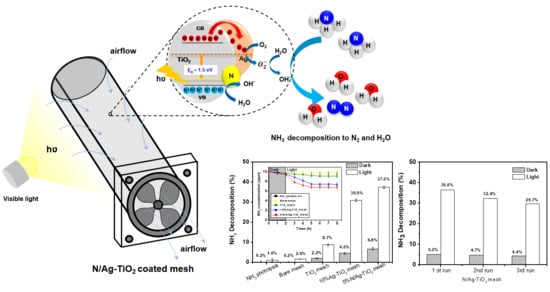
Enhanced Visible Light Photocatalytic Activity of N and Ag Doped and Co-Doped TiO2 Synthesized by Using an In-Situ Solvothermal Method for Gas Phase Ammonia Removal
Abstract
1. Introduction
2. Results and Discussion
2.1. Material Characteristics
2.1.1. Color and Surface Morphology
2.1.2. Specific Surface Area and Porosity
2.1.3. Crystallinity
2.1.4. The Surface Elemental Composition of the Photocatalysts
2.1.5. The Optical Property and Band Gap Energy
2.1.6. Electronic Structure
2.2. Photocatalytic Activities in Aqueous Phase and Gas Phase under Visible Light Irradiation
2.2.1. Photocatalytic Degradation of MB Dye Solution
2.2.2. Photocatalytic Decomposition of a Gas Phase NH3
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparations
3.3. Material Characterizations
3.4. The Photocatalytic Degradation of Methylene Blue (MB)
3.5. Immobilization of the Photocatalyst on Stainless Steel Mesh
3.6. Photocatalytic Decomposition of a Gas Phase Ammonia (NH3)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Estrada, J.M.; Kraakman, N.J.R.B.; Muñoz, R.; Lebrero, R. A Comparative Analysis of Odour Treatment Technologies in Wastewater Treatment Plants. Environ. Sci. Technol. 2011, 45, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Alinezhad, E.; Haghighi, M.; Rahmani, F.; Keshizadeh, H.; Abdi, M.; Naddafi, K. Technical and economic investigation of chemical scrubber and bio-filtration in removal of H2S and NH3 from wastewater treatment plant. J. Environ. Manag. 2019, 241, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Altomare, M.; Selli, E. Effects of metal nanoparticles deposition on the photocatalytic oxidation of ammonia in TiO2 aqueous suspensions. Catal. Today 2013, 209, 127–133. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.; Wang, S. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Boxi, S.S.; Paria, S. Visible light induced enhanced photocatalytic degradation of organic pollutants in aqueous media using Ag doped hollow TiO2 nanospheres. RSC Adv. 2015, 5, 37657–37668. [Google Scholar] [CrossRef]
- Liu, H.; Dong, X.; Liu, T.; Zhu, Z. In-situ fabrication of silver-modified TiO2 microspheres for enhanced visible light driven photocatalytic activities. Sol. Energy Mater. Sol. Cells 2015, 132, 86–93. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospect. JSAP Int. 2005, 44, 8269–8285. [Google Scholar]
- Pelaez, M.; Nolan, N.T.; Pillai, S.; Seery, M.; Falaras, P.; Kontos, A.G.; Dunlop, P.; Hamilton, J.; Byrne, J.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 Anatase with a Bandgap in the Visible Region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef]
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Mater. 2017, 3, 3–16. [Google Scholar] [CrossRef]
- Santos, L.M.; Machado, A.; França, M.D.; Borges, K.A.; Paniago, R.M.; Patrocinio, A.O. Structural characterization of Ag-doped TiO2 with enhanced photocatalytic activity. RSC Adv. 2015, 5, 103752–103759. [Google Scholar] [CrossRef]
- Albiter, E.; Valenzuela, M.; Alfaro, S.; Valverde-Aguilar, G.; Martínez-Pallares, F. Photocatalytic deposition of Ag nanoparticles on TiO2: Metal precursor effect on the structural and photoactivity properties. J. Saudi Chem. Soc. 2015, 19, 563–573. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G. Trends in non-metal doping of anatase TiO2: B, C, N and F. Catal. Today 2013, 206, 12–18. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, B.; Yuan, R. Doping a metal (Ag, Al, Mn, Ni and Zn) on TiO2 nanotubes and its effect on Rhodamine B photocatalytic oxidation. Environ. Eng. Res. 2015, 20, 329–335. [Google Scholar] [CrossRef]
- Parveen, B.; Hassan, M.U.; Khalid, Z.; Riaz, S.; Naseem, S. Room-temperature ferromagnetism in Ni-doped TiO2 diluted magnetic semiconductor thin films. J. Appl. Res. Technol. 2017, 15, 132–139. [Google Scholar] [CrossRef]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Katoueizadeh, E.; Zebarjad, S.M.; Janghorban, K. Synthesis and enhanced visible-light activity of N-doped TiO2 nano-additives applied over cotton textiles. J. Mater. Res. Technol. 2018, 7, 204–211. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Ali, T.; Ahmed, A.; Alam, U.; Uddin, I.; Tripathi, P.; Muneer, M. Enhanced photocatalytic and antibacterial activities of Ag-doped TiO2 nanoparticles under visible light. Mater. Chem. Phys. 2018, 212, 325–335. [Google Scholar] [CrossRef]
- Elsellami, L.; Dappozze, F.; Houas, A.; Guillard, C. Effect of Ag+ reduction on the photocatalytic activity of Ag-doped TiO2. Superlattices Microstruct. 2017, 109, 511–518. [Google Scholar] [CrossRef]
- García-Serrano, J.; Gómez-Hernández, E.; Ocampo-Fernández, M.; Pal, U. Effect of Ag doping on the crystallization and phase transition of TiO2 nanoparticles. Curr. Appl. Phys. 2009, 9, 1097–1105. [Google Scholar] [CrossRef]
- Kerkez, Ö.; Boz, İ. Efficient removal of methylene blue by photocatalytic degradation with TiO2 nanorod array thin films. React. Kinet. Mech. Catal. 2013, 110, 543–557. [Google Scholar] [CrossRef]
- Elghniji, K.; Ksibi, M.; Elaloui, E. Sol–gel reverse micelle preparation and characterization of N-doped TiO2: Efficient photocatalytic degradation of methylene blue in water under visible light. J. Ind. Eng. Chem. 2012, 18, 178–182. [Google Scholar] [CrossRef]
- Khan, M.; Gul, S.R.; Li, J.; Cao, W. Photocatalytic Degradation of Methylene Blue by Hydrothermally Prepared Ag-Doped TiO2 Under Visible Light Irradiations. JOM 2015, 67, 2104–2107. [Google Scholar] [CrossRef]
- Gupta, S.; Tripathi, M. A review on the synthesis of TiO2 nanoparticles by solution route. Chin. Sci. Bull. 2011, 12, 1639–1657. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Li, J.; Wu, Q.; Wu, J. Synthesis of Nanoparticles via Solvothermal and Hydrothermal Methods. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–28. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Kusano, D.; Emori, M.; Sakama, S. Influence of electronic structure on visible light photocatalytic activity of nitrogen doped TiO2. RSC Adv. 2017, 7, 1887–1898. [Google Scholar] [CrossRef]
- Krejcikova, S.; Matějová, L.; Koci, K.; Obalová, L.; Matěj, Z.; Čapek, L.; Solcova, O. Preparation and characterization of Ag-doped crystalline titania for photocatalysis applications. Appl. Catal. B Environ. 2012, 111–112, 119–125. [Google Scholar] [CrossRef]
- Quan, F.; Hu, Y.; Zhang, X.; Wei, C. Simple preparation of Mn-N-codoped TiO2 photocatalyst and the enhanced photocatalytic activity under visible light irradiation. Appl. Surf. Sci. 2014, 320, 120–127. [Google Scholar] [CrossRef]
- Scarpelli, F.; Mastropietro, T.; Poerio, T.; Godbert, N. Mesoporous TiO2 Thin Films: State of the Art. In Titanium Dioxide—Material for a Sustainable Environment; Yang, D., Ed.; IntechOpen: London, UK, 2018; pp. 57–80. [Google Scholar]
- Mogal, S.I.; Gandhi, V.G.; Mishra, M.K.; Tripathi, S.; Shripathi, T.; Joshi, P.A.; Shah, D.O. Single-Step Synthesis of Silver-Doped Titanium Dioxide: Influence of Silver on Structural, Textural, and Photocatalytic Properties. Ind. Eng. Chem. Res. 2014, 53, 5749–5758. [Google Scholar] [CrossRef]
- Akel, S.; Dillert, R.; Balayeva, N.O.; Boughaled, R.; Koch, J.; El Azzouzi, M.; Bahnemann, D.B.D. Ag/Ag2O as a Co-Catalyst in TiO2 Photocatalysis: Effect of the Co-Catalyst/Photocatalyst Mass Ratio. Catalysts 2018, 8, 647. [Google Scholar] [CrossRef]
- Singh, I.; Birajdar, B. Synthesis, characterization and photocatalytic activity of mesoporous Na-doped TiO2 nano-powder prepared via a solvent-controlled non-aqueous sol–gel route. RSC Adv. 2017, 7, 54053–54062. [Google Scholar] [CrossRef]
- Moghaddam, M.; Nasirian, S. Dependence of activation energy and lattice strain on TiO2 nanoparticles. Nanosci. Methods 2012, 1, 201–212. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, K.; Guo, X.; Shen, G.; Xiang, J. Synthesis and characterization of N-doped TiO2 loaded onto activated carbon fiber with enhanced visible-light photocatalytic activity. New J. Chem. 2014, 38, 6139–6146. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z.; Wan, J. Enhanced Photocatalytic Activity of Nitrogen Doped TiO2 Anatase Nano-Particle under Simulated Sunlight Irradiation. Energy Procedia 2012, 16, 598–605. [Google Scholar] [CrossRef]
- Li, G.; Li, J.; Li, G.; Jiang, G. N and Ti3+ co-doped 3D anatase TiO2 superstructures composed of ultrathin nanosheets with enhanced visible light photocatalytic activity. J. Mater. Chem. A 2015, 3, 22073–22080. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z.; Yang, L. Synthesis and characterization of N-doped TiO2 and its enhanced visible-light photocatalytic activity. Arab. J. Chem. 2016, 9 (Suppl. 2), S1706–S1711. [Google Scholar] [CrossRef]
- Lee, T.Y.; Lee, C.Y.; Chiu, H.T. Enhanced Photocatalysis from Truncated Octahedral Bipyramids of Anatase TiO2 with Exposed {001}/{101} Facets. ACS Omega 2018, 3, 10225–10232. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, S.; Wan, P.; Sun, J.; Hood, Z.D. Introducing Ti3+ defects based on lattice distortion for enhanced visible light photoreactivity in TiO2 microspheres. RSC Adv. 2017, 7, 32461–32467. [Google Scholar] [CrossRef]
- Xu, T.; Wang, M.; Wang, T. Effects of N Doping on the Microstructures and Optical Properties of TiO2. J. Wuhan Univ. Technol.-Mat. Sci. 2019, 34, 55–63. [Google Scholar] [CrossRef]
- Miyauchi, M.; Ikezawa, A.; Tobimatsu, H.; Irie, H.; Hashimoto, K. Zeta potential and photocatalytic activity of nitrogen doped TiO2 thin films. Phys. Chem. Chem. Phys. 2004, 6, 865–870. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Y. The Photocatalytic Property of Nitrogen-Doped TiO2 Nanoball Film. Int. J. Photoenergy 2013, 1–6. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; He, X.; Huang, X.; Hang, R.; Tang, B. Effects of silver concentrations on microstructure and properties of nanostructured titania films. Mater. Des. (1980–2015) 2015, 65, 600–605. [Google Scholar] [CrossRef]
- Zielinska, J.A.; Kowalska, E.; Sobczak, J. Silver-doped TiO2 prepared by microemulsion method: Surface properties, bio- and photoactivity. Sep. Purif. Technol. 2010, 45, 155–162. [Google Scholar] [CrossRef]
- Gannoruwa, A.; Ariyasinghe, B.; Bandara, J. The mechanism and material aspects of a novel Ag2O/TiO2 photocatalyst active in infrared radiation for water splitting. Catal. Sci. Technol. 2016, 6, 479–487. [Google Scholar] [CrossRef]
- Jung, H.-Y.; Yeo, I.-S.; Kim, T.-U.; Ki, H.-C.; Gu, H.-B. Surface plasmon resonance effect of silver nanoparticles on a TiO2 electrode for dye-sensitized solar cells. Appl. Surf. Sci. 2018, 432, 266–271. [Google Scholar] [CrossRef]
- Jin, M.; Nagaoka, Y.; Nishi, K.; Ogawa, K.; Nagahata, S.; Horikawa, T.; Katoh, M.; Tomida, T.; Hayashi, J. Adsorption properties and photocatalytic activity of TiO2 and La-doped TiO2. Adsorption 2008, 14, 257–263. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, B.; Wang, L.; Xing, M.; Lei, J. Provides a comprehensive description of the photocatalytic mechanism and factors affecting photocatalytic activity. In Photocatalysis: Fundamentals, Materials and Applications; Springer: Singapore, 2018; pp. 1–15. [Google Scholar]
- Lee, J.; Park, H.; Choi, W. Selective Photocatalytic Oxidation of NH3 to N2 on Platinized TiO2 in Water. Environ. Sci. Technol. 2002, 36, 5462–5468. [Google Scholar] [CrossRef]
- Kibasomba, P.M.; Dhlamini, S.; Maaza, M.; Liu, C.-P.; Rashad, M.M.; Rayan, D.A.; Mwakikunga, B. Strain and grain size of TiO2 nanoparticles from TEM, Raman spectroscopy and XRD: The revisiting of the Williamson-Hall plot method. Results Phys. 2018, 9, 628–635. [Google Scholar] [CrossRef]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 2012, 6, 6. [Google Scholar] [CrossRef]
- Suram, S.K.; Newhouse, P.F.; Gregoire, J.M. High Throughput Light Absorber Discovery, Part 1: An Algorithm for Automated Tauc Analysis. ACS Comb. Sci. 2016, 18, 673–681. [Google Scholar] [CrossRef] [PubMed]

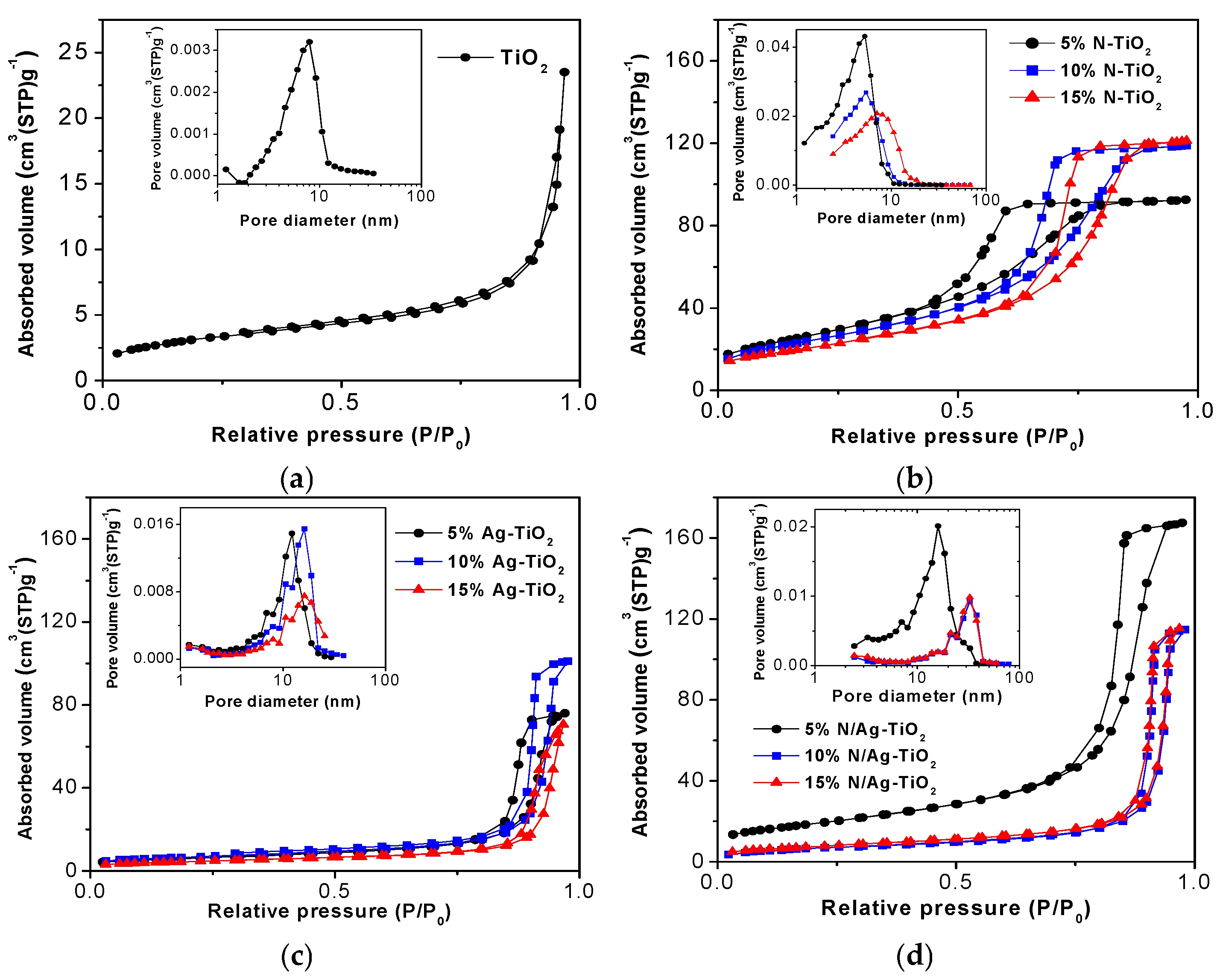




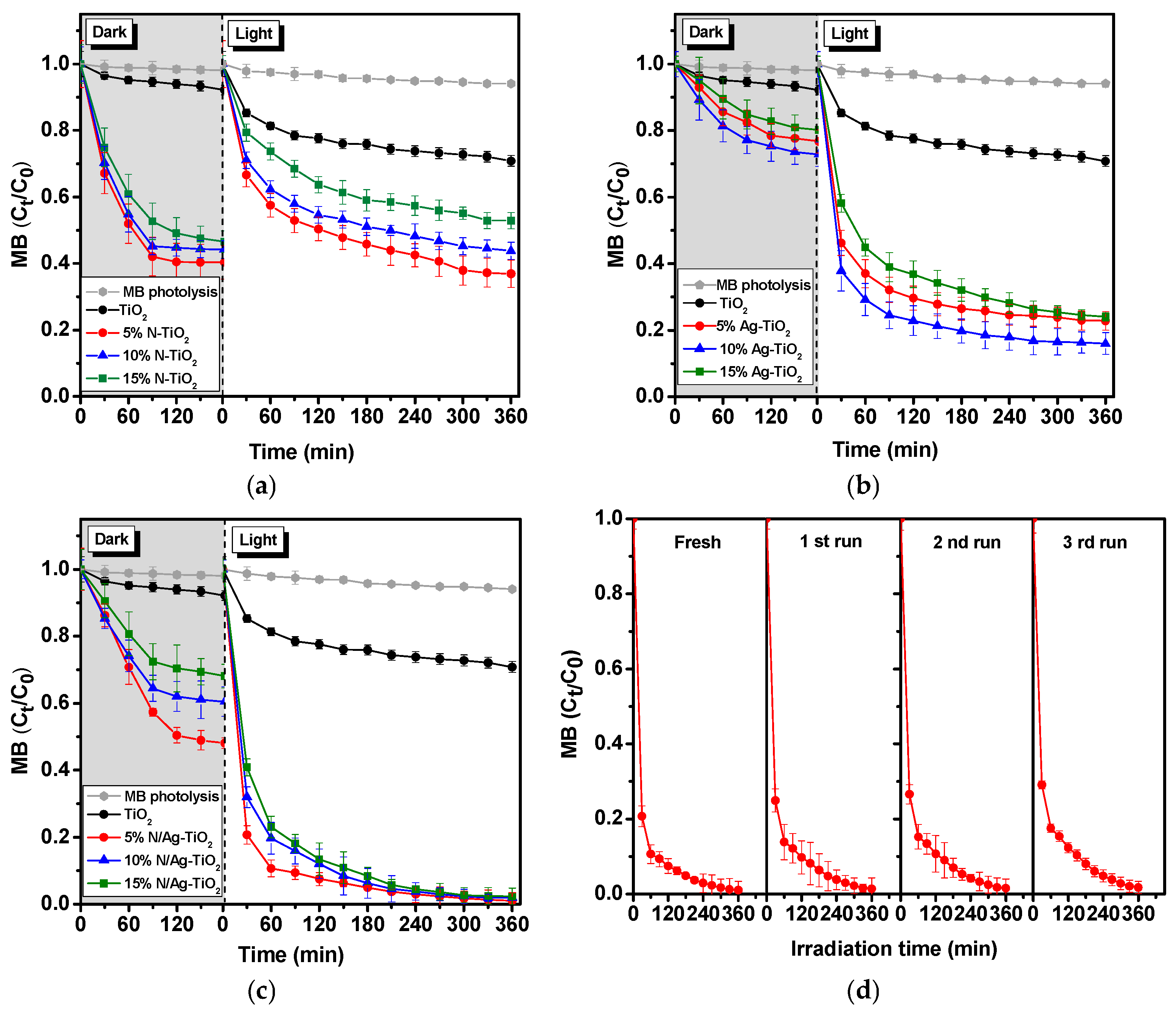




| Samples | Crystal Sizes | Lattice Strain | SBET (m2g−1) | VBJH (cm3g−1) | DBJH (nm) | Eg (eV) | % MB Reduction (Average ± SD) | Kapp (×10−5 min−1) | |
|---|---|---|---|---|---|---|---|---|---|
| DD-S (nm) | DW-H (nm) | ||||||||
| MB degradation by photolysis without a catalyst | 6 ± 0.01 | 0.0 | |||||||
| TiO2 | 61.48 | 57.11 | 0.0000 | 11.64 | 0.02 | 7.99 | 3.20 | 35.17 ± 0.02 | 1.5 |
| 5% N-TiO2 | 11.45 | 9.72 | −0.0120 | 122.80 | 0.19 | 5.87 | 3.02 | 63.05 ± 0.06 | 4.0 |
| 10% N-TiO2 | 14.85 | 10.15 | −0.0034 | 117.23 | 0.18 | 8.11 | 3.02 | 56.21 ± 0.04 | 3.2 |
| 15% N-TiO2 | 16.39 | 14.28 | −0.0040 | 101.91 | 0.14 | 9.83 | 3.00 | 47.10 ± 0.05 | 2.5 |
| 5% Ag-TiO2 | 18.78 | 18.52 | 0.0002 | 21.50 | 0.12 | 21.82 | 2.1 | 77.18 ± 0.03 | 5.7 |
| 10% Ag-TiO2 | 15.81 | 16.71 | 0.0007 | 23.60 | 0.16 | 26.54 | 1.7 | 84.00 ± 0.04 | 6.8 |
| 15% Ag-TiO2 | 15.53 | 12.35 | −0.0031 | 16.15 | 0.11 | 27.04 | 1.7 | 75.95 ± 0.04 | 5.5 |
| Co-doped materials 6 | |||||||||
| 5% N/Ag-TiO2 | 11.47 | 9.86 | −0.0017 | 81.16 | 0.26 | 15.97 | 1.5 | 98.82 ± 0.02 | 17.8 |
| 10% N/Ag-TiO2 | 15.08 | 15.06 | −0.0007 | 30.87 | 0.17 | 32.57 | 1.5 | 98.08 ± 0.03 | 17.2 |
| 15% N/Ag-TiO2 | 15.97 1 | 15.12 1 | −0.0007 2 | 29.01 3 | 0.17 3 | 30.88 3 | 1.6 4 | 97.65 ± 0.02 | 16.5 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirivallop, A.; Areerob, T.; Chiarakorn, S. Enhanced Visible Light Photocatalytic Activity of N and Ag Doped and Co-Doped TiO2 Synthesized by Using an In-Situ Solvothermal Method for Gas Phase Ammonia Removal. Catalysts 2020, 10, 251. https://doi.org/10.3390/catal10020251
Sirivallop A, Areerob T, Chiarakorn S. Enhanced Visible Light Photocatalytic Activity of N and Ag Doped and Co-Doped TiO2 Synthesized by Using an In-Situ Solvothermal Method for Gas Phase Ammonia Removal. Catalysts. 2020; 10(2):251. https://doi.org/10.3390/catal10020251
Chicago/Turabian StyleSirivallop, Adilah, Thanita Areerob, and Siriluk Chiarakorn. 2020. "Enhanced Visible Light Photocatalytic Activity of N and Ag Doped and Co-Doped TiO2 Synthesized by Using an In-Situ Solvothermal Method for Gas Phase Ammonia Removal" Catalysts 10, no. 2: 251. https://doi.org/10.3390/catal10020251
APA StyleSirivallop, A., Areerob, T., & Chiarakorn, S. (2020). Enhanced Visible Light Photocatalytic Activity of N and Ag Doped and Co-Doped TiO2 Synthesized by Using an In-Situ Solvothermal Method for Gas Phase Ammonia Removal. Catalysts, 10(2), 251. https://doi.org/10.3390/catal10020251