Development and Validation of a LC-MS/MS Method for Determination of Multi-Class Antibiotic Residues in Aquaculture and River Waters, and Photocatalytic Degradation of Antibiotics by TiO2 Nanomaterials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimal MS/MS Parameters
2.2. Chromatographic Optimization
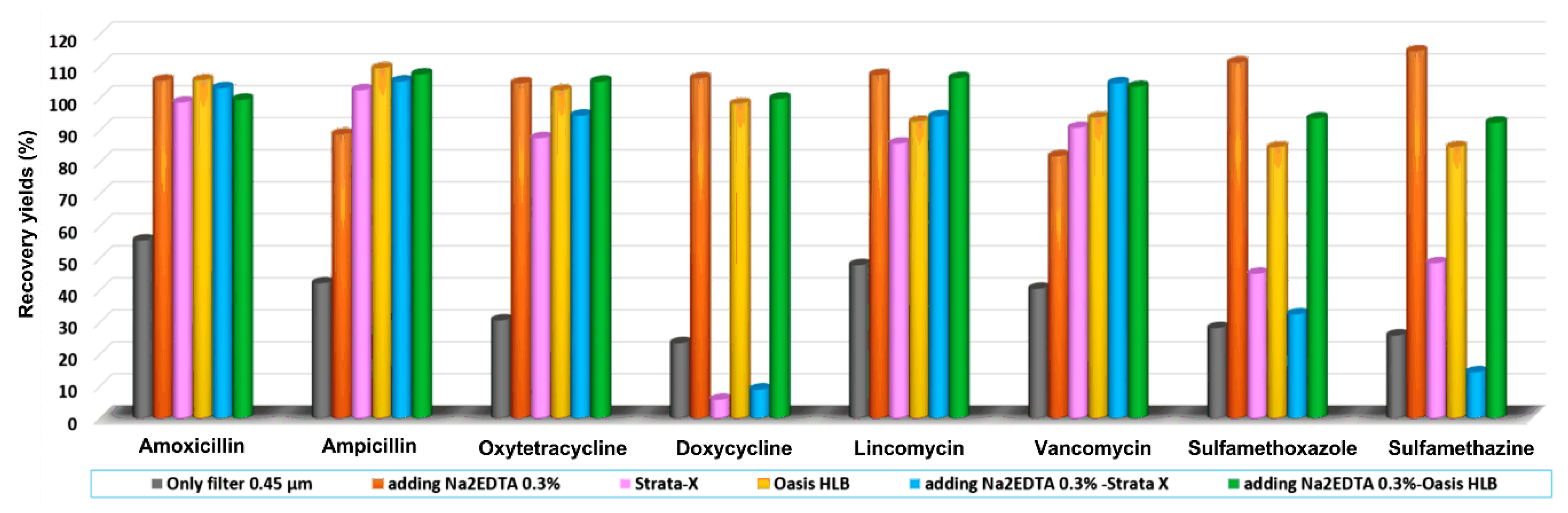
2.3. Optimization of Sample Preparation
2.4. Matrix Effects
2.5. Method Validation
2.6. Evaluation of the Antibiotic Residues in Aquaculture and River Water Samples
2.7. Photocatalytic Degradation of Antibiotics in Aquaculture Wastewater Samples by TiO2 Nanomaterials
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Collections
3.3. Sample Pre-Treatment
3.4. Liquid Chromatography/Mass Spectrometry Analysis
3.5. Method Validation
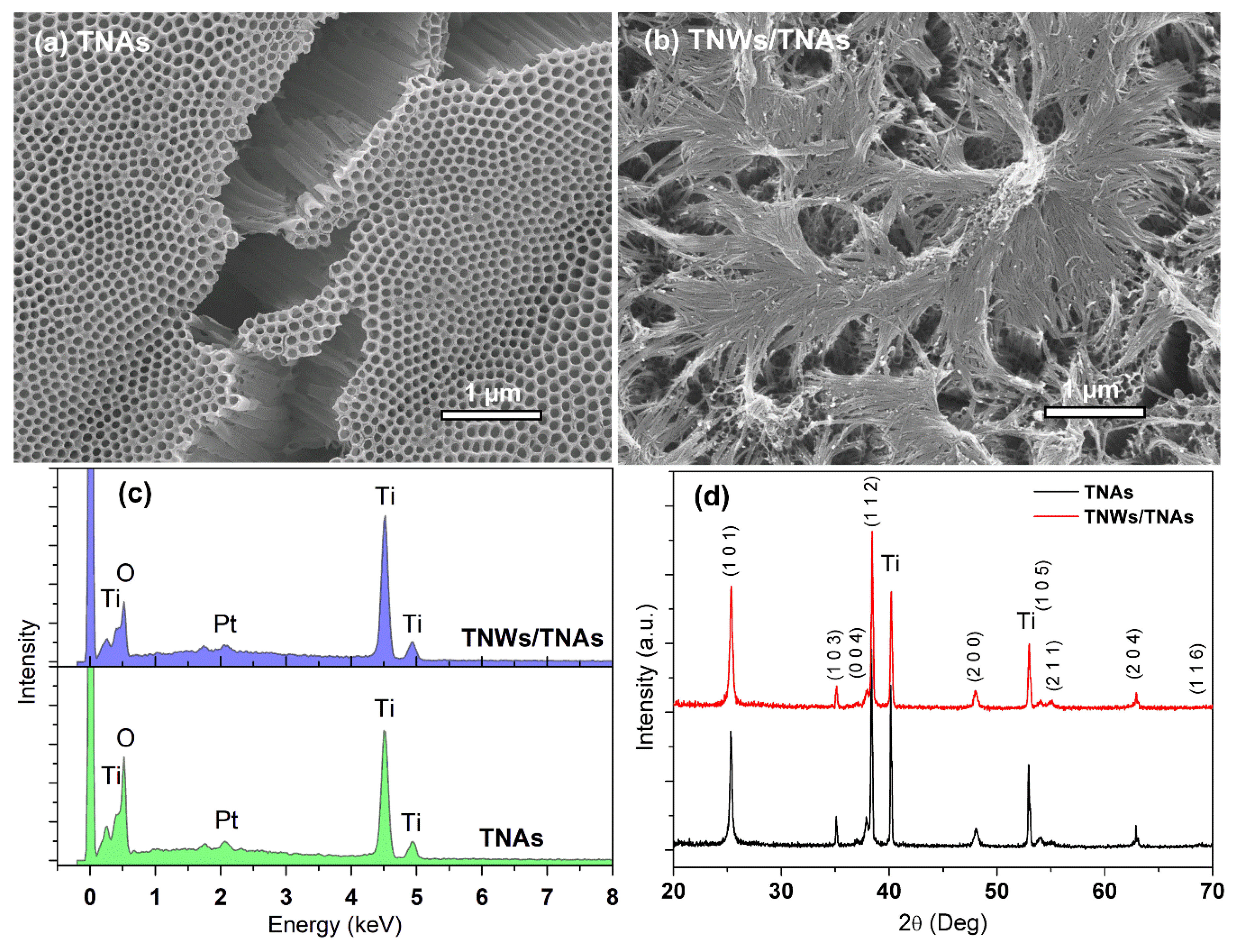
3.6. Fabricate of NanoTiO2 Materials
3.7. Photocatalytic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gelband, H.; Miller-Petrie, M.; Pant, S.; Gandra, S.; Levinson, J.; Barter, D.; White, A.; Laxminarayan, R. The State of the World’s Antibiotics 2015; Center for Disease Dynamics, Economics & Policy: Washington, DC, USA, 2015; pp. 1–79. [Google Scholar]
- European Centre for Disease Prevention and Control. Combating Resistance to Last-Line Antibiotics in the EU Still a Priority; Press Release: Brussels, Belgium, 2015; pp. 1–3. [Google Scholar]
- Kummerer, K. Resistance in the environment. J. Antimicrob. Chemother. 2004, 54, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, J.L. Antibiotics and antibiotic resistance genes in natural environments. Science 2008, 321, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Thuy, H.T.T.; Nguyen, T.D. The potential environmental risks of pharmaceuticals in Vietnamese aquatic systems: Case study of antibiotics and synthetic hormones. Environ. Sci. Pollut. Res. 2013, 20, 8132. [Google Scholar] [CrossRef] [PubMed]
- Jelic, A.; Petrovic, M.; Barcelo, D. Multi-residue method for trace level determination of pharmaceuticals in solid samples using pressurized liquid extraction followed by liquid chromatography/quadrupole-linear ion trap mass spectrometry. Talanta 2010, 80, 363–371. [Google Scholar] [CrossRef]
- Leung, H.W.; Minh, B.T.; Murphy, M.B.; Lam, J.C.W.; So, M.K.; Martin, M.; Lam, P.K.S.; Richardson, B.J. Distribution, fate and risk assessment of antibiotics in sewage treatment plants in Hong Kong, South China. Environ. Int. 2012, 42, 1–9. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Multi analyte method for the determination of pharmaceuticals in wastewater samples using solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 4229. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Borova, V.; Dasenaki, M.E.; Τhomaidis, Ν.S. Simultaneous determination of 148 pharmaceuticals and illicit drugs in sewage sludge based on ultrasound-assisted extraction and liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 4287–4297. [Google Scholar] [CrossRef] [Green Version]
- Wei, R.; Ge, F.; Huang, S.; Chen, M.; Wang, R. Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere 2011, 82, 1408–1414. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Directive 2000/60/EC of the European Parliament and of the council 23 October 2000 establishing a framework for community action in the field of water policy. Off. J. Eur. Commun. 2000, L327, 1–71. [Google Scholar]
- Vietnam Industrial Pollution Management Project (P113151). In Environmental and Social Management Framework; Ministry of Planning and Investment: Hanoi, Vietnam, 2015; pp. 29–58.
- Ngo, T.T.; Le, N.T.; Hoang, T.M.; Luong, D.H. Water Scarcity in Vietnam: A Point of View on Virtual Water Perspective. Water Resour. Manag. 2018, 32, 3579. [Google Scholar] [CrossRef]
- Wilder, M.; Nguyen, T.P. The Status of Aquaculture in the Mekong Delta Region of Vietnam: Sustainable Production and Combined Farming Systems. Fish. Sci. 2002, 68, 847–850. [Google Scholar] [CrossRef] [Green Version]
- Binh, V.N.; Dang, N.; Anh, N.T.K.; Ky, L.X.; Thai, P.K. Antibiotics in the aquatic environment of Vietnam: Sources, concentrations, risk and control strategy. Chemosphere 2018, 197, 438–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.T.H.; Rossi, P.; Dinh, H.D.K.; Pham, N.T.A.; Tran, P.A.; Ho, T.T.K.M.; Dinh, Q.T.; Alencastro, L.F.D. Analysis of antibiotic multi-resistant bacteria and resistance genes in the effluent of an intensive shrimp farm (Long An, Vietnam). J. Environ. Manag. 2018, 214, 149–156. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Murby, E.J.; Costanzo, S.D. Removal of antibiotics in conventional and advanced wastewater treatment: Implications for environmental discharge and wastewater recycling. Water Res. 2007, 41, 4164–4176. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T.; Xu, Z.; Fang, H.H.P. Rapid analysis of 21 antibiotics of multiple classes in municipal wastewater using ultra performance liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2009, 645, 64–72. [Google Scholar] [CrossRef]
- Opris, O.; Soran, M.-L.; Coman, V.; Copaciu, F.; Ristoiu, D. Determination of some frequently used antibiotics in waste waters using solid phase extraction followed by high performance liquid chromatography with diode array and mass spectrometry detection. Cent. Eur. J. Chem. 2013, 11, 1343–1351. [Google Scholar] [CrossRef]
- Cha, J.; Yang, S.; Carlson, K.H. Occurrence of β-lactam and polyether ionophore antibiotics in surface water, urban wastewater, and sediment. Geosyst. Eng. 2015, 18, 140–150. [Google Scholar] [CrossRef]
- Elhag, D.E.; Abdallah, B.S.; Hassan, M.; Suliman, A. Pharmaceutica ESI-LC/MS Method Development and Validation for the Determination of Some Selected Antibiotics in Hospital Wastewater. Pharm. Anal. Acta 2018, 9, 1–6. [Google Scholar]
- Rossmann, J.; Schubert, S.; Gurke, R.; Oertel, R.; Kirch, W. Simultaneous determination of most prescribed antibiotics in multiple urban wastewater by SPE-LC–MS/MS. J. Chromatogr. B 2014, 969, 162–170. [Google Scholar] [CrossRef]
- Shao, B.; Chen, D.; Zhang, J.; Wu, Y.; Sun, C. Determination of 76 pharmaceutical drugs by liquid chromatography–tandem mass spectrometry in slaughterhouse wastewater. J. Chromatogr. A 2009, 1216, 8312–8318. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Serna, R.; Perez, S.; Ginebreda, G.; Petrovic, M.; Barcelo, D. Fully automated determination of 74 pharmaceuticals in environmental and waste waters by online solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry. Talanta 2010, 83, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Carlson, K.H. Solid-phase extraction–high-performance liquid chromatography–ion trap mass spectrometry for analysis of trace concentrations of macrolide antibiotics in natural and waste water matrices. J. Chromatogr. A 2004, 1038, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Yunesian, M.; Nasseri, S.; Gholam, M.; Jalilzadeh, E.; Shoeibi, S.; Bidshahi, H.S.; Mesdaghinia, A. An optimized SPE-LC-MS/MS method for antibiotics residue analysis in ground, surface and treated water samples by response surface methodology- central composite design. J. Environ. Health Sci. Eng. 2017, 5, 21. [Google Scholar] [CrossRef]
- Pailler, J.-Y.; Krein, A.; Pfiste, L.; Hoffmann, L.; Guignard, C. Solid phase extraction coupled to liquid chromatography-tandem mass spectrometry analysis of sulfonamides, tetracyclines, analgesics and hormones in surface water and wastewater in Luxembourg. Sci. Total Environ. 2009, 407, 4736–4743. [Google Scholar] [CrossRef]
- Miao, X.-S.; Bishay, F.; Chen, M.; Metcalfe, C.D. Occurrence of Antimicrobials in the Final Effluents of Wastewater Treatment Plants in Canada. Environ. Sci. Technol. 2004, 38, 3533–3541. [Google Scholar] [CrossRef]
- Batt, L.A.; Aga, S.D. Simultaneous Analysis of Multiple Classes of Antibiotics by Ion Trap LC/MS/MS for Assessing Surface Water and Groundwater Contamination. Anal. Chem. 2005, 77, 2940–2947. [Google Scholar] [CrossRef]
- Yang, S.; Cha, J.; Carlson, K. Quantitative determination of trace concentrations of tetracycline and sulfonamide antibiotics in surface water using solid-phase extraction and liquid chromatography/ion trap tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 2131–2145. [Google Scholar] [CrossRef]
- Babic, S.; Pavlović, D.M.; Ašperger, D.; Periša, M.; Zrnčić, M.; Horvat, A.J.M.; Kaštelan-Macan, M. Determination of multi-class pharmaceuticals in wastewater by liquid chromatography—Tandem mass spectrometry (LC–MS–MS). Anal. Bioanal. Chem. 2010, 398, 1185–1194. [Google Scholar] [CrossRef]
- Zuccato, E.; Castiglioni, S.; Fanelli, R. Identification of the pharmaceuticals for human use contaminating the Italian aquatic environment. J. Hazzard. Mater. 2005, 122, 205–209. [Google Scholar] [CrossRef]
- Xu, W.-H.; Zhang, G.; Zou, S.-C.; Li, X.-D.; Liu, Y.-C. Determination of selected antibiotics in the Victoria Harbour and the Pearl River, South China using high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Environ. Pollut. 2007, 145, 672–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Managaki, S.; Murata, A.; Takada, H.; Tuyen, B.C.; Chiem, N.H. Distribution of macrolides, sulfonamides, and trimethoprim in tropical waters: Ubiquitous occurrence of veterinary antibiotics in the Mekong Delta. Environ. Sci. Technol. 2007, 41, 8004–8010. [Google Scholar] [CrossRef] [PubMed]
- Giang, C.N.D.; Sebesvari, Z.; Renaud, F.; Rosendahl, I.; Minh, Q.H.; Amelung, W. Occurrence and dissipation of the antibiotics sulfamethoxazole, sulfadiazine, trimethoprim, and enrofloxacin in the Mekong Delta, Vietnam. PLoS ONE 2015, 10, e0131855. [Google Scholar]
- Hoa, P.T.P.; Managaki, S.; Nakada, N.; Takada, H.; Shimizu, A.; Anh, D.H.; Viet, P.H.; Suzuki, S. Antibiotic contamination and occurrence of antibiotic-resistant bacteria in aquatic environments of northern Vietnam. Sci. Total Environ. 2011, 409, 2894–2901. [Google Scholar] [CrossRef]
- Castiglioni, S.; Bagnati, R.; Calamari, D.; Fanelli, R.; Zuccato, E. A multiresidue analytical method using solid-phase extraction and high-pressure liquid chromatography tandem mass spectrometry to measure pharmaceuticals of different therapeutic classes in urban wastewaters. J. Chromatogr. A 2005, 1092, 206–215. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. Multi-residue method for the determination of basic/neutral pharmaceuticals and illicit drugs in surface water by solid-phase extraction and ultra-performance liquid chromatography-positive electrospray ionisation tandem mass spectrometry. J. Chromatogr. A 2007, 1161, 132–145. [Google Scholar] [CrossRef]
- Teixeira, S.; Gurke, R.; Eckert, H.; Kuhn, K.; Fauler, J.; Cuniberti, G. Photocatalytic degradation of pharmaceuticals present in conventional treated wastewater by nanoparticle suspensions. J. Environ. Chem. Eng. 2016, 4, 287–292. [Google Scholar] [CrossRef]
- Russo, D.; Cochran, K.H.; Westerman, D.; Puma, G.L.; Marotta, R.; Andreozzi, R.; Richardson, S.D. Ultrafast photodegradation of isoxazole and isothiazolinones by UV254 and UV254/H2O2 photolysis in a microcapillary reactor. Water Res. 2020, 169, 115203. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Russo, D.; Tammaro, M.; Salluzzo, A.; Andreozzi, R.; Marotta, R. Modeling and validation of a modular multi-lamp photo-reactor for cetylpyridinium chloride degradation by UV and UV/H2O2 processes. Chem. Eng. J. 2019, 376, 120380. [Google Scholar] [CrossRef]
- Pal, P. Treatment and Disposal of Pharmaceutical Wastewater: Towards Sustainable Strategy. Sep. Purif. Rev. 2017, 47, 179–198. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on Fate and Mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Siciliano, A.; Russo, D.; Spasiano, D.; Marotta, R.; Race, M.; Fabbricino, M.; Galdiero, E.; Guida, M. Chronic toxicity of treated and untreated aqueous solutions containing imidazole-based ionic liquids and their oxydized by-products. Ecotoxicol. Environ. Saf. 2019, 180, 466–472. [Google Scholar]
- Babu, D.S.; Srivastava, V.; Nidheesh, P.V.; Kumar, M.S. Detoxification of water and wastewater by advanced oxidation processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- Russo, D.; Siciliano, A.; Guida, M.; Andreozzi, R.; Reis, N.M.; Puma, G.L.; Marotta, R. Removal of antiretroviral drugs stavudine and zidovudine in water under UV254 and UV254/H2O2 processes: Quantum yields, kinetics and ecotoxicology assessment. J. Hazard. Mater. 2018, 349, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Ahmad, J.; Flora, S.J.S. Application of advanced oxidation processes and toxicity assessment of transformation products. Environ. Res. 2018, 167, 223–233. [Google Scholar] [CrossRef]
- Lucchetti, R.; Siciliano, A.; Clarizia, L.; Russo, D.; Somma, H.D.; Natale, F.D.; Guida, M.; Andreozzi, R.; Marotta, R. Sacrificial photocatalysis: Removal of nitrate and hydrogen production by nano-copper-loaded P25 titania. A kinetic and ecotoxicological assessment. Environ. Sci. Pollut. Res. 2017, 24, 5898–5907. [Google Scholar] [CrossRef]
- Alharbi, S.K.; Price, W.E. Degradation and Fate of Pharmaceutically Active Contaminants by Advanced Oxidation Processes. Curr. Pollut. Rep. 2017, 3, 268–280. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Lin, Z.; Ye, M.; Wang, M. Multifunctional Photocatalytic Materials for Energy; Woodhead Publishing in Materials: Cambridge, MA, USA, 2018; pp. 187–213. [Google Scholar]
- Zhu, D.; Zhou, Q. Environmental Nanotechnology, Monitoring & Management Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100255. [Google Scholar]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- O’Shea, K.E.; Dionysiou, D.D. Advanced Oxidation Processes for Water Treatment. J. Phys. Chem. Lett. 2012, 3, 2112–2113. [Google Scholar] [CrossRef]
- Stefan, M.I. Advanced Oxidation Processes for Water Treatment: Fundamental and Applications; IWA Publishing: London, UK, 2017; pp. 1–710. [Google Scholar]
- Miranda-García, N.; Suárez, S.; Sánchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal. B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Hu, L.; Flanders, P.M.; Miller, P.L.; Strathmann, T.J. Oxidation of sulfamethoxazole and related antimicrobial agents by TiO2 photocatalysis. Water Res. 2007, 41, 2612–2626. [Google Scholar] [CrossRef]
- Li, D.; Shi, W. Recent developments in visible—Light photocatalytic degradation of antibiotics. Chin. J. Catal. 2016, 37, 792–798. [Google Scholar] [CrossRef]
- Le, P.H.; Hieu, L.T.; Lam, T.-N.; Hang, N.T.N.; Truong, N.V.; Tuyen, L.T.C.; Phong, P.T.; Leu, J. Enhanced Photocatalytic Performance of Nitrogen-Doped TiO2 Nanotube Arrays Using a Simple Annealing Process. Micromachines 2018, 9, 618. [Google Scholar] [CrossRef] [Green Version]
- Do, T.C.M.V.; Nguyen, D.Q.; Nguyen, K.T.; Le, P.H. TiO2 and Au-TiO2 Nanomaterials for Rapid Photocatalytic Degradation of Antibiotic Residues in Aquaculture Wastewater. Materials 2019, 12, 2434. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-Y.; Zhang, K.-Q.; Lai, Y. Fabrication, Modification, and Emerging Applications of TiO2 Nanotube Arrays by Electrochemical Synthesis: A Review. Int. J. Photoenergy 2013, 2013, 761971. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, G.; Guo, D.; Yu, L.; Zhang, W. Anodization Fabrication of Highly Ordered TiO2 Nanotubes. J. Phys. Chem. C 2009, 113, 12759–12765. [Google Scholar] [CrossRef]
- Hsu, M.-Y.; Hsu, H.-L.; Leu, J. TiO2 Nanowires on Anodic TiO2 Nanotube Arrays (TNWs/TNAs): Formation Mechanism and Photocatalytic Performance. J. Electrochem. Soc. 2012, 159, H722–H727. [Google Scholar] [CrossRef]
- MassBank Europe, High resolution Mass Spectral Database. Available online: https://massbank.eu/MassBank/ (accessed on 24 March 2020).
- Hu, F.-Y.; He, L.-M.; Yang, J.-W.; Bian, K.; Wang, Z.-N.; Yang, H.-C.; Liu, Y.-H. Determination of 26 veterinary antibiotics residues in water matrices by lyophilization in combination with LC–MS/MS. J. Chromatogr. B 2014, 949–950, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high performance liquid chromatography coupled to quadrupole linear ion trap tandem mass spectrometry. J. Chromatogr. A 2013, 1292, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Minh, T.B.; Leung, H.W.; Loi, I.H.; Chan, W.H.; So, M.K.; Mao, J.Q.; Choi, D.; Lam, J.C.W.; Zheng, G.; Martin, M.; et al. Antibiotics in the Hong Kong metropolitan area: Ubiquitous distribution and fate in Victoria Harbour. Mar. Pollut. Bull. 2009, 58, 1052–1062. [Google Scholar] [CrossRef]
- Shimizu, A.; Takada, H.; Koike, T.; Takeshita, A.; Saha, M.; Rinawati, N.; Murata, A.; Suzuki, T.; Suzuki, S.; Chiem, N.H.; et al. Ubiquitous occurrence of sulfonamides in tropical Asian waters. Sci. Total Environ. 2013, 452–453, 108–115. [Google Scholar] [CrossRef]
- Zhang, T.; Watson, D.G.; Azike, C.; Tettey, J.N.A.; Stearns, A.T.; Binning, A.R.; Payne, C.J. Determination of vancomycin in serum by liquid chromatography–high resolution full scan mass spectrometry. J. Chromatogr. B 2007, 857, 352–356. [Google Scholar] [CrossRef]
- Campagnolo, E.R.; Johnson, K.R.; Karpati, A.; Rubin, C.S.; Kolpin, D.W.; Meyer, M.T.; Esteban, J.E.; Currier, R.W.; Smith, K.; Thu, K.M.; et al. Antimicrobial residues in animal waste and water resources proximal to large-scale swine and poultry feeding operations. Sci. Total Environ. 2002, 299, 89–95. [Google Scholar] [CrossRef]
- Tong, L.; Li, P.; Wang, Y.; Zhu, K. Analysis of veterinary antibiotic residues in swine wastewater and environmental water samples using optimized SPE-LC/MS/MS. Chemosphere 2009, 74, 1090–1097. [Google Scholar] [CrossRef]
- Commission Decision (2002/657/EC) of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results. Off. J. Eur. Commun. 2002, L221, 8–36.
- Thuy, T.T.H.; Nga, L.P.; Loan, T.T.C. Antibiotic contaminants in coastal wetlands from Vietnamese shrimp farming. Sci. Total Environ. 2011, 18, 835–841. [Google Scholar] [CrossRef]
- Tuyen, L.T.C.; Jian, S.-R.; Tien, N.T.; Le, P.H. Nanomechanical and Material Properties of Fluorine-Doped Tin Oxide Thin Films Prepared by Ultrasonic Spray Pyrolysis: Effects of F-Doping. Materials 2019, 12, 1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.D.; Saini, K.K.; Kant, C.; Sharma, C.P.; Jain, S.C. Photodegradation of dye pollutant under UV light by nano-catalyst doped titania thin films. Appl. Catal. B Environ. 2008, 84, 233–240. [Google Scholar] [CrossRef]
- Kim, I.; Tanaka, H. Photodegradation characteristics of PPCPs in water with UV treatment. Environ. Int. 2009, 35, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Zhang, X.; Luong, D.; Oakes, K.D.; Servos, M.R.; Liang, R.; Kurdi, S.; Peng, P.; Zhou, Y. Adsorption and photocatalytic degradation kinetics of pharmaceuticals by TiO2 nanowires during water treatment. Waste Biomass Valor. 2012, 3, 443–449. [Google Scholar] [CrossRef]



| Compounds | Retention Time | Precusor Ion [M+H]+ | Product Ion 1 (Quantitative) | Product Ion 2 (Identified) | Cone Voltage (V) | Optimal Collision Energy-Product Ion 1 (V) | Optimal Collision Energy-Product Ion 2 (V) |
|---|---|---|---|---|---|---|---|
| Amoxicillin | 3.91 | 366.2 | 114 | 208.3 | 18 | 22 | 12 |
| Ampicillin | 4.55 | 350.3 | 106.1 | 114 | 28 | 20 | 30 |
| Oxy tetracycline | 5.33 | 461 | 426.1 | 443 | 34 | 20 | 12 |
| Doxycycline | 8.43 | 445.3 | 428 | 98.1 | 38 | 16 | 44 |
| Lincomycin | 4.19 | 407.3 | 126.2 | 359.2 | 44 | 28 | 18 |
| Vancomycin | 3.28 | 725.7 | 100.1 | 144.2 | 24 | 16 | 40 |
| Sulfamethoxazole | 8.06 | 254.2 | 92.1 | 108 | 28 | 28 | 24 |
| Sulfamethazine | 6.43 | 279.2 | 186 | 92 | 38 | 16 | 30 |
| Trimethoprim (IS-1) | 4.81 | 291.3 | 123.1 | 230.1 | 48 | 24 | 24 |
| Atenolol (IS-2) | 3.83 | 267.4 | 56.1 | 71.6 | 38 | 28 | 22 |
| Time (minutes) | %A | %B | %C |
|---|---|---|---|
| 0 | 24 | 13 | 63 |
| 5.3 | 24 | 13 | 63 |
| 5.8 | 5 | 94 | 1 |
| 10.5 | 5 | 94 | 1 |
| 11 | 24 | 13 | 63 |
| 15 | 24 | 13 | 63 |
| Parameter | AMOX | AMPI | OTC | DXC | LCM | VCM | SMZ | SMX |
|---|---|---|---|---|---|---|---|---|
| r2 | 0.998 | 0.998 | 0.998 | 0.995 | 0.999 | 0.995 | 0.999 | 0.997 |
| MDLs (ng·mL−1) | 0.05 | 0.02 | 0.02 | 0.05 | 0.025 | 0.07 | 0.025 | 0.01 |
| MQLs (ng·mL−1) | 0.16 | 0.07 | 0.05 | 0.15 | 0.06 | 0.20 | 0.07 | 0.03 |
| Precision a (intra-day) | 3.70 | 3.10 | 4.11 | 2.82 | 1.14 | 4.21 | 3.08 | 1.96 |
| Precision b (inter-day) | 4.73 | 3.94 | 4.30 | 3.48 | 1.60 | 4.11 | 4.61 | 3.70 |
| Accuracy c (40 ng·mL−1) | 87.36 | 87.04 | 96.35 | 96.51 | 100.47 | 91.57 | 105.6 | 101.24 |
| Accuracy c (20 ng·mL−1) | 89.51 | 89.06 | 94.36 | 90.28 | 99.77 | 88.87 | 103.45 | 99.81 |
| Accuracy c (10 ng·mL−1) | 88.65 | 87.67 | 92.33 | 89.75 | 98.99 | 87.03 | 101.68 | 100.24 |
| Antibiotics | CM-DD1–10 | BL-HB1–10 | CT-TL1–6 | AG-CT1–9 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Occ a | Range | Aver b | Occ | Range | Aver | Occ | Range | Aver | Occ | Range | Aver | |
| AMOX | 1/10 | 0.4 | 0.4 | 2/10 | 0.3–0.8 | 0.55 | n.d c | n.d | n.d | n.d | n.d | n.d |
| AMPI | 2/10 | 0.1–0.3 | 0.2 | 3/10 | 0.3–0.7 | 0.6 | n.d | n.d | n.d | n.d | n.d | n.d |
| DXC | 7/10 | 1.7–12.6 | 6.2 | 6/10 | 1.5–8.9 | 7.1 | 4/6 | 1.4–7.6 | 4.8 | 6/9 | 7.3–55.6 | 27.5 |
| OTC | 10/10 | 12–112.6 | 76.8 | 10/10 | 6.2–25.4 | 14.3 | 6/6 | 2.3–27.4 | 18.5 | 9/9 | 0.7–2.7 | 1.7 |
| LCM | 7/10 | 5.2–22.6 | 16.5 | 7/10 | 9.8–60.7 | 32.5 | 4/6 | 0.3–1.4 | 0.9 | 6/9 | 2.4–25.2 | 13.3 |
| VCM | 3/10 | 1.7–4.8 | 3.0 | 4/10 | 1.1–5.7 | 3.7 | n.d | n.d | n.d | 2/9 | 1.6–5.2 | 3.4 |
| SMX | 10/10 | 18.6–83.5 | 60.9 | 10/10 | 3.2–25.8 | 16.5 | 6/6 | 0.4–12.2 | 7.6 | 9/9 | 2.4–23.6 | 11.5 |
| SMZ | 7/10 | 6.8–20.5 | 10.4 | 8/10 | 1.1–15.5 | 8.7 | 4/6 | 1.6–10.8 | 6.5 | 7/9 | 7.2–48.4 | 24.6 |
| Antibiotics | Levels (ng·mL−1) | |||
|---|---|---|---|---|
| DD-River | BL-River | XS-Canal | BT-Canal | |
| AMOX | n.d a | n.d | n.d | n.d |
| AMPI | n.d | n.d | n.d | n.d |
| DXC | 0.2 | 0.3 | 0.2 | 0.4 |
| OTC | 1.1 | 0.1 | 0.2 | n.d |
| LCM | 0.1 | 0.3 | n.d | 0.1 |
| VCM | n.d | n.d | n.d | n.d |
| SMX | 0.8 | 0.3 | n.d | 0.1 |
| SMZ | 0.2 | 0.1 | 0.2 | 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, T.C.M.V.; Nguyen, D.Q.; Nguyen, T.D.; Le, P.H. Development and Validation of a LC-MS/MS Method for Determination of Multi-Class Antibiotic Residues in Aquaculture and River Waters, and Photocatalytic Degradation of Antibiotics by TiO2 Nanomaterials. Catalysts 2020, 10, 356. https://doi.org/10.3390/catal10030356
Do TCMV, Nguyen DQ, Nguyen TD, Le PH. Development and Validation of a LC-MS/MS Method for Determination of Multi-Class Antibiotic Residues in Aquaculture and River Waters, and Photocatalytic Degradation of Antibiotics by TiO2 Nanomaterials. Catalysts. 2020; 10(3):356. https://doi.org/10.3390/catal10030356
Chicago/Turabian StyleDo, Tho Chau Minh Vinh, Duy Quoc Nguyen, Tuan Duc Nguyen, and Phuoc Huu Le. 2020. "Development and Validation of a LC-MS/MS Method for Determination of Multi-Class Antibiotic Residues in Aquaculture and River Waters, and Photocatalytic Degradation of Antibiotics by TiO2 Nanomaterials" Catalysts 10, no. 3: 356. https://doi.org/10.3390/catal10030356
APA StyleDo, T. C. M. V., Nguyen, D. Q., Nguyen, T. D., & Le, P. H. (2020). Development and Validation of a LC-MS/MS Method for Determination of Multi-Class Antibiotic Residues in Aquaculture and River Waters, and Photocatalytic Degradation of Antibiotics by TiO2 Nanomaterials. Catalysts, 10(3), 356. https://doi.org/10.3390/catal10030356






