Greener Synthesis of Nitrogen-Containing Heterocycles in Water, PEG, and Bio-Based Solvents
Abstract
1. Introduction
Background and Methodology
2. Sustainable Chemistry
2.1. Water as Solvent
2.2. PEG as Solvent
2.3. Bio-Based Solvents
3. Five-Membered Aromatic Nitrogen Heterocycles
3.1. Formation of the Pyrrole ring
3.1.1. Reactions in Water
3.1.2. Reactions in PEG
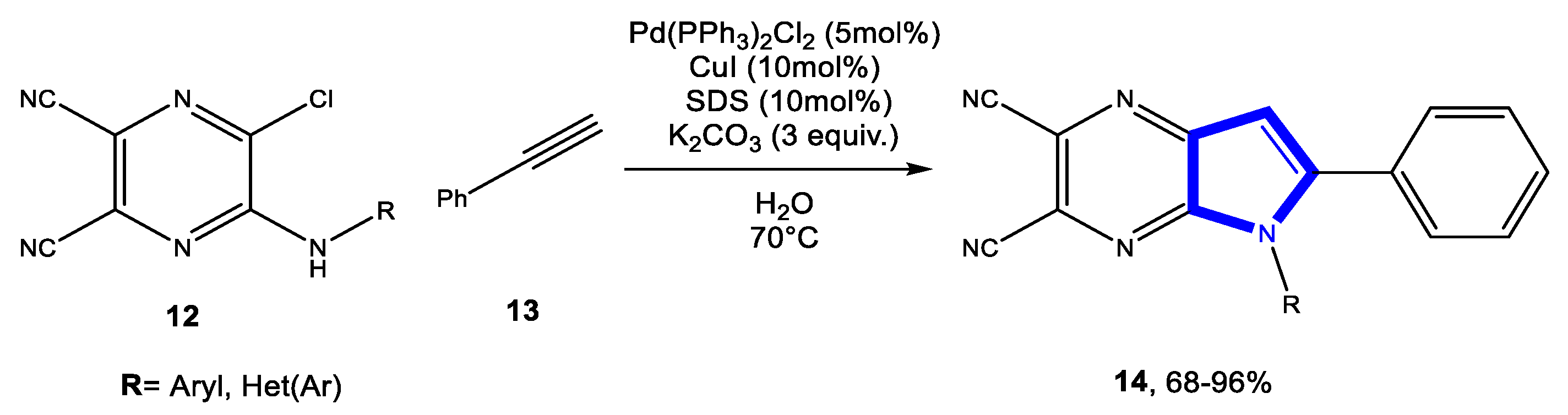
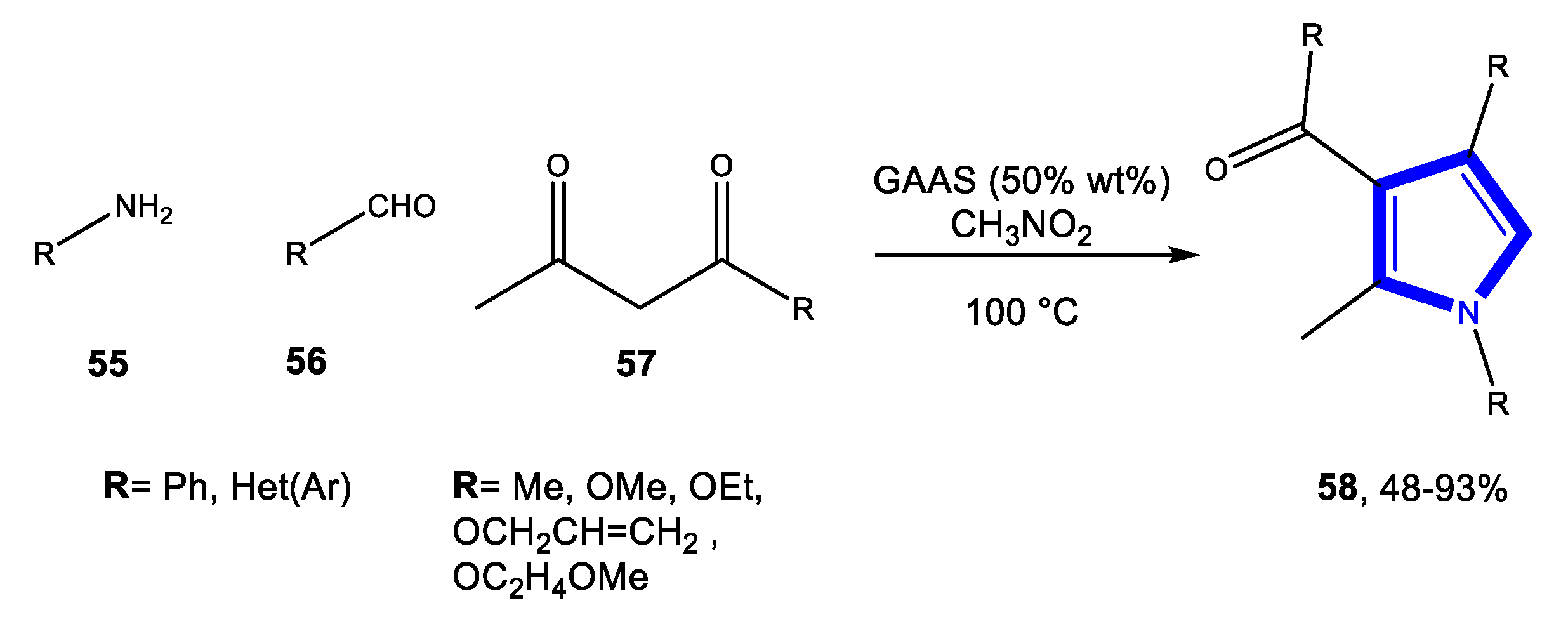
3.1.3. Reactions in Bio-Based Solvents
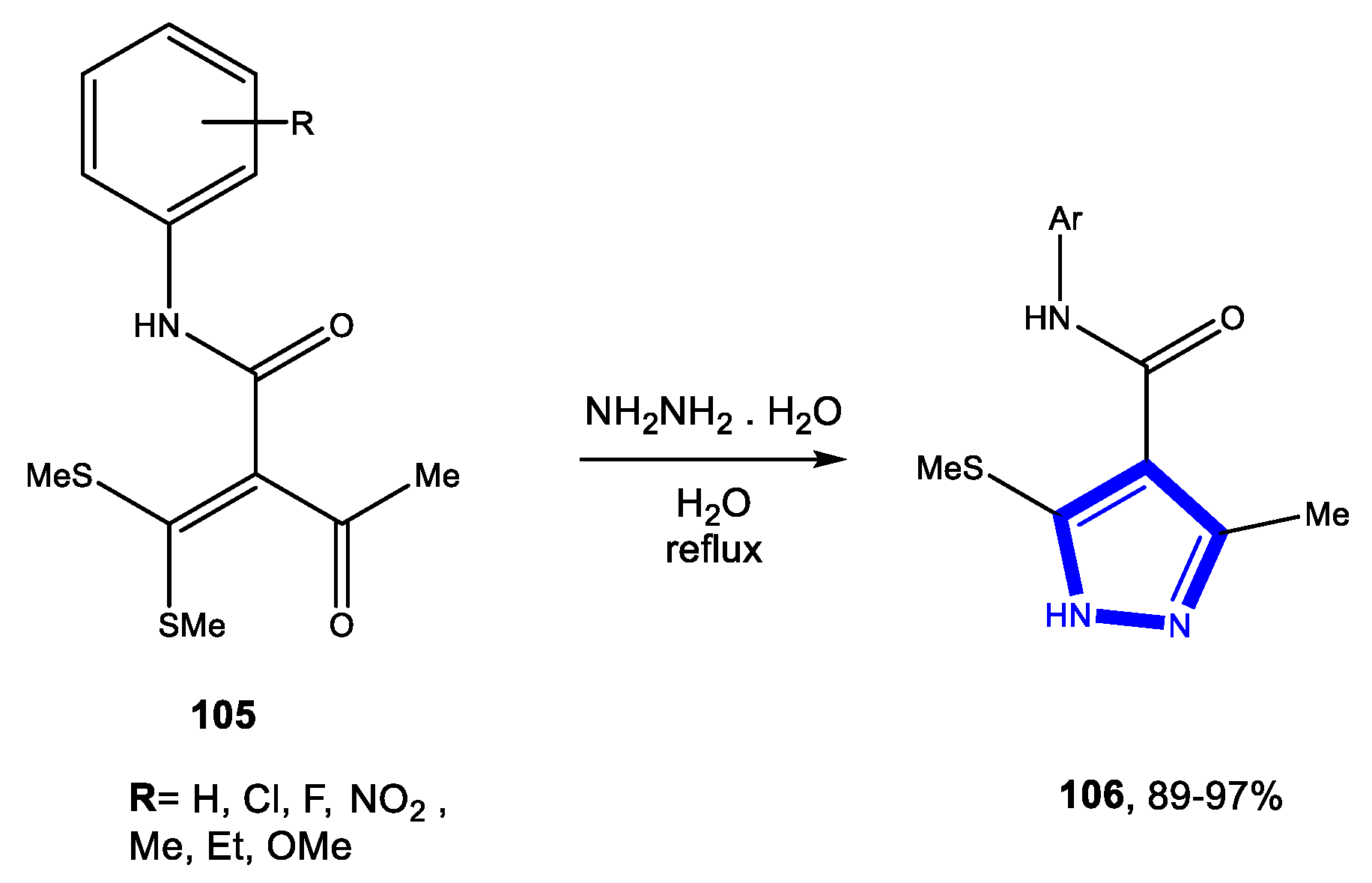
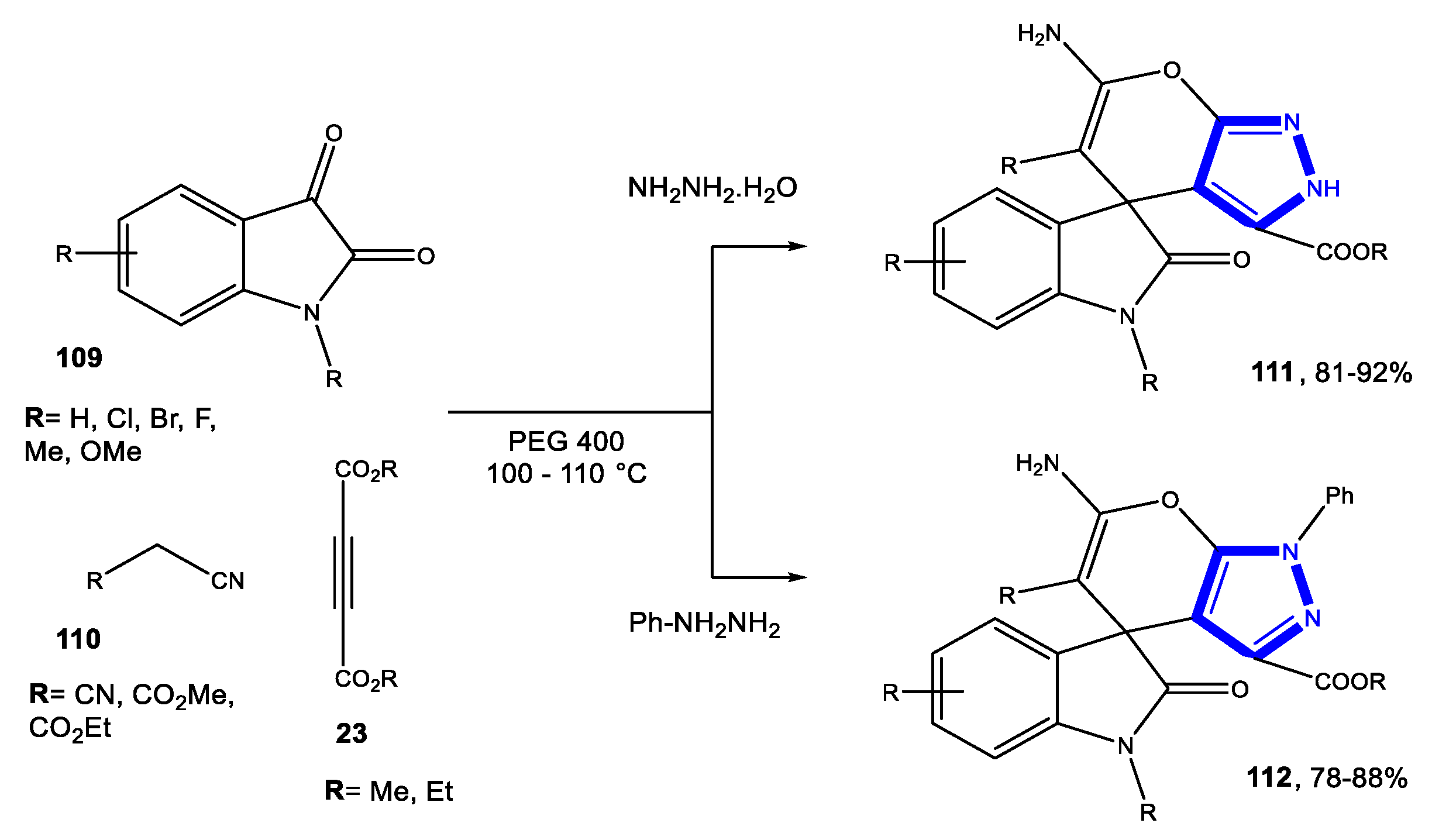
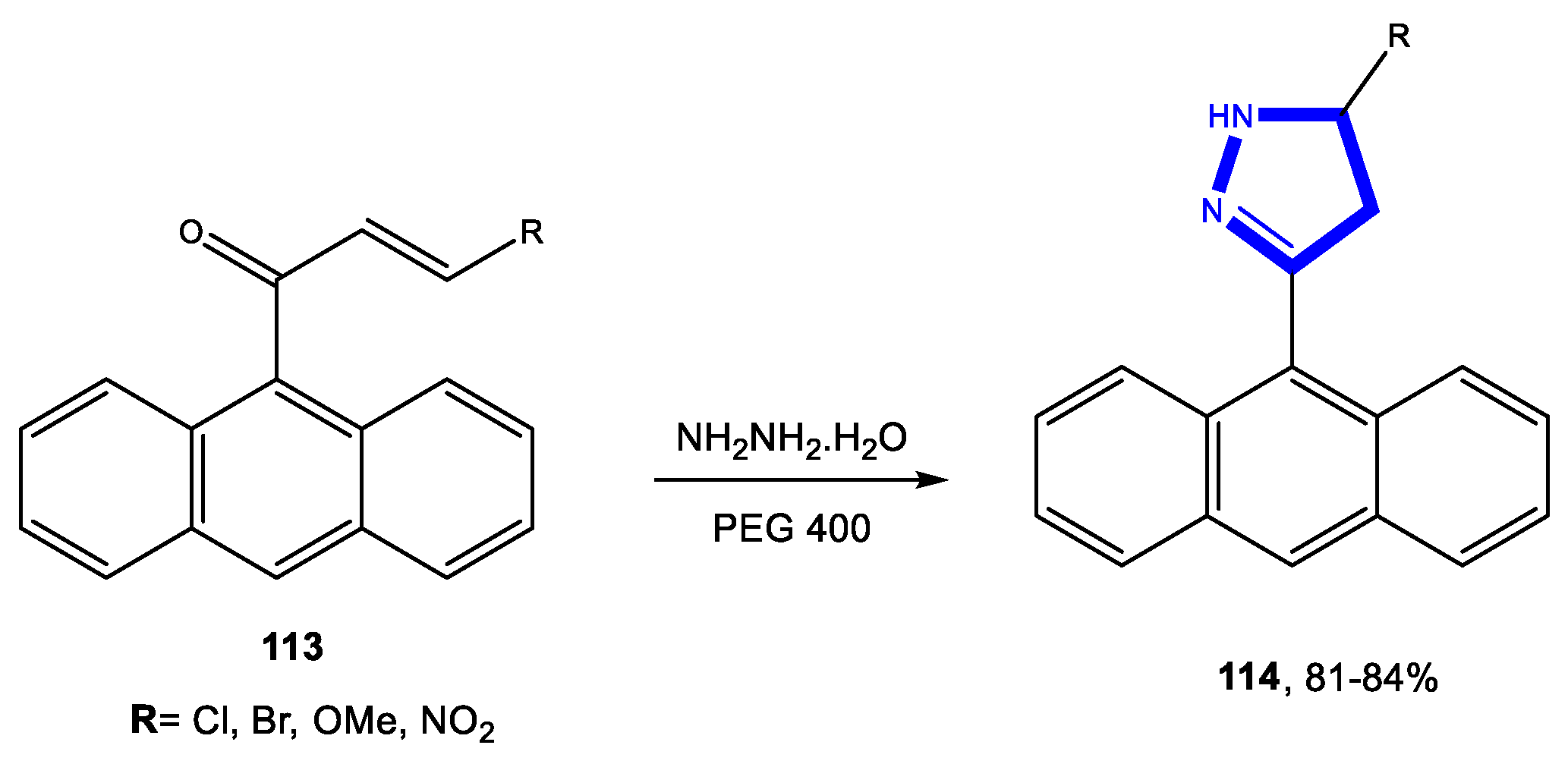
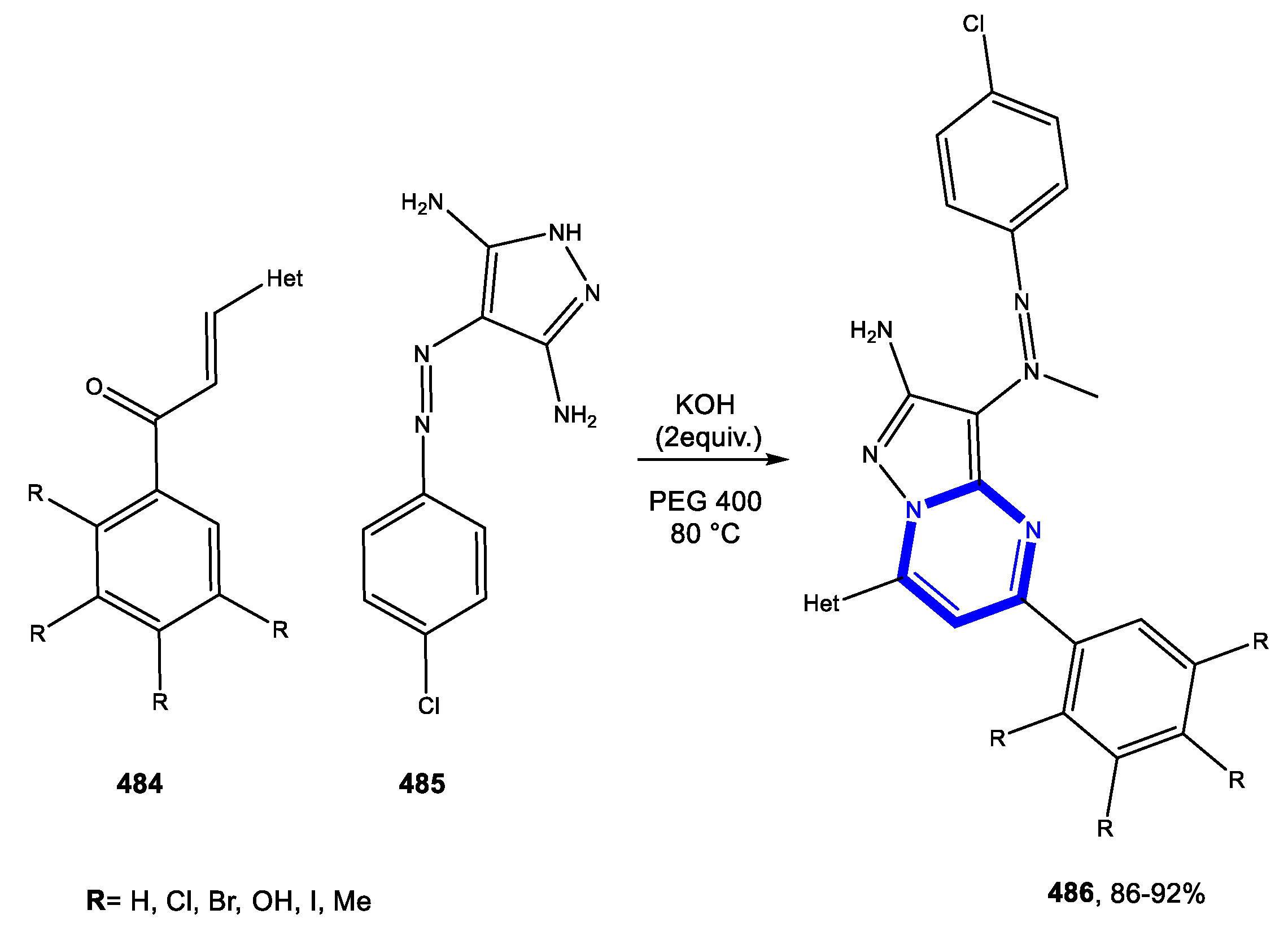
3.2. Formation of the Pyrazole Ring
3.2.1. Reactions in Water
3.2.2. Reactions in PEG
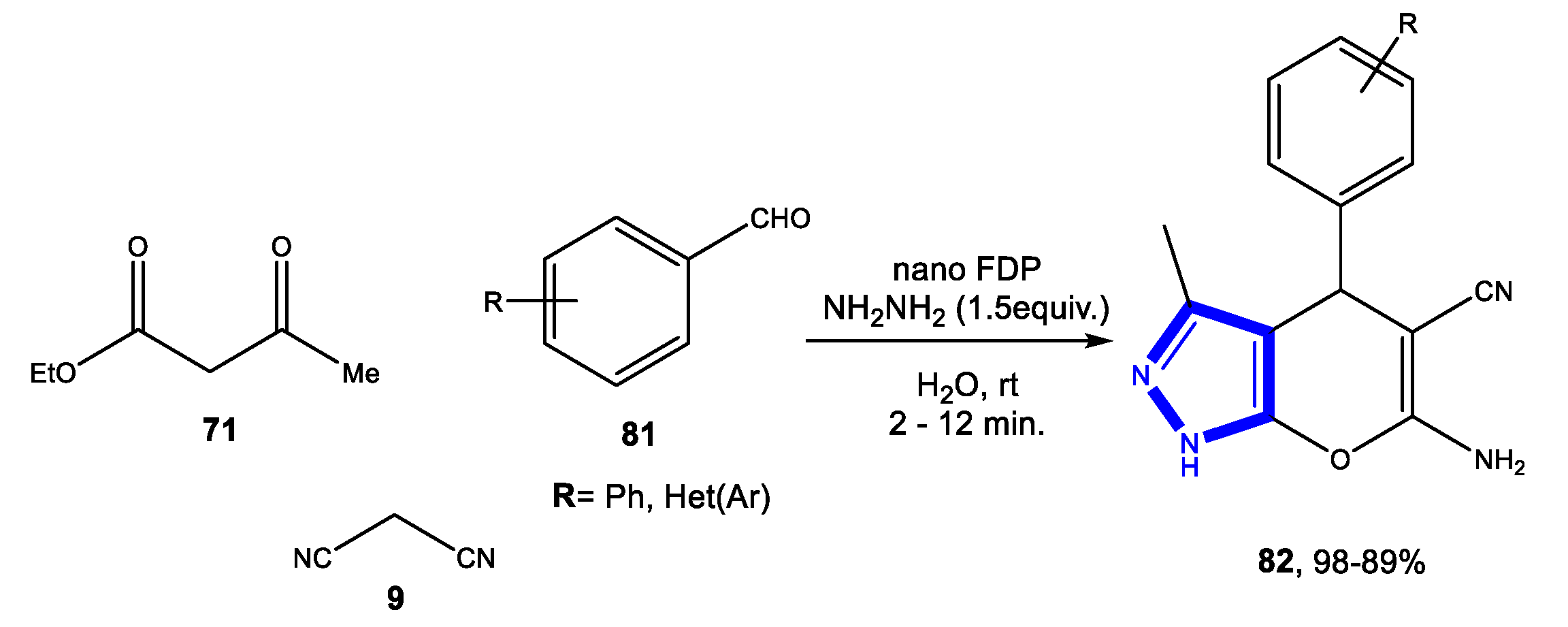
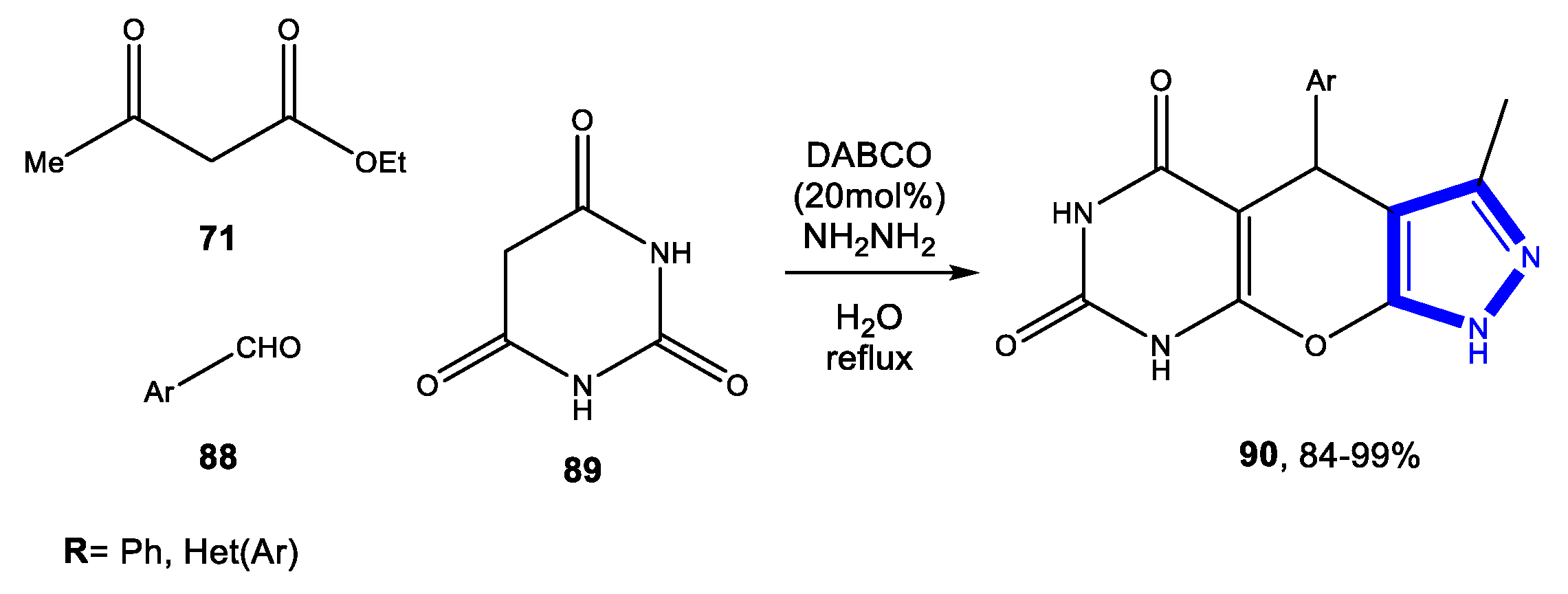
3.2.3. Reactions in Bio-Based Solvents
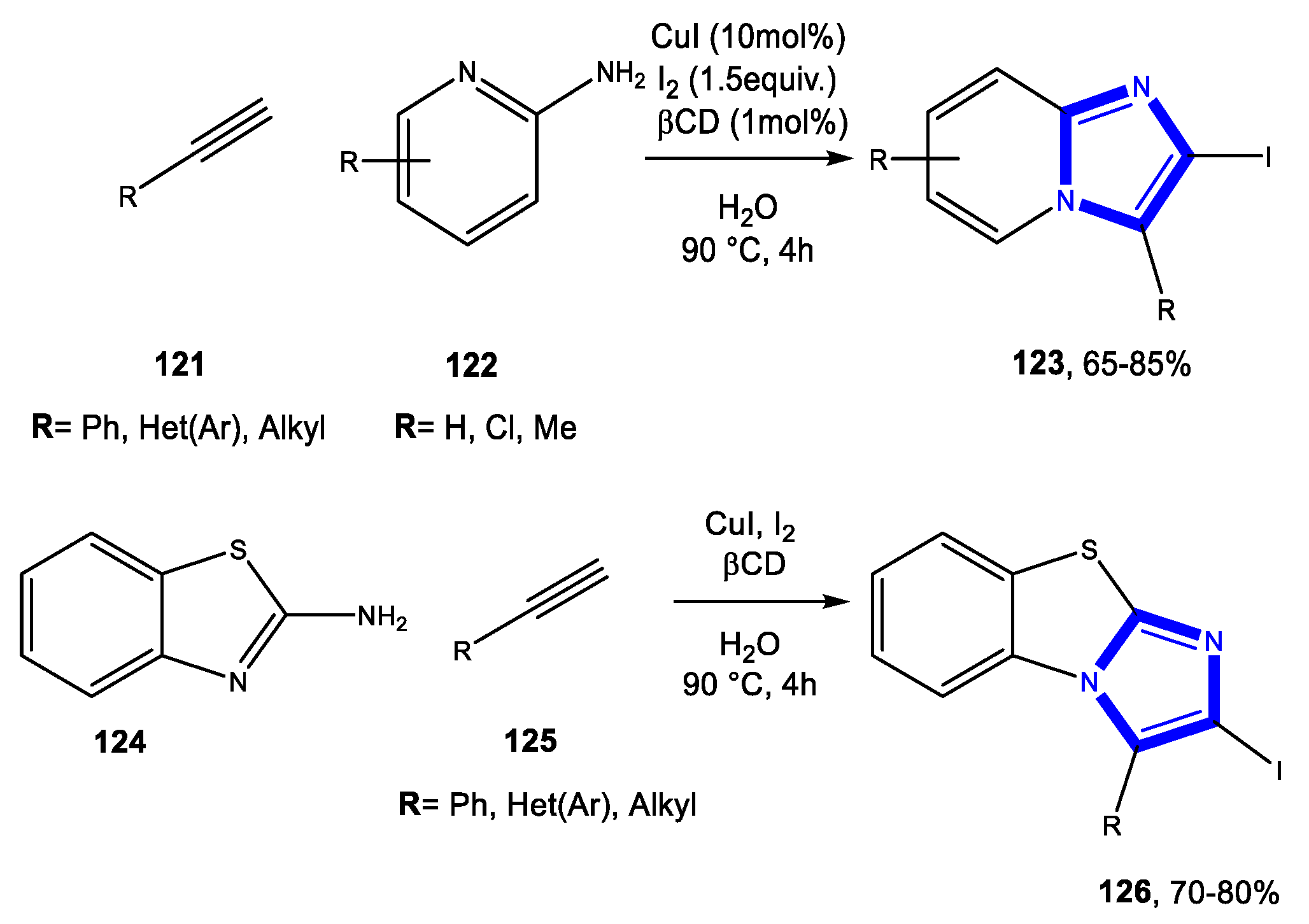
3.3. Formation of the Imidazole Ring
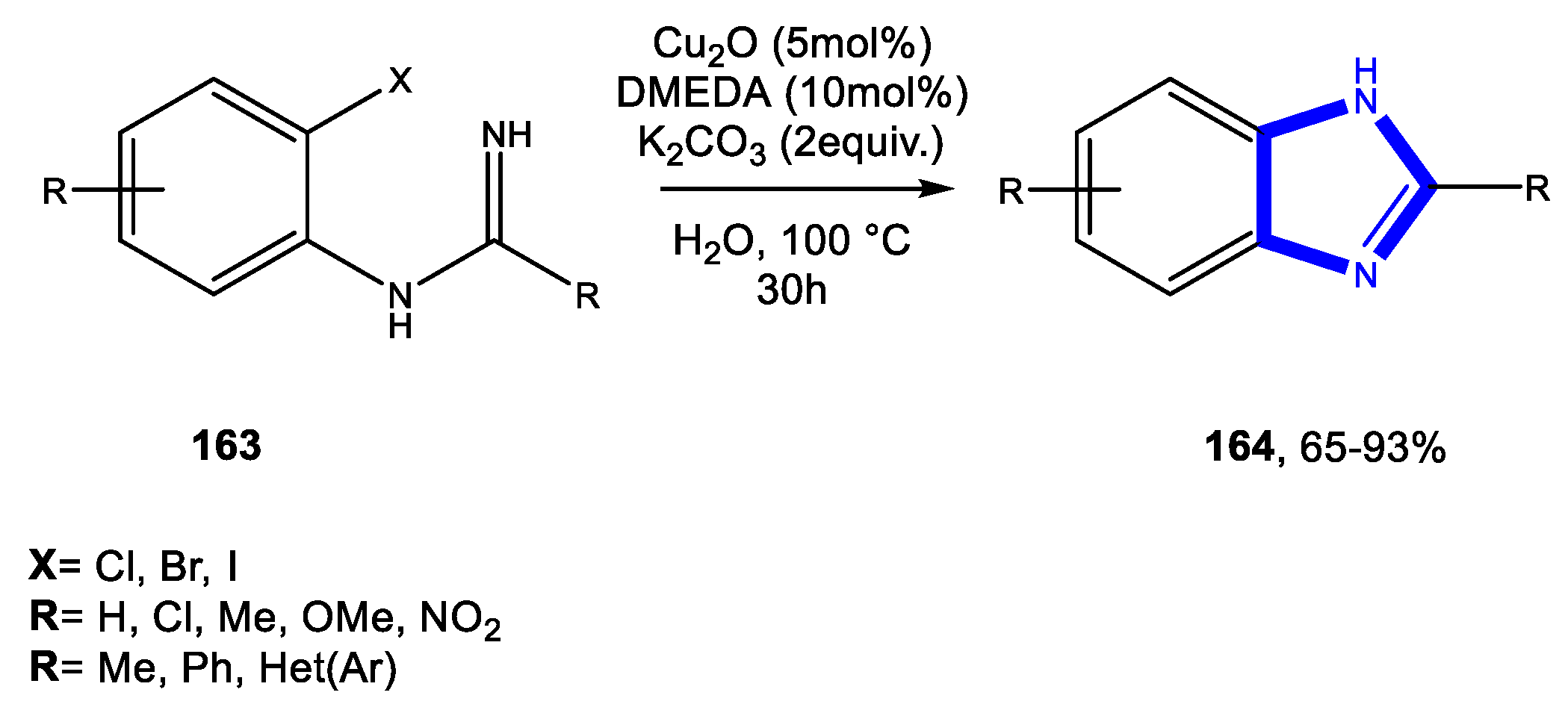
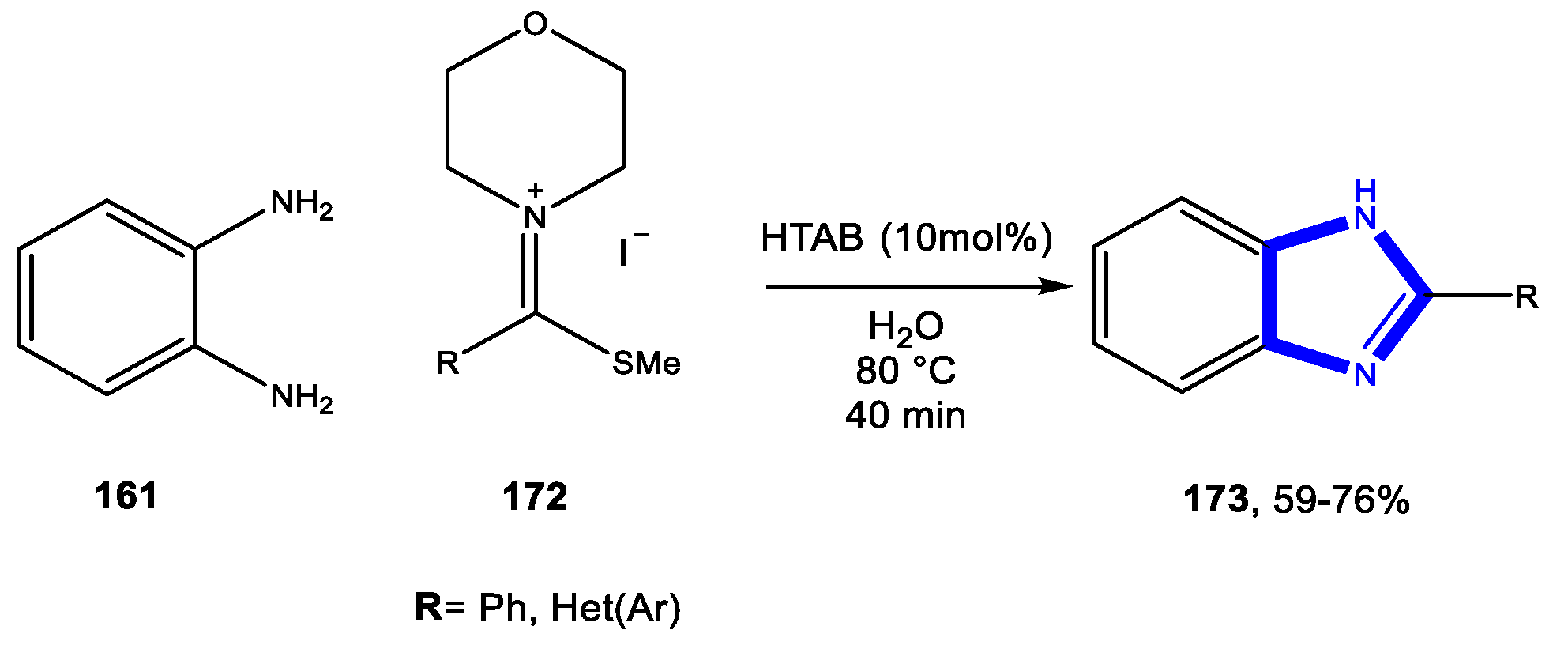
3.3.1. Reactions in Water
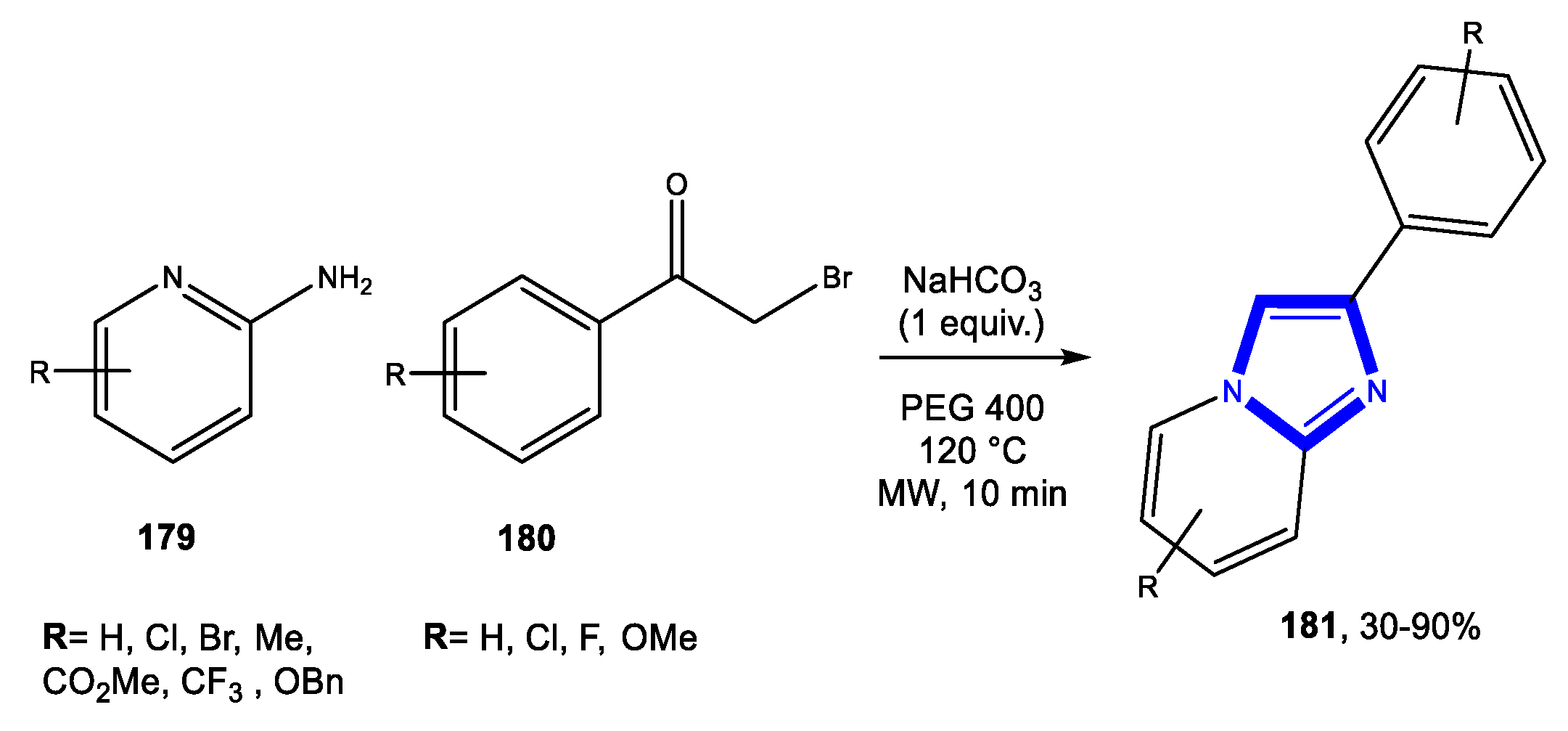
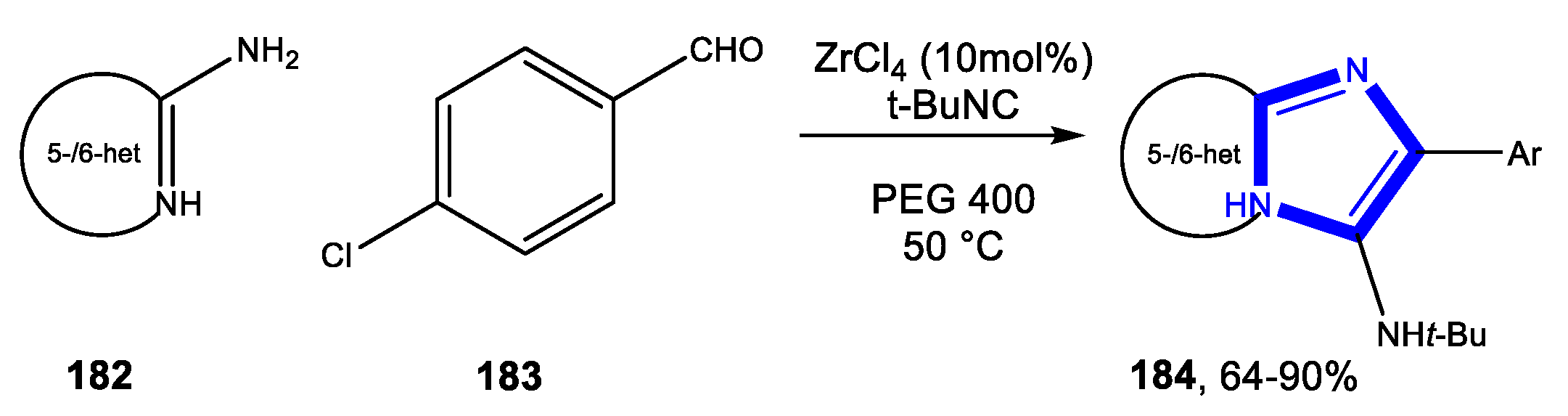
3.3.2. Reactions in PEG
3.3.3. Reactions in Bio-Based Solvents
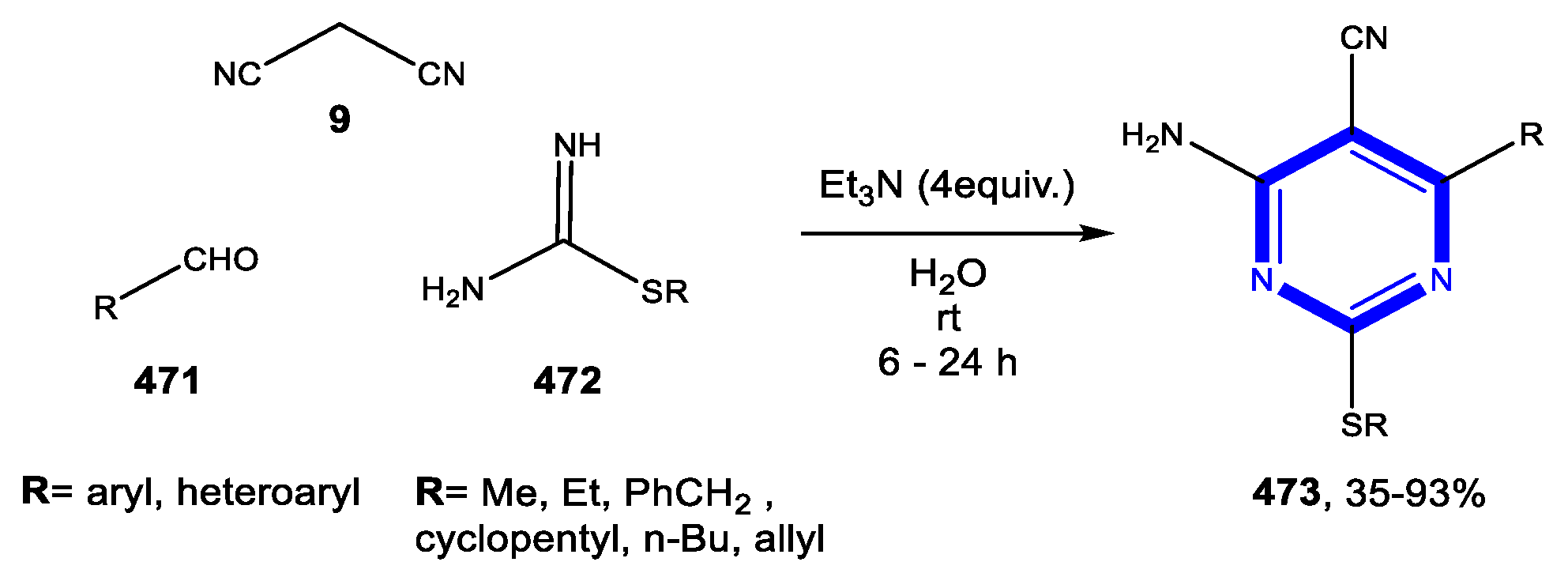
3.4. Formation of the Triazole Ring
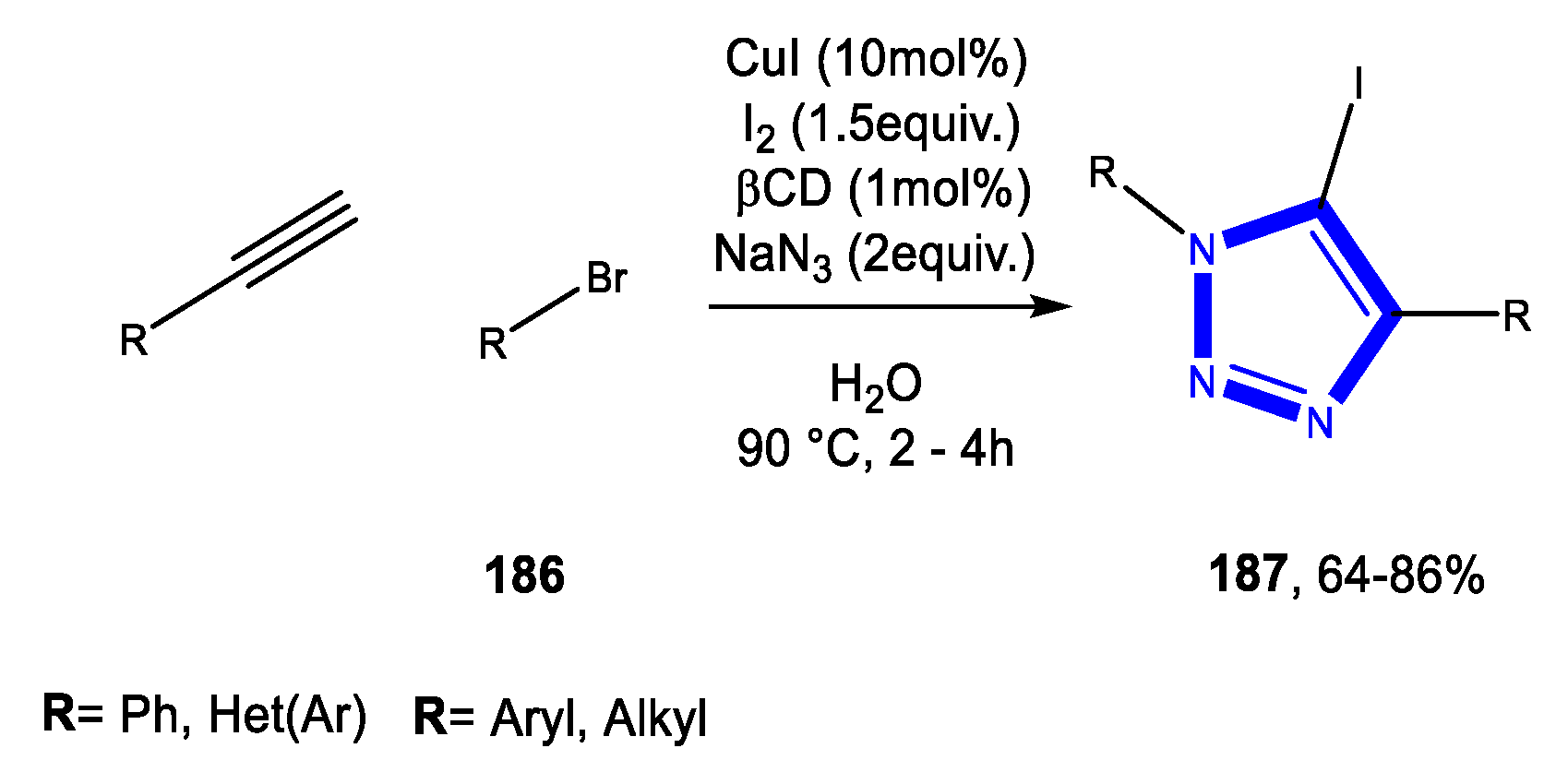
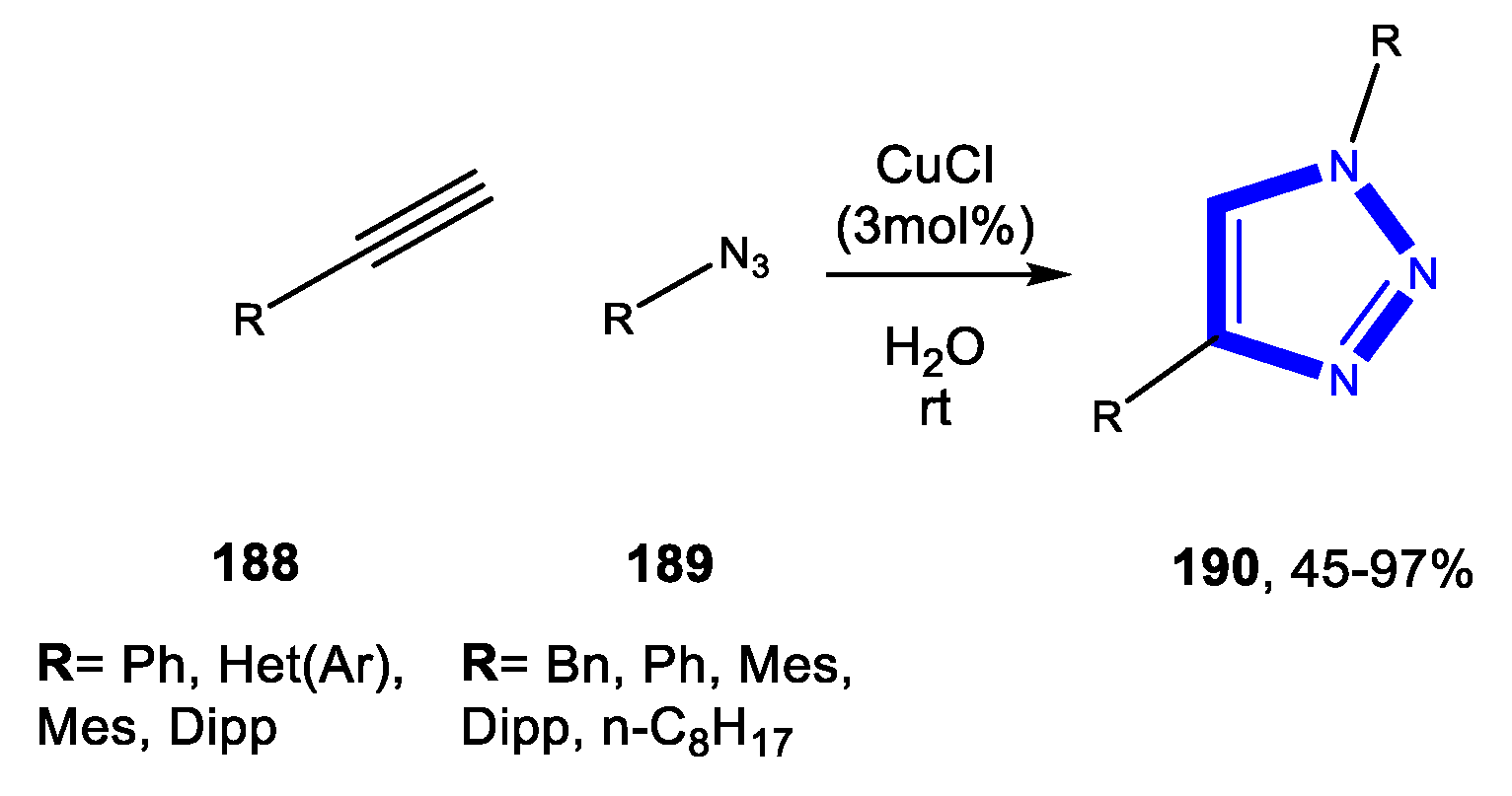
3.4.1. Reactions in Water
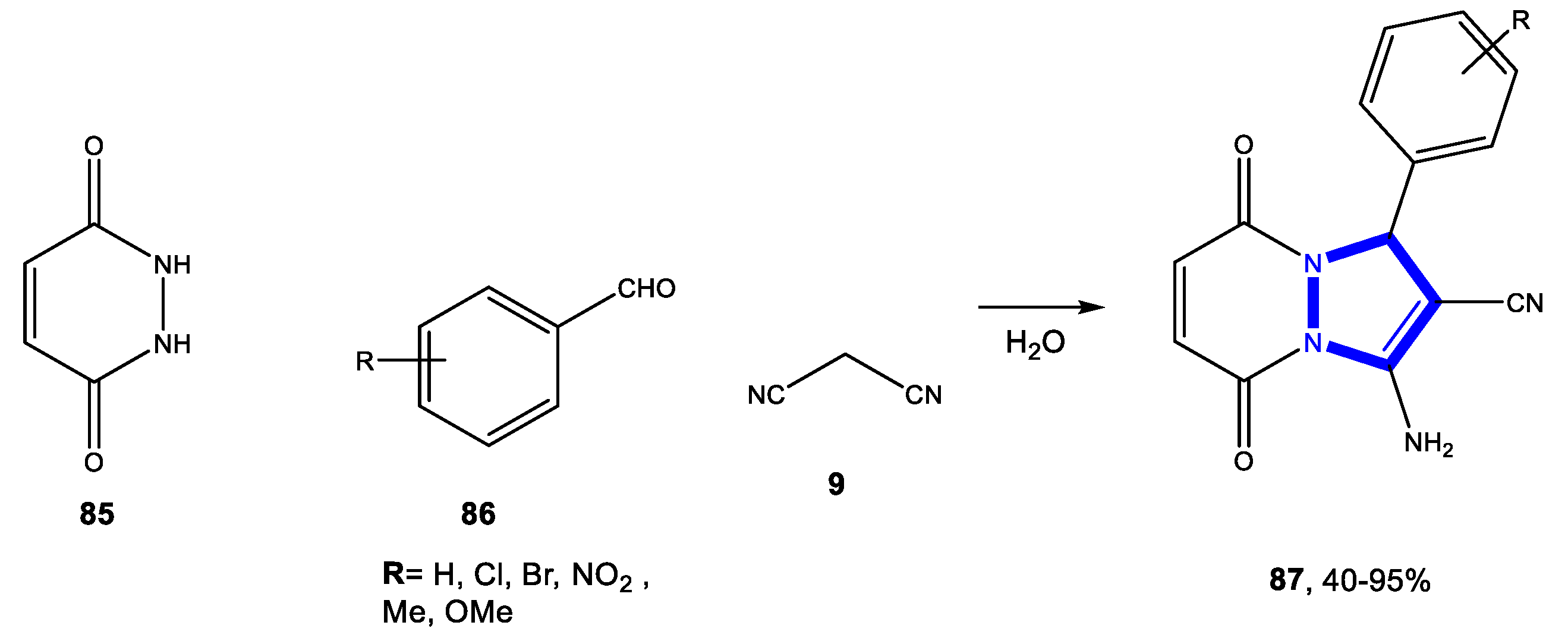
3.4.2. Reactions in PEG
3.5. Formation of the Tetrazole Ring
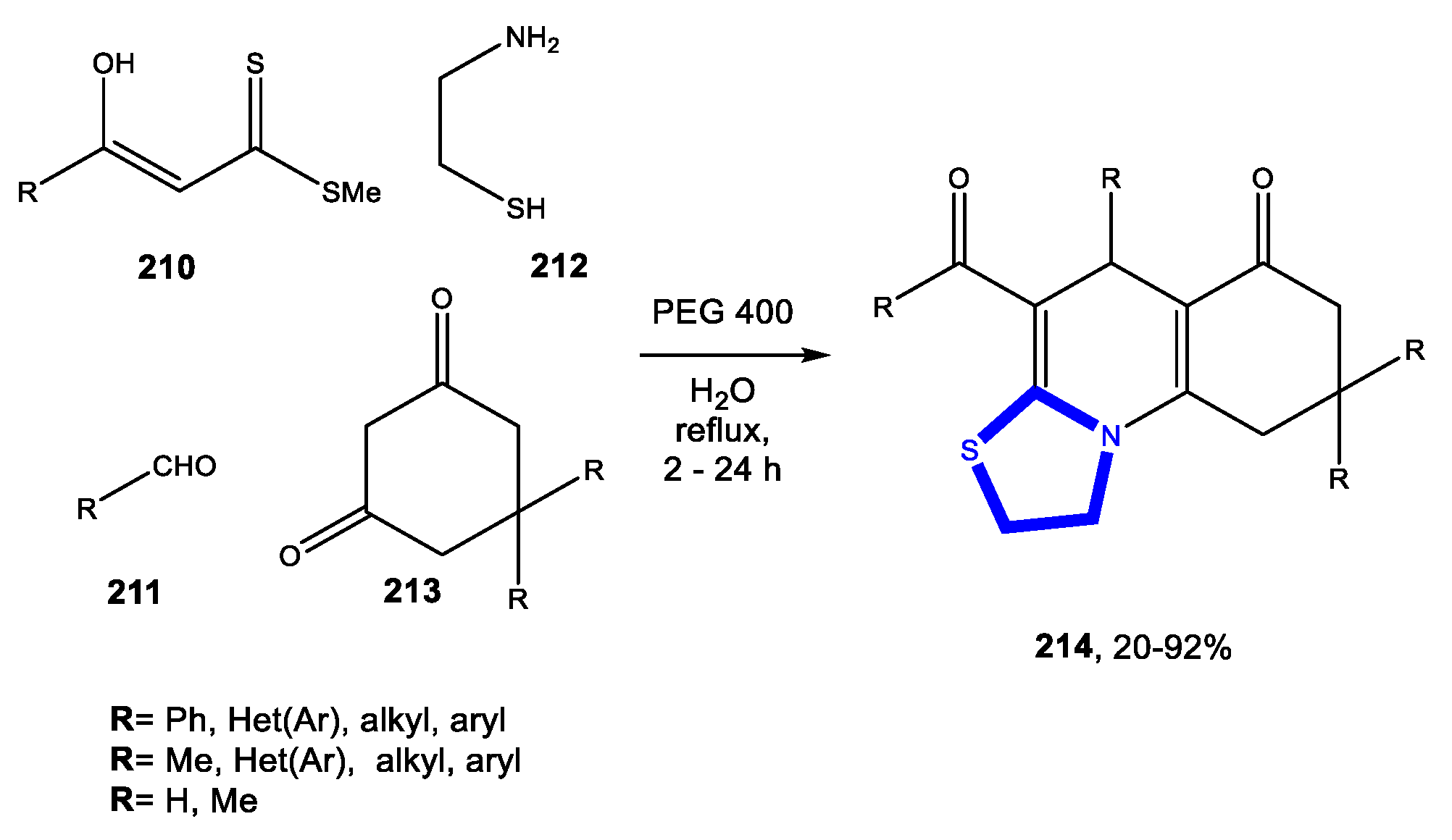
Reactions in Water
3.6. Formation of the Isothiazole Ring
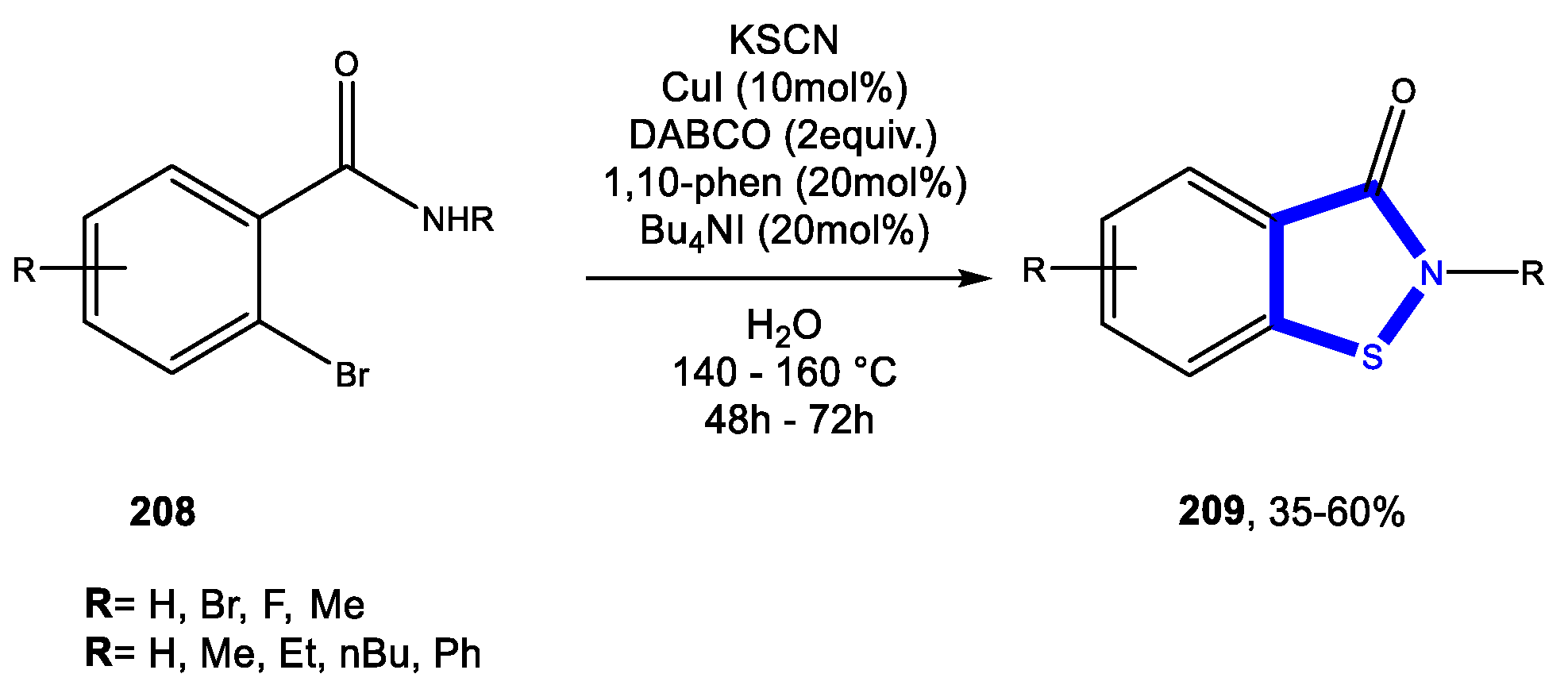
Reactions in Water
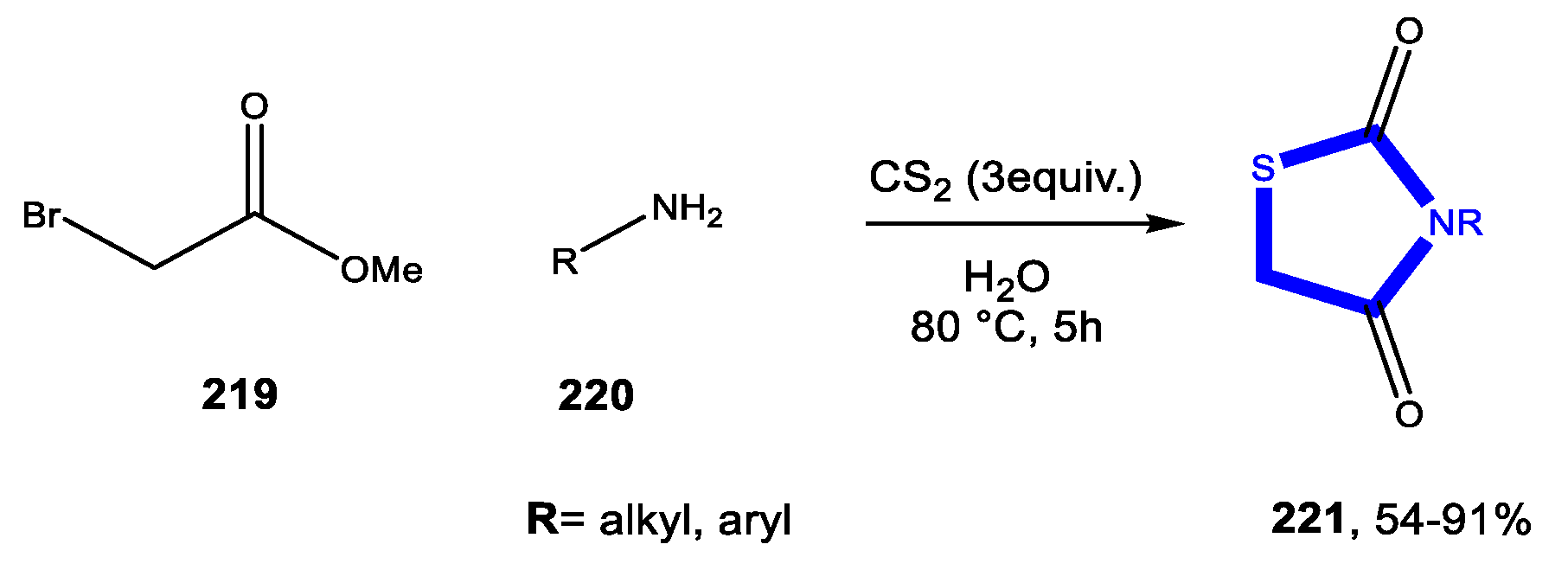
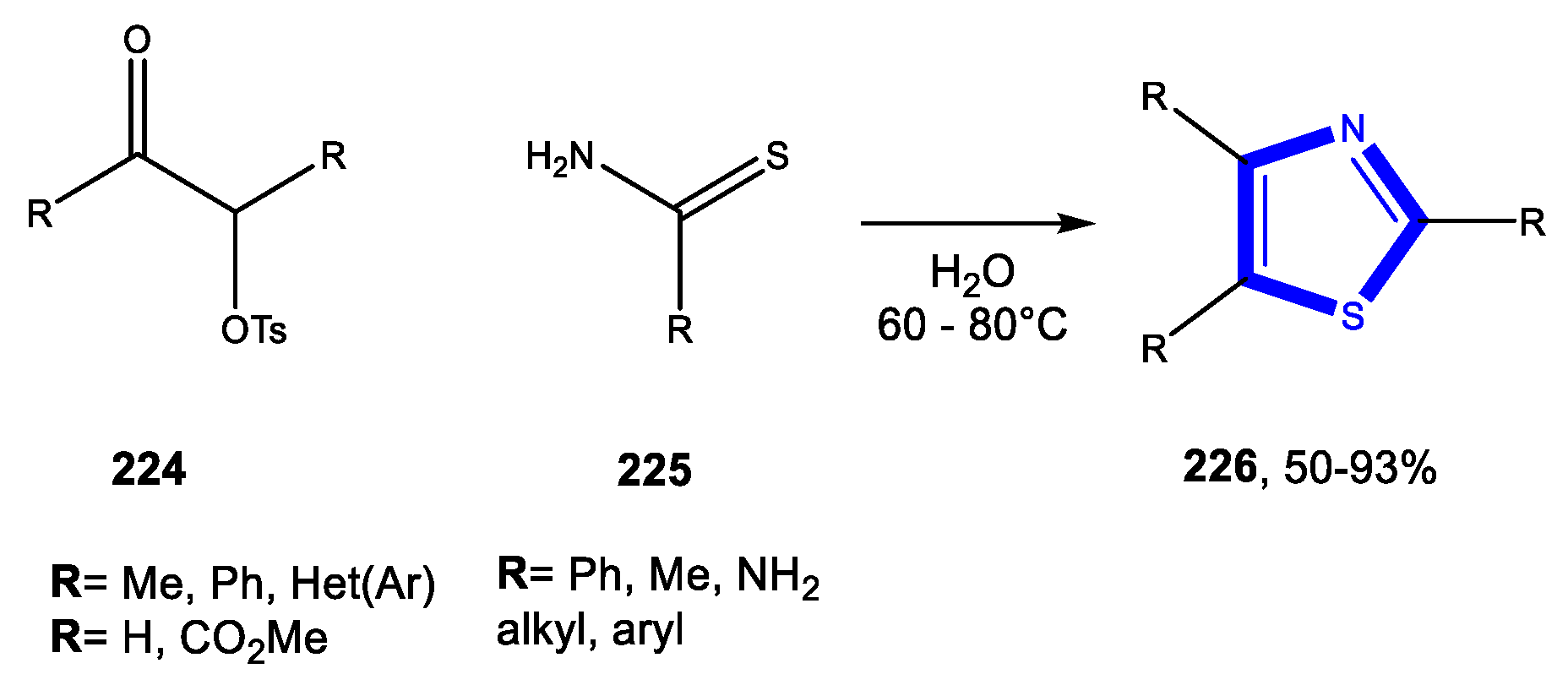
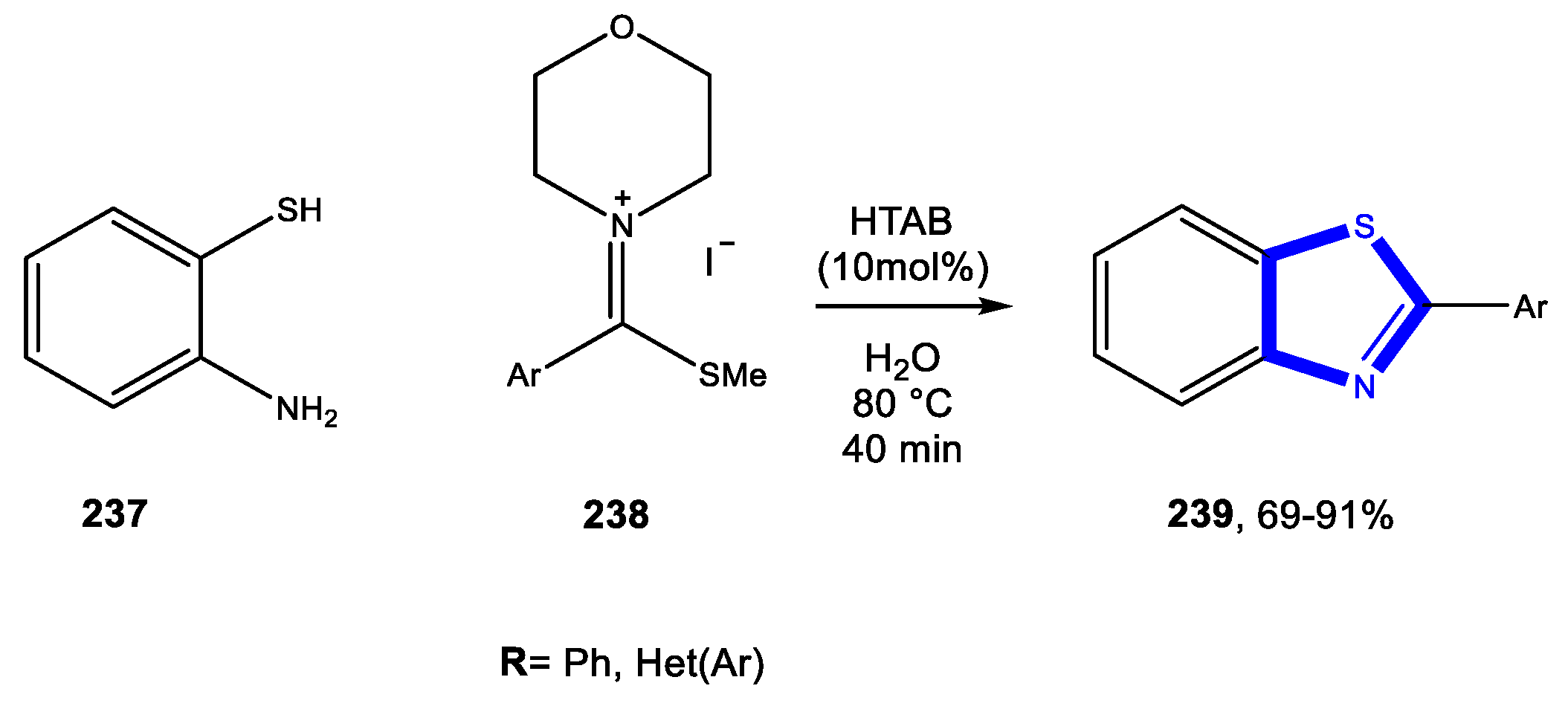
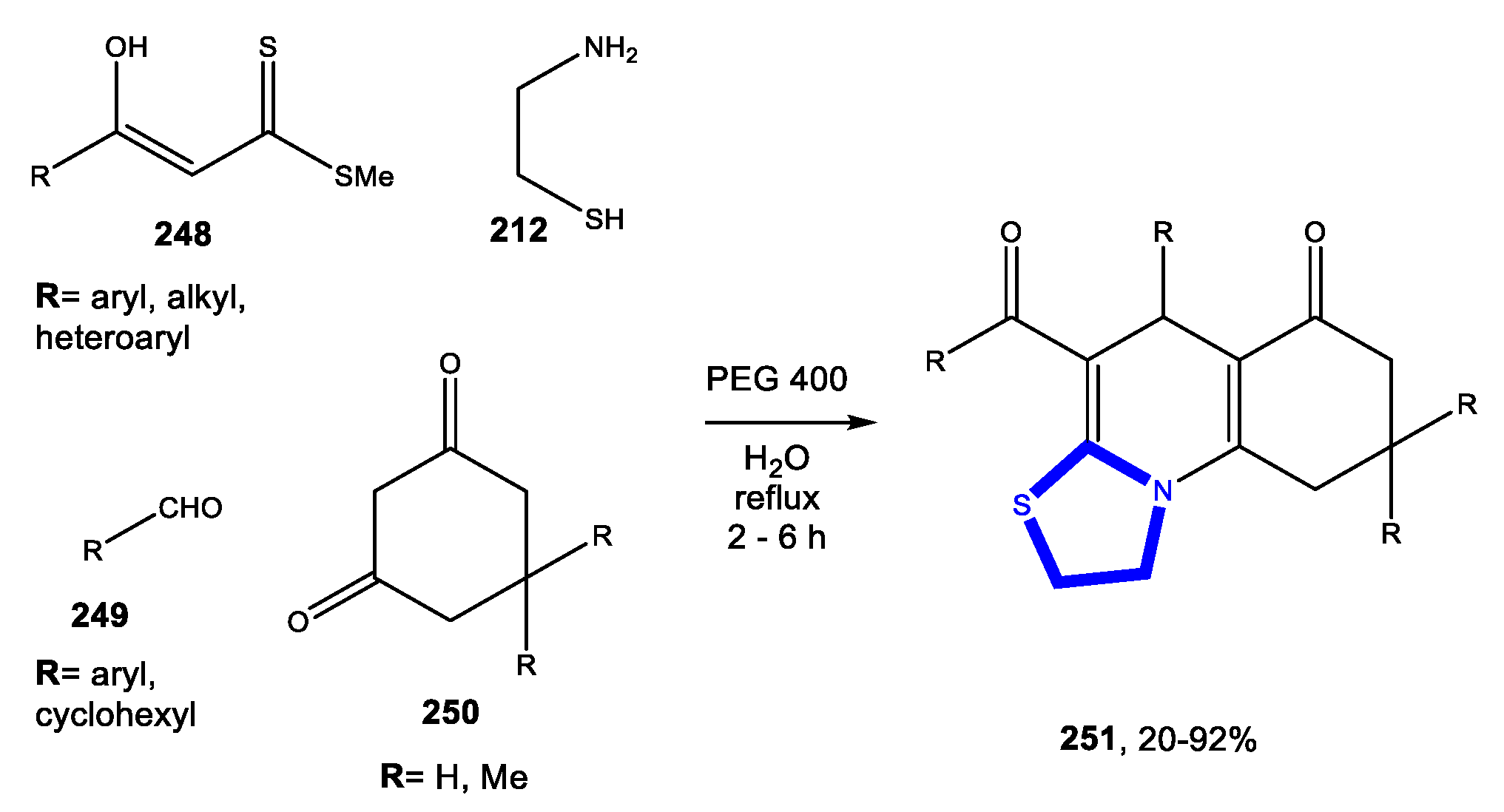
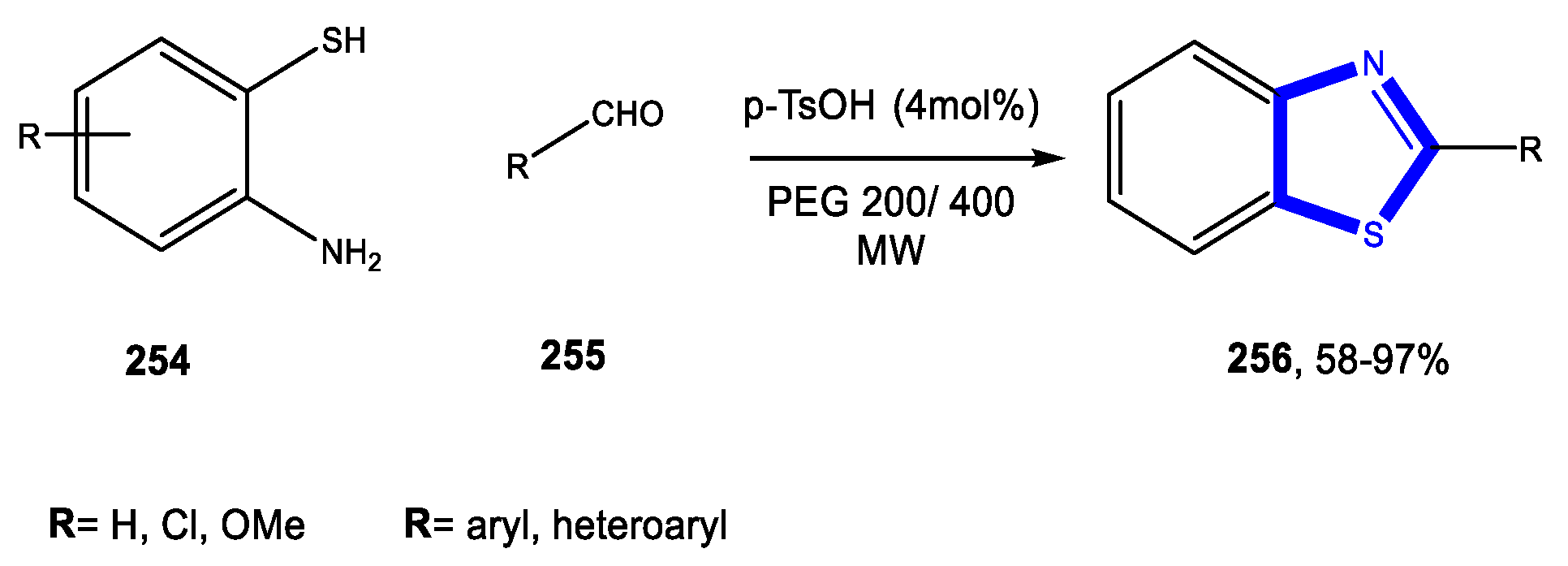
3.7. Formation of the Thiazole Ring
3.7.1. Reactions in Water
3.7.2. Reactions in PEG
3.7.3. Reactions in Bio-Based Solvents
3.8. Formation of the Thiadiazole Ring
Reactions in Water
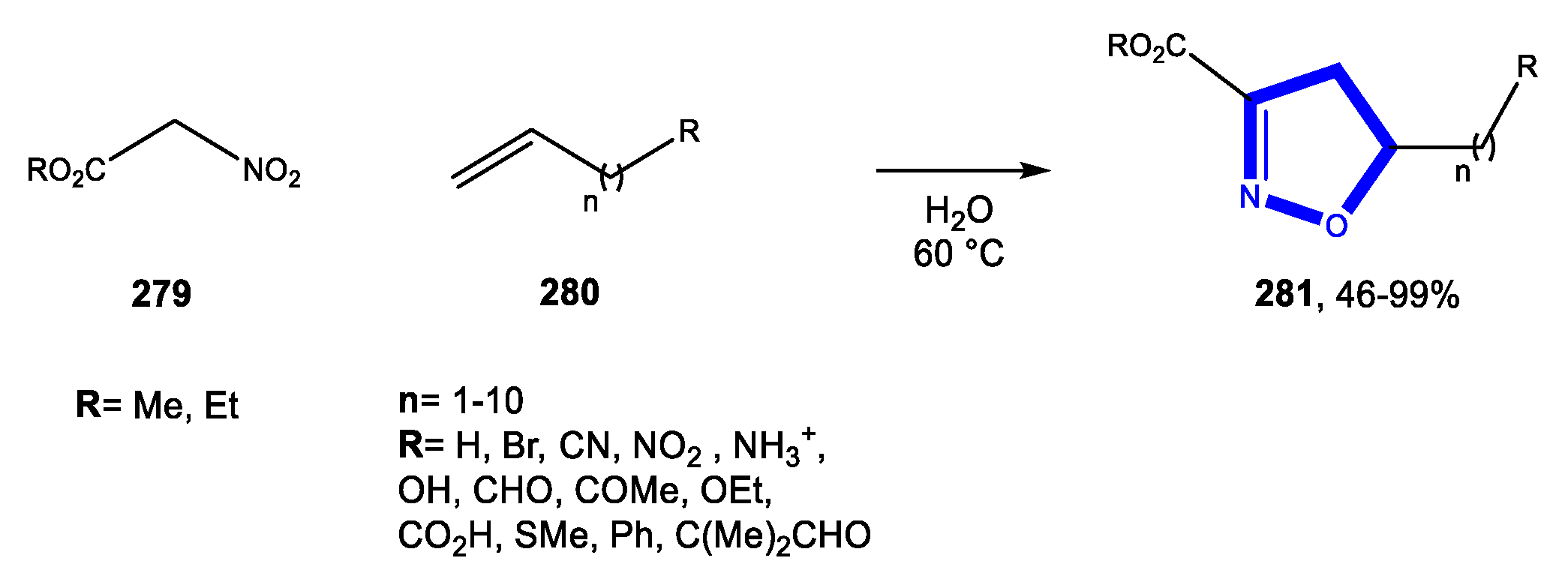
3.9. Formation of the Isoxazole Ring
3.9.1. Reactions in Water
3.9.2. Reactions in PEG
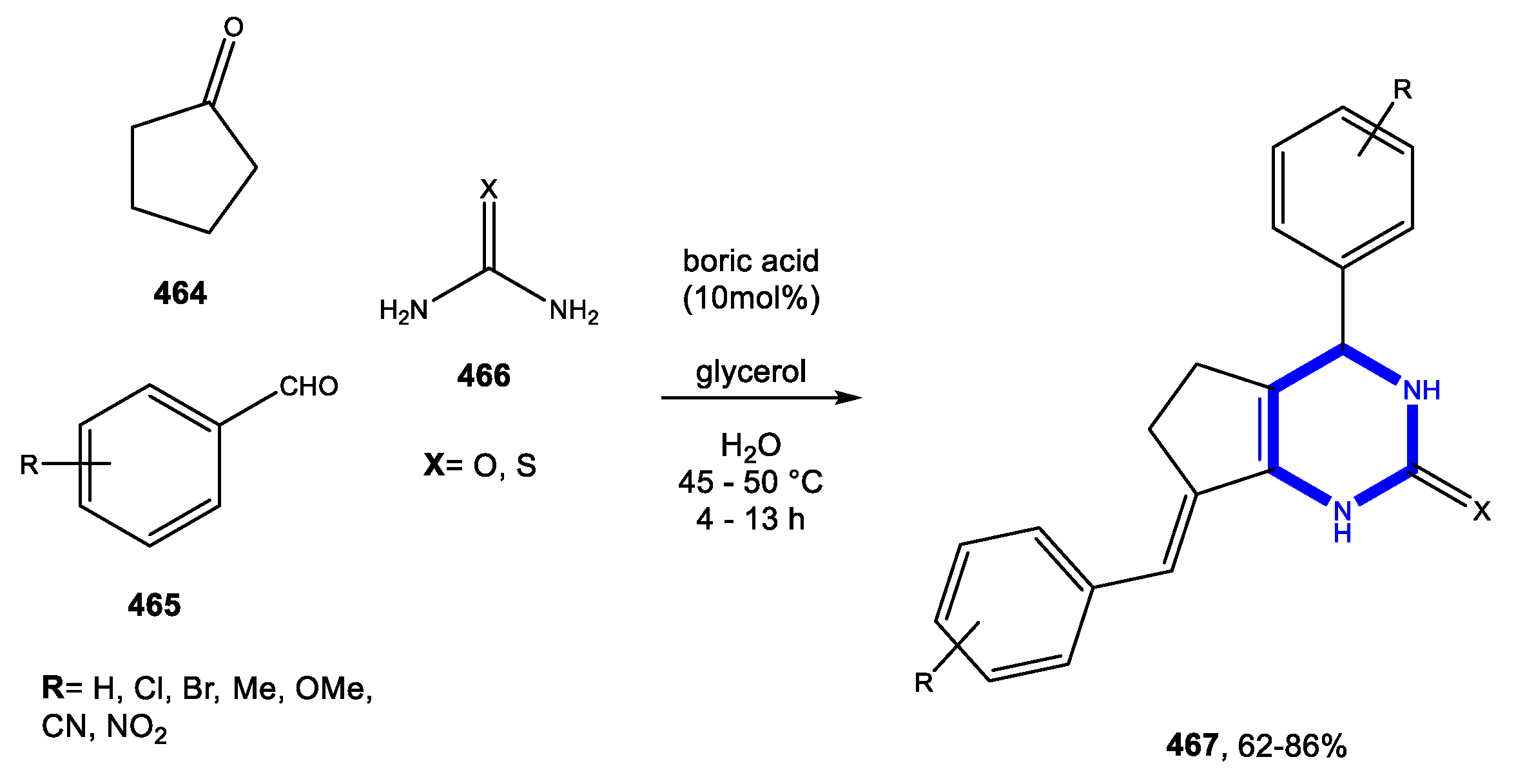
3.9.3. Reactions in Bio-Based Solvents
3.10. Formation of the Oxazole Ring
3.10.1. Reactions in Water
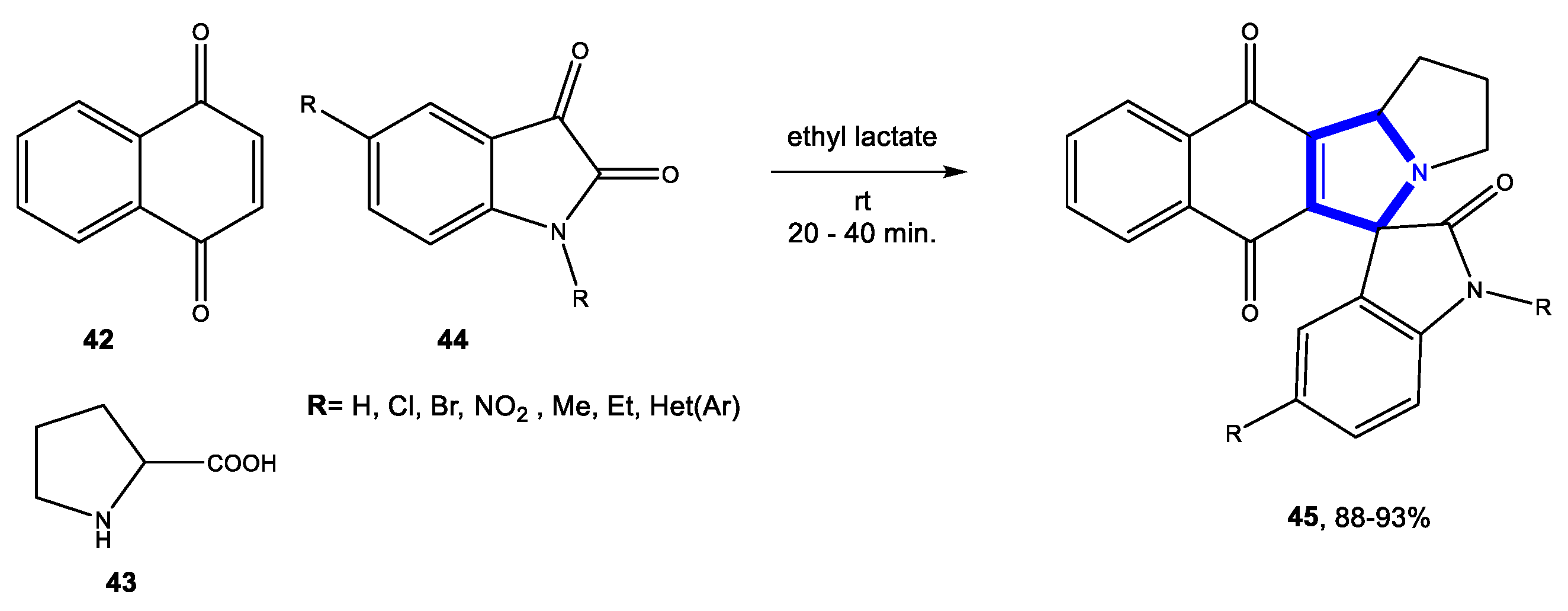
3.10.2. Reactions in Bio-Based Solvents
4. Six-Membered Aromatic Nitrogen Heterocycles
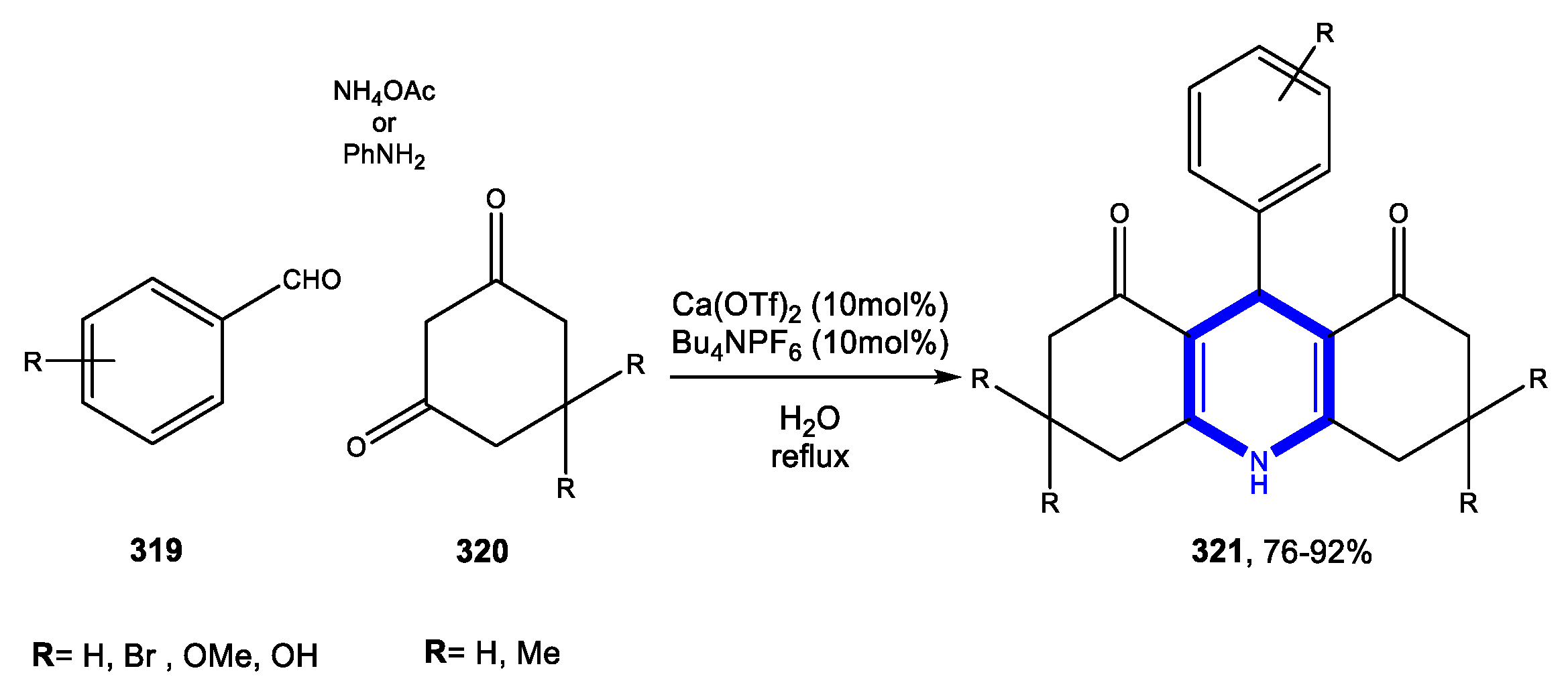
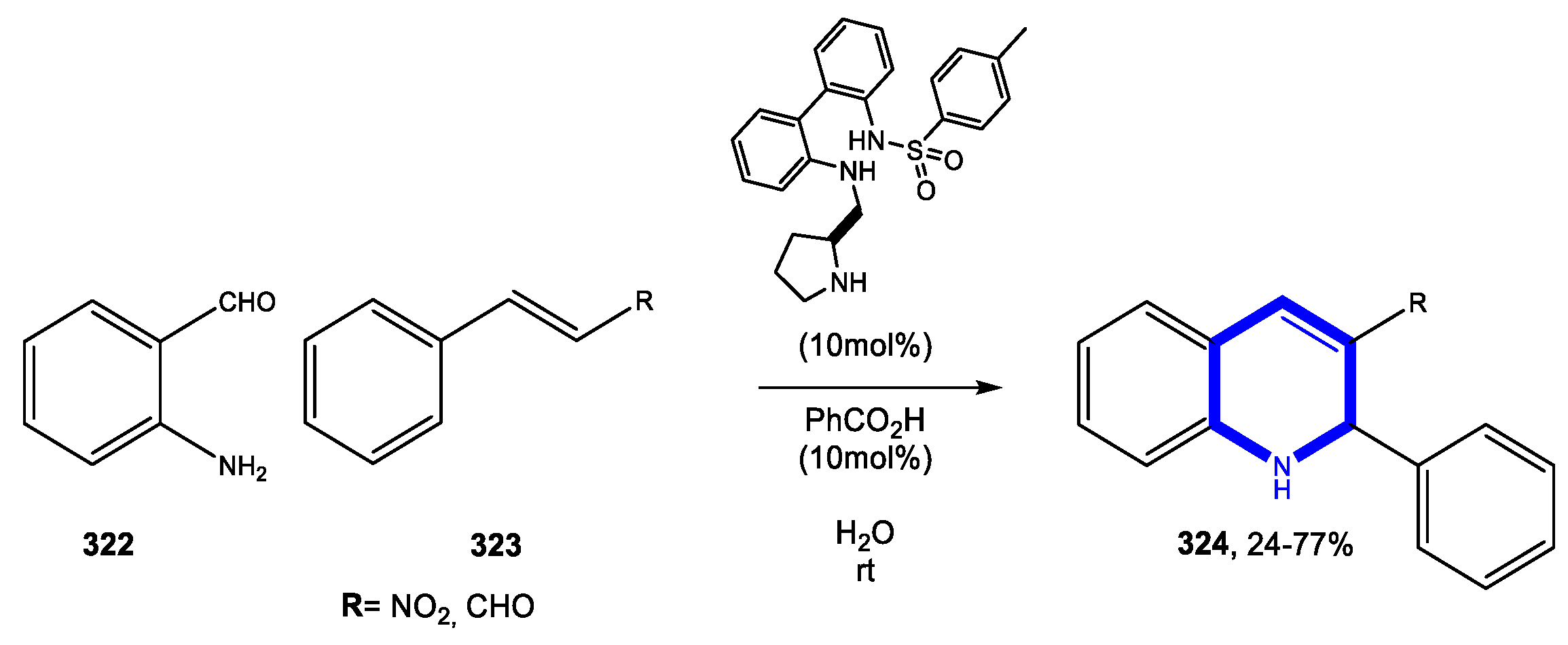
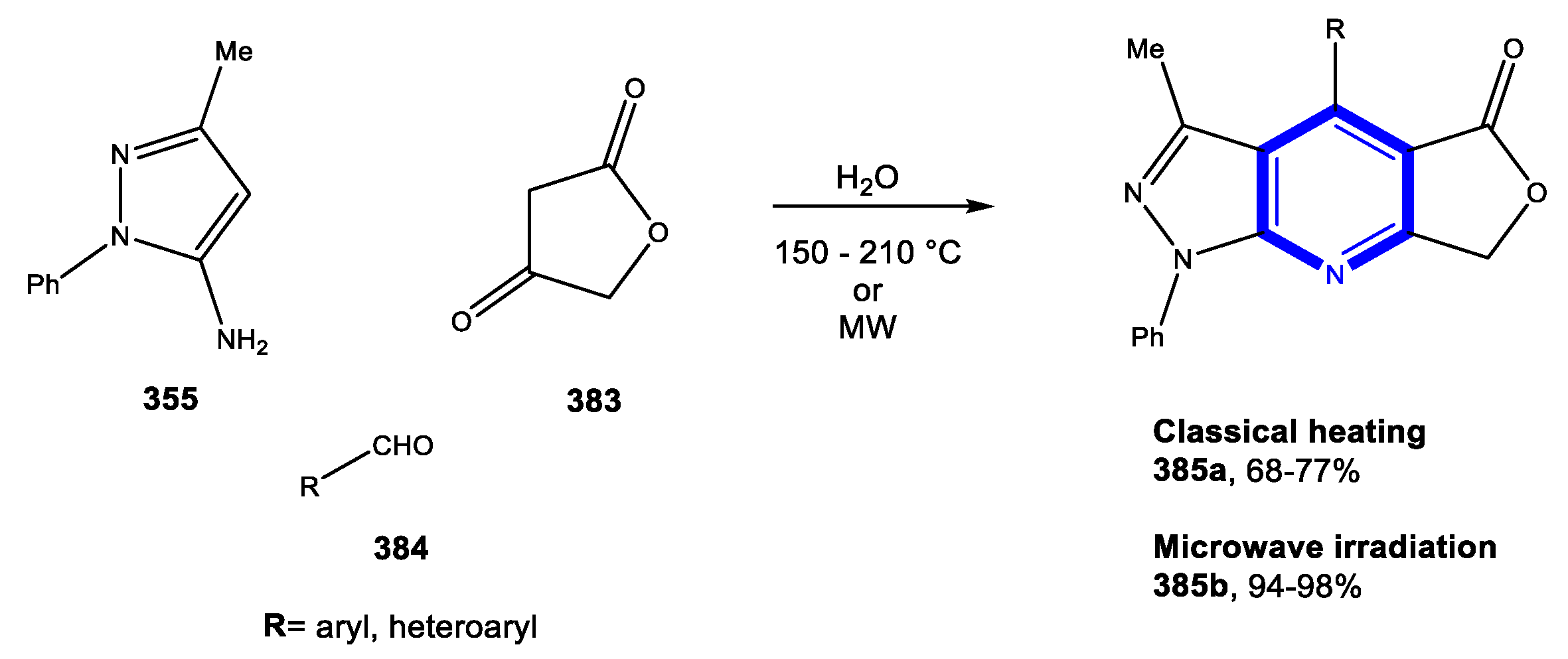
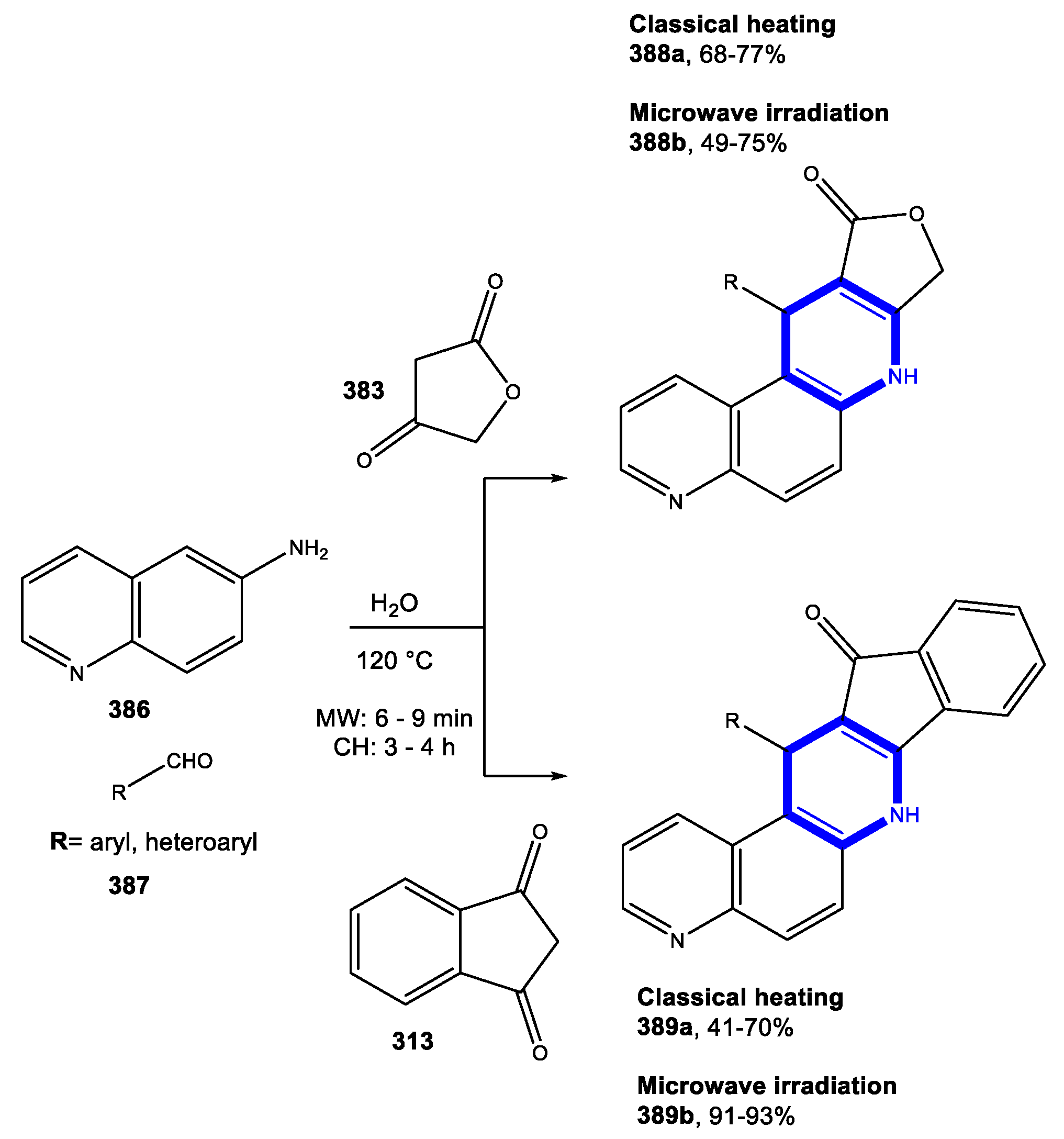
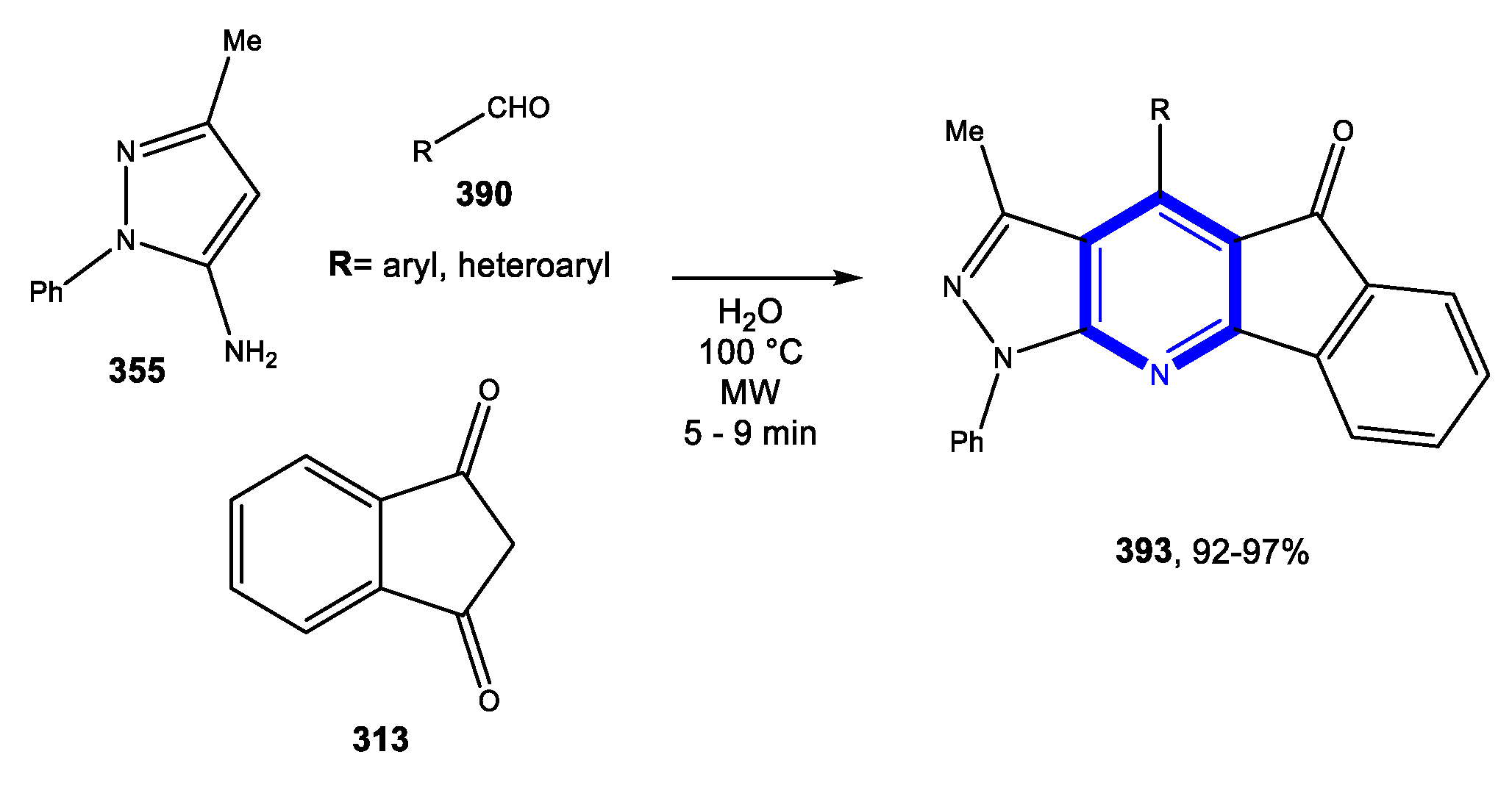
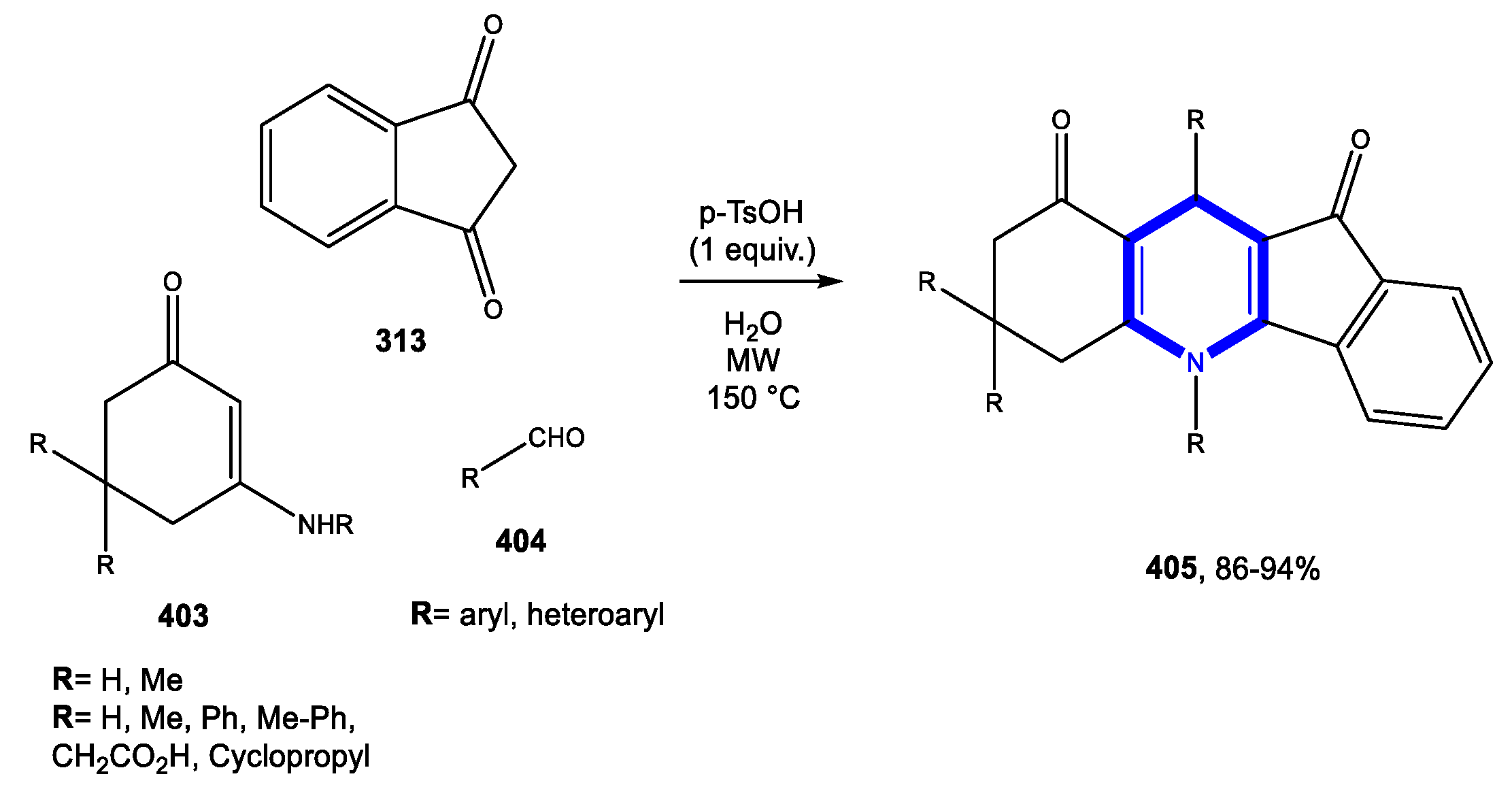
4.1. Formation of the Pyridine Ring
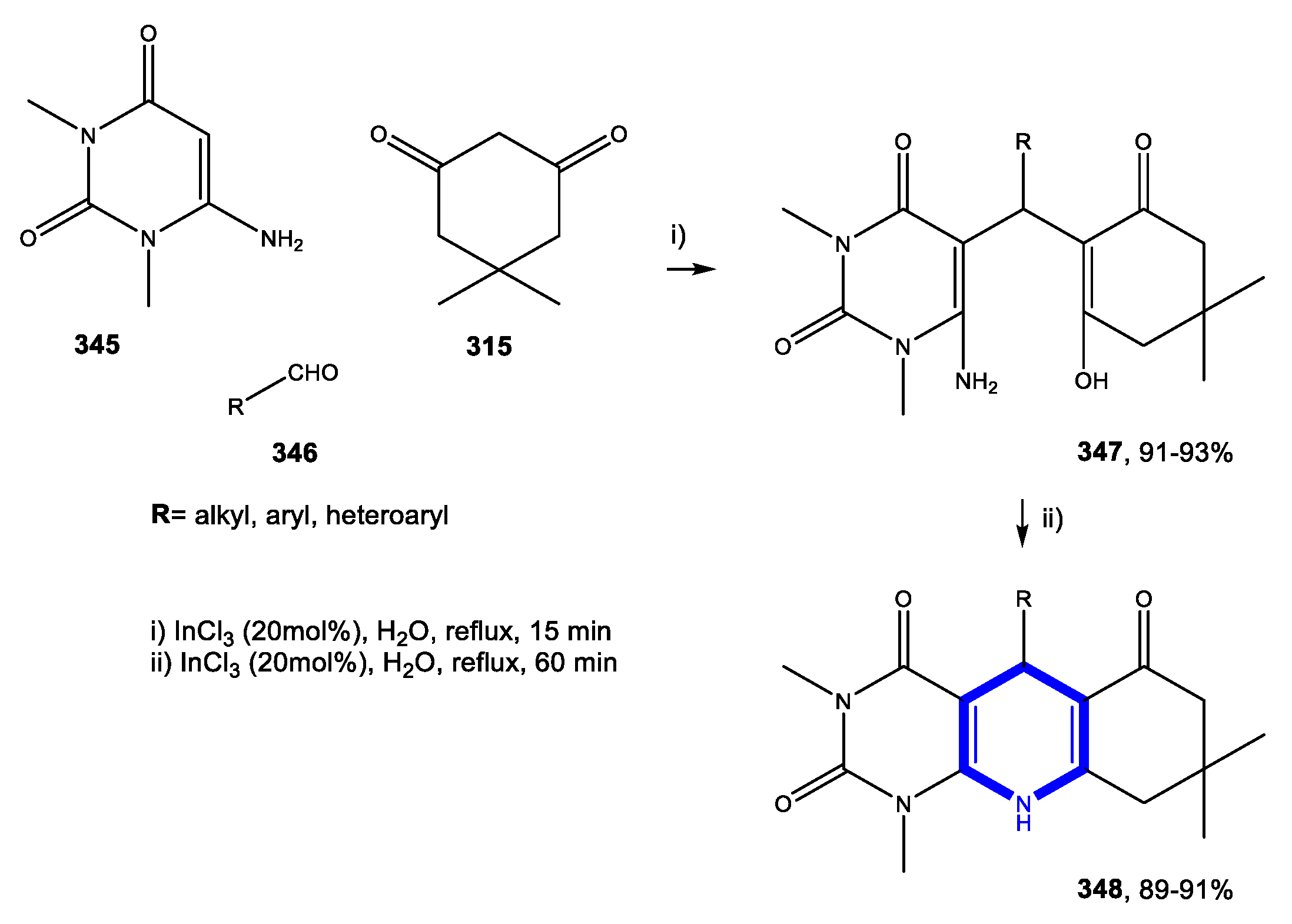
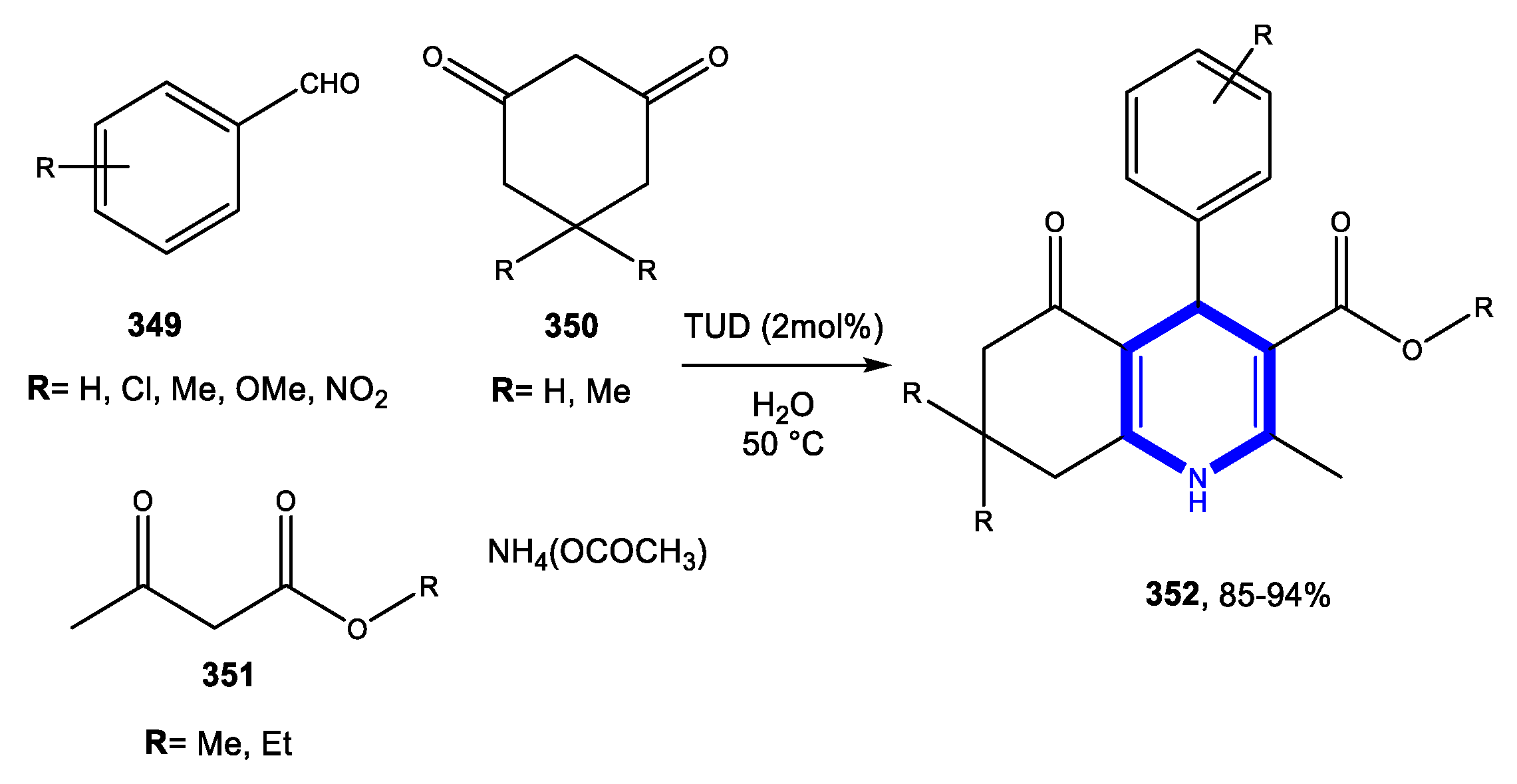
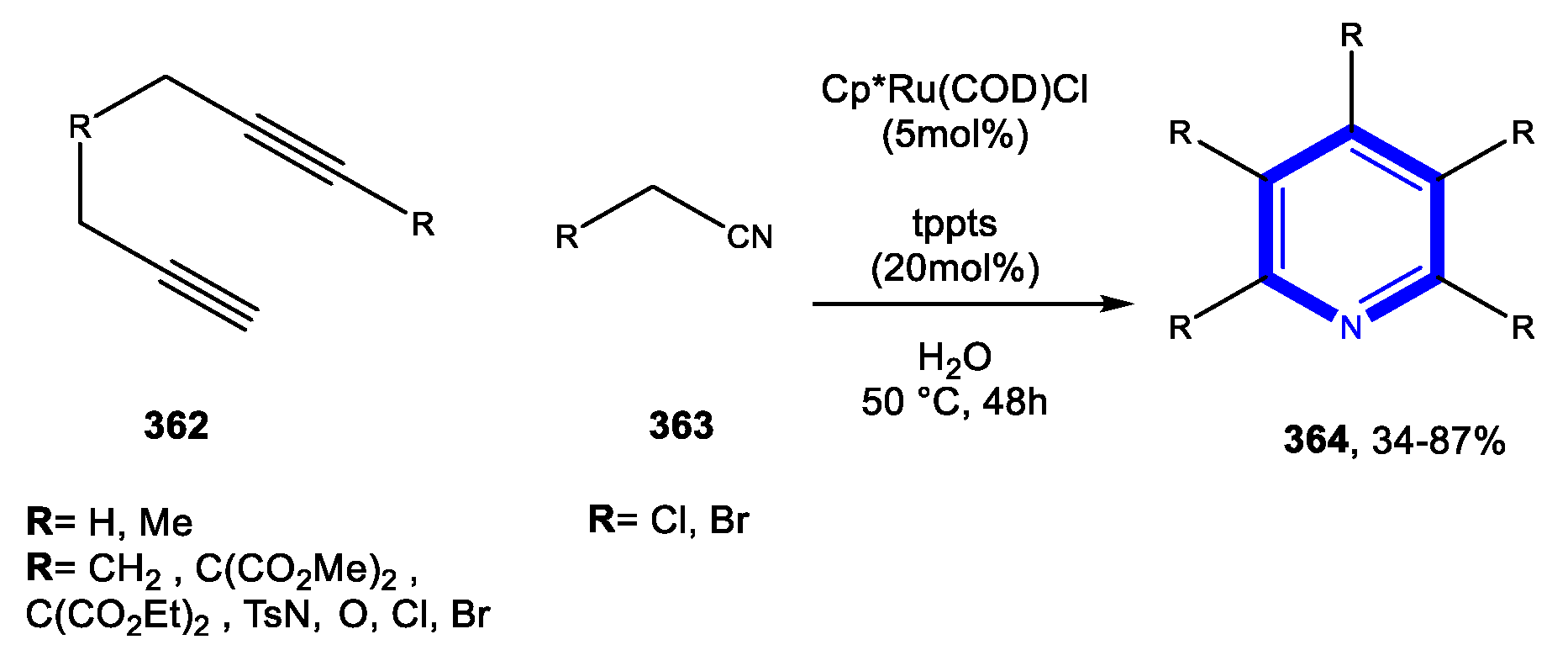
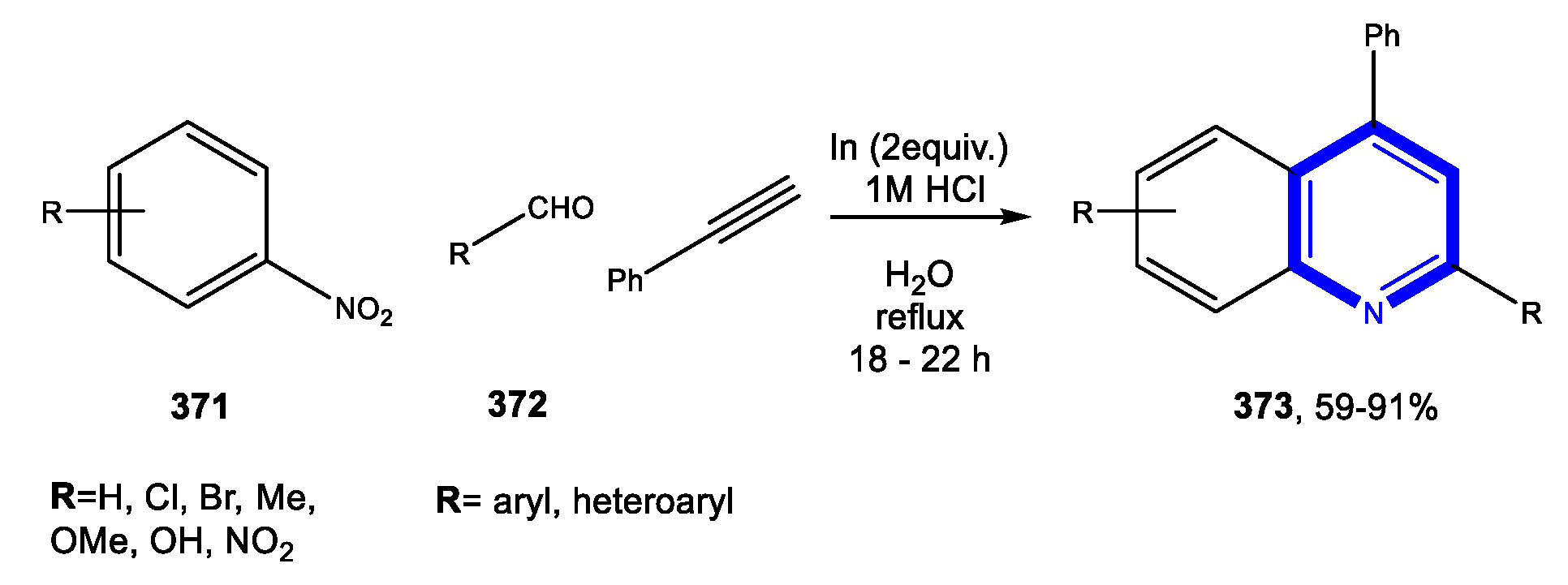
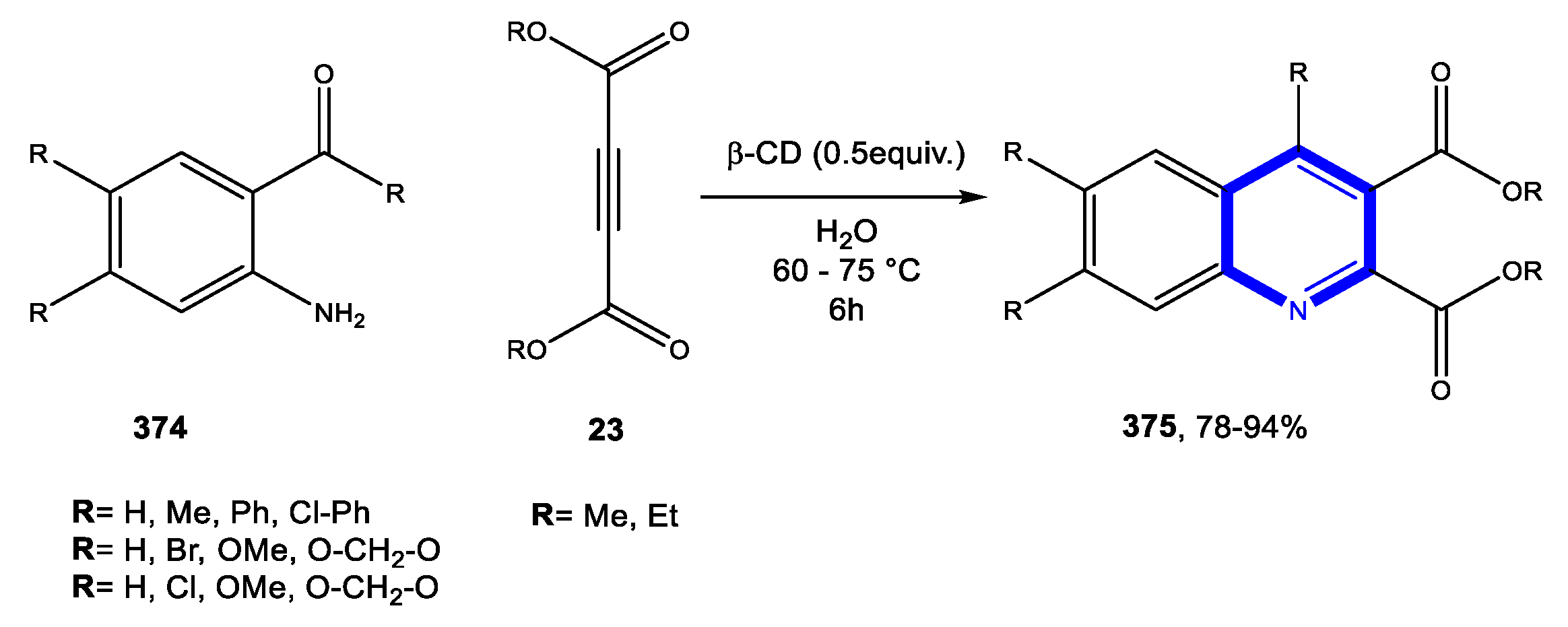
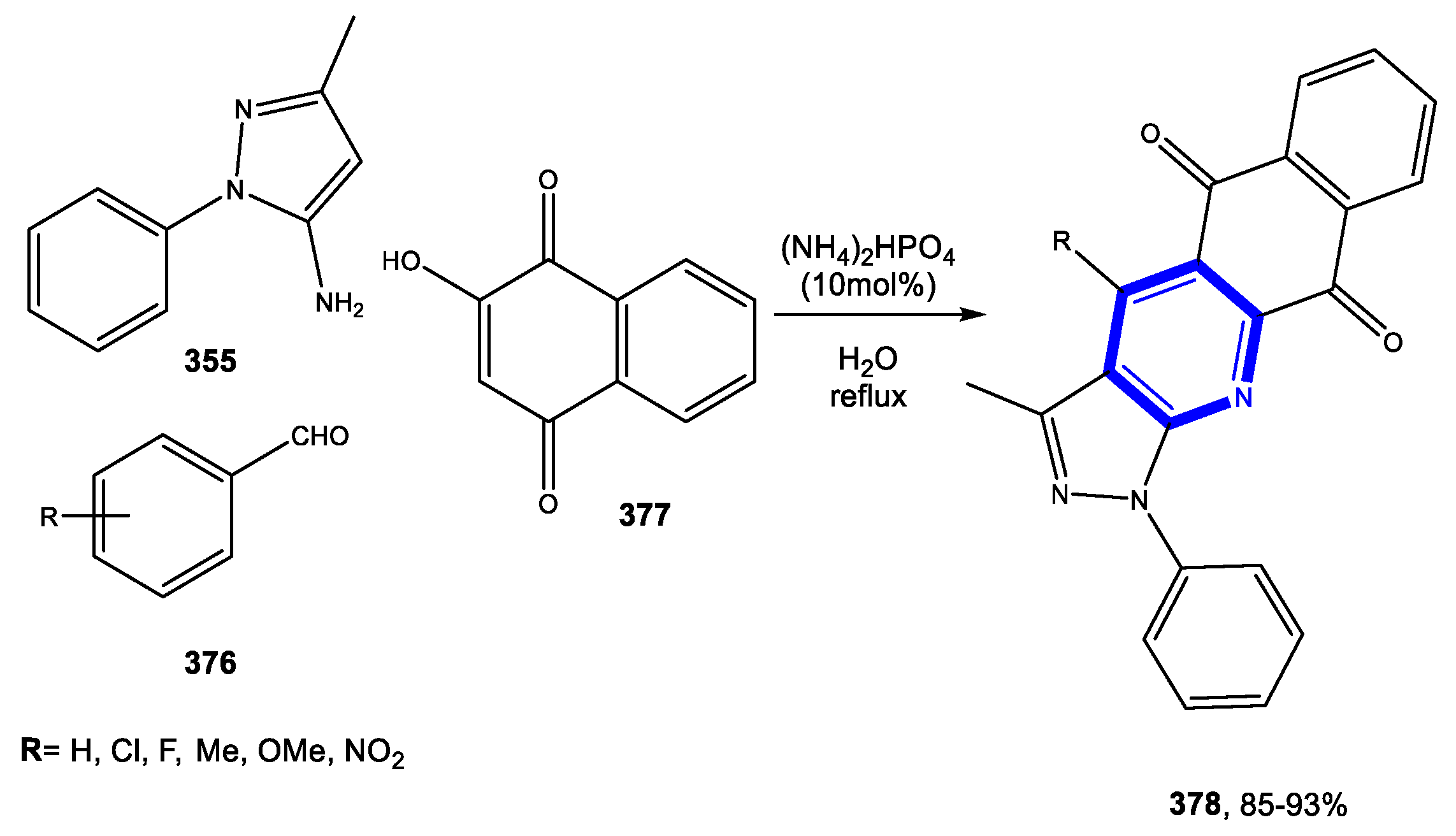
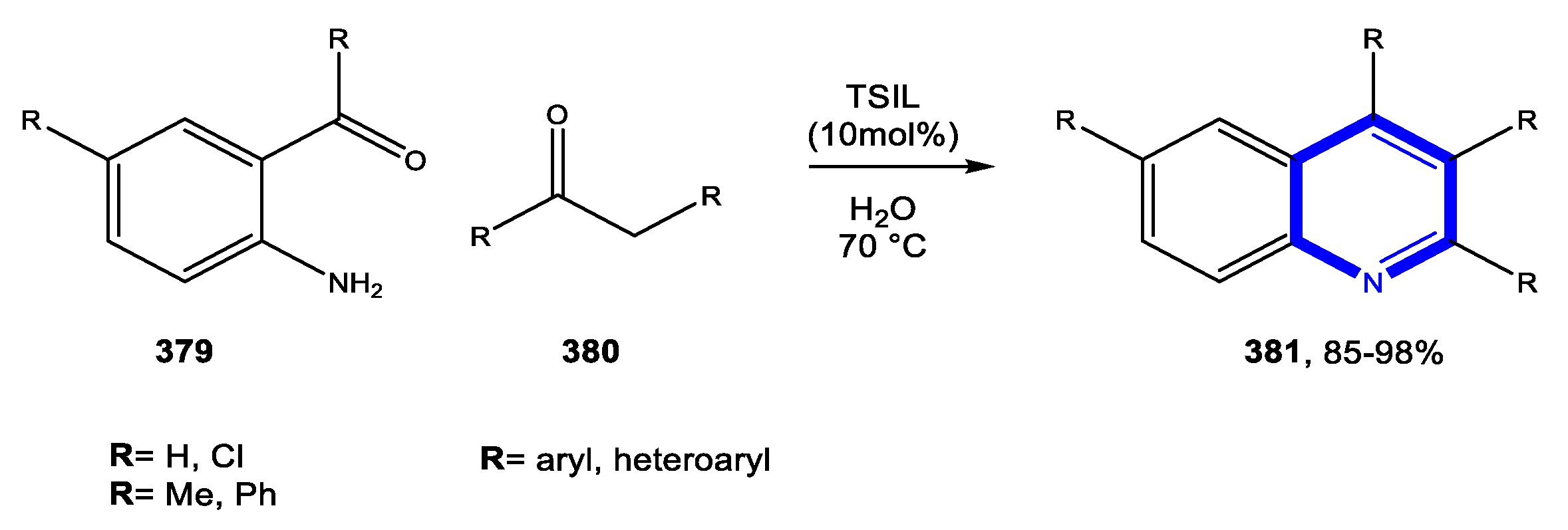
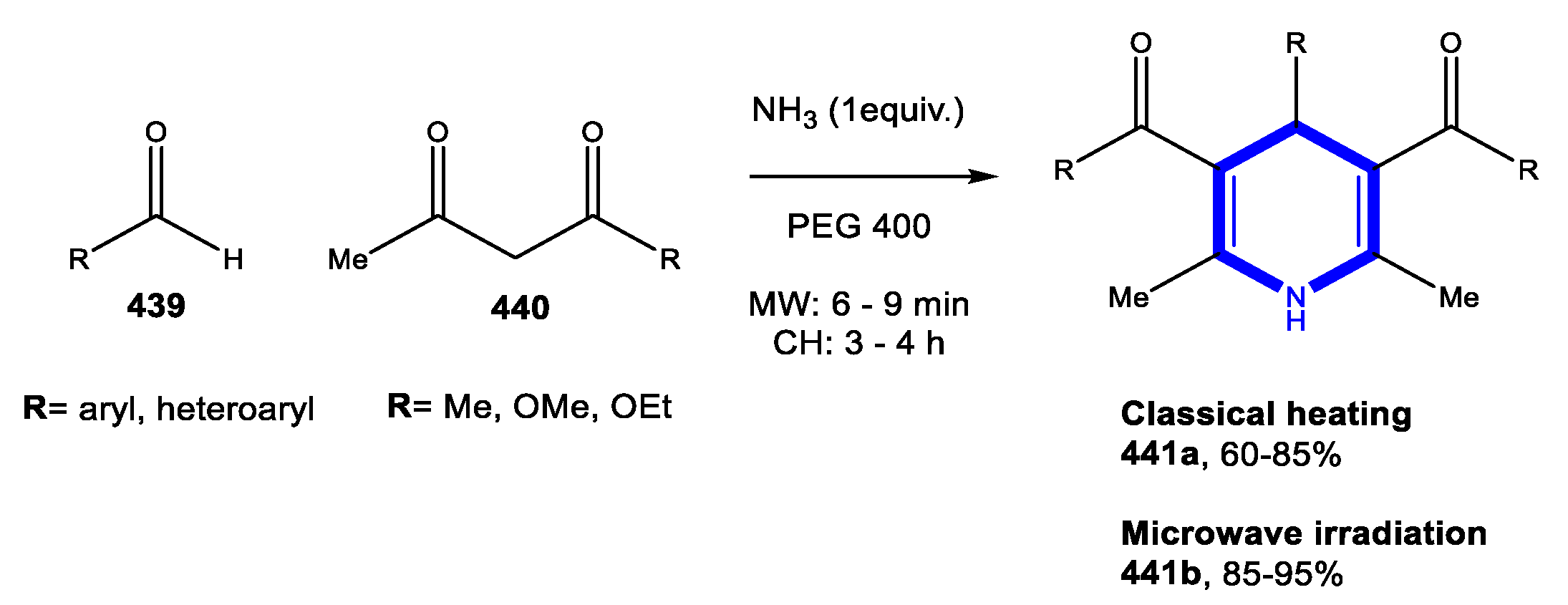
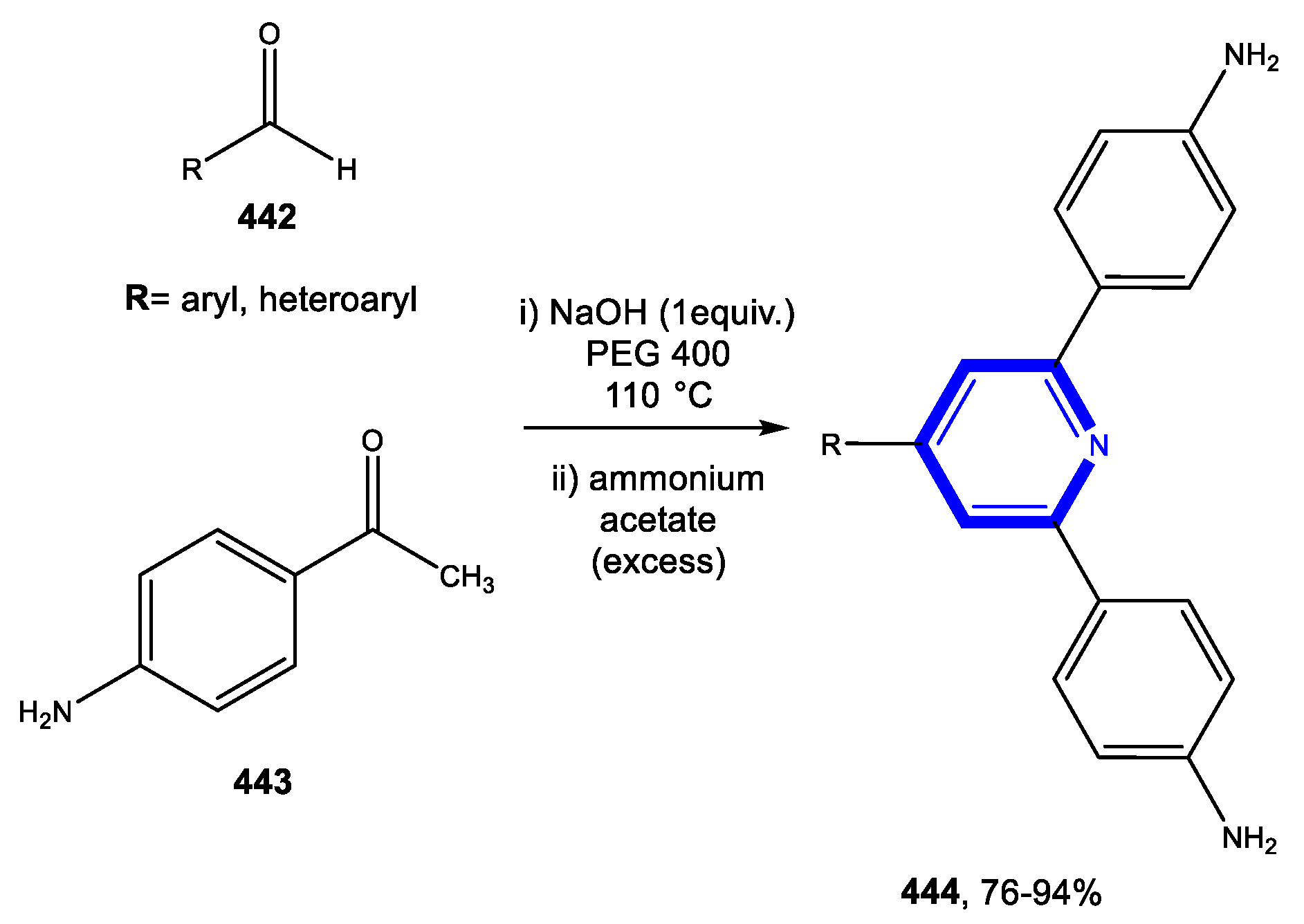
4.1.1. Reactions in Water
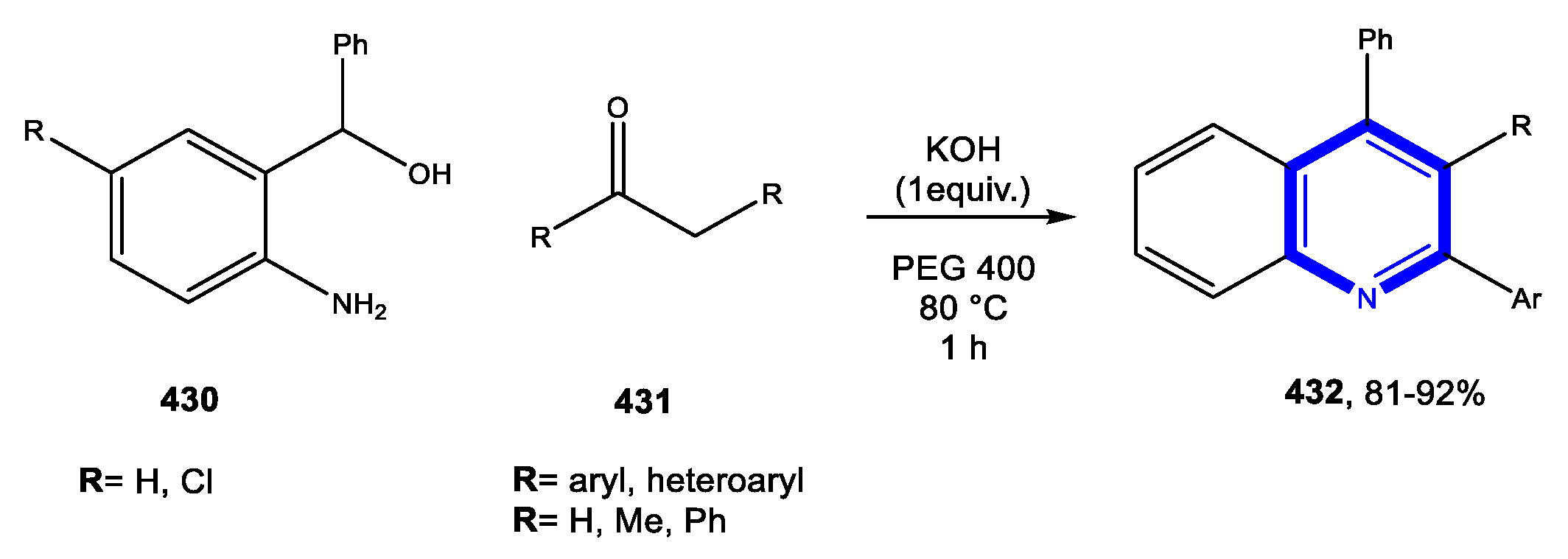
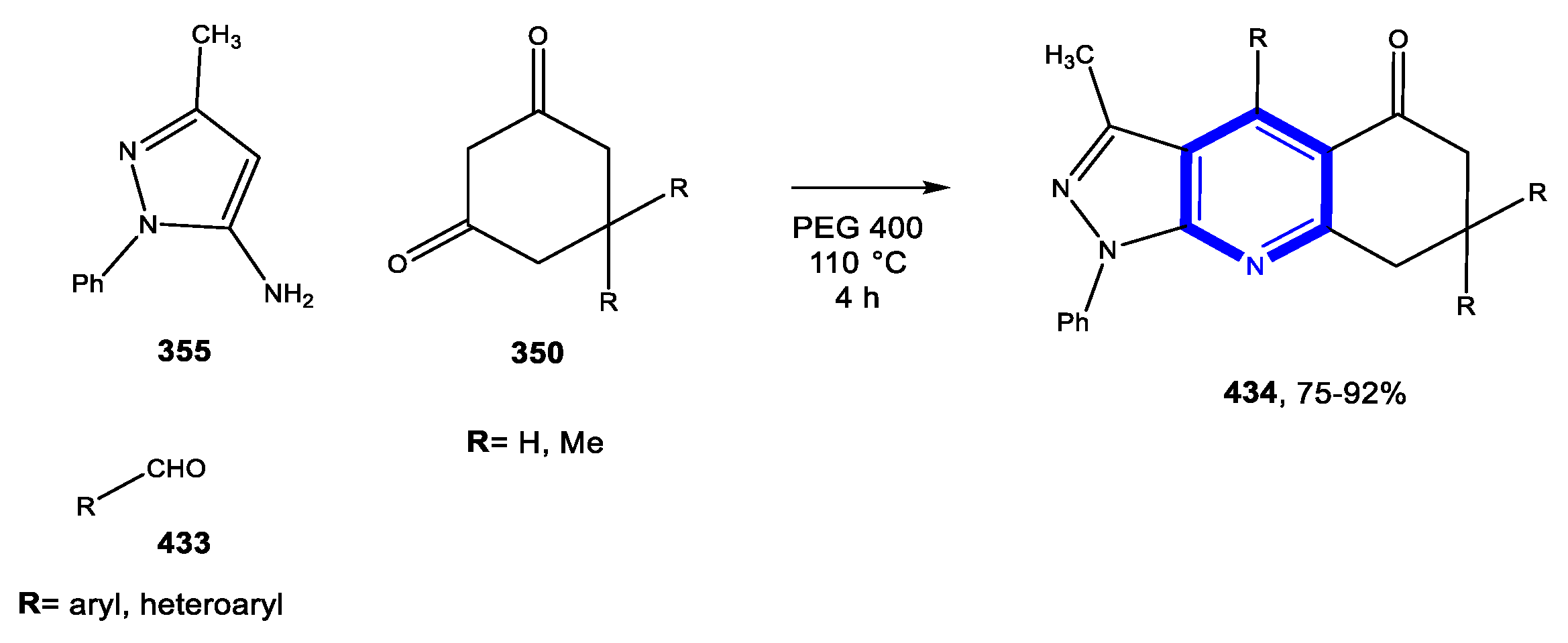
4.1.2. Reactions in PEG
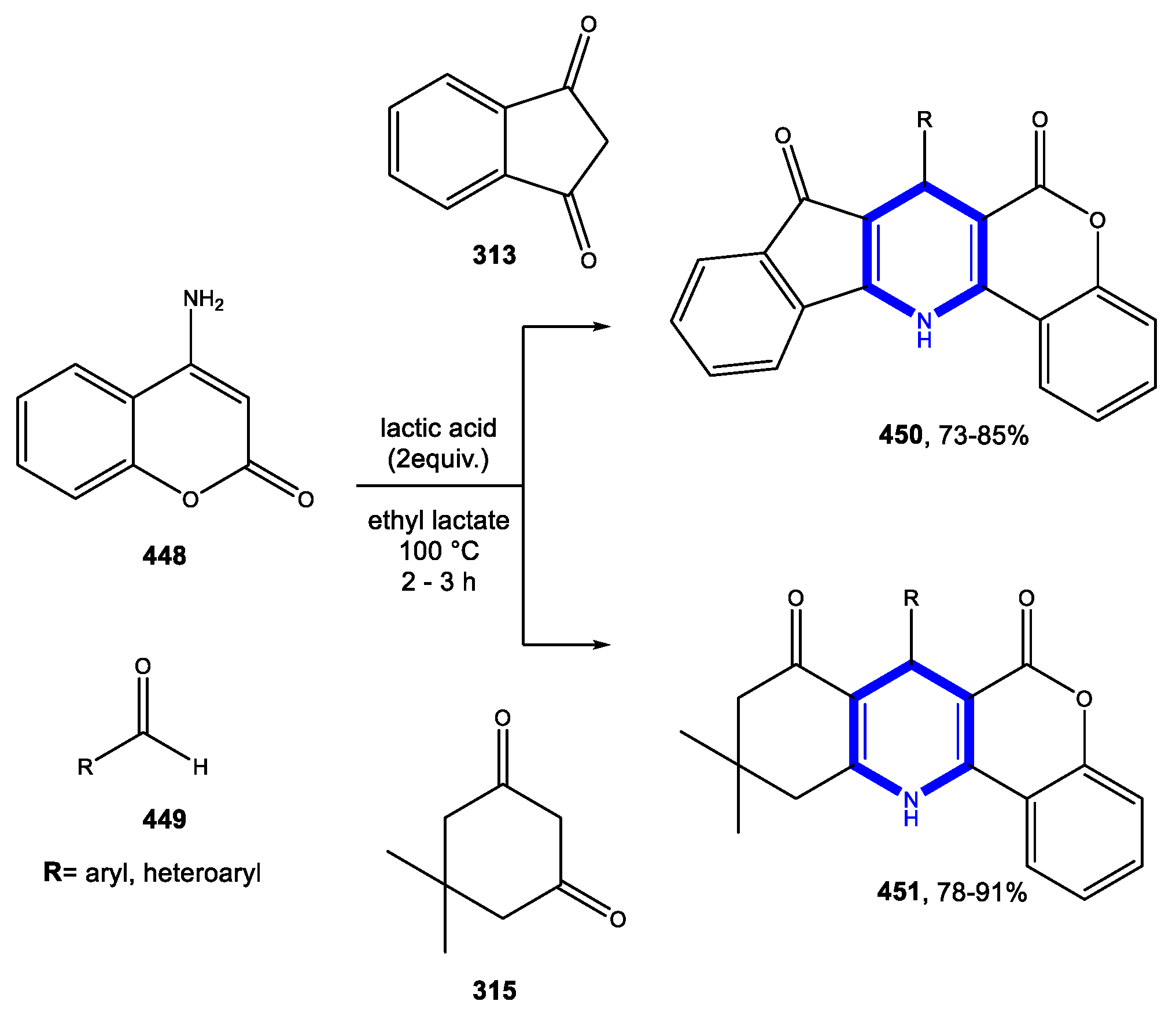
4.1.3. Reactions in Bio-Based Solvents
4.2. Formation of the Pyridazine Ring
Reactions in Water
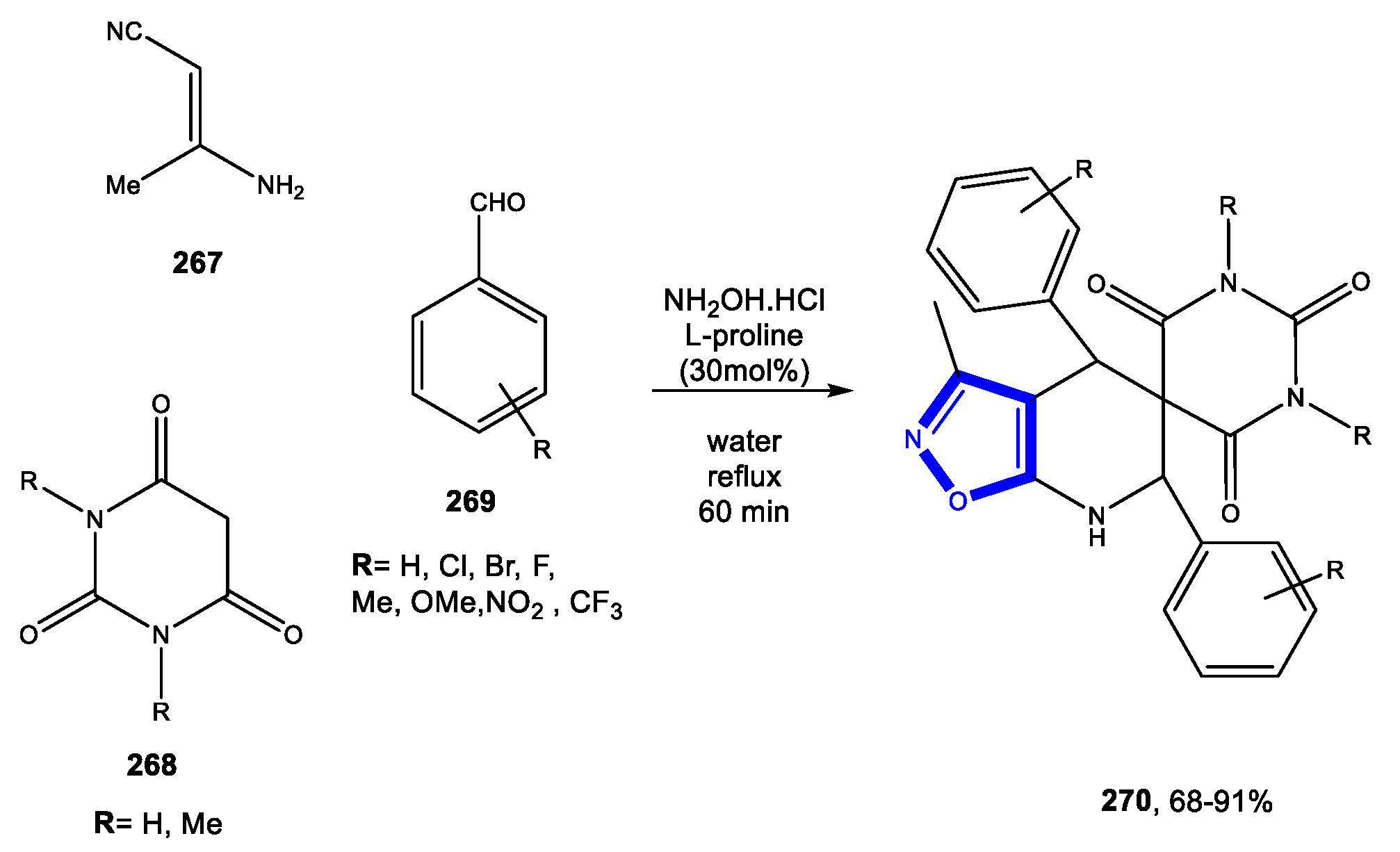
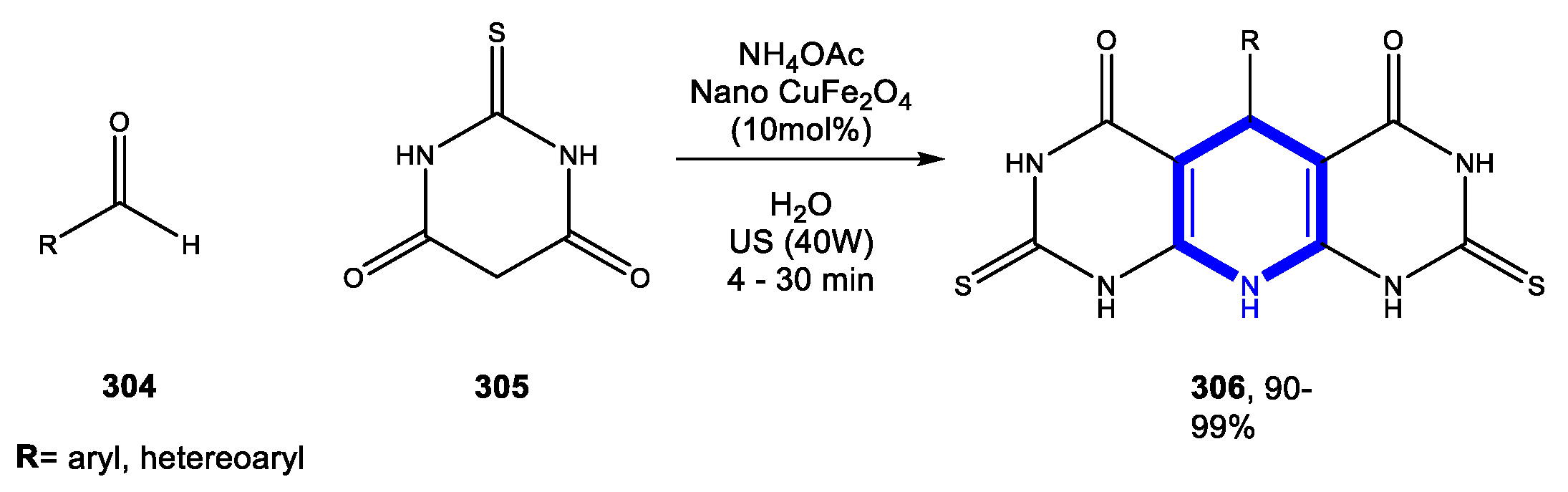
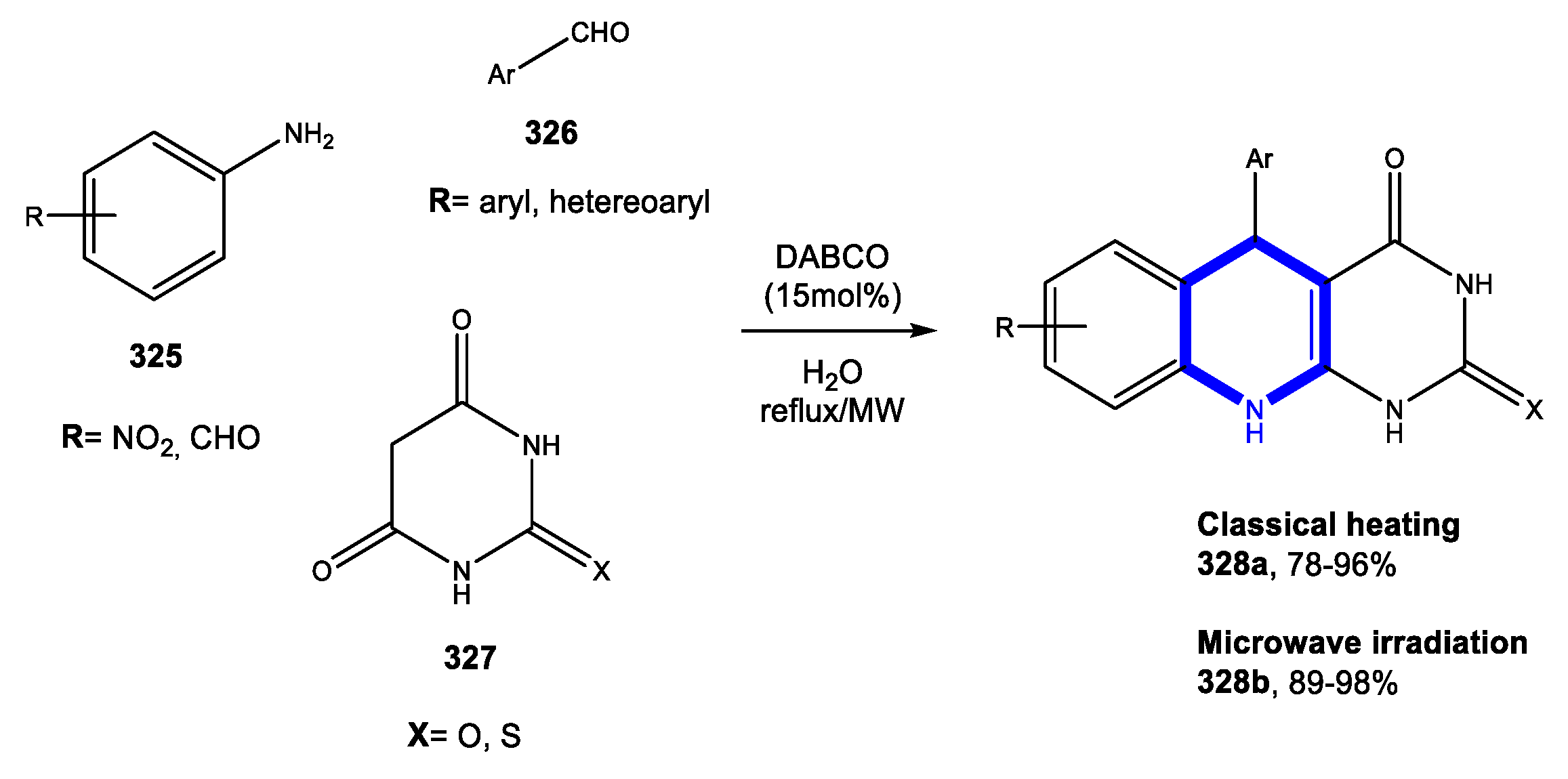
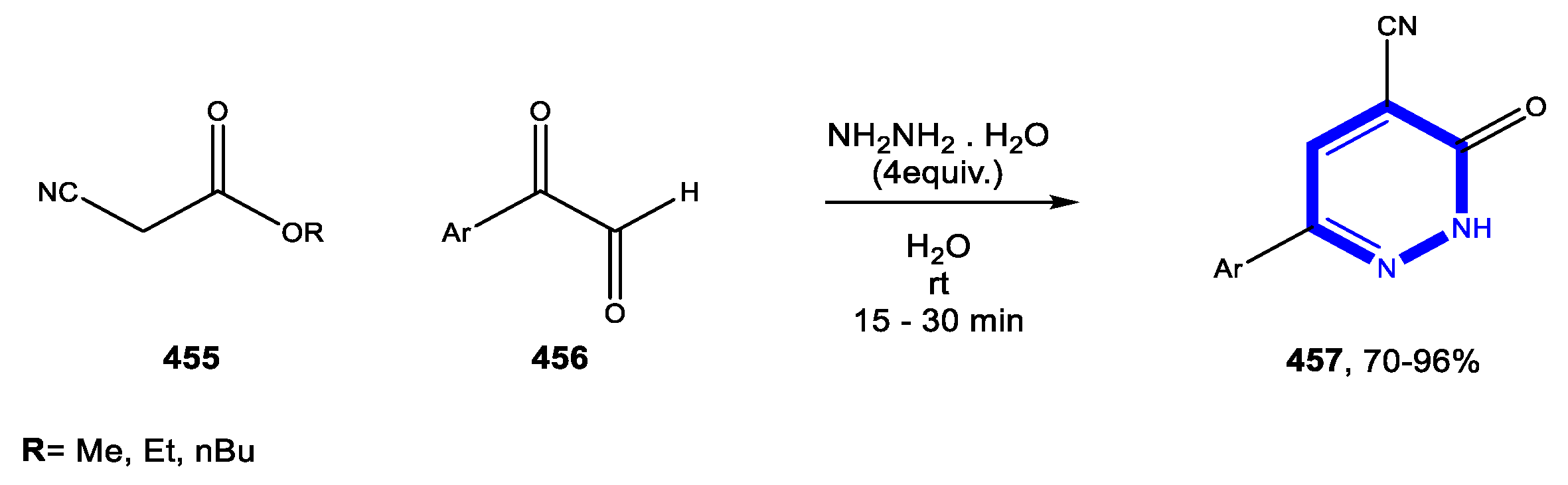
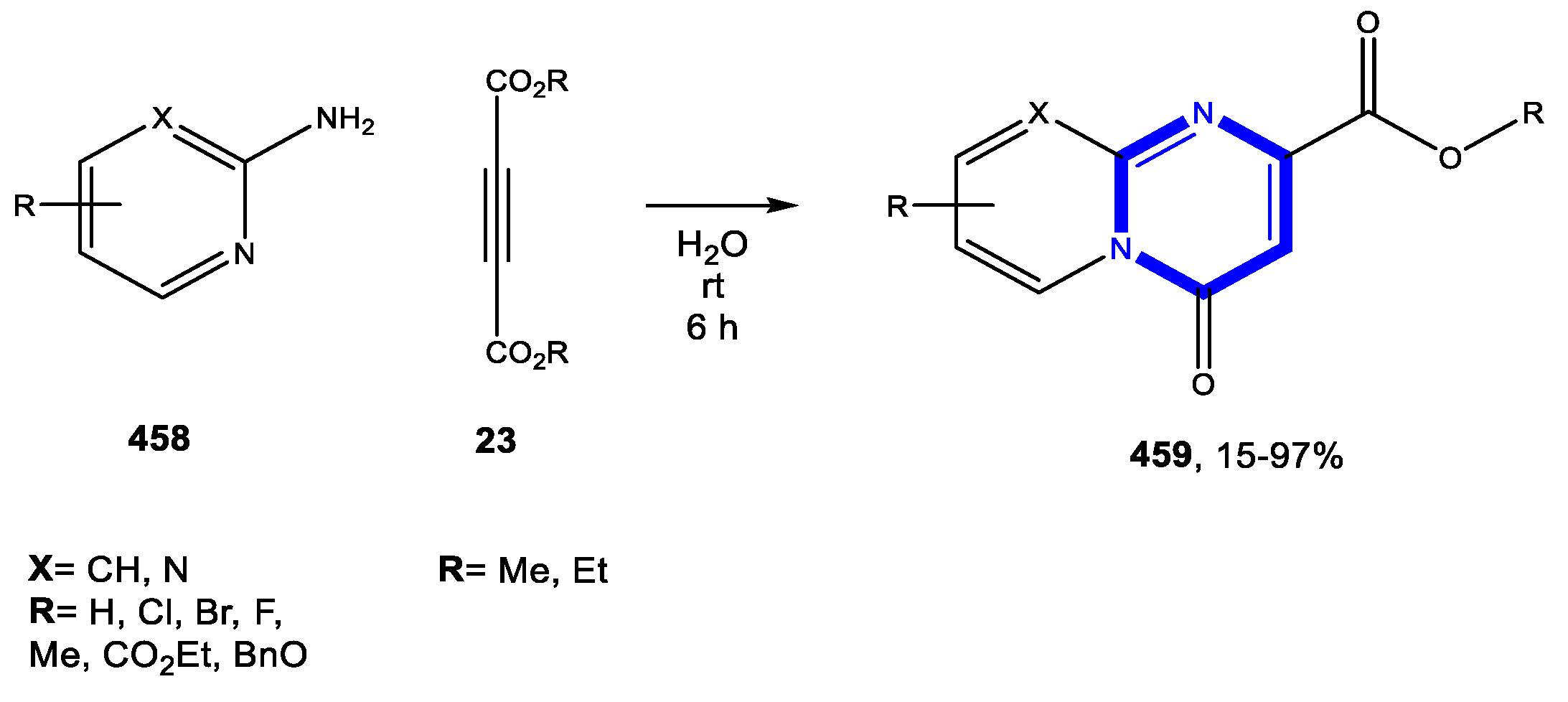
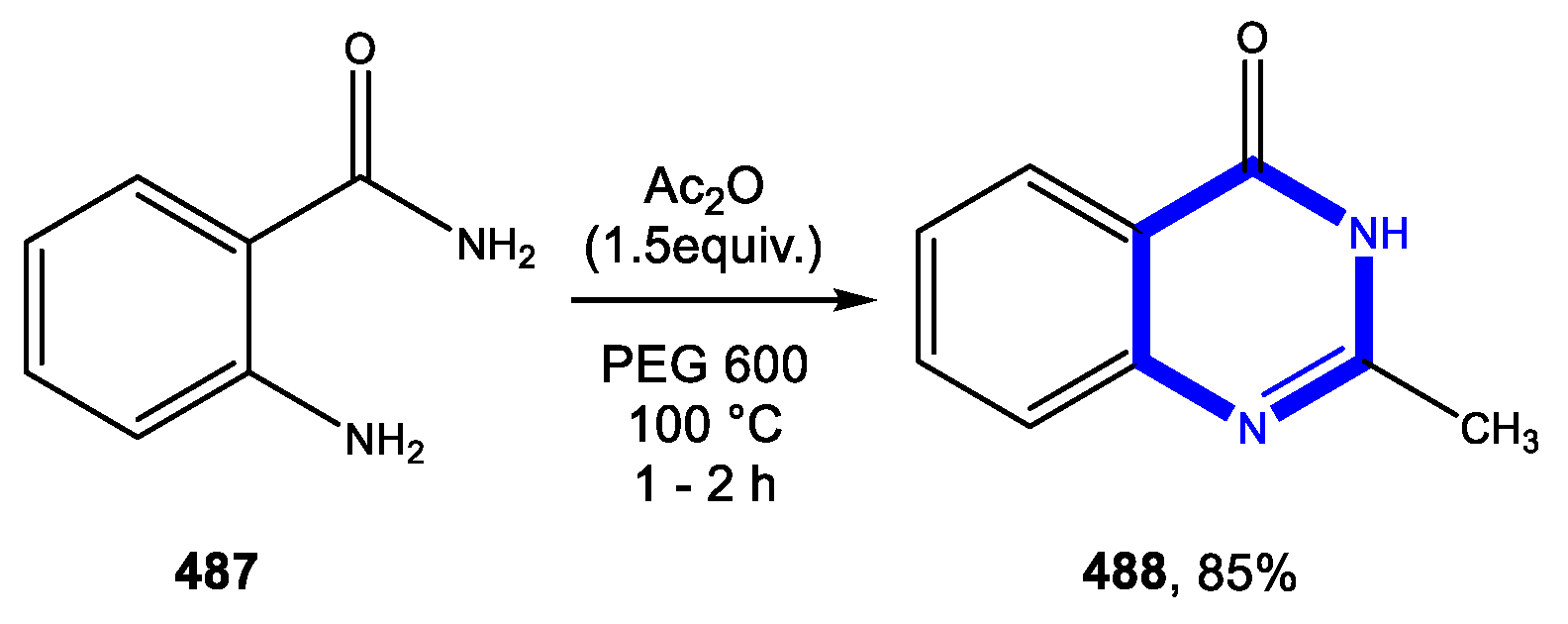
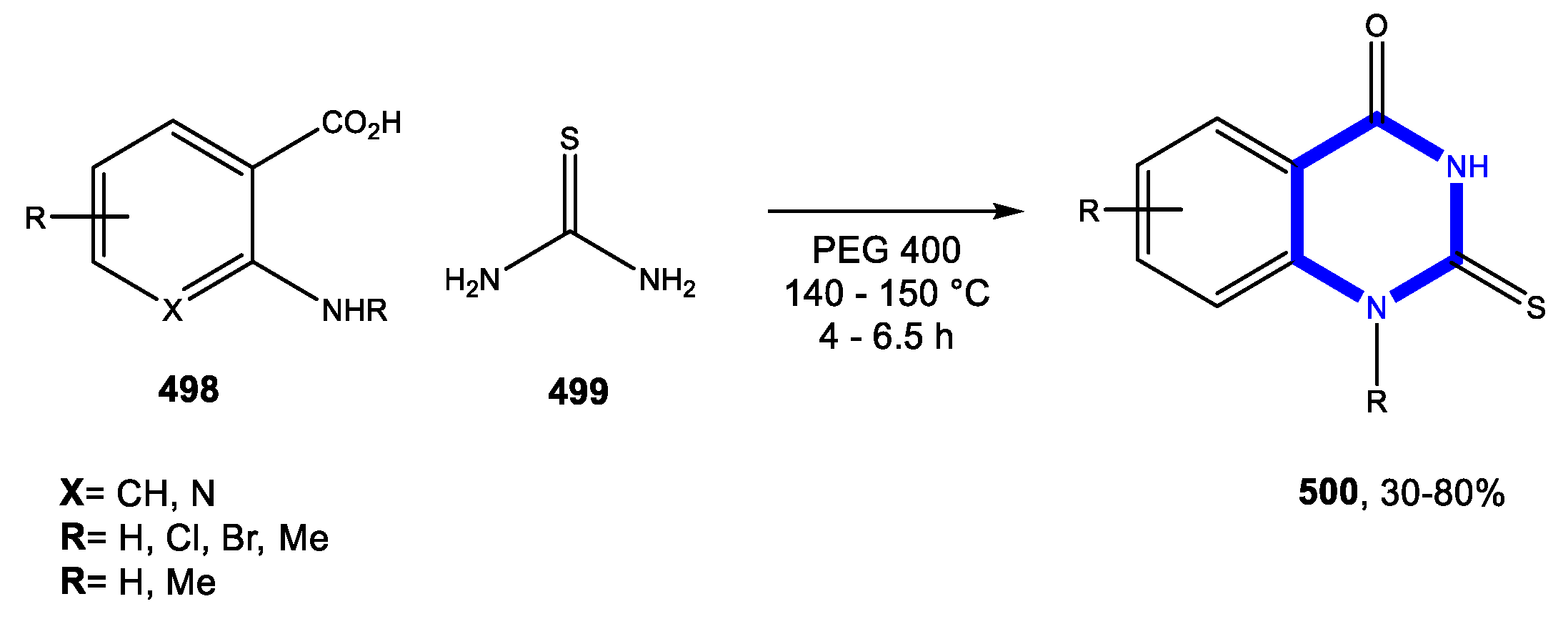
4.3. Formation of the Pyrimidine Ring
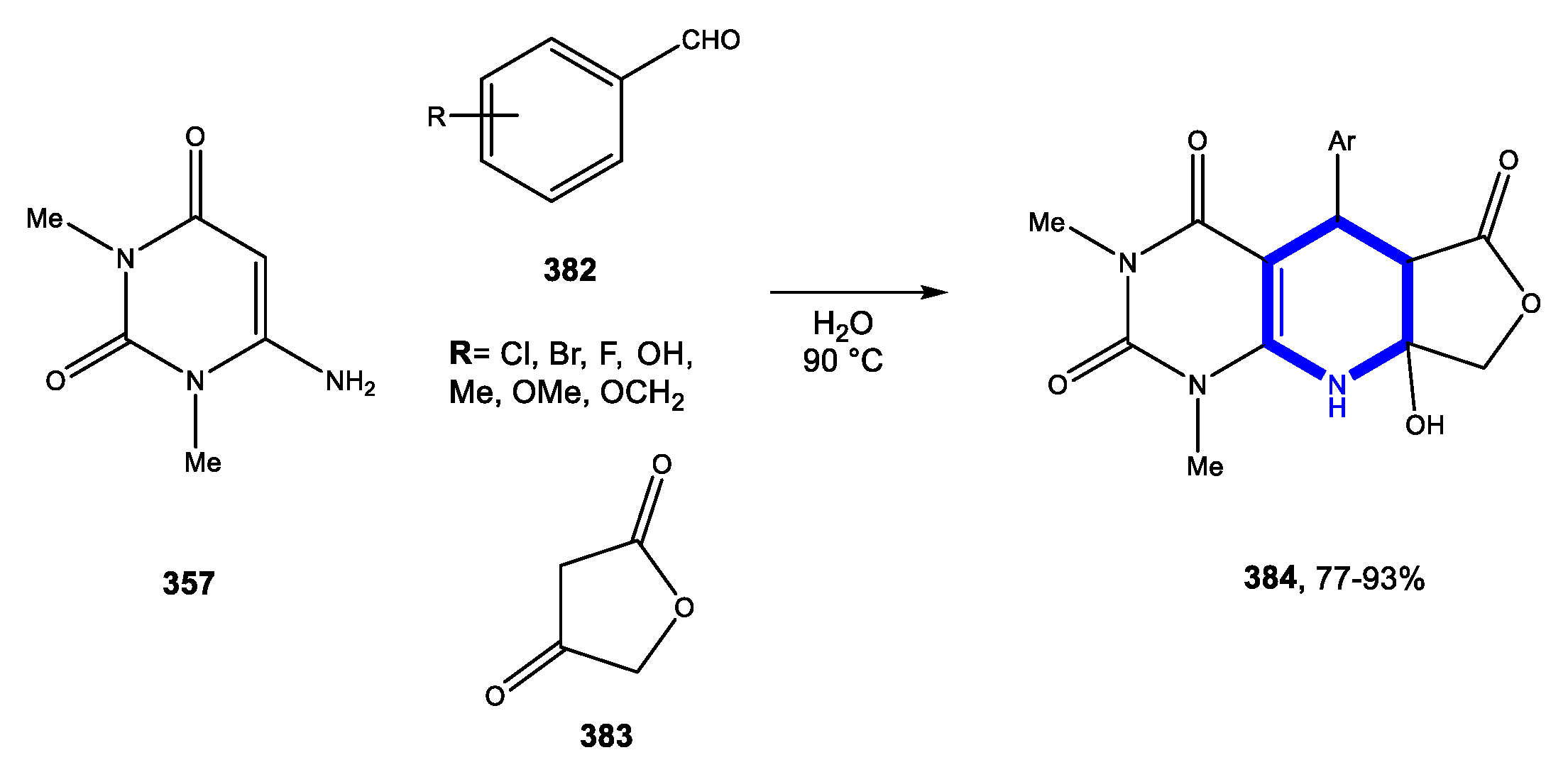
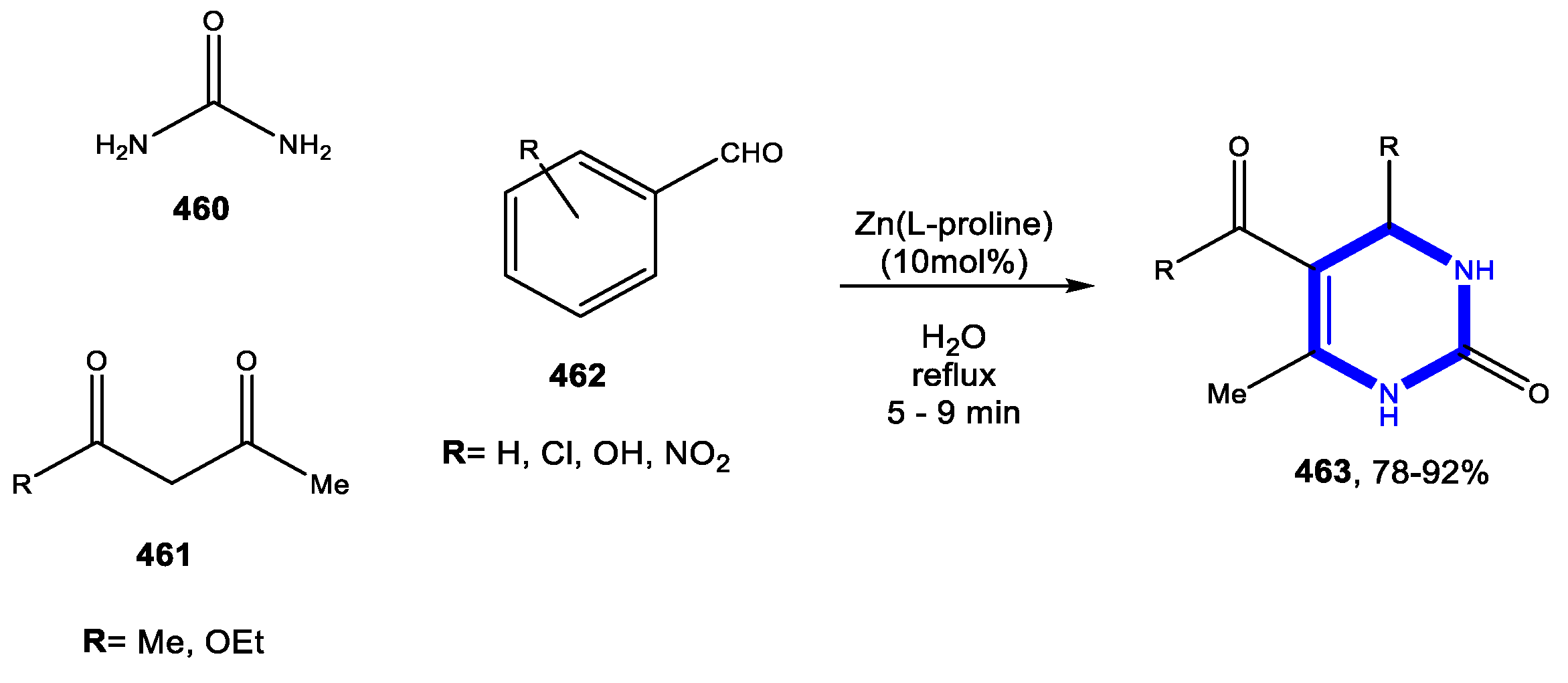
4.3.1. Reactions in Water
4.3.2. Reactions in PEG
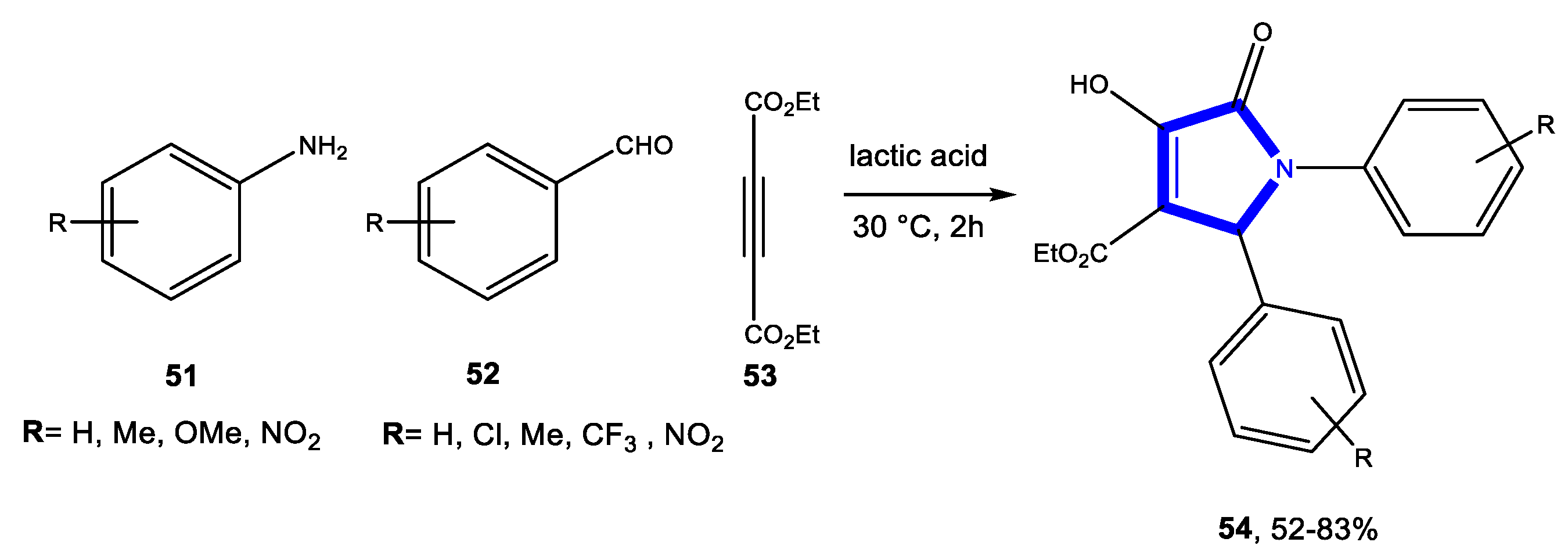
4.3.3. Reactions in Bio-Based Solvents
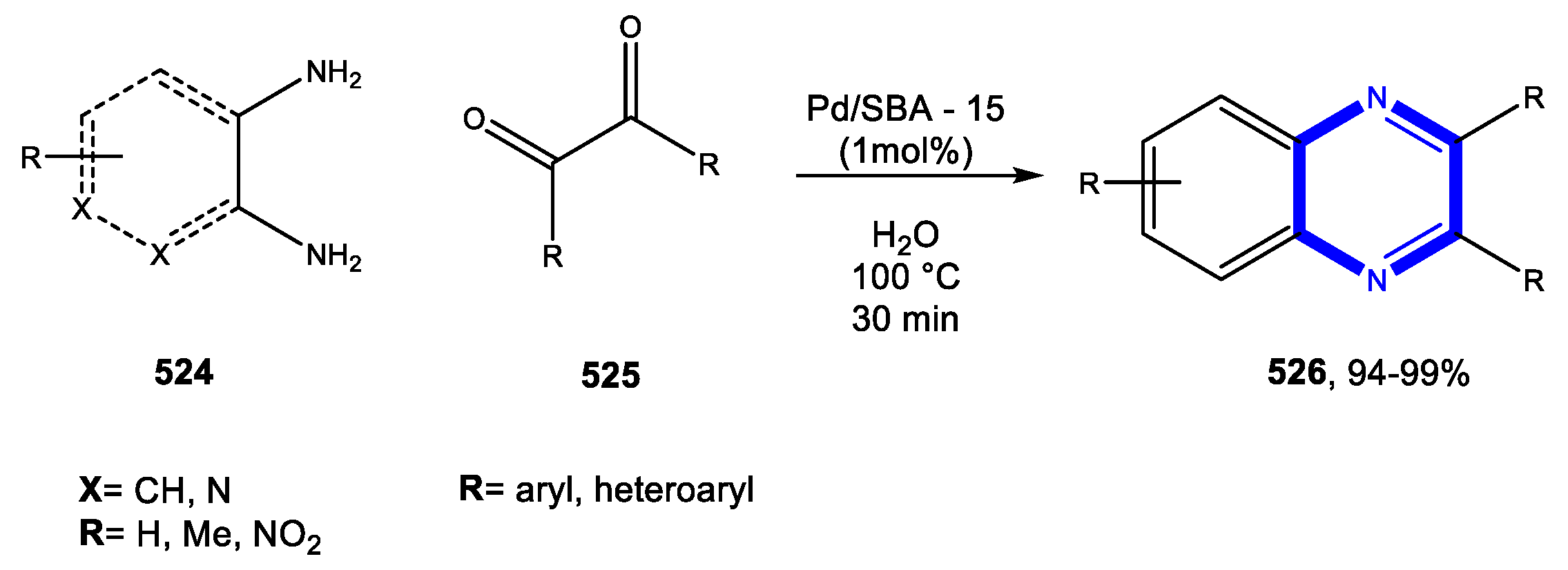
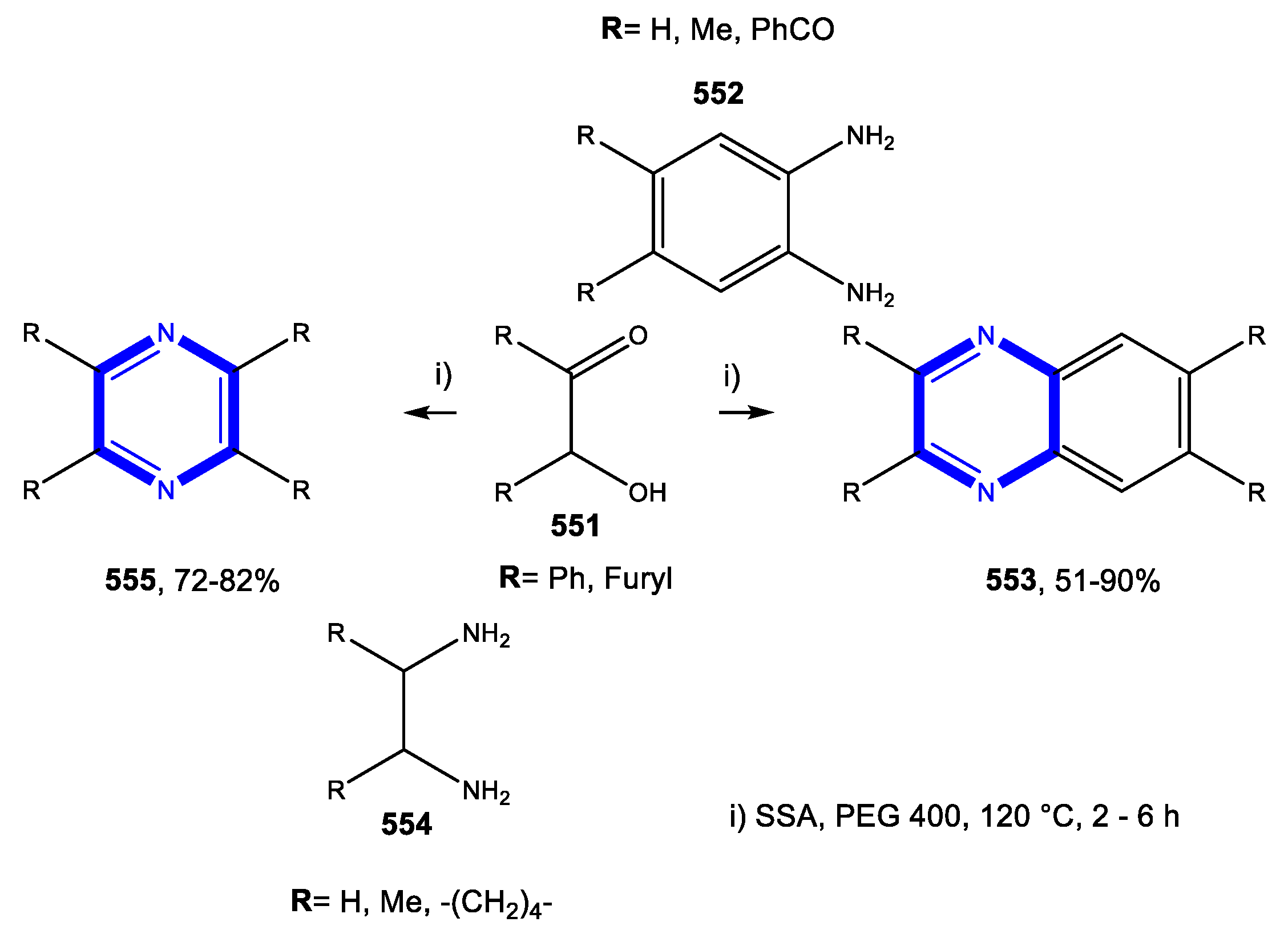
4.4. Formation of the Pyrazine Ring
4.4.1. Reactions in Water
4.4.2. Reactions in PEG
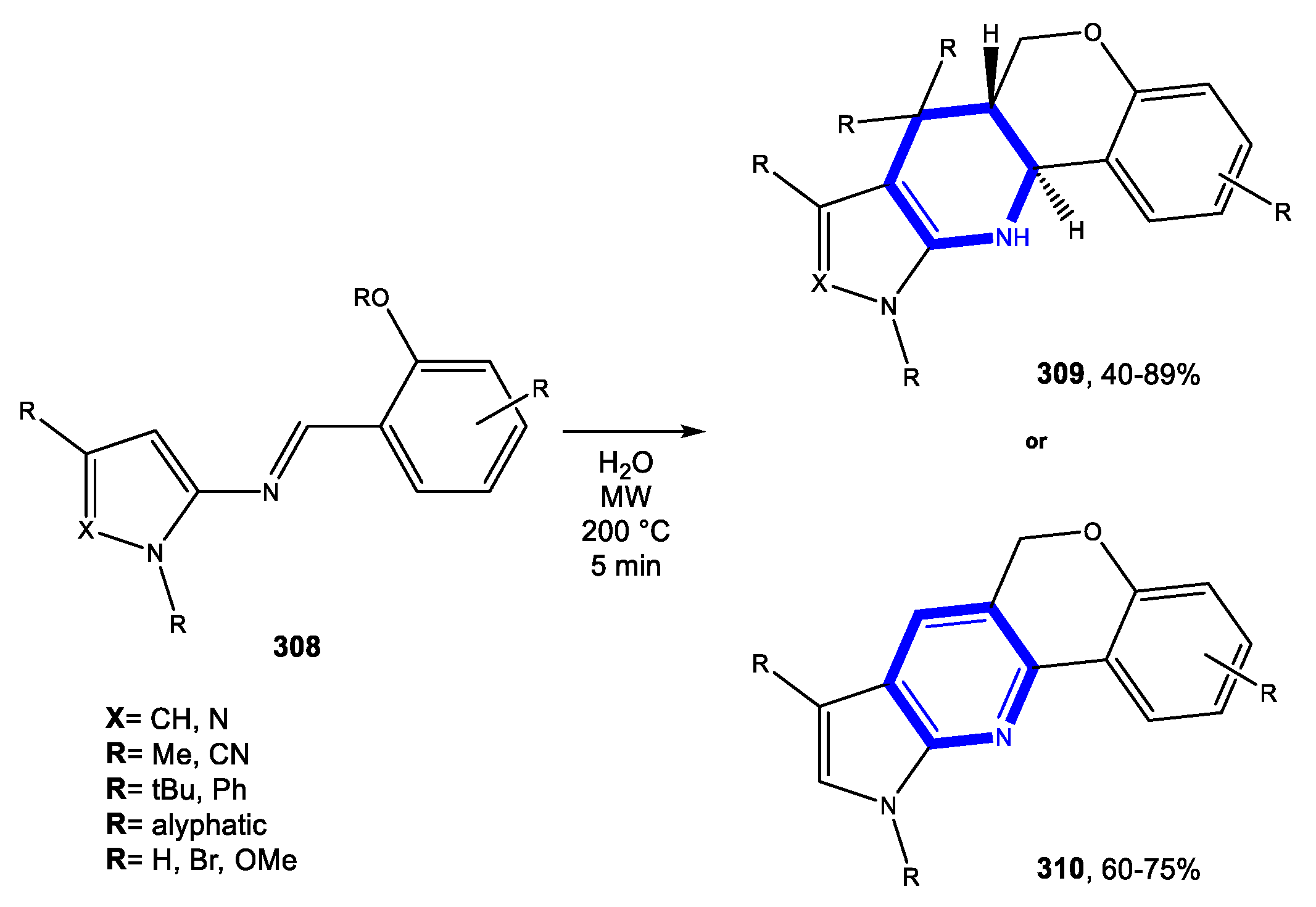
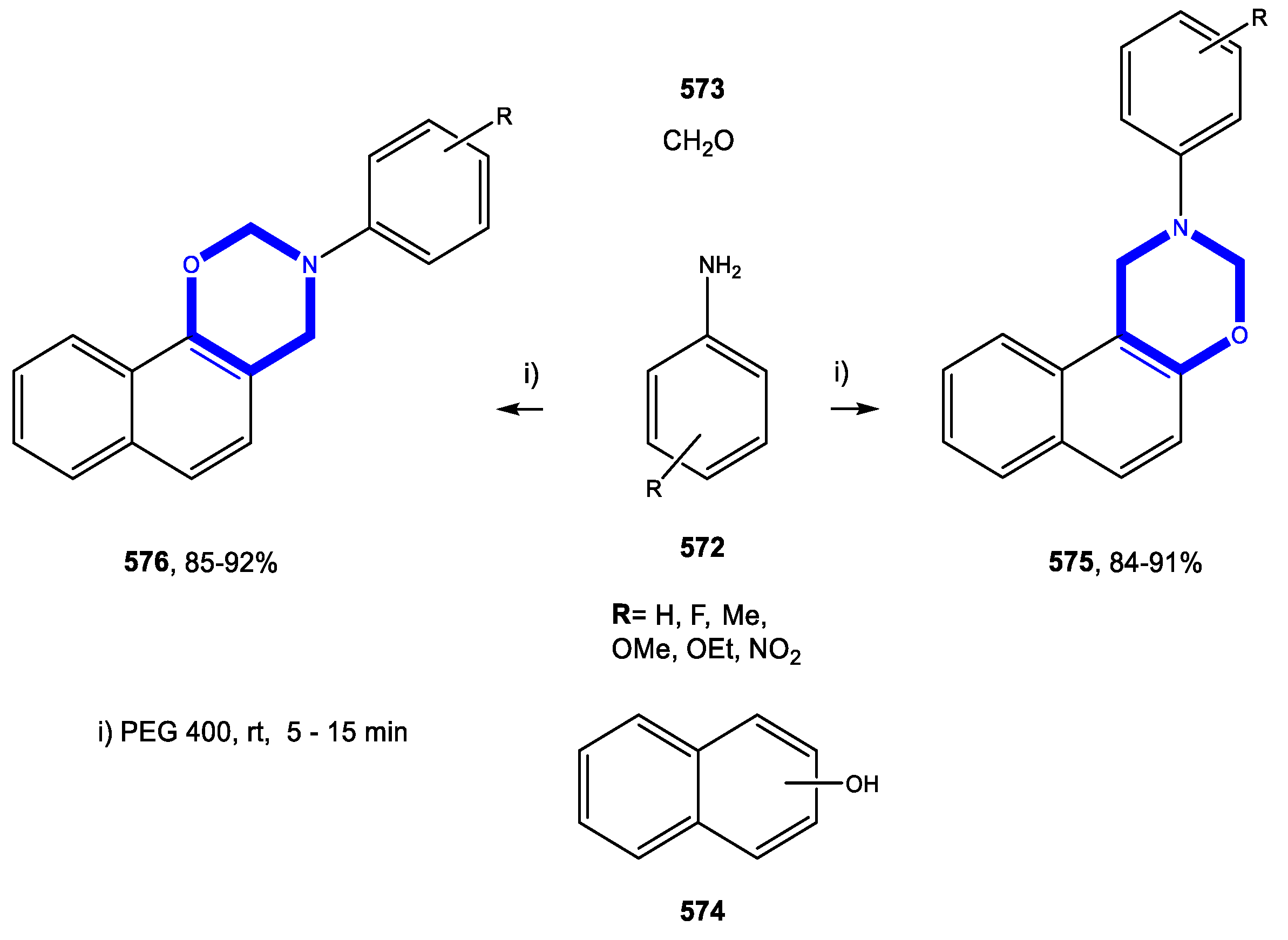
4.5. Formation of the Oxazine Ring
4.5.1. Reactions in Water
4.5.2. Reactions in PEG
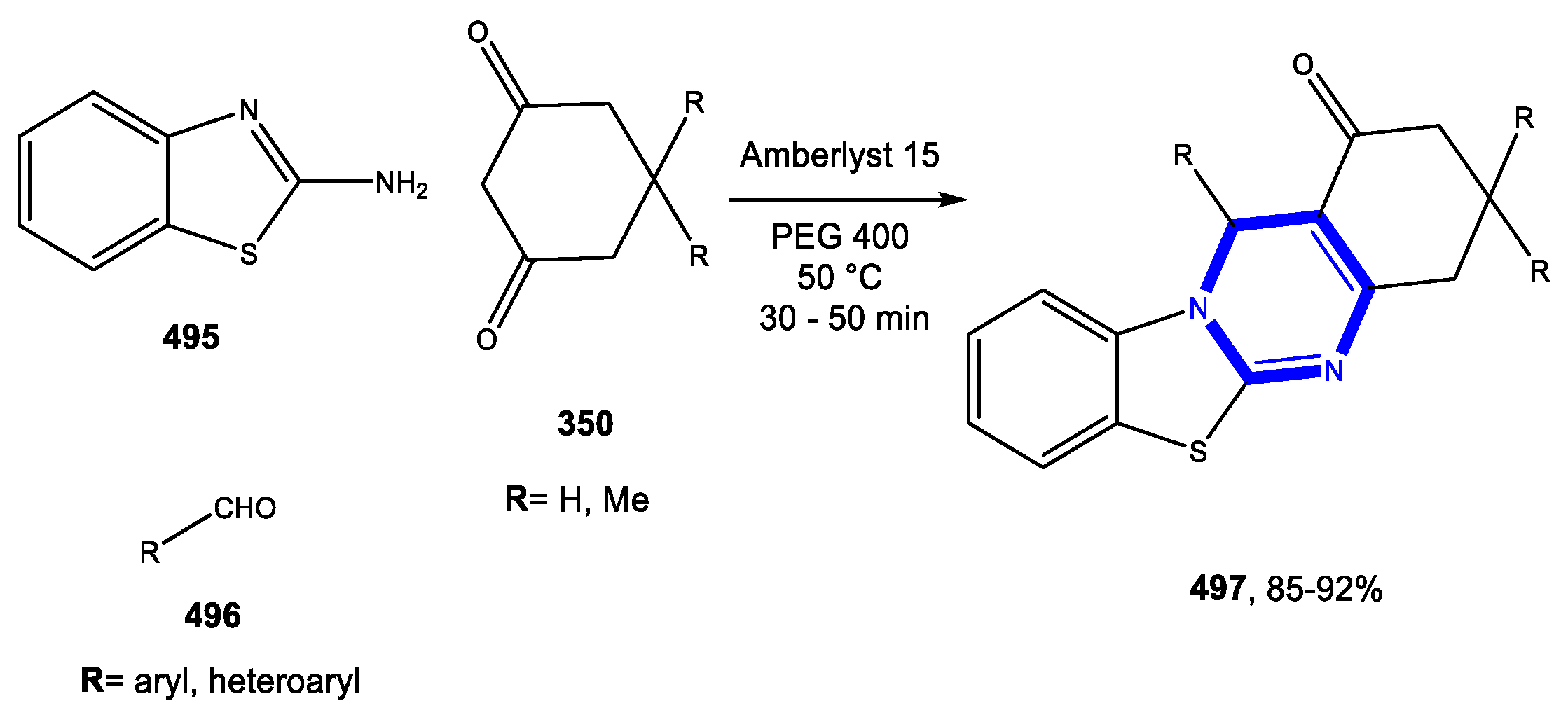
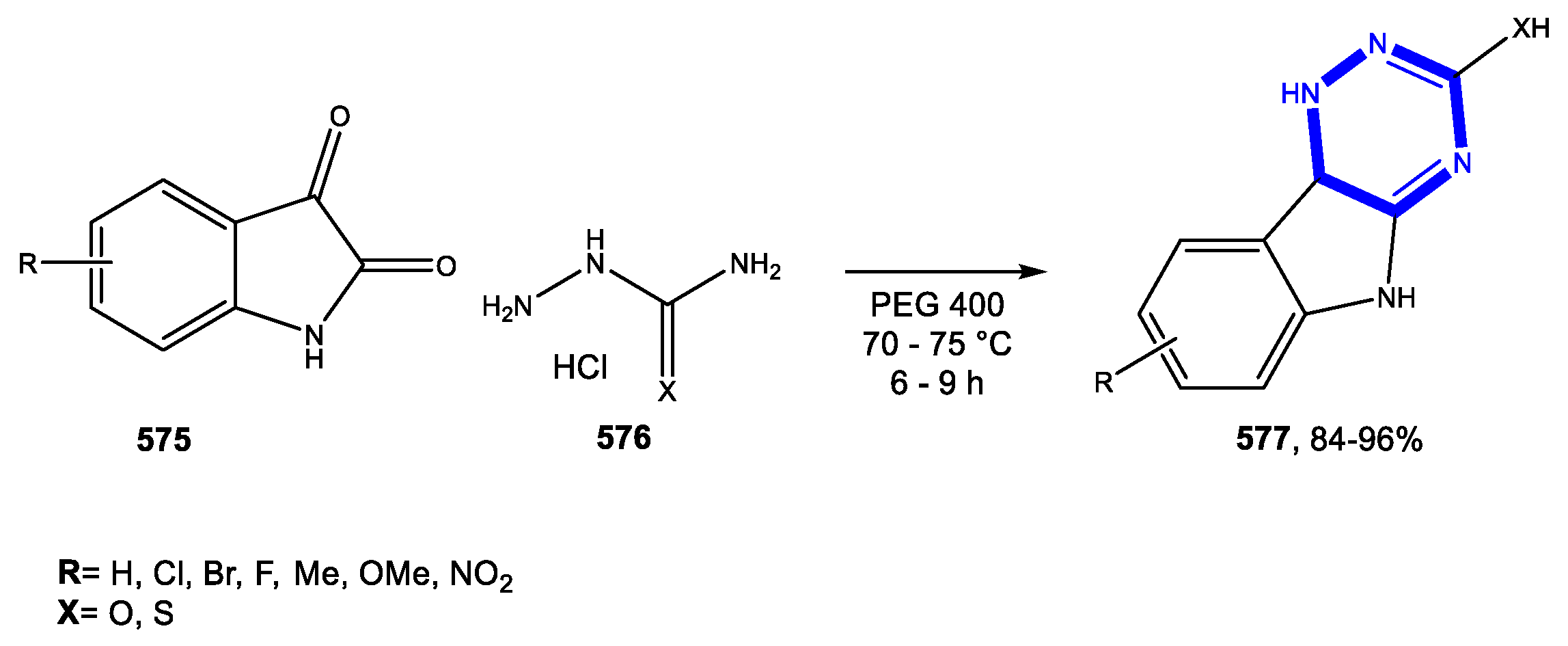
4.6. Formation of the Triazine and Thiadiazine Rings
Reactions in PEG
5. Seven-Membered Aromatic Nitrogen Heterocycles
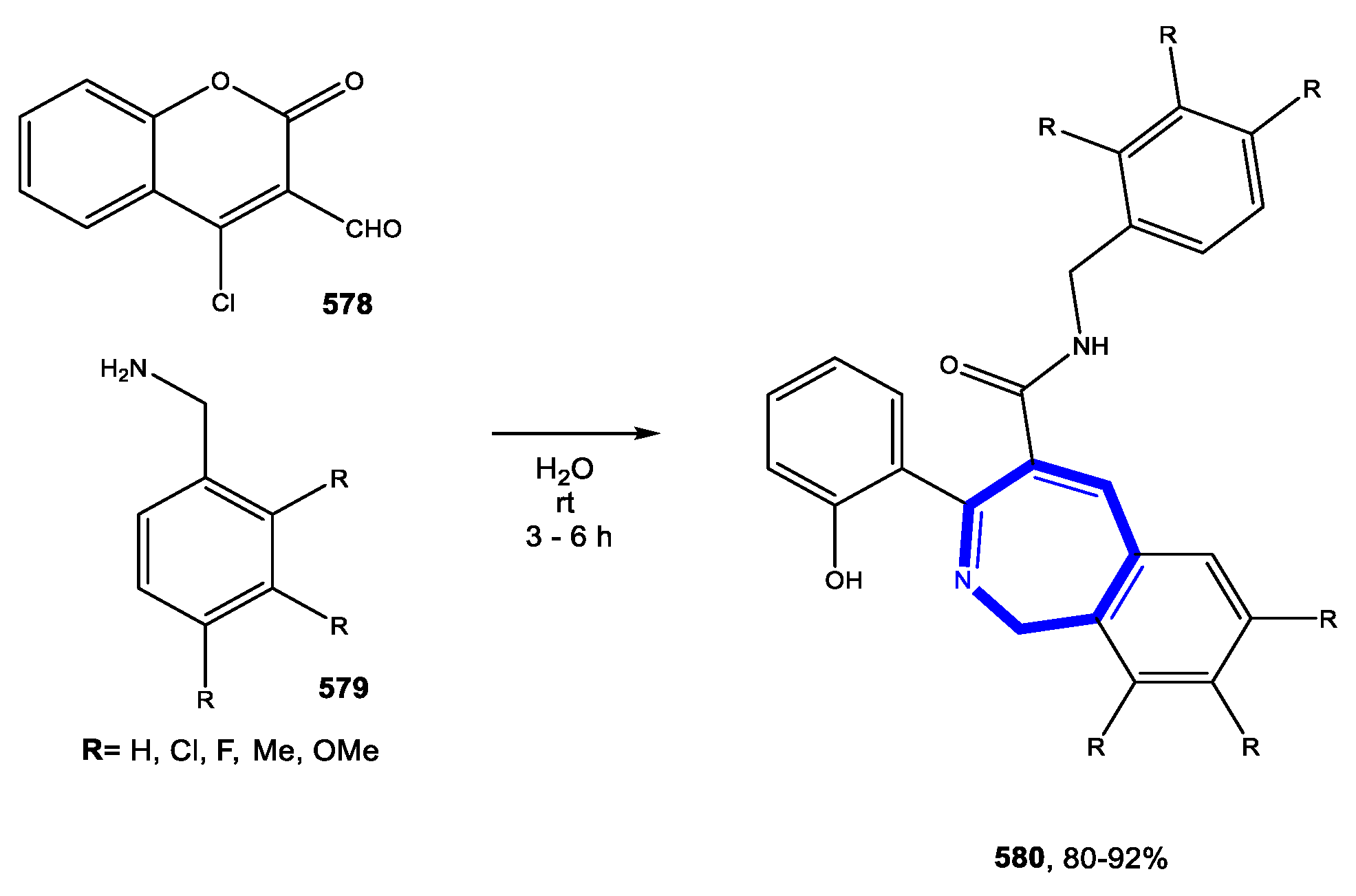
5.1. Formation of the Azepine Ring
5.1.1. Reactions in Water
5.1.2. Reactions in PEG
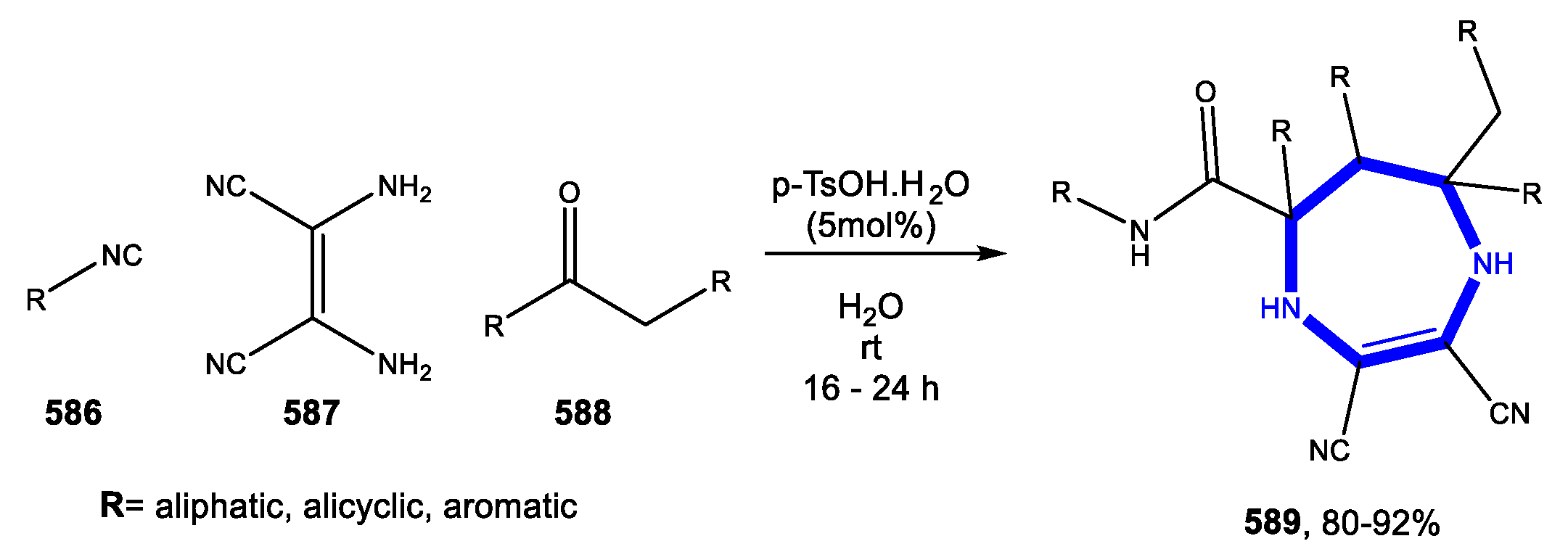
5.2. Formation of the Diazepine Ring
Reactions in Water
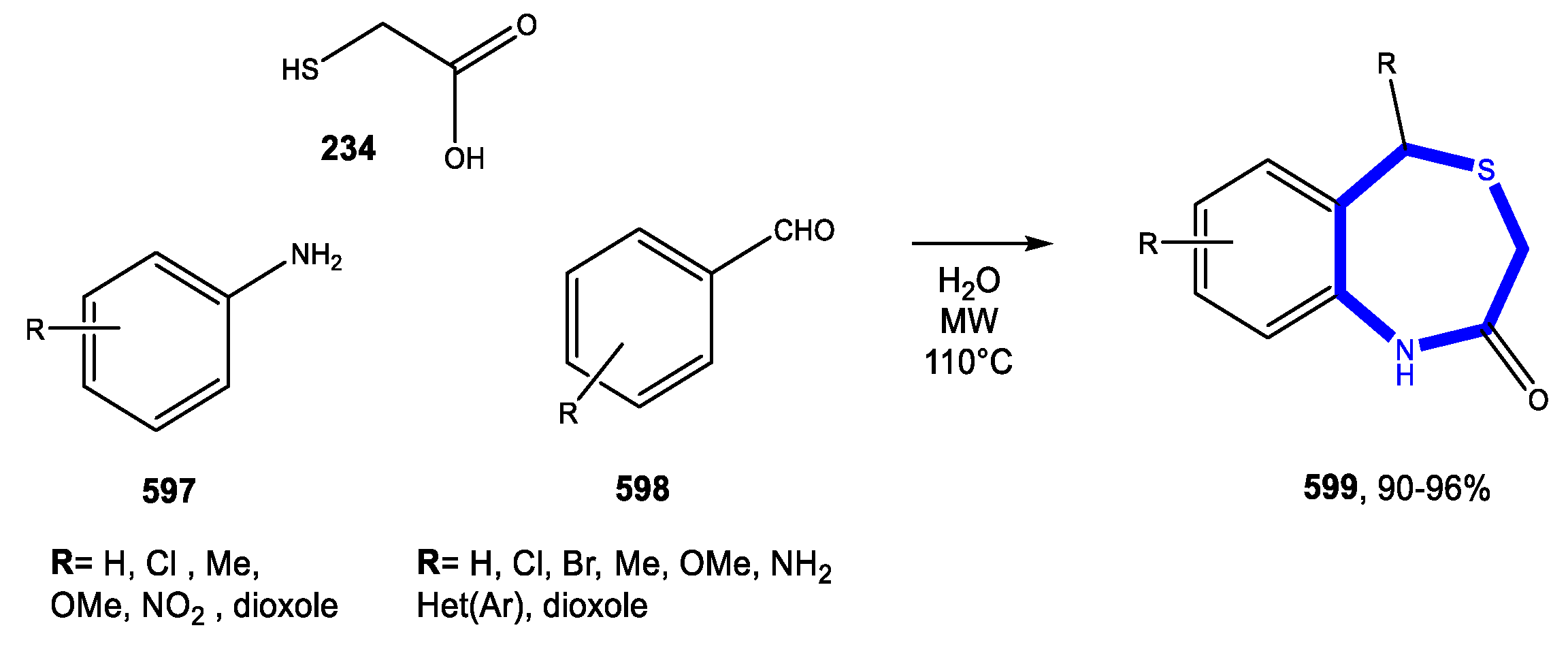
5.3. Formation of the Thiazepine Ring
5.3.1. Reactions in Water
5.3.2. Reactions in PEG
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Candeias, N.R.; Branco, L.C.; Gois, P.M.P.; Afonso, C.A.M.; Trindade, A.F. More Sustainable Approaches for the Synthesis of N-Based Heterocycles. Chem. Rev. 2009, 109, 2703–2802. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.S. Greener and Sustainable Trends in Synthesis of Organics and Nanomaterials. ACS Sustain. Chem. Eng. 2016, 4, 5866–5878. [Google Scholar] [CrossRef]
- Gupta, P.; Mahajan, A. Green chemistry approaches as sustainable alternatives to conventional strategies in the pharmaceutical industry. RSC Adv. 2015, 5, 26686–26705. [Google Scholar] [CrossRef]
- Varma, R.S. Journey on greener pathways: From the use of alternate energy inputs and benign reaction media to sustainable applications of nano-catalysts in synthesis and environmental remediation. Green Chem. 2014, 16, 2027–2041. [Google Scholar] [CrossRef]
- Deligeorgiev, T.; Gadjev, N.; Vasilev, A.; Kaloyanova, S.; Vaquero, J.J.; Alvarez-Builla, J. Green Chemistry in Organic Synthesis. Mini-Rev. Org. Chem. 2010, 7, 44–53. [Google Scholar]
- Hülsey, M.J.; Yang, H.; Yan, N. Sustainable Routes for the Synthesis of Renewable Heteroatom-Containing Chemicals. ACS Sustain. Chem. Eng. 2018, 65, 5694–5707. [Google Scholar] [CrossRef]
- Kaur, N.; Jangid, N.K.; Sharma, V. Metal-and nonmetal-catalyzed synthesis of five-membered S,N-heterocycles. J. Sulfur Chem. 2018, 39, 193–236. [Google Scholar] [CrossRef]
- Bhaskaruni, S.V.H.S.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A review on multi-component green synthesis of N-containing heterocycles using mixed oxides as heterogeneous catalysts. Arab. J. Chem. 2017. In press. [Google Scholar] [CrossRef]
- Batista, V.F.; Pinto, D.C.G.A.; Silva, A.M.S. Synthesis of Quinolines: A Green Perspective. ACS Sustain. Chem. Eng. 2016, 4, 4064–4078. [Google Scholar] [CrossRef]
- Frecentese, F.; Saccone, I.; Caliendo, G.; Corvino, A.; Fiorino, F.; Magli, E.; Perissutti, E.; Severino, B.; Santagada, V. Microwave Assisted Organic Synthesis of Heterocycles in Aqueous Media: Recent Advances in Medicinal Chemistry. Med. Chem. 2016, 12, 720–732. [Google Scholar] [CrossRef]
- Prashant, K.N.; Kumar, K.R. Green Synthesis of Benzimidazole Derivatives: An Overview of Bulk Drug Synthesis. Int. J. Pharmtech. Res. 2015, 8, 60–68. [Google Scholar]
- Kaur, N. A Review on the Synthesis of Six-Membered N,N-Heterocycles by Microwave Irradiation. Synth. Commun. 2015, 45, 1145–1182. [Google Scholar] [CrossRef]
- Chakrabarty, M.; Mukherji, A. Eco-Friendly Synthesis of Condensed Nitrogen Heterocycles: A Brief Experience from Our Group. J. Indian Chem. Soc. 2013, 90, 1681–1694. [Google Scholar] [CrossRef]
- Ivantsova, M.N.; Tokareva, M.I.; Mironov, M.A. Multicomponent interphase synthesis of heterocyclic compounds (review). Chem. Heterocycl. Comp. 2012, 48, 584–600. [Google Scholar] [CrossRef]
- Sharma, S.; Gangal, S.; Rauf, A. Green chemistry approach to the sustainable advancement to the synthesis of heterocyclic chemistry. Rasayan J. Chem. 2008, 1, 693–717. [Google Scholar]
- Bryson, T.A.; Gibson, J.M.; Stewart, J.J.; Voegtle, H.; Tiwari, A.; Dawson, J.H.; Marley, W.; Harmon, B. Synthesis of quinolines, pyridine ligands and biological probes in green media. Green Chem. 2003, 5, 177–180. [Google Scholar] [CrossRef]
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Gu, Y. Multicomponent reactions in unconventional solvents: State of the art. Green Chem. 2012, 14, 2091–2128. [Google Scholar] [CrossRef]
- Ranu, B.C.; Chattopadhyay, K. Eco-Friendly Synthesis of Fine Chemicals, Chap 5; Ballini, R., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2009; pp. 186–219. [Google Scholar]
- Rahmati, A.; Pashmforoush, N. Synthesis of various heterocyclic compounds via multi-component reactions in water. J. Iran. Chem. Soc. 2015, 12, 993–1036. [Google Scholar] [CrossRef]
- Gawande, M.B.; Bonifácio, V.D.B.; Luque, R.; Branco, P.S.; Varma, R.S. Benign by design: Catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem. Soc. Rev. 2013, 42, 5522–5551. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.S.; Jain, S.C. “In Water” Syntheses of Heterocyclic Compounds. Mini Rev. Org. Chem. 2011, 8, 455–464. [Google Scholar] [CrossRef]
- Pires, M.J.D.; Purificação, S.I.; Santos, A.S.; Marques, M.M.B. The Role of PEG on Pd-and Cu-Catalyzed Cross-Coupling Reactions. Synthesis 2017, 49, 2337–2350. [Google Scholar] [CrossRef]
- Colacino, E.; Martinez, J.; Lamaty, F.; Patrikeeva, L.S.; Khemchyan, L.L.; Ananikov, V.P.; Beletskaya, I.P. PEG as an alternative reaction medium in metal-mediated transformations. Coord. Chem. Rev. 2012, 256, 2893–2920. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Hashemi, M.M. Polyethylene glycol (PEG) as a green solvent for carbon–carbon bond formation reactions. J. Mol. Liq. 2015, 207, 73–79. [Google Scholar] [CrossRef]
- Byrne, F.; Jin, S.; Sherwood, J.; McElroy, C.R.; Farmer, T.J.; Clark, J.H.; Hunt, A.J. Bio-Based Solvents, 1st ed.; Chap 3; Jérôme, F., Luque, R., Eds.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2017; pp. 49–82. [Google Scholar]
- Paul, S.; Pradhan, K.; Das, A.R. Ethyl Lactate as a Green Solvent: A Promising Bio-compatible Media for Organic Synthesis. Curr. Green Chem. 2016, 3, 111–118. [Google Scholar] [CrossRef]
- Hulsbosch, J.; De Vos, D.E.; Binnemans, K.; Ameloot, R. Bio-based ionic liquids: Solvents for a green processing industry? ACS Sustain. Chem. Eng. 2016, 4, 2917–2931. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes–a review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Manabe, K.; Iimura, S.; Sun, X.M.; Kobayashi, S. Dehydration Reactions in Water. Brønsted Acid-Surfactant-Combined Catalyst for Ester, Ether, Thioether, and Dithioacetal Formation in Water. J. Am. Chem. Soc. 2002, 124, 11971–11978. [Google Scholar] [CrossRef]
- Tsukinoki, T.; Nagashima, S.; Mitoma, Y.; Tashiro, M. Organic reaction in water. Part 4. New synthesis of vicinal diamines using zinc powder-promoted carbon–carbon bond Formation. Green Chem. 2000, 2, 117–119. [Google Scholar] [CrossRef]
- Bigi, F.; Conforti, M.L.; Maggi, R.; Piccinno, A.; Sartori, G. Clean synthesis in water: Uncatalysed preparation of ylidenemalononitriles. Green Chem. 2000, 2, 101–103. [Google Scholar] [CrossRef]
- Tundo, P.; Anastas, P.; Black, D.S.; Breen, J.; Collins, T.; Memoli, S.; Miyamoto, J.; Polyakoff, M.; Tumas, W. Synthetic pathways and processes in green chemistry. Introductory overview. Pure Appl. Chem. 2000, 72, 1207–1228. [Google Scholar] [CrossRef]
- Diels, O.; Alder, K. The Diels-Alder Diene Synthesis. Ann. Chem. 1931, 490, 243–247. [Google Scholar] [CrossRef]
- Rideout, D.C.; Breslow, R. Hydrophobic Acceleration of Diels-Alder Reactions. J. Am. Chem. Soc. 1980, 102, 7816–7817. [Google Scholar] [CrossRef]
- Breslow, R. Hydrophobic Effects on Simple Organic Reactions in Water. Acc. Chem. Res. 1991, 24, 159–164. [Google Scholar] [CrossRef]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 7, 64–82. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Harris, J.M.; Zalipsky, S. Polyethylene Glycol: Chemistry and Biological Application; ACS Books: Washington, DC, USA, 1997. [Google Scholar]
- Harris, J.M. Poly(ethylene Glycol) Chemistry; Biotechnological and Biomedical Applications Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Sherwood, J. Opportunities for Bio-Based Solvents Created as Petrochemical and Fuel Products Transition towards Renewable Resources. Int. J. Mol. Sci. 2015, 16, 17101–17159. [Google Scholar] [CrossRef]
- Campos, J.F.; Scherrmann, M.-C.; Berteina-Raboin, S. Eucalyptol: A new solvent for the synthesis of heterocycles containing oxygen, sulfur and nitrogen. Green Chem. 2019, 21, 1531–1539. [Google Scholar] [CrossRef]
- Reddy, G.R.; Reddy, T.R.; Chary, R.G.; Joseph, S.C.; Mukherjee, S.; Pal, M. β-Cyclodextrin mediated MCR in water: Synthesis of dihydroisoindolo[2,1-a]quinazoline-5,11-dione derivatives under microwave irradiation. Tetrahedron Lett. 2013, 54, 6744–6746. [Google Scholar] [CrossRef]
- Suresh, R.; Muthusubramanian, S.; Nagaraj, M.; Manickam, G. Indium trichloride catalyzed regioselective synthesis of substituted pyrroles in water. Tetrahedron Lett. 2013, 54, 1779–1784. [Google Scholar] [CrossRef]
- Karamthulla, S.; Pal, S.; Khan, M.N.; Choudhury, L.H. Synthesis of novel spiro[indoline-3,79-pyrrolo[1,2-c]imidazole]-69-carbonitrile derivatives in water using a regioselective sequential three component reaction. RSC Adv. 2013, 3, 15576–15581. [Google Scholar] [CrossRef]
- Keivanloo, A.; Bakherad, M.; Nasr-Isfahani, H.; Esmaily, S. Highly efficient synthesis of 5,6-disubstituted-5H-pyrrolo[2,3-b]pyrazine-2,3-dicarbonitriles through a one-pot palladium-catalyzed coupling reaction/cyclization in water. Tetrahedron Lett. 2012, 53, 3126–3130. [Google Scholar] [CrossRef]
- Gogoi, S.; Dutta, M.; Gogoi, J.; Boruah, R.C. Microwave promoted synthesis of cycl[3.2.2]azines in water via a new three-component reaction. Tetrahedron Lett. 2011, 52, 813–816. [Google Scholar] [CrossRef]
- Yu, L.; Jiang, X.; Wang, L.; Li, Z.; Wu, D.; Zhou, X. Catalytic Alkynylation Coupling Reactions by Copper(II) Complex in Water and Its Applications to Domino Synthesis of 2-Arylindoles. Eur. J. Org. Chem. 2010, 29, 5560–5562. [Google Scholar] [CrossRef]
- Das, B.; Shinde, D.B.; Kanth, B.S.; Satyalakshmi, G. An Efficient Multicomponent Synthesis of Polysubstituted Pyrrolidines and Tetrahydropyrimidines Starting Directly from Nitro Compounds in Water. Synthesis 2010, 16, 2823–2827. [Google Scholar] [CrossRef]
- Barnard, T.M.; Vanier, G.S.; Collins, M.J. Scale-Up of the Green Synthesis of Azacycloalkanes and Isoindolines under Microwave Irradiation. Org. Process Res. Dev. 2006, 10, 1233–1237. [Google Scholar] [CrossRef]
- Demir, A.S.; Emrullahoglu, M. Zinc perchlorate catalyzed one-pot amination–annulation of a-cyanomethyl-b-ketoesters in water. Regioselective synthesis of 2-aminopyrrole-4-carboxylates. Tetrahedron 2006, 62, 1452–1458. [Google Scholar] [CrossRef]
- Yedukondalu, M.; Sridhar, R.; Luther, B.J.; Rao, M.V.B. Synthesis of Tetrahydro Carbazoles And Tetrahydro-Γ-Carbolines Catalyzed By PEG-400 As Recyclable Reaction Medium. J. Appl. Chem. 2014, 3, 1295–1305. [Google Scholar]
- Mishra, S.; Naskar, B.; Ghosh, R. CuCl catalyzed green and efficient one-pot synthesis of aminoindolizine frameworks via three-component reactions of aldehydes, secondary amines, and terminal alkynes in PEG. Tetrahedron Lett. 2012, 53, 5483–5487. [Google Scholar] [CrossRef]
- Dandia, A.; Jain, A.K.; Laxkar, A.K. Ethyl lactate as a promising bio based green solvent for the synthesis of spirooxindole derivatives via 1,3 dipolar cycloaddtion reaction. Tetrahedron Lett. 2013, 54, 3929–3932. [Google Scholar] [CrossRef]
- Wang, S.-F.; Guo, C.-L.; Cui, K.-K.; Zhu, Y.-T.; Ding, J.-X.; Zou, X.-Y.; Li, Y.-H. Lactic acid as an invaluable green solvent for ultrasound-assisted scalable synthesis of pyrrole derivatives. Ultrason. Sonochem. 2015, 26, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tana, J.-N.; Gu, Y. Lactic acid as an invaluable bio-based solvent for organic reactions. Green Chem. 2012, 14, 3304–3317. [Google Scholar] [CrossRef]
- Li, B.-L.; Li, P.-H.; Fang, X.-N.; Li, C.-X.; Sun, J.-L.; Mo, L.-P.; Zhang, Z.-H. One-pot four-component synthesis of highly substituted pyrroles in gluconic acid aqueous solution. Tetrahedron 2013, 69, 7011–7018. [Google Scholar] [CrossRef]
- Muthusamy, S.; Gangadurai, C. “On water” cascade synthesis of benzopyranopyrazoles and their macrocycles. Tetrahedron Lett. 2018, 59, 1501–1505. [Google Scholar] [CrossRef]
- Eswararao, S.V.; Venkataramireddy, V.; Sreenivasareddy, M.; Pramod, K. Water mediated one- pot- synthesis of 1h-pyrazolo[1,2-b]phthalazine-5,10-diones and 2h-indazolo[2,1-b]phthalazine-1,6,11(13h)-triones. Heterocycl. Lett. 2017, 7, 895–903. [Google Scholar]
- Sagir, H.; Rai, P.; Rahila; Tiwari, S.; Siddiqui, I.R. Iodine/Water Mediated Parallel Synthesis of Bioactive Diversely Substituted Pyrazolo-Pyrido-Pyrimidines and Its Spiro Analogues: An Enviro-Economic Approach. J. Heterocycl. Chem. 2017, 54, 397–405. [Google Scholar] [CrossRef]
- Maleki, B.; Nasiri, N.; Tayebee, R.; Khojastehnezhad, A.; Akhlaghi, H.A. Green Synthesis of Tetrahydrobenzo[b]pyrans, Pyrano [2, 3-c]pyrazoles and Spiro [indoline-3,4’-pyrano[2,3-c]pyrazoles Catalyzed by Nano-structured diphosphate in Water. RSC Adv. 2016, 6, 79128–79134. [Google Scholar] [CrossRef]
- Bakherad, M.; Keivanloo, A.; Gholizadeh, M.; Doosti, R.; Javanmardi, M. Using magnetized water as a solvent for a green, catalyst-free, and efficient protocol for the synthesis of pyrano[2,3-c]pyrazoles and pyrano[40,30:5,6]pyrazolo[2,3-d]pyrimidines. Res. Chem. Intermed. 2017, 43, 1013–1029. [Google Scholar] [CrossRef]
- Marković, V.; Joksović, M.D. “On water” synthesis of N-unsubstituted pyrazoles:semicarbazide hydrochloride as an alternative to hydrazine for preparation of pyrazole-3-carboxylate derivatives and 3,5-disubstituted pyrazoles. Green Chem. 2015, 17, 842–847. [Google Scholar] [CrossRef]
- Dam, B.; Saha, M.; Pal, A.K. Magnetically Recyclable Nano-FDP: A Novel, Efficient Nano-Organocatalyst for the One-Pot Multi-Component Synthesis of Pyran Derivatives in Water under Ultrasound Irradiation. Catal. Lett. 2015, 145, 1808–1816. [Google Scholar] [CrossRef]
- Srivastava, M.; Rai, P.; Singhb, J.; Singh, J. Efficient iodine-catalyzed one pot synthesis of highly functionalised pyrazoles in water. New J. Chem. 2014, 38, 302–307. [Google Scholar] [CrossRef]
- Khoraamabadi-zad, A.; Azadmanesh, M.; Karamian, R.; Asadbegyb, M.; Akbari, M. Triethanolamine as an inexpensive and efficient catalyst for the green synthesis of novel 1Hpyrazolo[1,2-a]pyridazine-5,8-diones under ultrasound irradiation in water and their antibacterial activity. RSC Adv. 2014, 4, 47721–47725. [Google Scholar] [CrossRef]
- Heravi, M.M.; Mousavizadeh, F.; Ghobadi, N.; Tajbakhsh, M. A green and convenient protocol for the synthesis of novel pyrazolopyranopyrimidines via a one-pot, four-component reaction in water. Tetrahedron Lett. 2014, 55, 1226–1228. [Google Scholar] [CrossRef]
- Sachdeva, H.; Saroj, R. ZnO Nanoparticles as an Efficient, Heterogeneous, Reusable, and Ecofriendly Catalyst for Four-Component One-Pot Green Synthesis of Pyranopyrazole Derivatives in Water. Sci. World J. 2013, 2013, 680671. [Google Scholar] [CrossRef]
- Wu, M.; Feng, Q.; Wan, D.; Ma, J. CTACl as Catalyst for Four-Component, One-Pot Synthesis of Pyranopyrazole Derivatives in Aqueous Medium. Synth. Commun. 2013, 43, 1721–1726. [Google Scholar] [CrossRef]
- Zonouz, A.M.; Eskandari, I.; Khavasi, H.R. A green and convenient approach for the synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates via a one-pot, multi-component reaction in water. Tetrahedron Lett. 2012, 53, 5519–5522. [Google Scholar] [CrossRef]
- Madjea, B.R.; Bharada, J.V.; Ubalea, M.B.; Shingareb, M.S. Alum promoted synthesis of 2H-indazolo[2,1-b]phthalazinetrione derivatives in water. Orbital Electron. J. Chem. 2012, 4, 202–208. [Google Scholar] [CrossRef]
- Savant, M.M.; Pansuriya, A.M.; Bhuva, C.V.; Kapuriya, N.; Patel, A.S.; Audichya, V.B.; Pipaliya, P.V.; Naliapara, Y.T. Water Mediated Construction of Trisubstituted Pyrazoles/Isoxazoles Library Using Ketene Dithioacetals. J. Comb. Chem. 2010, 12, 176–180. [Google Scholar] [CrossRef]
- Mali, J.R.; Pratap, U.R.; Jawale, D.V.; Mane, R.A. Water-mediated one-pot synthetic route for pyrazolo[3,4-b]quinolones. Tetrahedron Lett. 2010, 51, 3980–3982. [Google Scholar] [CrossRef]
- Karnakar, K.; Ramesh, K.; Reddy, K.H.V.; Kumar, B.S.P.A.; Nanubonula, J.B.; Nageswar, Y.V.D. A novel one pot four-component reaction for the efficient synthesis of spiro[indoline-3,40-pyrano-[2,3-c]pyrazole]-30-carboxylate and trifluoromethylated spiro[indole-3,40-pyrano[2,3-c]pyrazole] derivatives using recyclable PEG-400. New J. Chem. 2015, 39, 8978–8983. [Google Scholar] [CrossRef]
- Lavania, A.; Dasary, K.; Yadav, M.; Anand, A.V.K. Eco-friendly Synthesis and characterization of some (2-pyrazoline) and (2-isoxazoline) containing anthracene moiety by using PEG(400) as a Catalyst. J. Appl. Chem. 2013, 2, 1341–1346. [Google Scholar]
- Kidwai, M.; Chauhan, R.; Jahan, A. Efficient CAN catalyzed synthesis of 1H-indazolo[1,2-b]phthalazine-1,6,11-triones: An eco-friendly protocol. Chin. Sci. Bull. 2012, 57, 2273–2279. [Google Scholar] [CrossRef]
- Bhat, P.; Shridhar, G.; Ladage, S.; Ravishankar, L. An eco-friendly synthesis of 2-pyrazoline derivatives catalyzed by CeCl3·7H2O. J. Chem. Sci. 2017, 129, 1441–1448. [Google Scholar] [CrossRef]
- Dheer, D.; Rawal, R.K.; Singh, V.; Sangwan, P.L.; Das, P.; Shankar, R. b-CD/CuI catalyzed regioselective synthesis of iodo substituted 1,2,3-triazoles, imidazo[1,2-a]-pyridines and benzoimidazo[2,1-b]thiazoles in water and their functionalization. Tetrahedron 2017, 73, 4295–4306. [Google Scholar] [CrossRef]
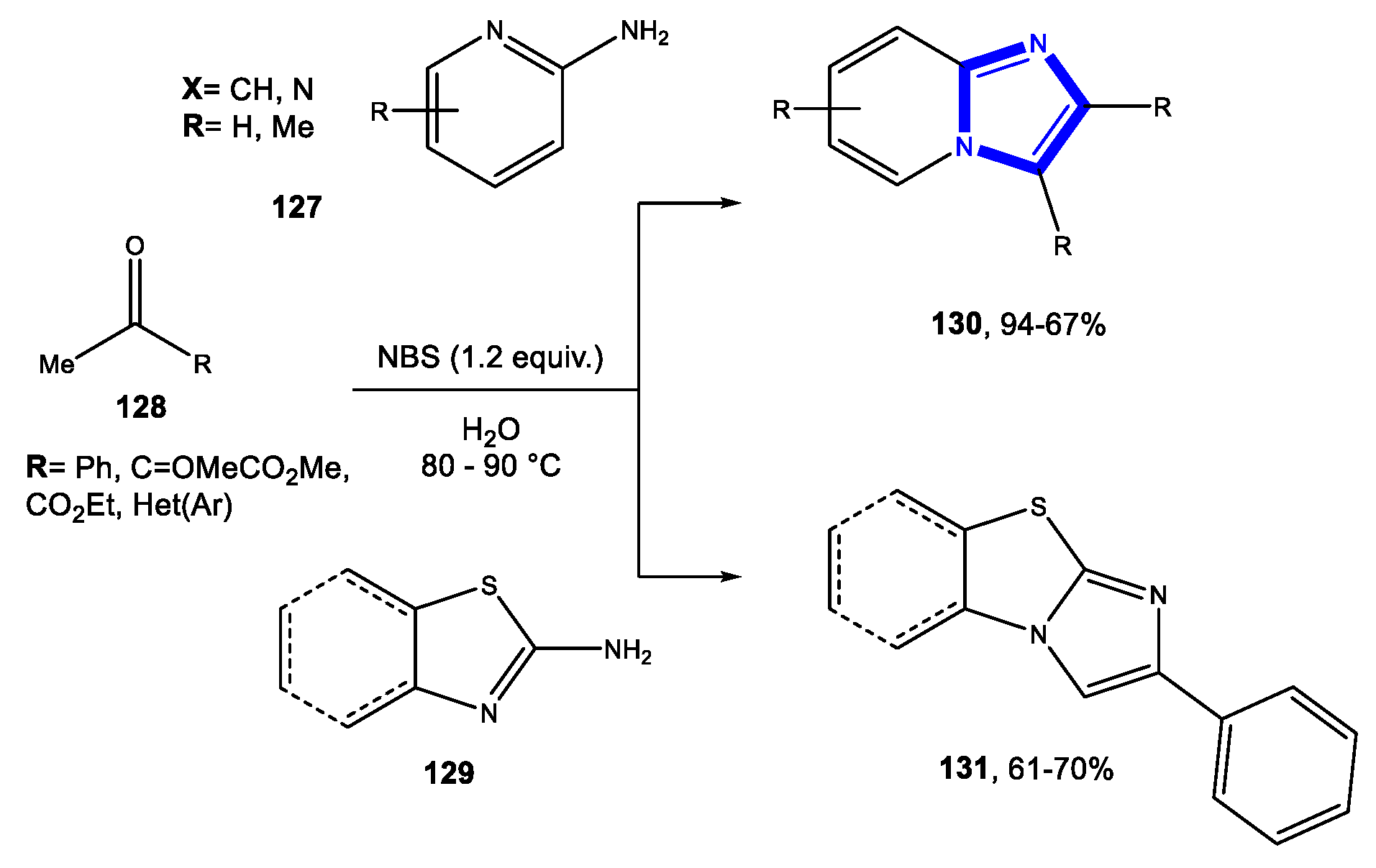
- Bhagat, S.B.; Telvekar, V.N. NBS mediated protocol for the synthesis of N-bridged fused heterocycles in water. Tetrahedron Lett. 2017, 58, 3662–3666. [Google Scholar] [CrossRef]
- Wagare, D.S.; Farooqui, M.; Keche, T.D.; Durrani, A. Efficient and green microwave-assisted one-pot synthesis of azaindolizines in PEG-400 and water. Synth. Commun. 2016, 46, 1741–1746. [Google Scholar] [CrossRef]
- Nikoofar, K.; Haghighi, M.; Lashanizadegan, M.; Ahmadvand, Z. ZnO nanorods: Efficient and reusable catalysts for the synthesis ofsubstituted imidazoles in water. J. Taibah Univ. Sci. 2015, 9, 570–578. [Google Scholar] [CrossRef]
- Chanu, L.G.; Singh, T.P.; Jang, Y.J.; Yoon, Y.-J.; Singh, O.M.; Lee, S.-G. Synthesis of Imidazo[1,2-a]pyridines and Pyrido[1,2-a]pyrimidines in Water and their SNAr Cyclizations. Bull. Korean Chem. Soc. 2014, 35, 994–1000. [Google Scholar] [CrossRef]
- Siddiqui, I.R.; Rai, P.; Rahila; Srivastava, A.; Shamim, S. Synthesis of imidazo[1,2-a]pyridine in the presence of iodine–water catalytic system. Tetrahedron Lett. 2014, 55, 1159–1163. [Google Scholar] [CrossRef]
- Peshkov, V.A.; Peshkov, A.A.; Pereshivko, O.P.; Hecke, K.V.; Zamigaylo, L.L.; Van der Eycken, E.V.; Gorobets, N.Y. Three-Component Reaction of a 2-Aminoazine, a 2-Oxoaldehyde, and a Cyclic 1,3-Dicarbonyl Compound for the Synthesis of Imidazo[1,2-a]azine Derivatives. ACS Comb. Sci. 2014, 16, 535–542. [Google Scholar] [CrossRef]
- Bangade, V.M.; Reddy, B.C.; Thakur, P.B.; Babu, B.M.; Meshram, H.M. DABCO catalyzed highly regioselective synthesis of fused imidazo-heterocycles in aqueous medium. Tetrahedron Lett. 2013, 54, 4767–4771. [Google Scholar] [CrossRef]
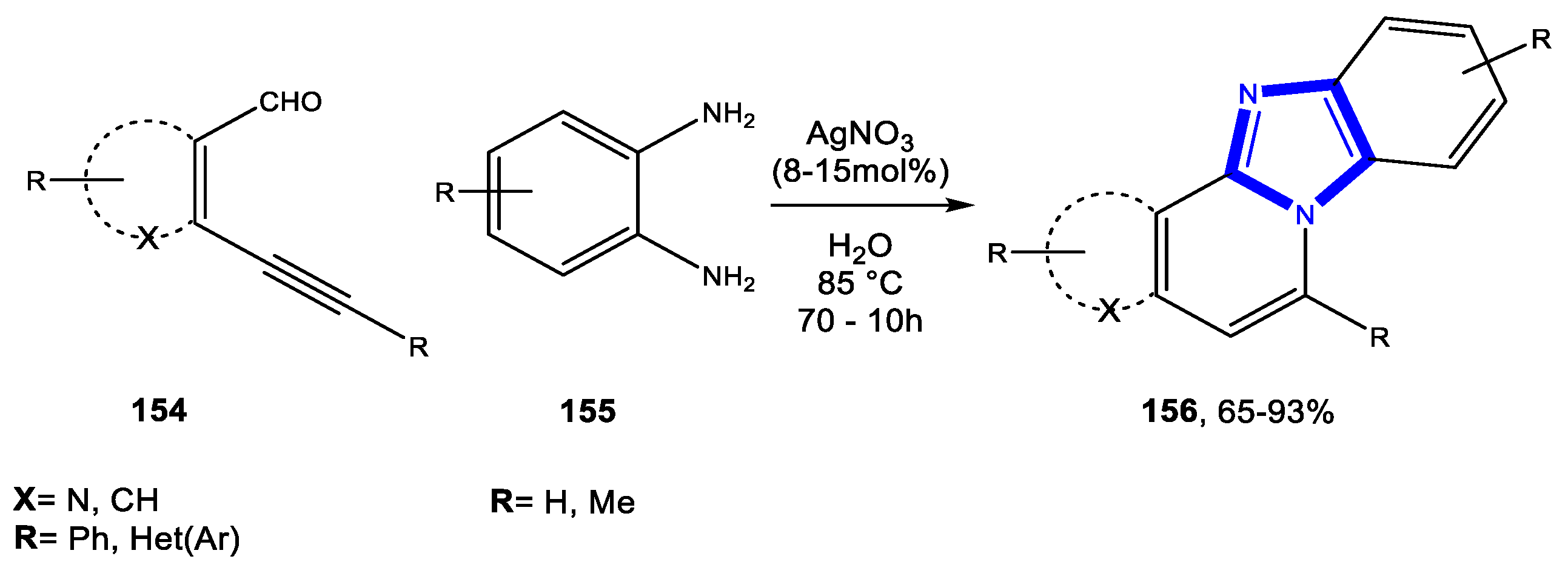
- Rustagi, V.; Tiwari, R.; Verma, A.K. AgI-Catalyzed Cascade Strategy: Regioselective Access to Diversely Substituted Fused Benzimidazo[2,1-a]isoquinolines, Naphthyridines, Thienopyridines, and Quinoxalines in Water. Eur. J. Org. Chem. 2012, 4590–4602. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, N.M.; Patel, G.; Gupta, S.; Varma, R.S. A facile and eco-friendly synthesis of diarylthiazoles and diarylimidazoles in water. Tetrahedron Lett. 2011, 52, 1983–1986. [Google Scholar] [CrossRef]
- Rustagi, V.; Aggarwal, T.; Verma, A.K. Highly efficient Ag(I)-catalyzed regioselective tandem synthesis of diversely substituted quinoxalines and benzimidazoles in water. Green Chem. 2011, 13, 1640–1643. [Google Scholar] [CrossRef]
- Peng, J.; Ye, M.; Zong, C.; Hu, F.; Feng, L.; Wang, X.; Wang, Y.; Chen, C. Copper-Catalyzed Intramolecular C-N Bond Formation: A Straightforward Synthesis of Benzimidazole Derivatives in Water. J. Org. Chem. 2011, 76, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Chanu, L.G.; Singh, O.M.; Jang, S.H.; Lee, S.-G. Regioselective Synthesis of Heterocyclic Ketene N,N-, N,O- and N,S –acetals in Aqueous Medium. Bull. Korean Chem. Soc. 2010, 31, 859–862. [Google Scholar] [CrossRef]
- Boeini, H.Z.; Najafabadi, K.H. Efficient One-Step Synthesis of Benzazoles in Aqueous Media. Eur. J. Org. Chem. 2009, 4926–4929. [Google Scholar] [CrossRef]
- Barbero, N.; Carril, M.; SanMartin, R.; Dominguez, E. Copper-catalyzed intramolecular N-arylation of ureas in water: A novel entry to benzoimidazolones. Tetrahedron 2008, 64, 7283–7288. [Google Scholar] [CrossRef]
- Mekala, R.S.; Balam, S.K.; Harinath, J.P.S.; Gajjal, R.R.; Cirandur, S.R. Polyethylene glycol (PEG-400): An efficient medium for the synthesis of 1,2-disubstituted benzimidazoles. Cogent Chem. 2015, 1, 1049932. [Google Scholar] [CrossRef]
- Hiebel, M.-A.; Fall, Y.; Scherrmann, M.-C.; Berteina-Raboin, S. Straightforward Synthesis of Various 2,3-Diarylimidazo[1,2-a]pyridines in PEG400 Medium through One-Pot Condensation and C–H Arylation. Eur. J. Org. Chem. 2014, 4643–4650. [Google Scholar] [CrossRef]
- Guchhait, S.K.; Madaan, C. An Efficient, Regioselective, Versatile Synthesis of N-Fused 2- and 3-Aminoimidazoles via Ugi-Type Multicomponent Reaction Mediated by Zirconium(IV) Chloride in Polyethylene Glycol-400. Synlett 2009, 4, 628–632. [Google Scholar] [CrossRef]
- Hiroki, H.; Ogata, K.; Fukuzawa, S.-I. l2et-teEr thynylpyridine-Promoted Rapid Copper(I) Chloride Catalyzed Azide–Alkyne Cycloaddition Reaction in Water. Synlett 2013, 24, 843–846. [Google Scholar] [CrossRef]
- Kshirsagar, S.S.; Mutha, M.M.; Shitre, M.R.; Baheti, K.G. Multicomponent stereo and regioselective reactions of beta lactams in water: Click reaction. OCAIJ 2013, 9, 229–235. [Google Scholar]
- Kumar, I.; Mir, N.A.; Rode, C.V.; Wakhloo, B.P. Intramolecular Huisgen [3+2] cycloaddition in water: Synthesis of fused pyrrolidine–triazoles. Tetrahedron Asymmetry 2012, 23, 225–229. [Google Scholar] [CrossRef]
- Kumar, B.S.P.A.; Reddy, K.H.V.; Madhav, B.; Ramesh, K.; Nageswar, Y.V.D. Magnetically separable CuFe2O4 nano particles catalyzed multicomponent synthesis of 1,4-disubstituted 1,2,3-triazoles in tap water using ‘click chemistry’. Tetrahedron Lett. 2012, 53, 4595–4599. [Google Scholar] [CrossRef]
- Reddy, A.S.; Reddy, M.N.; Swamy, K.C.K. A simple copper-catalysed tandem cyclisation of ynamides leading to triazolo-1,2,4-benzothiadiazine-1,1-dioxides in PEG-400 Medium. RSC Adv. 2014, 4, 28359–28367. [Google Scholar] [CrossRef]
- Saiprathima, P.; Srinivas, K.; Sridhar, B.; Rao, M.M. “On water” one-pot synthesis of quaternary centered 3-hydroxy-3-(1H-tetrazol-5-yl)indolin-2-ones. RSC Adv. 2013, 3, 7708–7712. [Google Scholar] [CrossRef]
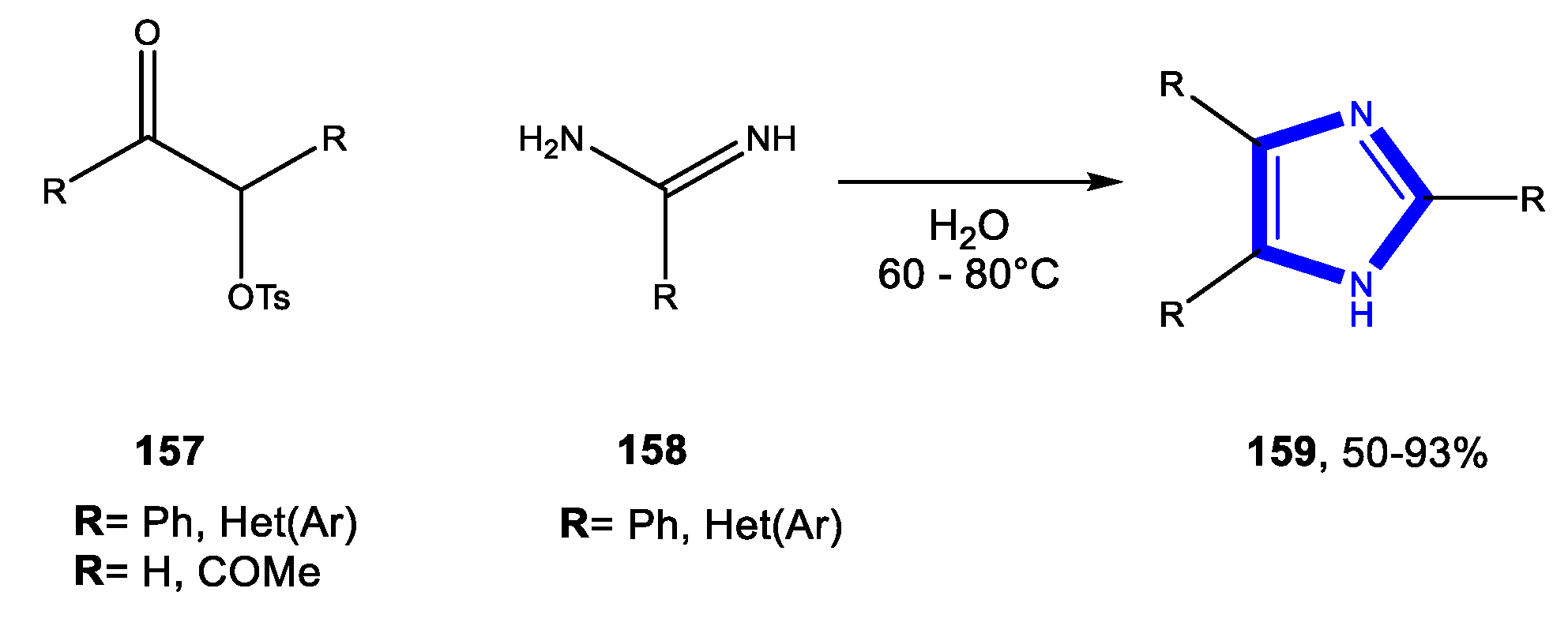
- Khalili, B.; Darabi, F.S.; Eftekhari-Sis, B.; Rimaz, M. Green chemistry: ZrOCl28H2O catalyzed regioselective synthesis of 5-amino-1-aryl-1H-tetrazoles from secondary arylcyanamides in water. Monatsh. Chem. 2013, 144, 1569–1572. [Google Scholar] [CrossRef]
- Habibi, D.; Nasrollahzadeh, M.; Faraji, A.R.; Bayat, Y. Efficient synthesis of arylaminotetrazoles in water. Tetrahedron 2010, 66, 3866–3870. [Google Scholar] [CrossRef]
- Wang, F.; Chen, C.; Deng, G.; Xi, C. Concise Approach to Benzisothiazol-3(2H)-one via Copper-Catalyzed Tandem Reaction of o-Bromobenzamide and Potassium Thiocyanate in Water. J. Org. Chem. 2012, 77, 4148–4151. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, A.; Ramulu, B.J.; Shukla, G.; Srivastava, A.; Verma, G.K.; Raghuvanshib, K.; Singh, M.S. Catalyst-free one-pot four-component domino reactions in water–PEG-400: Highly efficient and convergent approach to thiazoloquinoline scaffolds. Green Chem. 2015, 17, 950–958. [Google Scholar] [CrossRef]
- Sengoden, M.; Vijay, M.; Balakumara, E.; Punniyamurthy, T. Efficient Pyrrolidine Catalyzed Cycloaddition of Aziridines with Isothiocyanates, Isoselenocyanates and Carbon Disulfide “On Water”. RSC Adv. 2014, 4, 54149–54157. [Google Scholar] [CrossRef]
- Aryanasaba, F.; Shokrib, A.; Saidib, M.R. A simple approach to the synthesis of 3-substituted rhodanines and thiazolidine-2,4-diones. Sci. Iran. C 2013, 20, 1833–1838. [Google Scholar]
- Zhang, W.; Yue, Y.; Yu, D.; Song, L.; Xu, Y.-Y.; Tian, Y.-J.; Guo, Y.-J. 1,10-Phenanthroline-Catalyzed Tandem Reaction of 2-Iodoanilines with Isothiocyanates in Water. Adv. Synth. Catal. 2012, 354, 2283–2287. [Google Scholar] [CrossRef]
- Pathak, U.; Bhattacharyya, S.; Dhruwansh, V.; Pandey, L.K.; Tank, R.; Suryanarayana, M.V.S. Easy access to thiazolines and thiazines via tandem S-alkylation-cyclodeamination of thioamides/haloamines. Green Chem. 2011, 13, 1648–1651. [Google Scholar] [CrossRef]
- Yavari, I.; Sirouspour, M.; Souri, S. A one-pot synthesis of N-alkylthiazoline-2-thiones from CS2, primary amines, and 2-chloro-1,3-dicarbonyl compounds in water. Monatsh. Chem. 2010, 141, 49–52. [Google Scholar] [CrossRef]
- Tu, S.-J.; Cao, X.-D.; Hao, W.-J.; Zhang, X.-H.; Yan, S.; Wu, S.-S.; Han, Z.-G.; Shi, F. An efficient and chemoselective synthesis of benzo[e][1,4]thiazepin-2-(1H,3H,5H)-ones via a microwave-assisted multi-component reaction in water. Org. Biomol. Chem. 2009, 7, 557–563. [Google Scholar] [CrossRef]
- Potewar, T.M.; Ingale, S.A.; Srinivasan, K.V. Catalyst-free efficient synthesis of 2-aminothiazoles in water at ambient temperature. Tetrahedron 2008, 64, 5019–5022. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Rudrawar, S.; Jadhav, K.B.; Kaur, G.; Chankeshwara, S.V. “On water” organic synthesis: A highly efficient and clean synthesis of 2-aryl/heteroaryl/styryl benzothiazoles and 2-alkyl/aryl alkyl benzothiazolines. Green Chem. 2007, 9, 1335–1340. [Google Scholar] [CrossRef]
- Dumonteil, G.; Hiebel, M.-A.; Scherrmann, M.-C.; Berteina-Raboin, S. Iodine-catalyzed formation of substituted 2-aminobenzothiazole derivatives in PEG400. RSC Adv. 2016, 6, 73517–73521. [Google Scholar] [CrossRef]
- Jin, H.-L.; Cheng, T.-X.; Chen, J.-X. Cerium-catalyzed tandem synthesis of 2-substituted benzothiazoles in PEG. Appl. Organometal. Chem. 2011, 25, 238–240. [Google Scholar] [CrossRef]
- Deligeorgiev, T.G.; Kaloyanova, S.; Vasilev, A.; Vaquero, J.J. Novel Green Procedure for the Synthesis of 2-Arylbenzothiazoles Under Microwave Irradiation in Peg 200 Or Peg 400. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 2292–2302. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, J.; Xiao, H.; Liu, M.; Ding, J.; Wu, H. Efficient and Expeditious Synthesis of Di- and Trisubstituted Thiazoles in PEG Under Catalyst-Free Conditions. Synth. Commun. 2009, 39, 2895–2906. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Fang, Q.-S.; Zhou, J.; Song, Z.-B. Reusable proline-based ionic liquid catalyst for the simple synthesis of 2-arylbenzothiazoles in a biomass medium. Res. Chem. Intermed. 2016, 42, 2035–2045. [Google Scholar] [CrossRef]
- Halimehjani, A.Z.; Ashouri, A.; Marjani, K. Dithiocarbamates as an Efficient Intermediate for the Synthesis of Symmetrical Substituted 2,5-Diamino-1,3,4-thiadiazoles in Water. J. Heterocycl. Chem. 2012, 49, 939–942. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Zarnegar, Z.; Safari, J. Sonochemical synthesis of methyl-4-(hetero)arylmethylene isoxazole-5(4H)-ones using SnII-montmorillonite. Green Chem. Lett. Rev. 2018, 11, 78–85. [Google Scholar] [CrossRef]
- Dommaraju, Y.; Borthakura, S.; Prajapati, D. L-Proline-Catalysed One-Pot aza-Diels–Alder Reaction in Water: Regioselective Synthesis of Spiro(isoxazolo[5,4-b]pyridine-5,5′-pyrimidine) Derivatives. Synlett 2018, 29, 1195–1198. [Google Scholar] [CrossRef]
- Kiyani, H.; Ghorbani, F. Efficient tandem synthesis of a variety of pyranannulated heterocycles, 3,4-disubstituted isoxazol-5(4H)-ones, and a,b-unsaturated nitriles catalyzed by potassium hydrogen phthalate in water. Res. Chem. Intermed. 2015, 41, 7847–7882. [Google Scholar] [CrossRef]
- Chary, R.G.; Reddy, G.R.; Ganesh, Y.S.S.; Prasad, K.V.; Raghunadh, A.; Krishna, T.; Mukherjee, S.; Pal, M. Effect of Aqueous Polyethylene Glycol on 1,3-Dipolar Cycloaddition of Benzoylnitromethane/Ethyl 2-Nitroacetate with Dipolarophiles: Green Synthesis of Isoxazoles and Isoxazolines. Adv. Synth. Catal. 2014, 356, 160–164. [Google Scholar] [CrossRef]
- Trogu, E.; Vinattieri, C.; De Sarlo, F.; Machetti, F. Acid–Base-Catalysed Condensation Reaction in Water: Isoxazolines and Isoxazoles from Nitroacetates and Dipolarophiles. Chem. Eur. J. 2012, 18, 2081–2093. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Zhu, D.; Zhang, L.; Chua, P.J.; Zeng, X.; Zhong, G. Water—More Than Just a Green Solvent: A Stereoselective One-Pot Access to All-Chiral Tetrahydronaphthalenes in Aqueous Media. Chem. Eur. J. 2010, 16, 3842–3848. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.P.; Shridhar, G.; Ladage, S.; Ravishankar, L. A Facile Synthesis of Isoxazolone Derivatives Catalyzed by Cerium Chloride Heptahydrate in Ethyl Lactate as a Solvent: A Green Methodology. Curr. Green Chem. 2016, 3, 160–167. [Google Scholar] [CrossRef]
- Wallace, J.R.; Lieberman, D.L.; Hancock, M.T.; Pinhas, A.R. Conversion of an Aziridine to an Oxazolidinone Using a Salt and Carbon Dioxide in Water. J. Chem. Educ. 2005, 82, 1229–1230. [Google Scholar] [CrossRef]
- Balijapalli, U.; Thiyagarajan, M.D.; Manickam, S.; Sathiyanarayanan, K.I. Synthesis of T-shaped Oxazolonaphthoimidazo[1,2-a]pyridines using Lactic Acid as Bio–based Green Solvent: An Insight into Photophysical Studies. ChemistrySelect 2016, 1, 2900–2908. [Google Scholar] [CrossRef]
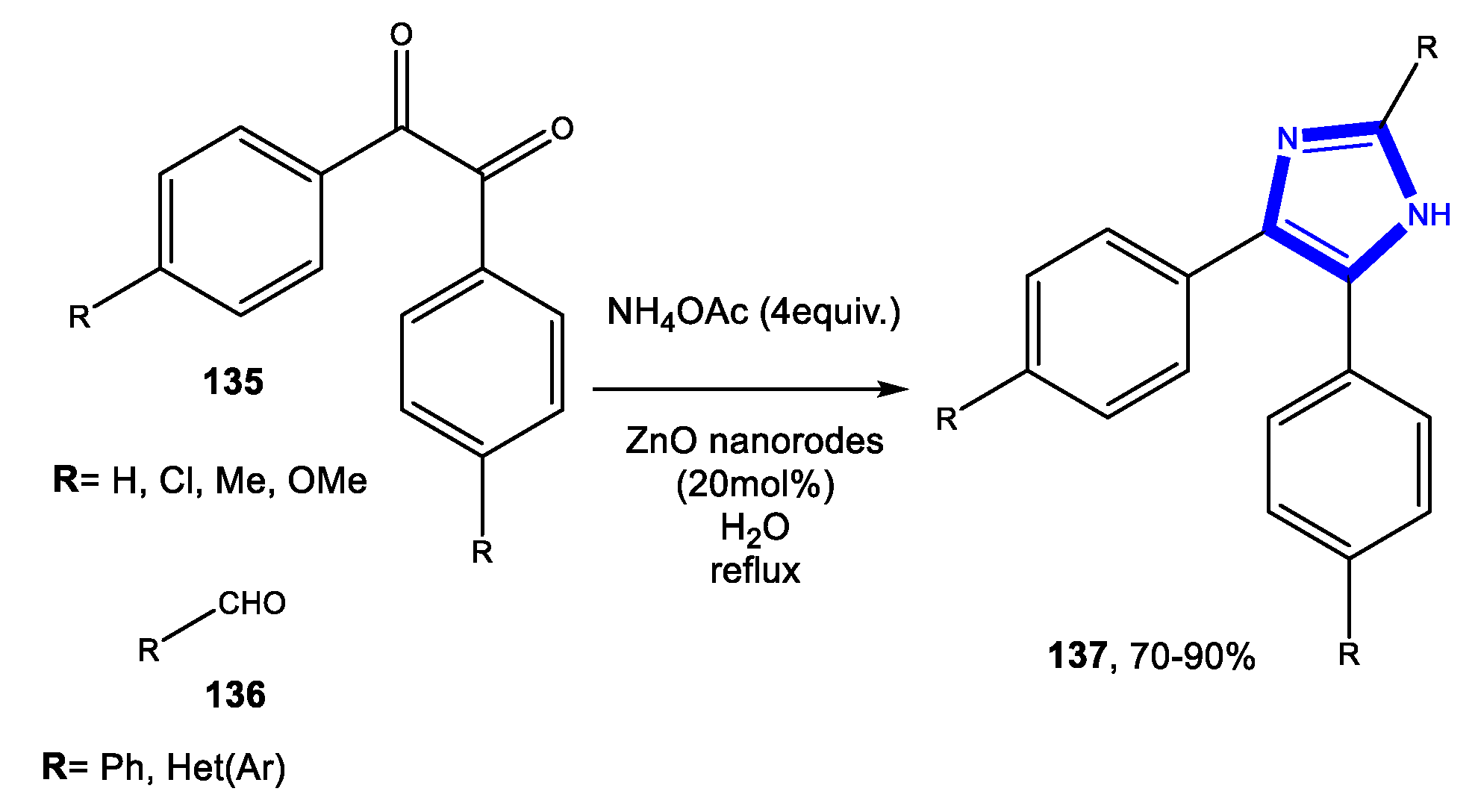
- Naeimi, H.; Didar, A. Efficient sonochemical green reaction of aldehyde, thiobarbituric acid and ammonium acetate using magnetically recyclable nanocatalyst in water. Ultrason Sonochem. 2017, 34, 889–895. [Google Scholar] [CrossRef]
- Lezana, N.; Matus-Pérez, M.; Galdámez, A.; Lührb, S.; Vilches -Herrera, M. Highly stereoselective and catalyst-free synthesis of annulated tetrahydropyridines by intramolecular imino-Diels–Alder reaction under microwave irradiation in water. Green Chem. 2016, 18, 3712–3717. [Google Scholar] [CrossRef]
- Abdelmoniem, A.M.; Hassaneen, H.M.E.; Abdelhamid, I.A. An Efficient One-pot Synthesis of Novel Spiro Cyclic 2-Oxindole Derivatives of Pyrimido[4,5-b]Quinoline, Pyrido[2,3-d:6,5-d′]Dipyrimidine and Indeno[2′,1′:5,6]Pyrido [2,3-d]Pyrimidine in Water. J. Heterocycl. Chem. 2016, 53, 2084–2090. [Google Scholar] [CrossRef]
- Yaragorla, S.; Singh, G.; Pareek, A. Highly stereoselective and catalyst-free synthesis of annulated tetrahydropyridines by intramolecular imino-Diels–Alder reaction under microwave irradiation in water. Indian J. Chem. 2015, 54, 1321–1326. [Google Scholar]
- Zhao, H.-W.; Yang, Z.; Yue, Y.-Y.; Li, H.-L.; Song, X.-Q.; Sheng, Z.-H.; Meng, W.; Guo, X.-Y. l Asymmetric Direct Michael Reactions of Cyclohexanone with Aromatic Nitroolefins in Water Catalyzed by Novel Axially Unfixed Biaryl-Based Bifunctional Organocatalysts. Synlett 2014, 25, 293–297. [Google Scholar] [CrossRef]
- Mosslemin, M.H.; Zarenezhad, E.; Shams, N.; Rad, M.N.S.; Anaraki-Ardakani, H.; Fayazipoor, R. Green synthesis of 5-aryl-(1H,3H,5H,10H)-pyrimido[4,5-b]quinoline-2,4-diones catalysed by 1,4-diazabicyclo[2.2.2]octane in water. J. Chem. Res. 2014, 38, 169–171. [Google Scholar] [CrossRef]
- Siddiqui, I.R.; Rai, P.; Rahila; Srivastava, A.; Srivastava, A.; Srivastava, A. Synthesis of fused pyridines in the presence of thiamine hydrochloride as an efficient and reusable catalyst in aqueous conditions. New J. Chem. 2013, 37, 3798–3804. [Google Scholar] [CrossRef]
- Nasseri, M.A.; Alavi, S.A.; Zakerinasab, B. PEG-SO3H as a catalyst in aqueous media: A simple, proficient and green approach for the synthesis of quinoline derivatives. J. Chem. Sci. 2013, 125, 109–116. [Google Scholar] [CrossRef]
- Makone, S.S.; Niwadange, S.N. An efficient and simple un-catalysed synthesis of 1,4-dihydropyridines in an aqueous media. Chem. Sin. 2012, 3, 1293–1296. [Google Scholar]
- Khurana, J.M.; Chaudhary, A.; Nand, B.; Lumb, A. Aqua mediated indium(III) chloride catalyzed synthesis of fused pyrimidines and pyrazoles. Tetrahedron Lett. 2012, 53, 3018–3022. [Google Scholar] [CrossRef]
- Kumar, N.; Verma, S.; Jain, S.L. Combined Thiourea DioxideWater: An Effective Reusable Catalyst for the Synthesis of Polyhydroquinolines via Hantzsch Multicomponent Coupling. Chem. Lett. 2012, 41, 920–922. [Google Scholar] [CrossRef]
- Rahmati, A.; Khalesi, Z. Catalyst free synthesis of fused pyrido[2,3-d]pyrimidines and pyrazolo[3,4-b]pyridines in water. Chin. Chem. Lett. 2012, 23, 1149–1152. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, L.; Yu, J.; Liu, M.; Qiu, J.; Fang, L.; Guo, F.; Tang, J. Synthesis of Quinolines via Friedländer Reaction in Water and under Catalyst-Free Conditions. Synthesis 2012, 44, 389–392. [Google Scholar] [CrossRef]
- Xu, F.; Wang, C.; Li, X.; Wan, B. Ruthenium-catalyzed [2+2+2] Cycloaddition of Diynes with Nitriles in Pure Water. ChemSusChem 2012, 5, 854–857. [Google Scholar] [CrossRef]
- Mukhopadhyay, C.; Das, P.; Butcher, R.J. An Expeditious and Efficient Synthesis of Highly Functionalized [1,6]-Naphthyridines under Catalyst-Free Conditions in Aqueous Medium. Org. Lett. 2011, 13, 4664–4667. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.; Muthusubramanian, S.; Bhuvanesh, N. A green protocol for the synthesis of conformationally rigid sulfur linked bisquinolines by double Friedlander reaction in water. New J. Chem. 2011, 35, 2607–2613. [Google Scholar] [CrossRef]
- Das, B.; Jangili, P.; Kashanna, J.; Kumar, R.A. Organic Reactions in Water: A Distinct Approach for the Synthesis of Quinoline Derivatives Starting Directly from Nitroarenes. Synthesis 2011, 20, 3267–3270. [Google Scholar] [CrossRef]
- Madhav, B.; Murthy, S.N.; Rao, K.R.; Nageswar, Y.V.D. An Improved Protocol for the Synthesis of Quinoline-2,3-dicarboxylates under Neutral Conditions via Biomimetic Approach. Helv. Chim. Acta 2010, 93, 257–260. [Google Scholar] [CrossRef]
- Wu, L.Q.; Dong, R.-Y.; Yang, C.-G.; Yan, F.-L. Synthesis of 4-Aryl-3-methyl-1-phenyl-1Hbenzo[h]pyrazolo[3,4-b]quinoline-5,10-diones Using Diammonium Hydrogen Phosphate as an Efficient and Versatile Catalyst in Water. J. Chin. Chem. Soc. 2010, 57, 19–23. [Google Scholar] [CrossRef]
- Akbari, J.; Heydari, A.; Kalhor, H.R.; Kohan, S.A. Sulfonic Acid Functionalized Ionic Liquid in Combinatorial Approach, a Recyclable and Water Tolerant-Acidic Catalyst for One-Pot Friedlander Quinoline Synthesis. J. Comb. Chem. 2010, 12, 137–140. [Google Scholar] [CrossRef]
- Shi, D.-Q.; Yao, H. Facile and Clean Synthesis of Furopyridine Derivatives via Three-Component Reaction in Aqueous Media Without Catalyst. Synth. Commun. 2009, 39, 2481–2491. [Google Scholar] [CrossRef]
- Shi, F.; Wang, Q.; Tu, S.; Zhou, J.; Jiang, B.; Li, C.; Zhou, D.; Shao, Q.; Cao, L. A Green and Efficient Synthesis of Furo[3,4-e]pyrazolo[3,4-b]-pyridine Derivatives in Water under Microwave Irradiation without Catalyst. J. Heterocycl. Chem. 2008, 45, 1103–1108. [Google Scholar] [CrossRef]
- Shi, F.; Zhou, D.; Tu, S.; Shao, Q.; Li, C.; Cao, L. An Efficient Microwave-assisted Synthesis Furo[3,4-b]-[4,7]phenanthroline and Indeno[2,1-b][4,7]phenanthroline Derivatives in Water. J. Heterocycl. Chem. 2008, 45, 1065–1070. [Google Scholar] [CrossRef]
- Shi, F.; Zhou, D.; Tu, S.; Li, C.; Cao, L.; Shao, Q. Pot, Atom and Step Economic Synthesis of Fused Three Heterocyclic Ring Compounds under Microwave Irradiation in Water. J. Heterocycl. Chem. 2008, 45, 1305–1310. [Google Scholar] [CrossRef]
- Shi, F.; Zhang, Y.; Tu, S.-J.; Zhou, D.-X.; Li, C.-M.; Shao, Q.-Q.; Cao, L.-J. A Green Approach to the Synthesis of Biologically Important Indeno[2,1-e]pyrazolo[5,4-b]pyridines via Microwave-assisted Multi-component Reactions in Water. Chin. J. Chem. 2008, 26, 1262–1266. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, R.A. Synthesis of polyhydroquinoline derivatives through unsymmetric Hantzsch reaction using organocatalysts. Tetrahedron 2007, 63, 1946–1952. [Google Scholar] [CrossRef]
- Wang, G.-W.; Jia, C.-S.; Dong, Y.-W. Benign and highly efficient synthesis of quinolines from 2-aminoarylketone or 2-aminoarylaldehyde and carbonyl compounds mediated by hydrochloric acid in water. Tetrahedron Lett. 2006, 47, 1059–1063. [Google Scholar] [CrossRef]
- Tu, S.-J.; Jiang, B.; Zhang, J.-Y.; Jia, R.-H.; Zhang, Y.; Yao, C.-S. Efficient and direct synthesis of poly-substituted indeno[1,2-b]quinolones assisted by p-toluene sulfonic acid using high-temperature water and microwave heating via one-pot, three-component reaction. Org. Biomol. Chem. 2006, 4, 3980–3985. [Google Scholar] [CrossRef] [PubMed]
- Loncaric, C.; Manabe, K.; Kobayashi, S. AgOTf-Catalyzed Aza-Diels-Alder Reactions of Danishefsky’s Diene with Imines in Water. Adv. Synth. Catal. 2003, 345, 475–477. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Wan, Y.; Huang, Y.; Huang, G.; Zhang, G. Ammonium Acetate-Promoted One-Pot Tandem Aldol Condensation/Aza-Addition Reactions: Synthesis of 2,3,6,7-Tetrahydro-1H-pyrrolo[3,2-c]pyridin-4(5H)-ones. ACS Omega 2017, 2, 6844–6851. [Google Scholar] [CrossRef] [PubMed]
- Maryamabadi, A.; Hasaninejad, A.; Nowrouzi, N.; Mohebbi, G.; Asghari, B. Application of PEG-400 as a green biodegradable polymeric medium for the catalyst-free synthesis of spiro-dihydropyridines and their use as acetyl and butyrylcholinesterase inhibitors. Bioorg. Med. Chem. 2016, 24, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Singh, V.; Das, P.; Choudhury, L.H. PEG-mediated one-pot multicomponent reactions for the efficient synthesis of functionalized dihydropyridines and their functional group dependent DNA cleavage activity. Bioorg. Chem. 2013, 48, 8–15. [Google Scholar] [CrossRef]
- Paidepala, H.; Nagendra, S.; Saddanappu, V.; Addlagatta, A.; Das, B. Catalyst-free efficient synthesis of polyhydroquinolines using polyethylene glycol as a solvent and evaluationof their cytotoxicity. Med. Chem. Res. 2014, 23, 1031–1036. [Google Scholar] [CrossRef]
- Wu, F.-W.; Hou, R.-S.; Wang, H.-M.; Kang, I.-J.; Chen, L.-C. Catalyst Free Indirect Friedländer Synthesis of Substituted Quinolines from Alcohols in PEG-400. J. Chin. Chem. Soc. 2012, 59, 535–539. [Google Scholar] [CrossRef]
- Karnakar, K.; Murthy, S.N.; Ramesh, K.; Satish, G.; Nanubolu, J.B.; Nageswar, Y.V.D. Polyethylene glycol (PEG-400): An efficient and recyclable reaction medium for the synthesis of pyrazolo[3,4-b]quinoline derivatives. Tetrahedron Lett. 2012, 53, 2897–2903. [Google Scholar] [CrossRef]
- Nalage, S.V.; Nikum, A.P.; Kalyankar, M.B.; Patil, V.S.; Patil, U.D.; Desale, K.R.; Patil, S.L.; Bhosale, S.V. One-Pot Four Component Synthesis of 4, 6-Disubstituted 3-Cyano-2-Pyridones in Polyethylene Glycol. Lett. Org. Chem. 2010, 7, 406–410. [Google Scholar] [CrossRef]
- Manvar, A.; Karia, D.; Trangadia, V.; Vekariya, N.; Shah, A. PEG-400 mediated and microwave assisted one pot three-component coupling reactions: Expedient and rapid synthesis of Hantzsch 1,4-dihydropyridines devoid of use of catalyst. OCAIJ 2007, 3, 166–169. [Google Scholar]
- Smith, N.M.; Raston, C.L.; Smith, C.B.; Sobolev, A.N. PEG mediated synthesis of amino-functionalised 2,4,6-triarylpyridines. Green Chem. 2007, 9, 1185–1190. [Google Scholar] [CrossRef]
- Paul, S.; Das, A.R. An efficient green protocol for the synthesis of coumarin fused highly decorated indenodihydropyridyl and dihydropyridyl derivatives. Tetrahedron Lett. 2012, 53, 2206–2210. [Google Scholar] [CrossRef]
- Rimaz, M.; Khalafy, J. A novel one-pot, three-component synthesis of alkyl 6-aryl-3-methylpyridazine-4-carboxylates in water. ARKIVOC 2010, 110–117. [Google Scholar] [CrossRef]
- Rimaz, M.; Khalafy, J.; Moghadam, P.N. A Regioselective One-Pot, Three Component Synthesis of 6-Aryl-4-cyano-3(2H)-pyridazinones in Water. Aust. J. Chem. 2010, 63, 1396–1401. [Google Scholar] [CrossRef]
- Yan, H.; Ma, Y.; Sun, Y.; Ma, C.; Wang, Y.; Ren, X.; Huang, G. Catalyst-free synthesis of alkyl 4-oxo-4H-pyrido[1,2-a]pyrimidine-2-carboxylate derivatives on water. Tetrahedron 2014, 70, 2761–2765. [Google Scholar] [CrossRef]
- Siddiqui, Z.N. Bis[(L)prolinato-N,O]Zn–water: A green catalytic system for the synthesis of 3,4-dihydropyrimidin-2 (1H)-ones via the Biginelli reaction. Comptes Rendus Chim. 2013, 16, 183–188. [Google Scholar] [CrossRef]
- Mukhopadhyay, C.; Datta, A. Synthesis of Substituted Pyrimidinones Catalyzed by Boric Acid and Glycerol in Aqueous Medium. Synth. Commun. 2013, 43, 438–449. [Google Scholar] [CrossRef]
- Ramesh, K.; Karnakar, K.; Satish, G.; Kumar, B.S.P.A.; Nageswar, Y.V.D. A concise aqueous phase supramolecular synthesis of 2-phenyl-2,3-dihydroquinazolin-4(1H)-one derivatives. Tetrahedron Lett. 2012, 53, 6936–6939. [Google Scholar] [CrossRef]
- Li, Q.-Y.; Ge, Z.-M.; Cheng, T.-M.; Li, R.-T. An efficient three-component, one-pot synthesis of 2-alkylthio-4-amino-5-cyano-6-aryl(alkyl)pyrimidines in water. Mol. Divers. 2012, 16, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ye, D.; Sun, H.; Guo, D.; Wang, J.; Huang, H.; Zhang, X.; Jianga, H.; Liu, H. Microwave-assisted synthesis of quinazolinone derivatives by efficient and rapid iron-catalyzed cyclization in water. Green Chem. 2009, 11, 1881–1888. [Google Scholar] [CrossRef]
- Xu, F.; Wang, J.J.; Tian, Y.P. New Procedure for One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones by Biginelli Reaction. Synth. Commun. 2008, 38, 1299–1310. [Google Scholar] [CrossRef]
- Hese, S.V.; Kamble, R.D.; Mogle, P.P.; Kadam, S.S.; Hebade, M.J.; Ambhore, A.N.; Dawane, B.S. Green synthesis and antimicrobial evaluation ofpyrido[1,2-a]pyrimidine3-carbonitrile derivatives. Pharma Chem. 2015, 7, 249–256. [Google Scholar]
- Konda, S.G. PEG-400: An efficient and recyclable reaction medium for the synthesis of pyrazolo[1,5-a]pyrimidines. Eur. J. Chem. 2014, 5, 676–680. [Google Scholar] [CrossRef]
- Reddy, B.S.; Naidu, A.; Dubey, P.K. PEG-600-mediated, green and efficient, tandem syntheses of N-subtituted-2-styrylquinazolin-4-ones. Green Chem. Lett. Rev. 2013, 6, 254–261. [Google Scholar] [CrossRef]
- Maddila, S.; Jonnalagadda, S.B.; Chunduri, V.; Lavanya, P. Solvent-free splendid one pot synthesis of 2-amino-6-(substitutedphenyl)-5-methylpyrimidin-4-ol using PEG-400. Heterocycl. Lett. 2012, 2, 37–42. [Google Scholar]
- Sharafi-Kolkeshvandi, M.; Nikpour, F. A facile and convenient approach for the one-pot synthesis of 2,4(1H,3H)-quinazolinediones. Chin. Chem. Lett. 2012, 23, 431–433. [Google Scholar] [CrossRef]
- Kidwai, M.; Chauhan, R.; Bhatnagar, D. Amberlyst-15® in PEG: A novel catalytic system for the facile and efficient one-pot synthesis of benzothiazolo-[2,3-b]-quinazolinone derivatives. Sci. China Chem. 2012, 55, 2154–2160. [Google Scholar] [CrossRef]
- Nikpour, F.; Mozafari, R.; Paibast, T. A facile and convenient method to the one-pot synthesis of 2-mercapto-4(3h)-quinazolinones. Heterocycles 2009, 78, 1569–1571. [Google Scholar] [CrossRef]
- Wang, X.; Quan, Z.; Zhang, Z. Michael additions of dihydropyrimidines and 2-amino-1,3,4-thiadiazoles to a,b-ethylenic compounds: Using polyethylene glycols as a green reaction media. Tetrahedron 2007, 63, 8227–8233. [Google Scholar] [CrossRef]
- Khandelwal, S.; Rajawat, A.; Kumar, M. An Efficient and Environmentally Benign One-pot Three- Component Domino Protocol for the Synthesis of Structurally Diverse Spiroquinazolines. Curr. Catal. 2015, 4, 214–223. [Google Scholar] [CrossRef]
- Khandelwal, S.; Rajawat, A.; Tailor, Y.K.; Kumar, M. Efficient and Environmentally Benign Diversity Oriented Synthesis of 2, 3-dihydroquinazolin-4(1H)-ones Using GAAS As a Bio-based Green Solvent. Curr. Green Chem. 2015, 2, 156–162. [Google Scholar] [CrossRef]
- Xu, Z.; Jianga, Y.; Zou, S.; Liu, Y. Bio-Based Solvent Mediated Synthesis of Dihydropyrimidinthiones Via Biginelli Reaction. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 791–795. [Google Scholar] [CrossRef]
- Rashidi, R.; Nasr-Esfahani, M.; Mohammadpoor-Baltork, I.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V. Highly selective synthesis of mono- and bis-4,5-dihydropyrrolo[1,2-a]quinoxalines catalyzed by sustainable supported acidic ionic liquid in water media. Monatsh. Chem. 2018, 149, 557–567. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, Y.; Sui, Y.; Liu, H.; Zhou, H. B2(OH)4-mediated one-pot synthesis of tetrahydroquinoxalines from 2-amino(nitro)anilines and 1, 2-dicarbonyl compounds in water. Org. Chem. Front. 2017, 4, 2175–2178. [Google Scholar] [CrossRef]
- Yazdani-Elah-Abadi, A.; Mohebat, R.; Maghsoodlou, M.-T. Theophylline as the catalyst for the diastereoselective synthesis of trans-1,2-dihydrobenzo[a]furo[2,3-c]phenazines in water. RSC Adv. 2016, 6, 84326–84333. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Malakooti, R.; Abtin, I.; Atashin, H. Palladium Schiff-base complex loaded SBA-15 as a novel nanocatalyst for the synthesis of 2,3-disubstituted quinoxalines and pyridopyrazine derivatives. Micropor. Mesopor. Mat. 2013, 169, 67–74. [Google Scholar] [CrossRef]
- Alinezhad, H.; Tajbakhsh, M.; Norouzi, M.; Baghery, S. An Efficient and Green Protocol for the Synthesis of 1,5-benzodiazepine and Quinoxaline Derivatives Using Protic Pyridinium Ionic Liquid as a Catalyst. World Appl. Sci. J. 2013, 22, 1711–1717. [Google Scholar] [CrossRef]
- Bardajee, G.R. ZrOCl28H2O in water: An efficient catalyst for rapid one-pot synthesis of pyridopyrazines, pyrazines and 2,3-disubstituted quinoxalines. Comptes Rendus Chim. 2013, 16, 872–877. [Google Scholar] [CrossRef]
- Feng, E.; Zhou, Y.; Zhao, F.; Chen, X.; Zhang, L.; Jiang, H.; Liu, H. Gold-catalyzed tandem reaction in water: An efficient and convenient synthesis of fused polycyclic indoles. Green Chem. 2012, 14, 1888–1895. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Liu, B.-K.; Chen, W.-Q.; Wu, Q.; Lin, X.-F. A green protocol for synthesis of benzo-fused N,S-, N,O- and N,N-heterocycles in water. Green Chem. 2008, 10, 972–977. [Google Scholar] [CrossRef]
- Srinivas, C.; Kumar, C.N.S.S.P.; Rao, V.J.; Palaniappan, S. Green Approach for the Synthesis of Quinoxaline Derivatives in Water Medium Using Reusable Polyaniline-sulfate Salt Catalyst and Sodium Laurylsulfate. Catal. Lett. 2008, 121, 291–296. [Google Scholar] [CrossRef]
- Khurana, J.M.; Chaudhary, A.; Lumb, A.; Nand, B. An expedient four-component domino protocol for the synthesis of novel benzo[a]phenazine annulated heterocycles and their photophysical studies. Green Chem. 2012, 14, 2321–2327. [Google Scholar] [CrossRef]
- Huang, T.; Jiang, D.; Chen, J.; Gao, W.; Ding, J.; Wu, H. Silica Sulfuric Acid (SSA)/Polyethylene Glycol (PEG) as a Recyclable System for the Synthesis of Quinoxalines and Pyrazines. Synth. Commun. 2011, 41, 3334–3343. [Google Scholar] [CrossRef]
- Nagarapu, L.; Mallepalli, R.; Arava, G.; Yeramanchi, L. Polyethylene glycol (PEG-400) mediated synthesis of quinoxalines. Eur. J. Chem. 2010, 1, 228–231. [Google Scholar] [CrossRef]
- Abdollahi–Irandegan, A.; Hassanabadi, A. Synthesis of 4-aryl-7-methyl-3,4-dihydropyrano[3,4-e][1,3]oxazine-2,5-dione in aqueous media. J. Chem. Res. 2016, 40, 727–728. [Google Scholar] [CrossRef]
- Rostami-Charati, F.; Hossaini, Z.; Gharaee, E.; Khalilzadeh, M.A. One-Pot Three-Component Synthesis of Oxazine Derivatives in Water. J. Heterocycl. Chem. 2013, 50, E174–E177. [Google Scholar] [CrossRef]
- Paira, R.; Paira, P.; Maity, A.; Mondal, S.; Hazra, A.; Sahu, K.B.; Naskar, S.; Saha, P.; Banerjee, M.; Mondal, N.B. Amberlite IRA 402(OH): An efficient mediator for the exclusive synthesis of fused tricyclic oxaza quinolinium salts. Tetrahedron Lett. 2010, 51, 3200–3204. [Google Scholar] [CrossRef]
- Shinde, P.V.; Kategaonkar, A.H.; Shingate, B.B.; Shingare, M.S. Polyethylene glycol (PEG) mediated expeditious synthetic route to 1,3-oxazine derivatives. Chin. Chem. Lett. 2011, 22, 915–918. [Google Scholar] [CrossRef]
- Sridhar, R.; Yedukondalu, M.; Prasad, K.R.S.; Rao, M.V.B. Polyethylene glycol mediated catalyst-free synthesis of 5,9b-dihydro-1H-[1,2,4] triazino[5,6-b]indole-3-ols/thioles. Pharma Chem. 2014, 6, 1–8. [Google Scholar]
- Prasad, J.V.; Prabhakar, M.; Manjulatha, K.; Rambabu, D.; Solomon, K.A.; Krishna, G.G.; Kumar, K.A. Efficient catalyst-free Domino approach for the synthesis of novel 2-benzazepine derivatives in water. Tetrahedron Lett. 2010, 51, 3109–3111. [Google Scholar] [CrossRef]
- Mallepalli, R.; Yeramanchi, L.; Bantu, R.; Nagarapu, L. Polyethylene Glycol (PEG-400) as an Efficient and Recyclable ReactionMedium for the One-Pot Synthesis of N-Substituted Azepines under Catalyst-Free Conditions. Synlett 2011, 18, 2730–2732. [Google Scholar] [CrossRef]
- Shaabani, A.; Maleki, A.; Mofakham, H.; Moghimi-Rad, J. A Novel One-Pot Pseudo-Five-Component Synthesis of 4,5,6,7-Tetrahydro-1H-1,4-diazepine-5-carboxamide Derivatives. J. Org. Chem. 2008, 73, 3925–3927. [Google Scholar] [CrossRef] [PubMed]
- Dandia, A.; Singh, R.; Joshi, J.; Maheshwari, S. Green and chemoselective synthesis of pyrazolo[3,4-e][1,4]thiazepines and evaluation of their anti-infective activities. Res. Chem. Intermed. 2015, 41, 4213–4226. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, X.; Wu, L. Facile Synthesis of 1,5-Benzothiazepines in Water Using Tetrabutylammonium Tribromide. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 573–579. [Google Scholar] [CrossRef]
- Lingampalle, D.L.; Netankar, P.D.; Jagrut, V.B.; Mane, R.A. PEG-400 mediated synthesis of 1, 5-benzothiazepines. J. Chem. Biol. Interfaces 2014, 4, 287–291. [Google Scholar]























































































































© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, J.F.; Berteina-Raboin, S. Greener Synthesis of Nitrogen-Containing Heterocycles in Water, PEG, and Bio-Based Solvents. Catalysts 2020, 10, 429. https://doi.org/10.3390/catal10040429
Campos JF, Berteina-Raboin S. Greener Synthesis of Nitrogen-Containing Heterocycles in Water, PEG, and Bio-Based Solvents. Catalysts. 2020; 10(4):429. https://doi.org/10.3390/catal10040429
Chicago/Turabian StyleCampos, Joana F., and Sabine Berteina-Raboin. 2020. "Greener Synthesis of Nitrogen-Containing Heterocycles in Water, PEG, and Bio-Based Solvents" Catalysts 10, no. 4: 429. https://doi.org/10.3390/catal10040429
APA StyleCampos, J. F., & Berteina-Raboin, S. (2020). Greener Synthesis of Nitrogen-Containing Heterocycles in Water, PEG, and Bio-Based Solvents. Catalysts, 10(4), 429. https://doi.org/10.3390/catal10040429





