octadecadienoic acid production by recombinant cells expressing Lactobacillus acidophilus 13-hydratase. J. Am. Oil Chem. Soc. 2016, 93, 649–655. [Google Scholar] [CrossRef]
- Davis, E.N.; Wallen, L.L.; Goodwin, J.C.; Rohwedder, W.K.; Rhodes, R.A. Microbial hydration of cis-9-alkenoic acids. Lipids 1969, 4, 356–362. [Google Scholar] [CrossRef]
- Hou, C.T. Conversion of linoleic acid to 10-hydroxy-12(Z)-octadecenoic acid by Flavobacterium sp. (NRRL B-14859). J. Am. Oil Chem. Soc. 1994, 71, 975–978. [Google Scholar] [CrossRef]
- Kaneshiro, T.; Huang, J.-K.; Weisleder, D.; Bagby, M.O. 10(R)-hydroxystearic acid production by a novel microbe, NRRL B-14797, isolated from compost. J. Ind. Microbiol. 1994, 13, 351–355. [Google Scholar] [CrossRef]
- Hudson, J.A.; MacKenzie, C.A.M.; Joblin, K.N. Conversion of oleic acid to 10-hydroxystearic acid by two species of ruminal bacteria. Appl. Microbiol. Biotechnol. 1995, 44, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Morvan, B.; Joblin, K.N. Hydration of oleic acid by Enterococcus gallinarum, Pediococcus acidilactici and Lactobacillus sp. Isolated from the rumen. Anaerobe 1999, 5, 605–611. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, M.S.; Chung, C.H.; Kim, C.T.; Kim, Y.S.; Kyung, K.H. Conversion of unsaturated food fatty acids into hydroxy fatty acids by lactic acid bacteria. J. Microbiol. Biotechnol. 2003, 13, 360–365. [Google Scholar]
- Kishimoto, N.; Yamamoto, I.; Toraishi, K.; Yoshioka, S.; Saito, K.; Masuda, H.; Fujita, T. Two distinct pathways for the formation of hydroxy fa from linoleic acid by lactic acid bacteria. Lipids 2003, 38, 1269–1274. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Song, Y.; Chen, Y.Q.; Zhang, H.; Chen, W. Myosin-cross-reactive antigens from four different lactic acid bacteria are fatty acid hydratases. Biotechnol. Lett. 2013, 35, 75–81. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, S.-H.; Kim, K.-R.; Park, J.-B.; Oh, D.-K. Production of 13S-hydroxy-9(Z)-octadecenoic acid from linoleic acid by whole recombinant cells expressing linoleate 13-hydratase from Lactobacillus acidophilus. J. Biotechnol. 2015, 208, 1–10. [Google Scholar] [CrossRef]
- Kim, K.-R.; Oh, H.-J.; Park, C.-S.; Hong, S.-H.; Park, J.-Y.; Oh, D.-K. Unveiling of novel regio-selective fatty acid double bond hydratases from Lactobacillus acidophilus involved in the selective oxyfunctionalization of mono- and di-hydroxy fatty acids. Biotechnol. Bioeng. 2015, 112, 2206–2213. [Google Scholar] [CrossRef]
- Hirata, A.; Kishino, S.; Park, S.-B.; Takeuchi, M.; Kitamura, N.; Ogawa, J. A novel unsaturated fatty acid hydratase toward C16 to C22 fatty acids from Lactobacillus acidophilus. J. Lipid Res. 2015, 56, 1340–1350. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Y.; Liang, N.Y.; Curtis, J.M.; Gänzle, M.G. Characterization of linoleate 10-hydratase of Lactobacillus plantarum and novel antifungal metabolites. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbach, S.H.; Goldin, B.R. Lactobacillus Strains and Methods of Selection. U.S. Patent 4839281, 13 June 1989. [Google Scholar]
- Liu, Y.-W.; Liong, M.-T.; Tsai, Y.-C. New perspectives of Lactobacillus plantarum as a probiotic: The gut-heart-brain axis. J. Microbiol. 2018, 56, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Forssten, S.D.; Salazar, N.; López, P.; Nikkilä, J.; Ouwehand, A.C.; Patterson, Á.; Ruas-Madiedo, P.; Suarez, A.; Gonzalez, S.; Gueimonde, M. Influence of a probiotic milk drink, containing Lactobacillus paracasei lpc-37, on immune function and gut microbiota in elderly subjects. Eur. J. Nutr. Food Safety 2011, 159–172. [Google Scholar]
- Opekun, A.R.; Gonzales, S.A.; Al-Saadi, M.A.; Graham, D.Y. Brief report: Lactobacillus bulgaricus GLB44 (Proviotic™) plus esomeprazole for Helicobacter pylori eradication: A pilot study. Helicobacter 2018, 23, e12476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbańska, M.; Gieruszczak-Białek, D.; Szajewska, H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment. Pharmacol. Ther. 2016, 43, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Drago, L.; Iemoli, E.; Rodighiero, V.; Nicola, L.; De Vecchi, E.; Piconi, S. Effects of Lactobacillus salivarius LS01 (DSM 22775) treatment on adult atopic dermatitis: A randomized placebo-controlled study. Int. J. Immunopathol. Pharmacol. 2011, 24, 1037–1048. [Google Scholar] [CrossRef]
- Selle, K.; Klaenhammer, T.R. Genomic and phenotypic evidence for probiotic influences of Lactobacillus gasseri on human health. FEMS Microbiol. Rev. 2013, 37, 915–935. [Google Scholar] [CrossRef] [Green Version]
- Bull, M.; Plummer, S.; Marchesi, J.; Mahenthiralingam, E. The life history of Lactobacillus acidophilus as a probiotic: A tale of revisionary taxonomy, misidentification and commercial success. FEMS Microbiol. Lett. 2013, 349, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.P.; Ba, Z.Y.; Sanders, M.E.; D’Amico, F.J.; Roberts, R.F.; Smith, K.H.; Merenstein, D.J. Safety of Bifidobacterium animalis subsp lactis (B. Lactis) strain BB-12-supplemented yogurt in healthy children. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Brenner, D.M.; Chey, W.D. Bifidobacterium infantis 35624: A novel probiotic for the treatment of irritable bowel syndrome. Rev. Gastroenterol. Disord. 2009, 9, 7–15. [Google Scholar]
- Wilcox, C.R.; Stuart, B.; Leaver, H.; Lown, M.; Willcox, M.; Moore, M.; Little, P. Effectiveness of the probiotic Streptococcus salivarius K12 for the treatment and/or prevention of sore throat: A systematic review. Clin. Microbiol. Infect. 2019, 25, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Urgesi, R.; Casale, C.; Pistelli, R.; Rapaccini, G.L.; De Vitis, I. A randomized double-blind placebo-controlled clinical trial on efficacy and safety of association of simethicone and Bacillus coagulans (Colinox ®) in patients with irritable bowel syndrome. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1344–1353. [Google Scholar] [PubMed]
- EFSA Panel on Dietetic Products, N.; Allergies. Scientific opinion on the substantiation of a health claim related to Saccharomyces cerevisiae var. boulardii CNCM I-3799 and reducing gastro-intestinal discomfort pursuant to article 13 (5) of regulation (EC) no 1924/2006. EFSA J. 2012, 10, 2801. [Google Scholar]
- Yang, W.; Dostal, L.; Rosazza, J.P.N. Stereospecificity of microbial hydrations of oleic acid to 10-hydroxystearic acid. Appl. Environ. Microbiol. 1993, 59, 281–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, S.; De Simeis, D. New insights on the baker’s yeast-mediated hydration of oleic acid: The bacterial contaminants of yeast are responsible for the stereoselective formation of (R)-10-hydroxystearic acid. J. Appl. Microbiol. 2018, 124, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Ebbers, E.J.; Ariaans, G.J.A.; Bruggink, A.; Zwanenburg, B. Controlled racemization and asymmetric transformation of α-substituted carboxylic acids in the melt. Tetrahedron Asymmetry 1999, 10, 3701–3718. [Google Scholar] [CrossRef]

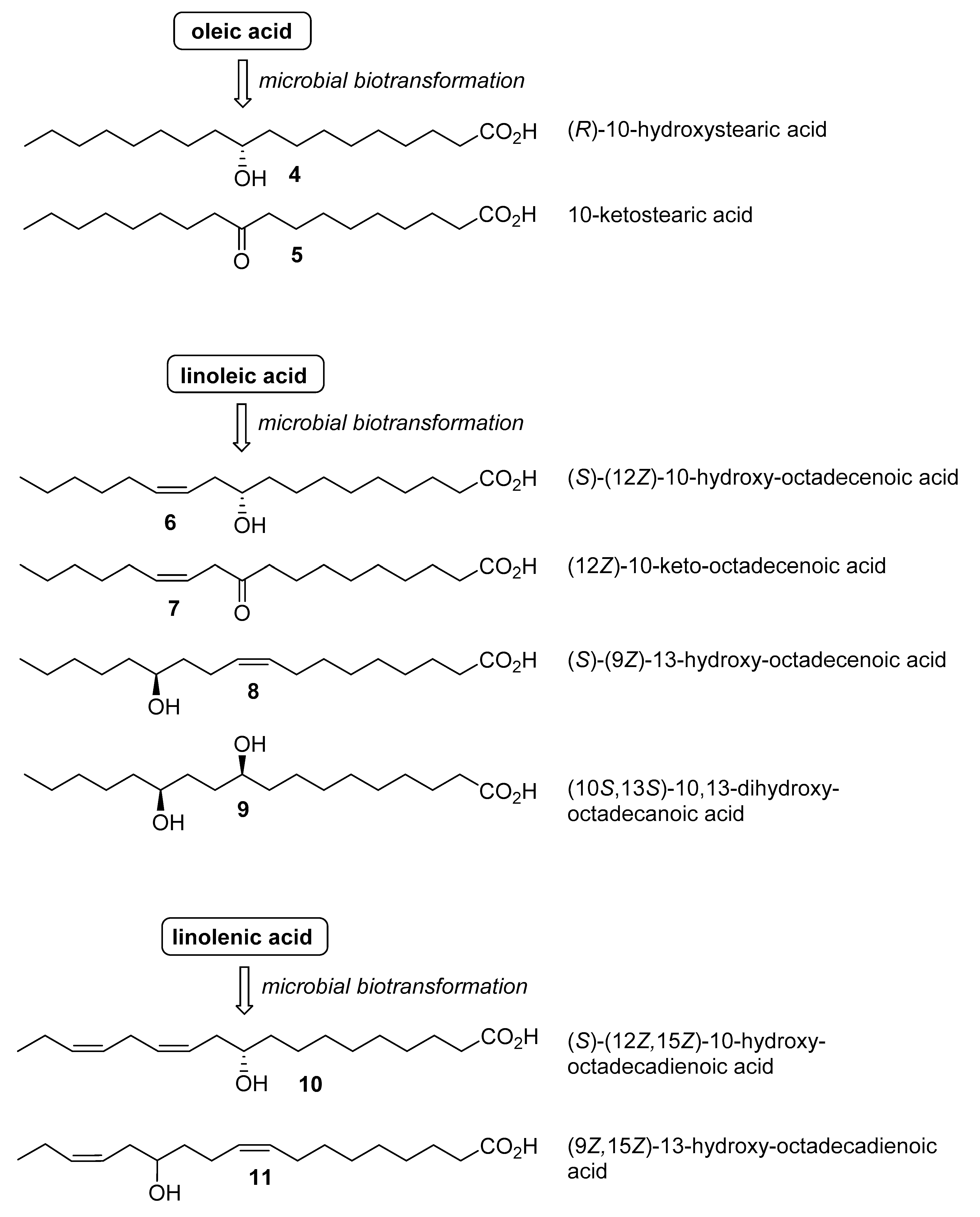
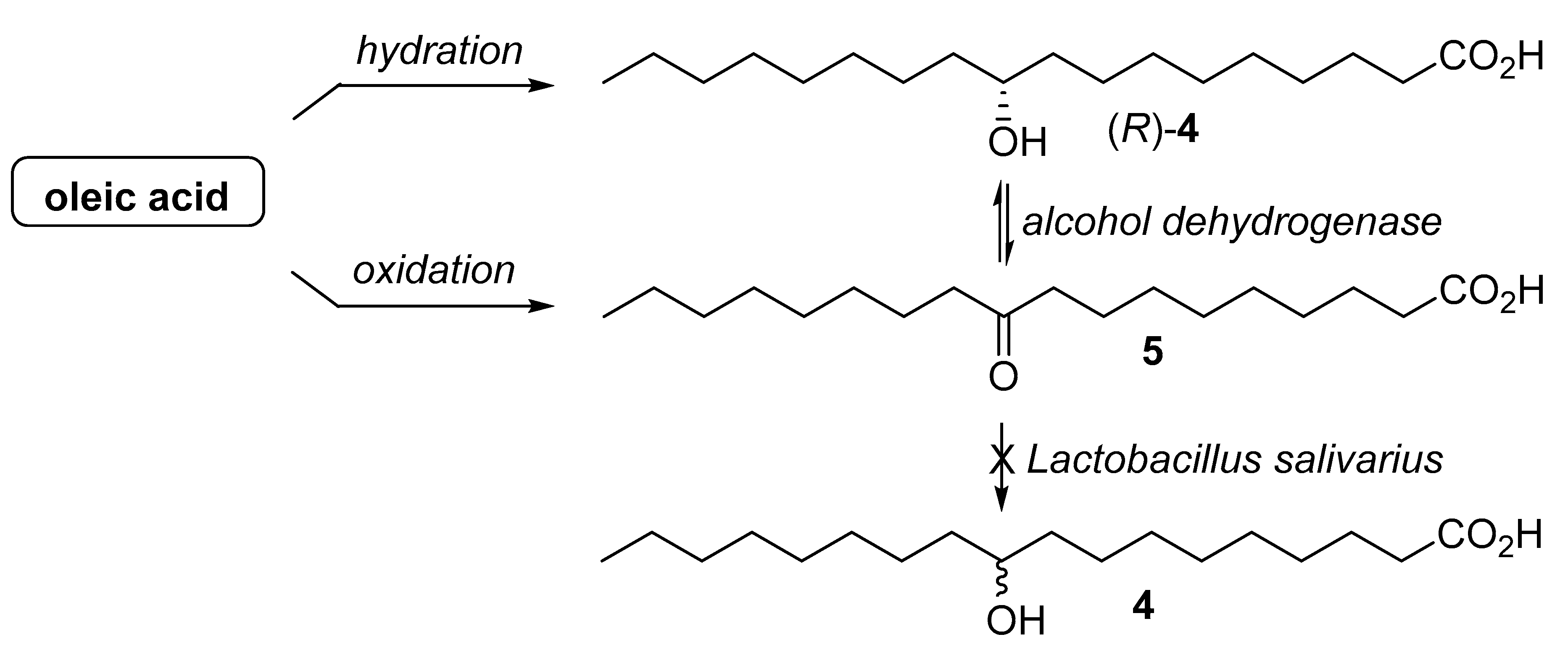
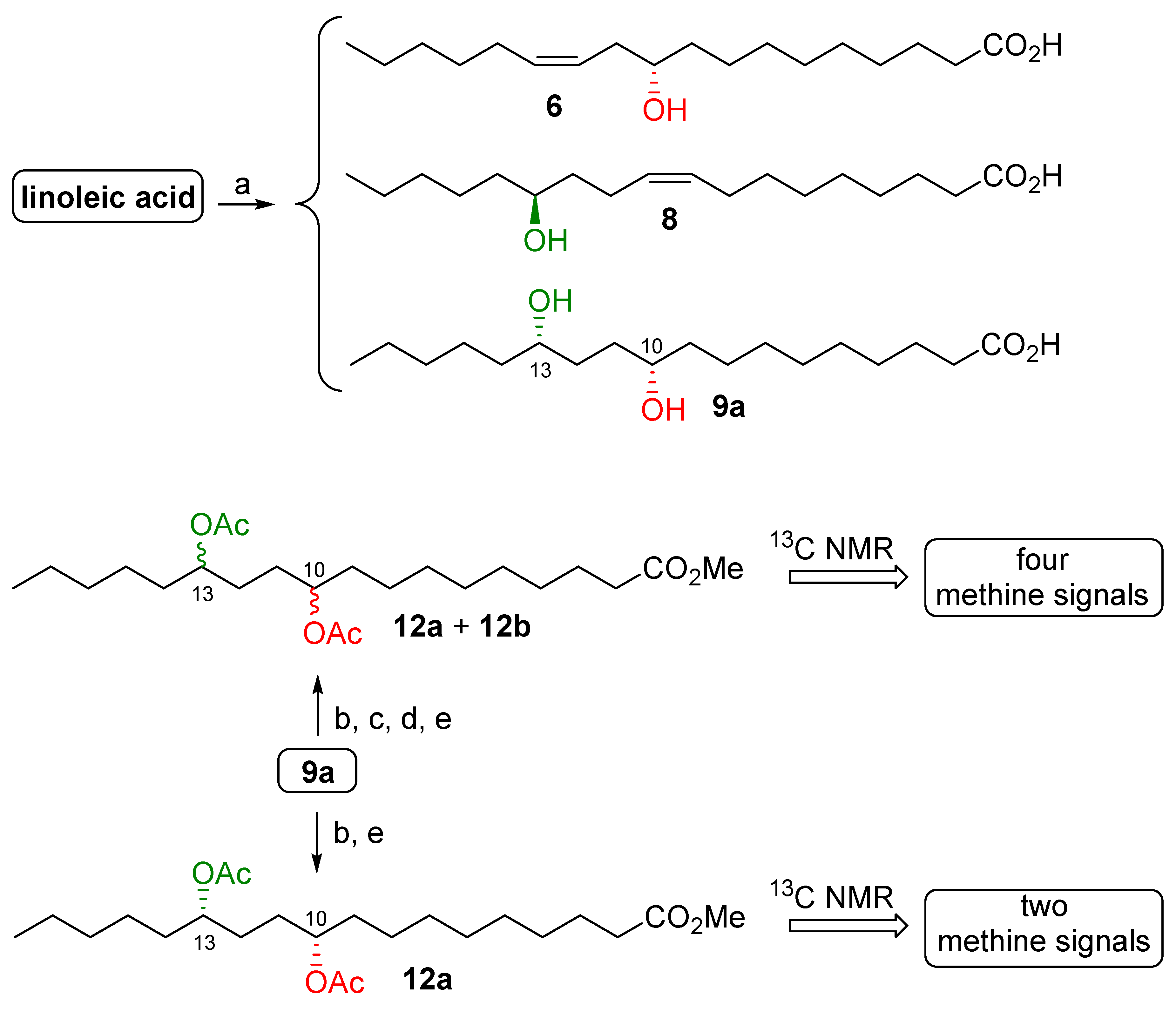
| Microorganism | Hydratase Activity 1 | ||
|---|---|---|---|
| Starting Fatty Acid | |||
| Oleic | Linoleic | Linolenic | |
| Lactobacillus rhamnosus ATCC 53103 | (R)-4 (ee > 95%; yield 46%) | (S)-6 (ee > 95%; yield 47%) | (S)-10 (ee > 95%; yield 36%) |
| Lactobacillus plantarum 299V | (R)-4 (ee > 95%; yield 52%) | (S)-6 (ee > 95%; yield 46%) | (S)-10 (ee > 95%; yield 52%) |
| Lactobacillus paracasei ATCC SD5275 | (R)-4 (ee > 95%; yield 45%) | (S)-6 (ee > 94%; yield 44%) | 10 low activity 2 |
| Lactobacillus bulgaricus GLB 44 | (R)-4 (ee > 95%; yield 48%) | (S)-6 (ee > 95%; yield 41%) | 10 low activity 2 |
| Lactobacillus reuteri DSM 17938 | (R)-4 (ee > 10%; yield 17%) | (S)-6 (ee > 78%; yield 5%) | no activity 3 |
| Lactobacillus salivarius DSM 22775 | (R)-4 (ee > 80%; yield 38%) 5 (6%) | (S)-6 (ee > 95%; yield 6%) | no activity 3 |
| Lactobacillus gasseri SFB | (R)-4 (ee > 95%; yield 16%) | 6 low activity 2 | no activity 3 |
| Lactobacillus acidophilus ATCC SD5212 | (R)-4 (ee > 95%; yield 45%) | (S)-6 (ee > 95%; yield 10%) (S)-8 (ee > 95%; yield 41%) (10S,13S)-9 (yield 20%) 4 | no activity 3 |
| Bifidobacterium animalis subsp. lactis DSM 15954 | (R)-4 (ee > 90%; yield 51%) | 6 low activity 2 | no activity 3 |
| Bifidobacterium infantis 35624 | 4 low activity 2 | no activity 3 | no activity 3 |
| Streptococcus salivarius ATCC BAA 1024 | (R)-4 (ee > 95%; yield 15%) | no activity 3 | no activity 3 |
| Bacillus coagulans Colinox® | no activity 3 | no activity 3 | no activity 3 |
| Saccharomyces boulardii CNCM I-3799 | no activity 3 | no activity 3 | no activity 3 |
| Microorganism | Hydratase Activity Versus Oleic Acid 1 | ||
|---|---|---|---|
| Experimental Conditions | |||
| Aerobic Flask 1 | Anaerobic Flask 1 | Bioreactor (Anaerobic) 1 | |
| Streptococcus salivarius ATCC BAA 1024 | 4 (yield 1%) | 4 (yield 5%) | (R)-4 (ee 95%; yield 15%) |
| Bacillus coagulans Colinox® | no activity 2 | no activity 2 | no activity 2 |
| Saccharomyces boulardii CNCM I-3799 | no activity 2 | no activity 2 | no activity 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, S.; De Simeis, D.; Castagna, A.; Valentino, M. The Fatty-Acid Hydratase Activity of the Most Common Probiotic Microorganisms. Catalysts 2020, 10, 154. https://doi.org/10.3390/catal10020154
Serra S, De Simeis D, Castagna A, Valentino M. The Fatty-Acid Hydratase Activity of the Most Common Probiotic Microorganisms. Catalysts. 2020; 10(2):154. https://doi.org/10.3390/catal10020154
Chicago/Turabian StyleSerra, Stefano, Davide De Simeis, Antonio Castagna, and Mattia Valentino. 2020. "The Fatty-Acid Hydratase Activity of the Most Common Probiotic Microorganisms" Catalysts 10, no. 2: 154. https://doi.org/10.3390/catal10020154
APA StyleSerra, S., De Simeis, D., Castagna, A., & Valentino, M. (2020). The Fatty-Acid Hydratase Activity of the Most Common Probiotic Microorganisms. Catalysts, 10(2), 154. https://doi.org/10.3390/catal10020154






