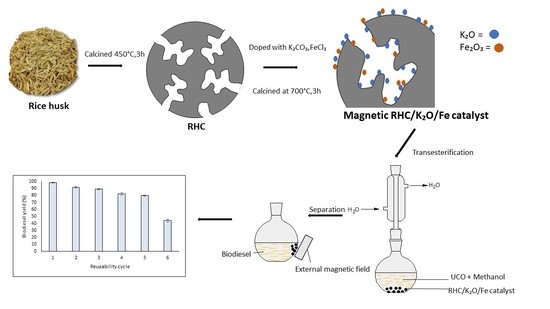
Supermagnetic Nano-Bifunctional Catalyst from Rice Husk: Synthesis, Characterization and Application for Conversion of Used Cooking Oil to Biodiesel
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Supermagnetic Nano-Bifunctional Catalysts
2.1.1. Crystallinity Investigation
2.1.2. Temperature Programmed Desorption (TPD)
2.1.3. Surface Area and Porosity Measurement
2.1.4. Thermal Gravimetric Analysis
2.1.5. Functional Group Analysis
2.1.6. Morphological and Energy Dispersive X-ray Spectroscopy Analysis
2.1.7. Vibrating Sampling Magnetometer Analysis
2.2. Influence of Process Parameters on the Transesterification of Used Cooking Oil
2.2.1. Effects of Catalyst Amount for Catalyst Screening and Catalytic Performance
2.2.2. Effects of Methanol to Oil Molar Ratio
2.2.3. Effects of Reaction Temperature
2.2.4. Effects of Reaction Duration
2.3. Reusability and Study of Deactivation of Supramagnetic RHC/K2O-20%/Fe-5% Nano-Bifunctional Catalyst
2.4. Comparative Study of Catalytic Activity with Literature Reported on Biochar Supported Catalysts
2.5. Biodiesel Confirmation Using 1H-Nuclear Magnetic Resonance (1H-NMR) and Fourier-Transform Infrared Spectroscopy (FTIR)
3. Materials and Method
3.1. Materials
3.2. Synthesis of Rice Husk Char
3.3. Preparation of Nano-Magnetic RHC/K2O/Fe Catalyst
3.4. Characterization of RHC/K2O/Fe Catalysts
3.5. Catalytical Activity Reactions Via Transesterification
3.6. Biodiesel Analysis
3.7. Catalyst Regeneration and Spent Catalyst Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jamil, F.; Al-Muhtaseb, A.H.; Al-Haj, L.; Al-Hinai, M.A.; Hellier, P.; Rashid, U. Optimization of oil extraction from waste “Date pits” for biodiesel production. Energy Convers. Manag. 2016, 117, 264–272. [Google Scholar] [CrossRef]
- Seffati, K.; Honarvar, B.; Esmaeili, H.; Esfandiari, N. Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel 2019, 235, 1238–1244. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Rashid, U.; Taufiq-Yap, Y.H.; Yaw, T.C.S.; Ismail, I. Synthesis of carbonaceous solid acid magnetic catalyst from empty fruit bunch for esterification of palm fatty acid distillate (PFAD). Energy Convers. Manag. 2019, 195, 480–491. [Google Scholar] [CrossRef]
- Oladipo, A.S.; Ajayi, O.A.; Oladipo, A.A.; Azarmi, S.L.; Nurudeen, Y.; Atta, A.Y.; Ogunyemi, S.S. Magnetic recyclable eggshell-based mesoporous catalyst for biodiesel production from crude neem oil: Process optimization by central composite design and artificial neural network. C. R. Chim. 2018, 21, 684–695. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Rashid, U.; Rehman, H.A.; Hussain, I.; Ibrahim, M.; Haider, M.S. Muskmelon (Cucumis melo) seed oil: A potential non-food oil source for biodiesel production. Energy 2011, 36, 5632–5639. [Google Scholar] [CrossRef]
- Mansir, N.; Teo, S.H.; Rabiu, I.; Taufiq-Yap, Y.H. Effective biodiesel synthesis from waste cooking oil and biomass residue solid green catalyst. Chem. Eng. J. 2018, 347, 137–144. [Google Scholar] [CrossRef]
- Mahesh, S.E.; Ramanathan, A.; Begum, K.M.M.S.; Narayanan, A. Biodiesel production from waste cooking oil using KBr impregnated CaO as catalyst. Energy Convers. Manag. 2015, 91, 442–450. [Google Scholar] [CrossRef]
- Sinha, D.; Murugavelh, S. Comparative studies on biodiesel production from Waste Cotton Cooking Oil using alkaline, calcined eggshell and pistachio shell catalyst. In Proceedings of the 2016 International Conference on Energy Efficient Technologies for Sustainability (ICEETS), Nagercoil, India, 7–8 April 2016; pp. 130–133. [Google Scholar]
- Chung, Z.L.; Tan, Y.H.; Chan, Y.S.; Kansedo, J.; Mubarak, N.M.; Ghasemi, M.; Abdullah, M.O. Life cycle assessment of waste cooking oil for biodiesel production using waste chicken eggshell derived CaO as catalyst via transesterification. Biocatal. Agric. Biotechnol. 2019, 21, 101317. [Google Scholar] [CrossRef]
- Wang, A.; Li, H.; Zhang, H.; Pan, H.; Yang, S. Efficient Catalytic Production of Biodiesel with Acid-Base Bifunctional Rod-Like Ca-B Oxides by the Sol-Gel Approach. Materials 2018, 12, 83. [Google Scholar] [CrossRef]
- Soltani, S.; Rashid, U.; Nehdi, I.A.; Al-Resayes, S.I.; Al-Muhtaseb, A.H. Sulfonated mesoporous zinc aluminate catalyst for biodiesel production from high free fatty acid feedstock using microwave heating system. J. Taiwan Inst. Chem. Eng. 2017, 70, 219–228. [Google Scholar] [CrossRef]
- Souza, R.D.; Vats, T.; Chattree, A.; Felix, P. Graphene supported magnetically separable solid acid catalyst for the single step conversion of waste cooking oil to biodiesel. Renew. Energy 2018, 126, 1064–1073. [Google Scholar] [CrossRef]
- Tantirungrotechai, J.; Thepwatee, S.; Yoosuk, B. Biodiesel synthesis over Sr/MgO solid base catalyst. Fuel 2013, 106, 279–284. [Google Scholar] [CrossRef]
- Rahmani Vahid, B.; Haghighi, M. Urea-nitrate combustion synthesis of MgO/MgAl2O4 nanocatalyst used in biodiesel production from sunflower oil: Influence of fuel ratio on catalytic properties and performance. Energy Convers. Manag. 2016, 126, 362–372. [Google Scholar] [CrossRef]
- Fan, M.; Wu, H.; Shi, M.; Zhang, P.; Jiang, P. Well-dispersive K2O—KCl alkaline catalyst derived from waste banana peel for biodiesel synthesis. Green Energy Environ. 2019, 4, 322–327. [Google Scholar] [CrossRef]
- Shahraki, H.; Entezari, M.H.; Goharshadi, E.K. Sono-synthesis of biodiesel from soybean oil by KF/γ-Al2O3 as a nano-solid-base catalyst. Ultrason. Sonochem. 2015, 23, 266–274. [Google Scholar] [CrossRef]
- Yatish, K.V.; Lalithamba, H.S.; Suresh, R.; Latha, H.K.E. Ochrocarpus longifolius assisted green synthesis of CaTiO3 nanoparticle for biodiesel production and its kinetic study. Renew. Energy 2020, 147, 310–321. [Google Scholar] [CrossRef]
- Argyle, M.; Bartholomew, C. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Thushari, I.; Babel, S.; Samart, C. Biodiesel production in an autoclave reactor using waste palm oil and coconut coir husk derived catalyst. Renew. Energy 2019, 134, 125–134. [Google Scholar] [CrossRef]
- Chellappan, S.; Aparna, K.; Chingakham, C.; Sajith, V.; Nair, V. Microwave assisted biodiesel production using a novel Brønsted acid catalyst based on nanomagnetic biocomposite. Fuel 2019, 246, 268–276. [Google Scholar] [CrossRef]
- Rezayan, A.; Taghizadeh, M. Synthesis of magnetic mesoporous nanocrystalline KOH/ZSM-5-Fe3O4 for biodiesel production: Process optimization and kinetics study. Process Saf. Environ. Prot. 2018, 117, 711–721. [Google Scholar] [CrossRef]
- Salimi, Z.; Hosseini, S.A. Study and optimization of conditions of biodiesel production from edible oils using ZnO/BiFeO3 nano magnetic catalyst. Fuel 2019, 239, 1204–1212. [Google Scholar] [CrossRef]
- Soltani, N.; Bahrami, A.; Pech-Canul, M.I.; González, L.A. Review on the physicochemical treatments of rice husk for production of advanced materials. Chem. Eng. J. 2015, 264, 899–935. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, W.; Lin, H.; Li, Y.; Lu, H.; Wang, Y. A green technology for the preparation of high capacitance rice husk-based activated carbon. J. Clean. Prod. 2016, 112, 1190–1198. [Google Scholar] [CrossRef]
- Daffalla, S.B.; Mukhtar, H.; Shaharun, M.S. Properties of activated carbon prepared from rice husk with chemical activation. Int. J. Glob. Environ. Issues 2012, 12, 107–129. [Google Scholar] [CrossRef]
- Shafini, M.S. A review on paddy residue based power generation: Energy, environment and economic perspective. Renew. Sustain. Energ. Rev. 2016, 59, 1089–1100. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Upgrading the rice husk char obtained by flash pyrolysis for the production of amorphous silica and high quality activated carbon. Bioresour. Technol. 2014, 170, 132–137. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, L.; Xing, S.; Luo, W.; Wang, Z.; Lv, P. Biodiesel production by a highly effective renewable catalyst from pyrolytic rice husk. J. Clean. Prod. 2018, 199, 772–780. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, Y.; Zhang, Z.; Ge, X.; Chen, M. Activated bio-chars derived from rice husk via one- and two-step KOH-catalyzed pyrolysis for phenol adsorption. Sci. Total Environ. 2019, 646, 1567–1577. [Google Scholar] [CrossRef]
- Chen, G.; Shan, R.; Shi, J.; Yan, B. Transesteri fi cation of palm oil to biodiesel using rice husk ash-based catalysts. Fuel Process. Technol. 2015, 133, 8–13. [Google Scholar] [CrossRef]
- Chen, K.; Wang, J.; Dai, Y.; Wang, P.; Liou, C.; Nien, C.; Wu, J.; Chen, C. Journal of the Taiwan Institute of Chemical Engineers Rice husk ash as a catalyst precursor for biodiesel production. J. Taiwan Inst. Chem. Eng. 2013, 44, 622–629. [Google Scholar] [CrossRef]
- Roschat, W.; Siritanon, T.; Yoosuk, B.; Promarak, V. Rice husk-derived sodium silicate as a highly efficient and low-cost basic heterogeneous catalyst for biodiesel production. Energy Convers. Manag. 2016, 119, 453–462. [Google Scholar] [CrossRef]
- Angin, D.; Şensöz, S. Effect of Pyrolysis Temperature on Chemical and Surface Properties of Biochar of Rapeseed (Brassica napus L.). Int. J. Phytoremed. 2014, 16, 684–693. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Wang, A.; Li, H.; Pan, H.; Zhang, H.; Xu, F.; Yu, Z.; Yang, S. Efficient and green production of biodiesel catalyzed by recyclable biomass-derived magnetic acids. Fuel Process. Technol. 2018, 181, 259–267. [Google Scholar] [CrossRef]
- Zhang, F.; Tian, X.F.; Fang, Z.; Shah, M.; Wang, Y.T.; Jiang, W.; Yao, M. Catalytic production of Jatropha biodiesel and hydrogen with magnetic carbonaceous acid and base synthesized from Jatropha hulls. Energy Convers. Manag. 2017, 142, 107–116. [Google Scholar] [CrossRef]
- Sano, N.; Yamada, K.; Tsunauchi, S.; Tamon, H. A novel solid base catalyst for transesterification of triglycerides toward biodiesel production: Carbon nanohorn dispersed with calcium ferrite. Chem. Eng. J. 2017, 307, 135–142. [Google Scholar] [CrossRef]
- Chellappan, S.; Nair, V.; Sajith, V.; Aparna, K. Synthesis, optimization and characterization of biochar based catalyst from sawdust for simultaneous esterification and transesterification. Chin. J. Chem. Eng. 2018, 26, 2654–2663. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, P.; Yang, L.; Xing, S.; Luo, W.; Wang, Z. Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel. Energy Convers. Manag. 2018, 160, 477–485. [Google Scholar] [CrossRef]
- Mendonça, I.M.; Paes, O.A.R.L.; Maia, P.J.S.; Souza, M.P.; Almeida, R.A.; Silva, C.C.; Duvoisin, S.; de Freitas, F.A. New heterogeneous catalyst for biodiesel production from waste tucumã peels (Astrocaryum aculeatum Meyer): Parameters optimization study. Renew. Energy 2019, 130, 103–110. [Google Scholar] [CrossRef]
- Li, X.F.; Zuo, Y.; Zhang, Y.; Fu, Y.; Guo, Q.X. In situ preparation of K2CO3 supported Kraft lignin activated carbon as solid base catalyst for biodiesel production. Fuel 2013, 113, 435–442. [Google Scholar] [CrossRef]
- Putra, M.D.; Irawan, C.; Udiatoro; Ristianingsih, Y.; Nata, I.F. A cleaner process for biodiesel production from waste cooking oil using waste materials as a heterogeneous catalyst and its kinetic study. J. Clean. Prod. 2018, 195, 1249–1258. [Google Scholar] [CrossRef]
- Mansir, N.; Teo, S.H.; Rashid, U.; Taufiq-Yap, Y.H. Efficient waste Gallus domesticus shell derived calcium-based catalyst for biodiesel production. Fuel 2018, 211, 67–75. [Google Scholar] [CrossRef]
- Ezzah-Mahmudah, S.; Lokman, I.M.; Saiman, M.I.; Taufiq-Yap, Y.H. Synthesis and characterization of Fe2O3/CaO derived from Anadara Granosa for methyl ester production. Energy Convers. Manag. 2016, 126, 124–131. [Google Scholar] [CrossRef]
- Dantas, J.; Leal, E.; Cornejo, D.R.; Kiminami, R.H.G.A.; Costa, A.C.F.M. Biodiesel production evaluating the use and reuse of magnetic nanocatalysts Ni0.5Zn0.5Fe2O4 synthesized in pilot-scale. Arab. J. Chem. 2018. [Google Scholar] [CrossRef]
- Gardy, J.; Rehan, M.; Hassanpour, A.; Lai, X.; Nizami, A.-S. Advances in nano-catalysts based biodiesel production from non-food feedstocks. J. Environ. Manag. 2019, 249, 109316. [Google Scholar] [CrossRef]
- Tan, Y.H.; Abdullah, M.O.; Nolasco Hipolito, C. Comparison of Biodiesel Production between Homogeneous and Heterogeneous Base Catalysts. Appl. Mech. Mater. 2016, 833, 71–77. [Google Scholar] [CrossRef]
- Zhang, P.; Han, Q.; Fan, M.; Jiang, P. Applied Surface Science Magnetic solid base catalyst CaO/CoFe2O4 for biodiesel production: Influence of basicity and wettability of the catalyst in catalytic performance. Appl. Surf. Sci. 2014, 317, 1125–1130. [Google Scholar] [CrossRef]
- Abdullah, M.O.; Nolasco-Hipolito, C.; Taufiq-Yap, Y.H. Waste ostrich- and chicken-eggshells as heterogeneous base catalyst for biodiesel production from used cooking oil: Catalyst characterization and biodiesel yield performance. Appl. Energy 2015, 160, 58–70. [Google Scholar]
- Teo, S.H.; Rashid, U.; Thomas Choong, S.Y.; Taufiq-Yap, Y.H. Heterogeneous calcium-based bimetallic oxide catalyzed transesterification of Elaeis guineensis derived triglycerides for biodiesel production. Energy Convers. Manag. 2017, 141, 20–27. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Kumar, T.; Halder, G. Recent advancement and prospective of heterogeneous carbonaceous catalysts in chemical and enzymatic transformation of biodiesel. Energy Convers. Manag. 2018, 167, 176–202. [Google Scholar] [CrossRef]
- Zhang, S.; Su, Y.; Zhu, S.; Zhang, H.; Zhang, Q. Effects of pretreatment and FeCl3preload of rice husk on synthesis of magnetic carbon composites by pyrolysis for supercapacitor application. J. Anal. Appl. Pyrolysis 2018, 135, 22–31. [Google Scholar] [CrossRef]
- Boz, N.; Degirmenbasi, N.; Kalyon, D.M. Transesterification of canola oil to biodiesel using calcium bentonite functionalized with K compounds. Appl. Catal. B Environ. 2013, 138, 236–242. [Google Scholar] [CrossRef]
- Balasundram, V.; Ibrahim, N.; Kasmani, R.M.; Hamid, M.K.A.; Isha, R.; Hasbullah, H.; Ali, R.R. Thermogravimetric catalytic pyrolysis and kinetic studies of coconut copra and rice husk for possible maximum production of pyrolysis oil. J. Clean. Prod. 2018, 167, 218–228. [Google Scholar] [CrossRef]
- Liou, T.H. Preparation and characterization of nano-structured silica from rice husk. Mater. Sci. Eng. A 2004, 364, 313–323. [Google Scholar] [CrossRef]
- Mansaray, K.G.; Ghaly, A.E. Thermal degradation of rice husks in nitrogen atmosphere. Bioresour. Technol. 1998, 65, 13–20. [Google Scholar] [CrossRef]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef]
- Yadav, M.; Sharma, Y.C. Process optimization and catalyst poisoning study of biodiesel production from kusum oil using potassium aluminum oxide as efficient and reusable heterogeneous catalyst. J. Clean. Prod. 2018, 199, 593–602. [Google Scholar] [CrossRef]
- Khelifi, S.; Ayari, F.; Hassan Chehimi, D.B.; Trabelsi-Ayadi, M. Synthesis and Characterization of Heterogeneous Catalysts and Comparison to Iron-ore. J. Chem. Eng. Process Technol. 2016, 7, 1–9. [Google Scholar]
- Santana Costa, J.A.; Paranhos, C.M. Systematic evaluation of amorphous silica production from rice husk ashes. J. Clean. Prod. 2018, 192, 688–697. [Google Scholar] [CrossRef]
- Touhami, D.; Zhu, Z.; Sinan, W.; Janaun, J.; Haywood, S. Journal of Environmental Chemical Engineering Characterization of rice husk-based catalyst prepared via conventional and microwave carbonisation. J. Environ. Chem. Eng. 2017, 5, 2388–2394. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Y.; Chen, C. Synthesis and characterization of magnetic LiFe5O8-LiFeO2 as a solid basic catalyst for biodiesel production. Catal. Commun. 2018, 106, 20–24. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Al-Tikrity, E.T.B.; Khalaf, A.M. Transesterification of non-edible oils over potassium acetate impregnated CaO solid base catalyst. Fuel 2018, 234, 81–93. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Haapaniemi, E.; Sillanpää, M. Nano-magnetic potassium impregnated ceria as catalyst for the biodiesel production. Renew. Energy 2019, 139, 1428–1436. [Google Scholar] [CrossRef]
- Yadav, M.; Mishra, N.; Sharma, N.; Chandra, S.; Kumar, D. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy Microwave assisted synthesis, characterization and biocidal activities of some new chelates of carbazole derived Schiff bases of cadmium and tin metals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 733–742. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Wang, Y.; Wang, T.; Zhou, S.; Hu, L.; Liu, T.; Elfalleh, W.; Yu, D. Structural characteristics of a Ni–Ag magnetic catalyst and its properties in soybean oil hydrogenation. Food Bioprod. Process. 2018, 109, 139–147. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A.; Subbarao, D. Biodiesel production from waste cooking oil using bifunctional heterogeneous solid catalysts. J. Clean. Prod. 2013, 59, 131–140. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.S.; Wang, X.J.; Jiang, J.Z.; Lin, H.; Han, S.; Pei, S.P. Mixed and ground KBr-impregnated calcined snail shell and kaolin as solid base catalysts for biodiesel production. Renew. Energy 2016, 93, 648–657. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, S.; Gong, W.; Wang, G.; Qiu, J.; Chen, H. Applied Catalysis A: General Synthesis, characterization and acid catalysis of solid acid from peanut shell. Appl. Catal. A Gen. 2014, 469, 284–289. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, A.; Sharma, Y.C. Biodiesel synthesis from microalgae (Anabaena PCC 7120) by using barium titanium oxide (Ba2TiO4) solid base catalyst. Bioresour. Technol. 2019, 287, 121357. [Google Scholar] [CrossRef]
- Naveenkumar, R.; Baskar, G. Biodiesel production from Calophyllum inophyllum oil using zinc doped calcium oxide (Plaster of Paris) nanocatalyst. Bioresour. Technol. 2019, 280, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Boro, J.; Konwar, L.J.; Thakur, A.J.; Deka, D. Ba doped CaO derived from waste shells of T striatula (TS-CaO) as heterogeneous catalyst for biodiesel production. Fuel 2014, 129, 182–187. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A.; Naeem, A.; Mahmood, T.; Ahmad, S.; Humayun, M.; Islam, M.G.U. Biodiesel production from date seed oil (Phoenix dactylifera L.) via egg shell derived heterogeneous catalyst. Chem. Eng. Res. Des. 2018, 132, 644–651. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Sayed, M.A. K+trapped kaolinite (Kaol/K+) as low cost and eco-friendly basic heterogeneous catalyst in the transesterification of commercial waste cooking oil into biodiesel. Energy Convers. Manag. 2018, 177, 468–476. [Google Scholar] [CrossRef]
- Essamlali, Y.; Amadine, O.; Fihri, A.; Zahouily, M. Sodium modified fluorapatite as a sustainable solid bi-functional catalyst for biodiesel production from rapeseed oil. Renew. Energy 2019, 133, 1295–1307. [Google Scholar] [CrossRef]
- Satyanarayana, C.V.; Srikant, D.; Gurav, H.R. Catalyst Deactivation and Regeneration. Ind. Catal. Process. Fine Spec. Chem. 2016, 5, 187–219. [Google Scholar]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H. Synthesis of Ti(SO4)O solid acid nano-catalyst and its application for biodiesel production from used cooking oil. Appl. Catal. A Gen. 2016, 527, 81–95. [Google Scholar] [CrossRef]
- Tariq, M.; Ali, S.; Ahmad, F.; Ahmad, M.; Zafar, M.; Khalid, N.; Khan, M.A. Identification, FT-IR, NMR (1H and 13C) and GC/MS studies of fatty acid methyl esters in biodiesel from rocket seed oil. Fuel Process. Technol. 2011, 92, 336–341. [Google Scholar] [CrossRef]
- Martínez, A.; Mijangos, G.E.; Romero-Ibarra, I.C.; Hernández-Altamirano, R.; Mena-Cervantes, V.Y. In-situ transesterification of Jatropha curcas L. seeds using homogeneous and heterogeneous basic catalysts. Fuel 2019, 235, 277–287. [Google Scholar] [CrossRef]
- Torres-Rodríguez, D.A.; Romero-Ibarra, I.C.; Ibarra, I.A.; Pfeiffer, H. Biodiesel production from soybean and Jatropha oils using cesium impregnated sodium zirconate as a heterogeneous base catalyst. Renew. Energy 2016, 93, 323–331. [Google Scholar] [CrossRef]
- Fernandes, S.A.; Cardoso, A.L.; Da Silva, M.J. A novel kinetic study of H3PW12O40—Catalyzed oleic acid esterification with methanol via 1H NMR spectroscopy. Fuel Process. Technol. 2012, 96, 98–103. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, V.; Sharma, Y.C. Methyl transesterification of waste cooking oil using a laboratory synthesized reusable heterogeneous base catalyst: Process optimization and homogeneity study of catalyst. Energy Convers. Manag. 2017, 148, 1438–1452. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Aziz, A.M.; Altamer, M.H. Potassium acetate supported on activated carbon for transesterification of new non-edible oil, bitter almond oil. Fuel 2016, 170, 130–140. [Google Scholar] [CrossRef]
- Li, J.; Liang, X. Magnetic solid acid catalyst for biodiesel synthesis from waste oil. Energy Convers. Manag. 2017, 141, 126–132. [Google Scholar] [CrossRef]
- Boonyuen, S.; Smith, S.M.; Malaithong, M.; Prokaew, A.; Cherdhirunkorn, B.; Luengnaruemitchai, A. Biodiesel production by a renewable catalyst from calcined Turbo jourdani (Gastropoda: Turbinidae) shells. J. Clean. Prod. 2018, 177, 925–929. [Google Scholar] [CrossRef]
- Mansir, N.; Hwa Teo, S.; Lokman Ibrahim, M.; Yun Hin, T.Y. Synthesis and application of waste egg shell derived CaO supported W-Mo mixed oxide catalysts for FAME production from waste cooking oil: Effect of stoichiometry. Energy Convers. Manag. 2017, 151, 216–226. [Google Scholar] [CrossRef]








| Catalyst | SBET (m2 g−1) a | Dp (nm) b | Vp (cm3 g−1) c | TPD-CO2 | TPD-NH3 | FAME Yield (%) d |
|---|---|---|---|---|---|---|
| Total Basicity (mmolg−1) | Total Acidity (mmolg−1) | |||||
| RH | 3.97 | 5.30 | 0.0097 | 2.07 | 15.57 | No reaction |
| RHC | 203.54 | 4.09 | 0.1644 | 2.71 | 37.34 | No reaction |
| RHC/K2O-15%/Fe-5% | 76.06 | 4.52 | 0.0632 | 3.34 | 37.36 | 67.29 |
| RHC/K2O-20%/Fe-5% | 57.89 | 4.70 | 0.0588 | 4.43 | 24.59 | 78.41 |
| RHC/K2O-20%/Fe-10% | 41.17 | 4.15 | 0.0486 | 2.07 | 30.15 | No reaction |
| RHC/K2O-25%/Fe-5% | 36.17 | 4.98 | 0.0474 | 2.69 | 17.22 | 75.27 |
| RHC/K2O-30%/Fe-5% | 27.62 | 4.77 | 0.0343 | 2.54 | 11.87 | 77.59 |
| Catalyst | SBET (m2 g−1) | Dp (nm) | Vp (cm3 g−1) | Total Basicity (mmolg−1) | Total Acidity (mmolg−1) |
|---|---|---|---|---|---|
| RHC/K2O-20%/Fe-5% | 57.89 | 4.70 | 0.0588 | 4.43 | 24.59 |
| Spent RHC/K2O-20%/Fe-5% | 2.78 | 14.66 | 0.0102 | 0.53 | 6.47 |
| Catalyst | Support | Reaction Condition | FAME Yield (%) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Catalyst Loading (wt %) | Duration (h) | Molar Ratio | Temperature (°C) | ||||
| CTPAC (K, P, CaMg) | Tucumã peels | 1 | 4 | 15:1 | 80 | 97.3 | [41] |
| K/BC-Fe2O3 | Bamboo charcoal | 2.5 | 1 | 8:1 | 60 | 98.0 | [19] |
| Ca2Fe2O5–CNH | Carbon nanohorn | - | 3 | - | - | 97.0 | [38] |
| 25K/AP-600 | Pomelo peel | 6 | 2.5 | 8:1 | 65 | 98.0 | [40] |
| RHC/K2O-20%/Fe-5% | Rice husk char | 4 | 4 | 12:1 | 75 | 98.6 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazmi, B.; Rashid, U.; Taufiq-Yap, Y.H.; Ibrahim, M.L.; Nehdi, I.A. Supermagnetic Nano-Bifunctional Catalyst from Rice Husk: Synthesis, Characterization and Application for Conversion of Used Cooking Oil to Biodiesel. Catalysts 2020, 10, 225. https://doi.org/10.3390/catal10020225
Hazmi B, Rashid U, Taufiq-Yap YH, Ibrahim ML, Nehdi IA. Supermagnetic Nano-Bifunctional Catalyst from Rice Husk: Synthesis, Characterization and Application for Conversion of Used Cooking Oil to Biodiesel. Catalysts. 2020; 10(2):225. https://doi.org/10.3390/catal10020225
Chicago/Turabian StyleHazmi, Balkis, Umer Rashid, Yun Hin Taufiq-Yap, Mohd Lokman Ibrahim, and Imededdine Arbi Nehdi. 2020. "Supermagnetic Nano-Bifunctional Catalyst from Rice Husk: Synthesis, Characterization and Application for Conversion of Used Cooking Oil to Biodiesel" Catalysts 10, no. 2: 225. https://doi.org/10.3390/catal10020225
APA StyleHazmi, B., Rashid, U., Taufiq-Yap, Y. H., Ibrahim, M. L., & Nehdi, I. A. (2020). Supermagnetic Nano-Bifunctional Catalyst from Rice Husk: Synthesis, Characterization and Application for Conversion of Used Cooking Oil to Biodiesel. Catalysts, 10(2), 225. https://doi.org/10.3390/catal10020225