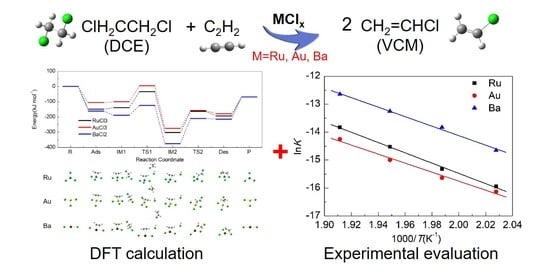
Catalytic Mechanism Comparison Between 1,2-Dichloroethane-Acetylene Exchange Reaction and Acetylene Hydrochlorination Reaction for Vinyl Chloride Production: DFT Calculations and Experiments
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection and Structure of Metal Chlorides
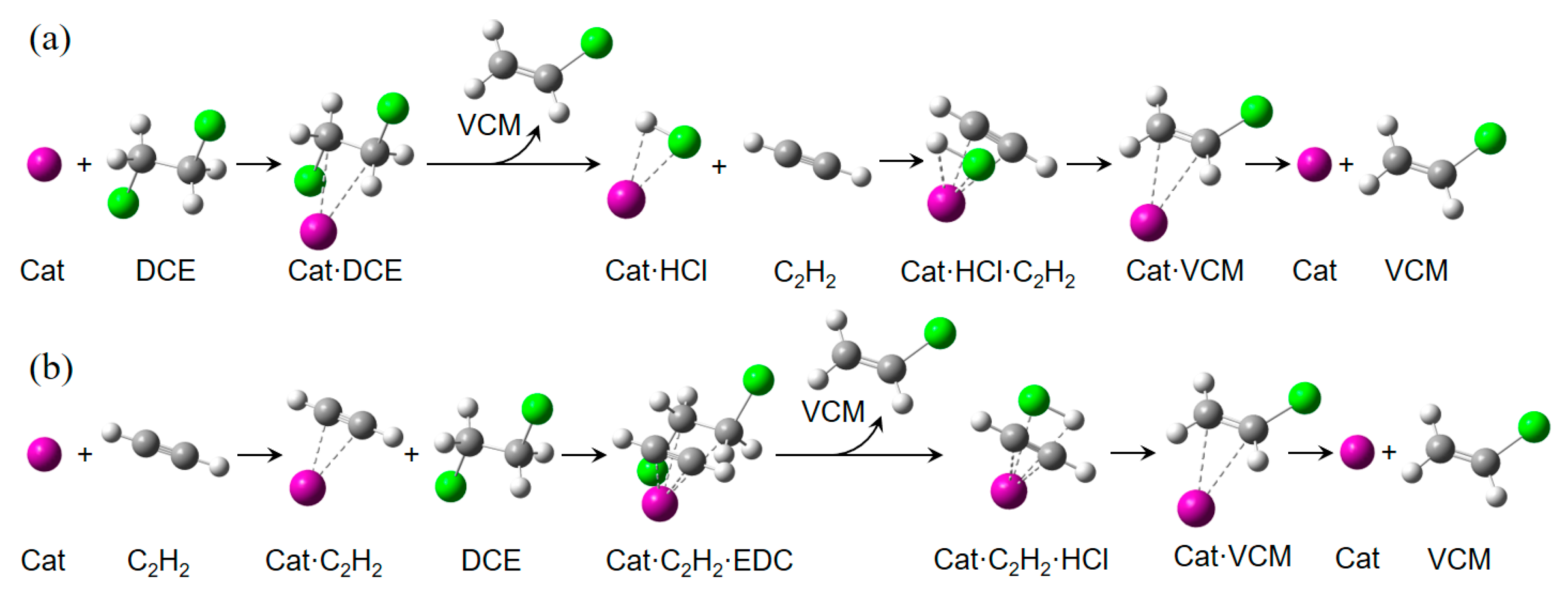
2.2. Possible Reaction Mechanisms
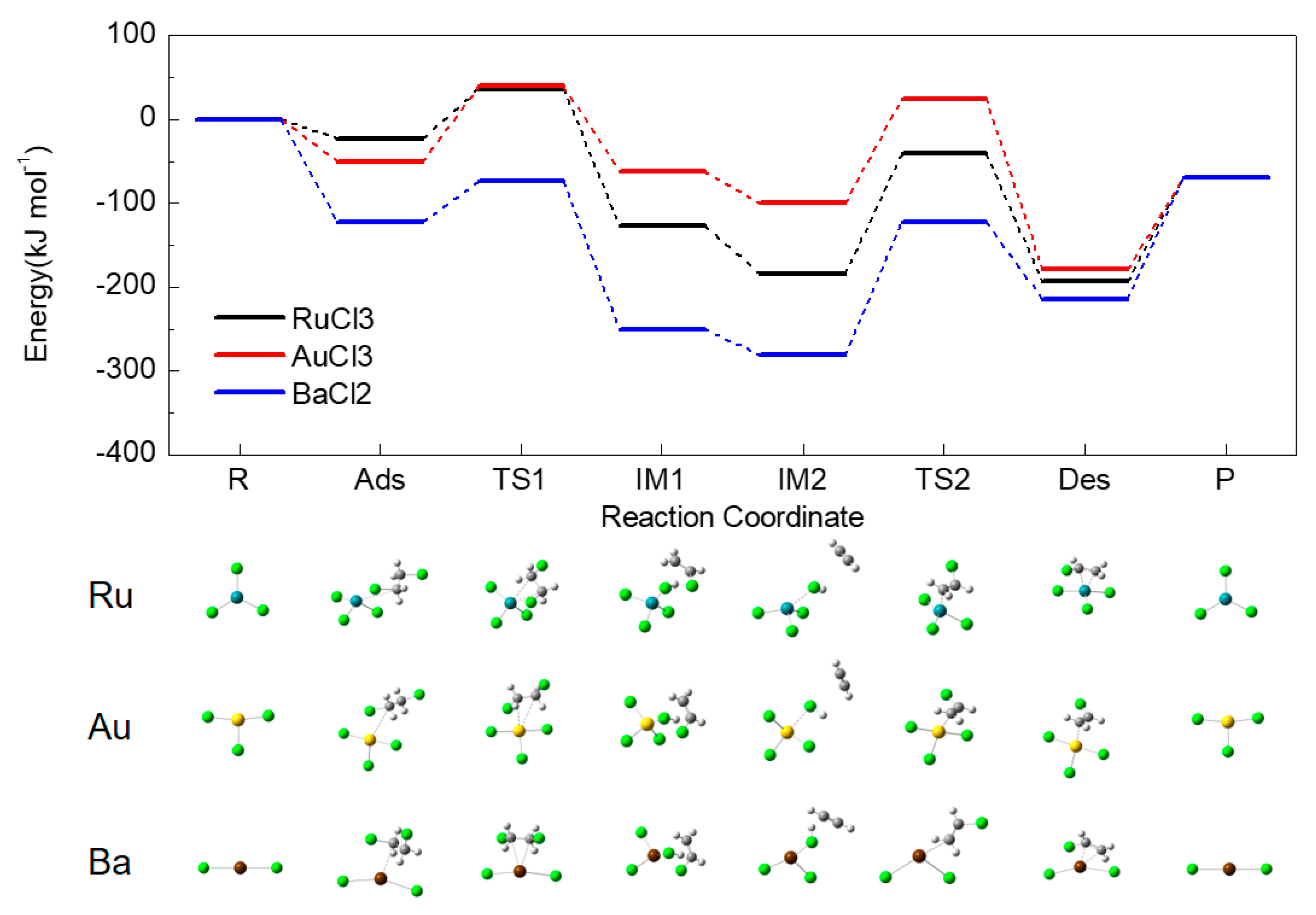
2.3. Adsorption Energy on Metal Chlorides
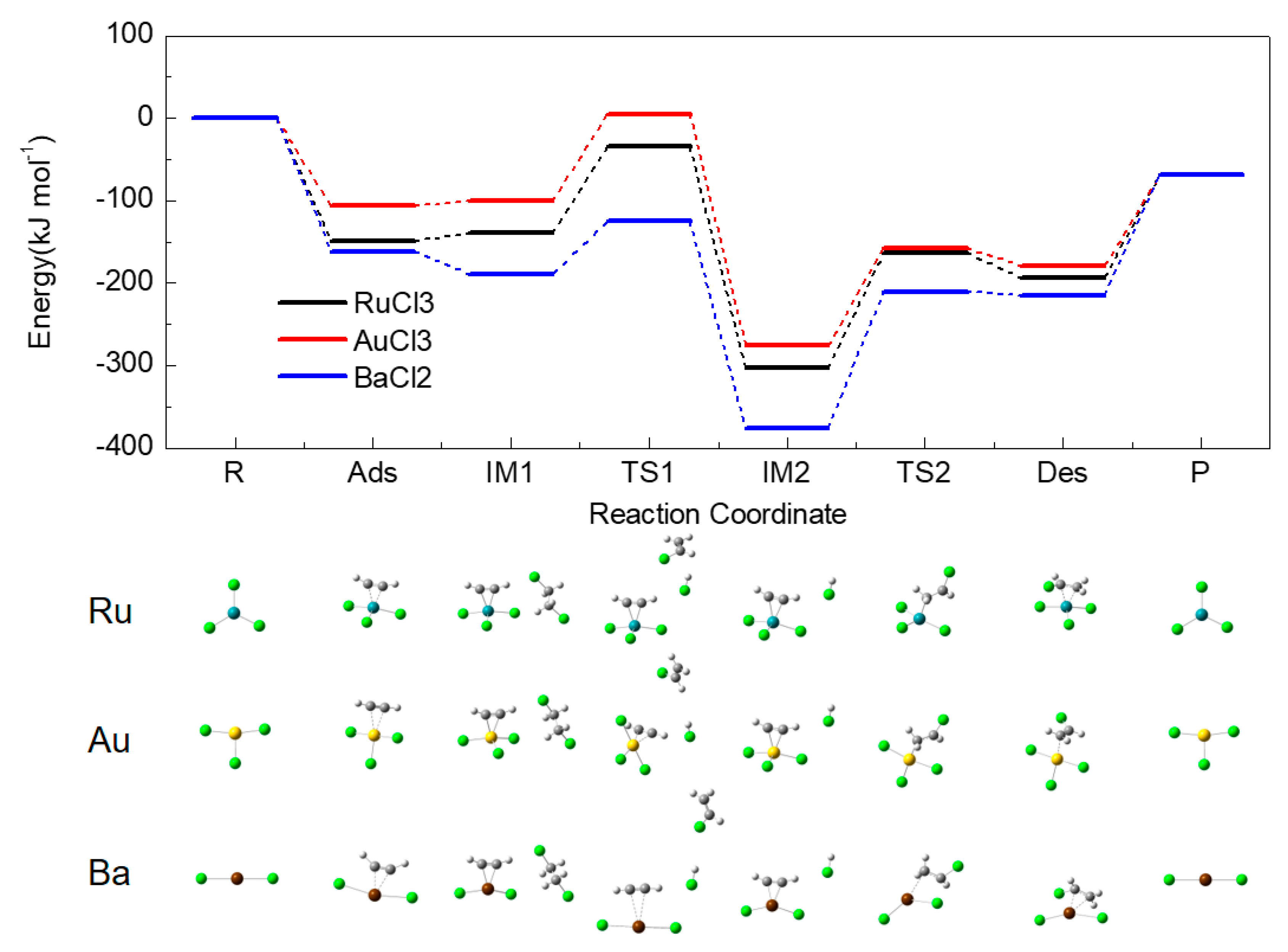
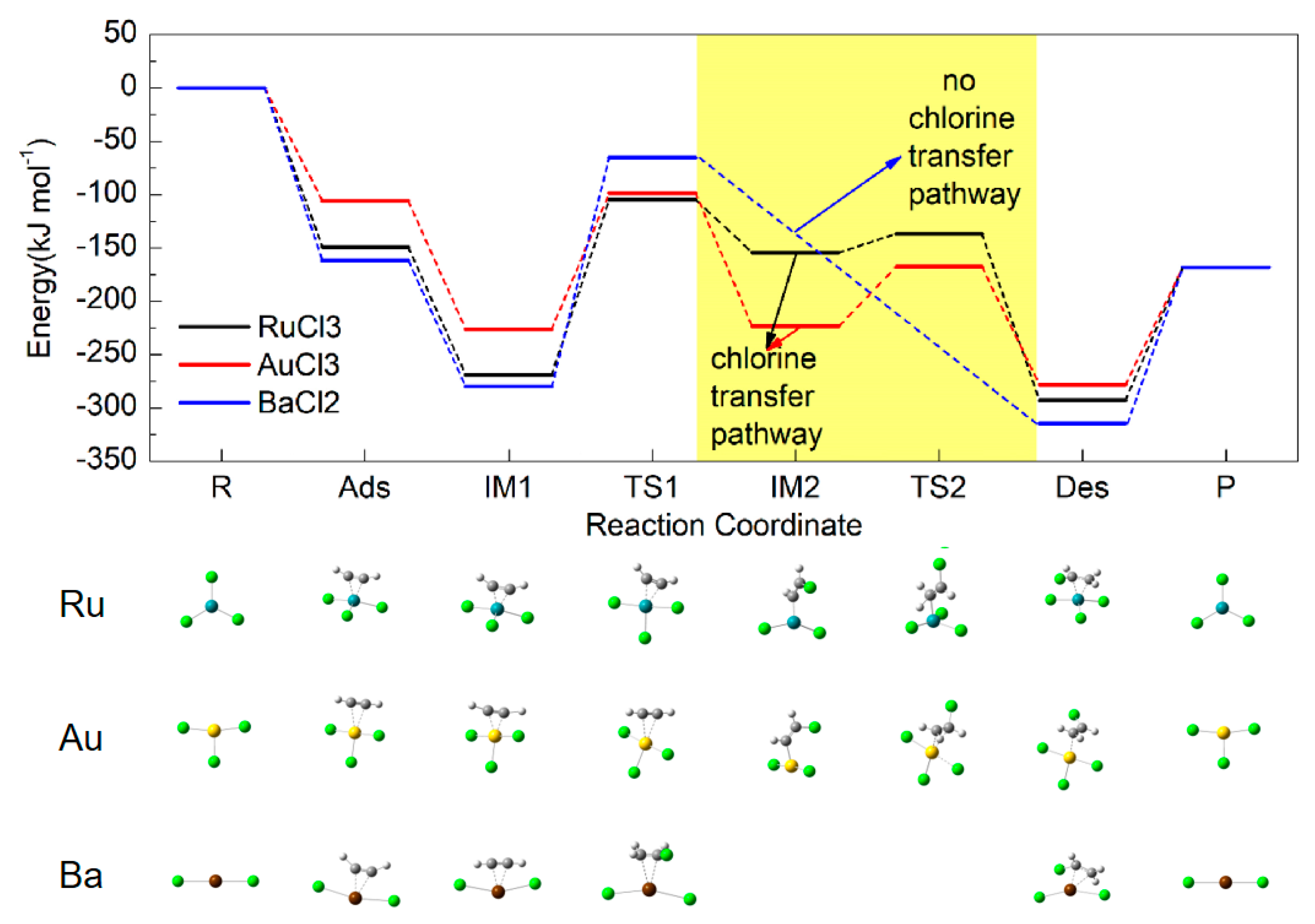
2.4. Energy Profiles
2.5. Activation Energy
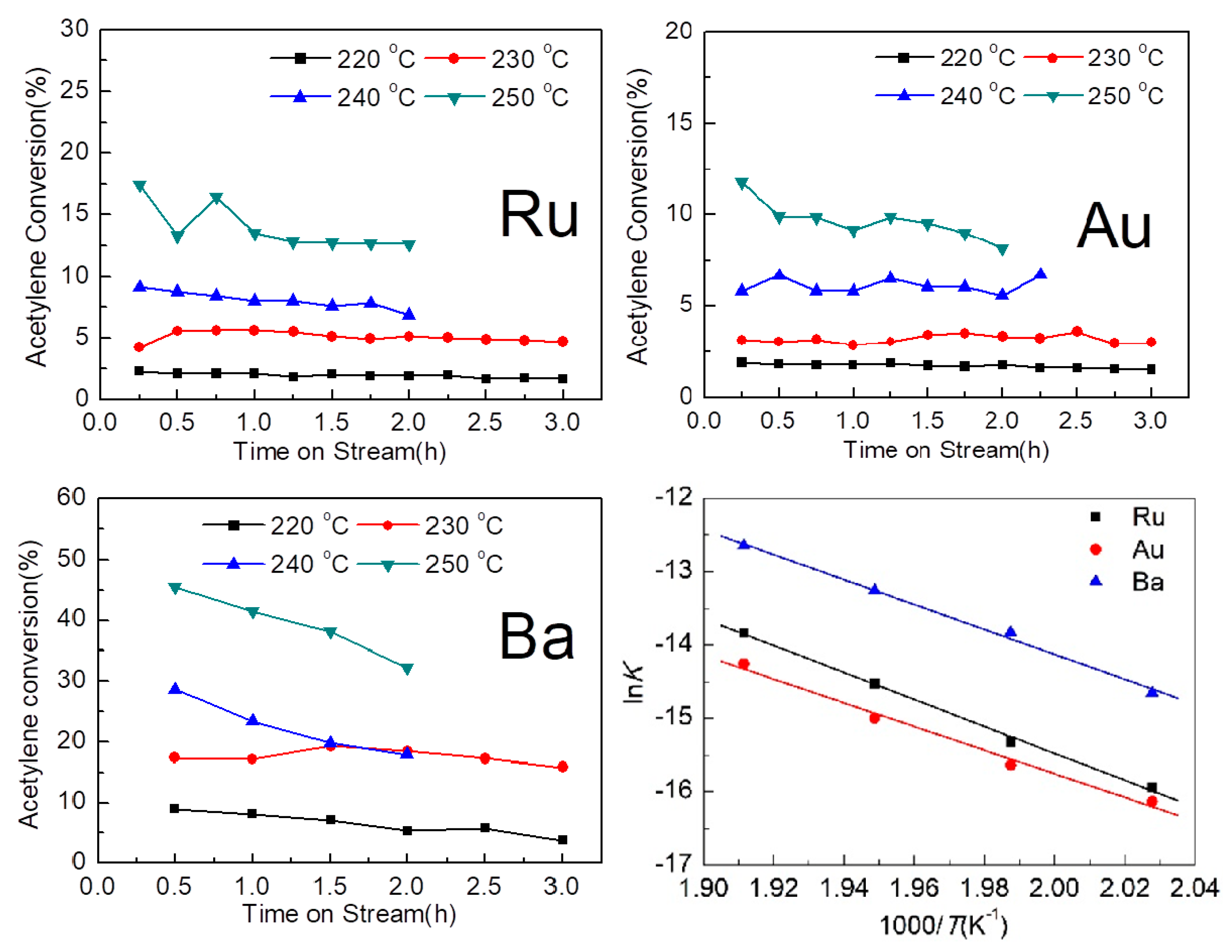
2.6. Comparison with Experimental Data
2.7. Comparison with Acetylene Hydrochlorination Process
3. Calculation Methodology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Z.; Yin, J.; Zhang, G.; Jin, W. The strategy of domestic PVC enterprise under new circumstance. Polyinyl Chloride 2015, 12, 43–44. [Google Scholar]
- Li, G.; Zhou, J. The non-mercury trend of acetylene based PVC industry. China Chlor Alkali 2017, 3, 20–22. [Google Scholar]
- Liu, G.; Ma, L.; Liu, J. Database Handbook of Physical Properties in Chemistry and Chemical Engineering; Chemical Industry Press: Beijing, China, 2002. [Google Scholar]
- Dean, J.A. Handbook of Chemistry and Physics, 70th ed.; McGrawHill, Inc.: New York, NY, USA, 1990. [Google Scholar]
- Wang, Y. The impact of mercury pollution on human health. Life Health 2004, 1, 21–23. [Google Scholar]
- Selin, H. Global environmental law and treaty-making on hazardous substances: The minamata convention and mercury abatement. Glob. Environ. Politics 2014, 14, 1–19. [Google Scholar] [CrossRef]
- Jiang, Z.; Wei, X.; Zhong, J. Jiang-Zhong new route for VCM production. Polyvinyl Chloride 2013, 41, 16–19. [Google Scholar]
- Argaman, N.; Makov, G. Density functional theory: An introduction. Am. J. Phys. 2000, 68, 69. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Zhong, J.; Wei, X. Jiang-Zhong new VCM production process introduction and perspective. China Chlor Alkali 2014, 1, 24–26. [Google Scholar]
- Zhao, W.; Li, W.; Zhang, J. Ru/N-AC catalyst to produce vinyl chloride from acetylene and 1,2-dichloroethane. Catal. Sci. Technol. 2016, 6, 1402–1409. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, K.; Si, J.; Li, C.; Luo, G. A ligand coordination approach for high reaction stability of an Au–Cu bimetallic carbon-based catalyst in the acetylene hydrochlorination process. Catal. Sci. Technol. 2016, 6, 1357–1366. [Google Scholar] [CrossRef]
- Wan, F.; Chao, S.; Guan, Q.; Wang, G.-C.; Li, W. Reaction mechanisms of acetylene hydrochlorination catalyzed by AuCl 3/C catalysts: A density functional study. Catal. Comm. 2017, 101, 120–124. [Google Scholar] [CrossRef]
- Han, Y.; Sun, M.; Li, W.; Zhang, J. Influence of chlorine coordination number on the catalytic mechanism of ruthenium chloride catalysts in the acetylene hydrochlorination reaction: A DFT study. Phys. Chem. Chem. Phys. 2015, 17, 7720–7730. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shen, S.W.B. Study on the effects of acetylene on an Au-Cu/C catalyst for acetylene hydrochlorination using Monte Carlo and DFT methods. React. Kinet. Mech. Catal. 2013, 110, 177–186. [Google Scholar] [CrossRef]
- Zhang, J.; He, Z.; Li, W.; Han, Y. Deactivation mechanism of AuCl3 catalyst in acetylene hydrochlorination reaction: A DFT study. RSC Adv. 2012, 2, 4814. [Google Scholar] [CrossRef]
- Straub, B.F. Gold(I) or gold(III) as active species in AuCl(3)-catalyzed cyclization/cycloaddition reactions? A DFT study. Chem. Commun. 2004, 1726–1728. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, M.; Zhang, H.; Dong, Y.; Li, X.; Li, W.; Zhang, J. Catalytic dehydrochlorination of 1,2-dichloroethane to produce vinyl chloride over N-doped coconut activated carbon. RSC Adv. 2015, 5, 104071–104078. [Google Scholar] [CrossRef]
- Shen, Z.; Zhao, H.; Liu, Y.; Kan, Z.; Xing, P.; Zhong, J.; Jiang, B. Mercury-free nitrogen-doped activated carbon catalyst: An efficient catalyst for the catalytic coupling reaction of acetylene and ethylene dichloride to synthesize the vinyl chloride monomer. React. Chem. Eng. 2018, 3, 34–40. [Google Scholar] [CrossRef]
- Hutchings, G.J. Vapor-phase hydrochlorination of acetylene—Correlation of catalytic activity of supported metal chloride catalysts. J. Catal. 1985, 96, 292–295. [Google Scholar] [CrossRef]
- Hutchings, G.J. Catalysis: A golden future. Gold Bull. 1996, 29, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, G.J. Nanocrystalline gold catalyst: A reflection on catalyst discovery and the nature of active sites. Gold Bull. 2009, 42, 8. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Jia, J.; Li, C.; Xu, H.; Zhou, J.; Luo, G.; Wei, F. A low content Au-based catalyst for hydrochlorination of C2H2and its industrial scale-up for future PVC processes. Green Chem. 2015, 17, 356–364. [Google Scholar] [CrossRef]
- Conte, M.; Carley, A.F.; Heirene, C.; Willock, D.J.; Johnston, P.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Hydrochlorination of acetylene using a supported gold catalyst: A study of the reaction mechanism. J. Catal. 2007, 250, 231–239. [Google Scholar] [CrossRef]
- Xu, H.; Meng, S.; Luo, G. Ionic liquids-coordinated Au catalysts for acetylene hydrochlorination: DFT approach towards reaction mechanism and adsorption energy. Catal. Sci. Technol. 2018, 8, 1176–1182. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.C.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
| Catalyst | Eads(DCE) | Eads(C2H2) | Eads(HCl) | Eads(VCM) |
|---|---|---|---|---|
| RuCl3 | −22.6 | −149.3 | 36.0 | −193.4 |
| AuCl3 | −51.4 | −106.0 | 39.1 | −179.0 |
| BaCl2 | −122.9 | −161.9 | −73.9 | −215.0 |
| Catalyst | DCE Decomposition | Acetylene-DCE Complex | Ea (exp) | Error (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Ea1 | Ea2 | ΔGRDS | Ea1 | Ea2 | ΔGRDS | |||
| RuCl3 | 58.6 | 142.9 | 185.5 | 105.4 | 139.6 | 189.1 | 152.8 | −19.2 |
| AuCl3 | 90.6 | 125.5 | 128.0 | 106.6 | 118.5 | 141.4 | 134.2 | −5.1 |
| BaCl2 | 49.0 | 159.7 | 176.6 | 64.8 | 165.8 | 167.3 | 141.3 | −15.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Man, B.; Luo, G. Catalytic Mechanism Comparison Between 1,2-Dichloroethane-Acetylene Exchange Reaction and Acetylene Hydrochlorination Reaction for Vinyl Chloride Production: DFT Calculations and Experiments. Catalysts 2020, 10, 204. https://doi.org/10.3390/catal10020204
Xu H, Man B, Luo G. Catalytic Mechanism Comparison Between 1,2-Dichloroethane-Acetylene Exchange Reaction and Acetylene Hydrochlorination Reaction for Vinyl Chloride Production: DFT Calculations and Experiments. Catalysts. 2020; 10(2):204. https://doi.org/10.3390/catal10020204
Chicago/Turabian StyleXu, Hao, Baochang Man, and Guohua Luo. 2020. "Catalytic Mechanism Comparison Between 1,2-Dichloroethane-Acetylene Exchange Reaction and Acetylene Hydrochlorination Reaction for Vinyl Chloride Production: DFT Calculations and Experiments" Catalysts 10, no. 2: 204. https://doi.org/10.3390/catal10020204
APA StyleXu, H., Man, B., & Luo, G. (2020). Catalytic Mechanism Comparison Between 1,2-Dichloroethane-Acetylene Exchange Reaction and Acetylene Hydrochlorination Reaction for Vinyl Chloride Production: DFT Calculations and Experiments. Catalysts, 10(2), 204. https://doi.org/10.3390/catal10020204