Synergy Effects of Cobalt Oxides on Ni/Co-Embedded Al2O3 for Hydrogen-Rich Syngas Production by Steam Reforming of Propane
Abstract
:1. Introduction
2. Results
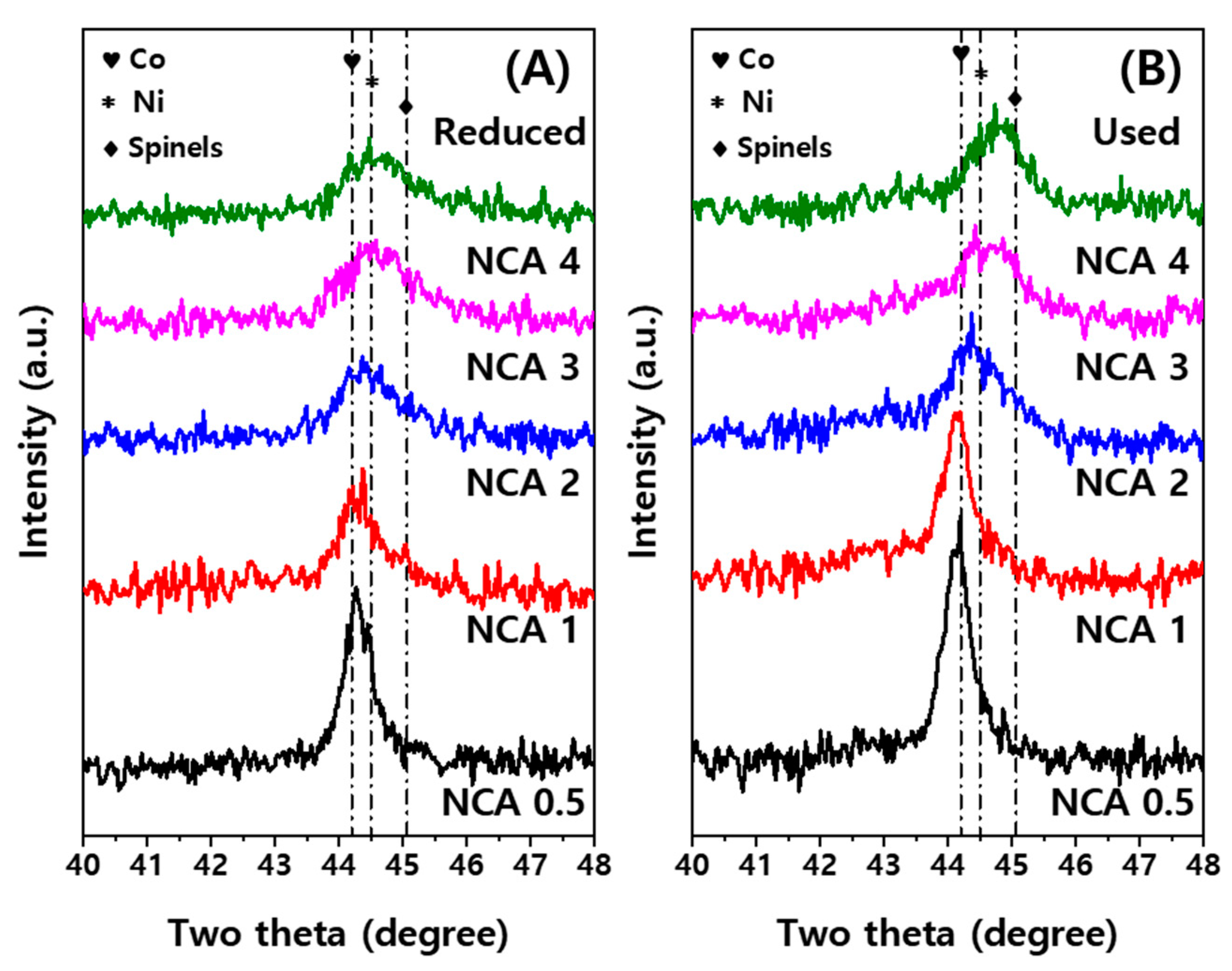
2.1. Physicochemical Properties of NCA Catalysts
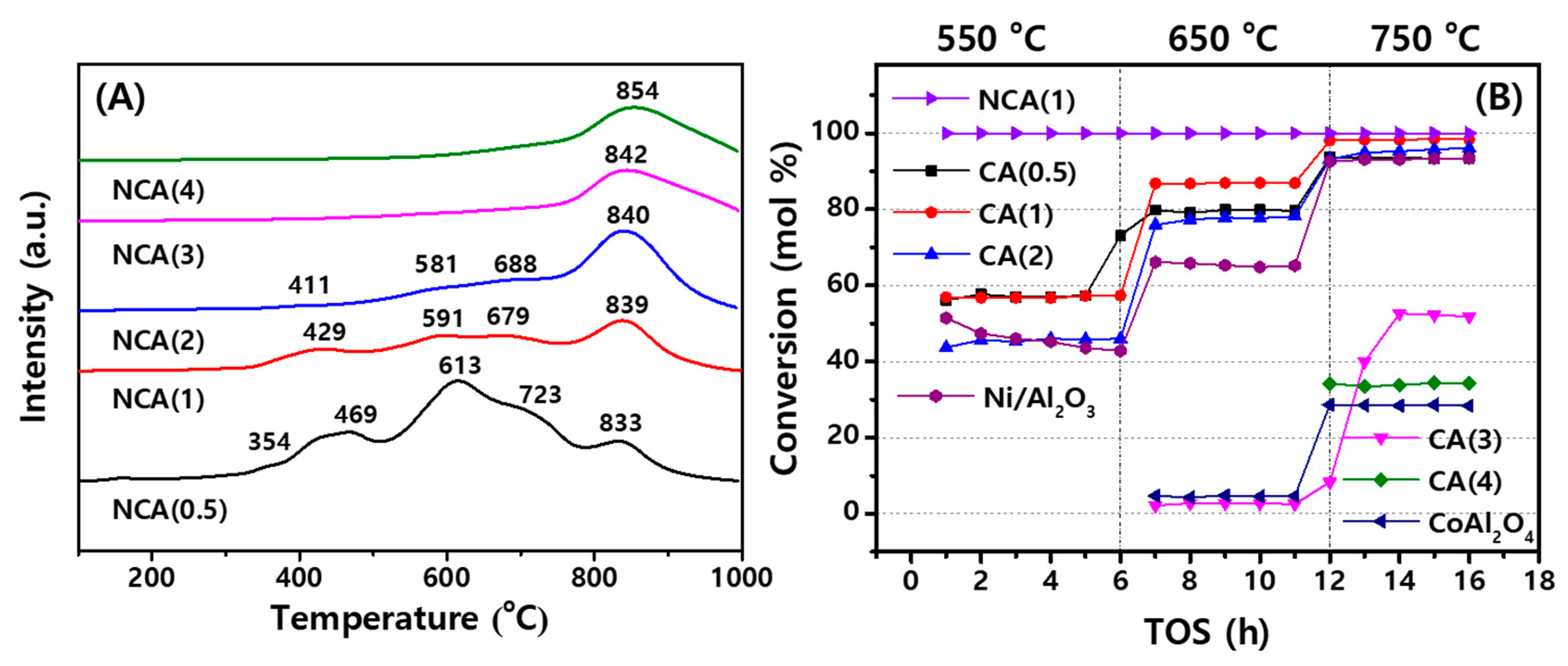
2.2. Reduction Properties of NCA Catalysts and Catalytic Activities of CA Supports Themselves
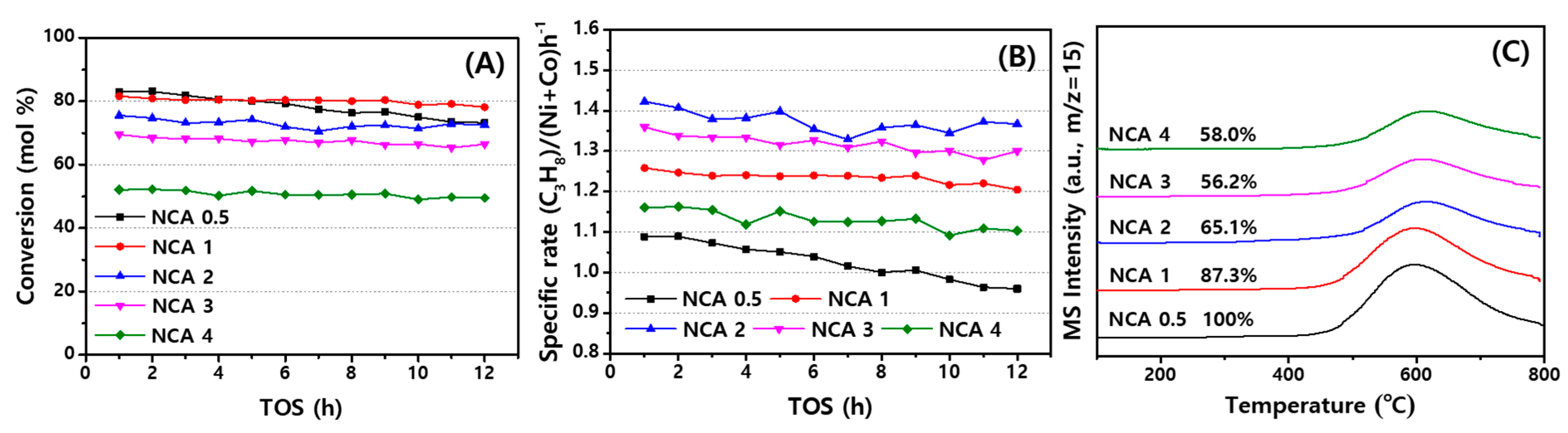
2.3. Catalytic Activities of NCA Catalysts with Coke Analysis
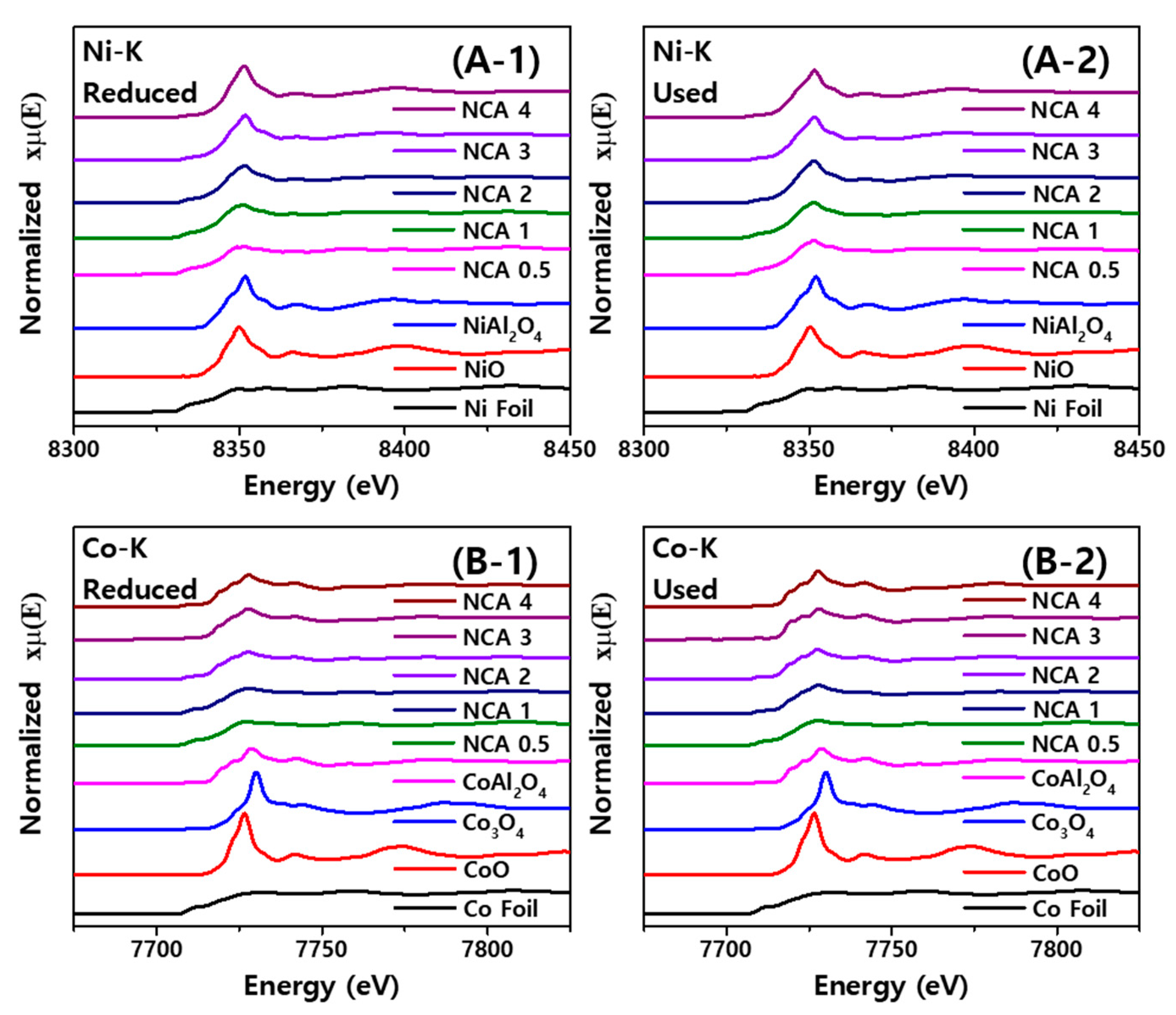
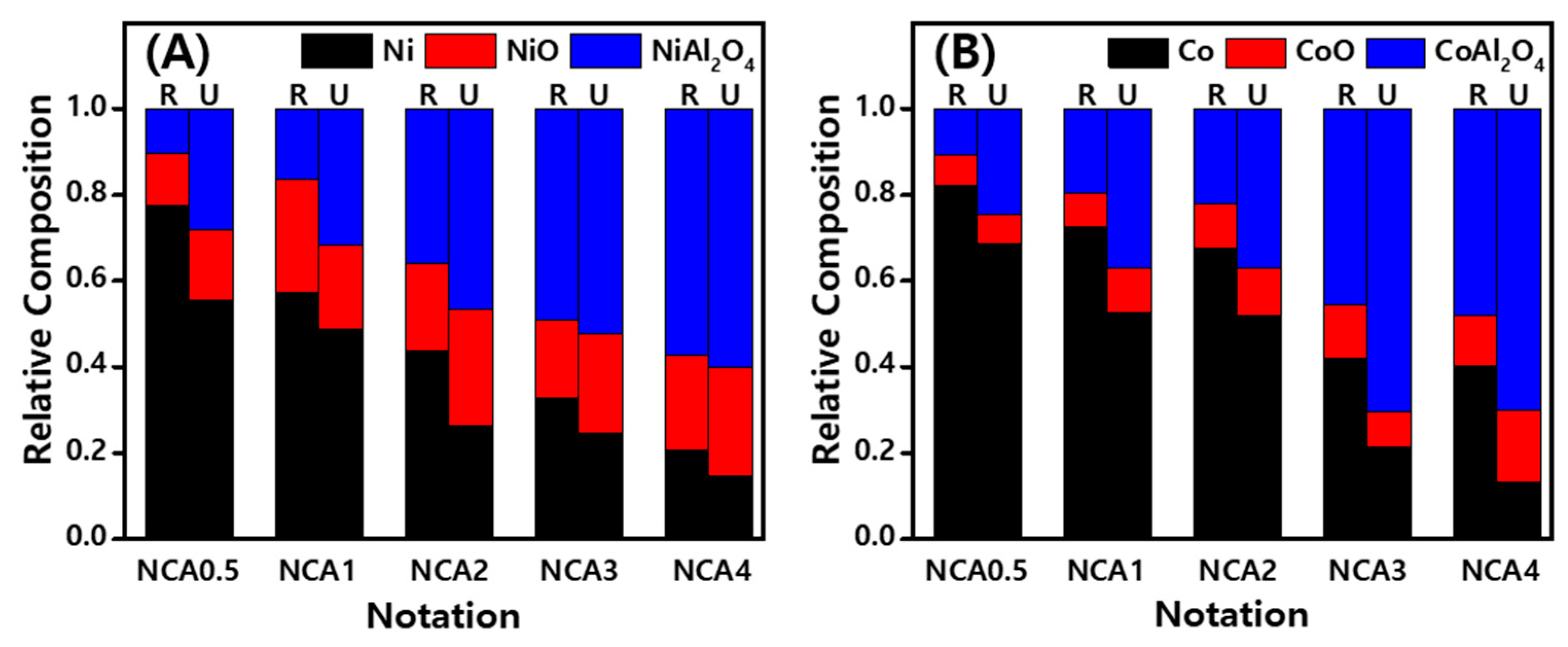
2.4. Relative Compositions of Active Metal (Oxides) Measured by X-ray Absorption Fine Structure (XAFS) Analysis
3. Discussion and Conclusions
4. Materials and Methods
4.1. Catalyst Preparation and Activity Measurement
4.2. Characterization of the CA Supports as Well as NCA Catalysts
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Song, C. Introduction to Hydrogen and Syngas Production and Purification Technologies in Hydrogen and Syngas Production and Purification Technologies; Liu, K., Song, C., Subramani, V., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 1–13. [Google Scholar]
- Ghenciu, A.F. Review of fuel processing catalysts for hydrogen production in PEM fuel cell systems. Curr. Opin. Solid State Mater. Sci. 2002, 6, 389–399. [Google Scholar] [CrossRef]
- Hardiman, K.M.; Ying, T.T.; Adesina, A.A.; Kennedy, E.M.; Dlugogorski, B.Z. Performance of a Co-Ni catalyst for propane reforming under low steam-to-carbon ratios. Chem. Eng. J. 2004, 102, 119–130. [Google Scholar] [CrossRef]
- Moon, D.J. Hydrogen production by catalytic reforming of gaseous hydrocarbons (Methane & LPG). Catal. Surv. Asia 2008, 12, 188–202. [Google Scholar]
- Tan, M.; Wang, X.; Hu, Y.; Shang, X.; Zhang, L.; Zou, X.; Ding, W.; Lu, X. Influence of nickel content on structural and surface properties, reducibility and catalytic behavior of mesoporous γ-alumina-supported Ni–Mg oxides for pre-reforming of liquefied petroleum gas. Catal. Sci. Technol. 2016, 6, 3049–3063. [Google Scholar] [CrossRef]
- Ávila-Neto, C.N.; Oliveira, K.D.; Oliveira, K.F.; Arouca, A.M.M.; Ferreira, R.A.R.; Hori, C.E. Interconnection between feed composition and Ni/Co ratio in (La-Ni-Co-O)-based perovskites and its effects on the stability of LPG steam reforming. Appl. Catal. A Gen. 2018, 550, 184–197. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Song, C. Spectroscopic characterization and catalytic activity of Rh supported on CeO2-modified Al2O3 for low-temperature steam reforming of propane. Catal. Today 2016, 263, 22–34. [Google Scholar] [CrossRef] [Green Version]
- Han, J.W.; Kim, C.; Park, J.S.; Lee, H. Highly coke-resistant ni nanoparticle catalysts with minimal sintering in dry reforming of methane. ChemSusChem 2014, 7, 451–456. [Google Scholar] [CrossRef]
- Jóźwiak, W.K.; Nowosielska, M.; Rynkowski, J. Reforming of methane with carbon dioxide over supported bimetallic catalysts containing Ni and noble metal: I. Characterization and activity of SiO2 supported Ni–Rh catalysts. Appl. Catal. A Gen. 2005, 280, 233–244. [Google Scholar] [CrossRef]
- Lucrédio, A.F.; Assaf, J.M.; Assaf, E.M. Methane conversion reactions on Ni catalysts promoted with Rh: Influence of support. Appl. Catal. A Gen. 2011, 400, 156–165. [Google Scholar] [CrossRef]
- García-Diéguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Alemany, L.J. RhNi nanocatalysts for the CO2 and CO2+H2O reforming of methane. Catal. Today 2011, 172, 136–142. [Google Scholar] [CrossRef]
- Park, K.S.; Son, M.; Park, M.-J.; Kim, D.H.; Kim, J.H.; Park, S.H.; Choi, J.-H.; Bae, J.W. Adjusted interactions of nickel nanoparticles with cobalt-modified MgAl2O4-SiC for an enhanced catalytic stability during steam reforming of propane. Appl. Catal. A Gen. 2018, 549, 117–133. [Google Scholar] [CrossRef]
- Szijjártó, G.P.; Pászti, Z.; Sajó, I.; Erdőhelyi, A.; Radnóczi, G.; Tompos, A. Nature of the active sites in Ni/MgAl2O4-based catalysts designed for steam reforming of ethanol. J. Catal. 2013, 305, 290–306. [Google Scholar] [CrossRef]
- Han, J.W.; Park, J.S.; Choi, M.S.; Lee, H. Uncoupling the size and support effects of Ni catalysts for dry reforming of methane. Appl. Catal. B Environ. 2017, 203, 625–632. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F. Coke-resistant Ni@SiO2 catalyst for dry reforming of methane. Appl. Catal. B Environ. 2015, 176–177, 513–521. [Google Scholar] [CrossRef]
- Bian, Z.; Kawi, S. Sandwich-Like Silica@Ni@Silica multicore-shell catalyst for the low-temperature dry reforming of methane: Confinement effect against carbon formation. ChemCatChem 2018, 10, 320–328. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Kawi, S. Sintering and Coke Resistant Core/Yolk Shell Catalyst for Hydrocarbon Reforming. ChemCatChem 2018, 11, 202–224. [Google Scholar] [CrossRef]
- Wang, C.; Sun, N.; Zhao, N.; Wei, W.; Zhang, J.; Zhao, T.; Sun, Y.; Sun, C.; Liu, H.; Snape, C.E. The properties of individual carbon residuals and their influence on the deactivation of Ni-CaO-ZrO2 catalysts in CH4 dry reforming. ChemCatChem 2014, 6, 640–648. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Thitsartarn, W.; Sutthiumporn, K.; Kawi, S. Inverse NiAl2O4 on LaAlO3–Al2O3: Unique catalytic structure for stable CO2 reforming of methane. J. Phys. Chem. C 2013, 117, 8120–8130. [Google Scholar] [CrossRef]
- Keshavarz, A.R.; Soleimani, M. Optimization of nano-sized Ni/MgAl2O4 catalyst synthesis by the surfactant-assisted deposition precipitation method for steam pre-reforming of natural gas. RSC Adv. 2016, 6, 61536–61543. [Google Scholar] [CrossRef]
- Zou, X.; Tian, Z.; Wang, X.; Tan, M.; Ding, W.; Lu, X. Formation of magnesium silicate on surface of silica for steam reforming of liquefied petroleum gas. Catal. Commun. 2015, 68, 116–119. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, D.; Lee, H.C.; Park, E.D. Steam reforming of liquid petroleum gas over Mn-promoted Ni/γ-Al2O3 catalysts. Korean J. Chem. Eng. 2010, 27, 1132–1138. [Google Scholar] [CrossRef]
- Crisafulli, C.; Scirè, S.; Minicò, S.; Solarino, L. Ni–Ru bimetallic catalysts for the CO2 reforming of methane. Appl. Catal. A Gen. 2002, 225, 1–9. [Google Scholar] [CrossRef]
- Misture, S.T.; McDevitt, K.M.; Glass, K.C.; Edwards, D.D.; Howe, J.Y.; Rector, K.D.; He, H.; Vogel, S.C. Sulfur-resistant and regenerable Ni/Co spinel-based catalysts for methane dry reforming. Catal. Sci. Technol. 2015, 5, 4565–4574. [Google Scholar] [CrossRef]
- Gao, X.; Tan, Z.; Hidajat, K.; Kawi, S. Highly reactive Ni-Co/SiO2 bimetallic catalyst via complexation with oleylamine/oleic acid organic pair for dry reforming of methane. Catal. Today 2017, 281, 250–258. [Google Scholar] [CrossRef]
- Tu, W.; Ghoussoub, M.; Singh, C.V.; Chin, Y.-H.C. Consequences of surface oxophilicity of Ni, Ni-Co, and Co clusters on methane activation. J. Am. Chem. Soc. 2017, 139, 6928–6945. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N. Effect of impurities on the growth kinetics of crystals. Cryst. Res. Technol. 2001, 36, 749–769. [Google Scholar] [CrossRef]
- Yuan, Q.; Yin, A.-X.; Luo, C.; Sun, L.-D.; Zhang, Y.-W.; Duan, W.-T.; Liu, H.-C.; Yan, C.-H. Facile synthesis for ordered mesoporous γ-aluminas with high thermal stability. J. Am. Chem. Soc. 2008, 130, 3465–3472. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, Q.; Li, J.; Zhu, Q. Effects of promoters and preparation procedures on reforming of methane with carbon dioxide over Ni/Al2O3 catalyst. Catal. Today 1996, 30, 147–155. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Watson, R.; Wang, X.; Ozkan, U. Deactivation characteristics of lanthanide-promoted sol–gel Ni/Al2O3 catalysts in propane steam reforming. J. Catal. 2005, 234, 496–508. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Oktar, O.; Ozkan, U.S. Effect of lanthanide promotion on catalytic performance of sol–gel Ni/Al2O3 catalysts in steam reforming of propane. J. Mol. Catal. A Chem. 2005, 241, 133–146. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, H.Y.; Cho, J.M.; Choi, J.-H.; Bae, J.W. Ni/M-Al2O3 (M = Sm, Ce or Mg) for combined steam and CO2 reforming of CH4 from coke oven gas. J. CO2 Util. 2017, 21, 211–218. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, A.R.; Park, M.-J.; Jo, J.M.; Lee, D.H.; Bae, J.W. Combined steam and CO2 reforming of CH4 using coke oven gas on nickel-based catalyst: Effects of organic acids to nickel dispersion and activity. Chem. Eng. J. 2015, 280, 771–781. [Google Scholar] [CrossRef]
- Ewbank, J.L.; Kovarik, L.; Kenvin, C.C.; Sievers, C. Effect of preparation methods on the performance of Co/Al2O3 catalysts for dry reforming of methane. Green Chem. 2014, 16, 885–896. [Google Scholar] [CrossRef]
- Shen, K.; Wang, X.; Zou, X.; Wang, X.; Lu, X.; Ding, W. Pre-reforming of liquefied petroleum gas over nickel catalysts supported on magnesium aluminum mixed oxides. Int. J. Hydrogen Energy 2011, 36, 4908–4916. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, X.; Wang, Z.; Zou, X.; Ding, W.; Lu, X. High catalytic performance and sustainability of the Ni/La2O3 catalyst for daily pre-reforming of liquefied petroleum gas under a low steam/carbon molar ratio. RSC Adv. 2014, 4, 14829–14832. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Wang, F.; Yuan, K.; Wang, L.; Wu, K.; Xu, G.; Chen, W. One-step synthesis of ordered mesoporous CoAl2O4 spinel-based metal oxides for CO2 reforming of CH4. RSC Adv. 2015, 5, 48256–48268. [Google Scholar] [CrossRef]
- Han, Y.K.; Ahn, C.-I.; Bae, J.W.; Kim, A.R.; Han, G.Y. Effects of Carbon Formation on Catalytic performance for CO2 reforming with methane on Ni/Al2O3 catalyst: Comparison of fixed-bed with fluidized-bed reactors. Ind. Eng. Chem. Res. 2013, 52, 13288–13296. [Google Scholar] [CrossRef]
- Lobo, L.S.; Figueiredo, J.L.; Bernardo, C.A. Carbon formation and gasification on metals. Bulk diffusion mechanism: A reassessment. Catal. Today 2011, 178, 110–116. [Google Scholar] [CrossRef]
- Amini, E.; Rezaei, M.; Nematollahi, B. Synthesis of mesoporous magnesium aluminate (MgAl2O4) nanopowder with high surface area with a novel and simple sol–gel method. J. Porous Mater. 2015, 22, 481–485. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [Green Version]
| Notation | XRF (wt%) | N2-Sorption 1 | XRD | CO Chemi. | ||||
|---|---|---|---|---|---|---|---|---|
| NiO | Co3O4 | Al2O3 | Sg (m2/g) | Pv (cm3/g) | Pd (nm) | Crystallite Size of Ni0 (Reduced/Used) (nm) | Dispersion (%) | |
| NCA(0.5) | 10.9 | 64.3 | 24.8 | 32 | 0.24 | 25.2 | 14.6/16.2 | 1.0 |
| NCA(1) | 10.6 | 50.6 | 38.8 | 46 | 0.36 | 27.6 | 9.2/12.9 | 2.4 |
| NCA(2) | 10.3 | 36.1 | 53.6 | 64 | 0.39 | 20.5 | 6.9/8.7 | 2.5 |
| NCA(3) | 11.9 | 28.4 | 59.7 | 78 | 0.43 | 15.3 | 6.3/7.4 | 3.6 |
| NCA(4) | 11.2 | 22.6 | 66.2 | 111 | 0.48 | 12.9 | 6.5/7.3 | 3.0 |
| Notation | Catalytic Performances 1 | |||
|---|---|---|---|---|
| Conversion (mol%) | Specific Rate [Moles of C3H8 Converted/(Moles of (Ni + Co)·h)] | Deactivation Rate (%/h) | Product Distribution (H2/CO/CO2/CH4) | |
| NCA(0.5) | 73.2 | 0.96 | 0.89 | 71.2/8.6/15.8/4.4 |
| NCA(1) | 78.1 | 1.20 | 0.31 | 70.1/9.0/15.7/5.2 |
| NCA(2) | 72.6 | 1.37 | 0.27 | 73.2/8.5/16.3/2.0 |
| NCA(3) | 66.5 | 1.30 | 0.28 | 72.5/7.6/16.8/3.1 |
| NCA(4) | 49.5 | 1.10 | 0.23 | 73.4/10.6/14.1/1.9 |
| Notation | Status | Linear Combination Fitting (LCF) Results by Using XANES Spectra | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ni | NiO | NiAl2O4 | R-Factor (%) | Co | CoO | CoAl2O4 | R-Factor (%) | ||
| NCA(0.5) | Reduced | 0.78 | 0.12 | 0.11 | 0.2 | 0.82 | 0.07 | 0.11 | 0.3 |
| Used | 0.55 | 0.17 | 0.28 | 0.9 | 0.69 | 0.07 | 0.25 | 0.2 | |
| NCA(1) | Reduced | 0.57 | 0.26 | 0.16 | 1.0 | 0.73 | 0.08 | 0.20 | 0.1 |
| Used | 0.49 | 0.20 | 0.32 | 0.2 | 0.53 | 0.10 | 0.37 | 0.2 | |
| NCA(2) | Reduced | 0.44 | 0.20 | 0.36 | 0.3 | 0.68 | 0.10 | 0.22 | 0.6 |
| Used | 0.27 | 0.27 | 0.47 | 0.2 | 0.52 | 0.11 | 0.37 | 0.8 | |
| NCA(3) | Reduced | 0.33 | 0.18 | 0.49 | 0.6 | 0.42 | 0.13 | 0.45 | 0.4 |
| Used | 0.25 | 0.23 | 0.52 | 0.1 | 0.21 | 0.08 | 0.70 | 1.4 | |
| NCA(4) | Reduced | 0.21 | 0.22 | 0.57 | 0.4 | 0.40 | 0.12 | 0.48 | 0.5 |
| Used | 0.15 | 0.25 | 0.60 | 0.2 | 0.13 | 0.17 | 0.70 | 1.0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.S.; Jeong, M.H.; Bae, J.W. Synergy Effects of Cobalt Oxides on Ni/Co-Embedded Al2O3 for Hydrogen-Rich Syngas Production by Steam Reforming of Propane. Catalysts 2020, 10, 461. https://doi.org/10.3390/catal10040461
Park KS, Jeong MH, Bae JW. Synergy Effects of Cobalt Oxides on Ni/Co-Embedded Al2O3 for Hydrogen-Rich Syngas Production by Steam Reforming of Propane. Catalysts. 2020; 10(4):461. https://doi.org/10.3390/catal10040461
Chicago/Turabian StylePark, Kyung Soo, Min Hye Jeong, and Jong Wook Bae. 2020. "Synergy Effects of Cobalt Oxides on Ni/Co-Embedded Al2O3 for Hydrogen-Rich Syngas Production by Steam Reforming of Propane" Catalysts 10, no. 4: 461. https://doi.org/10.3390/catal10040461
APA StylePark, K. S., Jeong, M. H., & Bae, J. W. (2020). Synergy Effects of Cobalt Oxides on Ni/Co-Embedded Al2O3 for Hydrogen-Rich Syngas Production by Steam Reforming of Propane. Catalysts, 10(4), 461. https://doi.org/10.3390/catal10040461





