Tunable Composition Aqueous-Synthesized Mixed-Phase TiO2 Nanocrystals for Photo-Assisted Water Decontamination: Comparison of Anatase, Brookite and Rutile Photocatalysts
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Mixed-Phase TiO2 Nanoparticles (NPs)
2.2. Characterization and Properties of Synthesized TiO2 Nanocrystals
Nanostructural Properties
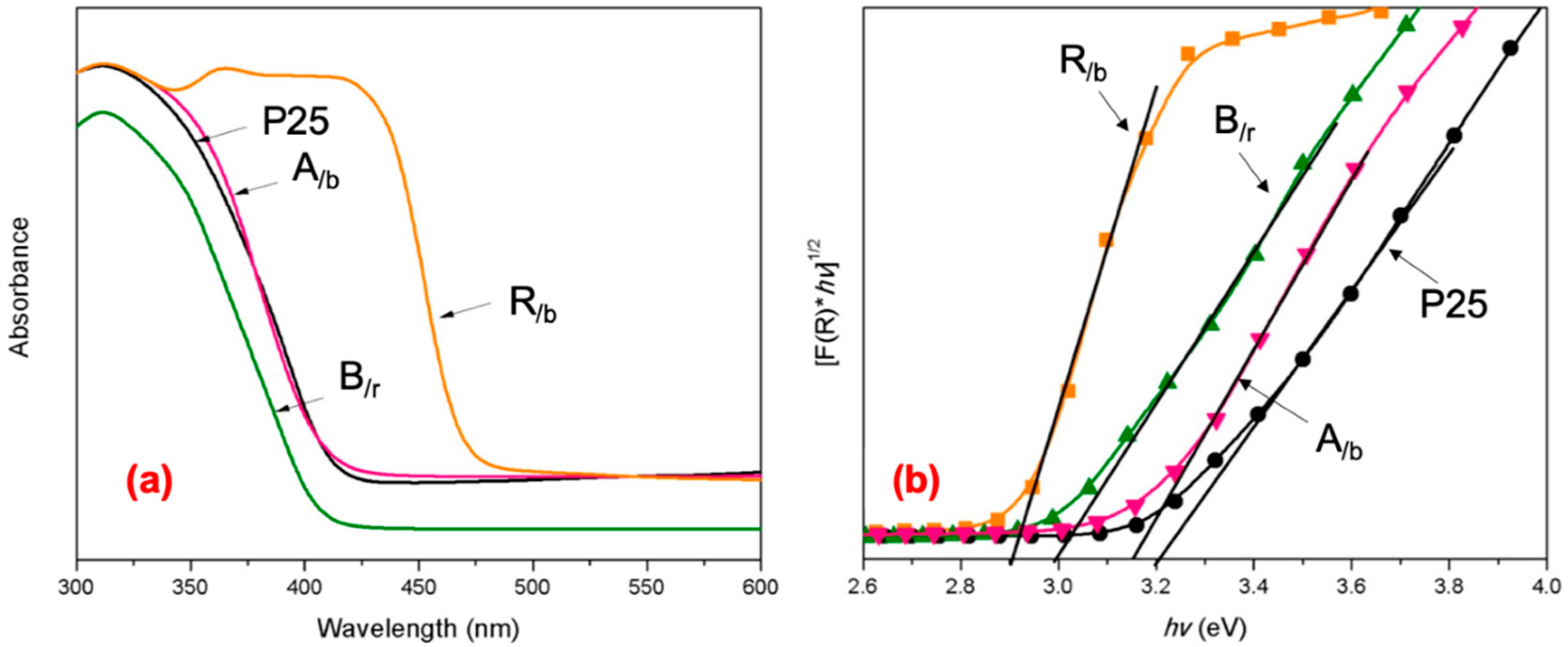
Light-Absorbing and Electronic Properties
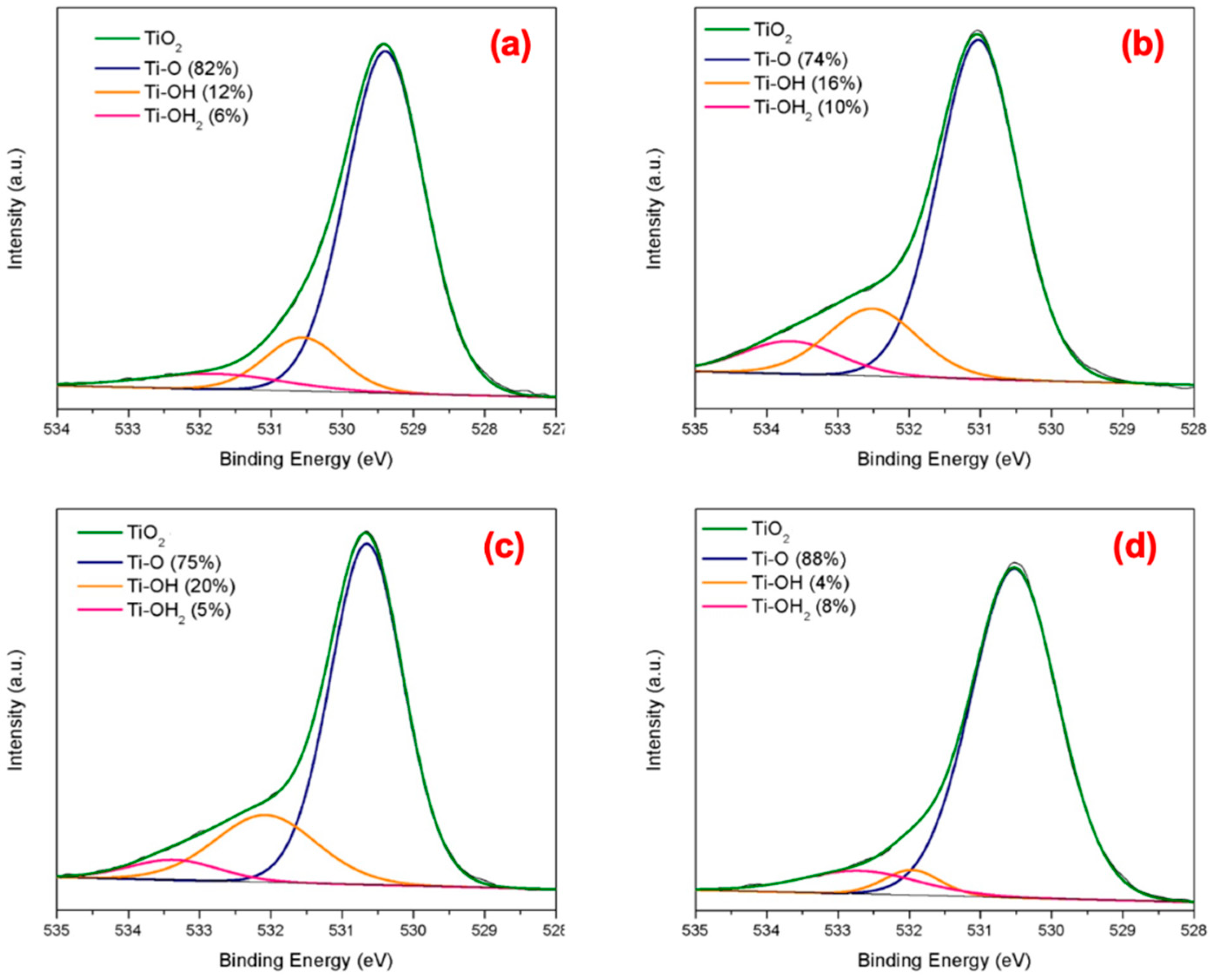
Surface Properties
2.3. Photocatalytic Evaluation of Mixed-Phase TiO2 Nanocrystals
Adsorption
Nano-TiO2 Pretreatment
Mixed-Phase vs. Single-Phase or Physically Mixed Photocatalysts
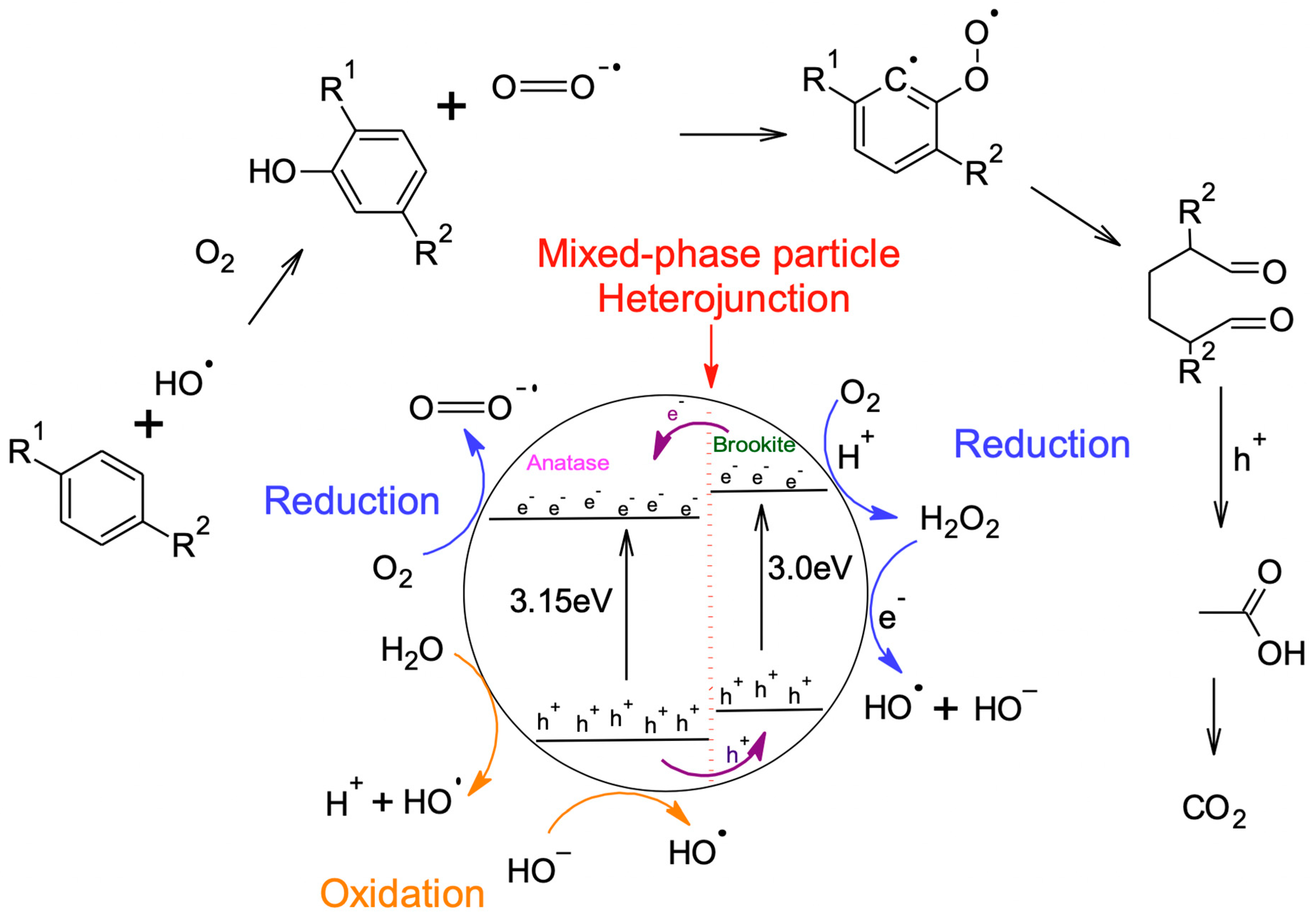
3. Discussion—Proposed Mechanism of Photocatalysis
4. Materials and Methods
4.1. Reagents—Chemicals
4.2. Synthesis of Mixed-Phase TiO2 Nanoparticles
4.3. Characterization Techniques
4.3.1. Photocatalyst Characterization
4.3.2. Solution Characterization
4.4. Evaluation of Photocatalytic Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Keane, D.A.; McGuigan, K.G.; Ibanez, P.F.; Polo-Lopez, M.I.; Byrne, J.A.; Dunlop, P.S.M.; O’Shea, K.; Dionysiou, D.D.; Pillai, S.C. Solar photocatalysis for water disinfection: Materials and reactor design. Catal. Sci. Technol. 2014, 4, 1211–1226. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Likodimos, V.; Chrysi, A.; Calamiotou, M.; Fernández-Rodríguez, C.; Doña-Rodríguez, J.; Dionysiou, D.; Falaras, P. Microstructure and charge trapping assessment in highly reactive mixed phase TiO 2 photocatalysts. Appl. Catal. B Environ. 2016, 192, 242–252. [Google Scholar] [CrossRef]
- Lee, K.E.; Charbonneau, C.; Demopoulos, G.P. Thin single screen-printed bifunctional titania layer photoanodes for high performing DSSCs via a novel hybrid paste formulation and process. J. Mater. Res. 2013, 28, 480–487. [Google Scholar] [CrossRef]
- Sussman, M.J.; Yasin, A.; Demopoulos, G.P. On the complex interplay of crystallinity and surface area effects on Li-ion intercalation and pseudocapacitive storage properties of nanocrystalline anatase. J. Power Sources 2014, 272, 58–67. [Google Scholar] [CrossRef]
- Pang, X.B.; Chen, C.C.; Ji, H.W.; Che, Y.K.; Ma, W.H.; Zhao, J.C. Unraveling the Photocatalytic Mechanisms on TiO2 Surfaces Using the Oxygen-18 Isotopic Label Technique. Molecules 2014, 19, 16291–16311. [Google Scholar] [CrossRef] [Green Version]
- Odling, G.; Robertson, N. Why is Anatase a Better Photocatalyst than Rutile? The Importance of Free Hydroxyl Radicals. ChemSusChem 2015, 8, 1838–1840. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Kosslick, H.; Ibad, M.F.; Fischer, C.; Bentrup, U.; Vuong, T.H.; Nguyen, L.Q.; Schulz, A. Photocatalytic Performance of Highly Active Brookite in the Degradation of Hazardous Organic Compounds Compared to Anatase and Rutile. Appl. Catal. B Environ. 2017, 200, 647–658. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.L.; Ho, J.H.; Jiang, Y.J.; Amal, R. Tuning Phase Composition of TiO2 by Sn4+ Doping for Efficient Photocatalytic Hydrogen Generation. Acs Appl. Mater. Interfaces 2015, 7, 23941–23948. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, B.; Borah, B.; Choudhury, A. Extending photocatalytic activity of TiO2 nanoparticles to visible region of illumination by doping of cerium. Photochem. Photobiol. 2012, 88, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Liu, L.; Andino, J.M.; Li, Y. Bicrystalline TiO2 with controllable anatase–brookite phase content for enhanced CO2 photoreduction to fuels. J. Mater. Chem. A 2013, 1, 8209. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wang, Q.; Dai, S.; Pan, J.H. Anatase and rutile in evonik aeroxide P25: Heterojunctioned or individual nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Ju, M.G.; Sun, G.; Wang, J.; Meng, Q.; Liang, W. Origin of high photocatalytic properties in the mixed-phase TiO2: A first-principles theoretical study. ACS Appl Mater Interfaces 2014, 6, 12885–12892. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Xu, Y. Explaining the High Photocatalytic Activity of a Mixed Phase TiO2: A Combined Effect of O2 and Crystallinity. J. Phys. Chem. C 2011, 115, 21161–21168. [Google Scholar] [CrossRef]
- Haque, F.Z.; Nandanwar, R.; Singh, P. Evaluating photodegradation properties of anatase and rutile TiO2 nanoparticles for organic compounds. Optik 2017, 128, 191–200. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the least known TiO2 photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef] [Green Version]
- Gai, L.; Duan, X.; Jiang, H.; Mei, Q.; Zhou, G.; Tian, Y.; Liu, H. One-pot synthesis of nitrogen-doped TiO2 nanorods with anatase/brookite structures and enhanced photocatalytic activity. CrystEngComm 2012, 14, 7662–7671. [Google Scholar] [CrossRef]
- Doudrick, K.; Monzón, O.; Mangonon, A.; Hristovski, K.; Westerhoff, P. Nitrate Reduction in Water Using Commercial Titanium Dioxide Photocatalysts (P25, P90, and Hombikat UV100). J. Environ. Eng. 2012, 138, 852–861. [Google Scholar] [CrossRef]
- Luo, Z.; Poyraz, A.S.; Kuo, C.H.; Miao, R.; Meng, Y.T.; Chen, S.Y.; Jiang, T.; Wenos, C.; Suib, S.L. Crystalline Mixed Phase (Anatase/Rutile) Mesoporous Titanium Dioxides for Visible Light Photocatalytic Activity. Chem. Mater. 2015, 27, 6–17. [Google Scholar] [CrossRef]
- Ide, Y.; Inami, N.; Hattori, H.; Saito, K.; Sohmiya, M.; Tsunoji, N.; Komaguchi, K.; Sano, T.; Bando, Y.; Golberg, D. Remarkable charge separation and photocatalytic efficiency enhancement through interconnection of TiO2 nanoparticles by hydrothermal treatment. Angew. Chem. Int. Ed. 2016, 55, 3600–3605. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, S.M.; Khedr, T.M.; Zhang, G.; Vogiazi, V.; Ismail, A.A.; O’Shea, K.; Dionysiou, D.D. Tailored synthesis of anatase–brookite heterojunction photocatalysts for degradation of cylindrospermopsin under UV–Vis light. Chem. Eng. J. 2017, 310, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Boehme, M.; Ensinger, W. Mixed Phase Anatase/rutile Titanium Dioxide Nanotubes for Enhanced Photocatalytic Degradation of Methylene-blue. Nano Micro Lett. 2011, 3, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Tay, Q.L.; Liu, X.F.; Tang, Y.X.; Jiang, Z.L.; Sum, T.C.; Chen, Z. Enhanced Photocatalytic Hydrogen Production with Synergistic Two-Phase Anatase/Brookite TiO2 Nanostructures. J. Phys. Chem. C 2013, 117, 14973–14982. [Google Scholar] [CrossRef]
- Benehkohal, N.P.; Gomez, M.A.; Gauvin, R.; Demopoulos, P.G. Enabling aqueous electrophoretic growth of adherent nanotitania mesoporous films via intrafilm cathodic deposition of hydrous zinc oxide. Electrochim. Acta 2013, 87, 169–179. [Google Scholar] [CrossRef]
- Caramazana-González, P.; Dunne, P.W.; Gimeno-Fabra, M.; Zilka, M.; Ticha, M.; Stieberova, B.; Freiberg, F.; McKechnie, J.; Lester, E.H. Assessing the life cycle environmental impacts of titania nanoparticle production by continuous flow solvo/hydrothermal syntheses. Green Chem. 2017, 19, 1536–1547. [Google Scholar] [CrossRef] [Green Version]
- Atitar, M.F.; Ismail, A.A.; Al-Sayari, S.; Bahnemann, D.; Afanasev, D.; Emeline, A. Mesoporous TiO2 nanocrystals as efficient photocatalysts: Impact of calcination temperature and phase transformation on photocatalytic performance. Chem. Eng. J. 2015, 264, 417–424. [Google Scholar] [CrossRef]
- Atitar, M.F.; Ismail, A.; Dillert, R.; Bahnemann, D.W. Photodegradation of herbicide imazapyr and phenol over mesoporous bicrystalline phases TiO2: A kinetic study. Catalysts 2019, 9, 640. [Google Scholar] [CrossRef] [Green Version]
- Yasin, A.; Guo, F.Q.; Demopoulos, G.P. Continuous-reactor, pH-controlled synthesis of multifunctional mesoporous nanocrystalline anatase aggregates. Chem. Eng. J. 2016, 287, 398–409. [Google Scholar] [CrossRef]
- Yasin, A.; Guo, F.; Sussman, M.J.; Gauvin, R.; Demopoulos, G.P. Steady-State, Scalable Production of Mesoporous Rutile and Brookite Particles and Their Use in Energy Conversion and Storage Cells. ChemNanoMat 2016, 2, 980–988. [Google Scholar] [CrossRef]
- Sussman, M.J.; Celikin, M.; Yasin, A.; Demopoulos, G.P. Mesoporous brookite nanoplatelets with superior lithium-ion intercalation stability. Electrochim. Acta 2014, 138, 215–223. [Google Scholar] [CrossRef]
- Lee, K.E.; Gomez, M.A.; Charbonneau, C.; Demopoulos, G.P. Enhanced surface hydroxylation of nanocrystalline anatase films improves photocurrent output and electron lifetime in dye sensitized solar cell photoanodes. Electrochim. Acta 2012, 67, 208–215. [Google Scholar] [CrossRef]
- Shirolkar, M.M.; Phase, D.; Sathe, V.; Rodriguez-Carvajal, J.; Choudhary, R.J.; Kulkarni, S.K. Relation between crystallinity and chemical nature of surface on wettability: A study on pulsed laser deposited TiO2 thin films. J. Appl. Phys. 2011, 109, 123512. [Google Scholar] [CrossRef]
- Iliev, M.N.; Hadjiev, V.G.; Litvinchuk, A.P. Raman and infrared spectra of brookite (TiO2): Experiment and theory. Vib. Spectrosc. 2013, 64, 148–152. [Google Scholar] [CrossRef]
- Hussain, M.; Ceccarelli, R.; Marchisio, D.L.; Fino, D.; Russo, N.; Geobaldo, F. Synthesis, characterization, and photocatalytic application of novel TiO2 nanoparticles. Chem. Eng. J. 2010, 157, 45–51. [Google Scholar] [CrossRef]
- Zhang, X.R.; Lin, Y.H.; He, D.Q.; Zhang, J.F.; Fan, Z.Y.; Xie, T.F. Interface junction at anatase/rutile in mixed-phase TiO2: Formation and photo-generated charge carriers properties. Chem. Phys. Lett. 2011, 504, 71–75. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.; Cab, C.; de Coss, R.D.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef]
- Charbonneau, C.; Gauvin, R.; Demopoulos, G. Aqueous solution synthesis of crystalline anatase nanocolloids for the fabrication of DSC photoanodes. J. Electrochem. Soc. 2011, 158, H224–H231. [Google Scholar] [CrossRef]
- López-Muñoz, M.J.; Revilla, A.; Alcalde, G. Brookite TiO2-based materials: Synthesis and photocatalytic performance in oxidation of methyl orange and As(III) in aqueous suspensions. Catal. Today 2015, 240, 138–145. [Google Scholar] [CrossRef]
- Sheng, H.; Ji, H.; Ma, W.; Chen, C.; Zhao, J. Direct four-electron reduction of O2 to H2O on TiO2 surfaces by pendant proton relay. Angew. Chem. Int. Ed. 2013, 52, 9686–9690. [Google Scholar] [CrossRef] [PubMed]
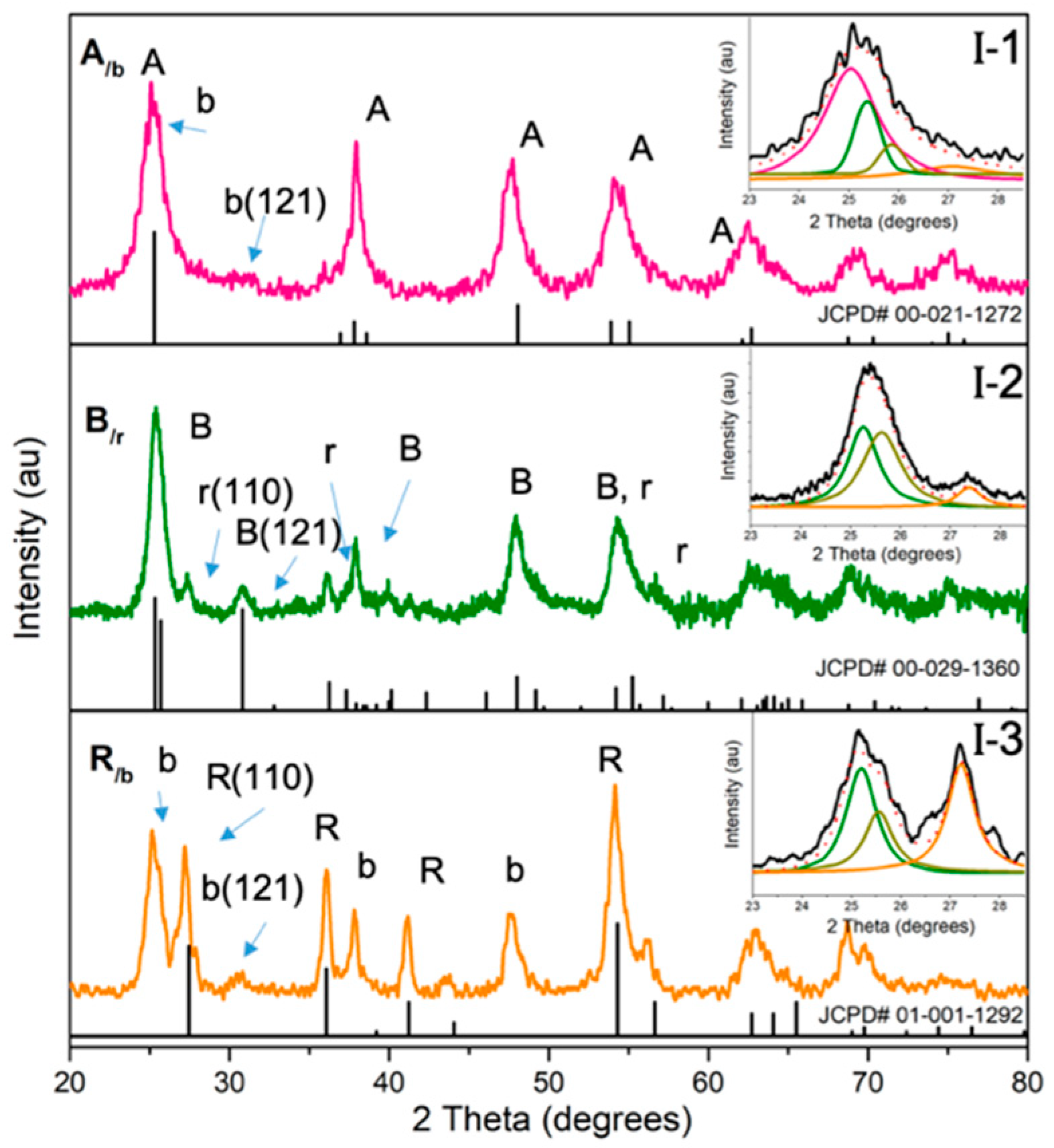
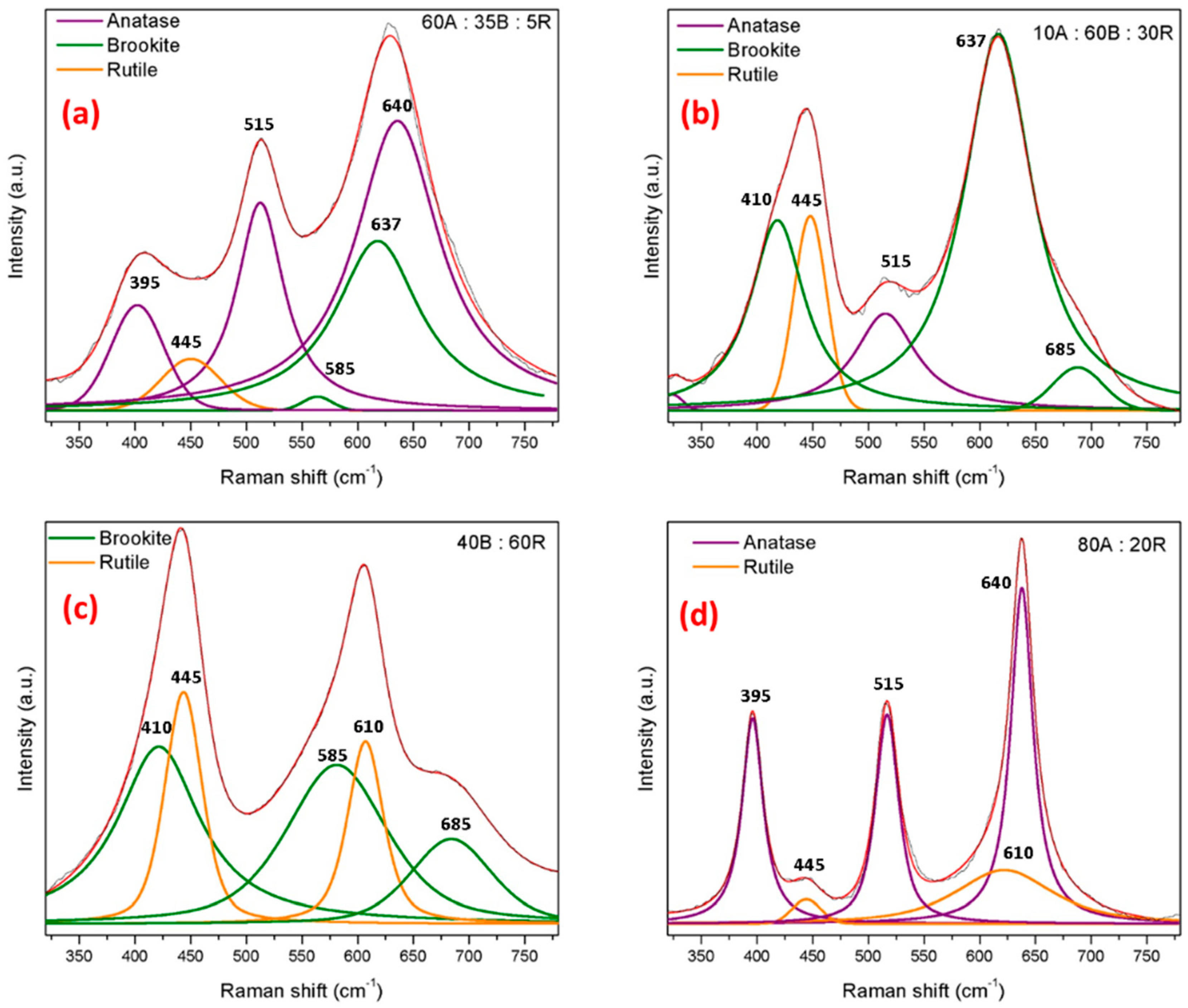
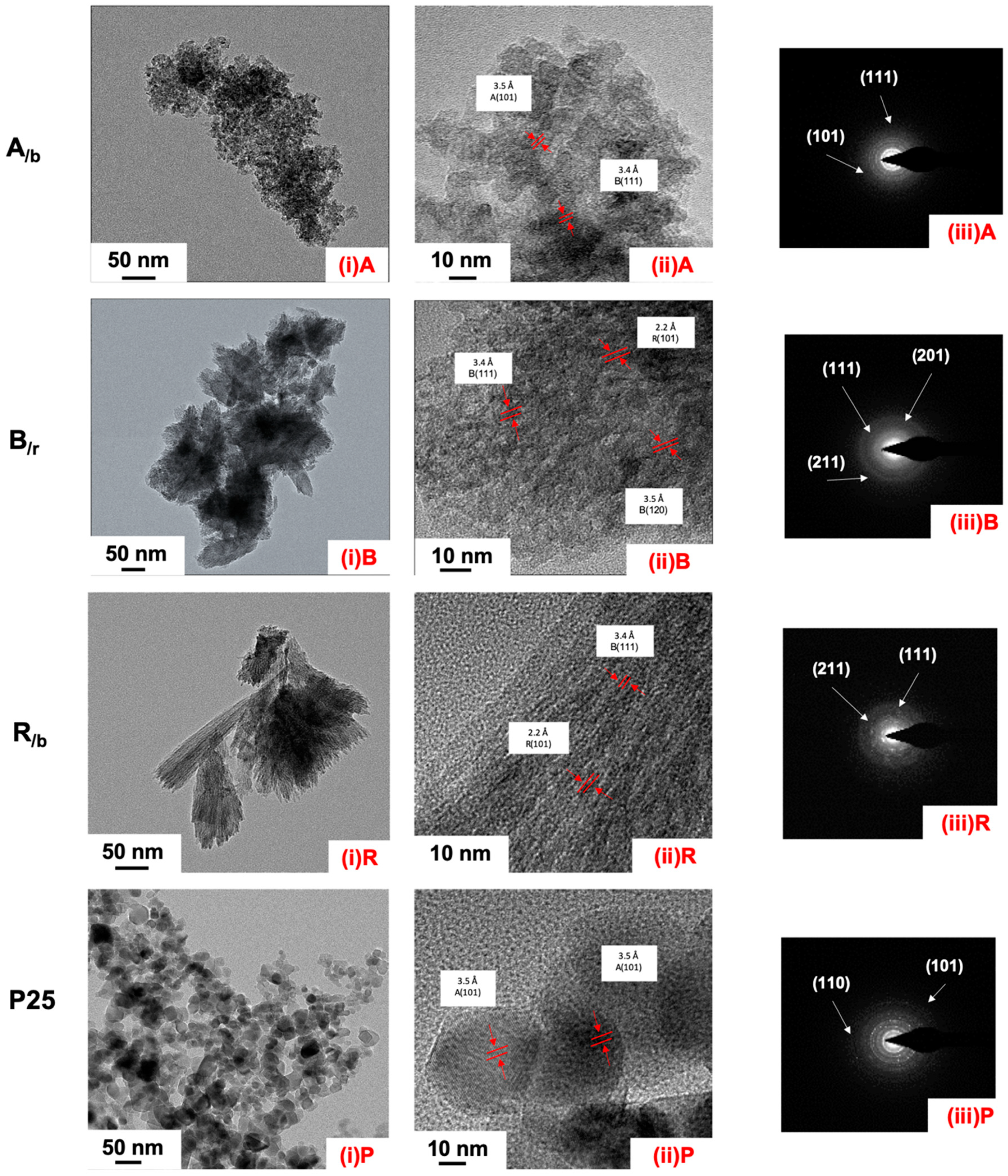
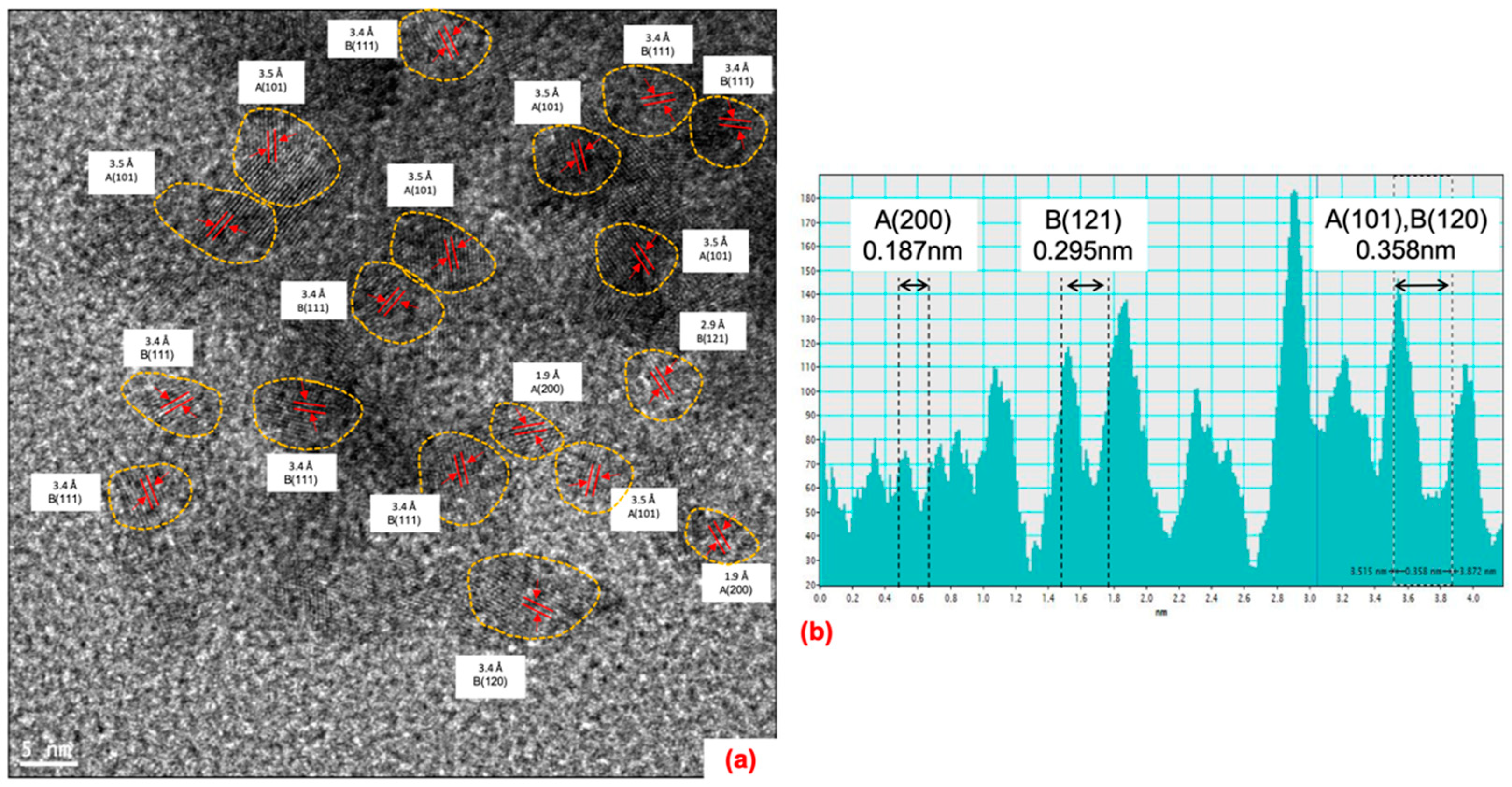


| Parameter | Single-Phase Anatase (HM-A) | A/b | Single-Phase Brookite (HM-B) | B/r | Single-Phase Rutile (HM-R) | R/b | ||||
| Product | ||||||||||
| Feed solution [TiCl4] | 0.1 M | 0.1 M | 0.1 M | 0.1 M | 0.5 M | 0.5 M | ||||
| Temperature | 80 °C | 80 °C | 80 °C | 80 °C | 80 °C | 80 °C | ||||
| Residence time | 30 min | 30 min | 60 min | 60 min | 60 min | 30 min | ||||
| Agitation | 800 rpm | 800 rpm | 800 rpm | 400 rpm | 800 rpm | 400 rpm | ||||
| pHsteady-state Base | pH 3 NH4OH | pH 2 NH4OH | 0.7–0.8 No | 0.7–0.8 No | <0.5 No | <0.5 No | ||||
| Phase (%) | XRD | XRD | Raman | XRD | XRD | Raman | XRD | XRD | Raman | |
| Anatase | 100 | 69 ± 1 | 60 | - | 12 ± 2 | 10 | - | - | - | |
| Brookite | - | 31 ± 1 | 35 | 94 | 63 ± 1 | 60 | - | 37 ± 2 | 40 | |
| Rutile | - | - | 5 | 6 | 25 ± 1 | 30 | 100 | 63 ± 2 | 60 | |
| TiO2 Products | BET Surface Area (m2 g−1) | BJH Pore Size (nm) | Size * (nm) | Eg (eV) | Adsorption Edge Wavelength, λ (nm) |
|---|---|---|---|---|---|
| A/b | 122 ± 12 | 45 | 5–10 | 3.15 | 422 |
| B/r | 110 ± 7 | 26 | 50–150 | 3.01 | 415 |
| R/b | 92 ± 5 | 4.3 | 200–500 | 2.9 | 455 |
| Commercial P25 | 49 ± 2 | 40 | 20–30 | 3.2 | 425 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalastara, K.; Guo, F.; Elouatik, S.; Demopoulos, G.P. Tunable Composition Aqueous-Synthesized Mixed-Phase TiO2 Nanocrystals for Photo-Assisted Water Decontamination: Comparison of Anatase, Brookite and Rutile Photocatalysts. Catalysts 2020, 10, 407. https://doi.org/10.3390/catal10040407
Chalastara K, Guo F, Elouatik S, Demopoulos GP. Tunable Composition Aqueous-Synthesized Mixed-Phase TiO2 Nanocrystals for Photo-Assisted Water Decontamination: Comparison of Anatase, Brookite and Rutile Photocatalysts. Catalysts. 2020; 10(4):407. https://doi.org/10.3390/catal10040407
Chicago/Turabian StyleChalastara, Konstantina, Fuqiang Guo, Samir Elouatik, and George P. Demopoulos. 2020. "Tunable Composition Aqueous-Synthesized Mixed-Phase TiO2 Nanocrystals for Photo-Assisted Water Decontamination: Comparison of Anatase, Brookite and Rutile Photocatalysts" Catalysts 10, no. 4: 407. https://doi.org/10.3390/catal10040407





