Eco-Friendly Mechanochemical Preparation of Ag2O–MnO2/Graphene Oxide Nanocomposite: An Efficient and Reusable Catalyst for the Base-Free, Aerial Oxidation of Alcohols
Abstract
:1. Introduction
2. Results and Discussion
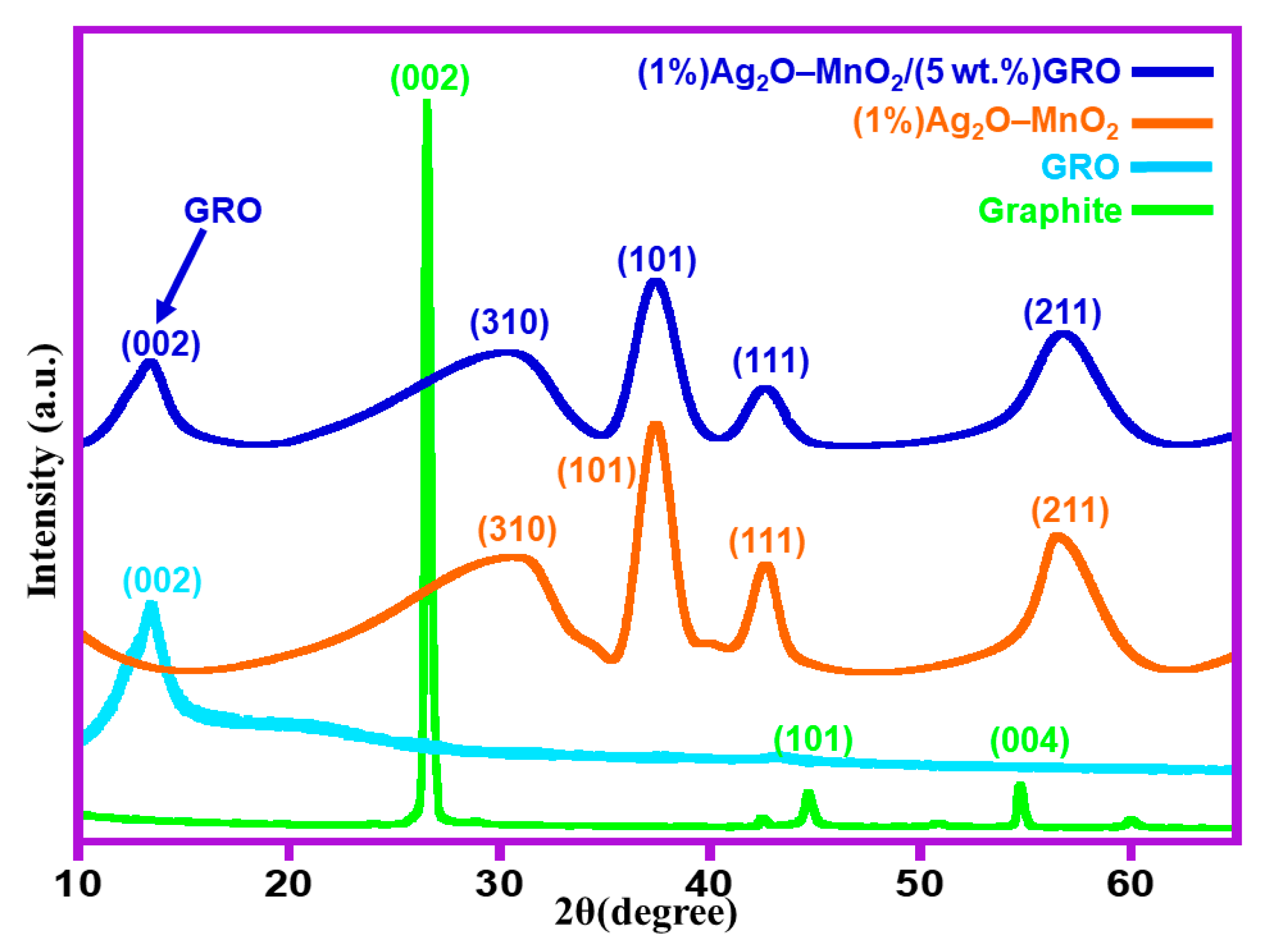
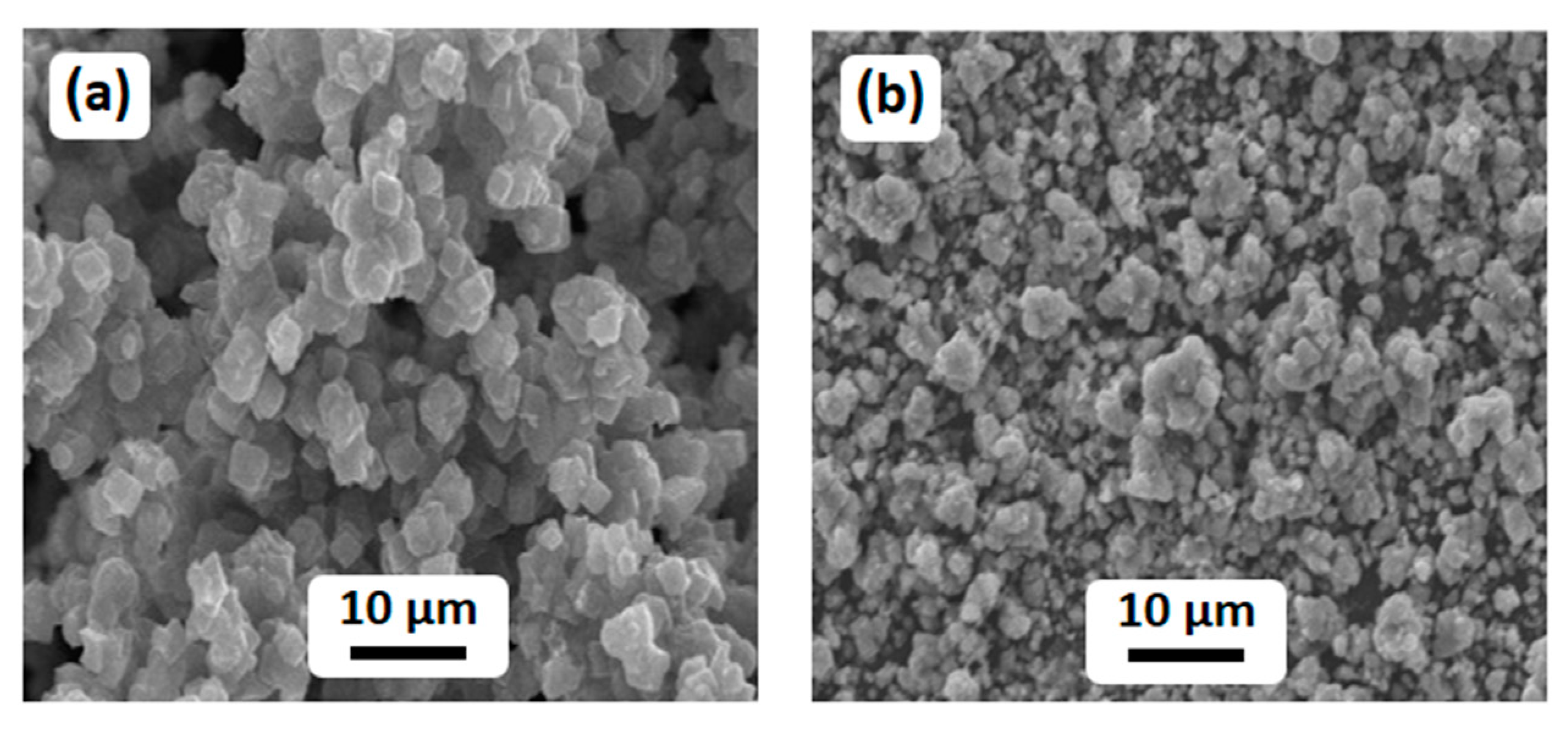
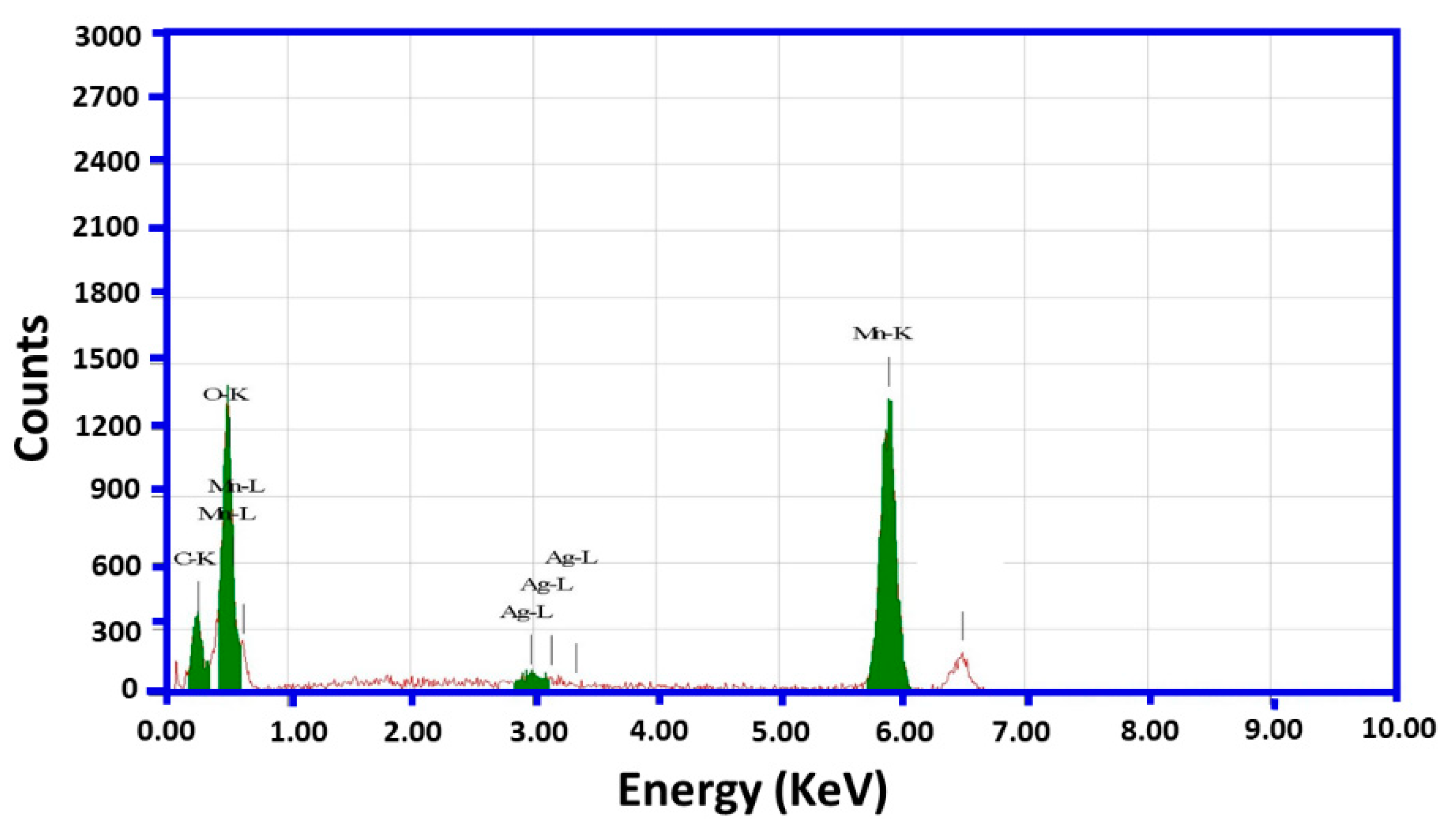
2.1. Characterizations
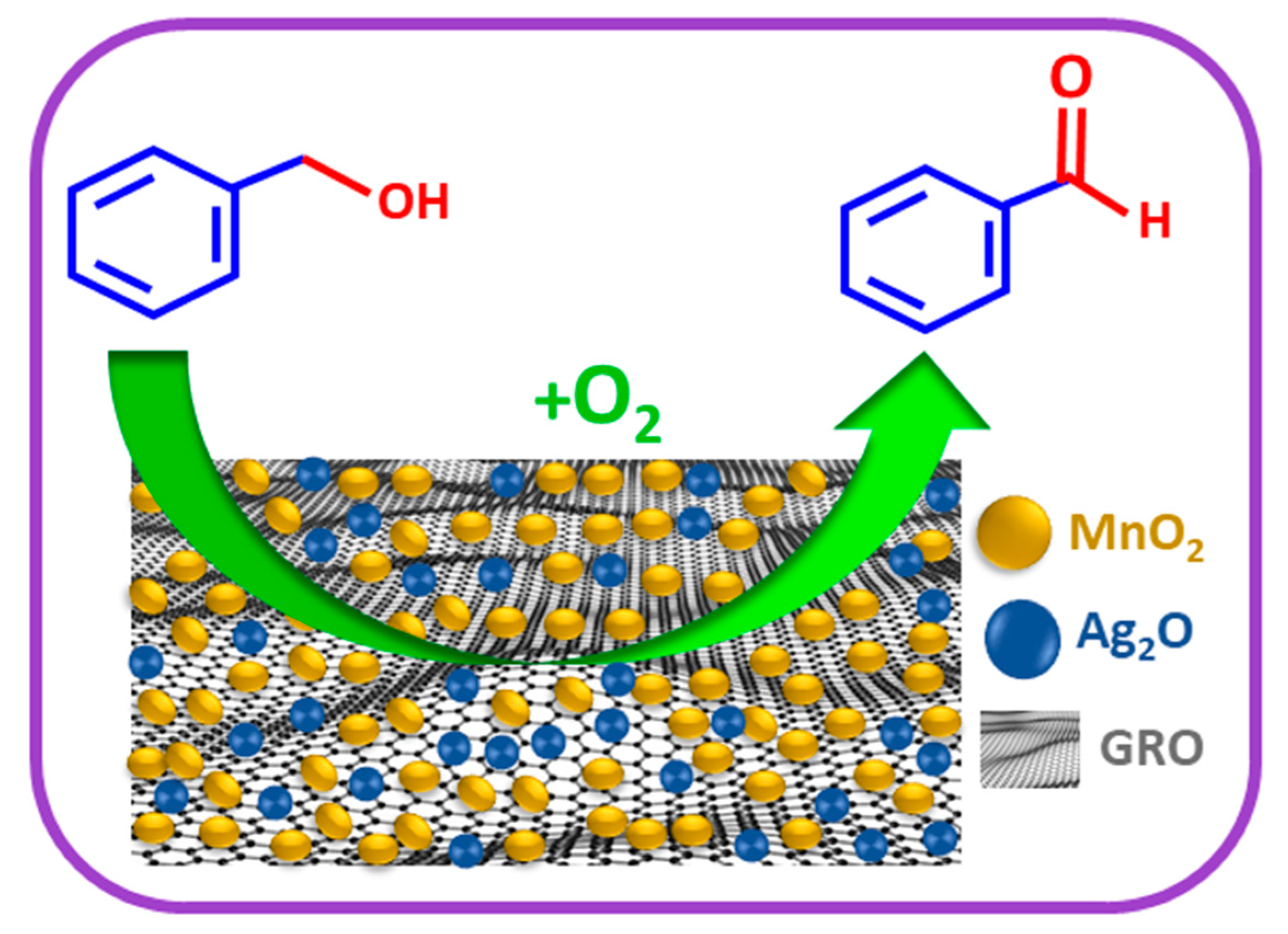
2.2. Catalytic Assessment
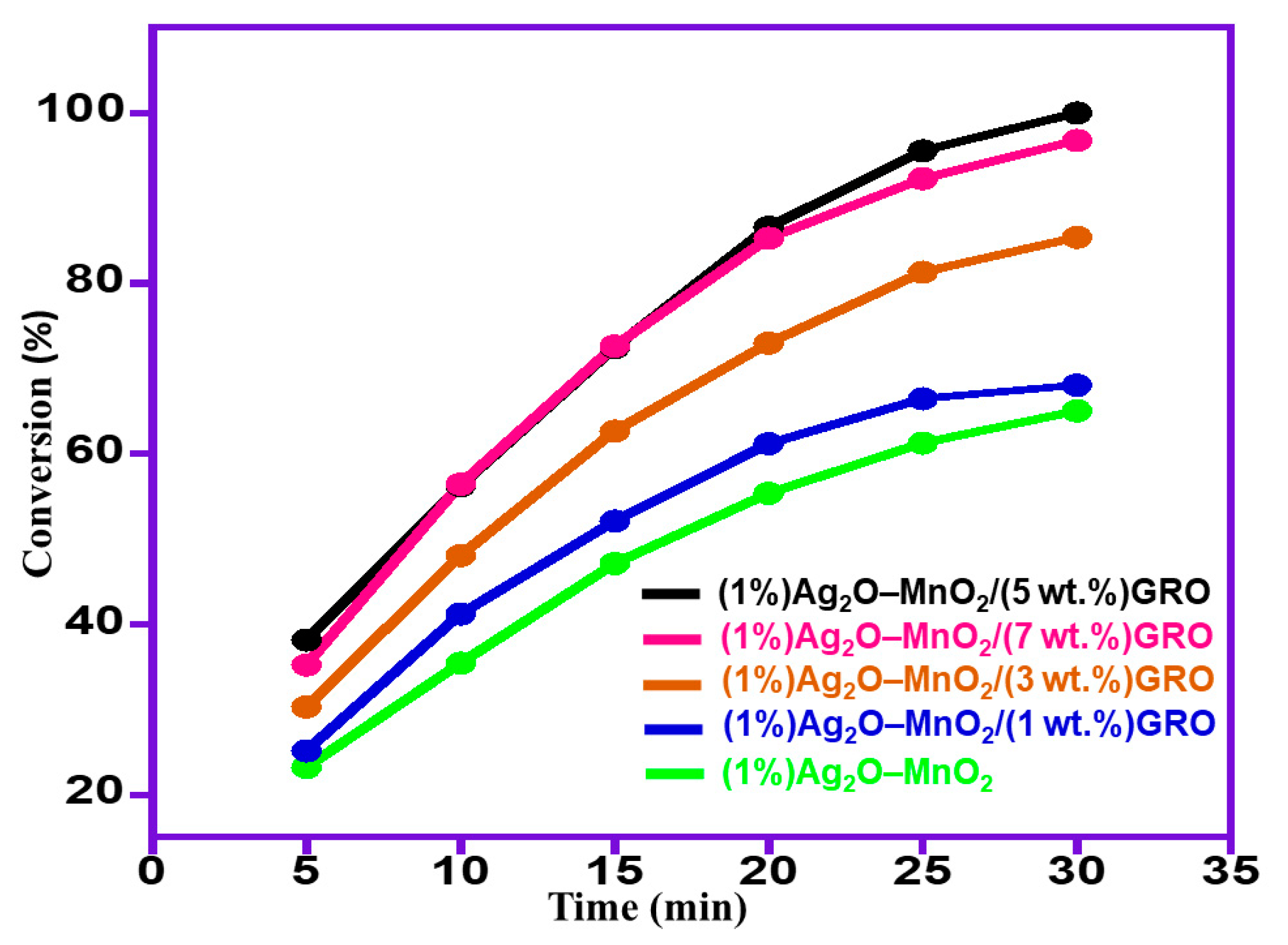
2.2.1. Influence of wt% GRO
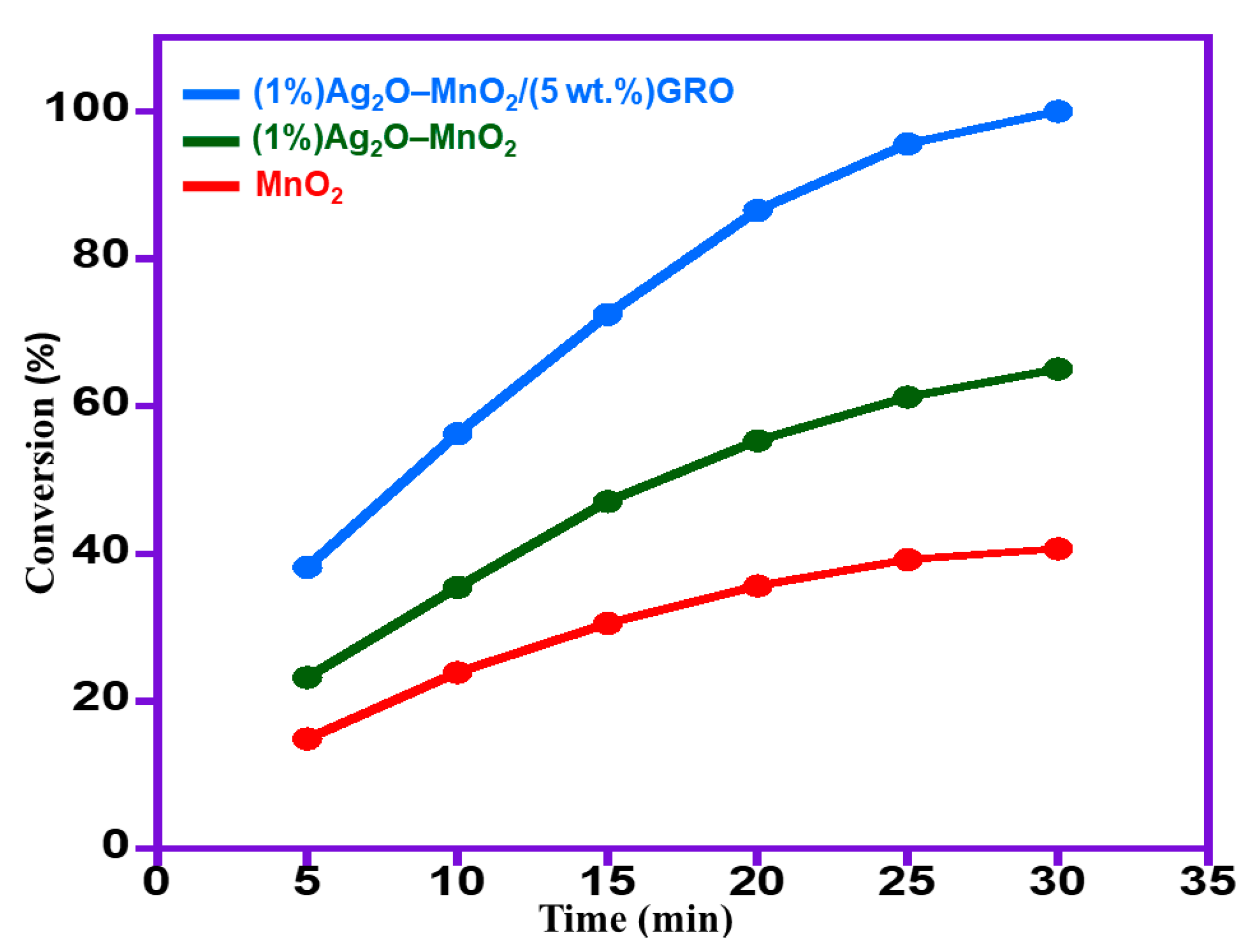
2.2.2. Role of Various Graphene Supports
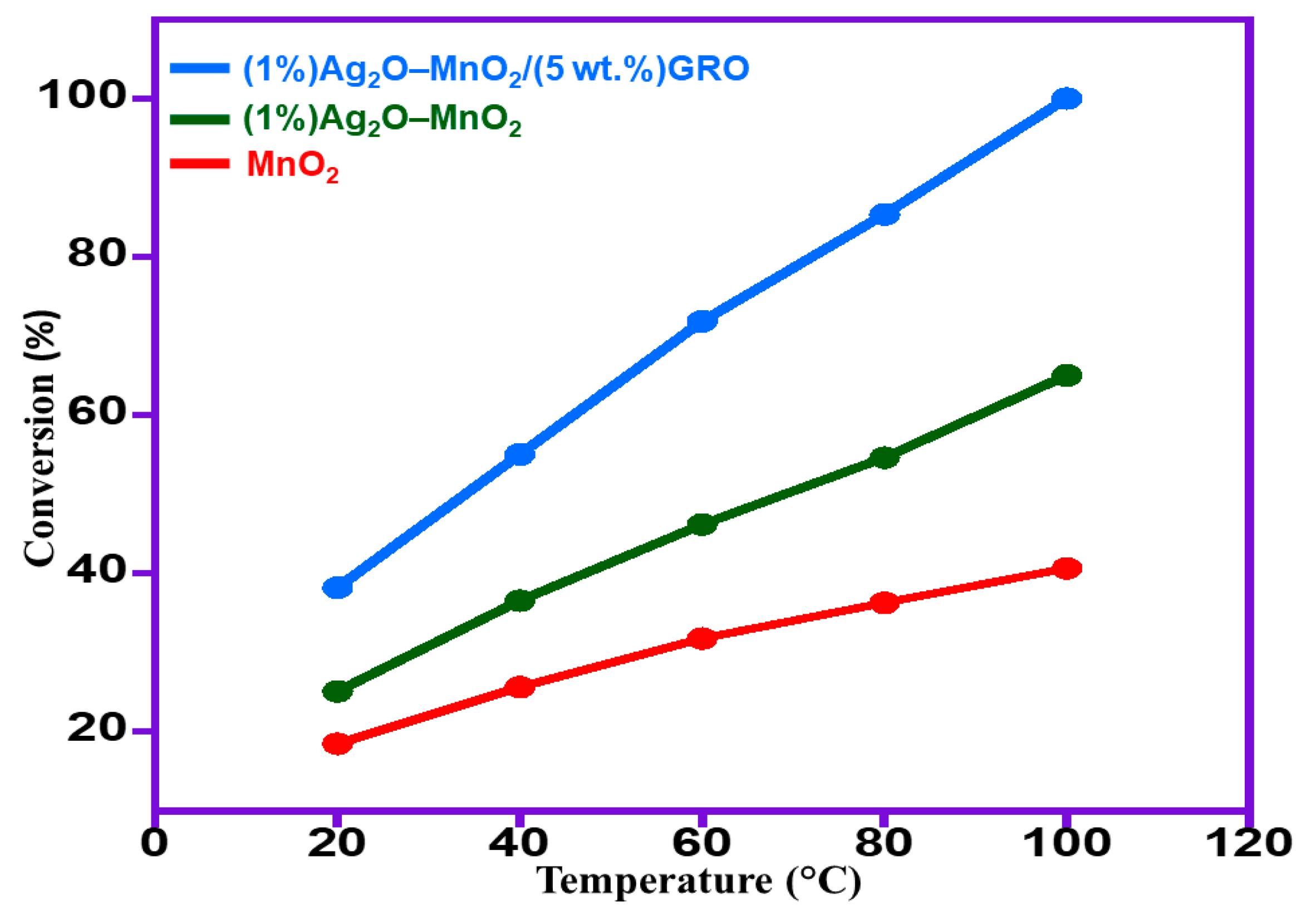
2.2.3. Impact of Temperature
2.2.4. Impact of Catalyst Dosage
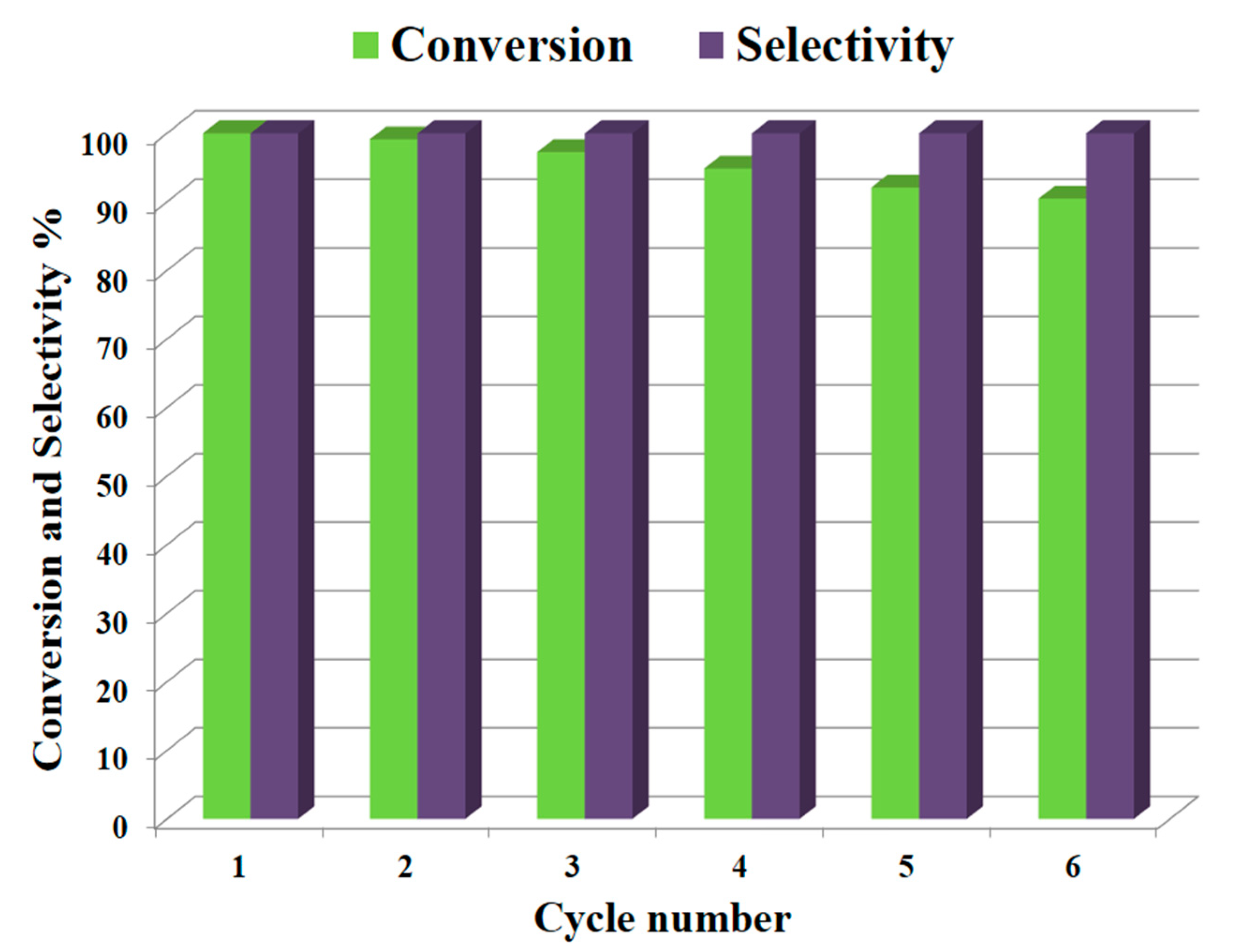
2.3. Reusability Tests
2.4. General Applicability
3. Experimental
3.1. Synthesis of GRO
3.2. Synthesis of Ag2O–MnO2 Nanoparticles
3.3. Synthesis of Ag2O–MnO2/GRO Nanocomposite
3.4. Catalyst Characterization
3.5. Catalytic Assessment
3.6. Reusability Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kolobova, E.; Kotolevich, Y.; Pakrieva, E.; Mamontov, G.; Farias, M.; Corberán, V.C.; Bogdanchikova, N.; Hemming, J.; Smeds, A.; Mäki-Arvela, P. Modified Ag/TiO2 systems: Promising catalysts for liquid-phase oxidation of alcohols. Fuel 2018, 234, 110–119. [Google Scholar] [CrossRef]
- Faqeeh, A.J.; Ali, T.T.; Basahel, S.N.; Narasimharao, K. Nanosized samarium modified Au-Ce0.5Zr0.5O2 catalysts for oxidation of benzyl alcohol. Mol. Catal. 2018, 456, 10–21. [Google Scholar] [CrossRef]
- Liu, K.; Qin, T.; Sun, Y.; Hou, C.; Cao, X.; Jiang, S. Synergistic effect between Ag and Mn3O4 in the gas phase oxidation of alcohols. Catal. Commun. 2018, 113, 15–18. [Google Scholar] [CrossRef]
- Dai, X.; Rasamani, K.D.; Wu, S.; Sun, Y. Enabling selective aerobic oxidation of alcohols to aldehydes by hot electrons in quantum-sized Rh nanocubes. Mater. Today Energy 2018, 10, 15–22. [Google Scholar] [CrossRef]
- Biella, S.; Rossi, M. Gas phase oxidation of alcohols to aldehydes or ketones catalysed by supported gold. Chem. Commun. 2003, 378–379. [Google Scholar] [CrossRef]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef] [Green Version]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, G.; Zhang, F. Multiple roles of graphene in heterogeneous catalysis. Chem. Soc. Rev. 2015, 44, 3023–3035. [Google Scholar] [CrossRef]
- Su, C.; Loh, K.P. Carbocatalysts: Graphene oxide and its derivatives. Acc. Chem. Res. 2013, 46, 2275–2285. [Google Scholar] [CrossRef]
- Su, C.; Acik, M.; Takai, K.; Lu, J.; Hao, S.-j.; Zheng, Y.; Wu, P.; Bao, Q.; Enoki, T.; Chabal, Y.J. Probing the catalytic activity of porous graphene oxide and the origin of this behaviour. Nat. Commun. 2012, 3, 1298. [Google Scholar] [CrossRef]
- Ali, A.A.; Madkour, M.; Sagheer, F.A.; Zaki, M.I.; Abdel Nazeer, A. Low-Temperature Catalytic CO Oxidation Over Non-Noble, Efficient Chromia in Reduced Graphene Oxide and Graphene Oxide Nanocomposites. Catalysts 2020, 10, 105. [Google Scholar] [CrossRef] [Green Version]
- Gopiraman, M.; Saravanamoorthy, S.; Deng, D.; Ilangovan, A.; Kim, I.S.; Chung, I.M. Facile mechanochemical synthesis of nickel/graphene oxide nanocomposites with unique and tunable morphology: Applications in heterogeneous catalysis and supercapacitors. Catalysts 2019, 9, 486. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Lu, X.; Tong, Y.; Zhang, Z.; Lei, J.; Nie, G.; Wang, C. Fabrication of highly dispersed palladium/graphene oxide nanocomposites and their catalytic properties for efficient hydrogenation of p-nitrophenol and hydrogen generation. Int. J. Hydrogen Energy 2014, 39, 9080–9086. [Google Scholar] [CrossRef]
- Shao, L.; Huang, X.; Teschner, D.; Zhang, W. Gold supported on graphene oxide: An active and selective catalyst for phenylacetylene hydrogenations at low temperatures. ACS Catal. 2014, 4, 2369–2373. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhu, J.; Yang, X.; Lu, L. Deposition of Co3O4 nanoparticles onto exfoliated graphite oxide sheets. J. Mater. Chem. 2008, 18, 5625–5629. [Google Scholar] [CrossRef]
- Adil, S.F.; Assal, M.E.; Shaik, M.R.; Kuniyil, M.; AlOtaibi, N.M.; Khan, M.; Sharif, M.; Alam, M.M.; Al-Warthan, A.; Mohammed, J.A. A Facile Synthesis of ZrOx-MnCO3/Graphene Oxide (GRO) Nanocomposites for the Oxidation of Alcohols using Molecular Oxygen under Base Free Conditions. Catalysts 2019, 9, 759. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Jin, W.; Zhao, Y.; Zhang, G.; Zhang, W. Enhanced catalytic degradation of methylene blue by α-Fe2O3/graphene oxide via heterogeneous photo-Fenton reactions. Appl. Catal. B Environ. 2017, 206, 642–652. [Google Scholar] [CrossRef]
- Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Khan, S.M.; Tremel, W.; Tahir, M.N.; Adil, S.F. A highly reduced graphene oxide/ZrOx–MnCO3 or–Mn2O3 nanocomposite as an efficient catalyst for selective aerial oxidation of benzylic alcohols. RSC Adv. 2017, 7, 55336–55349. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, S.; Mohan, B.C.; Vasudevan, S.V.; Vanaraj, R.; Rukmanikrishnan, B.; Periyasamy, T.; Asrafali, S.P. Selective Oxidation of Cyclohexane Using Graphene Oxide-Supported Ceria Nanocomposites with Exposed Active {1 0 0} Facets. ChemistrySelect 2017, 2, 6223–6230. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, J.; Zhou, L.; Long, D.; Feng, K.; Sun, X.; Zhong, J. Probing the electronic structure of M-graphene oxide (M= Ni, Co, NiCo) catalysts for hydrolytic dehydrogenation of ammonia borane. Appl. Surf. Sci. 2016, 362, 79–85. [Google Scholar] [CrossRef]
- Wang, C.; Hu, L.; Hu, Y.; Ren, Y.; Chen, X.; Yue, B.; He, H. Direct hydroxylation of benzene to phenol over metal oxide supported graphene oxide catalysts. Catal. Commun. 2015, 68, 1–5. [Google Scholar] [CrossRef]
- Diao, J.; Liu, H.; Wang, J.; Feng, Z.; Chen, T.; Miao, C.; Yang, W.; Su, D.S. Porous graphene-based material as an efficient metal free catalyst for the oxidative dehydrogenation of ethylbenzene to styrene. Chem. Commun. 2015, 51, 3423–3425. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Bai, C.; Zhang, W.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Catalytic epoxidation of olefins with graphene oxide supported copper (Salen) complex. Ind. Eng. Chem. Res. 2014, 53, 4232–4238. [Google Scholar] [CrossRef]
- Al-Marri, A.H.; Khan, M.; Shaik, M.R.; Mohri, N.; Adil, S.F.; Kuniyil, M.; Alkhathlan, H.Z.; Al-Warthan, A.; Tremel, W.; Tahir, M.N. Green Synthesis of Pd@ Graphene Nanocomposite: Catalyst for the Selective Oxidation of Alcohols. Arab. J. Chem. 2016, 9, 835–845. [Google Scholar] [CrossRef] [Green Version]
- Al-Marri, A.H.; Khan, M.; Khan, M.; Adil, S.F.; Al-Warthan, A.; Alkhathlan, H.Z.; Tremel, W.; Labis, J.P.; Siddiqui, M.R.H.; Tahir, M.N. Pulicaria glutinosa extract: A toolbox to synthesize highly reduced graphene oxide-silver nanocomposites. Int. J. Mol. Sci. 2015, 16, 1131–1142. [Google Scholar] [CrossRef] [Green Version]
- Rincon, E.; Garcia, A.; Romero, A.A.; Serrano, L.; Luque, R.; Balu, A.M. Mechanochemical preparation of novel polysaccharide-supported Nb2O5 catalysts. Catalysts 2019, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Zheng, Y.; Ye, F.; Deng, X.; Xu, X.; Liu, M.; Shao, Z. Multifunctional iron oxide nanoflake/graphene composites derived from mechanochemical synthesis for enhanced lithium storage and electrocatalysis. Acs Appl. Mater. Interfaces 2015, 7, 14446–14455. [Google Scholar] [CrossRef]
- Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Khan, M.; Al-Warthan, A.; Alharthi, A.I.; Varala, R.; Siddiqui, M.R.H.; Adil, S.F. Ag2O nanoparticles/MnCO3,–MnO2 or–Mn2O3/highly reduced graphene oxide composites as an efficient and recyclable oxidation catalyst. Arab. J. Chem. 2019, 12, 54–68. [Google Scholar] [CrossRef]
- Sandhya, P.K.; Jose, J.; Sreekala, M.S.; Padmanabhan, M.; Kalarikkal, N.; Thomas, S. Reduced graphene oxide and ZnO decorated graphene for biomedical applications. Ceram. Int. 2018, 44, 15092–15098. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, T.; Bi, L. Ru-containing polyoxometalate fabricated on graphene oxide: Preparation, characterization and catalytic activity. J. Solid State Chem. 2019, 275, 16–22. [Google Scholar] [CrossRef]
- Vithalani, R.; Patel, D.; Modi, C.K.; Som, N.N.; Jha, P.K.; Kane, S. Enhancing the potency of surface hydroxyl groups of graphene oxide for selective oxidation of benzyl alcohol. Diam. Relat. Mater. 2018, 90, 154–165. [Google Scholar] [CrossRef]
- Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Khan, M.; Kumar, J.; Alzahrani, A.Y.; Al-Warthan, A.; Al-Tamrah, S.A.; Siddiqui, M.R.H.; Hashmi, S.A. Silver-doped manganese based nanocomposites for aerial oxidation of alcohols. Mater. Express 2018, 8, 35–54. [Google Scholar] [CrossRef]
- Santra, S.; Hota, P.K.; Bhattacharyya, R.; Bera, P.; Ghosh, P.; Mandal, S.K. Palladium nanoparticles on graphite oxide: A recyclable catalyst for the synthesis of biaryl cores. ACS Catal. 2013, 3, 2776–2789. [Google Scholar] [CrossRef]
- Wang, Z.-l.; Xu, D.; Huang, Y.; Wu, Z.; Wang, L.-m.; Zhang, X.-b. Facile, mild and fast thermal-decomposition reduction of graphene oxide in air and its application in high-performance lithium batteries. Chem. Commun. 2012, 48, 976–978. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Wu, S.; Zou, Y.; Jia, M.; Zhang, W.; Yan, W.; Liu, G. Correlation between the microstructures of graphite oxides and their catalytic behaviors in air oxidation of benzyl alcohol. J. Colloid Interface Sci. 2014, 421, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Metin, Ö.; Kayhan, E.; Özkar, S.; Schneider, J.J. Palladium nanoparticles supported on chemically derived graphene: An efficient and reusable catalyst for the dehydrogenation of ammonia borane. Int. J. Hydrogen Energy 2012, 37, 8161–8169. [Google Scholar] [CrossRef]
- Han, G.; Liu, Y.; Kan, E.; Tang, J.; Zhang, L.; Wang, H.; Tang, W. Sandwich-structured MnO2/polypyrrole/reduced graphene oxide hybrid composites for high-performance supercapacitors. Rsc Adv. 2014, 4, 9898–9904. [Google Scholar] [CrossRef]
- Gil, S.; Muñoz, L.; Sánchez-Silva, L.; Romero, A.; Valverde, J.L. Synthesis and characterization of Au supported on carbonaceous material-based catalysts for the selective oxidation of glycerol. Chem. Eng. J. 2011, 172, 418–429. [Google Scholar] [CrossRef]
- Liu, S.; Yan, J.; He, G.; Zhong, D.; Chen, J.; Shi, L.; Zhou, X.; Jiang, H. Layer-by-layer assembled multilayer films of reduced graphene oxide/gold nanoparticles for the electrochemical detection of dopamine. J. Electroanal. Chem. 2012, 672, 40–44. [Google Scholar] [CrossRef]
- Rajesh, D.; Mahendiran, C.; Suresh, C.; Pandurangan, A.; Maiyalagan, T. Hydrothermal synthesis of three dimensional reduced graphene oxide-multiwalled carbon nanotube hybrids anchored with palladium-cerium oxide nanoparticles for alcohol oxidation reaction. Int. J. Hydrogen Energy 2019, 44, 4962–4973. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, Y.; Liu, J.; Wang, J.; Zhang, B.; Xiang, X. Ultrafine MnO2 nanoparticles decorated on graphene oxide as a highly efficient and recyclable catalyst for aerobic oxidation of benzyl alcohol. J. Colloid Interface Sci. 2016, 483, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wang, X.; Guan, N.; Li, L. Palladium on graphene as efficient catalyst for solvent-free aerobic oxidation of aromatic alcohols: Role of graphene support. Appl. Catal. B Environ. 2013, 136, 177–185. [Google Scholar] [CrossRef]
- Yu, X.; Huo, Y.; Yang, J.; Chang, S.; Ma, Y.; Huang, W. Reduced Graphene Oxide Supported Au Nanoparticles as an Efficient Catalyst for Aerobic Oxidation of Benzyl Alcohol. Appl. Surf. Sci. 2013, 280, 450–455. [Google Scholar] [CrossRef]
- Rana, S.; Jonnalagadda, S.B. Cu doped amine functionalized graphene oxide and its scope as catalyst for selective oxidation. Catal. Commun. 2017, 100, 183–186. [Google Scholar] [CrossRef]
- Feng, X.; Lv, P.; Sun, W.; Han, X.; Gao, L.; Zheng, G. Reduced graphene oxide-supported Cu nanoparticles for the selective oxidation of benzyl alcohol to aldehyde with molecular oxygen. Catal. Commun. 2017, 99, 105–109. [Google Scholar] [CrossRef]
- Ramirez-Barria, C.S.; Isaacs, M.; Parlett, C.; Wilson, K.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Ru nanoparticles supported on N-doped reduced graphene oxide as valuable catalyst for the selective aerobic oxidation of benzyl alcohol. Catal. Today 2019. [Google Scholar] [CrossRef]
- Zahed, B.; Hosseini-Monfared, H. A comparative study of silver-graphene oxide nanocomposites as a recyclable catalyst for the aerobic oxidation of benzyl alcohol: Support effect. Appl. Surf. Sci. 2015, 328, 536–547. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Shen, J.-J.; Wu, J.; Liang, X.-Y.; Xu, J.; Liu, P.; Xue, B.; Li, Y.-X. An amphiphilic graphene oxide-immobilized polyoxometalate-based ionic liquid: A highly efficient triphase transfer catalyst for the selective oxidation of alcohols with aqueous H2O2. Mol. Catal. 2017, 443, 262–269. [Google Scholar] [CrossRef]
- Jha, A.; Mhamane, D.; Suryawanshi, A.; Joshi, S.M.; Shaikh, P.; Biradar, N.; Ogale, S.; Rode, C.V. Triple nanocomposites of CoMn2O4, Co3O4 and reduced graphene oxide for oxidation of aromatic alcohols. Catal. Sci. Technol. 2014, 4, 1771–1778. [Google Scholar] [CrossRef]
- Liu, K.; Chen, T.; Hou, Z.; Wang, Y.; Dai, L. Graphene Oxide as Support for the Immobilization of Phosphotungstic Acid: Application in the Selective Oxidation of Benzyl Alcohol. Catal. Lett. 2014, 144, 314–319. [Google Scholar] [CrossRef]
- Rana, S.; Maddila, S.; Jonnalagadda, S.B. Synthesis and characterization of Pd (II) dispersed over diamine functionalized graphene oxide and its scope as a catalyst for selective oxidation. Catal. Sci. Technol. 2015, 5, 3235–3241. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, L.; An, Y.; Wang, X.; Xu, G.; Chen, Y.; Dai, L. Promotional synergistic effect of Sn doping into a novel bimetallic Sn-W oxides/graphene catalyst for selective oxidation of alcohols using aqueous H2O2 without additives. Appl. Catal. A Gen. 2018, 558, 26–33. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Ataee-Kachouei, T.; Moeini, F. A novel and active catalyst Ag/ZnO for oxidant-free dehydrogenation of alcohols. Mater. Res. Bull. 2015, 72, 98–105. [Google Scholar] [CrossRef]
- Borthakur, R.; Asthana, M.; Kumar, A.; Lal, R.A. Cooperative Catalysis by Polymetallic Copper–Zinc Complexes in the Efficient Oxidation of Alcohols under Solvent Free Condition. Inorg. Chem. Commun. 2014, 46, 198–201. [Google Scholar] [CrossRef]
- Assady, E.; Yadollahi, B.; Riahi Farsani, M.; Moghadam, M. Zinc polyoxometalate on activated carbon: An efficient catalyst for selective oxidation of alcohols with hydrogen peroxide. Appl. Organomet. Chem. 2015, 29, 561–565. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]





| Entry | Catalyst | Surface Area (m2.g−1) | Conv. (%) | Sp. Performance (mmol·g−1·h−1) | Sel. (%) |
|---|---|---|---|---|---|
| 1 | GRO | 85.4 | 3.2 | 0.43 | <99 |
| 2 | HRG | 78.2 | 3.0 | 0.39 | <99 |
| 3 | MnO2 | 39.3 | 40.7 | 5.4 | <99 |
| 4 | (1%)Ag2O–MnO2 | 84.3 | 65.0 | 8.6 | <99 |
| 5 | (1%)Ag2O–MnO2/(5 wt.%)HRG | 149.1 | 94.1 | 12.5 | <99 |
| 6 | (1%)Ag2O–MnO2/(5 wt.%)GRO | 158.7 | 100.0 | 13.3 | <99 |
| Entry | Catalyst | Conv. (%) | Sp. Performance (mmol·g−1·h−1) | Sel. (%) |
|---|---|---|---|---|
| 1 | GRO | 3.2 | 0.43 | <99 |
| 2 | (1%)Ag2O–MnO2 | 65.0 | 8.6 | <99 |
| 3 | (1%)Ag2O–MnO2/(1 wt.%)GRO | 68.1 | 9.1 | <99 |
| 4 | (1%)Ag2O–MnO2/(3 wt.%)GRO | 85.4 | 11.3 | <99 |
| 5 | (1%)Ag2O–MnO2/(5 wt.%)GRO | 100.0 | 13.3 | <99 |
| 6 | (1%)Ag2O–MnO2/(7 wt.%)GRO | 96.8 | 12.9 | <99 |
| Catalyst | Conv. (%) | Sel. (%) | t. | T. (°C) | Sp. Performance (mmol·g−1·h−1) | Ref. |
|---|---|---|---|---|---|---|
| Ag2O–MnO2/(5 wt.%)GRO | 100 | <99 | 30 min | 100 | 13.3 | This work |
| Ag2O–MnO2/(5 wt.%)HRG | 94.1 | <99 | 30 min | 100 | 12.5 | [28] |
| Cu@AGO | 92 | 99 | 3 h | 70 | 2.1 | [44] |
| CuNPs@rGO | <99 | 98.6 | 16 h | 80 | 8.3 | [45] |
| Ru(CO)/NrGO | 46 | <99 | 24 h | 90 | 6.4 | [46] |
| AgNPs/GRO | 33 | 55 | 24 h | 80 | 2.8 | [47] |
| AgNPs/rGO | 12 | 8 | 24 h | 80 | 1.0 | [47] |
| Im-PW/GRO | 90.8 | 99.2 | 7 h | 90 | 8.6 | [48] |
| Au/RGO | 65 | 93 | 8 h | 100 | 5.4 | [43] |
| Pd NPs/GRO | 36 | 34.1 | 6 h | 110 | 1.0 | [42] |
| 1%RGO–MnCoO | 78 | 100 | 2 h | 140 | 12.6 | [49] |
| GRO–N–PW | 76 | 99 | 6 h | 100 | 10.6 | [50] |
| MnO2/GRO | 97 | 100 | 3 h | 110 | 1.6 | [41] |
| Pd(II)-AAPTMS@GRO | 96 | 99 | 3 h | 60 | 2.1 | [51] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adil, S.F.; Assal, M.E.; Khan, M.; Shaik, M.R.; Kuniyil, M.; Sekou, D.; Dewidar, A.Z.; Al-Warthan, A.; Siddiqui, M.R.H. Eco-Friendly Mechanochemical Preparation of Ag2O–MnO2/Graphene Oxide Nanocomposite: An Efficient and Reusable Catalyst for the Base-Free, Aerial Oxidation of Alcohols. Catalysts 2020, 10, 281. https://doi.org/10.3390/catal10030281
Adil SF, Assal ME, Khan M, Shaik MR, Kuniyil M, Sekou D, Dewidar AZ, Al-Warthan A, Siddiqui MRH. Eco-Friendly Mechanochemical Preparation of Ag2O–MnO2/Graphene Oxide Nanocomposite: An Efficient and Reusable Catalyst for the Base-Free, Aerial Oxidation of Alcohols. Catalysts. 2020; 10(3):281. https://doi.org/10.3390/catal10030281
Chicago/Turabian StyleAdil, Syed Farooq, Mohamed E. Assal, Mujeeb Khan, Mohammed Rafi Shaik, Mufsir Kuniyil, Doumbia Sekou, Ahmed Z. Dewidar, Abdulrahman Al-Warthan, and Mohammed Rafiq H. Siddiqui. 2020. "Eco-Friendly Mechanochemical Preparation of Ag2O–MnO2/Graphene Oxide Nanocomposite: An Efficient and Reusable Catalyst for the Base-Free, Aerial Oxidation of Alcohols" Catalysts 10, no. 3: 281. https://doi.org/10.3390/catal10030281
APA StyleAdil, S. F., Assal, M. E., Khan, M., Shaik, M. R., Kuniyil, M., Sekou, D., Dewidar, A. Z., Al-Warthan, A., & Siddiqui, M. R. H. (2020). Eco-Friendly Mechanochemical Preparation of Ag2O–MnO2/Graphene Oxide Nanocomposite: An Efficient and Reusable Catalyst for the Base-Free, Aerial Oxidation of Alcohols. Catalysts, 10(3), 281. https://doi.org/10.3390/catal10030281









