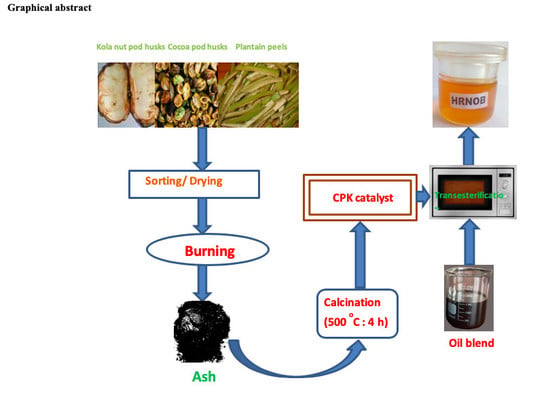
Sustainable Biodiesel Synthesis from Honne-Rubber-Neem Oil Blend with a Novel Mesoporous Base Catalyst Synthesized from a Mixture of Three Agrowastes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Calcined CPK Characterization
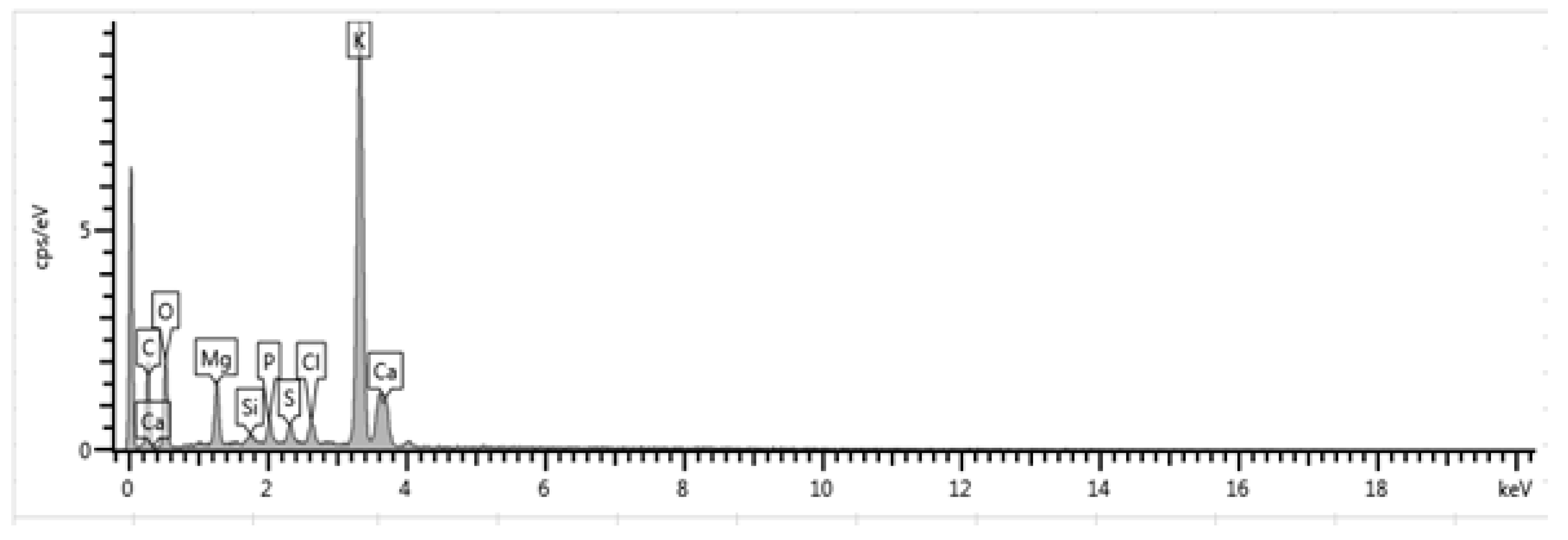
2.1.1. EDX Results on CPK
2.1.2. SEM Analysis of Calcined CPK
2.1.3. XRD Pattern of Calcined CPK
2.1.4. IR Spectrum for Calcined CPK
2.1.5. Surface Properties of Calcined CPK
2.2. Physicochemical Properties of Oil Blends
2.3. Results of Esterification of HRNO
2.4. Modelling Results of Transesterification Process
2.5. Interactive Effect of Process Parameters on HRNOB
2.6. Optimization Condition and Model Validation
2.7. Quality of HRNOB
2.8. Reusability Study of CPK Catalyst
3. Experimental
3.1. Materials
3.2. Preparation of CPK Catalyst
3.3. Characterization of Synthesized CPK Catalyst
3.4. Blending of Honne, Rubber Seed and Neem Oils
3.5. Model Development for HRNO Blend Transesterification
3.6. Esterification of HRNO Blend
3.7. Transesterification of Treated HRNO Blend
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sahoo, P.; Das, L. Process optimization for biodiesel production from Jatropha, Karanja and Polanga oils. Fuel 2009, 88, 1588–1594. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Thanh, L.T.; Okitsu, K.; Boi, L.V.; Maeda, Y. Catalytic technologies for biodiesel fuel production and utilization of glycerol: A review. Catalysts 2012, 2, 191–222. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Azid, A.; Umar, R.; Juahir, H.; Khatoon, H.; Endut, A. A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Renew. Sustain. Energy Rev. 2017, 70, 1040–1051. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Semwal, S.; Arora, A.K.; Badoni, R.P.; Tuli, D.K. Biodiesel production using heterogeneous catalysts. Bioresour. Technol. 2011, 102, 2151–2161. [Google Scholar] [CrossRef]
- Betiku, E.; Akintunde, A.M.; Ojumu, T.V. Banana peels as a biobase catalyst for fatty acid methyl esters production using Napoleon’s plume (Bauhinia monandra) seed oil: A process parameters optimization study. Energy 2016, 103, 797–806. [Google Scholar] [CrossRef]
- Boey, P.-L.; Maniam, G.P.; Hamid, S.A.; Ali, D.M.H. Utilization of waste cockle shell (Anadara granosa) in biodiesel production from palm olein: Optimization using response surface methodology. Fuel 2011, 90, 2353–2358. [Google Scholar] [CrossRef] [Green Version]
- Odude, V.O.; Adesina, A.J.; Oyetunde, O.O.; Adeyemi, O.O.; Ishola, N.B.; Etim, A.O.; Betiku, E. Application of Agricultural Waste-Based Catalysts to Transesterification of Esterified Palm Kernel Oil into Biodiesel: A Case of Banana Fruit Peel Versus Cocoa Pod Husk. Waste Biomass Valoriz. 2017, 1–12. [Google Scholar] [CrossRef]
- Liu, D.; Seeburg, D.; Kreft, S.; Bindig, R.; Hartmann, I.; Schneider, D.; Enke, D.; Wohlrab, S. Rice Husk Derived Porous Silica as Support for Pd and CeO2 for Low Temperature Catalytic Methane Combustion. Catalysts 2019, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Nisar, J.; Razaq, R.; Farooq, M.; Iqbal, M.; Khan, R.A.; Sayed, M.; Shah, A.; ur Rahman, I. Enhanced biodiesel production from Jatropha oil using calcined waste animal bones as catalyst. Renew. Energy 2017, 101, 111–119. [Google Scholar] [CrossRef]
- Mendonça, I.M.; Machado, F.L.; Silva, C.C.; Junior, S.D.; Takeno, M.L.; de Sousa Maia, P.J.; Manzato, L.; de Freitas, F.A. Application of calcined waste cupuaçu (Theobroma grandiflorum) seeds as a low-cost solid catalyst in soybean oil ethanolysis: Statistical optimization. Energy Convers. Manag. 2019, 200, 112095. [Google Scholar] [CrossRef]
- Nath, B.; Das, B.; Kalita, P.; Basumatary, S. Waste to value addition: Utilization of waste Brassica nigra plant derived novel green heterogeneous base catalyst for effective synthesis of biodiesel. J. Clean. Prod. 2019, 239, 118112. [Google Scholar] [CrossRef]
- Balajii, M.; Niju, S. Banana peduncle—A green and renewable heterogeneous base catalyst for biodiesel production from Ceiba pentandra oil. Renew. Energy 2020, 146, 2255–2269. [Google Scholar] [CrossRef]
- Gohain, M.; Laskar, K.; Paul, A.K.; Daimary, N.; Maharana, M.; Goswami, I.K.; Hazarika, A.; Bora, U.; Deka, D. Carica papaya stem: A source of versatile heterogeneous catalyst for biodiesel production and C–C bond formation. Renew. Energy 2020, 147, 541–555. [Google Scholar] [CrossRef]
- Pathak, G.; Das, D.; Rajkumari, K.; Rokhum, L. Exploiting waste: Towards a sustainable production of biodiesel using Musa acuminata peel ash as a heterogeneous catalyst. Green Chem. 2018, 20, 2365–2373. [Google Scholar] [CrossRef]
- Adeyi, O. Proximate composition of some agricultural wastes in Nigeria and their potential use in activated carbon production. J. Appl. Sci. Environ. Manag. 2010, 14. [Google Scholar] [CrossRef]
- Betiku, E.; Okeleye, A.A.; Ishola, N.B.; Osunleke, A.S.; Ojumu, T.V. Development of a Novel Mesoporous Biocatalyst Derived from Kola Nut Pod Husk for Conversion of Kariya Seed Oil to Methyl Esters: A Case of Synthesis, Modeling and Optimization Studies. Catal. Lett. 2019, 149, 1772–1787. [Google Scholar] [CrossRef]
- Osakwe, E.; Ani, I.; Akpan, U.; Olutoye, M. Kolanut pod husk as a biobase catalyst for fatty acid methyl ester production using Thevetia peruviana (Yellow oleander) seed oil. IOP Conf. Ser. 2018, 173, 012008. [Google Scholar] [CrossRef]
- Ofori-Boateng, C.; Lee, K.T. The potential of using cocoa pod husks as green solid base catalysts for the transesterification of soybean oil into biodiesel: Effects of biodiesel on engine performance. Chem. Eng. J. 2013, 220, 395–401. [Google Scholar] [CrossRef]
- Betiku, E.; Etim, A.O.; Pereao, O.; Ojumu, T.V. Two-step conversion of neem (Azadirachta indica) seed oil into fatty methyl esters using an heterogeneous biomass-based catalyst: An example of cocoa pod husk. Energy Fuels 2017, 31, 6182–6193. [Google Scholar] [CrossRef]
- Betiku, E.; Ajala, S.O. Modeling and optimization of Thevetia peruviana (yellow oleander) oil biodiesel synthesis via Musa paradisiacal (plantain) peels as heterogeneous base catalyst: A case of artificial neural network vs. response surface methodology. Ind. Crops Prod. 2014, 53, 314–322. [Google Scholar] [CrossRef]
- Etim, A.O.; Betiku, E.; Ajala, S.O.; Olaniyi, P.J.; Ojumu, T.V. Potential of Ripe Plantain Fruit Peels as an Ecofriendly Catalyst for Biodiesel Synthesis: Optimization by Artificial Neural Network Integrated with Genetic Algorithm. Sustainability 2018, 10, 707. [Google Scholar] [CrossRef] [Green Version]
- Meneghetti, S.M.P.; Meneghetti, M.R.; Serra, T.M.; Barbosa, D.C.; Wolf, C.R. Biodiesel production from vegetable oil mixtures: Cottonseed, soybean, and castor oils. Energy Fuels 2007, 21, 3746–3747. [Google Scholar] [CrossRef]
- Khalil, I.; Aziz, A.R.A.; Yusup, S.; Heikal, M.; El-Adawy, M. Response surface methodology for the optimization of the production of rubber seed/palm oil biodiesel, IDI diesel engine performance, and emissions. Biomass Convers. Biorefin. 2017, 7, 37–49. [Google Scholar] [CrossRef]
- Vinayaka, A.S.; Mahanty, B.; Rene, E.R.; Behera, S.K. Biodiesel production by transesterification of a mixture of pongamia and neem oils. Biofuels 2018. [Google Scholar] [CrossRef]
- Milano, J.; Ong, H.C.; Masjuki, H.; Silitonga, A.; Chen, W.-H.; Kusumo, F.; Dharma, S.; Sebayang, A. Optimization of biodiesel production by microwave irradiation-assisted transesterification for waste cooking oil-Calophyllum inophyllum oil via response surface methodology. Energy Convers. Manag. 2018, 158, 400–415. [Google Scholar] [CrossRef]
- Miraculas, G.A.; Bose, N.; Raj, R.E. Process parameter optimization for biodiesel production from mixed feedstock using empirical model. Sustain. Energy Technol. Assess. 2018, 28, 54–59. [Google Scholar]
- Qiu, F.; Li, Y.; Yang, D.; Li, X.; Sun, P. Biodiesel production from mixed soybean oil and rapeseed oil. Appl. Energy 2011, 88, 2050–2055. [Google Scholar] [CrossRef]
- Falowo, O.A.; Oloko-Oba, M.I.; Betiku, E. Biodiesel production intensification via microwave irradiation-assisted transesterification of oil blend using nanoparticles from elephant-ear tree pod husk as a base heterogeneous catalyst. Chem. Eng. Process.-Process Intensif. 2019, 140, 157–170. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Al-Tikrity, E.T.; Albadree, M.A. Biodiesel production from mixed non-edible oils, castor seed oil and waste fish oil. Fuel 2017, 210, 721–728. [Google Scholar] [CrossRef]
- Sharma, M.; Khan, A.A.; Puri, S.; Tuli, D. Wood ash as a potential heterogeneous catalyst for biodiesel synthesis. Biomass Bioenergy 2012, 41, 94–106. [Google Scholar] [CrossRef]
- Lukić, I.; Krstić, J.; Jovanović, D.; Skala, D. Alumina/silica supported K 2 CO 3 as a catalyst for biodiesel synthesis from sunflower oil. Bioresour. Technol. 2009, 100, 4690–4696. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.; Everett, D.; Haul, R.; Moscou, L.; Pierotti, L.; Rouquerol, J.; Siemieniewska, T. International union of pure and applied chemistry physical chemistry division reporting physisorption data for gas/soils systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Adewuyi, Y.G. Mesoporous nanocrystalline sulfated zirconia synthesis and its application for FFA esterification in oils. Appl. Catal. A Gen. 2013, 462, 196–206. [Google Scholar] [CrossRef]
- Storck, S.; Bretinger, H.; Maier, W.F. Characterization of micro-and mesoporous solids by physisorption methods and pore-size analysis. Appl. Catal. A Gen. 1998, 174, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Demirbaş, A. Relationships derived from physical properties of vegetable oil and biodiesel fuels. Fuel 2008, 87, 1743–1748. [Google Scholar]
- Wang, Y.; Ou, S.; Liu, P.; Zhang, Z. Preparation of biodiesel from waste cooking oil via two-step catalyzed process. Energy Convers. Manag. 2007, 48, 184–188. [Google Scholar] [CrossRef]
- Hamidreza, J.; Nor, A.; Amin, T.-K.; Noshadi, I. Microwave assisted biodiesel production from Jatropha curcas L. seed by two-step in situ process: Optimization using response surface methodology. Bioresour. Technol. 2013, 136, 565–573. [Google Scholar]
- Körbahti, B.K.; Rauf, M. Response surface methodology (RSM) analysis of photoinduced decoloration of toludine blue. Chem. Eng. J. 2008, 136, 25–30. [Google Scholar]
- Tan, Y.H.; Abdullah, M.O.; Nolasco-Hipolito, C.; Zauzi, N.S.A. Application of RSM and Taguchi methods for optimizing the transesterification of waste cooking oil catalyzed by solid ostrich and chicken-eggshell derived CaO. Renew. Energy 2017, 114, 437–447. [Google Scholar] [CrossRef]
- Nayak, M.G.; Vyas, A.P. Optimization of microwave-assisted biodiesel production from Papaya oil using response surface methodology. Renew. Energy 2019, 138, 18–28. [Google Scholar] [CrossRef]
- Silitonga, A.; Shamsuddin, A.; Mahlia, T.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.; Masjuki, H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Hsiao, M.-C.; Lin, C.-C.; Chang, Y.-H. Microwave irradiation-assisted transesterification of soybean oil to biodiesel catalyzed by nanopowder calcium oxide. Fuel 2011, 90, 1963–1967. [Google Scholar] [CrossRef]
- Gude, V.G.; Patil, P.; Martinez-Guerra, E.; Deng, S.; Nirmalakhandan, N. Microwave energy potential for biodiesel production. Sustain. Chem. Process. 2013, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Ong, H.C.; Masjuki, H.; Mahlia, T.; Silitonga, A.; Chong, W.; Leong, K. Optimization of biodiesel production and engine performance from high free fatty acid Calophyllum inophyllum oil in CI diesel engine. Energy Convers. Manag. 2014, 81, 30–40. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.; Masjuki, H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Sanford, S.D.; White, J.; Shah, P.; Wee, C.; Valverde, M.; Meier, G. Feedstock and Biodiesel Characteristics Report–2009; Renewable Energy Group: Ames, IA, USA, 2010. [Google Scholar]
- Oladipo, B.; Betiku, E. Process optimization of solvent extraction of seed oil from Moringa oleifera: An appraisal of quantitative and qualitative process variables on oil quality using D-optimal design. Biocatal. Agric. Biotechnol. 2019, 20, 101187. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Relationships between the Composition of Edible Oils and Lard and the Ratio of the Absorbance of Specific Bands of Their Fourier Transform Infrared Spectra. Role of Some Bands of the Fingerprint Region. J. Agric. Food Chem. 1998, 46, 1788–1793. [Google Scholar] [CrossRef]
- Latchubugata, C.S.; Kondapaneni, R.V.; Patluri, K.K.; Virendra, U.; Vedantam, S. Kinetics and Optimization Studies using Response Surface Methodology in Biodiesel Production using Heterogeneous Catalyst. Chem. Eng. Res. Des. 2018, 135, 129–139. [Google Scholar] [CrossRef]
- Rabelo, S.N.; Ferraz, V.P.; Oliveira, L.S.; Franca, A.S. FTIR analysis for quantification of fatty acid methyl esters in biodiesel produced by microwave-assisted transesterification. Int. J. Environ. Sci. Dev. 2015, 6, 964. [Google Scholar] [CrossRef] [Green Version]
- Soares, I.P.; Rezende, T.F.; Silva, R.C.; Castro, E.V.R.; Fortes, I.C. Multivariate calibration by variable selection for blends of raw soybean oil/biodiesel from different sources using fourier transform infrared spectroscopy (FTIR) spectra data. Energy Fuels 2008, 22, 2079–2083. [Google Scholar] [CrossRef]
- Dharma, S.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Silitonga, A.S.; Kusumo, F.; Mahlia, T.M.I. Optimization of biodiesel production process for mixed Jatropha curcas–Ceiba pentandra biodiesel using response surface methodology. Energy Convers. Manag. 2016, 115, 178–190. [Google Scholar] [CrossRef]
- Gimbun, J.; Ali, S.; Kanwal, C.; Shah, L.A.; Ghazali, N.H.M.; Cheng, C.K.; Nurdin, S. Biodiesel production from rubber seed oil using a limestone based catalyst. Adv. Mater. Phys. Chem. 2012, 2, 138–141. [Google Scholar] [CrossRef]
- Betiku, E.; Omilakin, O.R.; Ajala, S.O.; Okeleye, A.A.; Taiwo, A.E.; Solomon, B.O. Mathematical modeling and process parameters optimization studies by artificial neural network and response surface methodology: A case of non-edible neem (Azadirachta indica) seed oil biodiesel synthesis. Energy 2014, 72, 266–273. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Washington, DC, USA, 1990. [Google Scholar]












| Biowaste | Calcination Condition | Transesterification Condition | Biodiesel Yield | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Heat (°C) | Time (h) | Main Metallic Content | Oil | MeOH:Oil Molar Ratio | Catalyst (wt.%) | Temperature (°C) | Time (min) | |||
| Cocoa pod husk | 700 | 4 | K (59.2%), Mg (3%) | Neem | 0.73v/v | 0.65 | 65 | 57 | 99.3 | [21] |
| Cocoa pod husk | 650 | 4 | - | Soybean | 6:1 | 1 | 60 | 120 | 91.4 | [20] |
| Plantain peel (ripe) | 700 | 4 | K (51.02%), Mg (1.15%) | Neem | 0.73v/v | 0.65 | 65 | 57 | 99.2 | [23] |
| Plantain peel (unripe) | 500 | 3.5 | K (54.73%), Ca (1.13%), Al (3.42%) | Yellow oleander | 0.33v/v | 3 | 60 | 90 | 95.25 | [22] |
| Kola nut husk | 500 | 4 | K (47.14%), Ca (7.59%), Mg (5.32%) | Kariya | 6:1 | 3 | 65 | 75 | 98.67 | [18] |
| Kola nut husk | 600 | 3 | K (33.914%), Ca 25.382%), Mg (12.398%) | Yellow oleander | 6:1 | 1.5 | 60 | 90 | 84.5 | [19] |
| Banana peel | 700 | 4 | K (99.73%), Ca (0.03%), Na (0.19%), Mg (0.03%), Fe (0.01%) | Napoleon’s plume | 7.6:1 | 2.75 | 65 | 69.02 | 98.5 | [7] |
| Banana peel | Open air burning | NS | K (70.06%), Ca (9.54%) Mg (1.78%), Fe (1.49%) | Soybean | 6:1 | 0.7 | Room temperature | 240 | 98.95% | [16] |
| Banana peduncle | 700 | 4 | K K (68.37%), Mg K (4.66%), Ca K (7.09%) | Ceiba pentandra | 9.20:1 | 1.978 | 60 | 60 | 98.69% | [14] |
| CPK | 500 | 4 | K (47.67%), Ca (5.56%), Mg (4.21%) | Neem-rubber-honne oil blend | 12:1 | 1.16 | 150 W | 6 | 98.45 | Present study |
| Temperature (°C) | Composition (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | Mg | Si | P | S | Cl | K | Ca | Fe | Al | |
| 300 | 40.43 | 4.06 | 0.90 | 1.74 | 1.41 | 1.97 | 43.99 | 5.50 | 0.00 | 0.00 |
| 500 | 37.21 | 4.21 | 0.56 | 1.65 | 1.26 | 1.88 | 47.67 | 5.56 | 0.00 | 0.00 |
| 700 | 41.20 | 3.05 | 0.79 | 1.61 | 0.92 | 1.88 | 47.93 | 3.93 | 0.00 | 0.00 |
| 900 | 41.59 | 1.85 | 1.41 | 1.77 | 1.29 | 1.43 | 45.55 | 4.68 | 0.64 | 0.00 |
| 1100 | 45.30 | 0.81 | 2.82 | 5.20 | 1.17 | 1.03 | 43.90 | 0.00 | 0.00 | 0.75 |
| Properties | H20R60N20 | H40R40N20 | H60R20N20 | H20R40N40 | H40R20N40 | H20R20N60 | H33R33N33 |
|---|---|---|---|---|---|---|---|
| RI | 1.4726 ± 0 | 1.4732 ± 0 | 1.4743 ± 0 | 1.4738 ± 0 | 1.4745 ± 0.02 | 1.4732 ± 0.01 | 1.4737 ± 0 |
| Density (g/cm3) | 0.931 ± 0.01 | 0.93 ± 0.02 | 0.934 ± 0.2 | 0.935 ± 0.94 | 0.935 ± 0.01 | 0.938 ± 1.34 | 0.934 ± 0.01 |
| Viscosity (mm2/s) | 51.31 ± 1.67 | 49.06 ± 0.78 | 47.99 ± 0.99 | 65.08 ± 0.22 | 57.48 ± 1.15 | 66.15 ± 2.08 | 59.1 ± 0.18 |
| Acid Value (mg KOH/g) | 55.68 ± 2.2 | 47.01 ± 0.11 | 41.4 ± 0.11 | 42.53 ± 0.12 | 35.35 ± 0.12 | 31.97 ± 0.11 | 39.5 ± 0.46 |
| IV (g I2/100 g oil) | 90.62 ± 2.4 | 85.76 ± 1.46 | 87.72 ± 1.46 | 84.84 ± 2.46 | 82.38 ± 1.94 | 81.89 ± 2.42 | 83.4 ± 0.99 |
| SV (mg KOH/g) | 219.49 ± 0.70 | 236.32 ± 0.70 | 244.04 ± 0.34 | 254.55 ± 0.70 | 241.94 ± 0.61 | 206.17 ± 1.40 | 224.4 ± 0.48 |
| Calorific value (MJ/kg) | 39.07 ± 0.07 | 38.4 5 ± 0.05 | 38.11 ± 0.01 | 37.73 ± 0.06 | 38.26 ± 0.04 | 39.75 ± 0.02 | 39 ± 0.01 |
| Source of Variance | Sum of Squares | Degree of Freedom | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 282.08 | 9 | 31.34 | 87.33 | <0.0001 |
| A-MeOH: HRNO | 17.29 | 1 | 17.29 | 48.17 | 0.0010 |
| B-CPK loading | 0.99 | 1 | 0.99 | 2.77 | 0.1569 |
| C-Time | 4.87 | 1 | 4.87 | 13.56 | 0.0143 |
| AB | 5.60 | 1 | 5.60 | 15.60 | 0.0109 |
| AC | 20.69 | 1 | 20.69 | 57.65 | 0.0006 |
| BC | 41.79 | 1 | 41.79 | 116.44 | 0.0001 |
| A2 | 19.51 | 1 | 19.51 | 54.37 | 0.0007 |
| B2 | 2.10 | 1 | 2.10 | 5.86 | 0.0601 |
| C2 | 3.06 | 1 | 3.06 | 8.52 | 0.0331 |
| Lack of fit | 1.51 | 1 | 1.51 | 20.95 | 0.0102 |
| Fit statistics | |||||
| Standard deviation | 0.60 | ||||
| Mean | 87.65 | ||||
| Coefficient of variation (%) | 0.68 | ||||
| R2 | 0.9937 | ||||
| Adjusted R2 | 0.9823 | ||||
| Adequate Precision | 42.38 | ||||
| Mixed Oils | Nature of Oils | Ratio | Catalyst | Transesterification Condition | Yield | Ref |
|---|---|---|---|---|---|---|
| Cottonseed/castor | Both non-edible | 50:50 | NaOH | MeOH/oil/catalyst of 34:6:1 | 86% | [24] |
| Soybean/castor | Edible/non-edible | 25:75 | NaOH | MeOH/oil/catalyst of 34:6:1 | 87% | [24] |
| Palm oil/rubber seed | Edible/non-edible | 50:50 | KOH | 64 °C, 1 h, catalyst of 1.3, MeOH: Oil 6:1 | 97% | [25] |
| Pongamia/neem | Both non-edible | 70:30 | NaOH | 60–65 °C, catalyst of 0.67%, MeOH: Oil of 6:1 and 77 min, | 86.2% | [26] |
| Waste cooking/honne | Waste/non-edible | 70:30 | KOH | 100 °C, catalyst of 0.774 wt.%, 600 rpm, MeOH: Oil of 59.60 vol.% and 7.15 min under microwave irradiation | 97.65% | [27] |
| Pongamia/jatropha/honne | All are non-edible | 1:1:1 | KOH | 64 °C, catalyst of 1.17w/v, oil:MeOH of 2.5 v/v and 95 min | 98% | [28] |
| Soybean/rapeseed | Both edible | 50:50 | NaOH | 55 °C, catalyst of 0.8 wt.%, MeOH: Oil of 5:1 and 2 h | 94% | [29] |
| Rubber seed/neem | Both non-edible | 40:60 | Calcined ash of elephant ear pod husk | 150 W, MeOH: Oil 11.44:1, catalyst of 2.96 wt.% and 5.88 min under microwave irradiation | 98.77% | [30] |
| Castor/waste fish oil | Non-edible/waste | 50:50 | KOH | 32 °C, catalyst of 0.5 wt.%, MeOH: Oil of 8:1, 600 rpm and 30 min | 95.2 ± 2.5% | [31] |
| Honne/rubber seed/neem | All are non-edible | 1:1:1 | Calcined CPK | 150 W, MeOH: Oil 12:1, catalyst of 1.158 wt.% and 6 min under microwave irradiation | 98.45 wt.% | Present study |
| Properties | Unit | HRNO | HRNOB | Limit | |
|---|---|---|---|---|---|
| EN 14214 | ASTM D6751 | ||||
| Density (15 °C) | kg/m3 | 938 | 889 | 860–900 | 880 |
| Acid value | mg KOH/g | 31.97 | 0.45 | 0.5 max | 0.5 max |
| FFA content | % | 16.07 | 0.226 | NS | NS |
| Kinematic viscosity | mm2/s | 66.15 | 4.89 | 3.5–5 | 1.9–6 |
| Iodine value | g I2/100g oil | 81.89 | 44.9 | ˂120 | NS |
| SV | mg KOH/g | 206.17 | 190.74 | NS | NS |
| Cetane number | 51.34 | 64.8 | 51 min | 47 min | |
| Calorific Value | MJ/kg | 39.75 | 40.94 | 35 | NS |
| Pour Point | °C | ND | −6 | NS | −15 to 16 |
| Flash point | °C | ND | 128 | <120 | 100 to170 |
| Cloud point | °C | ND | 12 | NS | −3 to 12 |
| Wavenumber (cm−1) | Functional Group | Mode of Vibration | Intensity | Reference |
|---|---|---|---|---|
| 3450 | -C=O | Overtone | Weak | [18,50,51] |
| 3007 | =C-H | Stretching | Strong | [29,30,52] |
| 2926 | -C-H (CH2) | Asymmetric stretching vibration | Very strong | [14,18,50] |
| 2854 | -C-H (CH2) | Symmetric stretching vibration | Very strong | [14,18,29] |
| 1745 | -C=O | Stretching | Very strong | [14,18,51] |
| 1465 | -CH2 | Shear-type vibration | Medium | [30,51,52] |
| 1377 | -CH3 | Bending vibration, symmetric deformation | Medium | [30,51,52] |
| 1242 | -CH2 | Stretching | Medium | [30,52] |
| 1168 | C-O-C | Symmetric stretching vibration | strong | [14,18,29] |
| 721 | -CH2 | Bending out of plane, rocking vibration | Medium | [14,18,29] |
| Chain Length | Composition (%) | |||
|---|---|---|---|---|
| HRNOB | Honne | Rubber | Neem | |
| This Study | Ong et al. [47] | Gimbun et al. [56] | Betiku et al. [57] | |
| C14:0 | 0.22 | 0.1 | - | - |
| C16:0 | 15.73 | 14.2 | 10.29 | 13.98 |
| C16:1 | 0.12 | 0.3 | - | 0.39 |
| C18:0 | 18.65 | 15.9 | 8.68 | 6.25 |
| C18:1 | 40.42 | 39.8 | 20.07 | 45.00 |
| C18:2 | 20.15 | 28.1 | 58.2 | 32.46 |
| C18:3 | 2.84 | 0.2 | 0.8 | 0.6 |
| C20:0 | 1.55 | 0.8 | - | 0.8 |
| C22:0 | 0.54 | - | - | 0.5 |
| Run | MeOH: HRNO | CPK (wt.%) | Time (min) |
|---|---|---|---|
| (A) | (B) | (C) | |
| 1 | 9 | 3 | 1.17157 |
| 2 | 12 | 4 | 2 |
| 3 | 12 | 2 | 6 |
| 4 | 9 | 3 | 4 |
| 5 | 9 | 3 | 4 |
| 6 | 9 | 3 | 4 |
| 7 | 9 | 4.41421 | 4 |
| 8 | 6 | 2 | 2 |
| 9 | 9 | 3 | 4 |
| 10 | 9 | 1.58579 | 4 |
| 11 | 9 | 3 | 4 |
| 12 | 13.2426 | 3 | 4 |
| 13 | 4.75736 | 3 | 4 |
| 14 | 9 | 3 | 6.82843 |
| 15 | 6 | 4 | 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falowo, O.A.; Ojumu, T.V.; Pereao, O.; Betiku, E. Sustainable Biodiesel Synthesis from Honne-Rubber-Neem Oil Blend with a Novel Mesoporous Base Catalyst Synthesized from a Mixture of Three Agrowastes. Catalysts 2020, 10, 190. https://doi.org/10.3390/catal10020190
Falowo OA, Ojumu TV, Pereao O, Betiku E. Sustainable Biodiesel Synthesis from Honne-Rubber-Neem Oil Blend with a Novel Mesoporous Base Catalyst Synthesized from a Mixture of Three Agrowastes. Catalysts. 2020; 10(2):190. https://doi.org/10.3390/catal10020190
Chicago/Turabian StyleFalowo, Olayomi A., Tunde V. Ojumu, Omoniyi Pereao, and Eriola Betiku. 2020. "Sustainable Biodiesel Synthesis from Honne-Rubber-Neem Oil Blend with a Novel Mesoporous Base Catalyst Synthesized from a Mixture of Three Agrowastes" Catalysts 10, no. 2: 190. https://doi.org/10.3390/catal10020190
APA StyleFalowo, O. A., Ojumu, T. V., Pereao, O., & Betiku, E. (2020). Sustainable Biodiesel Synthesis from Honne-Rubber-Neem Oil Blend with a Novel Mesoporous Base Catalyst Synthesized from a Mixture of Three Agrowastes. Catalysts, 10(2), 190. https://doi.org/10.3390/catal10020190