Structurally and Compositionally Tunable Absorption Properties of AgCl@AgAu Nanocatalysts for Plasmonic Photocatalytic Degradation of Environmental Pollutants
Abstract
:1. Introduction
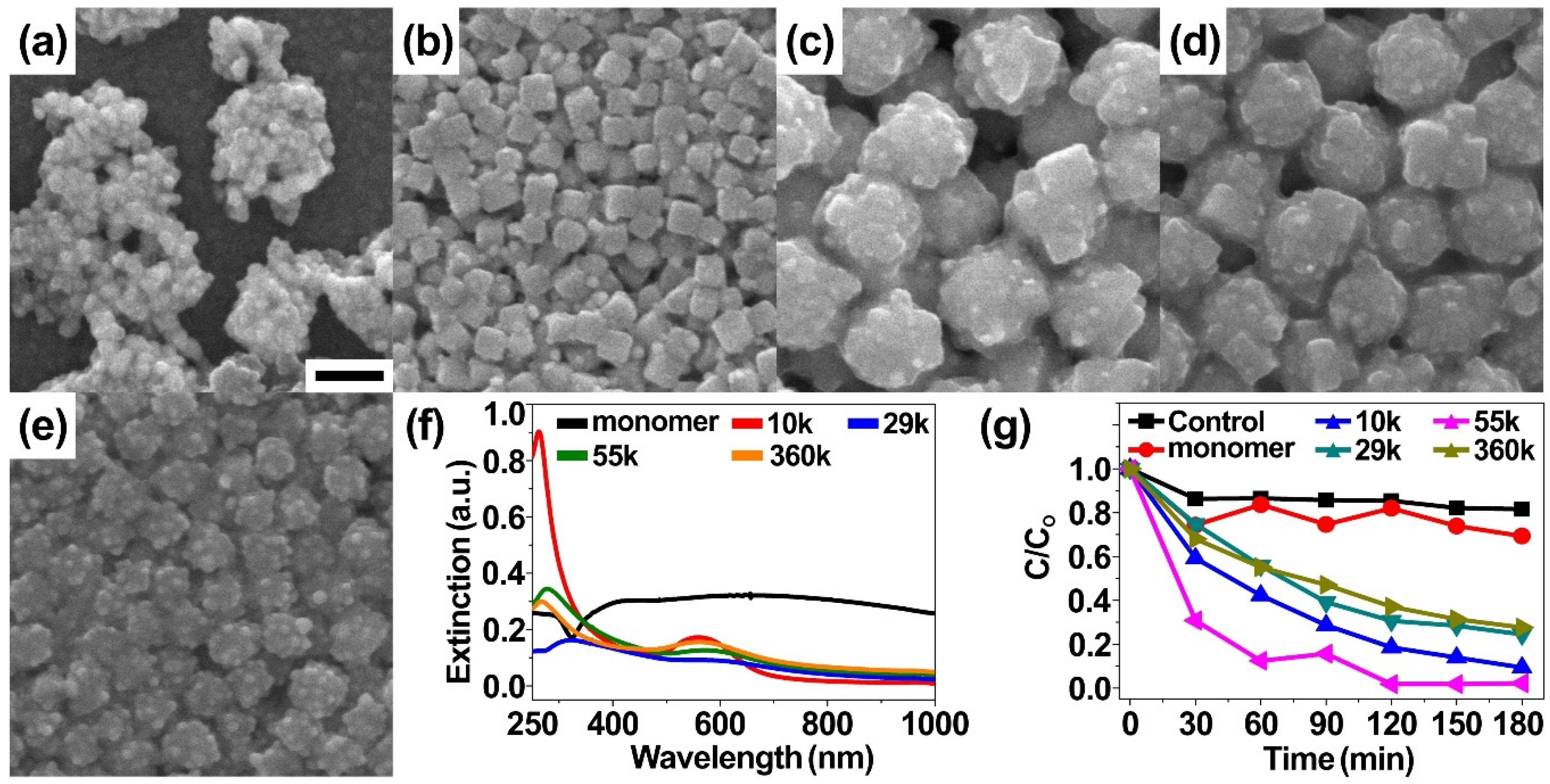
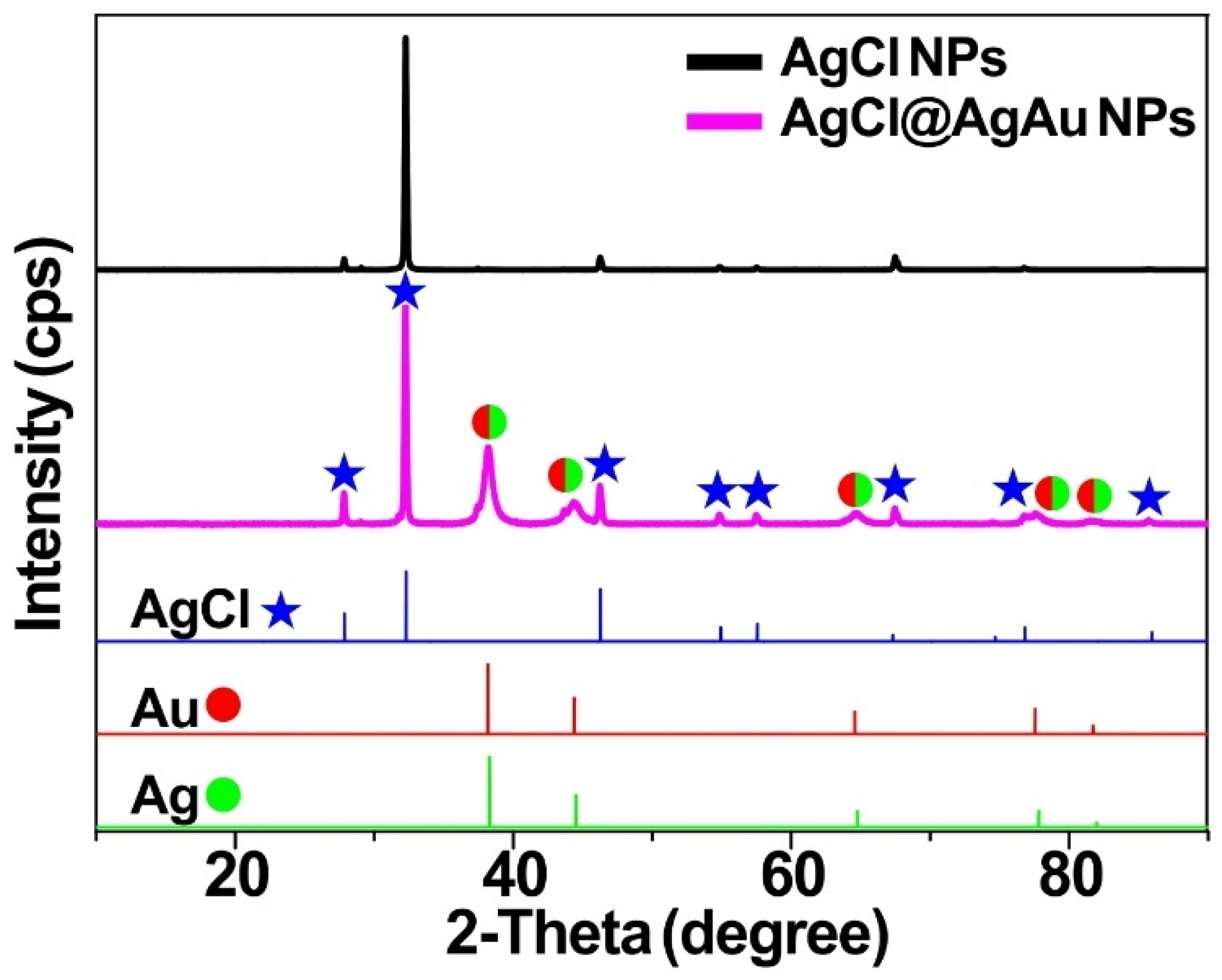
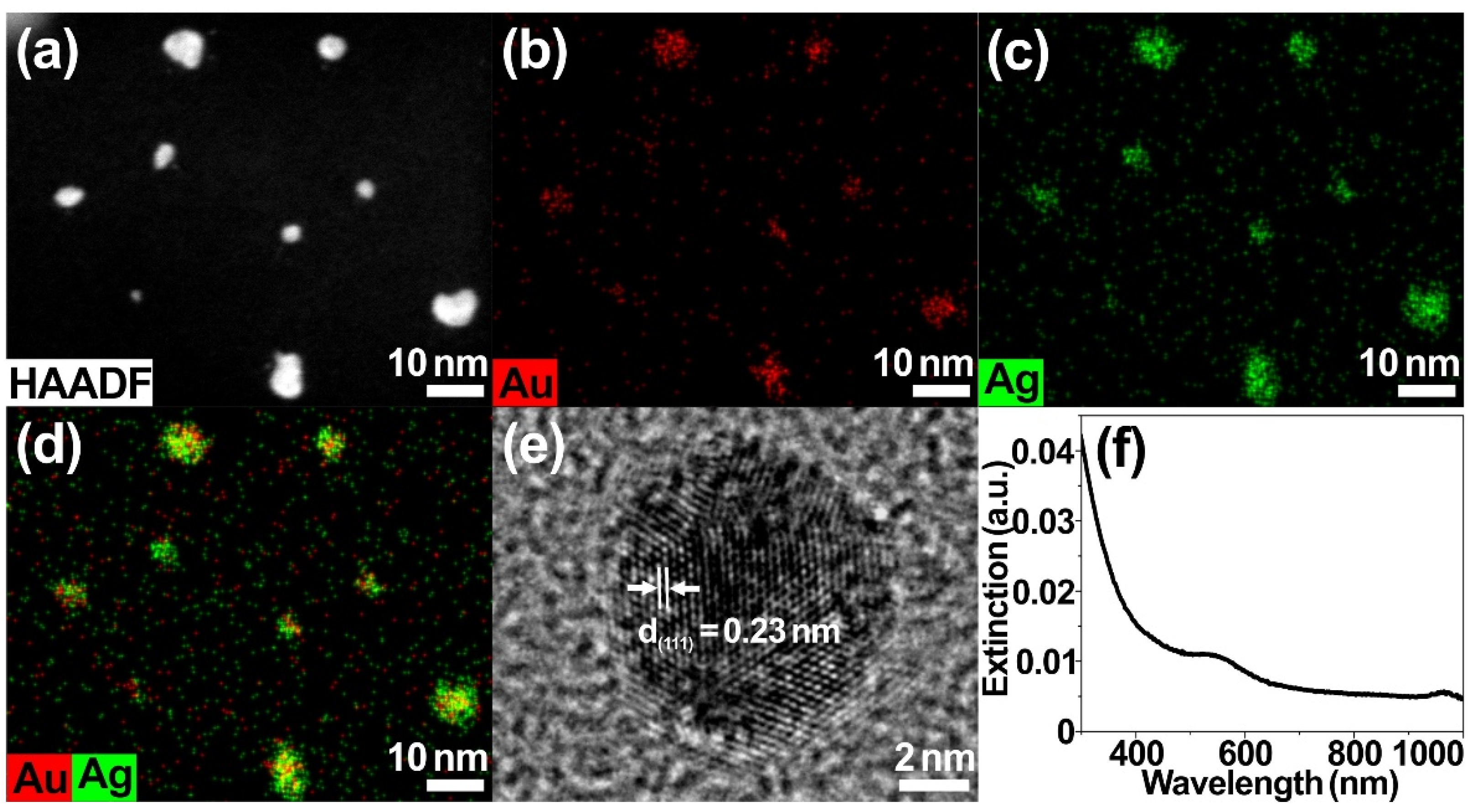
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis of AgCl@AgAu NPs
3.3. Synthesis of AgCl@AgAu NPs with Larger Metallic Bumps
3.4. Synthesis of Cubic AgCl@AgAu NPs
3.5. Synthesis of AgCl@AgAu NPs with Various Sizes
3.6. Synthesis of Various Shapes of AgCl@AgAu NPs
3.7. Photocatalytic Degradation of Organic dyes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zumdahl, S.S.; Zumdahl, S.A. Chemistry, 8th ed.; Cengage Learning: Boston, MA, USA, 2010. [Google Scholar]
- Garg, S.; Rong, H.Y.; Miller, C.J.; Waite, T.D. Chlorine-Mediated Regeneration of Semiconducting AgCl(s) Following Light-Induced Ag Formation: Implications to Contaminant Degradation. J. Phys. Chem. C 2016, 120, 5988–5996. [Google Scholar] [CrossRef]
- Polk, B.J.; Stelzenmuller, A.; Mijares, G.; MacCrehan, W.; Gaitan, M. Ag/AgCl microelectrodes with improved stability for microfluidics. Sens. Actuator B Chem. 2006, 114, 239–247. [Google Scholar] [CrossRef]
- Schurch, D.; Currao, A.; Sarkar, S.; Hodes, G.; Calzaferri, G. The Silver Chloride Photoanode in Photoelectrochemical Water Splitting. J. Phys. Chem. B 2002, 106, 12764–12775. [Google Scholar] [CrossRef]
- Joo, J.H.; Shin, H.; Kwon, K.; Hong, S.; Ryu, H.-J.; Choi, Y.; Lee, J.-S. Aqueous Synthesis of Highly Monodisperse Sub-100-nm AgCl Nanospheres/Cubes and Their Plasmonic Nanomesh Replicas as Visible-Light Photocatalysts and Single SERS Probes. Nanotechnology 2019, 30, 295604. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.-J.; Shin, H.; Oh, S.; Joo, J.H.; Choi, Y.; Lee, J.-S. Wrapping AgCl Nanostructures with Trimetallic Nanomeshes for Plasmon-Enhanced Catalysis and in Situ SERS Monitoring of Chemical Reactions. ACS Appl. Mater. Interfaces 2020, 12, 2842–2853. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.F.; Chen, P.L.; Xiao, D.; Chen, C.C.; Zhu, M.S.; Li, T.S.; Ma, W.G.; Liu, M.H. Spherical and Sheet like Ag/AgCl Nanostructures: Interesting Photocatalysts with Unusual Facet-Dependent yet SubstrateSensitive Reactivity. Langmuir 2015, 31, 602–610. [Google Scholar] [CrossRef]
- Kim, S.; Chung, H.; Kwon, J.H.; Yoon, H.G.; Kim, W. Facile Synthesis of Silver Chloride Nanocubes and Their Derivatives. Bull. Korean Chem. Soc. 2010, 31, 2918–2922. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Sun, Y.G. Ripening of bimodally distributed AgCl nanoparticles. J. Mater. Chem. 2011, 21, 11644–11650. [Google Scholar] [CrossRef]
- Zhou, S.; Li, J.H.; Gilroy, K.D.; Tao, J.; Zhu, C.L.; Yang, X.; Sun, X.J.; Xia, Y.N. Facile Synthesis of Silver Nanocubes with Sharp Corners and Edges in an Aqueous Solution. ACS Nano 2016, 10, 9861–9870. [Google Scholar] [CrossRef]
- Joo, J.H.; Kim, B.H.; Lee, J.S. Synthesis of Gold Nanoparticle-Embedded Silver Cubic Mesh Nanostructures Using AgCl Nanocubes for Plasmonic Photocatalysis. Small 2017, 13, 1701751. [Google Scholar] [CrossRef]
- Chew, W.S.; Pedireddy, S.; Lee, Y.H.; Tjiu, W.W.; Liu, Y.J.; Yang, Z.; Ling, X.Y. Nanoporous Gold Nanoframes with Minimalistic Architectures: Lower Porosity Generates Stronger Surface-Enhanced Raman Scattering Capabilities. Chem. Mat. 2015, 27, 7827–7834. [Google Scholar] [CrossRef]
- Pedireddy, S.; Lee, H.K.; Tjiu, W.W.; Phang, I.Y.; Tan, H.R.; Chua, S.Q.; Troadec, C.; Ling, X.Y. One-step synthesis of zero-dimensional hollow nanoporous gold nanoparticles with enhanced methanol electrooxidation performance. Nat. Commun. 2014, 5, 4947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spagnoletti, F.N.; Spedalieri, C.; Kronberg, F.; Giacometti, R. Extracellular biosynthesis of bactericidal Ag/AgCl nanoparticles for crop protection using the fungus Macrophomina phaseolina. J. Environ. Manag. 2019, 231, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Samimi, T. Green and simple synthesis route of Ag@AgCl nanomaterial using green marine crude extract and its application for sensitive and selective determination of mercury. Spectroc. Acta Part A Mol. Biomol. Spectr. 2019, 222, 117216. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.; Samadhiya, K.; Ghosh, A.; Anand, V.; Shirage, P.M.; Bala, K. Screening of microalgae for biosynthesis and optimization of Ag/AgCl nano hybrids having antibacterial effect. RSC Adv. 2019, 9, 25583–25591. [Google Scholar] [CrossRef] [Green Version]
- Gawali, P.; Jadhav, B.L. Synthesis of Ag/AgCl Nanoparticles and their action on Human Serum albumin: A fluorescence study. Process Biochem. 2018, 69, 106–122. [Google Scholar] [CrossRef]
- Zhang, H.J.; Chen, G.H.; Bahnemann, D.W. Photoelectrocatalytic materials for environmental applications. J. Mater. Chem. 2009, 19, 5089–5121. [Google Scholar] [CrossRef]
- Reddy, P.A.K.; Reddy, P.V.L.; Kwon, E.; Kim, K.H.; Akter, T.; Kalagara, S. Recent advances in photocatalytic treatment of pollutants in aqueous media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.X.; Bi, Y.P.; Umezawa, N.; Oshikiri, M.; Ye, J.H. Nano-photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Shen, S.H.; Guo, L.J.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.J.; Tan, L.L.; Chai, S.P.; Yong, S.T.; Mohamed, A.R. Facet-Dependent Photocatalytic Properties of TiO2-Based Composites for Energy Conversion and Environmental Remediation. Chemsuschem 2014, 7, 690–719. [Google Scholar] [CrossRef] [PubMed]
- Holton, J.C. Encyclopedia of Atmospheric Sciences, 1st ed.; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Zhang, Y.; Wang, T.; Zhou, M.; Wang, Y.; Zhang, Z.M. Hydrothermal preparation of Ag-TiO2 nanostructures with exposed {001}/{101} facets for enhancing visible light photocatalytic activity. Ceram. Int. 2017, 43, 3118–3126. [Google Scholar] [CrossRef]
- Anwer, S.; Bharath, G.; Iqbal, S.; Qian, H.M.; Masood, T.; Liao, K.; Cantwell, W.J.; Zhang, J.T.; Zheng, L.X. Synthesis of edge-site selectively deposited Au nanocrystals on TiO2 nanosheets: An efficient heterogeneous catalyst with enhanced visible-light photoactivity. Electrochim. Acta 2018, 283, 1095–1104. [Google Scholar] [CrossRef]
- Messih, M.F.A.; Ahmed, M.A.; Soltan, A.; Anis, S.S. Facile approach for homogeneous dispersion of metallic silver nanoparticles on the surface of mesoporous titania for photocatalytic degradation of methylene blue and indigo carmine dyes. J. Photochem. Photobiol. A Chem. 2017, 335, 40–51. [Google Scholar] [CrossRef]
- Khore, S.K.; Kadam, S.R.; Naik, S.D.; Kale, B.B.; Sonawane, R.S. Solar light active plasmonic Au@TiO2 nanocomposite with superior photocatalytic performance for H2 production and pollutant degradation. New J. Chem. 2018, 42, 10958–10968. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.T.; Zhao, B.; Hu, L.M. PVP protective mechanism of ultrafine silver powder synthesized by chemical reduction processes. J. Solid State Chem. 1996, 121, 105–110. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, G.H.; Nam, J.M. Directional Synthesis and Assembly of Bimetallic Nanosnowmen with DNA. J. Am. Chem. Soc. 2012, 134, 5456–5459. [Google Scholar] [CrossRef]
- Banica, R.; Ursu, D.; Nyari, T.; Kellenberger, A. Polyol synthesis of silver nanowires in the presence of silver chloride. J. Optoelectron. Adv. Mater. 2017, 19, 266–271. [Google Scholar]
- Wang, B.; Wang, D.D.; Zhao, S.; Huang, X.B.; Zhang, J.B.; Lv, Y.; Liu, X.C.; Lv, G.J.; Ma, X.J. Evaluate the ability of PVP to inhibit crystallization of amorphous solid dispersions by density functional theory and experimental verify. Eur. J. Pharm. Sci. 2017, 96, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Trasi, N.S.; Oucherif, K.A.; Litster, J.D.; Taylor, L.S. Evaluating the influence of polymers on nucleation and growth in supersaturated solutions of acetaminophen. CrystEngComm 2015, 17, 1242–1248. [Google Scholar] [CrossRef]
- Jukic, M.; Sviben, I.; Zoric, Z.; Milardovic, S. Effect of Polyvinylpyrrolidone on the Formation AgBr Grains in Gelatine Media. Croat. Chem. Acta 2012, 85, 269–276. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Mittal, A.; Saleh, T.A.; Nayak, A.; Agarwal, S.; Sikarwar, S. Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 12–17. [Google Scholar] [CrossRef]
- Rimer, J.D.; Chawla, A.; Le, T.T. Crystal Engineering for Catalysis. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 283–309. [Google Scholar] [CrossRef]
- Karkmaz, M.; Puzenat, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation of the alimentary azo dye amaranth mineralization of the azo group to nitrogen. Appl. Catal. B Environ. 2004, 51, 183–194. [Google Scholar] [CrossRef]
- Byeon, J.H.; Park, J.H.; Hwang, J.H. Spark generation of monometallic and bimetallic aerosol nanoparticles. J. Aerosol. Sci. 2008, 39, 888–896. [Google Scholar] [CrossRef]
- Ji, X.H.; Song, X.N.; Li, J.; Bai, Y.B.; Yang, W.S.; Peng, X.G. Size control of gold nanocrystals in citrate reduction: The third role of citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qian, K.; Bi, X.Z.; Huang, W.X. Influence of Speciation of Aqueous HAuCl4 on the Synthesis, Structure, and Property of Au Colloids. J. Phys. Chem. C 2009, 113, 6505–6510. [Google Scholar] [CrossRef]
- McKeehan, L.W. The crystal structure of silver-palladium and silver-gold alloys. Phys. Rev. 1922, 20, 424–432. [Google Scholar] [CrossRef] [Green Version]
- Storhoff, J.J.; Lazarides, A.A.; Mucic, R.C.; Mirkin, C.A.; Letsinger, R.L.; Schatz, G.C. What controls the optical properties of DNA-linked gold nanoparticle assemblies? J. Am. Chem. Soc. 2000, 122, 4640–4650. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Xiao, J.; Yan, J.H.; Liu, P.; Li, L.H.; Yang, G.W. Ag/AgCl plasmonic cubes with ultrahigh activity as advanced visible-light photocatalysts for photodegrading dyes. J. Mater. Chem. A 2015, 3, 7649–7658. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.L.; Wang, T.F.; Zhang, L.L. Silver chloride enwrapped silver grafted on nitrogen-doped reduced graphene oxide as a highly efficient visible-light-driven photocatalyst. J. Colloid Interface Sci. 2017, 505, 421–429. [Google Scholar] [CrossRef]
- Daupor, H.; Wongnawa, S. Urchinlike Ag/AgCl photocatalyst: Synthesis, characterization, and activity. Appl. Catal. A Gen. 2014, 473, 59–69. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J. Colloid Interface Sci. 2011, 362, 337–344. [Google Scholar] [CrossRef]
- Subash, B.; Krishnakumar, B.; Swaminathan, M.; Shanthi, M. Highly Efficient, Solar Active, and Reusable Photocatalyst: Zr-Loaded Ag-ZnO for Reactive Red 120 Dye Degradation with Synergistic Effect and Dye-Sensitized Mechanism. Langmuir 2013, 29, 939–949. [Google Scholar] [CrossRef]
- Khan, M.M.; Lee, J.; Cho, M.H. Au@TiO2 nanocomposites for the catalytic degradation of methyl orange and methylene blue: An electron relay effect. J. Ind. Eng. Chem. 2014, 20, 1584–1590. [Google Scholar] [CrossRef]
- Wang, C.C.; Li, J.R.; Lv, X.L.; Zhang, Y.Q.; Guo, G.S. Photocatalytic organic pollutants degradation in metal-organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Li, Z.X.; Luo, D.; Li, M.M.; Xing, X.F.; Ma, Z.Z.; Xu, H. Recyclable Fe3O4 Nanoparticles Catalysts for Aza-Michael Addition of Acryl Amides by Magnetic Field. Catalysts 2017, 7, 219. [Google Scholar] [CrossRef] [Green Version]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef] [PubMed]
- Davy, N.C.; Sezen-Edmonds, M.; Gao, J.; Lin, X.; Liu, A.; Yao, N.; Kahn, A.; Loo, Y.L. Pairing of near-ultraviolet solar cells with electrochromic windows for smart management of the solar spectrum. Nat. Energy 2017, 2, 17104. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, H.-J.; Kim, H.-L.; Joo, J.H.; Lee, J.-S. Structurally and Compositionally Tunable Absorption Properties of AgCl@AgAu Nanocatalysts for Plasmonic Photocatalytic Degradation of Environmental Pollutants. Catalysts 2020, 10, 405. https://doi.org/10.3390/catal10040405
Ryu H-J, Kim H-L, Joo JH, Lee J-S. Structurally and Compositionally Tunable Absorption Properties of AgCl@AgAu Nanocatalysts for Plasmonic Photocatalytic Degradation of Environmental Pollutants. Catalysts. 2020; 10(4):405. https://doi.org/10.3390/catal10040405
Chicago/Turabian StyleRyu, Han-Jung, Ha-Lin Kim, Jang Ho Joo, and Jae-Seung Lee. 2020. "Structurally and Compositionally Tunable Absorption Properties of AgCl@AgAu Nanocatalysts for Plasmonic Photocatalytic Degradation of Environmental Pollutants" Catalysts 10, no. 4: 405. https://doi.org/10.3390/catal10040405
APA StyleRyu, H. -J., Kim, H. -L., Joo, J. H., & Lee, J. -S. (2020). Structurally and Compositionally Tunable Absorption Properties of AgCl@AgAu Nanocatalysts for Plasmonic Photocatalytic Degradation of Environmental Pollutants. Catalysts, 10(4), 405. https://doi.org/10.3390/catal10040405





