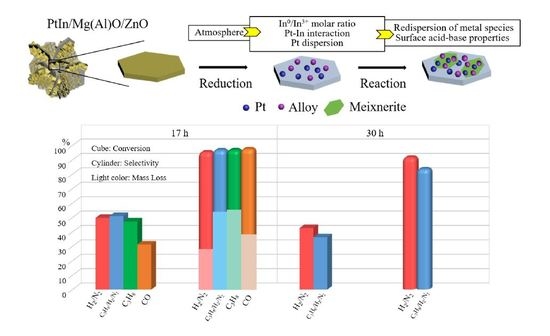
Effect of Reduction Atmosphere on Structure and Catalytic Performance of PtIn/Mg(Al)O/ZnO for Propane Dehydrogenation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Activity
2.2. Texture and Structure of Reduced Catalystic Systems
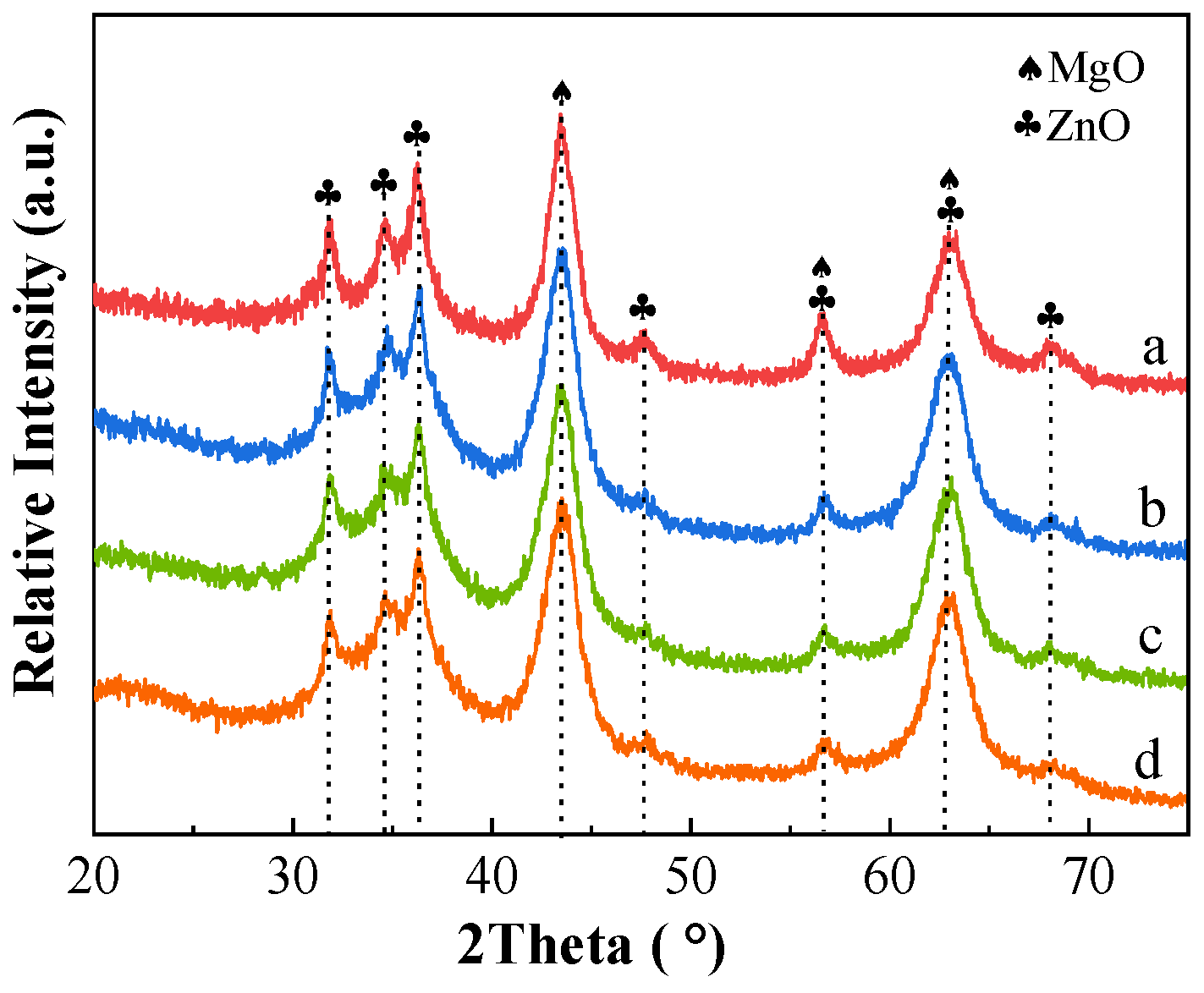
2.2.1. XRD
2.2.2. TEM
2.2.3. XPS
2.3. Analysis of The Used Catalytic System
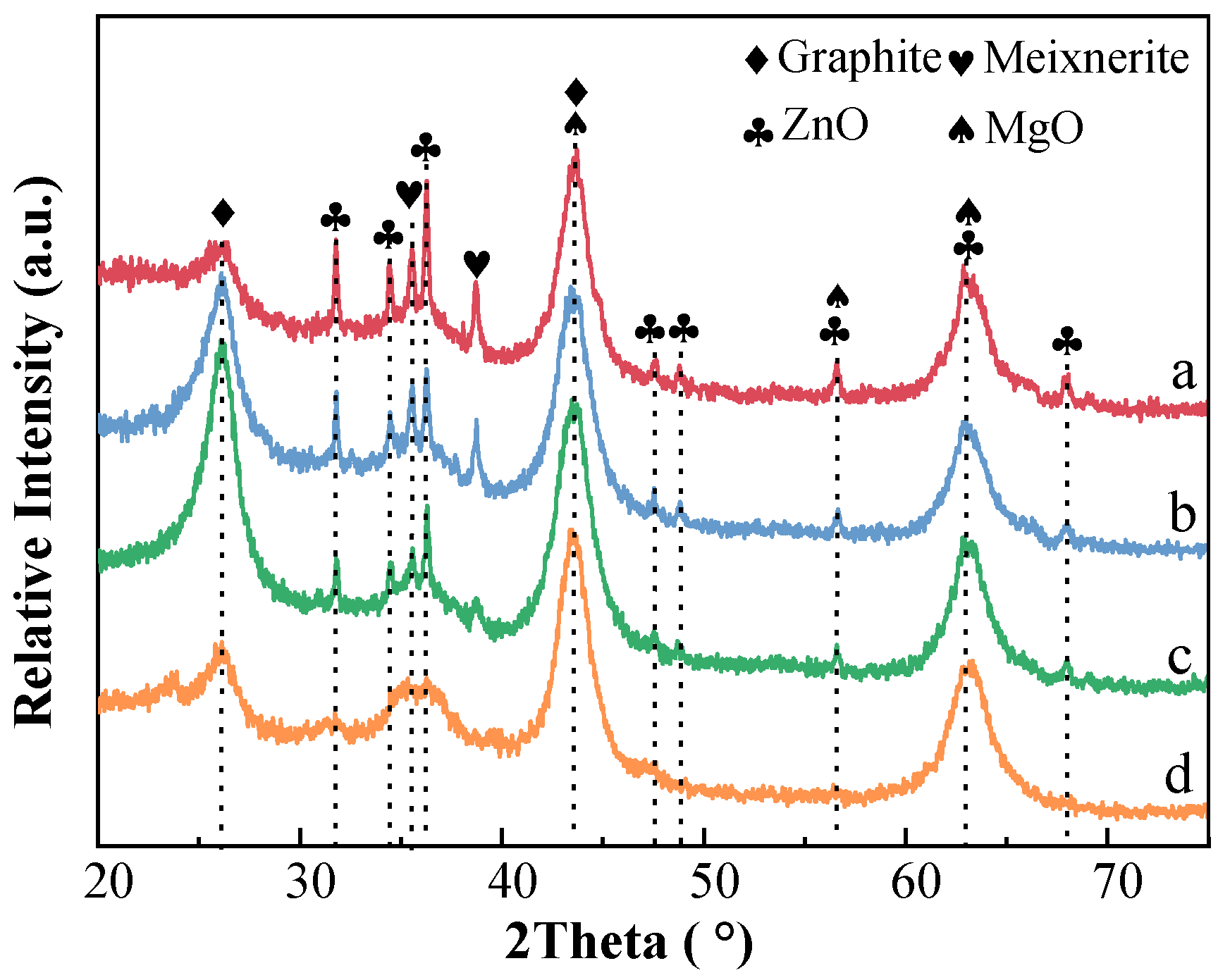
2.3.1. XRD
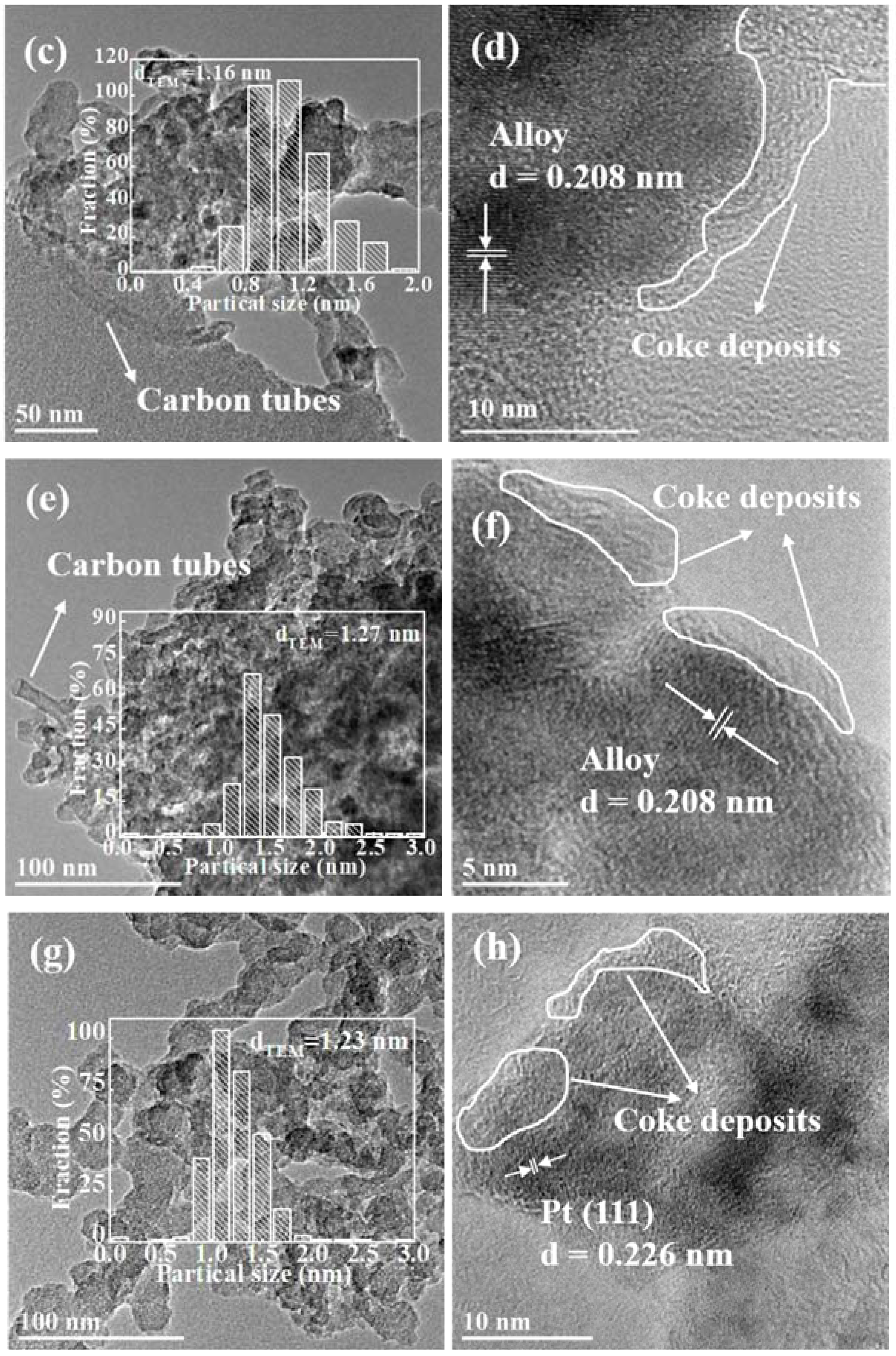
2.3.2. TEM
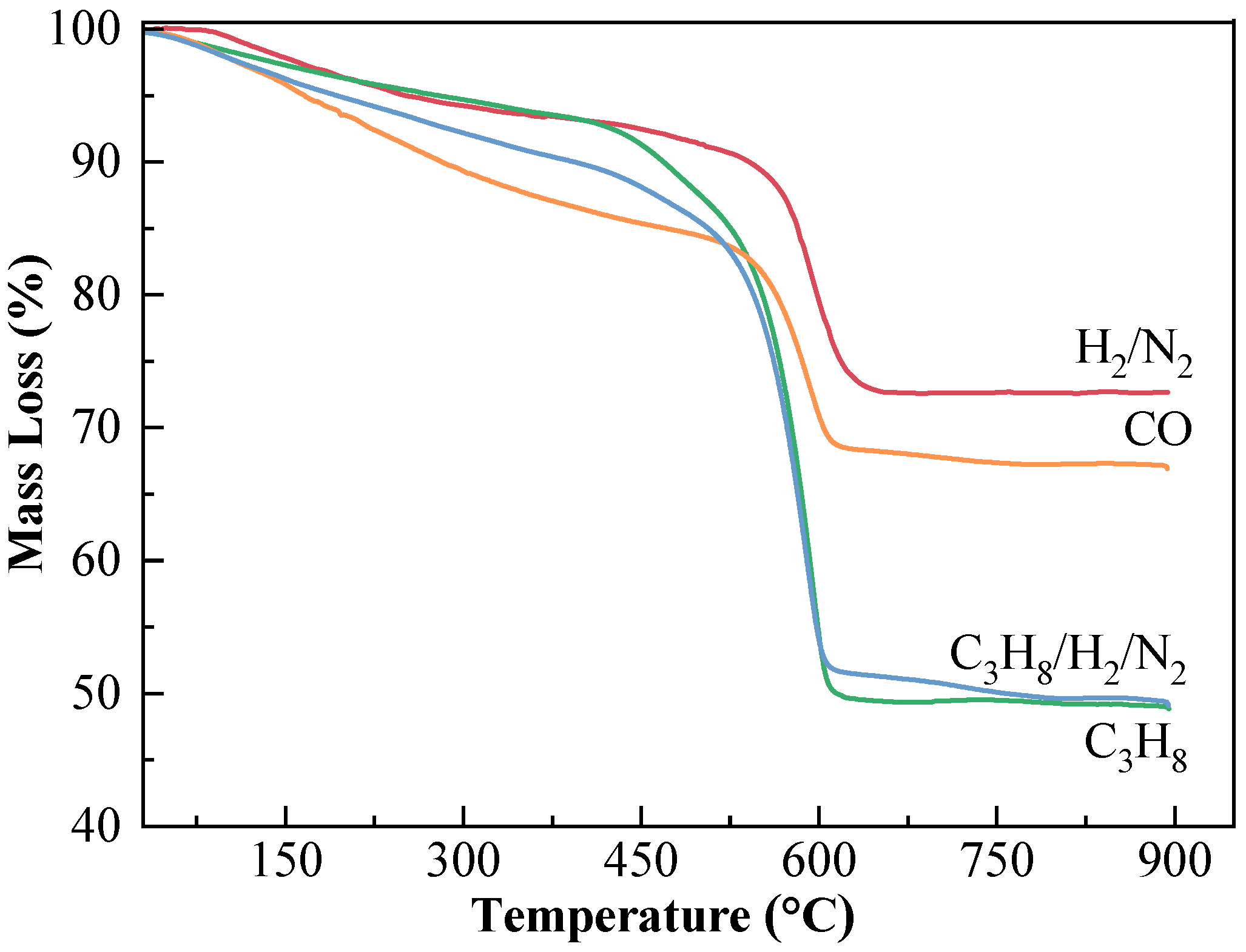
2.3.3. TG
3. Materials and Methods
3.1. Materials
3.2. Preparation of ZnO Precursor
3.3. Preparation of MAZ Support
3.4. Preparation and Reduction of PtIn/MAZ
3.5. Catalyst Characterizations
3.6. Catalytic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sun, P.; Siddiqi, G.; Chi, M.; Bell, A.T. Synthesis and characterization of a new catalyst Pt/Mg(Ga)(Al)O for alkane dehydrogenation. J. Catal. 2010, 274, 192–199. [Google Scholar] [CrossRef]
- Sahebdelfar, S.; Bijani, P.M.; Saeedizad, M.; Zangeneh, F.T.; Ganji, K. Modeling of adiabatic moving-bed reactor for dehydrogenation of isobutane to isobutene. Appl. Catal. A 2011, 395, 107–113. [Google Scholar] [CrossRef]
- Caeiro, G.; Carvalho, R.H.; Wang, X.; Lemos, M.A.N.D.; Lemos, F.; Guisnet, M.; Ramôa Ribeiro, F. Activation of C2-C4 alkanes over acid and bifunctional zeolite catalysts. J. Mol. Catal. A Chem. 2006, 255, 131–158. [Google Scholar] [CrossRef]
- Schäferhans, J.; Gómez-Quero, S.; Aneeva, D.V.; Rothenberg, G. Novel and effective copper-aluminum propane dehydrogenation catalysts. Chem. - Eur. J. 2011, 17, 12254–12256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckers, J.; Rothenberg, G. Sustainable selective oxidations using ceria-based materials. Green Chem. 2010, 12, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Bean Getsoian, A.; Schweitzer, N.M.; Das, U.; Kim, H.; Niklas, J.; Poluektov, O.; Curtiss, L.A.; Stair, P.C.; Miller, J.T.; et al. Selective propane dehydrogenation with single-site CoII on SiO2 by a non-redox mechanism. J. Catal. 2015, 322, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Pakhomov, N.A. Reversible and Irreversible Deactivation of Supported Bimetallic Catalysts for the Dehydrogenation of Lower Paraffins. Kinet. Catal. 2001, 42, 334–343. [Google Scholar] [CrossRef]
- De Miguel, S.; Castro, A.; Scelza, O.; Fierro, J.L.G.; Soria, J. FTIR and XPS study of supported PtSn catalysts used for light paraffins dehydrogenation. Catal. Lett. 1996, 36, 201–206. [Google Scholar] [CrossRef]
- Jablonski, E.L.; Castro, A.A.; Scelza, O.A.; de Miguel, S.R. Effect of Ga addition to Pt/Al2O3 on the activity, selectivity and deactivation in the propane dehydrogenation. Appl. Catal. A 1999, 183, 189–198. [Google Scholar] [CrossRef]
- Homs, N.; Llorca, J.; Riera, M.; Jolis, J.; Fierro, J.G.; Sales, J.; de la Piscina, P.R. Silica-supported PtSn alloy doped with Ga, In or, Tl: Characterization and catalytic behavior in n-hexane dehydrogenation. J. Mol. Catal. A Chem. 2003, 200, 251–259. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; Cao, Y.; He, H.; Fan, K.; Zhuang, J. Dehydrogenation of propane over In2O3-Al2O3 mixed oxide in the presence of carbon dioxide. J. Catal. 2010, 272, 101–108. [Google Scholar] [CrossRef]
- Hoang, D.L.; Farrage, S.A.F.; Radnik, J.; Pohl, M.M.; Schneider, M.; Lieske, H.; Martin, A. A comparative study of zirconia and alumina supported Pt and Pt–Sn catalysts used for dehydrocyclization of n-octane. Appl. Catal. A 2007, 333, 67–77. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, C.; Wang, Q.; Zhang, L.; Huang, A.; Ke, M.; Song, Z. Direct dehydrogenation of isobutane to isobutene over Zn-doped ZrO2 metal oxide heterogeneous catalysts. Catal. Sci. Technol. 2018, 8, 4916–4924. [Google Scholar] [CrossRef]
- Azzam, K.G.; Jacobs, G.; Shafer, W.D.; Davis, B.H. Dehydrogenation of propane over Pt/KL catalyst: Investigating the role of L-zeolite structure on catalyst performance using isotope labeling. Appl. Catal. A 2010, 390, 264–270. [Google Scholar] [CrossRef]
- Sun, P.; Siddiqi, G.; Vining, W.C.; Chi, M.; Bell, A.T. Novel Pt/Mg(In)(Al)O catalysts for ethane and propane dehydrogenation. J. Catal. 2011, 282, 165–174. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Yang, X.; Wang, X.; Xu, Y.; Zhang, L. Preparation of CeO2-Modified Mg(Al)O-Supported Pt–Cu Alloy Catalysts Derived from Hydrotalcite-Like Precursors and Their Catalytic Behavior for Direct Dehydrogenation of Propane. Trans. Tianjin Univ. 2019, 25, 169–184. [Google Scholar] [CrossRef]
- Zhu, Y.; An, Z.; Song, H.; Xiang, X.; Yan, W.; He, J. Lattice-Confined Sn (IV/II) Stabilizing Raft-Like Pt Clusters: High Selectivity and Durability in Propane Dehydrogenation. Acs Catal. 2017, 7, 6973–6978. [Google Scholar] [CrossRef]
- Gao, S.; Lu, T.; Li, S.; Zhong, H. The mechanism on the pH value influencing the property of glutamic acid/layered double hydroxide compounds. Colloids Surf. A 2009, 351, 26–29. [Google Scholar] [CrossRef]
- Xia, K.; Lang, W.; Li, P.; Long, L.; Yan, X.; Guo, Y. The influences of MgAl molar ratio on the properties of PtInMg(Al)O-x catalysts for propane dehydrogenation reaction. Chem. Eng. J. 2016, 284, 1068–1079. [Google Scholar] [CrossRef]
- Mascolo, G.; Mascolo, M.C. On the synthesis of layered double hydroxides (LDHs) by reconstruction method based on the “memory effect”. Micropor. Mesopor. Mater. 2015, 214, 246–248. [Google Scholar] [CrossRef]
- Xia, K.; Lang, W.; Li, P.; Yan, X.; Guo, Y. The properties and catalytic performance of PtIn/Mg(Al)O catalysts for the propane dehydrogenation reaction: Effects of pH value in preparing Mg(Al)O supports by the co-precipitation method. J. Catal. 2016, 338, 104–114. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Song, Z.; Liu, S.; Wang, J.; Zhang, L. Hierarchical PtIn/Mg(Al)O Derived from Reconstructed PtIn-hydrotalcite-like Compounds for Highly Efficient Propane Dehydrogenation. Catalysts 2019, 9, 767. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Sasaki, K.; Qiu, X. Structural Memory Effect of Mg–Al and Zn-Al layered Double Hydroxides in the Presence of Different Natural Humic Acids: Process and Mechanism. Langmuir 2018, 34, 5386–5395. [Google Scholar] [CrossRef] [PubMed]
- Ping, J.; Wang, Y.; Lu, Q.; Chen, B.; Chen, J.; Huang, Y.; Ma, Q.; Tan, C.; Yang, J.; Cao, X.; et al. Self-Assembly of Single-Layer CoAl-Layered Double Hydroxide Nanosheets on 3D Graphene Network Used as Highly Efficient Electrocatalyst for Oxygen Evolution Reaction. Adv. Mater. 2016, 28, 7640–7645. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Z.; Zhou, J.; Sui, Z.; Zhou, X. Hierarchical NiCo LDH-rGO/Ni Foam Composite as Electrode Material for High-Performance Supercapacitors. Trans. Tianjin Univ. 2019, 25, 266–275. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, J.; Ding, R.; Li, Y.; Wu, F.; Liu, J.; Huang, X. Co-Fe layered double hydroxide nanowall array grown from an alloy substrate and its calcined product as a composite anode for lithium-ion batteries. J. Mater. Chem. 2011, 21, 15969. [Google Scholar] [CrossRef]
- Zhu, Y.; An, Z.; He, J. Single-atom and small-cluster Pt induced by Sn (IV) sites confined in an LDH lattice for catalytic reforming. J. Catal. 2016, 341, 44–54. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Wang, L.; Wang, L.; Qin, H.; Qin, H.; Yang, Z.; Yang, Z. Fabrication and electrochemical performance of flower-like ZnAl LDH/SnO2 composites for zinc-nickel secondary batteries. Ionics 2019, 25, 1715–1724. [Google Scholar] [CrossRef]
- Wang, F.; Liu, M.; Zhang, X.; Lv, G.; Sun, M. In Situ Growth of 3D Hierarchical ZnO@NixCo1−x(OH)y Core/Shell Nanowire/Nanosheet Arrays on Ni Foam for High-Performance Aqueous Hybrid Supercapacitors. Trans. Tianjin Univ. 2018, 24, 201–211. [Google Scholar] [CrossRef]
- Shen, L.; Xia, K.; Lang, W.; Chu, L.; Yan, X.; Guo, Y. The effects of calcination temperature of support on PtIn/Mg(Al)O catalysts for propane dehydrogenation reaction. Chem. Eng. J. 2017, 324, 336–346. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, G.; Li, F. Synthesis of novel marigold-like carbonate-type Mg-Al layered double hydroxide micro-nanostructures via a two-step intercalation route. Mater. Lett. 2014, 116, 203–205. [Google Scholar] [CrossRef]
- Filez, M.; Redekop, E.A.; Poelman, H.; Galvita, V.V.; Meledina, M.; Turner, S.; Van Tendeloo, G.; Detavernier, C.; Marin, G.B. One-pot synthesis of Pt catalysts based on layered double hydroxides: An application in propane dehydrogenation. Catal. Sci. Technol. 2016, 6, 1863–1869. [Google Scholar] [CrossRef]
- Stepanova, L.N.; Belskaya, O.B.; Likholobov, V.A. Effect of the Nature of the Active-Component Precursor on the Properties of Pt/MgAlOx Catalysts in Propane and n-Decane Dehydrogenation. Kinet. Catal. 2017, 4, 400–409. [Google Scholar] [CrossRef]
- Wu, J.; Peng, Z.; Sun, P.; Bell, A.T. n-Butane dehydrogenation over Pt/Mg(In)(Al)O. Appl. Catal. A 2014, 470, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Larsson, M.; Hultén, M.; Blekkan, E.A.; Andersson, B. The Effect of Reaction Conditions and Time on Stream on the Coke Formed during Propane Dehydrogenation. J. Catal. 1996, 164, 44–53. [Google Scholar] [CrossRef]
- Airaksinen, S.M.K.; Krause, A.O.I.; Sainio, J.; Lahtinen, J.; Chao, K.; Ares, M.O.G.R. Reduction of chromia/alumina catalyst monitored by DRIFTS-mass spectrometry and TPR-Raman spectroscopy. Phys. Chem. Chem. Phys. 2003, 20, 4371–4377. [Google Scholar] [CrossRef] [Green Version]
- Hakuli, A.; Kytokivi, A.; Krause, A.O.I.; Suntola, T. Initial Activity of Reduced Chromia/Alumina Catalyst in n-Butane Dehydrogenation Monitored by On-Line FT-IR Gas Analysis. J. Catal. 1996, 161, 393–400. [Google Scholar] [CrossRef]
- Galvita, V.; Siddiqi, G.; Sun, P.; Bell, A.T. Ethane dehydrogenation on Pt/Mg(Al)O and PtSn/Mg(Al)O catalysts. J. Catal. 2010, 271, 209–219. [Google Scholar] [CrossRef]
- Santhosh Kumar, M.; Chen, D.; Walmsley, J.C.; Holmen, A. Dehydrogenation of propane over Pt-SBA-15: Effect of Pt particle size. Catal. Commun. 2008, 9, 747–750. [Google Scholar] [CrossRef]
- Chen, X.; Ge, M.; Li, Y.; Liu, Y.; Wang, J.; Zhang, L. Fabrication of highly dispersed Pt-based catalysts on γ-Al2O3 supported perovskite Nano islands: High durability and tolerance to coke deposition in propane dehydrogenation. Appl. Surf. Sci. 2019, 490, 611–621. [Google Scholar] [CrossRef]
- Xia, K.; Lang, W.; Li, P.; Yan, X.; Guo, Y. Analysis of the catalytic activity induction and deactivation of PtIn/Mg(Al)O catalysts for propane dehydrogenation reaction. Rsc Adv. 2015, 5, 64689–64695. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, F.; Liu, G.; Zeng, L.; Zhao, Z.J.; Gong, J. Effects of Ga doping on Pt/CeO2-Al2O3 catalysts for propane dehydrogenation. Aiche J. 2016, 62, 4365–4376. [Google Scholar] [CrossRef]
- Yu, J.; Wang, R.; Ren, S.; Sun, X.; Chen, C.; Ge, Q.; Fang, W.; Zhang, J.; Xu, H.; Su, D.S. The Unique Role of CaO in Stabilizing the Pt/Al2O3Catalyst for the Dehydrogenation of Cyclohexane. Chemcatchem 2012, 4, 1376–1381. [Google Scholar] [CrossRef]
- Mitchell, P.C.H.; Wass, S.A. Propane dehydrogenation over molybdenum hydrotalcite catalysts. Appl. Catal. A 2002, 225, 153–165. [Google Scholar] [CrossRef]
- Mokhtar, M.; Inayat, A.; Ofili, J.; Schwieger, W. Thermal decomposition, gas phase hydration and liquid phase reconstruction in the system Mg/Al hydrotalcite/mixed oxide: A comparative study. Appl. Clay Sci. 2010, 50, 176–181. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Abelló, S.; van der Pers, N.M. Memory Effect of Activated Mg-Al Hydrotalcite: In Situ XRD Studies during Decomposition and Gas-Phase Reconstruction. Chem. - Eur. J. 2007, 13, 870–878. [Google Scholar] [CrossRef]
- Airaksinen, S.M.K.; Harlin, M.E.; Krause, A.O.I. Kinetic Modeling of Dehydrogenation of Isobutane on Chromia/Alumina Catalyst. Ind. Eng. Chem. Res. 2002, 41, 5619–5626. [Google Scholar] [CrossRef]
- Derossi, S.; Ferraris, G.; Fremiotti, S.; Garrone, E.; Ghiotti, G.; Campa, M.C.; Indovina, V. Propane Dehydrogenation on Chromia/Silica and Chromia/Alumina catalysts. J. Catal. 1994, 148, 36–46. [Google Scholar] [CrossRef]
- Prinetto, F.; Ghiotti, G.; Durand, R.; Tichit, D. Investigation of Acid−Base Properties of Catalysts Obtained from Layered Double Hydroxides. J. Phys. Chem. B 2000, 104, 11117–11126. [Google Scholar] [CrossRef]
- Mounfield, W.P.; Claure, M.T.; Agrawal, P.K.; Jones, C.W.; Walton, K.S. Synergistic Effect of Mixed Oxide on the Adsorption of Ammonia with Metal-Organic Frameworks. Ind. Eng. Chem. Res. 2016, 22, 6492–6500. [Google Scholar] [CrossRef]
- Rao, K.K.; Gravelle, M.; Valente, J.S.; Figueras, F. Activation of Mg-Al Hydrotalcite Catalysts for Aldol Condensation Reactions. J. Catal. 1998, 173, 115–121. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Z.; Wu, T.; Zeng, L.; Gong, J. Nature of the Active Sites of VOx/Al2O3 Catalysts for Propane Dehydrogenation. Acs Catal. 2016, 6, 5207–5214. [Google Scholar] [CrossRef]
- Han, Z.; Li, S.; Jiang, F.; Wang, T.; Ma, X.; Gong, J. Propane dehydrogenation over Pt-Cu bimetallic catalysts: The nature of coke deposition and the role of copper. Nanoscale 2014, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]



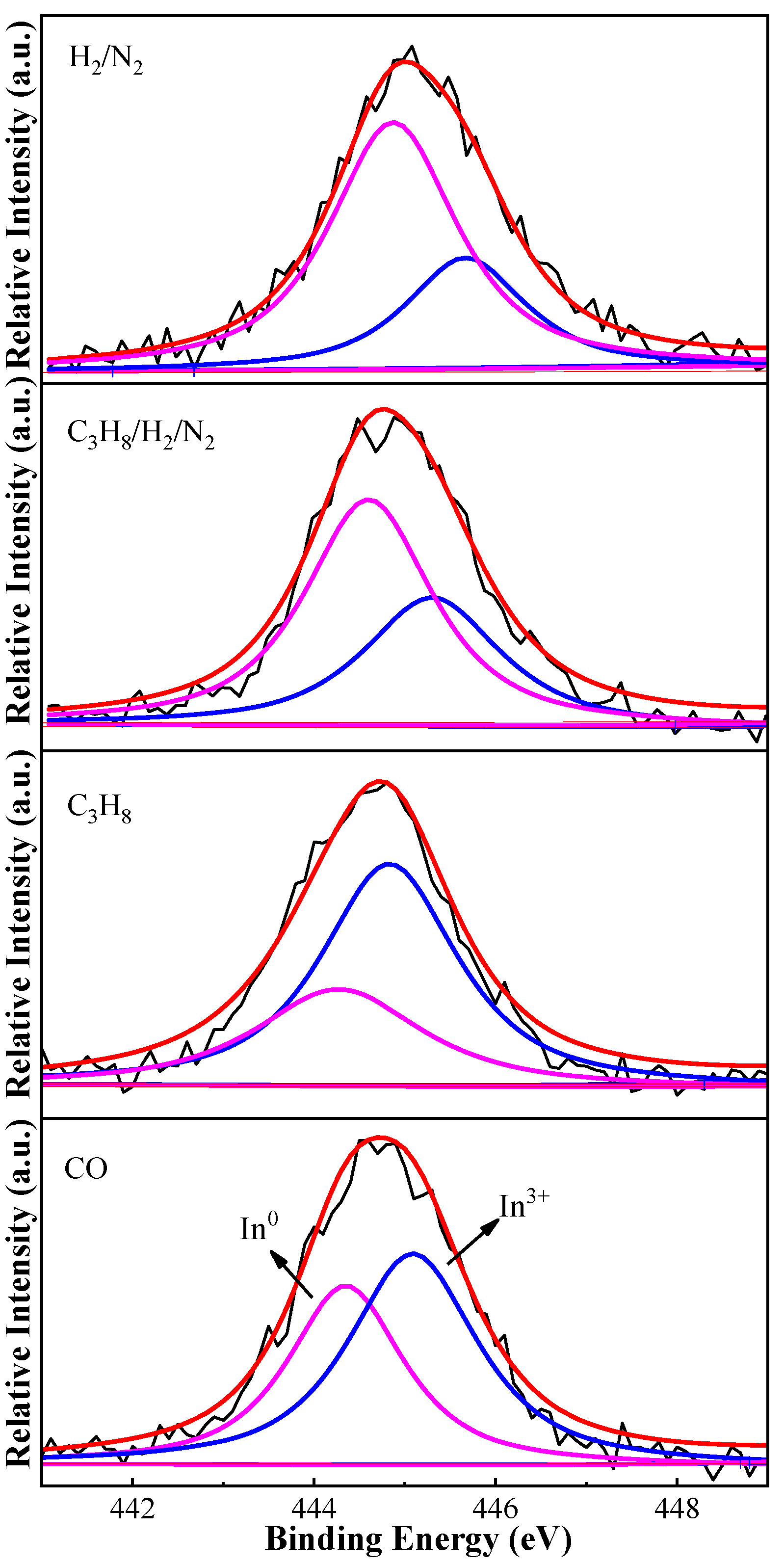

| Reduction Atmosphere | Binding Energy (eV) | In0/In3+ Molar Ratio b | Pt Dispersion c | |
|---|---|---|---|---|
| In0 | In3+ | |||
| H2/N2 | 444.9 | 445.7 | 2.50 | 48% |
| C3H8/H2/N2 | 444.5 | 445.2 | 1.22 | 34% |
| C3H8 | 444.3 | 444.8 | 0.59 | — |
| CO | 444.4 | 445.1 | 0.78 | — |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Song, Z.; Guo, M.; Li, X.; Lin, Y.; Zhang, L. Effect of Reduction Atmosphere on Structure and Catalytic Performance of PtIn/Mg(Al)O/ZnO for Propane Dehydrogenation. Catalysts 2020, 10, 485. https://doi.org/10.3390/catal10050485
Zhang M, Song Z, Guo M, Li X, Lin Y, Zhang L. Effect of Reduction Atmosphere on Structure and Catalytic Performance of PtIn/Mg(Al)O/ZnO for Propane Dehydrogenation. Catalysts. 2020; 10(5):485. https://doi.org/10.3390/catal10050485
Chicago/Turabian StyleZhang, Ming, Zhen Song, Mengquan Guo, Xiangxiang Li, Yanjun Lin, and Lihong Zhang. 2020. "Effect of Reduction Atmosphere on Structure and Catalytic Performance of PtIn/Mg(Al)O/ZnO for Propane Dehydrogenation" Catalysts 10, no. 5: 485. https://doi.org/10.3390/catal10050485
APA StyleZhang, M., Song, Z., Guo, M., Li, X., Lin, Y., & Zhang, L. (2020). Effect of Reduction Atmosphere on Structure and Catalytic Performance of PtIn/Mg(Al)O/ZnO for Propane Dehydrogenation. Catalysts, 10(5), 485. https://doi.org/10.3390/catal10050485