A Rapid Method for the Selection of Amidohydrolases from Metagenomic Libraries by Applying Synthetic Nucleosides and a Uridine Auxotrophic Host
Abstract
:1. Introduction
2. Results and Discussion
2.1. Functional Screening of Metagenomic Libraries
2.2. Bioinformatic Analysis of the Selected Clones
2.3. Substrate Specificity of the Selected Amidohydrolases
2.4. Catalytic Properties of the Amidohydrolase D8_RL
3. Materials and Methods
3.1. Materials
3.2. Functional Screening of Metagenomic Libraries
3.3. DNA Sequencing and Gene Annotation
3.4. Overexpression and Purification of Hydrolases
3.5. Enzymatic Activity of Hydrolases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Locey, K.J.; Lennon, J.T. Scaling laws predict global microbial diversity. Proc. Natl. Acad. Sci. USA 2016, 113, 5970–5975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.M.; Seo, C.; Ji, M.; Paik, M.-J.; Myung, S.-W.; Kim, J. Effective soil extraction method for cultivating previously uncultured soil bacteria. Appl. Environ. Microbiol. 2018, 84, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colin, P.-Y.; Kintses, B.; Gielen, F.; Miton, C.M.; Fischer, G.; Mohamed, M.F.; Hyvönen, M.; Morgavi, D.P.; Janssen, D.B.; Hollfelder, F. Ultrahigh-throughput discovery of promiscuous enzymes by picodroplet functional metagenomics. Nat. Commun. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T. The fourth wave of biocatalysis is approaching. Philos. Trans. R. Soc. A 2018, 376, 20170063. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, P.; Eck, J. Metagenomics and industrial applications. Nat. Rev. Microbiol. 2005, 3, 510. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, C.C.C.R. Whole cell biocatalysts: Essential workers from Nature to the industry. Microb. Biotechnol. 2017, 10, 250–263. [Google Scholar] [CrossRef] [Green Version]
- Galperin, M.Y.; Koonin, E.V. From complete genome sequence to ‘complete’ understanding? Trends Biotechnol. 2010, 28, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Montella, S.; Amore, A.; Faraco, V. Metagenomics for the development of new biocatalysts to advance lignocellulose saccharification for bioeconomic development. Crit. Rev. Biotechnol. 2016, 36, 998–1009. [Google Scholar] [CrossRef]
- Ngara, T.R.; Zhang, H. Recent Advances in Function-based Metagenomic Screening. Genom. Proteom. Bioinform. 2018, 16, 405–415. [Google Scholar] [CrossRef]
- Aučynaitė, A.; Rutkienė, R.; Tauraitė, D.; Meškys, R.; Urbonavičius, J. Discovery of bacterial deaminases that convert 5-Fluoroisocytosine into 5-Fluorouracil. Front. Microbiol. 2018, 9, 2375. [Google Scholar] [CrossRef]
- Aučynaitė, A.; Rutkienė, R.; Tauraitė, D.; Meškys, R.; Urbonavičius, J. Identification of a 2′-O-Methyluridine nucleoside hydrolase using the metagenomic libraries. Molecules 2018, 23, 2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbelienė, N.; Kutanovas, S.; Meškienė, R.; Gasparavičiūtė, R.; Tauraitė, D.; Koplūnaitė, M.; Meškys, R. Application of the uridine auxotrophic host and synthetic nucleosides for a rapid selection of hydrolases from metagenomic libraries. Microb. Biotechnol. 2019, 12, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, K. A breakthrough in enzyme technology to fight penicillin resistance—Industrial application of penicillin amidase. Appl. Microbiol. Biotechnol. 2016, 100, 3825–3839. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-P.; Wu, Z.-M.; Tang, X.-L.; Hao, C.-L.; Zheng, R.-C.; Zheng, Y.-G. Continuous production of aprepitant chiral intermediate by immobilized amidase in a packed bed bioreactor. Bioresour. Technol. 2019, 274, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Bhatia, S.K.; Bhatia, R.K.; Bhalla, T.C. Bench scale production of nicotinic acid using a versatile amide-hydrolysing Geobacillus subterraneus RL-2a isolated from thermal spring of Manikaran, India. J. Mol. Catal. B Enzym. 2014, 105, 58–65. [Google Scholar] [CrossRef]
- Tang, X.-L.; Jin, J.-Q.; Wu, Z.-M.; Jin, L.-Q.; Zheng, R.-C.; Zheng, Y.-G. Structure-based engineering of amidase from Pantoea sp. for efficient 2-Chloronicotinic acid biosynthesis. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.-M.; Cai, X.; Liu, Z.-Q.; Zheng, Y.-G. Identification and characterization of an amidase from Leclercia adecarboxylata for efficient biosynthesis of L-phosphinothricin. Bioresour. Technol. 2019, 289, 121658. [Google Scholar] [CrossRef]
- Ghonemy, D.H.E. Microbial amidases and their industrial applications: A Review. J. Med. Microbiol. Diagn. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Gabor, E.M.; de Vries, E.J.; Janssen, D.B. Construction, characterization, and use of small-insert gene banks of DNA isolated from soil and enrichment cultures for the recovery of novel amidases. Environ. Microbiol. 2004, 6, 948–958. [Google Scholar] [CrossRef] [Green Version]
- Krieg, L.; Ansorge-Schumacher, M.B.; Kula, M.-R. Screening for Amidases: Isolation and Characterization of a novel D-amidase from Variovorax paradoxus. Adv. Synth. Catal. 2002, 344, 965–973. [Google Scholar] [CrossRef]
- Kang, X.-M.; Zhang, X.-J.; Hong, L.-L.; Peng, F.; Liu, Z.-Q.; Zheng, Y.-G. Establishment of a novel high-throughput screening method for the detection and quantification of L-phosphinothricin produced by a biosynthesis approach. Process Biochem. 2019, 76, 136–141. [Google Scholar] [CrossRef]
- Tannières, M.; Beury-Cirou, A.; Vigouroux, A.; Mondy, S.; Pellissier, F.; Dessaux, Y.; Faure, D. A Metagenomic study highlights phylogenetic proximity of quorum-quenching and xenobiotic-degrading amidases of the AS-family. PLoS ONE 2013, 8, e65473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, R.-C.; Zheng, Y.-G.; Shen, Y.-C. A screening system for active and enantioselective amidase based on its acyl transfer activity. Appl. Microbiol. Biotechnol. 2007, 74, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Pérez, R.; García-Saura, A.G.; Jebbar, M.; Golyshin, P.N.; Sánchez-Ferrer, Á. Combined whole-cell high-throughput functional screening for identification of new nicotinamidases/pyrazinamidases in metagenomic/polygenomic libraries. Front. Microbiol. 2016, 7, 1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Časaitė, V.; Sadauskas, M.; Vaitekūnas, J.; Gasparavičiūtė, R.; Meškienė, R.; Skikaitė, I.; Sakalauskas, M.; Jakubovska, J.; Tauraitė, D.; Meškys, R. Engineering of a chromogenic enzyme screening system based on an auxiliary indole-3-carboxylic acid monooxygenase. MicrobiologyOpen 2019, 8, e00795. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Miyazaki, K. Product-induced gene expression, a product-responsive reporter assay used to screen metagenomic libraries for enzyme-encoding genes. Appl. Environ. Microbiol. 2010, 76, 7029–7035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aučynaitė, A.; Rutkienė, R.; Gasparavičiūtė, R.; Meškys, R.; Urbonavičius, J. A gene encoding a DUF523 domain protein is involved in the conversion of 2-thiouracil into uracil. Environ. Microbiol. Rep. 2018, 10, 49–56. [Google Scholar] [CrossRef]
- Ireton, G.C.; McDermott, G.; Black, M.E.; Stoddard, B.L. The structure of Escherichia coli cytosine deaminase11Edited by I. A. Wilson. J. Mol. Biol. 2002, 315, 687–697. [Google Scholar] [CrossRef] [Green Version]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef] [Green Version]
- UniProt Consortium. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [Green Version]
- Pratt, R.F.; McLeish, M.J. Structural Relationship between the Active Sites of β-Lactam-Recognizing and Amidase Signature Enzymes: Convergent Evolution? Biochemistry 2010, 49, 9688–9697. [Google Scholar] [CrossRef] [PubMed]
- Labahn, J.; Neumann, S.; Büldt, G.; Kula, M.-R.; Granzin, J. An alternative mechanism for Amidase Signature enzymes. J. Mol. Biol. 2002, 322, 1053–1064. [Google Scholar] [CrossRef]
- Bracey, M.H.; Hanson, M.A.; Masuda, K.R.; Stevens, R.C.; Cravatt, B.F. Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling. Science 2002, 298, 1793–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S. Structure of malonamidase E2 reveals a novel Ser-cisSer-Lys catalytic triad in a new serine hydrolase fold that is prevalent in nature. EMBO J. 2002, 21, 2509–2516. [Google Scholar] [CrossRef] [Green Version]
- Curnow, A.W.; Hong, K.; Yuan, R.; Kim, S.; Martins, O.; Winkler, W.; Henkin, T.M.; Söll, D. Glu-tRNAGln amidotransferase: A novel heterotrimeric enzyme required for correct decoding of glutamine codons during translation. Proc. Natl. Acad. Sci. USA 1997, 94, 11819–11826. [Google Scholar] [CrossRef] [Green Version]
- Seibert, C.M.; Raushel, F.M. Structural and catalytic diversity within the amidohydrolase superfamily †. Biochemistry 2005, 44, 6383–6391. [Google Scholar] [CrossRef]
- Sugrue, E.; Fraser, N.J.; Hopkins, D.H.; Carr, P.D.; Khurana, J.L.; Oakeshott, J.G.; Scott, C.; Jackson, C.J. Evolutionary expansion of the amidohydrolase superfamily in bacteria in pesponse to the synthetic compounds molinate and diuron. Appl. Environ. Microbiol. 2015, 81, 2612–2624. [Google Scholar] [CrossRef] [Green Version]
- de las Rivas, B.; Rodríguez, H.; Anguita, J.; Muñoz, R. Bacterial tannases: Classification and biochemical properties. Appl. Microbiol. Biotechnol. 2019, 103, 603–623. [Google Scholar] [CrossRef] [Green Version]
- Crepin, V.F.; Faulds, C.B.; Connerton, I.F. Functional classification of the microbial feruloyl esterases. Appl. Microbiol. Biotechnol. 2004, 63, 647–652. [Google Scholar] [CrossRef]
- Dilokpimol, A.; Mäkelä, M.R.; Aguilar-Pontes, M.V.; Benoit-Gelber, I.; Hildén, K.S.; de Vries, R.P. Diversity of fungal feruloyl esterases: Updated phylogenetic classification, properties, and industrial applications. Biotechnol. Biofuels 2016, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.M.; Mota, T.R.; Oliva, B.; Segato, F.; Marchiosi, R.; Ferrarese-Filho, O.; Faulds, C.B.; dos Santos, W.D. Feruloyl esterases: Biocatalysts to overcome biomass recalcitrance and for the production of bioactive compounds. Bioresour. Technol. 2019, 278, 408–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, A.; Jana, A.; Pati, B.R.; Mondal, K.C.; Das Mohapatra, P.K. Characterization of Tannase Protein Sequences of Bacteria and Fungi: An In Silico Study. Protein J. 2012, 31, 306–327. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Hori, A.; Kawamoto, K.; Thangudu, R.R.; Ishida, T.; Igarashi, K.; Samejima, M.; Yamada, C.; Arakawa, T.; Wakagi, T.; et al. Crystal structure of a feruloyl esterase belonging to the tannase family: A disulfide bond near a catalytic triad. Proteins Struct. Funct. Bioinforma. 2014, 82, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Palm, G.J.; Reisky, L.; Böttcher, D.; Müller, H.; Michels, E.A.P.; Walczak, M.C.; Berndt, L.; Weiss, M.S.; Bornscheuer, U.T.; Weber, G. Structure of the plastic-degrading Ideonella sakaiensis MHETase bound to a substrate. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Iyer, L.M.; Burroughs, A.M.; Aravind, L. The ASCH superfamily: Novel domains with a fold related to the PUA domain and a potential role in RNA metabolism. Bioinforma. Oxf. Engl. 2006, 22, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Collavin, L.; Buzzai, M.; Saccone, S.; Bernard, L.; Federico, C.; DellaValle, G.; Brancolini, C.; Schneider, C. cDNA Characterization and Chromosome Mapping of the Human GAS2 Gene. Genomics 1998, 48, 265–269. [Google Scholar] [CrossRef]
- Kim, B.-N.; Shin, M.; Ha, S.C.; Park, S.-Y.; Seo, P.-W.; Hofmann, A.; Kim, J.-S. Crystal structure of an ASCH protein from Zymomonas mobilis and its ribonuclease activity specific for single-stranded RNA. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Stanislauskienė, R.; Laurynėnas, A.; Rutkienė, R.; Aučynaitė, A.; Tauraitė, D.; Meškienė, R.; Urbelienė, N.; Kaupinis, A.; Valius, M.; Kaliniene, L.; et al. YqfB protein from Escherichia coli: An atypical amidohydrolase active towards N4-acylcytosine derivatives. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Petersen, E.I.; Valinger, G.; Sölkner, B.; Stubenrauch, G.; Schwab, H. A novel esterase from Burkholderia gladioli which shows high deacetylation activity on cephalosporins is related to β-lactamases and dd-peptidases. J. Biotechnol. 2001, 89, 11–25. [Google Scholar] [CrossRef]
- Ohlhoff, C.W.; Kirby, B.M.; Van Zyl, L.; Mutepfa, D.L.R.; Casanueva, A.; Huddy, R.J.; Bauer, R.; Cowan, D.A.; Tuffin, M. An unusual feruloyl esterase belonging to family VIII esterases and displaying a broad substrate range. J. Mol. Catal. B Enzym. 2015, 118, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, L.; Stephens, A.; Nam, S.-Z.; Rau, D.; Kübler, J.; Lozajic, M.; Gabler, F.; Söding, J.; Lupas, A.N.; Alva, V. A Completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J. Mol. Biol. 2018, 430, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Berman, H.M.; Bhikadiya, C.; Bi, C.; Chen, L.; Di Costanzo, L.; Christie, C.; Dalenberg, K.; Duarte, J.M.; Dutta, S.; et al. RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019, 47, D464–D474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Dong, S.; Chen, D.; Rui, Q.; Guo, J.; Wang, D.; Jiang, J. Potential of esterase DmtH in transforming plastic additive dimethyl terephthalate to less toxic mono-methyl terephthalate. Ecotoxicol. Environ. Saf. 2020, 187, 109848. [Google Scholar] [CrossRef]
- Barth, M.; Oeser, T.; Wei, R.; Then, J.; Schmidt, J.; Zimmermann, W. Effect of hydrolysis products on the enzymatic degradation of polyethylene terephthalate nanoparticles by a polyester hydrolase from Thermobifida fusca. Biochem. Eng. J. 2015, 93, 222–228. [Google Scholar] [CrossRef]
- Luo, Z.-H.; Wu, Y.-R.; Chow, R.K.K.; Luo, J.-J.; Gu, J.-D.; Vrijmoed, L.L.P. Purification and characterization of an intracellular esterase from a Fusarium species capable of degrading dimethyl terephthalate. Process Biochem. 2012, 47, 687–693. [Google Scholar] [CrossRef]
- Jakubovska, J.; Tauraite, D.; Birštonas, L.; Meškys, R. N4-acyl-2′-deoxycytidine-5′-triphosphates for the enzymatic synthesis of modified DNA. Nucleic Acids Res. 2018, 46, 5911–5923. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-García, S.; García-García, M.I.; García-Carmona, F. An improved method to measure lipase activity in aqueous media. Anal. Biochem. 2017, 530, 104–106. [Google Scholar] [CrossRef] [PubMed]

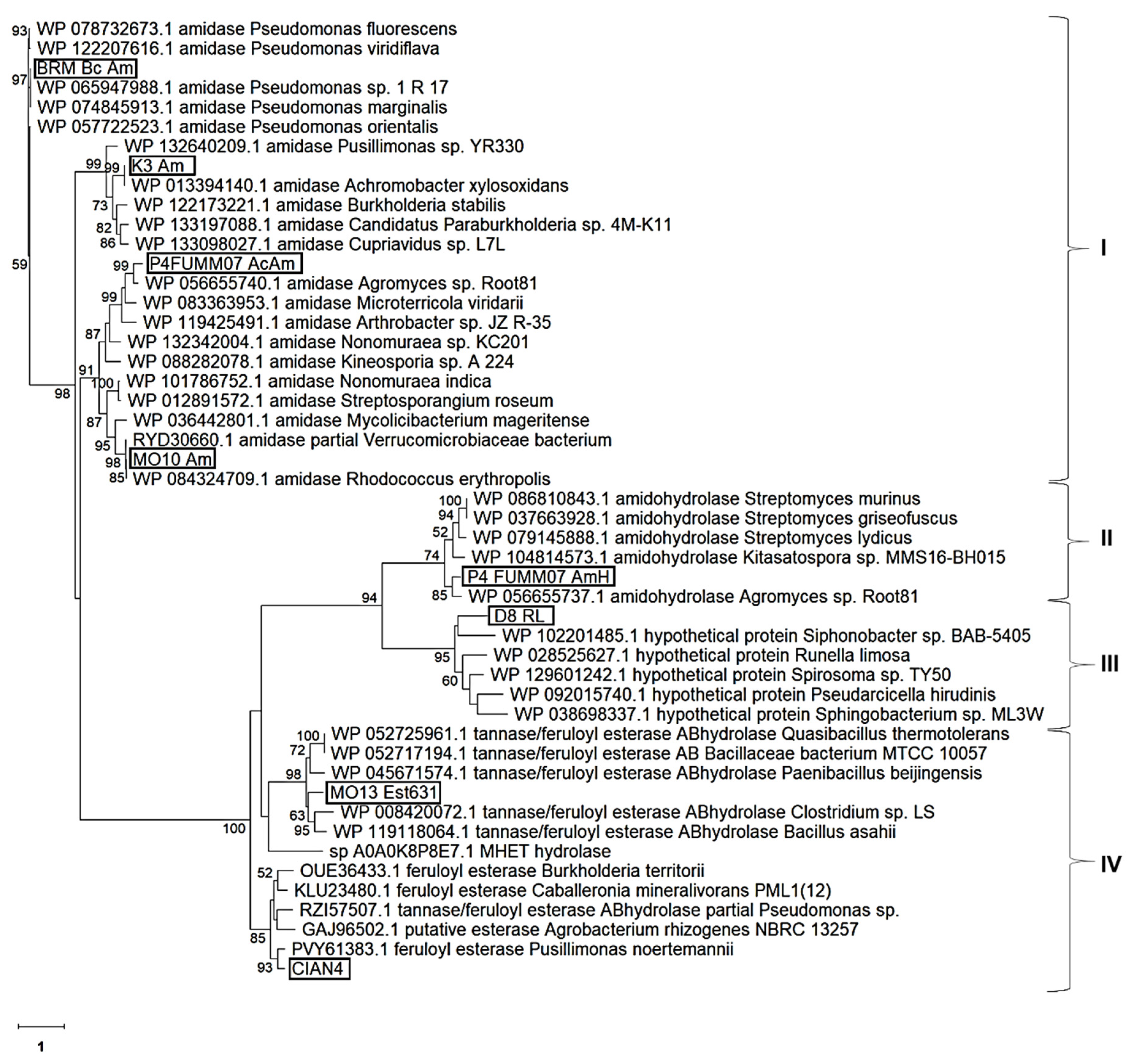

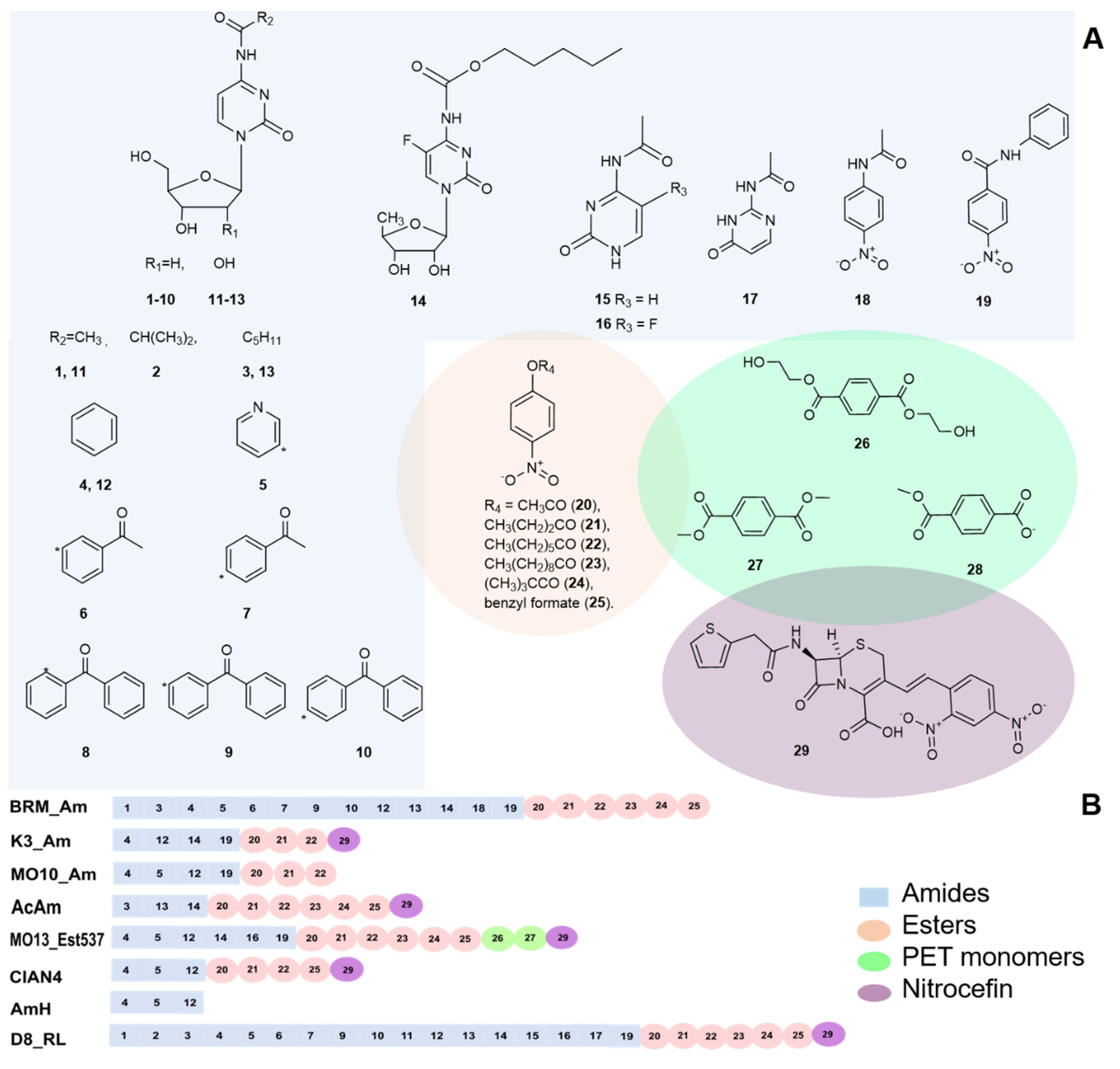
| Selected Amidohydrolase, GenBank Accession No. | The Nearest Homologue Genus/Species, GenBank Accession No., Protein Name | Identity (%) |
|---|---|---|
| BRM_Am, MN734430 | Pseudomonas sp. 1 R 17, WP_065947988.1, amidase | 99 |
| K3_Am, MN734429 | Achromobacter xylosoxidans, WP_013394140.1, amidase | 97 |
| MO10_Am, MN734431 | Rhodococcus erythropolis, WP_084324709.1, amidase | 99 |
| P4FUMM07_AcAm, MN734432 | Agromyces sp. Root80, Wp056655740.1, amidase | 84 |
| P4FUMM07_AmH, MN734432 | Agromyces sp. Root81, Wp056655731.1, amidase | 80 |
| CIAN4, MN734434 | Pusillimonas noertemannii, PVY61383.1, feruloyl esterase | 73 |
| MO13_Est631, MN734432 | Bacillus asahii, WP_119118064.1, tannase/feruloyl esterase family alpha/beta hydrolase | 56 |
| D8_RL, MN734435 | Runella limosa, WP_028525627.1, hypothetical protein | 40 |
| Enzyme | Substrate | KM (M) | kcat (s−1) | kcat/KM(M−1s−1) |
|---|---|---|---|---|
| D8_RL | 1 | (3.0 ± 1) × 10−4 | 0.08 ± 0.01 | (2.65 ± 0.9) × 102 |
| YqfB | (4.0 ± 0.04) × 10−4 | 101 ± 1 | (2.30 ± 0.02) × 105 | |
| D8_RL | 11 | N.D. | N.D. | (6.5 ± 3.4) × 101 |
| YqfB | (6.2 ± 0.1) × 10−5 | 157 ± 1 | (2.2 ± 0.1) × 106 | |
| D8_RL | 4 | (5.0 ± 0.2) × 10−5 | 0.37± 0.2 | (6.9 ± 0.6) × 103 |
| YqfB | (3.0 ± 0.01) × 10−4 | 0.07 ± 0.01 | (2.3 ± 0.1) × 102 | |
| D8_RL | 12 | (3.7 ± 0.1) × 10−5 | 0.3 ± 0.03 | (5.7 ± 0.4) × 103 |
| YqfB | (5.1 ± 0.03) × 10−3 | 1.8 ± 0.1 | (3.5 ± 0.7) × 103 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbelienė, N.; Meškienė, R.; Tiškus, M.; Stanislauskienė, R.; Aučynaitė, A.; Laurynėnas, A.; Meškys, R. A Rapid Method for the Selection of Amidohydrolases from Metagenomic Libraries by Applying Synthetic Nucleosides and a Uridine Auxotrophic Host. Catalysts 2020, 10, 445. https://doi.org/10.3390/catal10040445
Urbelienė N, Meškienė R, Tiškus M, Stanislauskienė R, Aučynaitė A, Laurynėnas A, Meškys R. A Rapid Method for the Selection of Amidohydrolases from Metagenomic Libraries by Applying Synthetic Nucleosides and a Uridine Auxotrophic Host. Catalysts. 2020; 10(4):445. https://doi.org/10.3390/catal10040445
Chicago/Turabian StyleUrbelienė, Nina, Rita Meškienė, Matas Tiškus, Rūta Stanislauskienė, Agota Aučynaitė, Audrius Laurynėnas, and Rolandas Meškys. 2020. "A Rapid Method for the Selection of Amidohydrolases from Metagenomic Libraries by Applying Synthetic Nucleosides and a Uridine Auxotrophic Host" Catalysts 10, no. 4: 445. https://doi.org/10.3390/catal10040445





