Biocatalytic Synthesis of Calycosin-7-O-β-D-Glucoside with Uridine Diphosphate–Glucose Regeneration System
Abstract
:1. Introduction
2. Results and Discussion
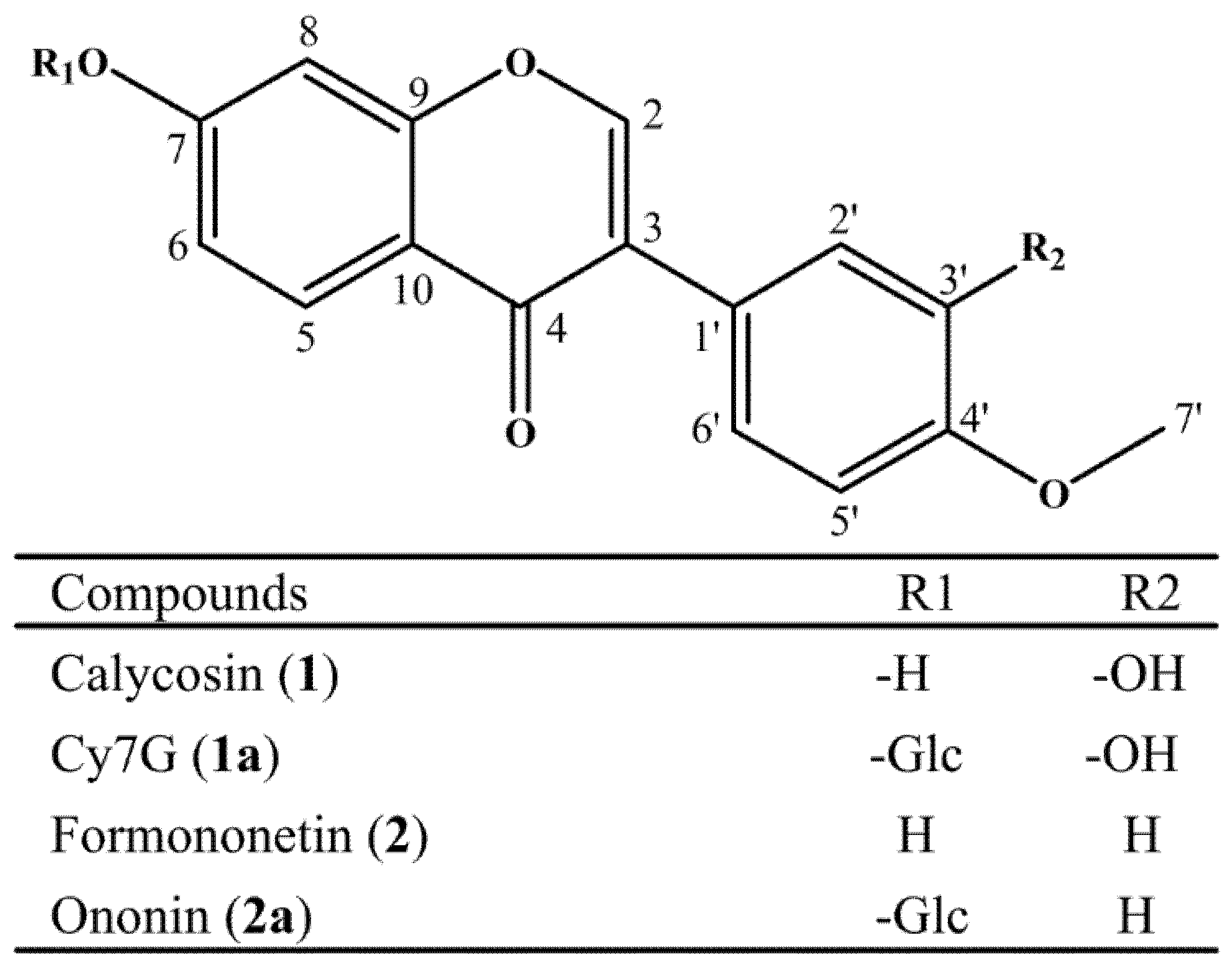
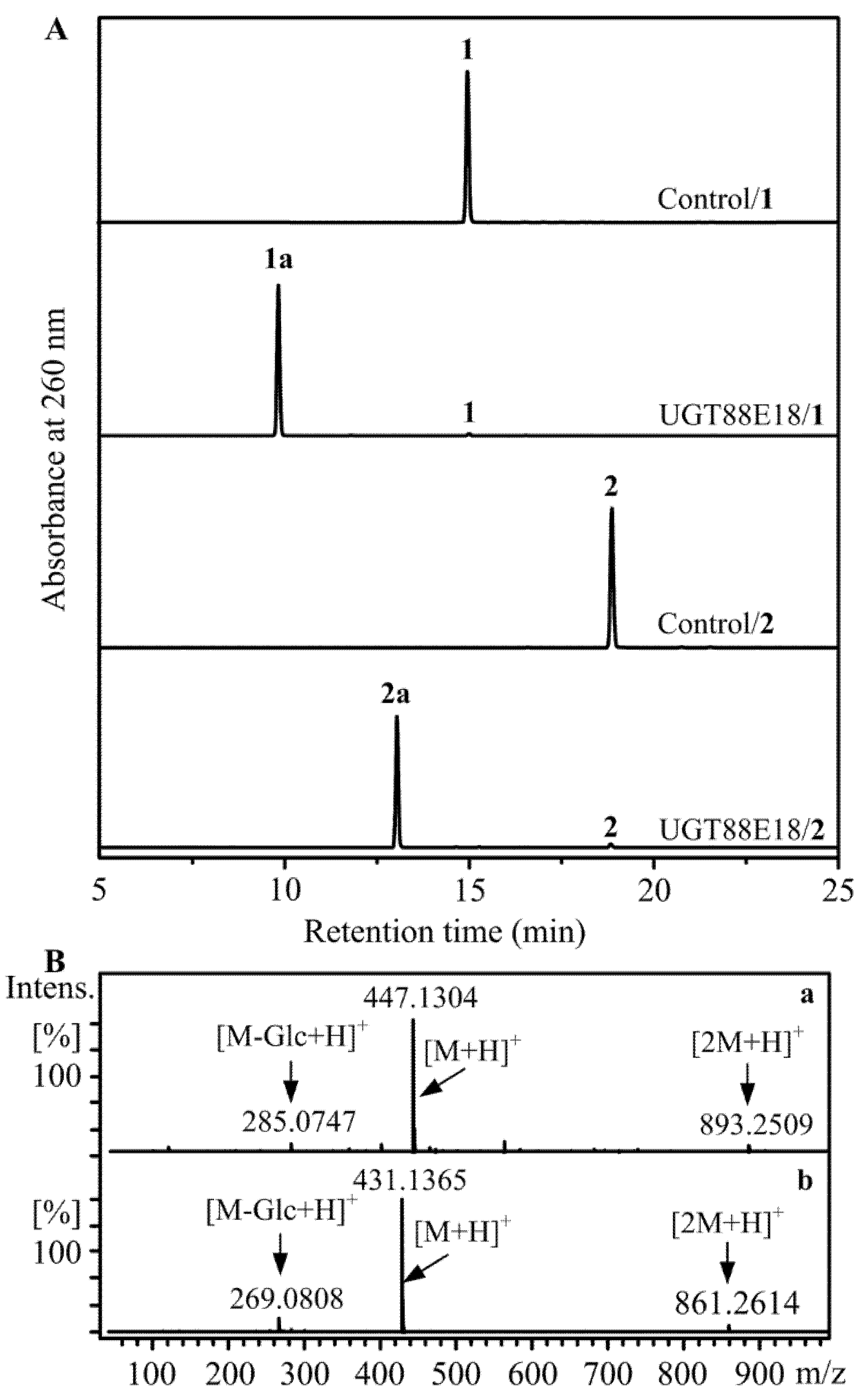
2.1. Regioselective Glucosylation of Calycosin and Formononetin by UGT88E18
2.2. Kinetic Analysis of UGT88E18 Toward Formononetin and Calycosin
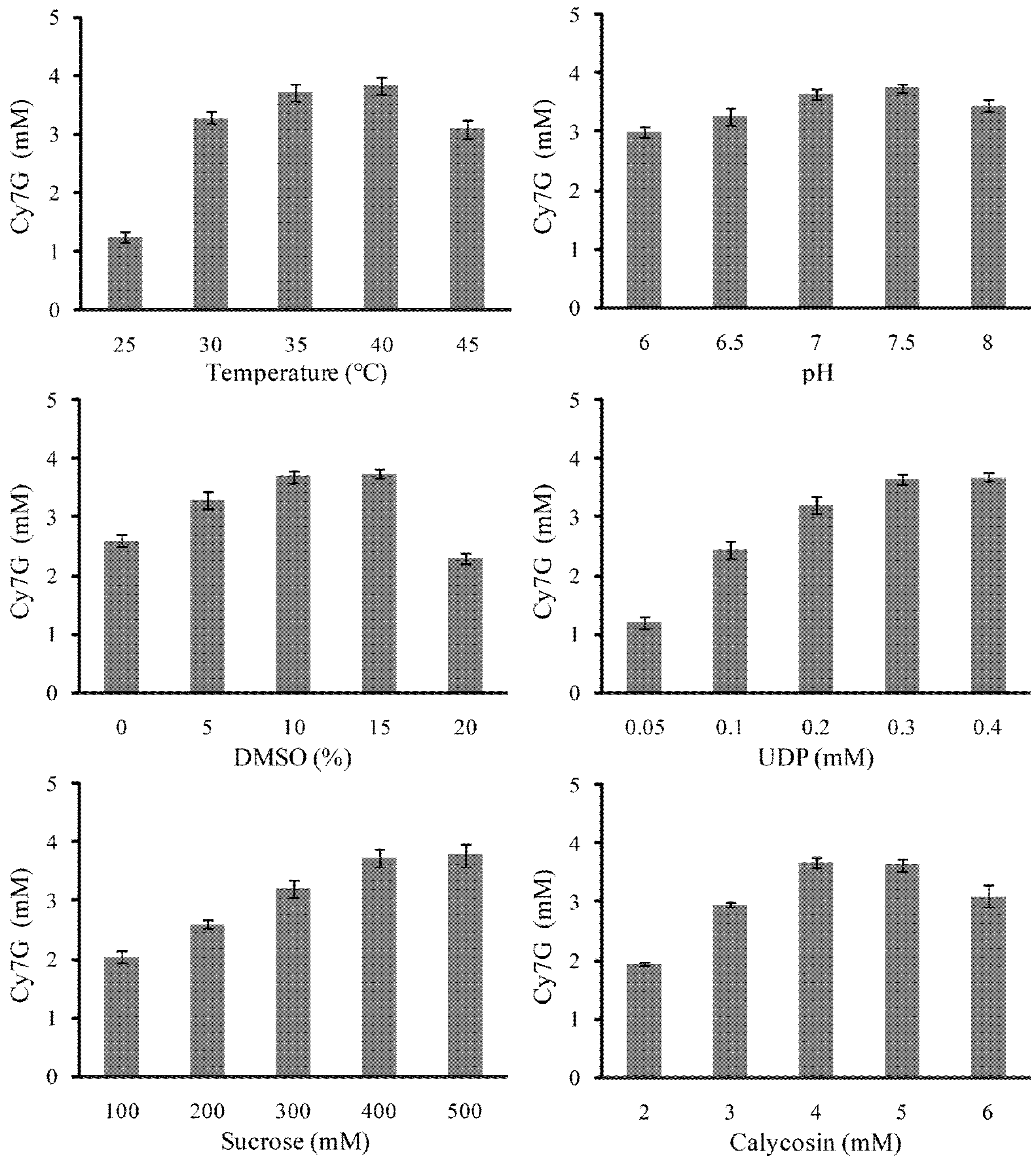
2.3. Optimization of UGT88E18–SuSy Cascade Reaction for Cy7G Synthesis
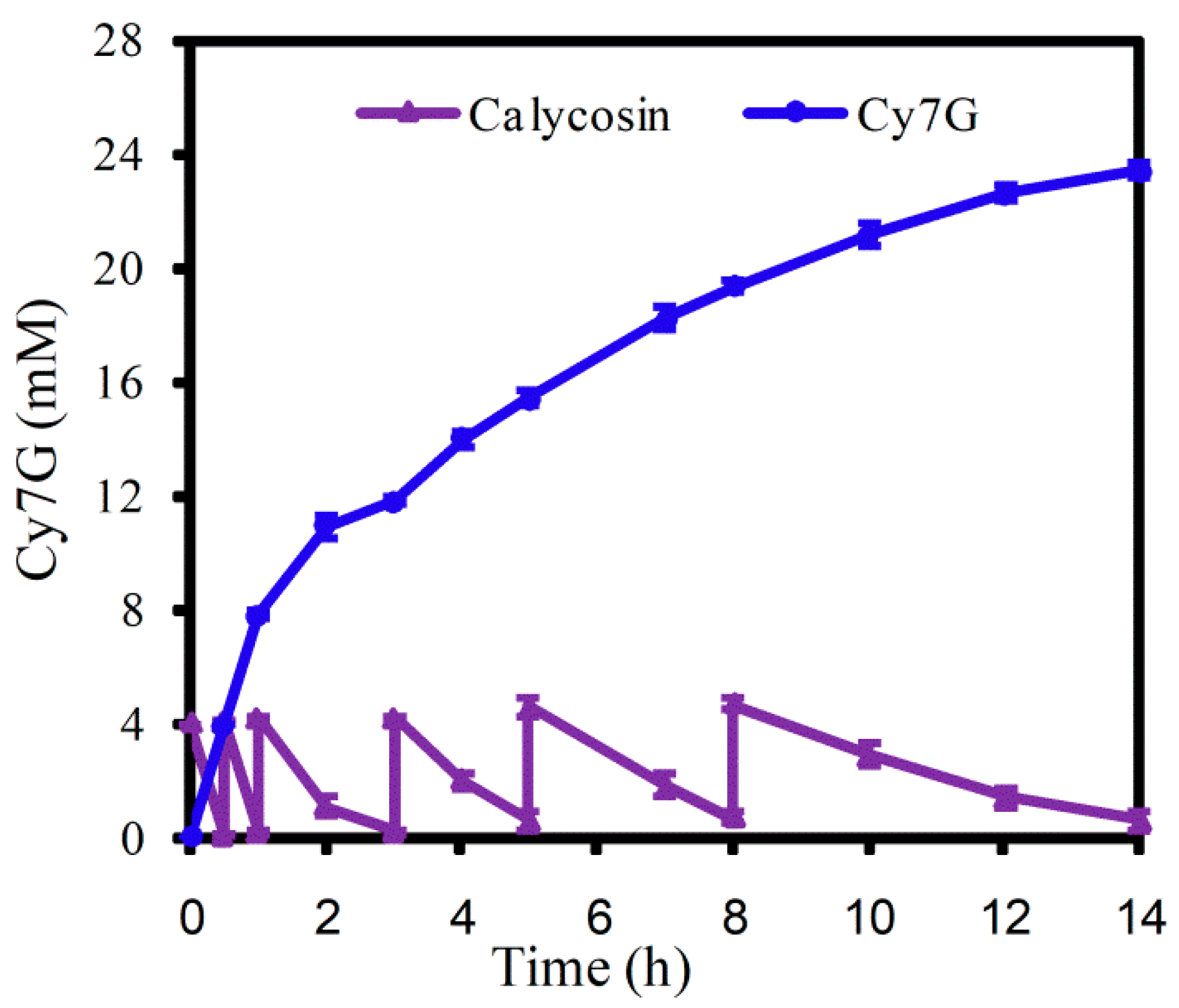
2.4. Fed-Batch Synthesis of Cy7G by UGT88E18–SuSy Cascade Reaction
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Expression and Purification of Recombinant Proteins
3.3. Enzyme Activity
3.4. Kinetic Parameters of UGT88E18
3.5. Optimization of UGT88E18–SuSy Cascade Reaction
3.6. Fed-Batch Synthesis of Cy7G
3.7. Purification and Structural Elucidation of the Glucosylated Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chiang, C.M.; Wang, T.Y.; Yang, Z.Y.; Wu, J.Y.; Chang, T.S. Production of new isoflavone glucosides from glycosylation of 8-hydroxydaidzein by glycosyltransferase from Bacillus subtilis ATCC 6633. Catalysts 2018, 9, 387. [Google Scholar] [CrossRef] [Green Version]
- Hertog, M.G.; Hollman, P.C.; Putte, B.V. Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices. J. Agric. Food Chem. 1993, 41, 1242–1246. [Google Scholar] [CrossRef]
- Hertog, M.G.; Hollman, P.C.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Saraei, R.; Marofi, F.; Naimi, A.; Talebi, M.; Ghaebi, M.; Javan, N.; Salimi, O.; Hassanzadeh, A. Leukemia therapy by flavonoids: Future and involved mechanisms. J. Cell. Physiol. 2019, 234, 8203–8220. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.P.; Gurung, R.B.; Parajuli, P.; Koirala, N.; Sohng, J.K. Assessing acceptor substrate promiscuity of YjiC-mediated glycosylation toward flavonoids. Carbohyd. Res. 2014, 393, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Hu, Z.M.; Song, W.; Wang, Z.L.; He, J.B.; Shi, X.M.; Cui, Q.H.; Qiao, X.; Ye, M. Diversity of O-glycosyltransferases contributes to the biosynthesis of flavonoid and triterpenoid glycosides in Glycyrrhiza uralensis. ACS Synth. Biol. 2019, 8, 1858–1866. [Google Scholar] [CrossRef]
- Rha, C.S.; Kim, E.R.; Kim, Y.J.; Jung, Y.S.; Kim, D.O.; Park, C.S. Simple and efficient production of highly soluble daidzin glycosides by amylosucrase from Deinococcus geothermalis. J. Agric. Food Chem. 2019, 67, 12824–12832. [Google Scholar] [CrossRef]
- Sangeetha, K.S.; Umamaheswari, S.; Reddy, C.U.M.; Kalkura, S.N. Flavonoids: Therapeutic potential of natural pharmacological agents. Int. J. Pharm. Sci. Res. 2016, 7, 3924. [Google Scholar]
- Li, L.; Zheng, S.H.; Brinckmann, J.A.; Fu, J.; Zeng, R.; Huang, L.F.; Chen, S.L. Chemical and genetic diversity of Astragalus mongholicus grown in different eco-climatic regions. PLoS ONE 2017, 12, e0184791. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.W.; Yu, Q.T.; Li, P.; Li, S.L.; Wang, Y.X.; Sheng, L.H.; Yi, L. Quality evaluation of Radix Astragali through a simultaneous determination of six major active isoflavonoids and four main saponins by high-performance liquid chromatography coupled with diode array and evaporative light scattering detectors. J. Chromatogr. A 2006, 1134, 162–169. [Google Scholar] [CrossRef]
- Jian, J.; Sun, L.J.; Cheng, X.; Hu, X.F.; Liang, J.C.; Chen, Y. Calycosin-7-O-β-d-glucopyranoside stimulates osteoblast differentiation through regulating the BMP/WNT signaling pathways. Acta Pharm. Sin. B 2015, 5, 454–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.Q.; Xue, X.L.; Yang, Y.; Ma, W.; Han, Y.; Qin, X.M. Multiple biological defects caused by calycosin-7-O-β-D-glucoside in the nematode Caenorhabditis elegans are associated with the activation of oxidative damage. J. Appl. Toxicol. 2018, 38, 801–809. [Google Scholar] [CrossRef]
- Xiao, W.H.; Han, L.J.; Shi, B. Isolation and purification of flavonoid glucosides from Radix Astragali by high-speed counter-current chromatography. J. Chromatogr. B 2009, 877, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.H.; Li, J.; Yang, J.G.; Zhu, Y.M.; Men, Y.; Zeng, Y.; Cai, Y.; Dong, C.X.; Dai, Z.B.; Zhang, X.L.; et al. Use of a promiscuous glycosyltransferase from Bacillus subtilis 168 for the enzymatic synthesis of novel protopanaxatriol-type ginsenosides. J. Agric. Food Chem. 2018, 66, 943–949. [Google Scholar] [CrossRef]
- Xie, K.B.; Dou, X.X.; Chen, R.D.; Chen, D.W.; Fang, C.; Xiao, Z.Y.; Dai, J.G. Two novel fungal phenolic UDP glycosyltransferases from Absidia coerulea and Rhizopus japonicus. Appl. Environ. Microbiol. 2017, 83, e03103–e03116. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.W.; Fan, S.; Chen, R.D.; Xie, K.B.; Yin, S.; Sun, L.L.; Liu, J.M.; Yang, L.; Kong, J.Q.; Yang, Z.Y.; et al. Probing and engineering key residues for bis-C-glycosylation and promiscuity of a C-glycosyltransferase. ACS Catal. 2018, 8, 4917–4927. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.N.; Liu, X.F.; Guo, F.; Ma, C.X.; Liang, J.H.; Feng, X.D.; Li, C. Mining of sucrose synthases from Glycyrrhiza uralensis and their application in the construction of an efficient UDP-recycling system. J. Agric. Food Chem. 2019, 67, 11694–11702. [Google Scholar] [CrossRef]
- Gutmann, A.; Nidetzky, B. Unlocking the potential of leloir glycosyltransferases for applied biocatalysis: Efficient synthesis of uridine 5′-diphosphate-glucose by sucrose synthase. Adv. Synth. Catal. 2016, 358, 3600–3609. [Google Scholar] [CrossRef]
- Gutmann, A.; Lepak, A.; Diricks, M.; Desmet, T.; Nidetzky, B. Glycosyltransferase cascades for natural product glycosylation: Use of plant instead of bacterial sucrose synthases improves the UDP-glucose recycling from sucrose and UDP. Biotechnol. J. 2017, 12, 1600557. [Google Scholar] [CrossRef]
- Nidetzky, B.; Gutmann, A.; Zhong, C. Leloir glycosyltransferases as biocatalysts for chemical production. ACS Catal. 2018, 8, 6283–6300. [Google Scholar] [CrossRef]
- Wang, X.; Fan, R.Y.; Li, J.; Li, C.F.; Zhang, Y.S. Molecular cloning and functional characterization of a novel (iso) flavone 4′, 7-O-diglucoside glucosyltransferase from Pueraria lobata. Front. Plant Sci. 2016, 7, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, Z.B.; Li, C.F.; Gou, J.B.; Zhang, Y.S. Molecular cloning and characterization of an isoflavone 7-O-glucosyltransferase from Pueraria lobata. Plant Cell Rep. 2014, 33, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Funaki, A.; Waki, T.; Noguchi, A.; Kawai, Y.; Yamashita, S.; Takahashi, S.; Nakayama, T. Identification of a highly specific isoflavone 7-O-glucosyltransferase in the soybean (Glycine max (L.) Merr.). Plant Cell Physiol. 2015, 56, 1512–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.H.; Bao, Y.M.; Wei, C.L.; Li, J.A. Studies of chemical constituents and their antioxidant activities from Astragalus mongholicus Bunge. Biomed. Environ. Sci. 2005, 18, 297. [Google Scholar]
- Dai, L.H.; Li, J.; Yang, J.G.; Men, Y.; Zeng, Y.; Cai, Y.; Sun, Y.X. Enzymatic synthesis of novel glycyrrhizic acid glucosides using a promiscuous Bacillus glycosyltransferase. Catalysts 2018, 8, 615. [Google Scholar] [CrossRef] [Green Version]
- Achnine, L.; Huhman, D.V.; Farag, M.A.; Sumner, L.W.; Blount, J.W.; Dixon, R.A. Genomics-based selection and functional characterization of triterpene glycosyltransferases from the model legume Medicago truncatula. Plant J. 2005, 41, 875–887. [Google Scholar] [CrossRef] [Green Version]
- Bungaruang, L.; Gutmann, A.; Nidetzky, B. Leloir glycosyltransferases and natural product glycosylation: Biocatalytic synthesis of the C-glucoside nothofagin, a major antioxidant of redbush herbal tea. Adv. Synth. Catal. 2013, 355, 2757–2763. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Ding, F.Y.; Shao, W.M.; He, B.F.; Wang, G.J. Regulated preparation of crocin-1 or crocin-2′ triggered by the cosolvent DMSO using Bs-GT/At-SuSy one-pot reaction. J. Agric. Food Chem. 2019, 67, 12496–12501. [Google Scholar] [CrossRef]
- Dai, L.H.; Liu, C.; Li, J.; Dong, C.X.; Yang, J.G.; Dai, Z.B.; Zhang, X.L.; Sun, Y.X. One-pot synthesis of ginsenoside Rh2 and bioactive unnatural ginsenoside by coupling promiscuous glycosyltransferase from Bacillus subtilis 168 to sucrose synthase. J. Agric. Food Chem. 2018, 66, 2830–2837. [Google Scholar] [CrossRef]
- Dai, L.H.; Li, J.; Yao, P.Y.; Zhu, Y.M.; Men, Y.; Zeng, Y.; Yang, J.G.; Sun, Y.X. Exploiting the aglycon promiscuity of glycosyltransferase Bs-YjiC from Bacillus subtilis and its application in synthesis of glycosides. J. Biotechnol. 2017, 248, 69–76. [Google Scholar] [CrossRef]
- Diricks, M.; Bruyn, F.D.; Daele, P.V.; Walmagh, M.; Desmet, T. Identification of sucrose synthase in nonphotosynthetic bacteria and characterization of the recombinant enzymes. Appl. Microbiol. Biotechnol. 2015, 99, 8465–8474. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.J.; Chen, A.N.; Zhao, L.G.; Cao, F.L.; Ding, G.; Xiao, W. One-pot synthesis of hyperoside by a three-enzyme cascade using a UDP-galactose regeneration system. J. Agric. Food Chem. 2017, 65, 6042–6048. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.M.; Xue, J.; Min, J.; Qin, L.J.; Zhang, J.K.; Dai, L.H. Biocatalytic synthesis of ginsenoside Rh2 using Arabidopsis thaliana glucosyltransferase-catalyzed coupled reactions. J. Biotechnol. 2020, 309, 107–112. [Google Scholar] [CrossRef] [PubMed]
| Substrate | Km (μM) | kcat (s−1) | kcat/ Km (s−1 M−1) |
|---|---|---|---|
| Calycosin | 18.67 ± 1.36 | 7.67 ± 0.13 | 4.11 × 105 |
| Formononetin | 30.64 ± 2.30 | 5.39 ± 0.11 | 1.76 × 105 |
| Entry | UGT88E18 (mU mL−1) | SuSy (mU mL−1) | Cy7G (mM) |
|---|---|---|---|
| 1 | 50 | 150 | 1.30 |
| 2 | 100 | 150 | 2.56 |
| 3 | 150 | 150 | 3.35 |
| 4 | 200 | 150 | 3.78 |
| 5 | 200 | 50 | 1.82 |
| 6 | 200 | 100 | 3.31 |
| 7 | 200 | 150 | 3.75 |
| 8 | 200 | 200 | 3.80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Min, J.; Qu, Y.; Zhang, X.; Zhang, J.; Yu, X.; Dai, L. Biocatalytic Synthesis of Calycosin-7-O-β-D-Glucoside with Uridine Diphosphate–Glucose Regeneration System. Catalysts 2020, 10, 258. https://doi.org/10.3390/catal10020258
Hu Y, Min J, Qu Y, Zhang X, Zhang J, Yu X, Dai L. Biocatalytic Synthesis of Calycosin-7-O-β-D-Glucoside with Uridine Diphosphate–Glucose Regeneration System. Catalysts. 2020; 10(2):258. https://doi.org/10.3390/catal10020258
Chicago/Turabian StyleHu, Yumei, Jian Min, Yingying Qu, Xiao Zhang, Juankun Zhang, Xuejing Yu, and Longhai Dai. 2020. "Biocatalytic Synthesis of Calycosin-7-O-β-D-Glucoside with Uridine Diphosphate–Glucose Regeneration System" Catalysts 10, no. 2: 258. https://doi.org/10.3390/catal10020258
APA StyleHu, Y., Min, J., Qu, Y., Zhang, X., Zhang, J., Yu, X., & Dai, L. (2020). Biocatalytic Synthesis of Calycosin-7-O-β-D-Glucoside with Uridine Diphosphate–Glucose Regeneration System. Catalysts, 10(2), 258. https://doi.org/10.3390/catal10020258





