The Effect of AgInS2, SnS, CuS2, Bi2S3 Quantum Dots on the Surface Properties and Photocatalytic Activity of QDs-Sensitized TiO2 Composite
Abstract
:1. Introduction
2. Results and Discussion
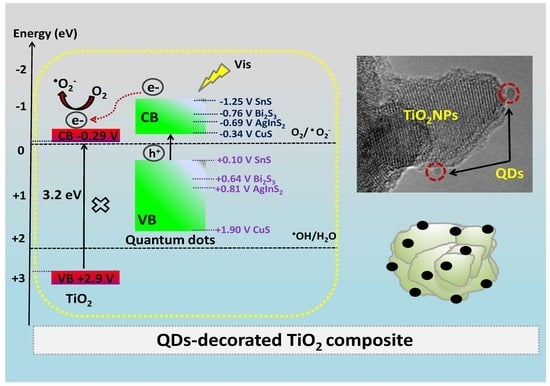
2.1. Morphology
2.2. UV-Vis Spectra
2.3. Photoluminescence Properties
2.4. XRD Analysis
2.5. FT-IR Analysis
2.6. Photocatalytic Activity in the Gas Phase
3. Experimental
3.1. Materials and Instruments
3.2. Sample Preparation
3.2.1. TiO2 NPs Preparation
3.2.2. Quantum Dots Preparation
3.2.3. Preparation of Quantum Dot-sensitized TiO2 Composites
3.3. Measurement of Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bajorowicz, B.; Kobylański, M.P.; Malankowska, A.; Mazierski, P.; Nadolna, J.; Pieczyńska, A.; Zaleska-Medynska, A. 4—Application of metal oxide-based photocatalysis. In Metal Oxide-Based Photocatalysis; Zaleska-Medynska, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 211–340. [Google Scholar]
- Nath, R.K.; Zain, M.F.M.; Jamil, M. An environment-friendly solution for indoor air purification by using renewable photocatalysts in concrete: A review. Renew. Sustain. Energy Rev. 2016, 62, 1184–1194. [Google Scholar] [CrossRef]
- Ren, H.; Koshy, P.; Chen, W.-F.; Qi, S.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, F.; Sang, Y.; Liu, H. Full-Spectrum Solar-Light-Activated Photocatalysts for Light–Chemical Energy Conversion. Adv. Energy Mater. 2017, 7, 1700473. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Low, J.; Long, R.; Kong, T.; Zhu, J.; Xiong, Y. Heterogeneous Single-Atom Photocatalysts: Fundamentals and Applications. Chem. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kampouri, S.; Stylianou, K.C. Dual-Functional Photocatalysis for Simultaneous Hydrogen Production and Oxidation of Organic Substances. ACS Catal. 2019, 9, 4247–4270. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Di, T.; Xu, Q.; Ho, W.; Tang, H.; Xiang, Q.; Yu, J. Review on Metal Sulphide-based Z-scheme Photocatalysts. ChemCatChem 2019, 11, 1394–1411. [Google Scholar] [CrossRef]
- Liu, B.; Li, X.; Zhao, Q.; Ke, J.; Tadé, M.; Liu, S. Preparation of AgInS2/TiO2 composites for enhanced photocatalytic degradation of gaseous o-dichlorobenzene under visible light. Appl. Catal. B Environ. 2016, 185, 1–10. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Z.; Zhang, P.; Jin, H.; Ding, Y. Natural assembly of a ternary Ag–SnS–TiO2 photocatalyst and its photocatalytic performance under simulated sunlight. RSC Adv. 2018, 8, 13408–13416. [Google Scholar] [CrossRef] [Green Version]
- Chandra, M.; Bhunia, K.; Pradhan, D. Controlled Synthesis of CuS/TiO2 Heterostructured Nanocomposites for Enhanced Photocatalytic Hydrogen Generation through Water Splitting. Inorg. Chem. 2018, 57, 4524–4533. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Umar, A.; Anderson, W.A.; Kansal, S.K. Facile synthesis of CdS/TiO2 nanocomposite and their catalytic activity for ofloxacin degradation under visible illumination. J. Photochem. Photobiol. A Chem. 2018, 360, 34–43. [Google Scholar] [CrossRef]
- Al-Fahdi, T.; Al Marzouqi, F.; Kuvarega, A.T.; Mamba, B.B.; Al Kindy, S.M.Z.; Kim, Y.; Selvaraj, R. Visible light active CdS@TiO2 core-shell nanostructures for the photodegradation of chlorophenols. J. Photochem. Photobiol. A Chem. 2019, 374, 75–83. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Z.; Wang, K.; Pu, S.; Yang, S.; Yang, L. A Facile Synthesis of TiO2–CdS Heterostructures With Enhanced Photocatalytic Activity. Catal. Lett. 2017, 147, 2581–2591. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Nengzi, L.-C.; Li, B.; Gou, J.; Cheng, X. Synthesis of SnS/TiO2 nano-tube arrays photoelectrode and its high photoelectrocatalytic performance for elimination of 2,4,6-trichlorophenol. Sep. Purif. Technol. 2019, 228, 115742. [Google Scholar] [CrossRef]
- Yadav, S.K.; Jeevanandam, P. Synthesis of Ag2S–TiO2 nanocomposites and their catalytic activity towards rhodamine B photodegradation. J. Alloys Compd. 2015, 649, 483–490. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, S.; Wang, G.; Xie, Z.; Zhang, Z. Role of Ag2S coupling on enhancing the visible-light-induced catalytic property of TiO2 nanorod arrays. Sci. Rep. 2016, 6, 19754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Zhang, J.; Zhu, B.; Yu, J.; Xu, J. CuInS2 sensitized TiO2 hybrid nanofibers for improved photocatalytic CO2 reduction. Appl. Catal. B Environ. 2018, 230, 194–202. [Google Scholar] [CrossRef]
- Li, C.; Xi, Z.; Fang, W.; Xing, M.; Zhang, J. Enhanced photocatalytic hydrogen evolution activity of CuInS2 loaded TiO2 under solar light irradiation. J. Solid State Chem. 2015, 226, 94–100. [Google Scholar] [CrossRef]
- Shen, F.; Que, W.; Liao, Y.; Yin, X. Photocatalytic Activity of TiO2 Nanoparticles Sensitized by CuInS2 Quantum Dots. Ind. Eng. Chem. Res. 2011, 50, 9131–9137. [Google Scholar] [CrossRef]
- Kim, J.; Kang, M. High photocatalytic hydrogen production over the band gap-tuned urchin-like Bi2S3-loaded TiO2 composites system. Int. J. Hydrog. Energy 2012, 37, 8249–8256. [Google Scholar] [CrossRef]
- Lu, J.; Han, Q.; Wang, Z. Synthesis of TiO2/Bi2S3 heterojunction with a nuclear-shell structure and its high photocatalytic activity. Mater. Res. Bull. 2012, 47, 1621–1624. [Google Scholar] [CrossRef]
- Yang, L.; Sun, W.; Luo, S.; Luo, Y. White fungus-like mesoporous Bi2S3 ball/TiO2 heterojunction with high photocatalytic efficiency in purifying 2,4-dichlorophenoxyacetic acid/Cr(VI) contaminated water. Appl. Catal. B Environ. 2014, 156, 25–34. [Google Scholar] [CrossRef]
- Bajorowicz, B.; Kobylański, M.P.; Gołąbiewska, A.; Nadolna, J.; Zaleska-Medynska, A.; Malankowska, A. Quantum dot-decorated semiconductor micro- and nanoparticles: A review of their synthesis, characterization and application in photocatalysis. Adv. Colloid Interface Sci. 2018, 256, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Chen, Z.; Qin, L.; Yang, L.; Zhu, L.; Tang, P.; Gao, T.; Huang, Y.; Sha, Z.; et al. Visible light induced photocatalysis on CdS quantum dots decorated TiO2 nanotube arrays. Appl. Catal. A Gen. 2015, 498, 159–166. [Google Scholar] [CrossRef]
- Liu, L.; Hui, J.; Su, L.; Lv, J.; Wu, Y.; Irvine, J.T.S. Uniformly dispersed CdS/CdSe quantum dots co-sensitized TiO2 nanotube arrays with high photocatalytic property under visible light. Mater. Lett. 2014, 132, 231–235. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Men, Y.; Bian, Z. TiO2 mesocrystal with exposed (001) facets and CdS quantum dots as an active visible photocatalyst for selective oxidation reactions. Appl. Catal. B Environ. 2016, 187, 115–121. [Google Scholar] [CrossRef]
- Ge, L.; Liu, J. Synthesis and photocatalytic performance of novel CdS quantum dots sensitized Bi2WO6 photocatalysts. Mater. Lett. 2011, 65, 1828–1831. [Google Scholar] [CrossRef]
- Mao, L.; Jiang, W.; He, L.; Fang, P. Photocatalytic activity of TiO2 sensitized by CdS quantum dots under visible-light irradiation. Wuhan Univ. J. Nat. Sci. 2011, 16, 313–318. [Google Scholar] [CrossRef]
- Mazierski, P.; Nadolna, J.; Nowaczyk, G.; Lisowski, W.; Winiarski, M.J.; Klimczuk, T.; Kobylański, M.P.; Jurga, S.; Zaleska-Medynska, A. Highly Visible-Light-Photoactive Heterojunction Based on TiO2 Nanotubes Decorated by Pt Nanoparticles and Bi2S3 Quantum Dots. J. Phys. Chem. C 2017, 121, 17215–17225. [Google Scholar] [CrossRef]
- Qian, X.; Yue, D.; Tian, Z.; Reng, M.; Zhu, Y.; Kan, M.; Zhang, T.; Zhao, Y. Carbon quantum dots decorated Bi2WO6 nanocomposite with enhanced photocatalytic oxidation activity for VOCs. Appl. Catal. B Environ. 2016, 193, 16–21. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Ângelo, J.; Girão, A.V.; Trindade, T.; Andrade, L.; Mendes, A. N-doped carbon quantum dots/TiO2 composite with improved photocatalytic activity. Appl. Catal. B Environ. 2016, 193, 67–74. [Google Scholar] [CrossRef]
- Bajorowicz, B.; Nadolna, J.; Lisowski, W.; Klimczuk, T.; Zaleska-Medynska, A. The effects of bifunctional linker and reflux time on the surface properties and photocatalytic activity of CdTe quantum dots decorated KTaO3 composite photocatalysts. Appl. Catal. B Environ. 2017, 203, 452–464. [Google Scholar] [CrossRef]
- Marchelek, M.; Grabowska, E.; Klimczuk, T.; Lisowski, W.; Zaleska-Medynska, A. Various types of semiconductor photocatalysts modified by CdTe QDs and Pt NPs for toluene photooxidation in the gas phase under visible light. Appl. Surf. Sci. 2017, 393, 262–275. [Google Scholar] [CrossRef]
- Cheraghizade, M.; Jamali-Sheini, F.; Yousefi, R.; Niknia, F.; Mahmoudian, M.R.; Sookhakian, M. The effect of tin sulfide quantum dots size on photocatalytic and photovoltaic performance. Mater. Chem. Phys. 2017, 195, 187–194. [Google Scholar] [CrossRef]
- Chaudhary, G.R.; Bansal, P.; Mehta, S.K. Recyclable CuS quantum dots as heterogeneous catalyst for Biginelli reaction under solvent free conditions. Chem. Eng. J. 2014, 243, 217–224. [Google Scholar] [CrossRef]
- Cui, W.; An, W.; Liu, L.; Hu, J.; Liang, Y. Novel Cu2O quantum dots coupled flower-like BiOBr for enhanced photocatalytic degradation of organic contaminant. J. Hazard. Mater. 2014, 280, 417–427. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Solar Energy Mater. Solar Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Yen, Y.C.; Chen, J.Z.; Lu, Y.J.; Gwo, S.; Lin, K.J. Chain-network anatase/TiO2 (B) thin film with improved photocatalytic efficiency. Nanotechnology 2014, 25, 235602. [Google Scholar] [CrossRef]
- Grabowska, E.; Marchelek, M.; Klimczuk, T.; Trykowski, G.; Zaleska-Medynska, A. Noble metal modified TiO2 microspheres: Surface properties and photocatalytic activity under UV–vis and visible light. J. Mol. Catal. A Chem. 2016, 423, 191–206. [Google Scholar] [CrossRef]
- Saraf, L.V.; Patil, S.I.; Ogale, S.B.; Sainkar, S.R.; Kshirsager, S.T. Synthesis of Nanophase TiO2 by Ion Beam Sputtering and Cold Condensation Technique. Int. J. Mod. Phys. B 1998, 12, 2635–2647. [Google Scholar] [CrossRef]
- Gołąbiewska, A.; Malankowska, A.; Jarek, M.; Lisowski, W.; Nowaczyk, G.; Jurga, S.; Zaleska-Medynska, A. The effect of gold shape and size on the properties and visible light-induced photoactivity of Au-TiO2. Appl. Catal. B Environ. 2016, 196, 27–40. [Google Scholar] [CrossRef]
- Chang, J.-Y.; Wang, G.-Q.; Cheng, C.-Y.; Lin, W.-X.; Hsu, J.-C. Strategies for photoluminescence enhancement of AgInS2 quantum dots and their application as bioimaging probes. J. Mater. Chem. 2012, 22, 10609–10618. [Google Scholar] [CrossRef]
- Deepa, K.G.; Nagaraju, J. Growth and photovoltaic performance of SnS quantum dots. Mater. Sci. Eng. B 2012, 177, 1023–1028. [Google Scholar] [CrossRef]
- Li, S.; Ge, Z.-H.; Zhang, B.-P.; Yao, Y.; Wang, H.-C.; Yang, J.; Li, Y.; Gao, C.; Lin, Y.-H. Mechanochemically synthesized sub-5nm sized CuS quantum dots with high visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2016, 384, 272–278. [Google Scholar] [CrossRef]
- Ijaz, S.; Ehsan, M.F.; Ashiq, M.N.; He, T. Synthesis of a Bi2S3/CeO2 nanocatalyst and its visible light-driven conversion of CO2 into CH3OH and CH4. Catal. Sci. Technol. 2015, 5, 5208–5215. [Google Scholar] [CrossRef]
- Derric, M.; Stulik, D.; Landry, J. Infrared Spectroscopy in Conservation Science; Getty Publications: Los Angeles, CA, USA, 2000. [Google Scholar]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, A.P. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Mugundan, S.; Rajamannan, B.; Viruthagiri, G.; Shanmugam, N.; Gobi, R.; Praveen, P. Synthesis and characterization of undoped and cobalt-doped TiO2 nanoparticles via sol–gel technique. Appl. Nanosci. 2015, 5, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Shen, S.; Wang, X.; Zhang, Y.; Xu, H.; Zhang, T.; Wang, Q. Controlled synthesis of AgInS2 nanocrystals and their application in organic–inorganic hybrid photodetectors. CrystEngComm 2013, 15, 6443–6447. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Ghanbari, D.; Davar, F. Shape selective hydrothermal synthesis of tin sulfide nanoflowers based on nanosheets in the presence of thioglycolic acid. J. Alloys Compd. 2010, 492, 570–575. [Google Scholar] [CrossRef]
- Mariappan, R.; Mahalingam, T.; Ponnuswamy, V. Preparation and characterization of electrodeposited SnS thin films. Optik 2011, 122, 2216–2219. [Google Scholar] [CrossRef]
- Zhou, T.; Pang, W.K.; Zhang, C.; Yang, J.; Chen, Z.; Liu, H.K.; Guo, Z. Enhanced Sodium-Ion Battery Performance by Structural Phase Transition from Two-Dimensional Hexagonal-SnS2 to Orthorhombic-SnS. ACS Nano 2014, 8, 8323–8333. [Google Scholar] [CrossRef] [PubMed]
- Tank, N.S.; Parikh, K.D.; Joshi, M.J. Synthesis and characterization of copper sulphide (CuS) nano particles. AIP Conf. Proc. 2017, 1837, 040018. [Google Scholar]
- Riyaz, S.; Parveen, A.; Azam, A. Microstructural and optical properties of CuS nanoparticles prepared by sol–gel route. Perspect. Sci. 2016, 8, 632–635. [Google Scholar] [CrossRef] [Green Version]
- Salavati-Niasari, M.; Behfard, Z.; Amiri, O.; Khosravifard, E.; Hosseinpour-Mashkani, S.M. Hydrothermal Synthesis of Bismuth Sulfide (Bi2S3) Nanorods: Bismuth(III) Monosalicylate Precursor in the Presence of Thioglycolic Acid. J. Clust. Sci. 2013, 24, 349–363. [Google Scholar] [CrossRef]
- Lee, K.T.; Lin, C.H.; Lu, S.Y. SnO2 quantum dots synthesized with a carrier solvent assisted interfacial reaction for band-structure engineering of TiO2 photocatalysts. J. Phys. Chem. C 2014, 118, 14457–14463. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Hsu, Y.-J. Interfacial charge carrier dynamics of type-II semiconductor nanoheterostructures. Appl. Catal. B Environ. 2013, 130, 93–98. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Kubacka, A.; Fernández-García, M. Effect of g-C3N4 loading on TiO2-based photocatalysts: UV and visible degradation of toluene. Catal. Sci. Technol. 2014, 4, 2006–2015. [Google Scholar] [CrossRef]
- Young, C.; Lim, T.M.; Chiang, K.; Scott, J.; Amal, R. Photocatalytic oxidation of toluene and trichloroethylene in the gas-phase by metallised (Pt, Ag) titanium dioxide. Appl. Catal. B Environ. 2008, 78, 1–10. [Google Scholar] [CrossRef]
- Cao, L.; Gao, Z.; Suib, S.L.; Obee, T.N.; Hay, S.O.; Freihaut, J.D. Photocatalytic Oxidation of Toluene on Nanoscale TiO2 Catalysts: Studies of Deactivation and Regeneration. J. Catal. 2000, 196, 253–261. [Google Scholar] [CrossRef]
- Méndez-Román, R.; Cardona-Martínez, N. Relationship between the formation of surface species and catalyst deactivation during the gas-phase photocatalytic oxidation of toluene. Catal. Today 1998, 40, 353–365. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Fu, X.; Wang, C.; Ni, M.; Leung, M.K.H.; Wang, X.; Fu, X. Hydrogen Production over Titania-Based Photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Jia, H.; Yang, D.; Xiao, P.; Fan, X.; Zheng, Z.; Kim, H.-K.; Wamer, W.G.; Yin, J.-J. Composition Directed Generation of Reactive Oxygen Species in Irradiated Mixed Metal Sulfides Correlated with Their Photocatalytic Activities. ACS Appl. Mater. Interfaces 2015, 7, 16440–16449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyauchi, M.; Shiga, Y.; Srinivasan, N.; Atarashi, D.; Sakai, E. Ubiquitous quantum dot-sensitized nanoporous film for hydrogen production under visible-light irradiation. Mater. Chem. Phys. 2015, 160, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, L.; Fu, Z.; Cui, F. Carbon quantum dots decorated CuS nanocomposite for effective degradation of methylene blue and antibacterial performance. J. Mol. Liq. 2018, 268, 578–586. [Google Scholar] [CrossRef]
- Bajorowicz, B.; Kowalska, E.; Nadolna, J.; Wei, Z.; Endo, M.; Ohtani, B.; Zaleska-Medynska, A. Preparation of CdS and Bi2S3 quantum dots co-decorated perovskite-type KNbO3 ternary heterostructure with improved visible light photocatalytic activity and stability for phenol degradation. Dalton Trans. 2018, 47, 15232–15245. [Google Scholar] [CrossRef]
- Ruan, C.; Zhang, Y.; Lu, M.; Ji, C.; Sun, C.; Chen, X.; Chen, H.; Colvin, V.L.; Yu, W.W. White Light-Emitting Diodes Based on AgInS₂/ZnS Quantum Dots with Improved Bandwidth in Visible Light Communication. Nanomaterials 2016, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Malankowska, A.; Kobylański, M.P.; Mikolajczyk, A.; Cavdar, O.; Nowaczyk, G.; Jarek, M.; Lisowski, W.; Michalska, M.; Kowalska, E.; Ohtani, B.; et al. TiO2 and NaTaO3 Decorated by Trimetallic Au/Pd/Pt Core–Shell Nanoparticles as Efficient Photocatalysts: Experimental and Computational Studies. ACS Sustain. Chem. Eng. 2018, 6, 16665–16682. [Google Scholar] [CrossRef]










| Sample Label | Loading of QDs (wt%) | Preparation Method | Toluene Degradation after 1 h Irradiation (%) | |
|---|---|---|---|---|
| λmax = 415 nm | λmax = 375 nm | |||
| TiO2 NPs | 0 | hydrothermal | 45.1 | 79.1 |
| AgInS2 | 0 | hot-injection | 56.2 | 59.3 |
| TiO2/AgInS2_5 | 5 | adsorption/calcination | 71.1 | 78.6 |
| TiO2/AgInS2_10 | 10 | adsorption/calcination | 70.7 | 86.3 |
| TiO2/AgInS2_15 | 15 | adsorption/calcination | 56.1 | 89.2 |
| SnS | 0 | sonochemical | 14.8 | 6.50 |
| TiO2/SnS_5 | 5 | adsorption/calcination | 13.7 | 87.9 |
| TiO2/SnS_10 | 10 | adsorption/calcination | 34.6 | 79.9 |
| TiO2/SnS_15 | 15 | adsorption/calcination | 36.6 | 91.1 |
| CuS | 0 | microwave | 25.9 | 28.1 |
| TiO2/CuS_5 | 5 | adsorption/calcination | 24.7 | 37.3 |
| TiO2/CuS_10 | 10 | adsorption/calcination | 36.5 | 46.3 |
| TiO2/CuS_15 | 15 | adsorption/calcination | 40.2 | 45.6 |
| Bi2S3 | 0 | hot-injection | 21.8 | 35.2 |
| TiO2/Bi2S3_5 | 5 | adsorption/calcination | 29.8 | 56.3 |
| TiO2/Bi2S3_10 | 10 | adsorption/calcination | 29.7 | 52.5 |
| TiO2/Bi2S3_15 | 15 | adsorption/calcination | 34.7 | 49.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malankowska, A.; Kulesza, D.; Sowik, J.; Cavdar, O.; Klimczuk, T.; Trykowski, G.; Zaleska-Medynska, A. The Effect of AgInS2, SnS, CuS2, Bi2S3 Quantum Dots on the Surface Properties and Photocatalytic Activity of QDs-Sensitized TiO2 Composite. Catalysts 2020, 10, 403. https://doi.org/10.3390/catal10040403
Malankowska A, Kulesza D, Sowik J, Cavdar O, Klimczuk T, Trykowski G, Zaleska-Medynska A. The Effect of AgInS2, SnS, CuS2, Bi2S3 Quantum Dots on the Surface Properties and Photocatalytic Activity of QDs-Sensitized TiO2 Composite. Catalysts. 2020; 10(4):403. https://doi.org/10.3390/catal10040403
Chicago/Turabian StyleMalankowska, Anna, Daria Kulesza, Jakub Sowik, Onur Cavdar, Tomasz Klimczuk, Grzegorz Trykowski, and Adriana Zaleska-Medynska. 2020. "The Effect of AgInS2, SnS, CuS2, Bi2S3 Quantum Dots on the Surface Properties and Photocatalytic Activity of QDs-Sensitized TiO2 Composite" Catalysts 10, no. 4: 403. https://doi.org/10.3390/catal10040403
APA StyleMalankowska, A., Kulesza, D., Sowik, J., Cavdar, O., Klimczuk, T., Trykowski, G., & Zaleska-Medynska, A. (2020). The Effect of AgInS2, SnS, CuS2, Bi2S3 Quantum Dots on the Surface Properties and Photocatalytic Activity of QDs-Sensitized TiO2 Composite. Catalysts, 10(4), 403. https://doi.org/10.3390/catal10040403