Abstract
The development of efficient and non-toxic photocatalysts with a full spectrum response is a primary strategy in the area of photocatalytically mediated pollutant elimination. Herein, we report the preparation of novel nitrogen and iron co-doped carbon dots/gear-shaped WO3 (N,Fe-CDs/G-WO3) with significantly improved broad-spectrum utilization. Characterization results demonstrated that the gear-shaped G-WO3, decorated by N,Fe-CDs with excellent electron transfer/reservoir properties, possessed abundant oxygen vacancies, had large specific surface areas, had multiple light-reflections and had a narrow band gap. As a result, the N,Fe-CDs/G-WO3 composite exhibited excellent photocatalytic activity towards the degradation of water contaminants under full spectrum irradiation. For example, the photodegradative efficiencies of rhodamine B (RhB) reached 81.4%, 97.1%, and 75% in 2 h, under ultraviolet, visible, and near-infrared (UV, vis, and NIR) light irradiation, respectively. Moreover, the N,Fe-CDs/G-WO3 composite also exhibited an outstanding photocatalytic degradation efficiency for other dyes, pharmaceuticals, and personal care products (PPCPs) like methylene blue (MB), ciprofloxacin (CIP), tetracycline hydrochloride (TCH), and oxytetracycline (OTC) (91.1%, 70.5%, 54.5%, and 47.8% in 3 h, respectively). The radical trapping experiments indicated that h+ and ·OH were the main reactive oxidative species (ROS), and the conversion between Fe (III) and Fe (II) played a key role in the photocatalytic reactions. Such a N,Fe-CD decorated material with brilliant photocatalytic activity has tremendous potential for application in environmental remediation.
1. Introduction
With the continuous process of industrialization and modernization in recent years, more and more organic contaminants, such as antibiotics, have been discharged, which causes increasingly serious environmental problems. Hence, diverse sewage treatment technologies, including various physical, biological, and oxidative methods, have been applied to the removal of such persistent organic pollutants in the water phase [1,2]. Among them, the photocatalytic degradation of organic contaminants has been considered as a promising technique due to its cleanliness, high efficiency, recyclability, and environmental benignity [3,4]. However, the low visible light and infrared ray utilization of conventional photocatalysts has hindered its irradiation utilization efficiency. For instance, most semiconductor materials can only use ultraviolet rays, which account for only 4% of the total sunlight energy [5,6]. Consequently, great efforts have been dedicated to develop high-efficiency and visible light active photocatalysts in the past years. To date, a wide variety of semiconductor materials, including WO3, ZnO, TiO2, g-C3N4, SnO2, and CdS, have been applicated as photocatalysts. Among them, WO3 has been widely used in catalysts, photocatalysts, photoelectrodes, battery electrodes, photochromic devices, and gas sensors, owing to its excellent properties such as non-toxicity, stability, low cost, and absorption in the visible light region [7,8,9,10]. However, pure WO3 shows a relatively narrow band gap (2.2–3.0 eV), and only a small portion of visible light can be used; in addition, the rapid photoelectron-hole recombination, the low electron migration capability, and the interfacial redox reactions rate have also limited the photocatalytic application of WO3 [11,12,13,14,15]. Two main approaches have been manifested to address these issues: (1) controlling the synthesis of novel and complex unique nanostructures and (2) decorating with metal or nonmetal composites. In terms of structural control, the novel nanostructure with a large surface area can provide a special channel for electron transfer, thus improving the activity of the photocatalyst. Meanwhile, for some photocatalysts, the structure does not play a primary role in improving the light absorption capacity. Therefore, further modification of WO3 is necessary.
Recently, carbon dots (CDs), as a new type of carbon nanomaterial with sizes under 10 nm, have attracted increasing interest due to their unique properties including water solubility, up-conversion ability, low toxicity, excellent physicochemical stability, and light absorption properties [2,16,17]. Owing to their unique properties, CDs have recently been applied to enhance the photocatalytic activities of WO3 [18,19,20]. However, the deficiencies of low light utilization and poor electron transfer ability have also limited the application of CDs/WO3. To overcome these issues, our previous work used N-doped CDs to improve the light-harvesting and electron transfer/reservoir capacity of CDs [21,22]. Meanwhile, previous work has demonstrated that metallic elements, especially transition metals (Fe) with more empty orbits, easily lose electrons and become conducive to electron migration, and the doping of transition metals might confer new physicochemical properties to CDs, such as catalytic properties [23,24]. On the other hand, the fluorescence of CDs can be quenched due to chelation with metal ions like Fe, which greatly enhances the charge transfer ability [25,26,27,28]. Therefore, we surmised that the co-doping of N and Fe might maximally enhance the light absorption and electron transfer/reservoir properties of CDs. To date, there have been few references about the synthesis of N,Fe co-doped CDs and their applications, particularly as biosensors and chemical catalysts [24,29]. There are no reports of N,Fe co-doped CD decorated WO3 being used as a photocatalyst in photocatalytic degradation.
Herein, we firstly report a facile one-step hydrothermal method for the synthesis of gear-shaped WO3 (G-WO3) with a higher surface area and narrower band gap. Taking folic acid and ferric trichloride hexahydrate as C and Fe sources, N,Fe-CDs were successfully synthesized by a simple hydrothermal process. Subsequently, N,Fe-CDs/G-WO3 composites were prepared by the hydrothermal method. Through characterization and comparison, the physico-chemical properties of composite materials were systematically analyzed. Their photocatalytic activities were investigated through the degradation of several target pollutants (Table S1), including rhodamine B (RhB), methylene blue (MB), tetracycline hydrochloride (TCH), ciprofloxacin (CIP), and oxytetracycline (OTC), under different types of light irradiation such as with ultraviolet, visible, and near-infrared (UV, vis, and NIR), and the results indicated that the composite has excellent photocatalytic activity, stability, and reusability. To the best of our knowledge, we are the first to apply metal-non-metal co-doped CDs combined with WO3 for photocatalysis. This work provides a new insight for the efficient utilization and conversion of solar energy in future research.
2. Results and Discussion
2.1. Formation of Gear-Shaped WO3 (G-WO3) and N,Fe-CDs/G-WO3
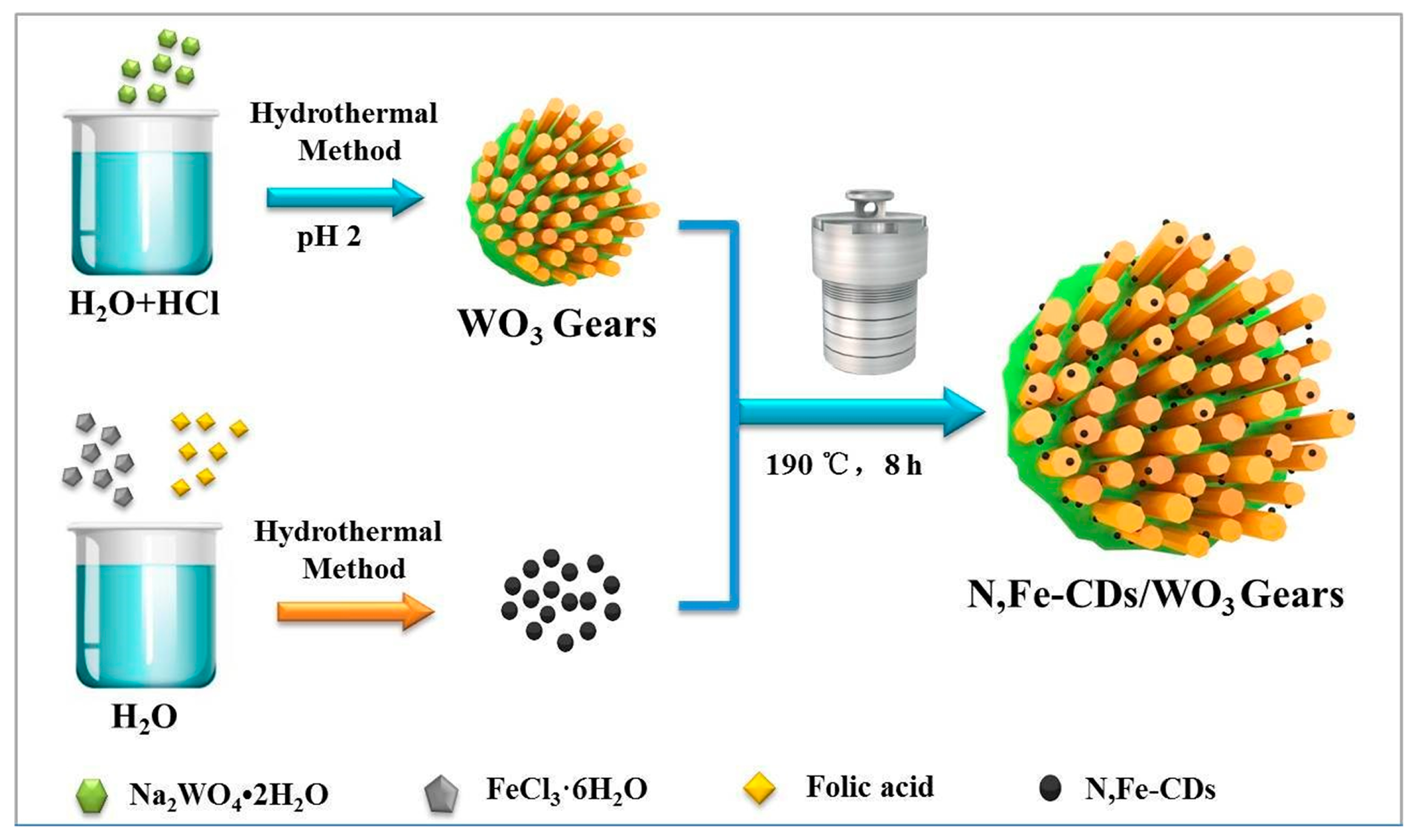
Scheme 1 illustrates the process of synthesizing gear-shaped WO3 decorated with N,Fe-CDs. The gear-shaped WO3 was prepared in HCl solution, taking sodium tungstate dihydrate as the precursor. The N,Fe-CDs were successfully synthesized via a simple hydrothermal method using folic acid and ferric trichloride hexahydrate as C and Fe sources. Finally, N,Fe-CDs/G-WO3 composites with good monodispersity, stable crystals, and large Brunner-Emmet-Teller (BET) surface areas were obtained by a simple hydrothermal process.
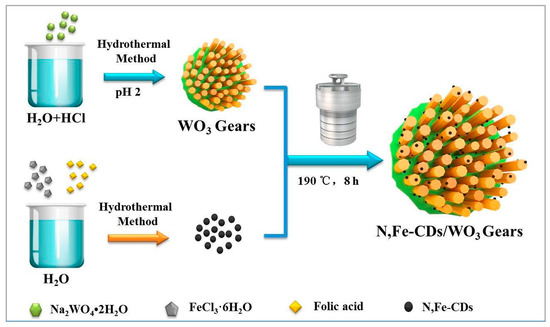
Scheme 1.
A schematic illustration of the fabrication of the gear-shaped WO3 (G-WO3) and N,Fe-CDs/G-WO3.
2.2. Characterization of Synthesized Samples
2.2.1. Morphological Analysis
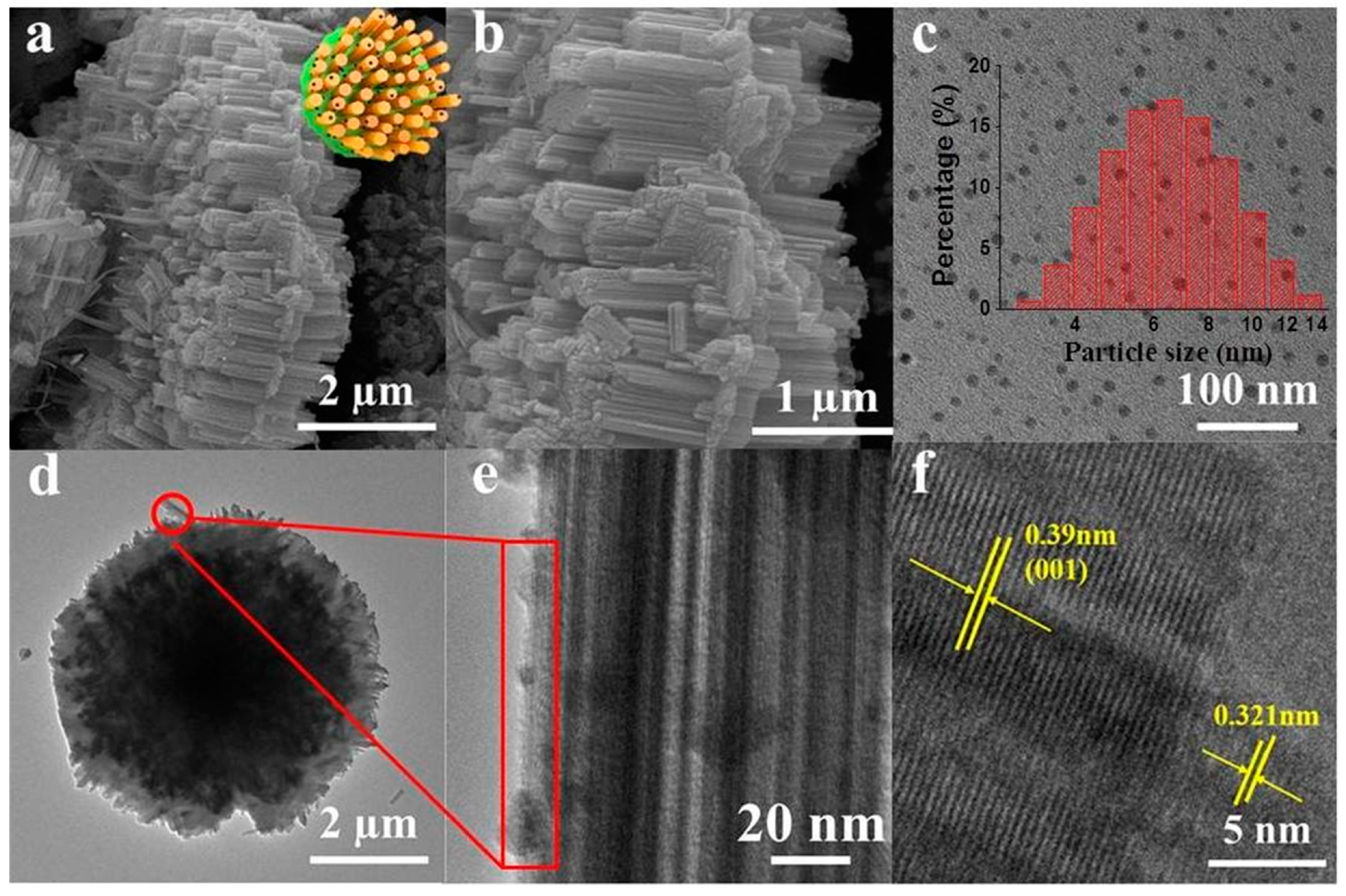
As shown in Figure 1a,b,d, the SEM and TEM images of G-WO3 showed a 3-D gear-like structure consisting of a plate with many protruding nanorods on its surface, making it possible to possess high surface areas and reflect the incident light during irradiation. The particle diameters of the N,Fe-CDs ranged from 3.5 to 13.5 nm, with an average diameter of 6.5 nm (Figure 1c). N,Fe-CDs could be clearly observed on the surface of G-WO3, as shown in Figure 1e. As illustrated in Figure 1f, the high resolution transmission electron microscopy images (HRTEM) of G-WO3 displayed an obvious lattice fringe with a lattice distance of 0.39 nm corresponding to the (001) lattice plane of WO3 [30], and the N,Fe-CDs displayed an obvious lattice fringe with a lattice spacing of 0.32 nm.
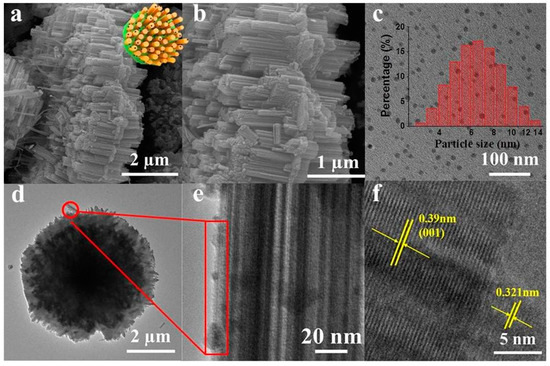
Figure 1.
SEM and TEM of N,Fe-CDs; N,Fe-CDs/G-WO3-0.6 composite. (a,b) SEM of N,Fe-CDs/G-WO3-0.6; (c) TEM of N,Fe-CDs with the inset for the size distribution; (d) TEM of N,Fe-CDs/G-WO3-0.6; (e,f) HRTEM of N,Fe-CDs/G-WO3-0.6.
2.2.2. FT-IR Analysis
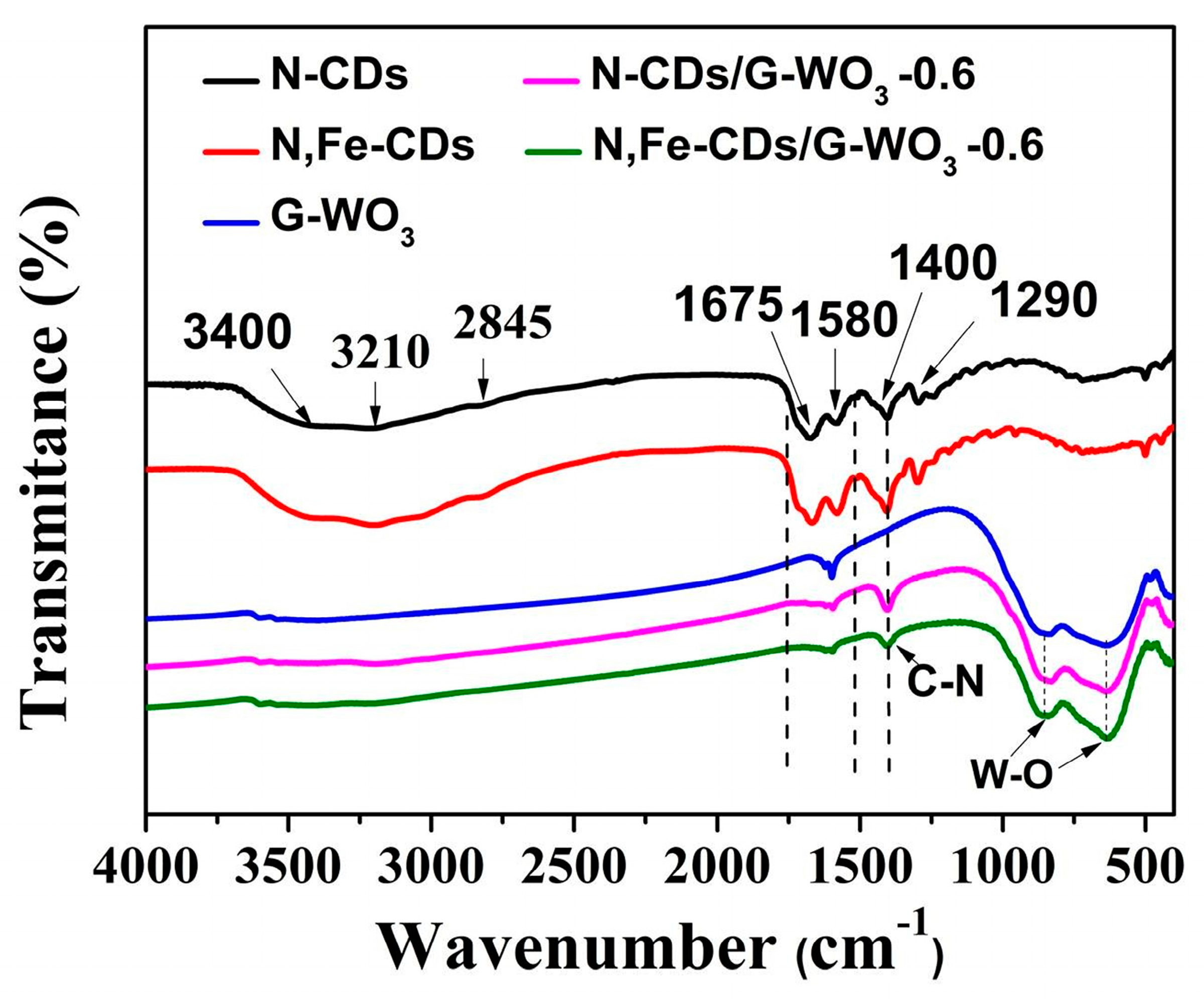
As shown in the FT-IR spectrum of the N-CDs and N,Fe-CDs (Figure 2), the absorption bands at around 3400 cm−1 were ascribed to the O-H stretching vibration, the bands at 3210 cm−1 were due to N-H stretching mode, and the bands located at 2840 and 1290 cm−1 were assigned to the C-H vibration. In addition, the bands found at 1675, 1580, and 1400 cm−1 corresponded to v (C=O), δ (N-H), and v (C-N), respectively [31,32,33]. No obvious characteristic bands of Fe in N,Fe-CDs could be observed because of the low content. In the FT-IR spectrum of the pure WO3, the bands appearing at 3400 and 1650 cm−1 were assigned to the O-H stretching vibration of surface-adsorbed water. The set of bands between 600 and 1000 cm−1 can be ascribed to the W-O bending vibration of WO3 [34]. The FT-IR spectra of N-CDs/G-WO3-0.6 and N,Fe-CDs/G-WO3-0.6 showed the same bands. It is worth noting that bands located at 1400 cm−1 can be observed, which could be associated with the stretching vibrations of C-N. In addition, the bands broadened in the region between 1500 and 1650 cm−1, a consequence of the CDs’ structures [11,31]. The above results indicate that CDs were successfully decorated onto the surfaces of WO3.
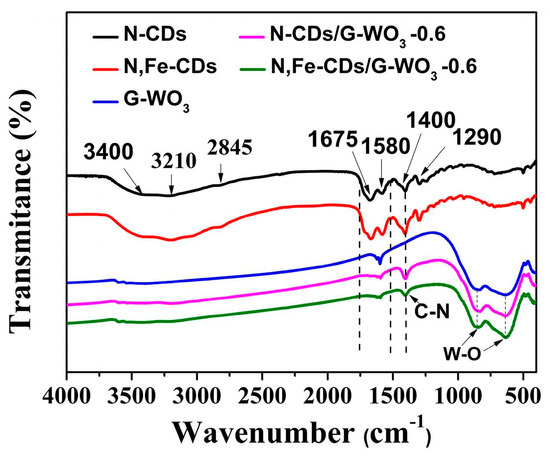
Figure 2.
The FT-IR spectra of N-CDs, N,Fe-CDs, pure G-WO3, N-CDs/G-WO3-0.6, and N,Fe-CDs/G-WO3-0.6.
2.2.3. BET Analysis
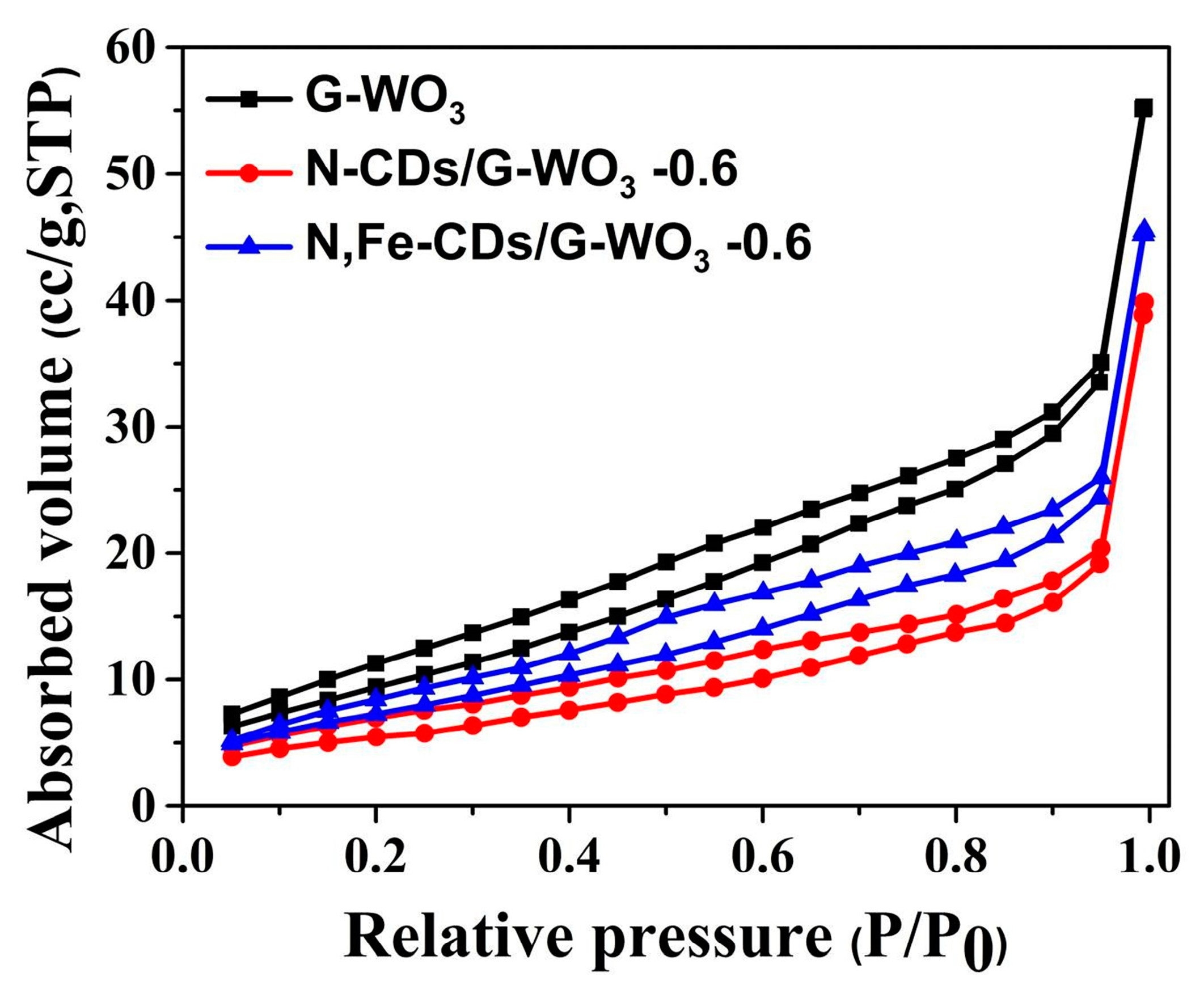
To clearly investigate the surface adsorption properties of the as-synthesized samples, the BET test and Barrett-Joyner-Halenda (BJH) analysis were carried out (Figure 3). The BET surface area of pure G-WO3 (36.039 m2/g STP) is nearly six-fold larger than that of commercial WO3 (6.135 m2/g STP), revealing that G-WO3 possesses more interfacial active sites. After doping with N-CDs or N,Fe-CDs, the surface area of G-WO3 decreased, which was attributable to the internal combination. The space between the interlayers of G-WO3 could accommodate CDs, and the vacant spaces between these protruding nanorods on G-WO3 made it more mesoporous, allowing it to better couple with CDs.
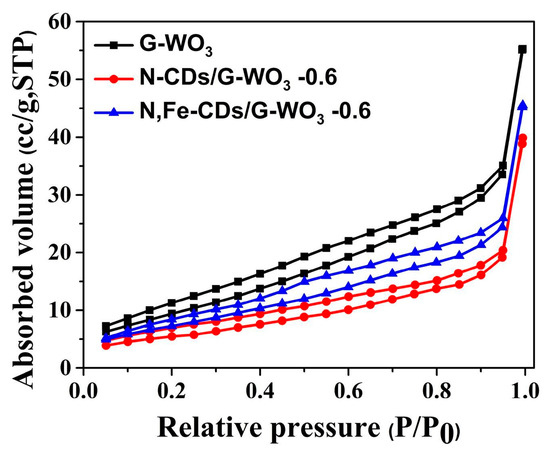
Figure 3.
The N2 absorption-desorption isotherms of pure G-WO3 and their N-CD or N,Fe-CD composites.
2.2.4. Phase Structures and Elemental State Analysis
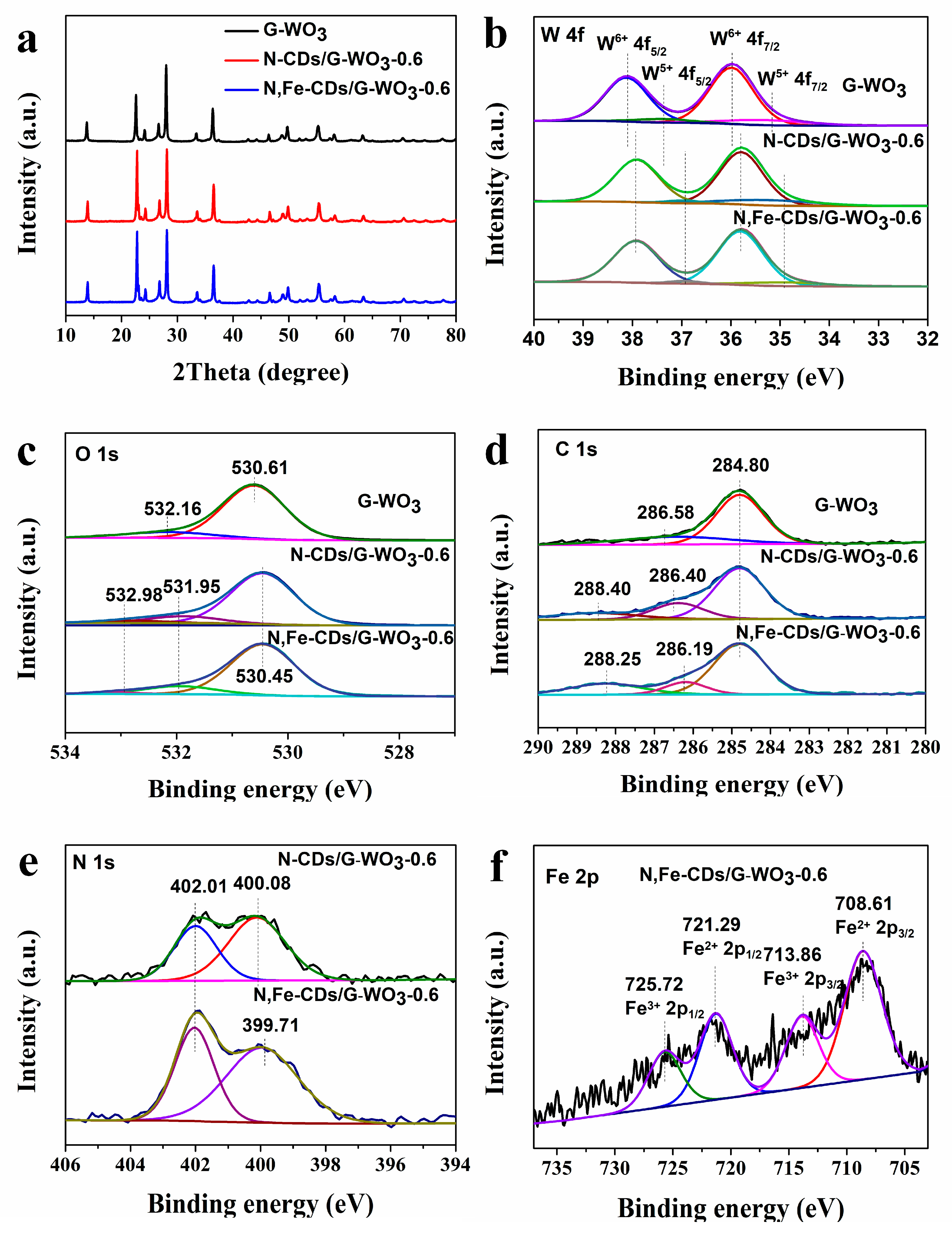
The XRD patterns of the as-synthesized samples are presented in Figure 4a. The main diffraction peaks of G-WO3 appeared at 13.8°, 22.6°, 28.0°, 36.3°, and 49.7°, demonstrating that the G-WO3 was in the hexagonal crystalline phase (JCPDS No. 33-1387) [30]. No diffraction peaks for the CDs could be detected in the composites because of their low content. In addition, the XRD patterns of G-WO3 and its composites showed no significant differences, indicating that the addition of CDs has almost no influence on the growth of G-WO3.
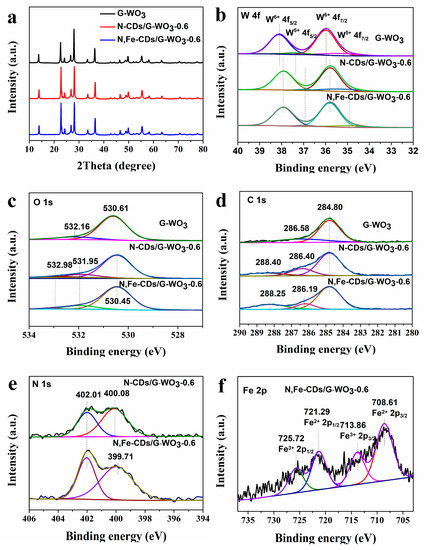
Figure 4.
XRD patterns (a) and high-resolution XPS spectra of G-WO3 and its composites W 4f (b), O 1s (c), C 1s (d), N 1s (e), and Fe 2p (f).
The surface chemical compositions of as-synthesized samples were further analyzed and confirmed using X-ray photoelectron spectroscopy (XPS). Compared with pure G-WO3, the co-existence of C, N, O, W, and Fe could be demonstrated in the full XPS spectrum of N,Fe-CDs/G-WO3-0.6 (Figure S1). Two valence states of tungsten (W) in all three samples could be confirmed by the W 4f spectrum (Figure 4b). The strong peaks at 38.12 eV for 4f5/2 and 35.99 eV for 4f7/2 were attributed to W6+, while the shoulder peaks at 37.37 eV for 4f5/2 and 35.28 eV for 4f7/2 were considered as W5+, demonstrating that G-WO3 has abundant oxygen vacancies [35]. Compared with the pure G-WO3, the W6+ and W5+ peaks of the N-CDs/G-WO3-0.6 and N,Fe-CDs/G-WO3-0.6 composites all showed an obvious shift to lower binding energies. This suggested that the surface chemical environment of W in the WO3/CDs changed, which could be caused by the different chemical interactions between carbon dots and G-WO3 [36]. For the O 1s spectra in Figure 4c, the strong peak of pure G-WO3 at 530.61 eV was related to the binding energy of W-O and WO=, and G-WO3 decorated with carbon dots exhibited shifts from 530.61 eV to 530.45 eV [37]. The peaks appearing at 532.16 eV and 532.98 eV corresponded to the oxygens of the hydroxyl groups or water bonded on the material’s surface, while the new peak for N-CDs or N,Fe-CDs/G-WO3 composites located at 531.95 eV was assigned to the C–O bond from CDs [21,38]. The C 1s spectra of G-WO3 (Figure 4d) was resolved into two peaks at 284.80 eV and 286.56 eV, which could be attributed to the surface adsorbed carbon and the C–O bond, respectively [37]. Compared with the pure WO3 (G), the C 1s peaks of N-CDs/G-WO3-0.6 or N,Fe-CDs/G-WO3-0.6 at about 286 eV and 288 eV were assigned to the binding energies of C-N and C=O from the CDs [29,39]. Besides, as shown in Figure 4e, compared to the pure G-WO3, the N 1s peaks at around 402 eV and 400 eV were obvious in the N-CDs/G-WO3-0.6 and N,Fe-CDs/G-WO3-0.6 composites, corresponding to the nitrogen atoms of N-H and C-N bonds, respectively [40]. The differences of C 1s and N 1s between N-CDs/G-WO3-0.6 and N,Fe-CDs/G-WO3-0.6 were attributed to the coordination of iron ions with the surface groups of N-CDs [26,27]. In the high-resolution Fe 2p spectrum (Figure 4f), there were two valence states of Fe, corresponding to Fe2+ 2p1/2 (721.29 eV), Fe3+ 2p1/2 (725.72 eV), Fe2+ 2p3/2 (708.61 eV), and Fe3+ 2p3/2 (713.86 eV), respectively. This indicated that iron in the CDs might act as a multi-electron transfer center [12,29].
2.2.5. The Optical Absorption and Photoluminescence Properties
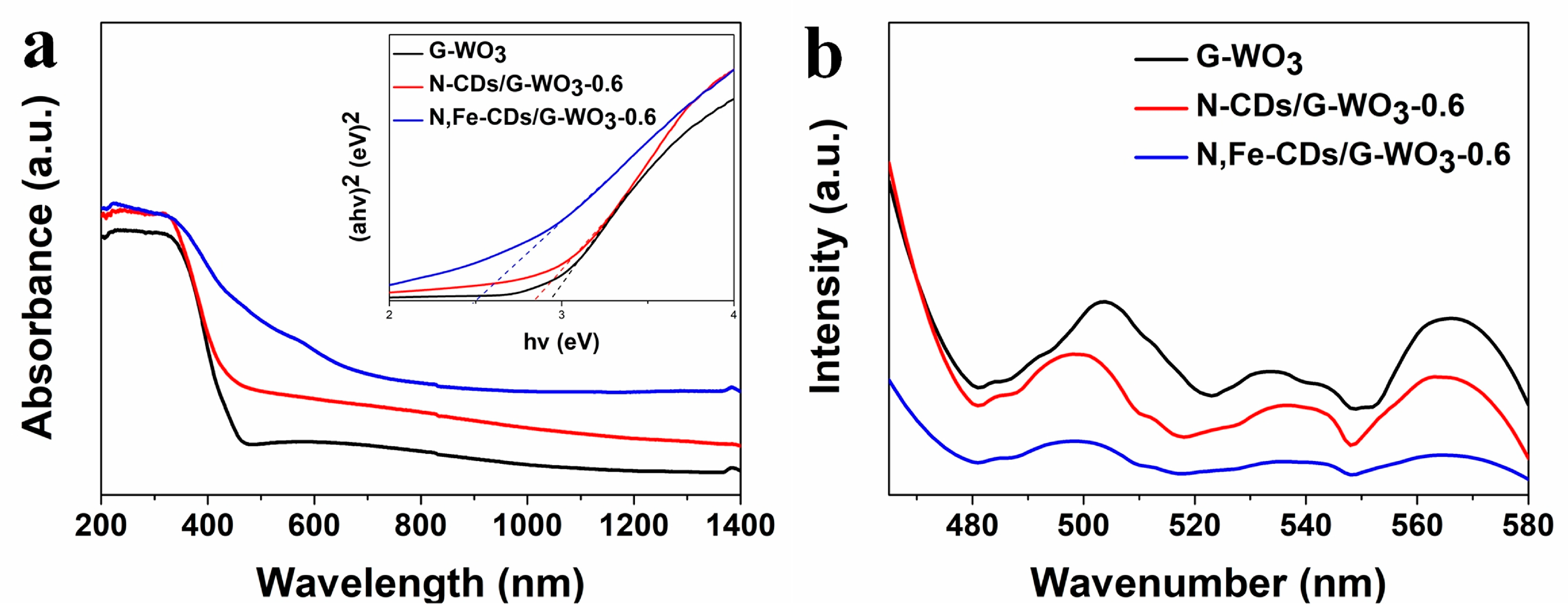
The optical band gaps (Eg) of the pure WO3 and CDs/WO3 composites were calculated according to this equation: αhν = A(hν-Eg)n/2, where α, h, and ν are the absorption coefficient, Planck constant, and light frequency, respectively. Here, n was equal to 1 for a direct-band-gap material [41]. The UV-vis-NIR diffuse reflectance spectra (DRS) are shown in Figure 5a. The G-WO3 exhibited a full spectrum absorption in the region from 200 to 1400 nm, which was primarily attributed to the oxygen vacancy [42]. Moreover, the special gear-shaped structure could enhance the UV-vis-NIR light harnessing ability and photocatalytic activity due to the multiple reflections of the incident light during irradiation [43,44]. N-CDs/G-WO3-0.6, with an energy gap of 2.83 eV, performed a broader absorption than pure G-WO3, indicating that the decoration of N-CDs could improve the optical absorption capacity of WO3, which is in agreement with other reports [31,45]. Compared with N-CD decoration, N,Fe-CDs/G-WO3-0.6, with an energy gap of 2.50 eV, exhibited a narrower band gap and broader-solar-spectrum absorption in the UV-vis-NIR regions, which demonstrated that N,Fe-CD decorated G-WO3 could use more solar energy and generate more e−/h+ pairs under UV-vis-NIR light irradiation.
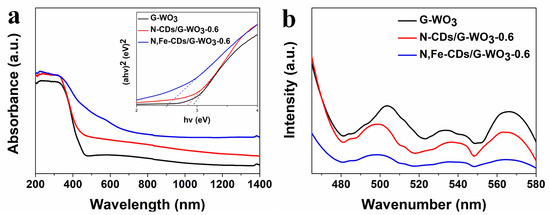
Figure 5.
The ultraviolet (UV)-visible (vis)-near-infrared (NIR) diffuse reflectance spectra (DRS) (a) with the inset for band gaps, and the PL spectra (b) of pure G-WO3, N-CDs/G-WO3-0.6, and N,Fe-CDs/G-WO3-0.6, respectively.
As shown in Figure 5b, the PL intensity of G-WO3 decreased significantly after decorating with CDs, especially with N,Fe-CDs, which indicated that the N,Fe-CDs on the G-WO3 surface acted as an electron acceptor and effectively inhibited the recombination of the photo-generated e-/h+ pairs [31].
2.3. Photocatalytic Performance
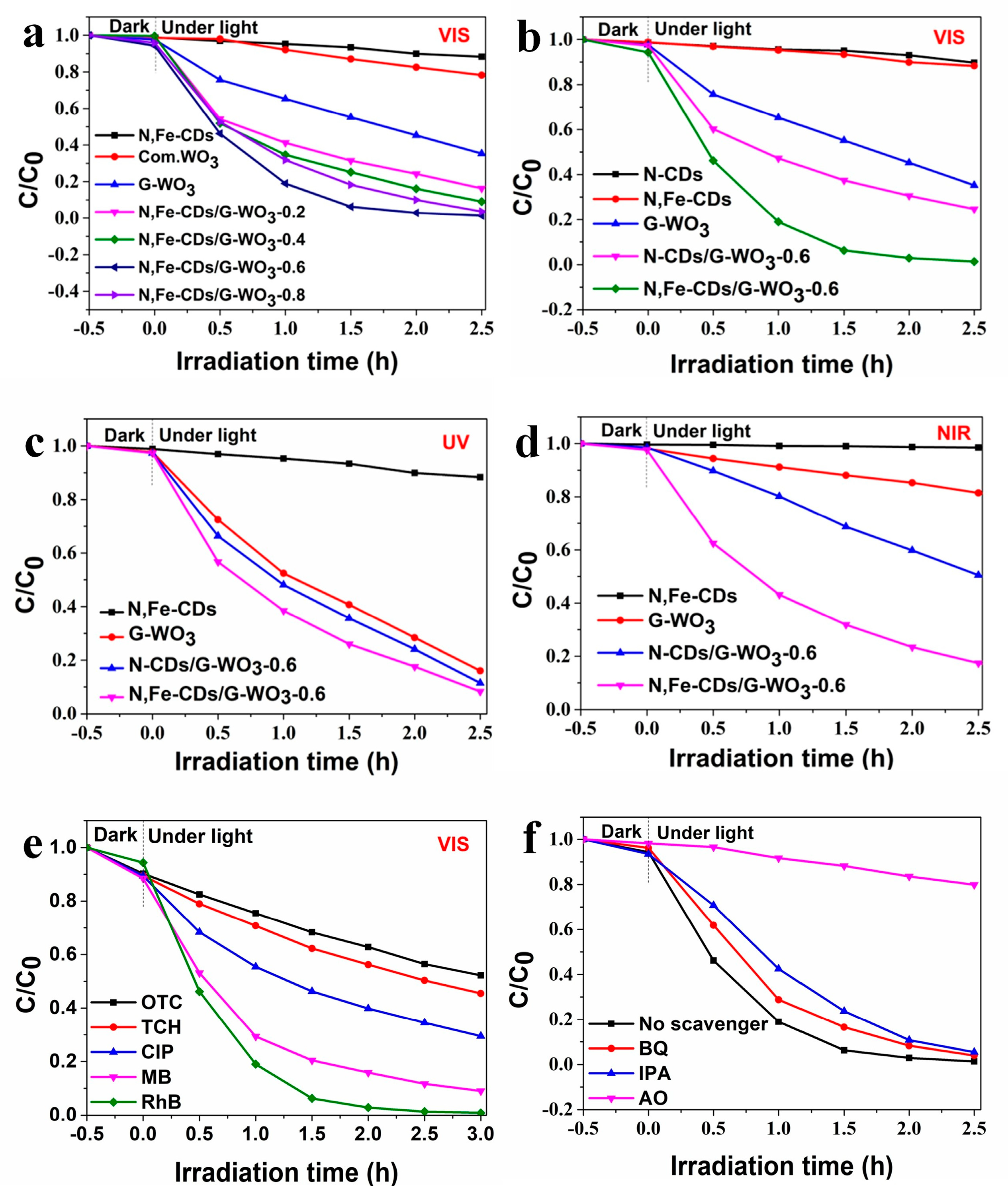
The photocatalytic performance of the WO3/CDs composites were evaluated via the photocatalytic degradation of RhB under UV-vis-NIR light irradiation. As shown in Figure 6a, under visible light irradiation, the G-WO3 with different amounts of N,Fe-CDs exhibited improved photocatalytic activity, and the RhB degradation rate initially increased and then decreased as the N,Fe-CDs amount was increased. The overloaded N,Fe-CDs increased the surface thickness, which hindered the transfer of photoinduced electrons, and also prevented the exposure of the active sites. The N,Fe-CDs/G-WO3-0.6 composite presented the maximum degradation efficiency of 97.1% under visible light irradiation for 2 h. In addition, the effects of different factors like catalyst dosage, RhB concentration, pH values, and ions on the photocatalytic degradation of RhB were also investigated. A catalyst dosage of 50 mg, RhB concentration of 10 mg/L, and pH of 3 were adopted as the optimal reaction conditions (Figure S2a–f). As exhibited in Figure 6b, compared to that of pure G-WO3 and N-CDs/G-WO3-0.6, the photocatalytic activity of Fe-CDs/G-WO3-0.6 was significantly enhanced.
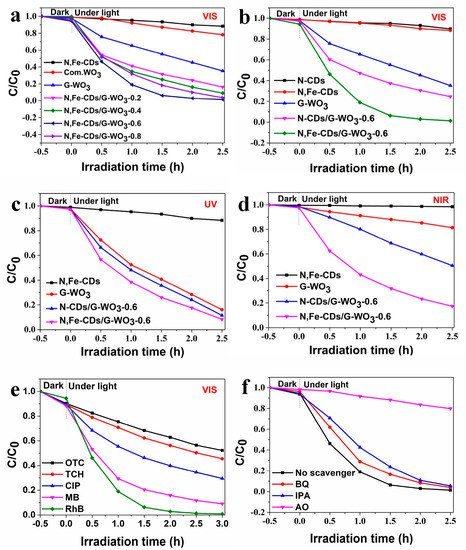
Figure 6.
(a) The degradation efficiencies for rhodamine B (RhB) of N,Fe-CDs/G-WO3 composites under visible light; (b) The degradation efficiencies for RhB of N-CDs/G-WO3-0.6 and N,Fe-CDs/G-WO3-0.6 composites under visible light; (c,d) The degradation efficiencies for RhB in the presence of N,Fe-CDs/G-WO3-0.6 under UV and NIR light, respectively; (e) The degradation efficiencies for various pollutants of the N,Fe-CDs/G-WO3-0.6 composite; (f) The effects of different scavengers on the photocatalytic efficiency of N,Fe-CDs/WO3 (G)-0.6.
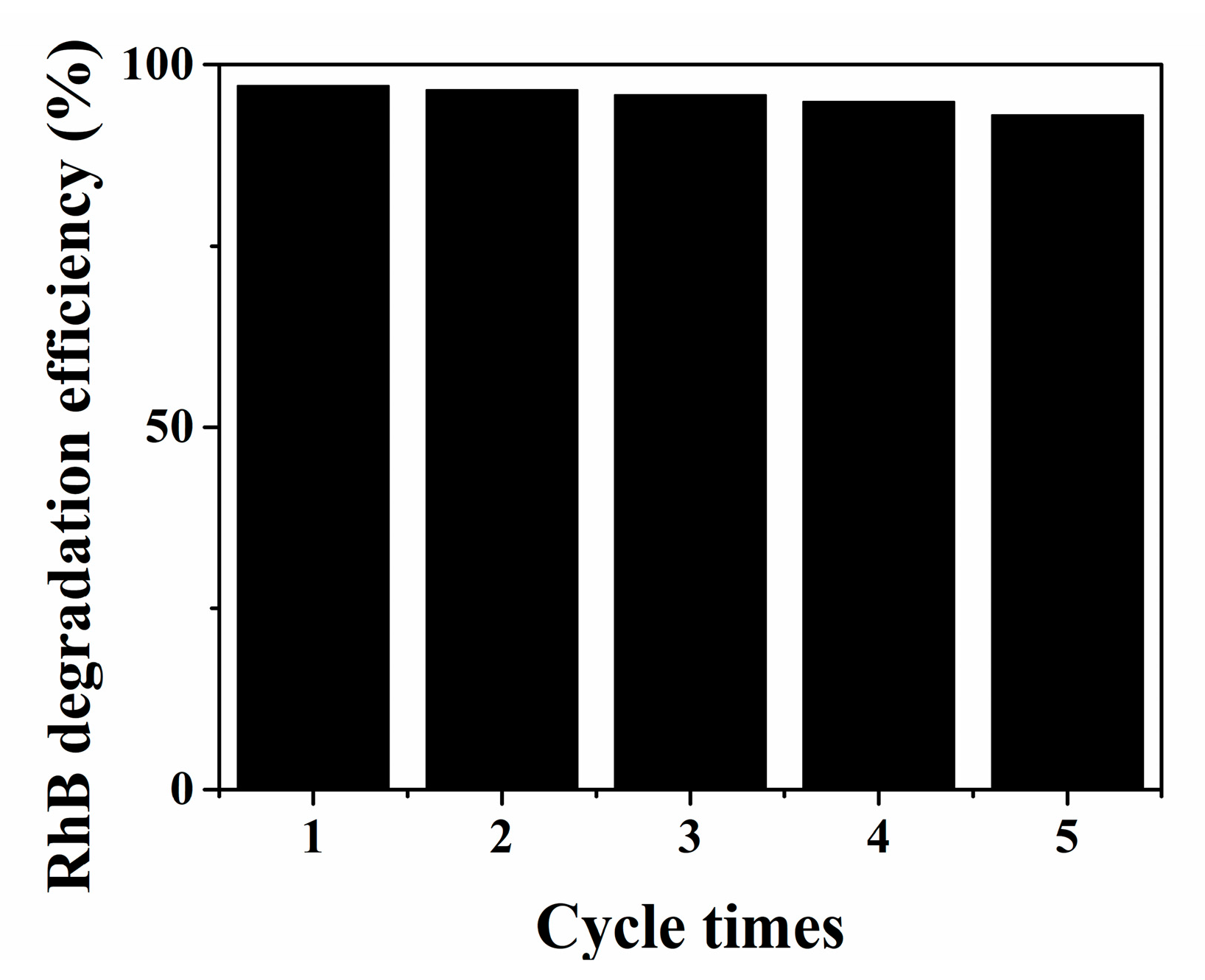
As illustrated in Figure S3, under visible light irradiation, the N,Fe-CDs/G-WO3-0.6 composite exhibited the best photocatalytic activity, and the RhB photodegradation kinetics can be well fitted by a pseudo-first-order kinetic model [46], where the RhB degradation rate constant over the N,Fe-CDs/G-WO3-0.6 was 1.72 h−1, which is about 20 fold higher than for the commercial WO3. The photodegradation efficiencies of RhB under UV and NIR light irradiation for 2 h were 81.4% and 75.0%, respectively (Figure 6c,d). A stability test of N,Fe-CDs/G-WO3-0.6 was conducted under visible light irradiation (shown in Figure 7). After recycling five times, the photocatalytic activities of the material remained almost unchanged and the photodegradation efficiencies only decreased from 97.1% to 91.6% under visible light irradiation in 2 h, suggesting that the as-prepared composite was stable and reusable.
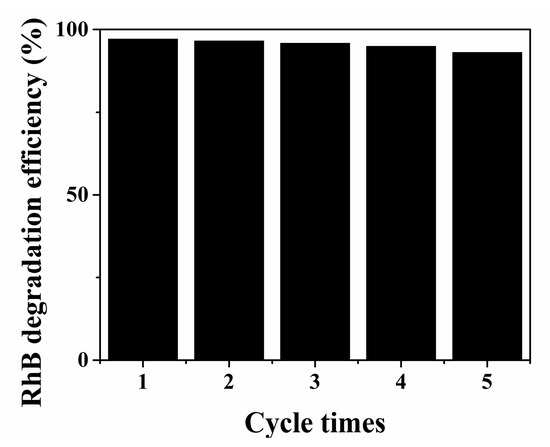
Figure 7.
The stability and reusability of N,Fe-CDs/G-WO3-0.6.
To investigate the universal application of N,Fe-CDs/G-WO3-0.6, four other organics, MB, CIP, TCH, and OTC, were selected as target contaminants. As shown in Figure 6e, the synthesized material exhibited good photodegradation efficiencies for MB, CIP, TCH, and OTC (91.1%, 70.5%, 54.5%, and 47.8% in 3 h, respectively. Apparently, the removal efficiencies for dyes (RhB and MB) were higher than those for colorless drugs, and one possible reason was the photosensitization effect of dye degradation. However, on the whole, the Fe-CDs/G-WO3-0.6 composite exhibited considerable activity for degrading different organic contaminants.
The photogenerated ·OH, ·O2−, and h+ are three typical active species for the degradation of pollutants during the photocatalytic reactions [47,48]. To investigate the mechanisms of the photocatalytic process involving N,Fe-CDs/G-WO3-0.6, various scavengers were used to analyze the active species under visible light. In this experiment, in which isopropyl alcohol (IPA) was used as a ·OH scavenger to eliminate the ·OH, benzoquinone (BQ) and ammonium oxalate (AO) were applied to capture ·O2− and h+, respectively [49,50]. As shown in Figure 6f and Figure S4, when IPA and BQ were added, the additions of IPA, BQ, and AO all provoked the partial inhibition of RhB photodegradation activity, and the degradation rate constants decreased from 1.72 h−1 to 1.23, 1.05, and 0.0781 h−1, respectively. This indicated that the percentages of inhibition of h+, ·OH, and ·O2−, were 54.6%, 39.0%, and 28.5%, respectively, suggesting that ·O2−, ·OH, and h+ all participated in the reaction on different levels and that h+ played a primary role in the reaction. The photogenerated h+ could not only directly break down surface absorbed pollutants, but also convert H2O to ·OH for indirect degradation processes [49].
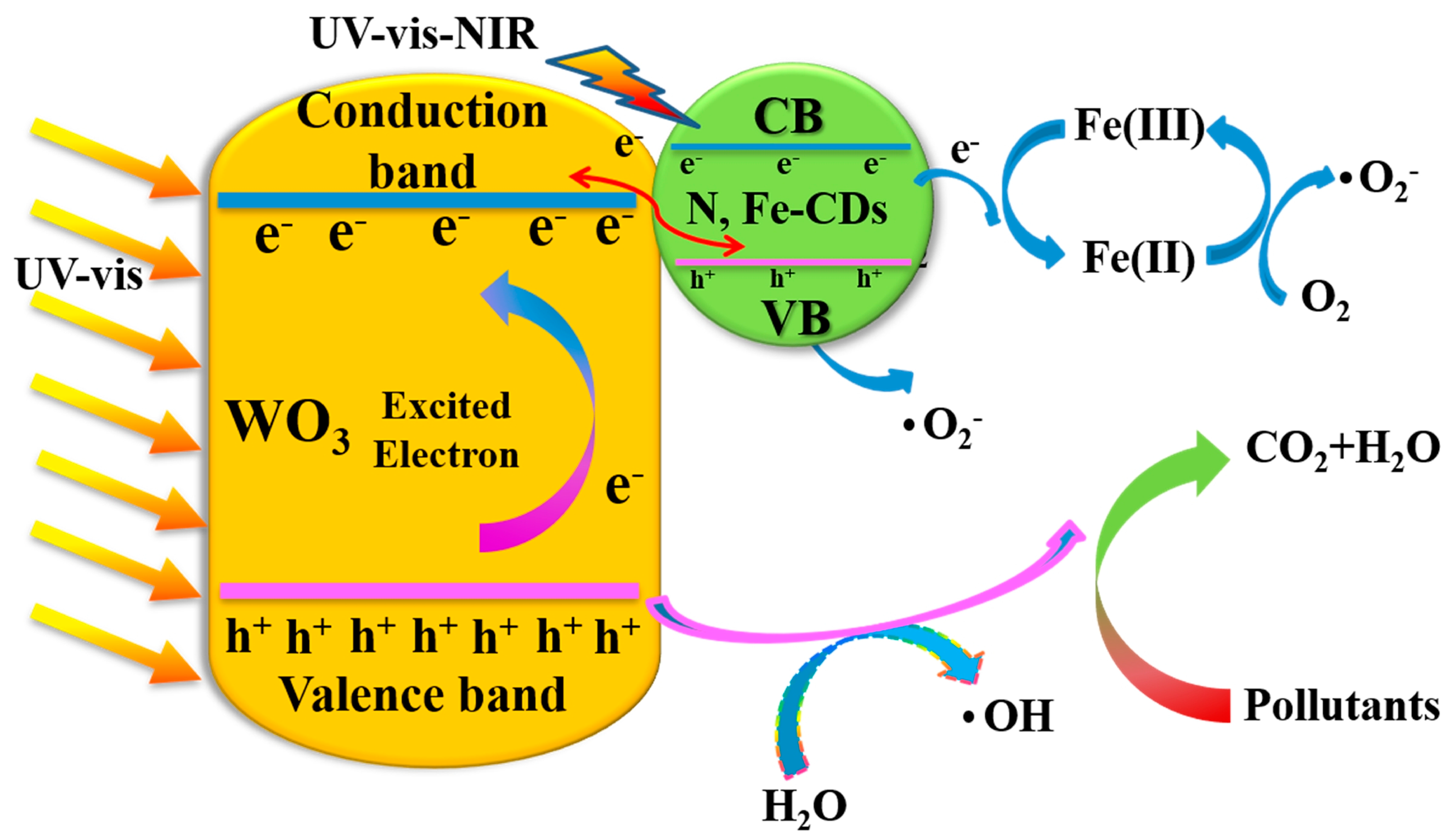
2.4. Possible Mechanism of Photocatalysis
Based on the results of the active species trapping experiments, a possible photocatalytic mechanism for the N,Fe-CDs/G-WO3-0.6 composite is proposed in Figure 8. Under solar irradiation, both WO3 and N,Fe-CDs are excited and photogenerated electrons transition from the valence band (VB) to the conduction band (CB). Then, the electrons on the CB of WO3 transfer to the N,Fe-CDs and couple with holes on the VB of N,Fe-CDs via the hetero-interface, leaving the holes to directly degrade pollutants into CO2 and H2O or convert H2O to ·OH for indirect degradation processes, which significantly facilitates the separation of e−/h+ pairs [22,31]. Finally, the photogenerated electrons from N,Fe-CDs can directly transfer from the VB to the Fe (III) as active sites for multi-electron reactions, which efficiently accelerates the transmission between WO3 and N,Fe-CDs. As e− are transferred to Fe (III), Fe (III) can convert to Fe (II), while Fe (II) can easily revert to Fe (III) with the reduction of O2 to ·O2− [12,51]. Thus, the N,Fe-CDs can not only act as oxygen reduction sites but also significantly inhibit electron-hole recombination.
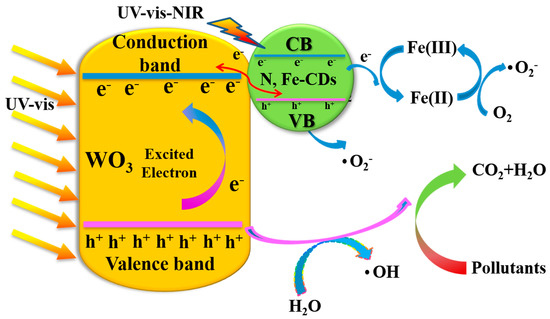
Figure 8.
The possible photocatalytic mechanism of N,Fe-CDs/G-WO3-0.6.
3. Materials and Methods
3.1. Preparation of Gear-Shaped WO3
WO3 gears, abbreviated to G-WO3, were prepared by using a simple hydrothermal process. Typically, 1.0 g Na2WO4·2H2O was dissolved in 80 mL of deionized water with ultrasound for 20 min. After complete dissolution, the pH values were adjusted to 2 using concentrated hydrochloric acid (HCl, 36%) with continuous stirring. Then, the solution was transferred to a 100 mL Teflon-lined stainless-steel autoclave which was maintained at 180 °C for 12 h. After cooling down to room temperature, the precipitates were washed two times with deionized water and ethanol and then dried in an oven at 80 °C for 6 h.
3.2. Preparation of N-CDs and N,Fe-CDs
N-CDs were synthesized by a one-step hydrothermal method. In a typical experiment, 0.3 g Folic acid (FA)was dissolved in 30 mL deionized water under vigorous stirring for 20 min. The solution was then transferred to a 100 mL Teflon-lined stainless-steel autoclave, which was kept at 200 °C for 4 h.
N,Fe-CDs were prepared as follows: 0.3 g FA and 0.03 g FeCl3·6H2O were mixed into 30 mL of deionized water. The mixed solution was continuously stirred for 20 min and then transferred into a 100 mL Teflon-lined autoclave, which was heated at 200 °C for 4 h.
After cooling down to room temperature, the solutions above were filtered with 0.22 μm aqueous microporous membranes and purified with dialysis membranes (MWCO of 1000) for 6 h. Finally, the solutions were freeze-dried to get powder for the further applications.
3.3. Preparation of N-CDs/WO3 and N,Fe-CDs/WO3 Composites
The composites were prepared via a hydrothermal method. Firstly, 0.1 g WO3 was dispersed in the two kinds of CD solution (20 mL) respectively. The different concentrations of CD solution used were 0.2, 0.4, 0.6, 0.8, and 1.0 g/L, respectively. After sonication for 10 min, the suspensions were transferred to a 50 mL Teflon-lined autoclave, which was maintained at 190 °C for 8 h. Finally, the composites were obtained by centrifugation to remove supernatants and dried at 80 °C. A series of N-CDs/WO3 or N,Fe-CDs/WO3 composites were obtained by adding different concentrations of CDs, which were denoted as N-CDs/WO3-X (X = 0.2–1.0) or N,Fe-CDs/WO3-X (X = 0.2–1.0), respectively. In this paper, we used N,Fe-CDs/WO3 (G)-0.6, due to it having the highest photocatalytic activity.
3.4. Photodegradation Experiments
The photocatalytic reactions were processed on a photoreactor (XPA-7, Nanjing XUJ Co. ltd., Nanjing, China) equipped with a 500 W gold halide lamp (coupled with a 420 nm cut-off filter) as the visible light source; a 300 W high pressure mercury lamp as the UV light source, with a 365 nm filter; and a 500 W xenon lamp as the NIR light source, with a 780 nm cut-off filter. The photocatalytic degradations of RhB, MB, TCH, CIP, and OTC were conducted to evaluate the activities of the synthesized photocatalysts. The temperature of the aqueous phase was accurately controlled at 25 ± 1 °C by the circulating cooling water system. Typically, 50 mg of photocatalysts were dispersed into 50 mL of RhB aqueous solution (10 mg/L) in a quartz tube, with pH values of about 3, and stirred in the dark for 30 min to ensure that adsorption/desorption equilibrium was reached. Then, 4 mL of the suspension were taken out every half hour, and 1 mL of sodium thiosulfate solution (1.24 g/mL) was added as a quenching agent, which was filtered using 0.22 μm aqueous microporous membranes. The concentration of RhB was measured using the UV–vis spectrophotometer (Shimadzu, UV-2600), according to its absorbance at 554 nm. The contents of MB (10 mg/L), TCH (20 mg/L), CIP (20 mg/L), and OTC (20 mg/L) were determined by the same method except for measuring the absorbances at 664, 354, 277, and 353 nm, respectively.
4. Conclusions
In summary, we successfully synthesized the N,Fe-CDs/G-WO3 composite via a facile hydrothermal method for the first time. The N,Fe-CDs/G-WO3 composite showed full spectrum absorption and photocatalytic activity superior to that of commercial WO3, pure G-WO3, and N-CDs/G-WO3 composites, and could efficiently degrade RhB, MB, CIP, TCH, and OTC under visible light irradiation. The radical trapping experiments indicated that the conversion between Fe (III) and Fe (II) played a key role in the photocatalytic reactions. The enhanced photocatalytic activity was mainly attributed to an improved light harvesting capability due to the modification of N,Fe-CDs and the special gear-shaped structure of WO3, which caused multiple reflections of light. This work provides new insights for the efficient utilization of solar energy for environmental remediation.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/10/4/416/s1, Figure S1: X-ray photoelectron spectroscopy (XPS) survey spectra of N-CDs/G-WO3-0.6 and N,Fe-CDs/G-WO3-0.6, Figure S2: Degradation efficiencies of the RhB solution under different factors. (a) catalyst dosage; (b) concentration of RhB; (c) pH; (d–g) different ions like NO3−, Ca2+, and Cu2+, Figure S3: The kinetic fit for the degradation of RhB with the different samples under visible light, Figure S4: The kinetic fit for the degradation of RhB with the different scavengers under visible light, Table S1: Molecular formulas and structures of several organic pollutants studied in this wok.
Author Contributions
Conceptualization, T.N. and G.L.; Data curation, T.N., Q.L., X.C. and Z.Y. (Zhibin Yang); Formal analysis, Q.L., F.W., Y.W. and Z.Y. (Zhijun Yang); Investigation, Y.Y., X.C., Z.Y. (Zhijun Yang), K.C. and G.L.; Methodology, F.W., Z.Y. (Zhibin Yang), Y.W. and K.C.; Writing—original draft, T.N., Q.L. and G.L.; Writing—review & editing, T.N., Q.L., Y.Y. and G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 21677040), Natural Science Foundation of Henan (No. 182300410118), the Key Scientific Research Projects of Universities of Henan Province (No. 17A150047).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kasiri, M.B.; Aleboyeh, H.; Aleboyeh, A. Degradation of Acid Blue 74 using Fe-ZSM5 zeolite as a heterogeneous photo-Fenton catalyst. Appl. Catal. B Environ. 2008, 84, 9–15. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Asadi, A.; Sillanpää, M.; Farhadian, N. Application of carbon quantum dots to increase the activity of conventional photocatalysts: A systematic review. J. Mol. Liq. 2018, 271, 857–871. [Google Scholar] [CrossRef]
- Li, R.; Yang, J.; Xu, S.; Zhou, Y.; Wang, X.; Peng, H.; Du, J. Preparation of Gd-Doped TiO2 Nanotube Arrays by Anodization Method and Its Photocatalytic Activity for Methyl Orange Degradation. Catalysts 2020, 10, 298. [Google Scholar] [CrossRef]
- Daneshvar, N.; Khataee, A.R.; Rasoulifard, M.H.; Pourhassan, M. Biodegradation of dye solution containing Malachite Green: Optimization of effective parameters using Taguchi method. J. Hazard. Mater. 2007, 143, 214–219. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Tian, J.; Leng, Y.; Zhao, Z.; Xia, Y.; Sang, Y.; Hao, P.; Zhan, J.; Li, M.; Liu, H. Carbon quantum dots/hydrogenated TiO2 nanobelt heterostructures and their broad spectrum photocatalytic properties under UV, visible, and near-infrared irradiation. Nano Energy 2015, 11, 419–427. [Google Scholar] [CrossRef]
- Yoon, H.; Mali, M.G.; Kim, M.W.; Al-Deyab, S.S.; Yoon, S.S. Electrostatic spray deposition of transparent tungsten oxide thin-film photoanodes for solar water splitting. Catal. Today 2016, 260, 89–94. [Google Scholar] [CrossRef]
- Mano, T.; Nishimoto, S.; Kameshima, Y.; Miyake, M. Water treatment efficacy of various metal oxide semiconductors for photocatalytic ozonation under UV and visible light irradiation. Chem. Eng. J. 2015, 264, 221–229. [Google Scholar] [CrossRef]
- Lin, C.F.; Wu, C.H.; Onn, Z.N. Degradation of 4-chlorophenol in TiO2, WO3, SnO2, TiO2/WO3 and TiO2/SnO2 systems. J. Hazard. Mater. 2008, 154, 1033–1039. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Navale, Y.H.; Navale, S.T.; Stadler, F.J.; Ramgir, N.S.; Patil, V.B. Hybrid polyaniline-WO3 flexible sensor: A room temperature competence towards NH3 gas. Sens. Actuators B Chem. 2019, 288, 279–288. [Google Scholar] [CrossRef]
- Mehmood, F.; Iqbal, J.; Jan, T.; Mansoor, Q. Structural, Raman and photoluminescence properties of Fe doped WO3 nanoplates with anti cancer and visible light driven photocatalytic activities. J. Alloys Compd. 2017, 728, 1329–1337. [Google Scholar] [CrossRef]
- Ma, G.; Chen, Z.; Chen, Z.; Jin, M.; Meng, Q.; Yuan, M.; Wang, X.; Liu, J.M.; Zhou, G. Constructing novel WO3/Fe(III) nanofibers photocatalysts with enhanced visible-light-driven photocatalytic activity via interfacial charge transfer effect. Mater. Today 2017, 3, 45–52. [Google Scholar] [CrossRef]
- Liu, Y.; Ya, Y.; Li, W.; Han, S.; Liu, C. Photoelectrochemical properties and photocatalytic activity of nitrogen-doped nanoporous WO3 photoelectrodes under visible light. Appl. Surf. Sci. 2012, 258, 5038–5045. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Du, Y.; Jiang, H.; Zhou, D.; Dong, S. Carbon nanodots/WO3 nanorods Z-scheme composites: Remarkably enhanced photocatalytic performance under broad spectrum. Appl. Catal. B Environ. 2017, 209, 253–264. [Google Scholar] [CrossRef]
- An, X.; Yu, J.C.; Wang, Y.; Hu, Y.; Yu, X.; Zhang, G. WO3 nanorods/graphene nanocomposites for high-efficiency visible-light-driven photocatalysis and NO2 gas sensing. J. Mater. Chem. 2012, 22, 8525–8531. [Google Scholar] [CrossRef]
- Sakdaronnarong, C.; Sangjan, A.; Boonsith, S.; Kim, D.C.; Shin, H.S. Recent developments in synthesis and photocatalytic applications of carbon dots. Catalysts 2020, 10, 320. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Liu, J.; Yang, Q.; Zhang, B.; Gao, Y.; Wang, Y.; Lu, G. Dual functional N- and S-co-doped carbon dots as the sensor for temperature and Fe3+ ions. Sens. Actuators B Chem. 2017, 242, 1272–1280. [Google Scholar] [CrossRef]
- Song, B.; Wang, T.; Sun, H.; Shao, Q.; Zhao, J.; Song, K.; Hao, L.; Wang, L.; Guo, Z. Two-step hydrothermally synthesized carbon nanodots/WO3 photocatalysts with enhanced photocatalytic performance. Dalton Trans. 2017, 46, 15769–15777. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Xiong, Y.; Zhou, D.; Dong, S. An environmentally friendly Z-scheme WO3 /CDots/CdS heterostructure with remarkable photocatalytic activity and anti-photocorrosion performance. J. Catal. 2017, 356, 1–13. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Wang, X.; Mu, L.; Yuan, M.; Liu, B.; Shi, H. Carbon dots-decorated Na2W4O13 composite with WO3 for highly efficient photocatalytic antibacterial activity. J. Hazard. Mater. 2018, 359, 1–8. [Google Scholar] [CrossRef]
- Wang, F.; Chen, P.; Feng, Y.; Xie, Z.; Liu, Y.; Su, Y.; Zhang, Q.; Wang, Y.; Yao, K.; Lv, W.; et al. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-Light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B Environ. 2017, 207, 103–113. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.; Wang, Y.; Li, J.; Jin, X.; Zhang, Q.; Li, R.; Yan, S.; Liu, H.; Feng, Y.; et al. Construction of novel Z-scheme nitrogen-doped carbon dots/{0 0 1} TiO2 nanosheet photocatalysts for broad-spectrum-driven diclofenac degradation: Mechanism insight, products and effects of natural water matrices. Chem. Eng. J. 2019, 356, 857–868. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Y.; Tsai, P.; Wang, J.; Chen, X. Metal ions doped carbon quantum dots: Synthesis, physicochemical properties, and their applications. TrAC Trends Anal. Chem. 2018, 103, 87–101. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Rathi, A.K.; Gawande, M.B.; Hola, K.; Goswami, A.; Kalytchuk, S.; Karakassides, M.A.; Kouloumpis, A.; Gournis, D.; Deligiannakis, Y.; et al. Fe(III)-functionalized carbon dots—Highly efficient photoluminescence redox catalyst for hydrogenations of olefins and decomposition of hydrogen peroxide. Appl. Mater. Today 2017, 7, 179–184. [Google Scholar] [CrossRef]
- Gao, X.; Du, C.; Zhuang, Z.; Chen, W. Carbon quantum dots-based nanoprobes for metal ions detection. J. Mater. Chem. C 2016, 4, 6927–6945. [Google Scholar] [CrossRef]
- Teng, X.; Ma, C.; Ge, C.; Yan, M.; Yang, J.; Zhang, Y.; Morais, P.C.; Bi, H. Green synthesis of nitrogen-doped carbon dots from konjac flour with “off–on” fluorescence by Fe3+ and L-lysine for bioimaging. J. Mater. Chem. B 2014, 2, 4631–4639. [Google Scholar] [CrossRef]
- Li, G.; Lv, N.; Bi, W.; Zhang, J.; Ni, J. Nitrogen-doped carbon dots as a fluorescent probe suitable for sensing Fe3+ under acidic conditions. New J. Chem. 2016, 40, 10213–10218. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Park, S.J.; Zhang, Y.; Kim, T.; Chae, S.; Park, M.; Kim, H.Y. One-step synthesis of robust nitrogen-doped carbon dots: Acid-evoked fluorescence enhancement and their application for Fe3+ detection. J. Mater. Chem. A 2015, 3, 17747–17754. [Google Scholar] [CrossRef]
- Yang, W.; Huang, T.; Zhao, M.; Luo, F.; Weng, W.; Wei, Q.; Lin, Z.; Chen, G. High peroxidase-like activity of iron and nitrogen co-doped carbon dots and its application in immunosorbent assay. Talanta 2017, 164, 1–6. [Google Scholar] [CrossRef]
- Yousaf, A.B.; Imran, M.; Zaidi, S.J.; Kasak, P. Highly efficient photocatalytic Z-scheme hydrogen production over oxygen-deficient WO3–x nanorods supported Zn0.3Cd0.7S heterostructure. Sci. Rep. 2017, 7, 6574. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Wang, X.; Mai, J.; Zhao, W.; Ding, Z.; Fang, Y. Construction of Z-scheme tungsten trioxide nanosheets-nitrogen-doped carbon dots composites for the enhanced photothermal synergistic catalytic oxidation of cyclohexane. Appl. Catal. B Environ. 2019, 259, 118063. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Xian, M.; Dong, C.; Shuang, S. Folic acid-conjugated green luminescent carbon dots as a nanoprobe for identifying folate receptor-positive cancer cells. Talanta 2018, 183, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cen, Y.; Sohail, M.; Xu, G.; Wei, F.; Shi, M.; Xu, X.; Song, Y.; Ma, Y.; Hu, Q. A ratiometric fluorescence universal platform based on N, Cu codoped carbon dots to detect metabolites participating in H2O2-generation reactions. ACS Appl. Mater. Interfaces 2017, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Liu, Y.; Liu, Q.; Liu, Z.; Yang, H.; Lei, B.; Zhuang, J.; Hu, C. Size-controlled synthesis of fluorescent tungsten oxide quantum dots via one-pot ethanol-thermal strategy for ferric ions detection and bioimaging. Sens. Actuators B Chem. 2018, 255, 290–298. [Google Scholar] [CrossRef]
- Xi, G.; Ouyang, S.; Li, P.; Ye, J.; Ma, Q.; Su, N.; Bai, H.; Wang, C. Ultrathin W18O49 nanowires with diameters below 1 nm: Synthesis, near-infrared absorption, photoluminescence, and photochemicalreduction of carbon dioxide. Angew. Chem. 2012, 124, 2445–2449. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ge, Y.; Li, H.; Ji, H.; Xu, H.; Zhang, Q.; Li, H.; Li, M. Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight. Appl. Catal. B Environ. 2015, 168–169, 51–61. [Google Scholar] [CrossRef]
- Shen, J.Y.; Zhang, L.; Ren, J.; Wang, J.C.; Yao, H.C.; Li, Z.J. Highly enhanced acetone sensing performance of porous C-doped WO3 hollow spheres by carbon spheres as templates. Sens. Actuators B Chem. 2017, 239, 597–607. [Google Scholar] [CrossRef]
- Perfecto, T.M.; Zito, C.A.; Mazon, T.; Volanti, D.P. Flexible room-temperature volatile organic compound sensors based on reduced graphene oxide–WO3·0.33H2O nano-needles. J. Mater. Chem. C 2018, 6, 2822–2829. [Google Scholar] [CrossRef]
- Yan, F.; Kong, D.; Fu, Y.; Ye, Q.; Wang, Y.; Chen, L. Construction of carbon nanodots/tungsten trioxide and their visible-light sensitive photocatalytic activity. J. Colloid Interface Sci. 2016, 466, 268–274. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Zhang, R.; Li, W.; Li, P.; Wang, X.; Zhou, Y. Enhanced visible-light photocatalytic performance of a monolithic tungsten oxide/graphene oxide aerogel for nitric oxide oxidation. Chin. J. Catal. 2018, 39, 646–653. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Lu, L.; Wu, G.; Chen, W. Self-regenerated solar-driven photocatalytic water-splitting by urea derived graphitic carbon nitride with platinum nanoparticles. Chem. Commun. 2012, 48, 8826–8828. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xiao, Y.; Xie, W.; Wang, Y.; Hu, Z.; Zhang, W.; Zhao, H. Facile strategy for synthesizing non-stoichiometric monoclinic structured tungsten trioxide (WO3−x) with plasma resonance absorption and enhanced photocatalytic activity. Nanomaterials 2018, 8, 553. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, J.; Jiang, G. Facile synthesis of spiny mesoporous titania tubes with enhanced photocatalytic activity. Chem. Commun. 2011, 47, 7443–7445. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, R.; Liu, Y.; Huang, H.; Yu, H.; Ming, H.; Lian, S.; Lee, S.T.; Kang, Z. Carbon quantum dots/Cu2O composites with protruding nanostructures and their highly efficient (near) infrared photocatalytic behavior. J. Mater. Chem. 2012, 22, 17470. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, X.; Liu, S.; Zhou, Y.; Chang, B.; Zhang, S.; Fan, H.; Yang, B. N doped carbon dot modified WO3 nanoflakes for efficient photoelectrochemical water oxidation. Adv. Mater. Interfaces 2019, 6, 1801653. [Google Scholar] [CrossRef]
- Fu, S.; Liu, X.; Yan, Y.; Li, L.; Liu, H.; Zhao, F.; Zhou, J. Few-layer WS2 modified BiOBr nanosheets with enhanced broad-spectrum photocatalytic activity towards various pollutants removal. Sci. Total Environ. 2019, 694, 133756. [Google Scholar] [CrossRef]
- Fu, J.; Kyzas, G.Z.; Cai, Z.; Deliyanni, E.A.; Liu, W.; Zhou, D. Photocatalytic degradation of phenanthrene by graphite oxide-TiO2-Sr(OH)2/SrCO3 nanocomposite under solar irradiation: Effects of water quality parameters and predictive modeling. Chem. Eng. J. 2018, 335, 290–300. [Google Scholar] [CrossRef]
- Sapkota, K.P.; Lee, I.; Hanif, M.A.; Islam, M.A.; Akter, J.; Hahn, J.R. Enhanced visible-light photocatalysis of nanocomposites of copper oxide and single-walled carbon nanotubes for the degradation of methylene blue. Catalysts 2020, 10, 297. [Google Scholar] [CrossRef]
- Cai, Z.; Hao, X.; Sun, X.; Du, P.; Liu, W.; Fu, J. Highly active WO3 @anatase-SiO2 aerogel for solar-light-driven phenanthrene degradation: Mechanism insight and toxicity assessment. Water Res. 2019, 162, 369–382. [Google Scholar] [CrossRef]
- Xiao, T.; Tang, Z.; Yang, Y.; Tang, L.; Zhou, Y.; Zou, Z. In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics. Appl. Catal. B Environ. 2018, 220, 417–428. [Google Scholar] [CrossRef]
- Song, H.; Li, Y.; Lou, Z.; Xiao, M.; Hu, L.; Ye, Z.; Zhu, L. Synthesis of Fe-doped WO3 nanostructures with high visible-light-driven photocatalytic activities. Appl. Catal. B Environ. 2015, 166–167, 112–120. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).