Fatty Acid Hydratases: Versatile Catalysts to Access Hydroxy Fatty Acids in Efficient Syntheses of Industrial Interest
Abstract
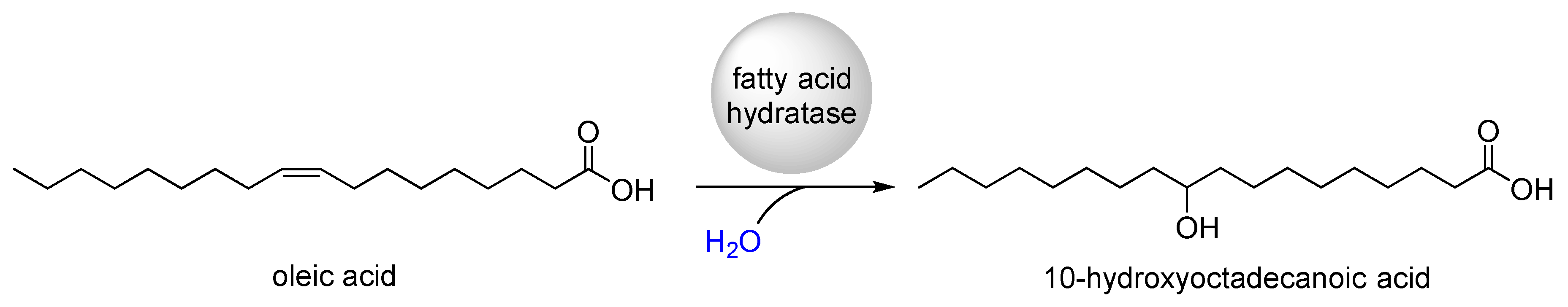
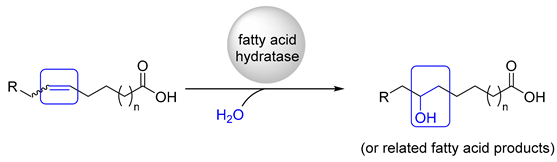
:1. Introduction
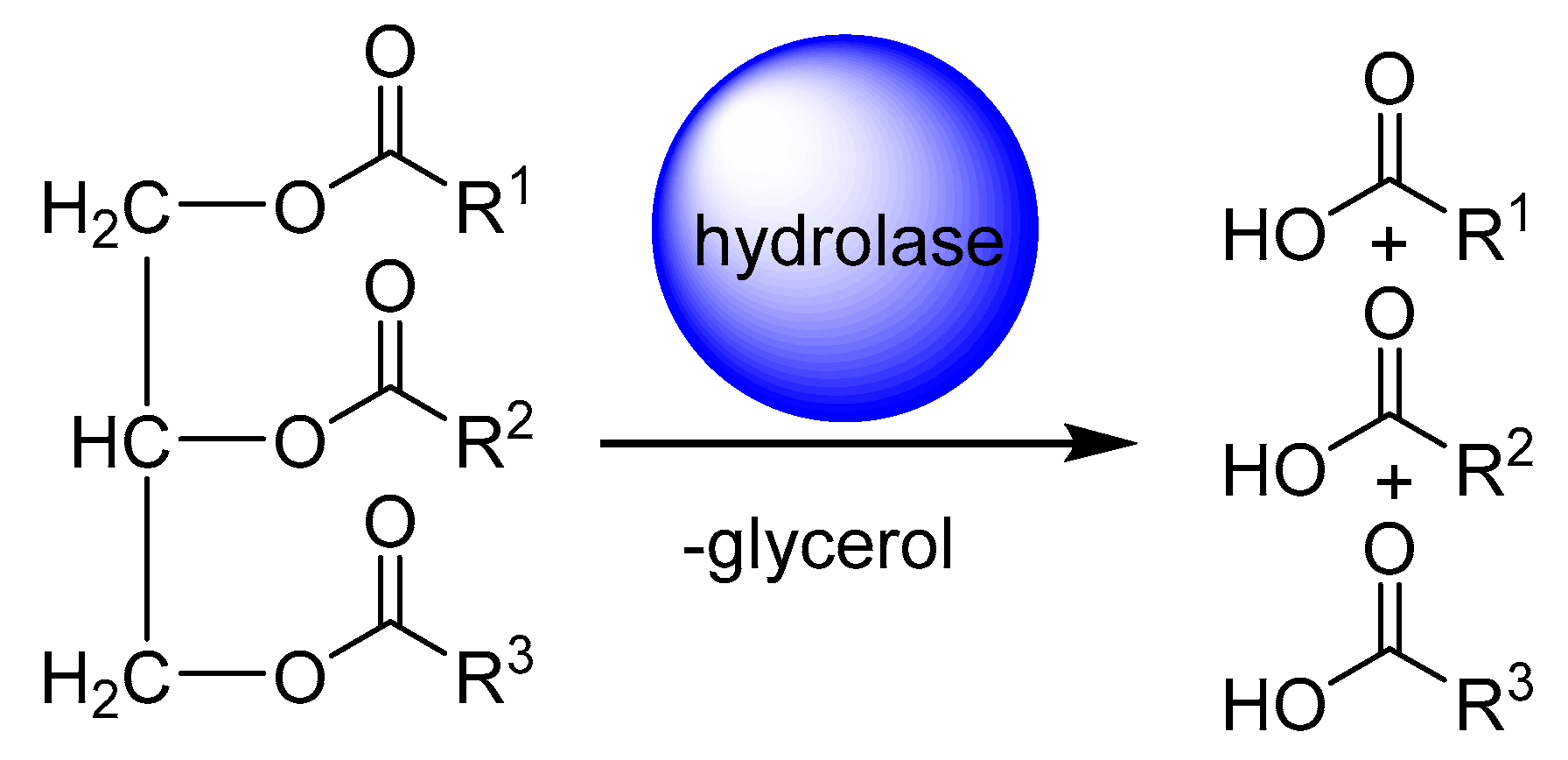
2. Application of Fatty Acid Hydratase in Synthesis and Process Development
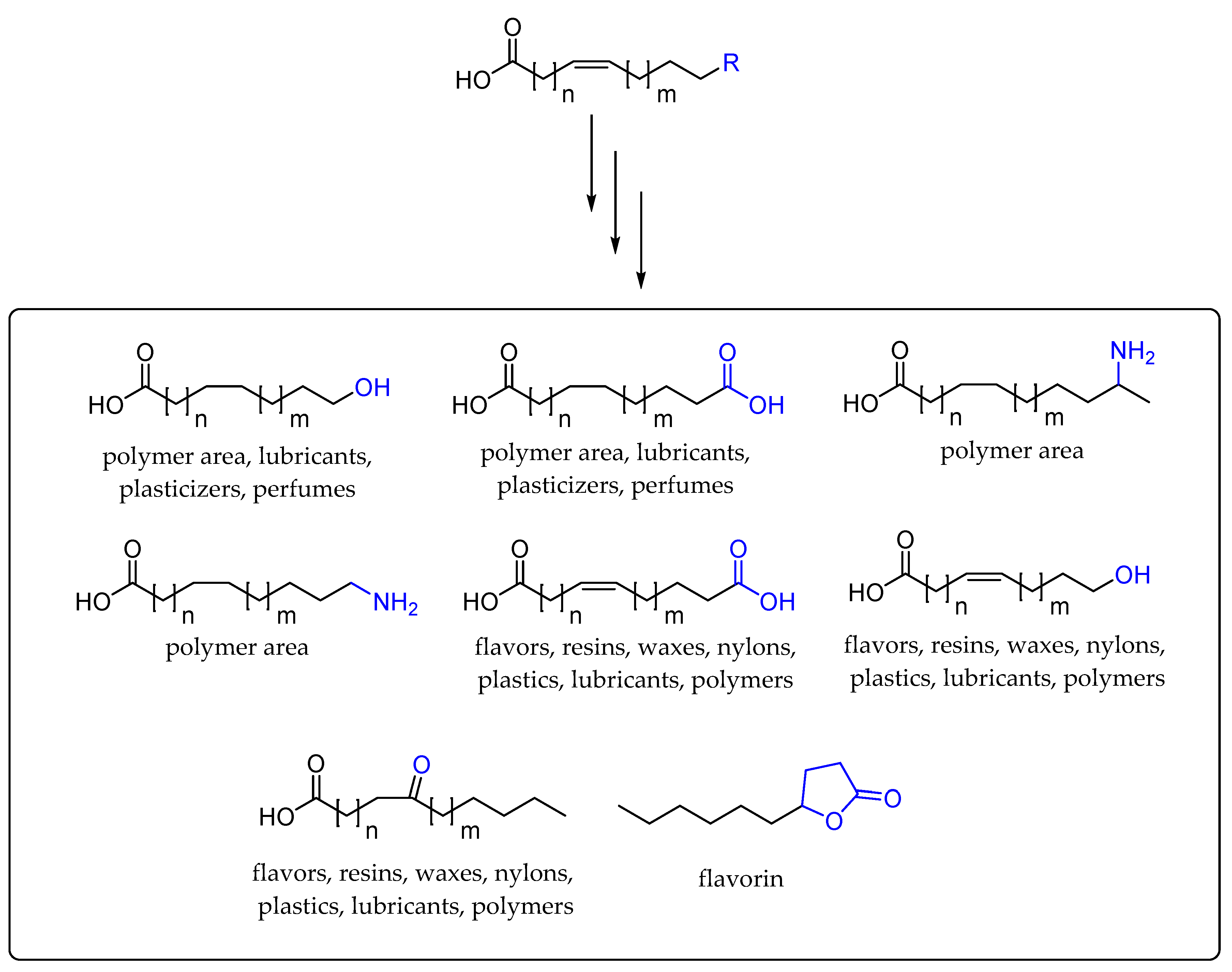
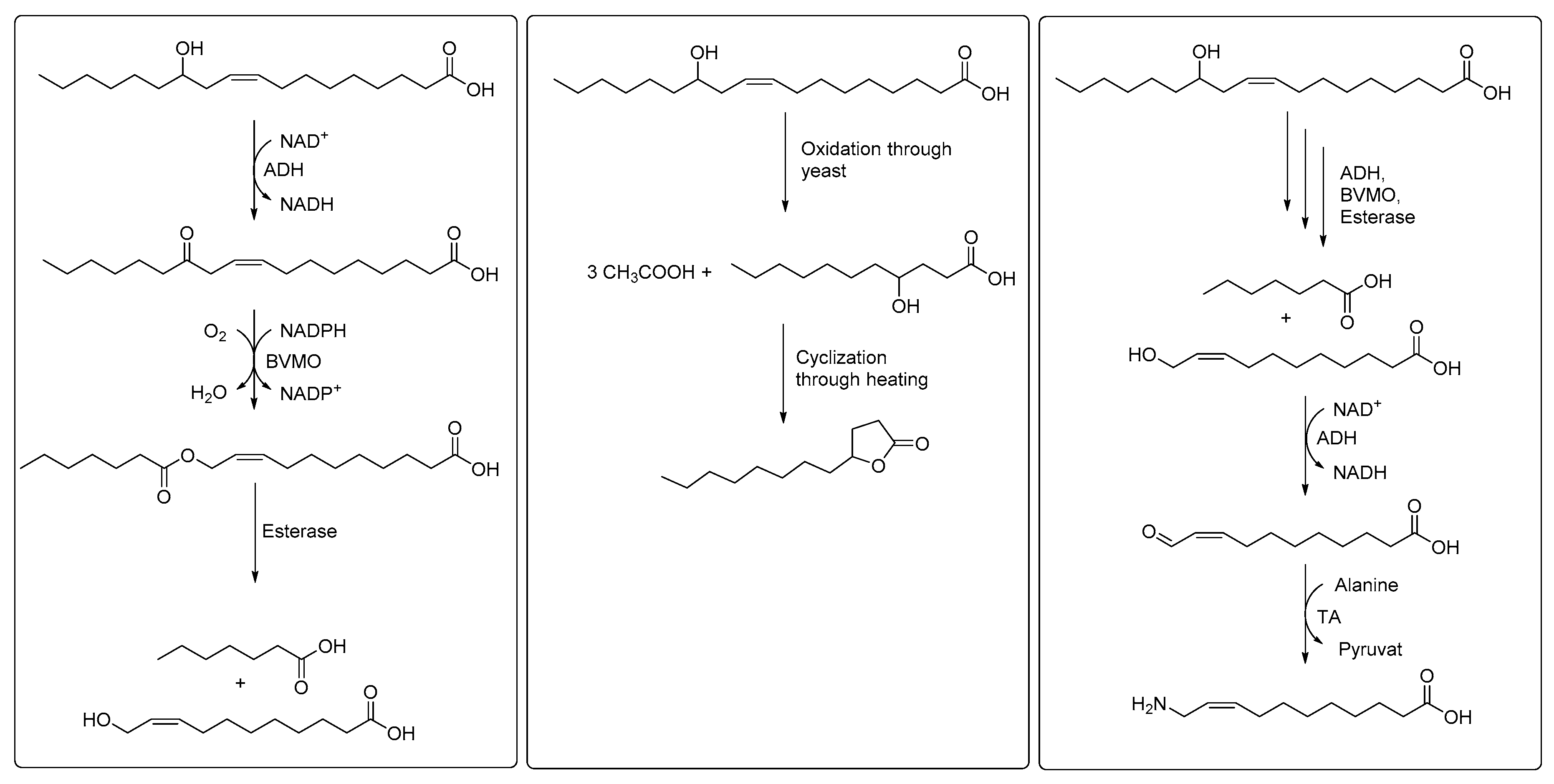
3. Cascade Processes Involving Fatty Acid Hydratase
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Liu, F.; Cai, J.; Xie, U.W.; Long, T.E.; Turner, S.R.; Lyons, A.; Gross, R.A. Polymers from Fatty Acids: Poly (Co-Hydroxyl Tetradecanoic Acid) Synthesis and Physico-Mechanical Studies. ACS Symp. Ser. 2012, 1105, 131–150. [Google Scholar] [CrossRef]
- Świzdor, A.; Panek, A.; Milecka-Tronina, N.; Kołek, T. Biotransformations Utilizing β-Oxidation Cycle Reactions in the Synthesis of Natural Compounds and Medicines. Int. J. Mol. Sci. 2012, 13, 16514–16543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, S.; Wubbolts, M.G.; Sanglard, D.; Witholt, B. Production of Chiral Hydroxy Long Chain Fatty Acids by Whole Cell Biocatalysis of Pentadecanoic Acid with an E. Coli Recombinant Containing Cytochrome P450BM-3 Monooxygenase. Tetrahedron Asymmetry 2002, 9, 2832–2844. [Google Scholar] [CrossRef]
- Ashby, R.D.; Solaiman, D.K.Y.; Liu, C.K.; Strahan, G.; Latona, N. Sophorolipid-Derived Unsaturated and Epoxy Fatty Acid Estolides as Plasticizers for Poly (3-Hydroxybutyrate). Am. Oil Chem. Soc. 2016, 93, 347–358. [Google Scholar] [CrossRef]
- Hu, J.; Jin, Z.; Chen, T.Y.; Polley, J.D.; Cunningham, M.F.; Gross, R.A. Anionic Polymerizable Surfactants from Biobased ω-Hydroxy Fatty Acids. Macromolecules 2014, 47, 113–120. [Google Scholar] [CrossRef]
- Mutlu, H.; Meier, M.A.R. Castor Oil as a Renewable Resource for the Chemical Industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30. [Google Scholar] [CrossRef]
- Hou, C.T. Biotechnology for Fats and Oils: New Oxygenated Fatty Acids. New Biotechnol. 2009, 26, 2–10. [Google Scholar] [CrossRef]
- Patel, V.R.; Dumancas, G.G.; Viswanath, L.C.K.; Maples, R.; Subong, B.J.J. Castor Oil: Properties, Uses, and Optimization of Processing Parameters in Commercial Production. Lipid Insights 2016, LPI-S40233. [Google Scholar] [CrossRef] [Green Version]
- Knothe, G.; Weisleder, D.; Bagby, M.O.; Peterson, R.E. Hydroxy Fatty Acids through Hydroxylation of Oleic Acid with Selenium Dioxide/Tert.-Butylhydroperoxide. J. Am. Oil Chem. Soc. 1993, 70, 401–404. [Google Scholar] [CrossRef]
- Mountanea, O.G.; Limnios, D.; Kokotou, M.G.; Bourboula, A.; Kokotos, G. Asymmetric Synthesis of Saturated Hydroxy Fatty Acids and Fatty Acid Esters of Hydroxy Fatty Acids. Eur. J. Org. Chem. 2019, 10, 2010–2019. [Google Scholar] [CrossRef]
- Köckritz, A.; Martin, A. Oxidation of Unsaturated Fatty Acid Derivatives and Vegetable Oils. Eur. J. Lipid Sci. Technol. 2008, 110, 812–824. [Google Scholar] [CrossRef]
- Kim, K.R.; Oh, H.J.; Park, C.S.; Hong, S.H.; Park, J.Y.; Oh, D.K. Unveiling of Novel Regio-Selective Fatty Acid Double Bond Hydratases from Lactobacillus Acidophilus Involved in the Selective Oxyfunctionalization of Mono- and Di-Hydroxy Fatty Acids. Biotechnol. Bioeng. 2015, 112, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Van de Loo, F.; Broun, P.; Turner, S.; Somervillet, C. An oleate 12-hydroxylase from Ricinus communis L. is a fatty acyl desaturase homolog. Proc. Natl. Acad. Sci. USA 1995, 92, 6743–6747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodkorb, D.; Gottschall, M.; Marmulla, R.; Lüddeke, F.; Harder, J. Linalool Dehydratase-Isomerase, a Bifunctional Enzyme in the Anaerobic Degradation of Monoterpenes. J. Biol. Chem. 2010, 285, 30436–30442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiger, S.; Mazet, A.; Sandmann, G. Heterologous Expression, Purification, and Enzymatic Characterization of the Acyclic Carotenoid 1, 2-Hydratase from Rubrivivax Gelatinosus. Arch. Biochem. Biophys. 2003, 414, 51–58. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Enzymes in Lipid Modification. Annu. Rev. Food Sci. Technol. 2018, 9, 116–127. [Google Scholar] [CrossRef]
- Engleder, M.; Pichler, H. On the Current Role of Hydratases in Biocatalysis. Appl. Microbiol. Biotechnol. 2018, 102, 5841–5858. [Google Scholar] [CrossRef] [Green Version]
- Wallen, L.L.; Benedict, R.G.; Jackson, W.R. The Microbiological Production from Oleic of 10-Hydroxystearic Acid from Oleic Acid. Arch. Biochem. Biophys. 1962, 99, 249–253. [Google Scholar] [CrossRef]
- Kim, K.-R.; Oh, D.-K. Production of hydroxy fatty acids by microbial fatty acid-hydroxylation enzymes. Biotechnol. Adv. 2013, 31, 1473–1485. [Google Scholar] [CrossRef]
- Wallen, L.L.; Davis, E.N.; Wu, Y.V.; Rohwedder, W.K. Stereospecific Hydration of Unsaturated Fatty Acids by Bacteria. Lipids 1971, 6, 745–750. [Google Scholar] [CrossRef]
- Niehaus, W.G.; Schroepfer, G.J. The Reversible Hydration of Oleic Acid to 10D-Hydroxystearic Acid. Biochem. Biophys. Res. Commun. 1965, 21, 271–275. [Google Scholar] [CrossRef]
- Volkov, A.; Liavonchanka, A.; Kamneva, O.; Fiedler, T.; Goebel, C.; Kreikemeyer, B.; Feussner, I. Myosin Cross-Reactive Antigen of Streptococcus Pyogenes M49 Encodes a Fatty Acid Double Bond Hydratase That Plays a Role in Oleic Acid Detoxification and Bacterial Virulence. J. Biol. Chem. 2010, 285, 10353–10361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.Y.; Liang, N.Y.; Curtis, J.M.; Gänzle, M.G. Characterization of Linoleate 10-Hydratase of Lactobacillus plantarum and Novel Antifungal Metabolites. Front. Microbiol. 2016, 7, 1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosberg-Cody, E.; Liavonchanka, A.; Göbel, C.; Ross, R.P.; O’Sullivan, O.; Fitzgerald, G.F.; Feussner, I.; Stanton, C. Myosin-Cross-Reactive Antigen (MCRA) Protein from Bifidobacterium Breve Is a FAD-Dependent Fatty Acid Hydratase Which Has a Function in Stress Protection. BMC Biochem. 2011, 12, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engleder, M.; Pavkov-Keller, T.; Emmerstorfer, A.; Hromic, A.; Schrempf, S.; Steinkellner, G.; Wriessnegger, T.; Leitner, E.; Strohmeier, G.A.; Kaluzna, I.; et al. Structure-Based Mechanism of Oleate Hydratase from Elizabethkingia Meningoseptica. ChemBioChem 2015, 16, 1730–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiseni, A.; Arends, I.W.C.E.; Otten, L.G. New Cofactor-Independent Hydration Biocatalysts: Structural, Biochemical, and Biocatalytic Characteristics of Carotenoid and Oleate Hydratases. ChemCatChem 2015, 7, 29–37. [Google Scholar] [CrossRef]
- Villeneuve, P.; Muderhwa, J.M.; Graille, J.; Haas, M.J. Customizing Lipases for Biocatalysis: A Survey of Chemical, Physical and Molecular Biological Approaches. J. Mol. Catal. B Enzym. 2000, 9, 113–148. [Google Scholar] [CrossRef]
- Goldberg, K.; Schroer, K.; Lütz, S.; Liese, A. Biocatalytic Ketone Reduction—A Powerful Tool for the Production of Chiral Alcohols-Part II: Whole-Cell Reductions. Appl. Microbiol. Biotechnol. 2007, 76, 249–255. [Google Scholar] [CrossRef]
- Breuer, M.; Ditrich, K.; Habicher, T.; Hauer, B.; Keßeler, M.; Stürmer, R.; Zelinski, T. Industrial Methods for the Production of Optically Active Intermediates. Angew. Chem. Int. Ed. 2004, 43, 788–824. [Google Scholar] [CrossRef]
- Strohmeier, G.A.; Pichler, H.; May, O.; Gruber-Khadjawi, M. Application of Designed Enzymes in Organic Synthesis. Chem. Rev. 2011, 111, 4141–4164. [Google Scholar] [CrossRef]
- Abdelraheem, E.M.M.; Busch, H.; Hanefeld, U.; Tonin, F. Biocatalysis explained: From pharmaceutical to bulk chemical production. React. Chem. Eng. 2019, 4, 1878–1894. [Google Scholar] [CrossRef] [Green Version]
- Jemli, S.; Ayadi-Zouari, D.; Hlima, H.B.; Bejar, S. Biocatalysts: Application and engineering for industrial purposes. Crit. Rev. Biotechnol. 2016, 36, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Seelbach, K.; Wandrey, C. Industrial Biotransformations; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar] [CrossRef] [Green Version]
- Yamada, H.; Nagasawa, T. Production of Useful Amides by Enzymatic Hydration of Nitriles. Agric. Chem. 1990, 613, 142–154. [Google Scholar] [CrossRef]
- Miyazawa, T. Enzymatic Resolution of Amino Acids via Ester Hydrolysis. Amino Acids 1999, 16, 191–213. [Google Scholar] [CrossRef] [PubMed]
- Brugging, A.; Roos, E.C.; De Vroom, E. Penicillin Acylase in the Industrial Production of β-Lactam Antibiotics. Org. Process Res. Dev. 1998, 2, 128–133. [Google Scholar] [CrossRef]
- Joo, Y.C.; Seo, E.S.; Kim, Y.S.; Kim, K.R.; Park, J.B.; Oh, D.K. Production of 10-Hydroxystearic Acid from Oleic Acid by Whole Cells of Recombinant Escherichia Coli Containing Oleate Hydratase from Stenotrophomonas Maltophilia. J. Biotechnol. 2012, 158, 17–23. [Google Scholar] [CrossRef]
- Fabritius, D.; Schäfer, H.J.; Steinbüchel, A. Identification and Production of 3-Hydroxy-Δ9-Cis-1, 18-Octadecenedioic Acid by Mutants of Candida Tropicalis. Appl. Microbiol. Biotechnol. 1996, 45, 342–348. [Google Scholar] [CrossRef]
- El-Sharkawy, S.H.; Yang, W.; Dostal, L.; Rosazza, J.P.N. Microbial Oxidation of Oleic Acid. Appl. Environ. Microbiol. 1992, 58, 2116–2122. [Google Scholar] [CrossRef] [Green Version]
- Hudson, J.A.; MacKenzie, C.A.M.; Joblin, K.N. Conversion of Oleic Acid to 10-Hydroxystearic Acid by Two Species of Ruminal Bacteria. Appl. Microbiol. Biotechnol. 1995, 44, 1–6. [Google Scholar] [CrossRef]
- Kaneshiro, T.; Kuo, T.M.; Nakamura, L.K. Conversion of Unsaturated Fatty Acids by Bacteria Isolated from Compost. Curr. Microbiol. 1999, 38, 250–255. [Google Scholar] [CrossRef]
- Koritala, S.; Hou, C.T.; Hesseltine, C.W.; Bagby, M.O. Microbial Conversion of Oleic Acid to 10-Hydroxystearic Acid. Appl. Microbiol. Biotechnol. 1989, 32, 299–304. [Google Scholar] [CrossRef]
- Shinha, T.; Ahuja, R. Bacteremia due to Elizab. Meningoseptica 2015, 2, 13–15. [Google Scholar] [CrossRef] [Green Version]
- Bevers, L.E.; Pinkse, M.W.H.; Verhaert, P.D.E.M.; Hagen, W.R. Oleate Hydratase Catalyzes the Hydration of a Nonactivated Carbon-Carbon Bond. J. Bacteriol. 2009, 191, 5010–5012. [Google Scholar] [CrossRef] [Green Version]
- Ryan, R.P.; Monchy, S.; Cardinale, M.; Taghavi, S.; Crossman, L.; Avison, M.B.; Berg, G.; van der Lelie, D.; Dow, J.M. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat. Rev. Microbiol. 2019, 7, 514–525. [Google Scholar] [CrossRef]
- Kim, B.N.; Yeom, S.J.; Oh, D.K. Conversion of Oleic Acid to 10-Hydroxystearic Acid by Whole Cells of Stenotrophomonas Nitritireducens. Biotechnol. Lett. 2011, 33, 993–997. [Google Scholar] [CrossRef]
- Gallegos-Monterrosa, R.; Maróti, G.; Bálint, B.; Kovács, A.T. Draft Genome Sequence of the Soil Isolate Lysinibacillus fusiformis M5, a Potential Hypoxanthine Producer. Genome Announc. 2016, 4, e01272-16. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.N.; Joo, Y.C.; Kim, Y.S.; Kim, K.R.; Oh, D.K. Production of 10-Hydroxystearic Acid from Oleic Acid and Olive Oil Hydrolyzate by an Oleate Hydratase from Lysinibacillus fusiformis. Appl. Microbiol. Biotechnol. 2012, 95, 929–937. [Google Scholar] [CrossRef]
- Jeon, E.Y.; Lee, J.H.; Yang, K.M.; Joo, Y.C.; Oh, D.K.; Park, J.B. Bioprocess Engineering to Produce 10- Hydroxystearic Acid from Oleic Acid by Recombinant Escherichia Coli Expressing the Oleate Hydratase Gene of Stenotrophomonas Maltophilia. Process Biochem. 2012, 47, 941–947. [Google Scholar] [CrossRef]
- Seo, M.H.; Kim, K.R.; Oh, D.K. Production of a Novel Compound, 10, 12-Dihydroxystearic Acid from Ricinoleic Acid by an Oleate Hydratase from Lysinibacillus fusiformis. Appl. Microbiol. Biotechnol. 2013, 97, 8987–8995. [Google Scholar] [CrossRef]
- De Angelis, M.; Gobbetti, M. Lactobacillus spp.: General Characteristics. Ref. Modul. Food Sci. 2016. [Google Scholar] [CrossRef]
- Kishino, S.; Takeuchi, M.; Park, S.B.; Hirata, A.; Kitamura, N.; Kunisawa, J.; Kiyono, H.; Iwamoto, R.; Isobe, Y.; Arita, M.; et al. Polyunsaturated Fatty Acid Saturation by Gut Lactic Acid Bacteria Affecting Host Lipid Composition. Proc. Natl. Acad. Sci. USA 2013, 110, 17808–17813. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Chen, H.; Song, Y.; Chen, Y.Q.; Zhang, H.; Chen, W. Myosin-Cross-Reactive Antigens from Four Different Lactic Acid Bacteria Are Fatty Acid Hydratases. Biotechnol. Lett. 2013, 35, 75–81. [Google Scholar] [CrossRef]
- Volkov, A.; Khoshnevis, S.; Neumann, P.; Herrfurth, C.; Wohlwend, D.; Ficner, R.; Feussner, I. Crystal Structure Analysis of a Fatty Acid Double-Bond Hydratase from Lactobacillus Acidophilus. Acta Cryst. Sect. D Biol. Cryst. 2013, 69, 648–657. [Google Scholar] [CrossRef]
- Jo, Y.S.; An, J.U.; Oh, D.K. γ-Dodecelactone Production from Safflower Oil via 10-Hydroxy-12 (z)-Octadecenoic Acid Intermediate by Whole Cells of Candida Boidinii and Stenotrophomonas Nitritireducens. J. Agric. Food Chem. 2014, 62, 6736–6745. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, S.H.; Kim, K.R.; Park, J.B.; Oh, D.K. Production of 13S-Hydroxy-9 (Z)-Octadecenoic Acid from Linoleic Acid by Whole Recombinant Cells Expressing Linoleate 13-Hydratase from Lactobacillus acidophilus. J. Biotechnol. 2015, 208, 1–10. [Google Scholar] [CrossRef]
- Hirata, A.; Kishino, S.; Park, S.-B.; Takeuchi, M.; Kitamura, N.; Ogawa, J. A Novel Unsaturated Fatty Acid Hydratase toward C16 to C22 Fatty Acids from Lactobacillus acidophilus. J. Lipid Res. 2015, 56, 1340–1350. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, M.; Kishino, S.; Park, S.B.; Hirata, A.; Kitamura, N.; Saika, A.; Ogawa, J. Efficient Enzymatic Production of Hydroxy Fatty Acids by Linoleic Acid Δ9 Hydratase from Lactobacillus Plantarum AKU 1009a. J. Appl. Microbiol. 2016, 120, 1282–1288. [Google Scholar] [CrossRef] [Green Version]
- Schmid, J.; Steiner, L.; Fademrecht, S.; Pleiss, J.; Otte, K.B.; Hauer, B. Biocatalytic Study of Novel Oleate Hydratases. J. Mol. Catal. B Enzym. 2016, 133, 243–249. [Google Scholar] [CrossRef]
- Choi, J.H.; Seo, M.J.; Lee, K.T.; Oh, D.K. Biotransformation of Fatty Acid-Rich Tree Oil Hydrolysates to Hydroxy Fatty Acid-Rich Hydrolysates by Hydroxylases and Their Feasibility as Biosurfactants. Biotechnol. Bioprocess Eng. 2017, 22, 709–716. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant Oil Renewable Resources as Green Alternatives in Polymer Science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef]
- Heo, S.H.; Hou, C.T.; Kim, B.S. Production of Oxygenated Fatty Acids from Vegetable Oils by Flavobacterium sp. Strain DS5. New Biotechnol. 2009, 26, 105–108. [Google Scholar] [CrossRef]
- Kang, W.R.; Seo, M.J.; Shin, K.C.; Park, J.B.; Oh, D.K. Gene Cloning of an Efficiency Oleate Hydratase from Stenotrophomonas Nitritireducens for Polyunsaturated Fatty Acids and Its Application in the Conversion of Plant Oils to 10-Hydroxy Fatty Acids. Biotechnol. Bioeng. 2017, 114, 74–82. [Google Scholar] [CrossRef]
- Jeon, E.Y.; Seo, J.H.; Kang, W.R.; Kim, M.J.; Lee, J.H.; Oh, D.K.; Park, J.B. Simultaneous Enzyme/Whole-Cell Biotransformation of Plant Oils into C9 Carboxylic Acids. ACS Catal. 2016, 6, 7547–7553. [Google Scholar] [CrossRef]
- Kim, S.K.; Park, Y.C. Biosynthesis of ω-Hydroxy Fatty Acids and Related Chemicals from Natural Fatty Acids by Recombinant Escherichia Coli. Appl. Microbiol. Biotechnol. 2019, 103, 191–199. [Google Scholar] [CrossRef]
- Song, J.W.; Lee, J.H.; Bornscheuer, U.T.; Park, J.B. Microbial Synthesis of Medium-Chain α, ω-Dicarboxylic Acids and ω-Aminocarboxylic Acids from Renewable Long-Chain Fatty Acids. Adv. Synth. Catal. 2014, 356, 1782–1788. [Google Scholar] [CrossRef]
- Oh, H.J.; Kim, S.U.; Song, J.W.; Lee, J.H.; Kang, W.R.; Jo, Y.S.; Kim, K.R.; Bornscheuer, U.T.; Oh, D.K.; Park, J.B. Biotransformation of Linoleic Acid into Hydroxy Fatty Acids and Carboxylic Acids Using a Linoleate Double Bond Hydratase as Key Enzyme. Adv. Synth. Catal. 2015, 357, 408–416. [Google Scholar] [CrossRef]
- Lee, D.S.; Song, J.W.; Voß, M.; Schuiten, E.; Akula, R.K.; Kwon, Y.U.; Bornscheuer, U.; Park, J.B. Enzyme Cascade Reactions for the Biosynthesis of Long Chain Aliphatic Amines from Renewable Fatty Acids. Adv. Synth. Catal. 2019, 361, 1359–1367. [Google Scholar] [CrossRef]
- Wu, Y.X.; Pan, J.; Yu, H.L.; Xu, J.H. Enzymatic Synthesis of 10-Oxostearic Acid in High Space-Time Yield via Cascade Reaction of a New Oleate Hydratase and an Alcohol Dehydrogenase. J. Biotechnol. X 2019. [Google Scholar] [CrossRef]
- Song, J.W.; Jeon, E.Y.; Song, D.H.; Jang, H.Y.; Bornscheuer, U.T.; Oh, D.K.; Park, J.B. Multistep Enzymatic Synthesis of Long-Chain α, ω-Dicarboxylic and ω-Hydroxycarboxylic Acids from Renewable Fatty Acids and Plant Oils. Angew. Chem. Int. Ed. 2013, 52, 2534–2537. [Google Scholar] [CrossRef]
- Kim, S.U.; Kim, K.R.; Kim, J.W.; Kim, S.; Kwon, Y.U.; Oh, D.K.; Park, J.B. Microbial Synthesis of Plant Oxylipins from γ-Linolenic Acid through Designed Biotransformation Pathways. J. Agric. Food Chem. 2015, 63, 2773–2781. [Google Scholar] [CrossRef]
- Koppireddi, S.; Seo, J.H.; Jeon, E.Y.; Chowdhury, P.S.; Jang, H.Y.; Park, J.B.; Kwon, Y.U. Combined Biocatalytic and Chemical Transformations of Oleic Acid to ω-Hydroxynonanoic Acid and α, ω-Nonanedioic Acid. Adv. Synth. Catal. 2016, 358, 3084–3092. [Google Scholar] [CrossRef]
- Cha, H.J.; Seo, E.J.; Song, J.W.; Jo, H.J.; Kumar, A.R.; Park, J.B. Simultaneous Enzyme/Whole-Cell Biotransformation of C18 Ricinoleic Acid into (R)-3-Hydroxynonanoic Acid, 9-Hydroxynonanoic Acid, and 1, 9-Nonanedioic Acid. Adv. Synth. Catal. 2018, 360, 696–703. [Google Scholar] [CrossRef]
- Farbood, M.I.; Morris, J.A.; McLean, L.B. Fermentation Process for Preparing 10-Hydroxy-C18-Carboxylic Acid and Gamma-Dodecalactone Derivatives. EP078388A25, 1 December 1994. [Google Scholar]
- Park, A.K.; Lee, G.H.; Kim, D.W.; Jang, E.H.; Kwon, H.T.; Chi, Y.M. Crystal structure of oleate hydratase from Stenotrophomonas sp. KCTC 12332 reveals conformational plasticity surrounding the FAD binding site. Biochem. Biophys. Res. Commun. 2018, 499, 772–776. [Google Scholar] [CrossRef]
- Lorenzen, J.; Driller, R.; Waldow, A.; Qoura, F.; Loll, B.; Brück, T. Rhodococcus erythropolis Oleate Hydratase: A New Member in the Oleate Hydratase Family Tree—Biochemical and Structural Studies. ChemCatChem 2018, 10, 407–414. [Google Scholar] [CrossRef]
- Demming, R.M.; Hammer, S.C.; Nestl, B.M.; Gergel, S.; Fademrecht, S.; Pleiss, J.; Hauer, B. Asymmetric Enzymatic Hydration of Unactivated, Aliphatic Alkenes. Angew. Chem. Int. Ed. 2019, 58, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Eser, B.E.; Poborsky, M.; Dai, R.; Kishino, S.; Ljubic, A.; Takeuchi, M.; Jacobsen, C.; Ogawa, J.; Kristensen, P.; Guo, Z. Rational Engineering of Hydratase from Lactobacillus Acidophilus Reveals Critical Residues Directing Substrate Specificity and Regioselectivity. ChemBioChem 2019. [Google Scholar] [CrossRef]
- Engleder, M.; Strohmeier, G.A.; Weber, H.; Steinkellner, G.; Leitner, E.; Müller, M.; Mink, D.; Schürmann, M.; Gruber, K.; Pichler, H. Evolving the Promiscuity of Elizabethkingia Meningoseptica Oleate Hydratase for the Regio- and Stereoselective Hydration of Oleic Acid Derivatives. Angew. Chem. Int. Ed. 2019, 58, 7480–7484. [Google Scholar] [CrossRef] [Green Version]
| Strain (Source of Fatty Acid Hydratase) | Product | Conv./% | Product-Amount/g L−1 | PRODUCTIVITY/g L−1 h−1 | Substrate Loading |
|---|---|---|---|---|---|
| Candida tropicalis DSM 3152 (wild-type) [38] | 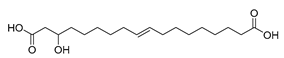 3-hydroxy-Δ9-cis-1,18- octadecenedioic acid | - | 19.4 | 0.8 | 70 mL d−1 |
| Stenotrophomonas nitritireducens (wild-type) [46] | 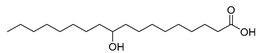 10-hydroxystearic acid | - | 31.5 | 7.9 | 15 g/L |
| Stenotrophomonas maltophilia (exp. in E. coli) [37] | 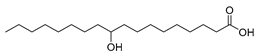 10-hydroxystearic acid | 98 | 49.0 | 12.3 | 50 g/L |
| Lysinibacillus fusiformis (exp. in E. coli) [48] |  10-hydroxystearic acid | 94 | 40.0 | 384 | 40 g/L |
| Stenotrophomonas maltophilia (exp. in E. coli) [49] | 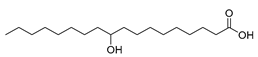 10-hydroxystearic acid | 91 | 46.0 | 197 | 50 g/L |
| Lysinibacillus fusiformis (exp. in E. coli) [50] |  10,12-dihydroxystearic acid | 90 | 13.5 | 108 | 15 g/L |
| Stenotrophomonas nitritireducens (exp. in E. coli) [55] |  10-hydroxy-12(Z)- octadecenoic acid 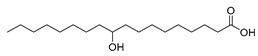 10-hydroxysteric acid | 89; 88 | 5.0, 0.85 | 102, 22 | 7.5 g/L |
| Lactobacillus acidophilus (exp. in E. coli) [56] |  13-hydroxy-9(Z)-octadecenoic acid | 79 | 79.0 | 631 | 100 g/L |
| Lactobacillus plantarum (exp. in E. coli) [58] | 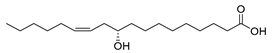 (S)-10-hydoxy-cis-12-octadecenoic acid | 98 | 280.0 | 552 | 90 g/L |
| Stenotrophomonas maltophilia, Lactobacillus acidophilus (exp. in E. coli) [60] |  10-monohydroxy fatty acids 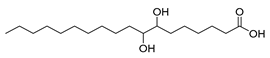 7,8-dihydroxy fatty acids | 65, 81 | 21.7, 13.3 | - | 50 mL (reaction volume) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löwe, J.; Gröger, H. Fatty Acid Hydratases: Versatile Catalysts to Access Hydroxy Fatty Acids in Efficient Syntheses of Industrial Interest. Catalysts 2020, 10, 287. https://doi.org/10.3390/catal10030287
Löwe J, Gröger H. Fatty Acid Hydratases: Versatile Catalysts to Access Hydroxy Fatty Acids in Efficient Syntheses of Industrial Interest. Catalysts. 2020; 10(3):287. https://doi.org/10.3390/catal10030287
Chicago/Turabian StyleLöwe, Jana, and Harald Gröger. 2020. "Fatty Acid Hydratases: Versatile Catalysts to Access Hydroxy Fatty Acids in Efficient Syntheses of Industrial Interest" Catalysts 10, no. 3: 287. https://doi.org/10.3390/catal10030287
APA StyleLöwe, J., & Gröger, H. (2020). Fatty Acid Hydratases: Versatile Catalysts to Access Hydroxy Fatty Acids in Efficient Syntheses of Industrial Interest. Catalysts, 10(3), 287. https://doi.org/10.3390/catal10030287






