Current Trends in MXene-Based Nanomaterials for Energy Storage and Conversion System: A Mini Review
Abstract
1. Introduction
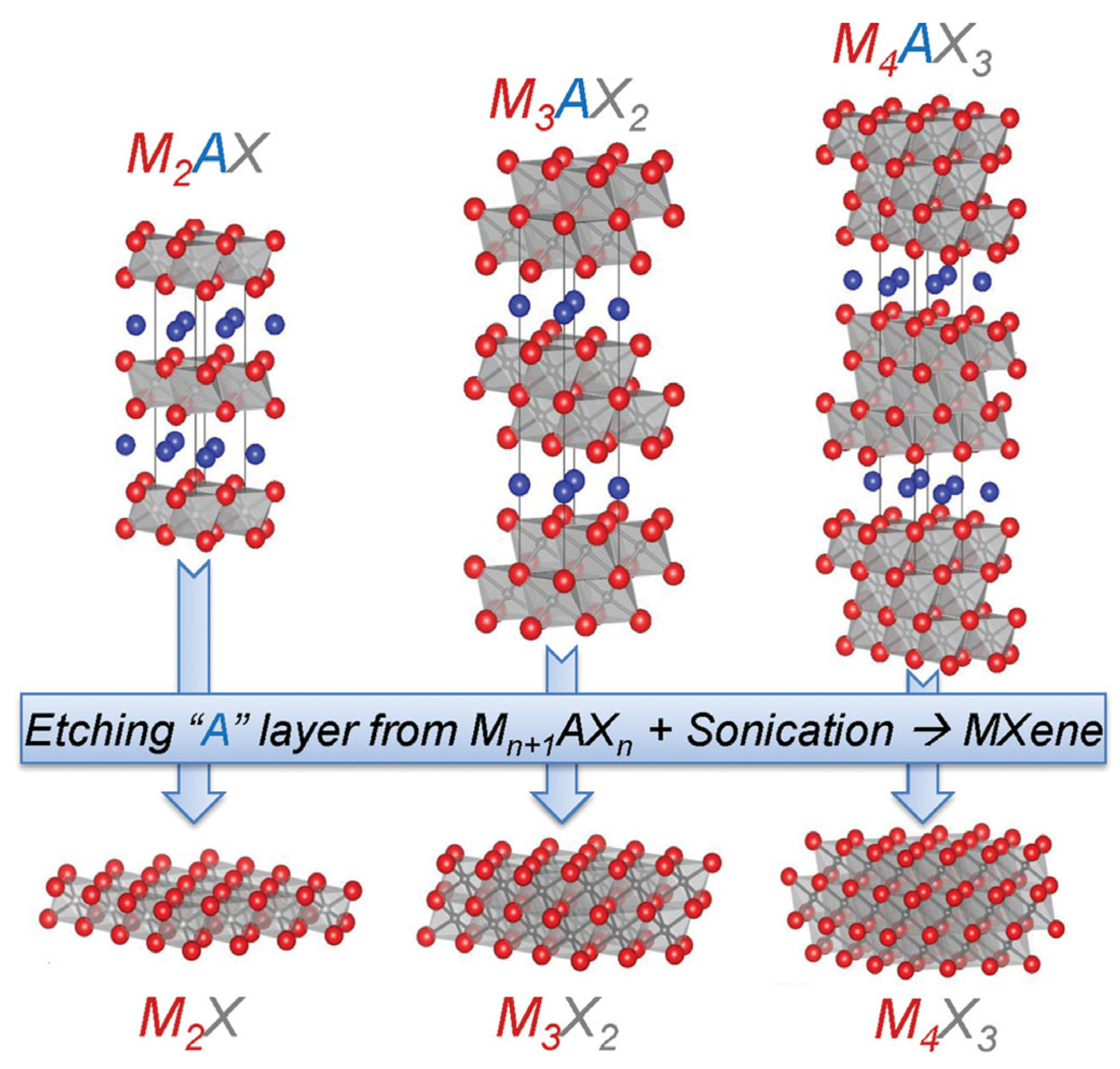
2. Synthesis and Characterization of MXenes
2.1. Synthesis
2.1.1. Synthesis Method
2.1.2. MXene Preparation
2.2. Characterization
3. Fundamental Properties of MXene with Relevance in Energy Storage System
3.1. Electronic and Electrical Properties
3.2. Morphological Properties
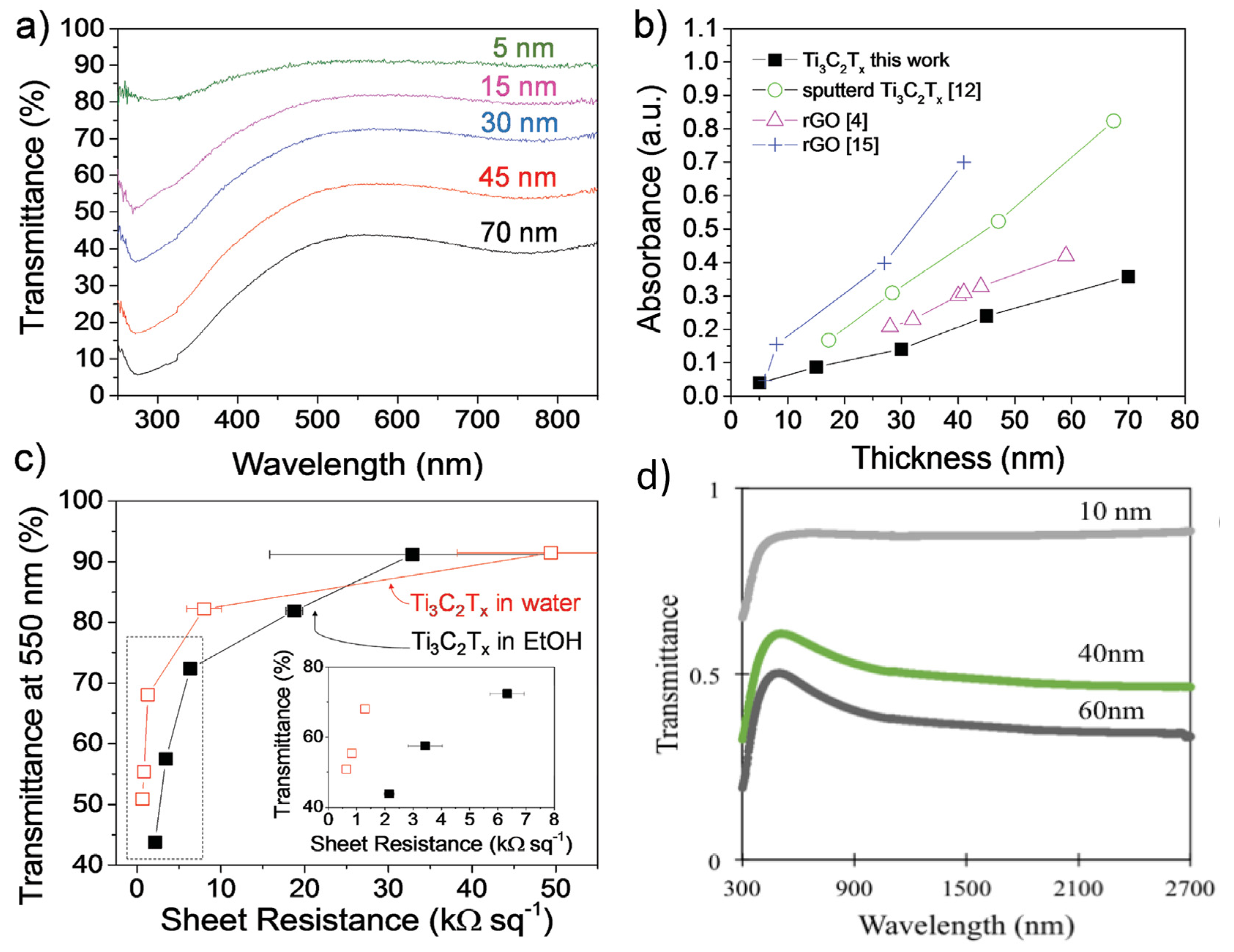
3.3. Optical Properties
4. MXene for Energy Storage and Conversion Systems
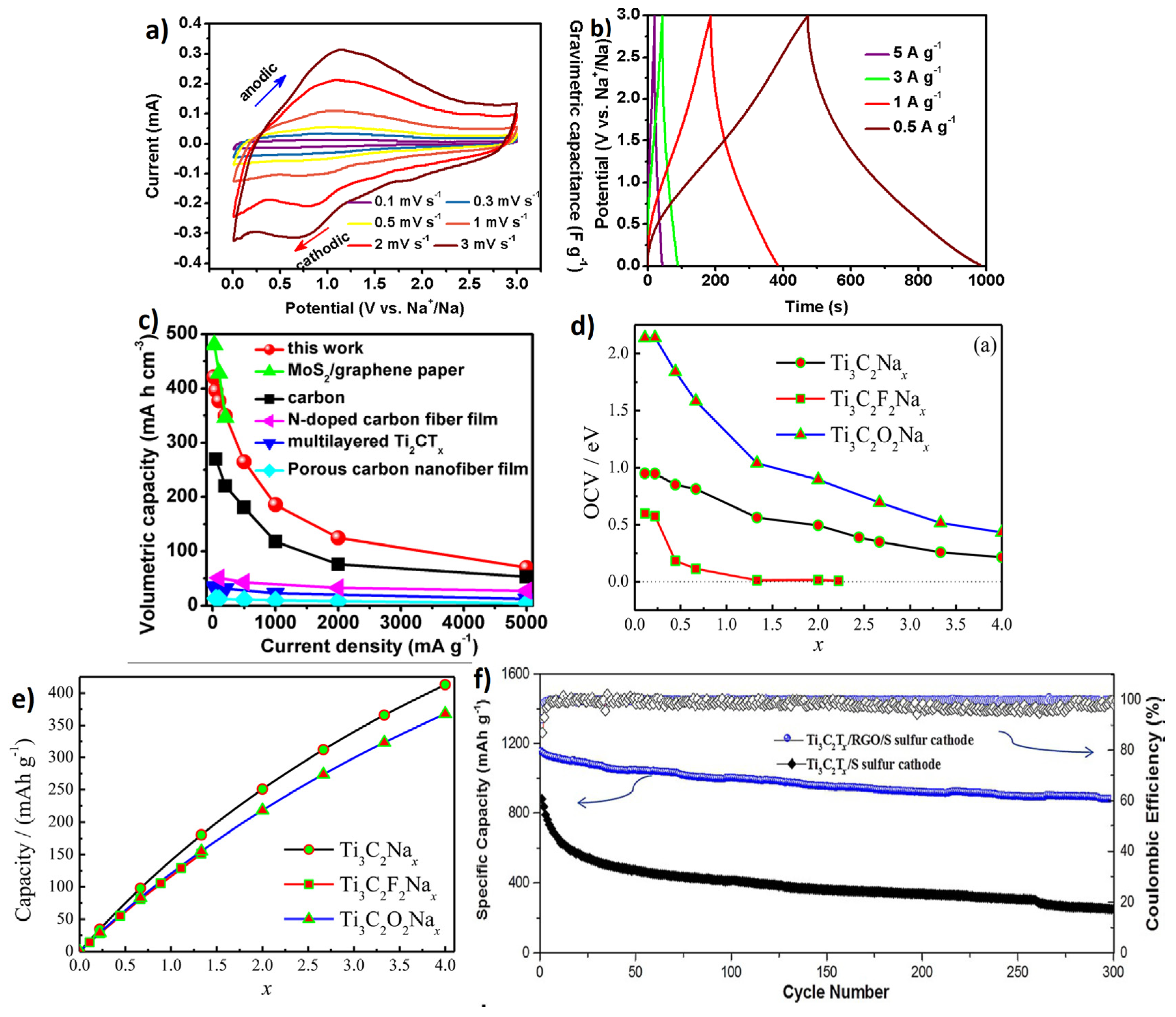
4.1. MXene for Energy Storage: Batteries
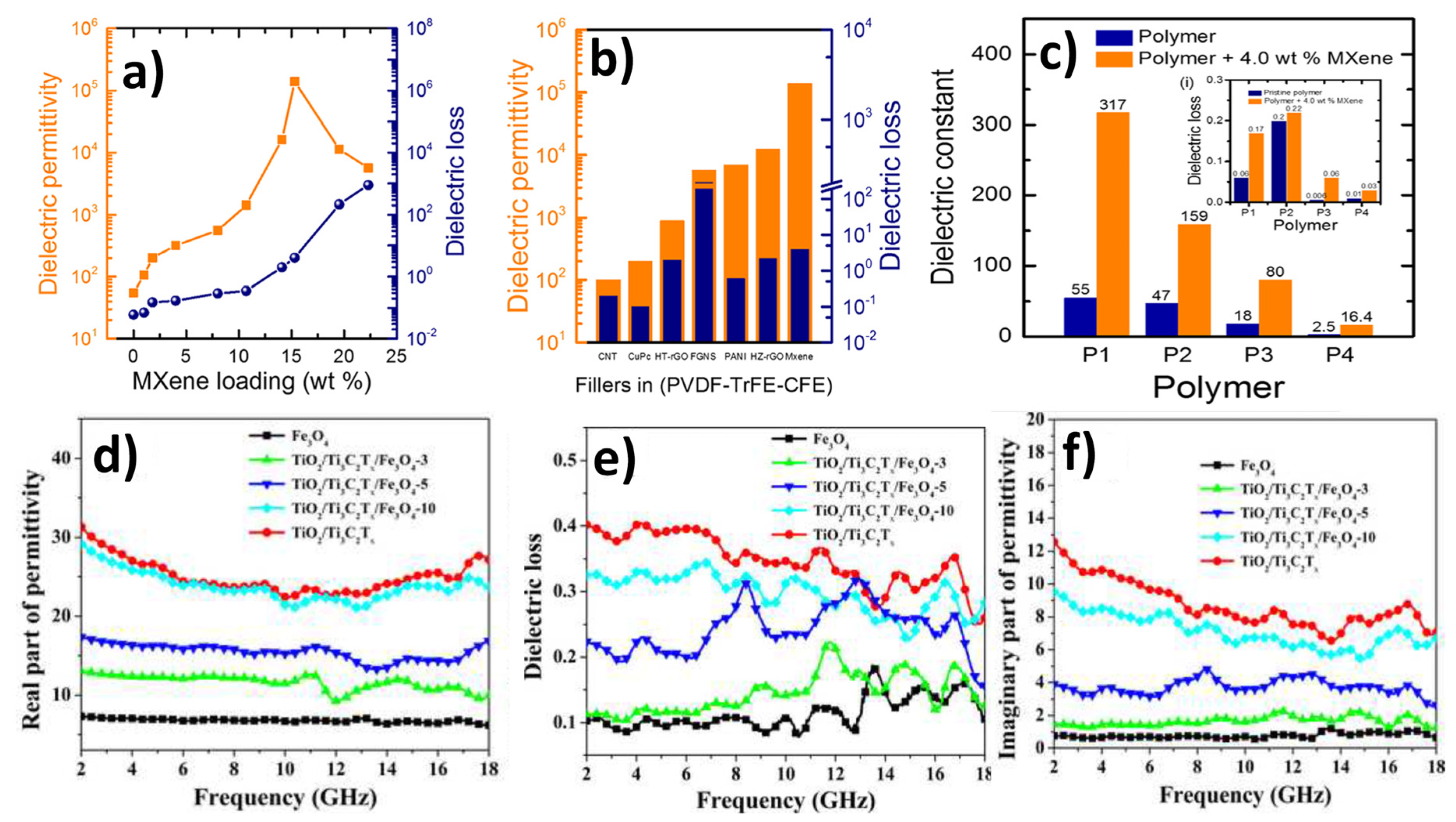
4.2. MXene for Energy Storage: Supercapacitors/Dielectrics
4.3. MXene for CO2 Conversion
4.4. MXene for Proton Reduction
4.5. MXene for Oxygen Reduction Reaction
5. Concluding Remarks and Prospective
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, Q.; Zhou, Z.; Chen, Z. Graphene-related nanomaterials: Tuning properties by functionalization. Nanoscale 2013, 5, 4541–4551. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zhou, Z. Graphene-analogous low -dimensional materials. Prog. Mater. Sci. 2013, 58, 1244–1315. [Google Scholar] [CrossRef]
- Naguib, M.; Gogotsi, Y. Synthesis of two -dimensional materials by selective extraction. Acc. Chem. Res. 2015, 48, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Shein, I.R.; Ivanovskii, A.L. Graphene-like nanocarbides and nanonitrides of d metals (MXenes): Synthesis. properties and simulation. Micro Nano Lett. 2013, 8, 59–62. [Google Scholar] [CrossRef]
- Karlsson, L.H.; Birch, J.; Halim, J.; Barsoum, M.W.; Persson, P.O. Atomically resolved structural and chemical investigation of single MXene sheets. Nano Lett. 2015, 15, 4955–4960. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 72–75. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of Two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Sobolčiak, P.; Tanvir, A.; Sadasivuni, K.K.; Krupa, I. Piezoresistive Sensors Based on Electrospun Mats Modified by 2D Ti3C2Tx MXene. Sensors 2019, 19, 4589–4597. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, J.; Zhu, L.; Sun, Z. MXene: A promisingphotocatalyst for water splitting. J. Mater. Chem. A 2016, 4, 11446–11452. [Google Scholar] [CrossRef]
- Wen, M.Q.; Xiong, T.; Zang, Z.G.; Wei, W.; Tang, X.S.; Dong, F. Synthesis of MoS 2/g-C3N4 nanocomposites with enhanced visible-light photocatalytic activity for the removal of nitric oxide (NO). Opt. Express 2016, 24, 10205–10212. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.; Lee, C.; Tai, N. Biomass-derived three-dimensional carbon framework for a flexible fibrous supercapacitor and its application as a wearable smart textile. RSC Adv. 2020, 10, 6960–6972. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An effective 2D Light-to-Heat conversion material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef] [PubMed]
- Bhimanapati, G.R.; Lin, Z.; Meunier, V.; Jung, Y.; Cha, J.; Das, S.; Xiao, D.; Son, Y.; Strano, M.S.; Cooper, V.R.; et al. Recent advances in two-dimensional materials beyond Graphene. ACS Nano 2015, 9, 11509–11539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, H.; Zou, G.; Fernandez, C.; Liu, B.; Zhang, Q.; Hu, J.; Peng, Q. Self–Reduction synthesis of new MXene/Ag composites with unexpected electrocatalytic activity. ACS Sustain. Chem. Eng. 2016, 4, 6763–6771. [Google Scholar] [CrossRef]
- Xue, Q.; Pei, Z.; Huang, Y.; Zhu, M.; Tang, Z.; Li, H.; Huang, Y.; Li, N.; Zhang, H.; Zhi, C. Mn3O4 nanoparticles on layer-structured Ti3C2 MXene towards the oxygen reduction reaction and zinc-air batteries. J. Mater. Chem. A 2017, 5, 20818–20823. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, B.; Li, S.; Zhou, L.; Lai, L.; Wang, Z.; Zhao, S.; Han, M.; Gao, K.; Lu, M.; et al. Interdiffusion reaction-assisted hybridization of two–dimensional metal-organic frameworks and Ti3C2Tx nanosheets for electrocatalytic oxygen evolution. ACS Nano 2017, 11, 5800–5807. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, F.; Yu, J.; Deng, Q.; Zhang, F.; Wang, G. Titanium carbide (Ti3C2Tx) MXene: A novel precursor to amphiphilic carbide-derived graphene quantum dots for fluorescent ink, light-emitting composite and bioimaging. Carbon 2017, 118, 50–57. [Google Scholar] [CrossRef]
- Kota, S.; Chen, Y.; Wang, J.; May, S.J.; Radovic, M.; Barsoum, M.W. Synthesis and characterization of the atomic laminate Mn2AlB2. J. Eur. Ceram. Soc. 2018, 38, 5333–5340. [Google Scholar] [CrossRef]
- Zang, X.; Wang, J.; Yijiang, Q.; Wang, T.; He, C.; Shao, Q. Enhancing Capacitance Performance of Ti3C2Tx MXene as Electrode Materials of Supercapacitor: From Controlled Preparation to Composite Structure Construction. Nano Micro Lett. 2020, 12, 77. [Google Scholar] [CrossRef]
- Nyamdelger, S.; Ochirkhuyag, T.; Sangaa, D.; Odkhuu, D. First-principles prediction of a two-dimensional vanadium carbide (MXene) as the anode for lithium ion batteries. Phys. Chem. Chem. Phys. 2020, 22, 5807–5818. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Anasori, B.; Gogotsi, Y.; Alshareef, H.N. Thermoelectric properties of two dimensional molybdenum-based MXenes. Chem. Mater. 2017, 29, 6472–6479. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Hedhili, M.N.; Gogotsi, Y.; Alshareef, H.N. H2O2 assisted room temperature oxidation of Ti2C MXene for Li-ion battery anodes. Nanoscale 2016, 8, 7580–7587. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Sun, D.F.; Huang, Q.; Yang, J.; Barsoum, M.W.; Yan, X.B. Carbon nanofiber bridged two-dimensional titanium carbide as a superior anode for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 14096–14100. [Google Scholar] [CrossRef]
- Ren, C.E.; Zhao, M.-Q.; Makaryan, T.; Halim, J.; Boota, M.; Kota, S.; Anasori, B.; Barsoum, M.W.; Gogotsi, Y. Porous Two-Dimensional Transition Metal Carbide (MXene) Flakes for High-Performance Li-Ion Storage. ChemElectroChem 2016, 3, 689–693. [Google Scholar] [CrossRef]
- Zhao, S.; Meng, X.; Zhu, K.; Du, F.; Chen, G.; Wei, Y.; Gogotsi, Y.; Gao, Y. Li-ion uptake and increase in interlayer spacing of Nb4C3 MXene. Energy StorageMater. 2017, 8, 42–48. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Torelli, M.; Ren, C.E.; Ghidiu, M.; Ling, Z.; Anasori, B.; Barsoum, M.W.; Gogotsi, Y. 2D titanium carbide and transition metal oxides hybrid electrodes for Li-ion storage. Nano Energy 2016, 30, 603–613. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Gogotsi, Y.; Alshareef, H.N. Atomic layer deposition of SnO2 on MXene for Li-ion battery anodes. Nano Energy 2017, 34, 249–256. [Google Scholar] [CrossRef]
- Shen, C.; Wang, L.; Zhou, A.; Zhang, H.; Chen, Z.; Hu, Q.; Qin, G. MoS2-Decorated Ti3C2 MXene nanosheet as anode material in lithium-ion batteries. J. Electrochem. Soc. 2017, 164, A2654. [Google Scholar] [CrossRef]
- Ling, Z.; Ren, C.E.; Zhao, M.-Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676–16681. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New two-dimensional niobium and vanadium carbides as promising materials for li-ion batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Li, C.; Yang, J.; Tang, H.; Xue, M. Synthesis of a new graphene-like transition metal carbide by deintercalating Ti3AlC2. Mater. Lett. 2013, 109, 295–298. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M.; Lu, J.; Smith, C.R.; Naslund, L.A.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; et al. Transparent conductive two dimensional titanium carbide epitaxial thin films. Chem. Mater. 2014, 26, 2374–2381. [Google Scholar] [CrossRef]
- Ghidiu, M.; Naguib, M.; Shi, C.; Mashtalir, O.; Pan, L.M.; Zhang, B.; Yang, J.; Gogotsi, Y.; Billinge, S.J.L.; Barsoum, M.W. Synthesis and characterization of two-dimensional Nb4C3 (MXene). Chem. Commun. 2014, 50, 9517–9520. [Google Scholar] [CrossRef]
- Feng, Y.; Deng, Q.; Peng, C.; Hu, J.; Li, Y.; Wu, Q.; Xu, Z. An ultrahigh discharged energy density achieved in an inhomogeneous PVDF dielectric composite filled with 2D MXene nanosheets via interface engineering. J. Mater. Chem. C 2018, 6, 13283–13292. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Persson, I.; El Ghazaly, A.; Tao, Q.; Halim, J.; Kota, S.; Darakchieva, V.; Palisaitis, J.; Barsoum, M.W.; Rosen, J.; Persson, P.O.Å. Tailoring structure, composition, and energy storage properties of MXenes from selective etching of in-plane, chemically orderedMAX phases. Small 2018, 1703676, 1–7. [Google Scholar]
- Tu, S.; Jiang, Q.; Zhang, J.; He, X.; Hedhili, M.N.; Zhang, X.; Alshareef, H.N. Enhancement of Dielectric Permittivity of Ti3C2Tx MXene/Polymer Composites by Controlling Flake Size and Surface Termination. ACS Appl. Mater. Interfaces 2019, 30, 27358–27362. [Google Scholar] [CrossRef]
- Tu, S.; Jiang, Q.; Zhang, X.; Husam Alshareef, N. Large Dielectric Constant Enhancement in MXene Percolative Polymer Composites. ACS Nano 2018, 12, 3369–3377. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Zhang, Q.; Wang, D. Titanium carbide MXene: Synthesis, electrical and optical properties and their applications in sensors and energy storage devices. Nanomater. Nanotechnol. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Seyedin, S.; Yanza, E.R.S.; Razal, J.M. Knittable energy storing fiber with high volumetric performance made from predominantly MXene nanosheets. J. Mater. Chem. A 2017, 5, 24076–24082. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Z.; Fang, B.; Huang, T.; Cai, S.; Chen, H.; Liu, Y.; Gopalsamy, K.; Gao, W.; Gao, C. MXene/graphene hybrid fibers for high performance flexible supercapacitors. J. Mater. Chem. A 2017, 5, 22113–22119. [Google Scholar] [CrossRef]
- Li, H.; Hou, Y.; Wang, F.; Lohe, M.R.; Zhuang, X.; Niu, L.; Feng, X. Flexible all-solid-state supercapacitors with high volumetric capacitances boosted by solution processable MXene and electrochemically exfoliated graphene. Adv. Energy Mater. 2017, 7, 1601847. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, W. High-Throughput Calculation Investigations on the Electrocatalytic Activity of Codoped Single Metal–Nitrogen Embedded in Graphene for ORR Mechanism. Electrocatalysis 2020. [Google Scholar] [CrossRef]
- Lin, H.; Chen, L.; Lu, X.; Yao, H.; Chen, Y.; Shi, J. Two-dimensional titanium carbide MXenes as efficient non-noble metal electrocatalysts for oxygen reduction reaction. Sci. China Mater. 2019, 62, 662–670. [Google Scholar] [CrossRef]
- Zeng, Z.; Yan, Y.; Chen, J.; Zan, P.; Tian, Q.; Chen, P. Boosting the Photocatalytic Ability of Cu2O Nanowires for CO2 Conversion by MXene Quantum Dots. Adv. Funct. Mater. 2018, 1806500. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.S.; Cheng, B.; Ho, W.K.; Yu, J.G. Sulfur-doped g -C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B 2015, 176, 44–52. [Google Scholar] [CrossRef]
- Zhao, H.; Lv, J.; Sang, J.; Zhu, L.; Zheng, P.; Andrew, G.; Tan, L. A Facile Method to Construct MXene/CuO Nanocomposite with Enhanced Catalytic Activity of CuO on Thermal Decomposition of Ammonium Perchlorate. Materials 2018, 11, 2457. [Google Scholar] [CrossRef]
- Yang, X.; Jia, Q.; Duan, F.; Hu, B.; Wang, M.; He, L.; Song, Y.; Zhang, Z. Multiwall carbonnanotubes loaded with MoS2 quantum dots and MXene quantum dots: Non-Pt bifunctional catalyst for the methanol oxidation and oxygen reduction reactions in alkaline solution. Appl. Surf. Sci. 2017, 464, 78–87. [Google Scholar] [CrossRef]
- Yu, X.; Yin, W.; Wang, T.; Zhang, Y. Decorating g-C3N4 Nanosheets with Ti3C2 MXene Nanoparticles for Efficient Oxygen Reduction Reaction. Langmuir 2019, 35, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhou, Y.; Xu, W.; Huang, D.; Wang, Z.; Hong, M. Fabrication and thermal stability of two-dimensional carbide Ti3C2 nanosheets. Ceram. Int. 2016, 42, 8419–8424. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Estili, M.; Sakka, Y. Two-dimensional molybdenum carbides: Potential thermoelectric materials of the MXene family. Phys. Chem. Chem. Phys. 2014, 16, 7841–7849. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.H.; Yin, J.; Zhou, Y.; Huang, Q.; Luo, K.; Lang, J.; Francisco, J.S.; He, J.; Du, S. Intrinsic structural, electrical, thermal and mechanicalproperties of the promising conductor Mo2C MXene. J. Phys. Chem. C 2016, 120, 15082–15088. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Zhang, J.; Li, G.; Huang, H.; Zhang, X.; Jiang, Q. Enhancement of the electrical properties of MXene Ti3C2 nanosheets by post-treatments of alkalization and calcination. Mater. Lett. 2015, 160, 537–540. [Google Scholar] [CrossRef]
- Zhang, C.J.; Anasori, B.; Seral-ascaso, A.; Park, S.; Mcevoy, N.; Shmeliov, A.; Duesberg, G.S.; Coleman, J.N.; Gogotsi, Y.; Nicolosi, V. Transparent, flexible and conductive2D titanium carbide (MXene) films with high volumetric capacitance. Adv. Mater. 2017, 29, 1702678. [Google Scholar] [CrossRef]
- Maleski, K.; Ren, C.E.; Zhao, M.; Anasori, B.; Gogotsi, Y. Size-dependent physical and electrochemical properties of two-dimensional MXene flakes. ACS Appl. Mater. Interfaces 2018, 10, 24491–24498. [Google Scholar] [CrossRef]
- Muckley, E.S.; Naguib, M.; Ivanov, I.N. Multi-modal, ultrasensitive, wide-range humidity sensing with Ti3C2 film. Nanoscale 2018, 10, 21689–21695. [Google Scholar] [CrossRef]
- Muckley, E.S.; Naguib, M.; Wang, H.W.; Vlcek, L.; Osti, N.C.; Sacci, R.L.; Sang, X.; Unocic, R.R.; Xie, Y.; Tyagi, M.; et al. Multimodality of structural, electrical and gravimetricresponses of intercalated MXenes to water. ACS Nano 2017, 11, 11118–11126. [Google Scholar] [CrossRef] [PubMed]
- Hantanasirisakul, K.; Gogotsi, Y. Electronic and optical properties of 2D transition metal carbides and nitrides (MXenes). Adv. Mater. 2018, 30, 1804779. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/graphene films for ultrafast supercapacitors with outstanding volumetric capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- He, J.; Liu, S.; Deng, L.; Shan, D.; Cao, C.; Luo, H.; Yan, S. Tunable electromagnetic and enhanced microwave absorption properties in CoFe2O4 decorated Ti3C2 MXene composites. Appl. Surf. Sci. 2020, 504, 144210. [Google Scholar] [CrossRef]
- Tariq, A.; Ali, S.I.; Akinwande, D.; Rizwan, S. Efficient Visible-Light Photocatalysis of 2D-MXene Nanohybrids with Gd3+ and Sn4+-Codoped Bismuth Ferrite. ACS Omega 2018, 3, 13828–13836. [Google Scholar] [CrossRef] [PubMed]
- Lee Ying, G.; Kota, S.; Dillon, A.D.; Fafarman, A.T.; Barsoum, M.W. Conductive transparent V2CTx (MXene) films. FlatChem. 2018, 8, 25–30. [Google Scholar] [CrossRef]
- Hantanasirisakul, K.; Zhao, M.Q.; Urbankowski, P.; Halim, J.; Anasori, B.; Kota, S.; Ren, C.E.; Barsoum, M.W.; Gogotsi, Y. Fabrication of Ti 3C2Tx MXene transparent thin films with tunable optoelectronic properties. Adv. Electron. Mater. 2016, 2, 1600050. [Google Scholar] [CrossRef]
- Karthik, K.; Pushpa, S.; Madhukara Naik, M.; Vinuth, M. Influence of Sn and Mn on structural, optical and magnetic properties of spray pyrolysed CdS thin films. Mater. Res. Innovation. 2020, 24, 82–86. [Google Scholar] [CrossRef]
- Xing, C.; Chen, S.; Liang, X.; Liu, Q.; Qu, M.; Zou, Q.; Li, J.; Tan, H.; Liu, L.; Fan, D.; et al. Two-Dimensional MXene (Ti3C2)-integrated cellulose hydrogels: Toward smart three-dimensional network nanoplatforms exhibiting light-induced swelling and bimodal photothermal/chemotherapy anticancer activity. ACS Appl. Mater. Interfaces 2018, 10, 27631–27643. [Google Scholar] [CrossRef]
- Berdiyorov, G.R. Optical properties of functionalized Ti3C2T2 (T = F, O, OH) MXene: First-principles calculations. AIP Adv. 2016, 6, 055105. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Hu, Q.; Zheng, M.; Chi, Y.; Qin, X.; Pang, H.; Xu, Q. MXene 2D layered electrode materials for energy storage. Prog. Nat. Sci. Mater. Int. 2018, 28, 133–147. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Y.; Zhang, J.; Hou, L.; Sun, J.; Yuan, C. Facile construction of ultrathin SnOx nanosheets decorated MXene (Ti3C2) nanocomposite towards Li-ion batteries as high performance anode materials. Electrochim. Acta 2019, 295, 237–245. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2018, 48, 72–133. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Guo, X.; Wu, W.; Wang, G. 2D metal carbides and nitrides (MXenes) as high-performance electrode materials for lithium-based batteries. Adv. Energy Mater. 2018, 8, 1801897. [Google Scholar] [CrossRef]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A promisingtransition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Shen, P.W. Are MXenes promising anode materials for Li ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) monolayer. J. Am. Chem. Soc. 2012, 134, 16909–16916. [Google Scholar] [CrossRef]
- Xie, Y.; Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y.; Yu, X.Q.; Nam, K.W.; Yang, X.Q.; Kolesnikov, A.I.; Kent, P.R.C. Role of surface structure on li-ion energy storage capacity of two-dimensional transition-metal carbides. J. Am. Chem. Soc. 2014, 136, 6385–6394. [Google Scholar] [CrossRef]
- Xie, Y.; Dall’Agnese, Y.; Naguib, M.; Gogotsi, Y.; Barsoum, M.W.; Zhuang, H.L.; Kent, P.R.C. Prediction and characterization of MXene nanosheet anodes for nonlithium- ion batteries. ACS Nano 2014, 8, 9606–9615. [Google Scholar] [CrossRef]
- Eames Islam, C.M.S. Ion intercalation into two dimensional transition-metal carbides: Global screening for new high-capacity battery materials. J. Am. Chem. Soc. 2014, 136, 16270–16276. [Google Scholar] [CrossRef]
- Burke, A. R&D considerations for the performance and application of electrochemical capacitors. Electrochim. Acta 2007, 53, 1083–1091. [Google Scholar]
- Xie, X.; Zhao, M.Q.; Anasori, B.; Maleski, K.; Ren, C.E.; Li, J.; Byles, B.W.; Pomerantseva, E.; Wang, G.; Gogotsi, Y. Porous heterostructured MXene /carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices. Nano Energy 2016, 26, 513–523. [Google Scholar] [CrossRef]
- Bao, W.; Tang, X.; Guo, X.; Choi, S.; Wang, C.; Gogotsi, Y.; Wang, G. Porous cryo-dried MXene for efficient capacitive deionization. Joule 2018, 2, 778–787. [Google Scholar] [CrossRef]
- Yu, Y.X. Prediction of mobility, enhanced storagecapacity and volumechange during sodiation on interlayer-expanded functionalized Ti3C2 MXene anode materials for sodium-ion batteries. J. Phys. Chem. C 2016, 120, 5288–5296. [Google Scholar] [CrossRef]
- Bao, W.; Xie, X.; Xu, J.; Guo, X.; Song, J.; Wu, W.; Su, D.; Wang, G. Confined sulfur in 3D MXene/reduced graphene oxide hybrid nanosheets for lithium-sulfur battery. Chem. A Eur. J. 2017, 23, 12613–12619. [Google Scholar] [CrossRef]
- Yan, B.; Lu, C.; Zhang, P.; Chen, J.; He, W.; Tian, W.; Zhang, W.; Sun, Z.M. Oxygen/sulfur decorated 2D MXene V2C for promising lithium ion battery anodes. Mater. Today Commun. 2020, 22, 100713. [Google Scholar] [CrossRef]
- Illa, M.P.; Khandelwal, M.; Sharma, C.S. Bacterial cellulose-derived carbon nanofibers as anode for lithium-ion batteries. Emerg. Mater. 2018, 1, 105–120. [Google Scholar] [CrossRef]
- Mashtalir, O.; Lukatskaya, M.R.; Zhao, M.-Q.; Barsoum, M.W.; Gogotsi, Y. Amine-Assisted Delamination of Nb2C MXene for Li-Ion Energy Storage Devices. Adv. Mater. 2015, 27, 3501–3506. [Google Scholar] [CrossRef]
- Luo, J.; Tao, X.; Zhang, J.; Xia, Y.; Huang, H.; Zhang, L.; Gan, Y.; Liang, C.; Zhang, W. Sn4+ Ion Decorated Highly Conductive Ti3C2 MXene: Promising Lithium-Ion Anodes with Enhanced Volumetric Capacity and Cyclic Performance. ACS Nano 2016, 10, 2491–2499. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, Z.; Guo, J.; Liu, B.; Zhang, Q.; Fernandez, C.; Peng, Q. Synthesis of MXene/Ag composites for extraordinary long cycle lifetime lithium storage at high rates. ACS Appl. Mater. Interfaces 2016, 8, 22280–22286. [Google Scholar] [CrossRef]
- Pan, X.; Shinde, N.M.; Lee, M.; Kim, D.; Ho Kim, K.; Kang, M. Controlled nanosheet morphology of titanium carbide Ti3C2Tx MXene via drying methods and its electrochemical analysis. J. Solid State Electrochem. 2020, 24, 675–686. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Syamsai, R.; Kollu, P.; Kwan Jeong, S.A. Nirmala Grace. Synthesis andproperties of 2D-titanium carbide MXene sheets towards electrochemical energy storage applications. Ceram. Int. 2017, 43, 13119–13126. [Google Scholar] [CrossRef]
- Okubo, M.; Sugahara, A.; Kajiyama, S.; Yamada, A. MXene as a charge storage host. Acc. Chem. Res. 2018, 51, 591–599. [Google Scholar] [CrossRef]
- Bak, S.M.; Qiao, R.; Yang, W.; Lee, S.; Yu, X.; Anasori, B.; Lee, H.; Gogotsi, Y.; Yang, X.Q. Na-ion intercalation and charge storage mechanism in 2D vanadium carbide. Adv. Energy Mater. 2017, 7, 1700959. [Google Scholar] [CrossRef]
- Zhan, C.; Naguib, M.; Lukatskaya, M.; Kent, P.R.C.; Gogotsi, Y.; Jiang, D. Understanding the MXene pseudocapacitance. J. Phys. Chem. Lett. 2018, 9, 1223–1228. [Google Scholar] [CrossRef]
- Weiming, W.; Changsong, Z.; Shaogang, H.; Shubin, Y. Synthesis of MXenes and MXenes-containing composites for energy storage and conversions. Chin. J. Appl. Chem. 2018, 35, 317–327. [Google Scholar]
- Xia, Q.X.; Fu, J.; Yun, J.M.; Mane, R.S.; Kim, K.H. High volumetric energy density annealed-MXene-nickel oxide/MXene asymmetric supercapacitor. RSC Adv. 2017, 7, 11000–11011. [Google Scholar] [CrossRef]
- Chang H, Q.; Zhang, G.H.; Chou K, C. Topochemical synthesis of two-dimensional molybdenum carbide (Mo2C) via Na2CO3-Assited carbothermal reduction of 2H–MoS2. Mater. Chem. Phys. 2020, 244, 122713. [Google Scholar] [CrossRef]
- Tao, Q.; Dahlqvist, M.; Lu, J.; Kota, S.; Meshkian, R.; Halim, J.; Palisaitis, J.; Hultman, L.; Barsoum, M.W.; Persson, P.O.Å.; et al. Two-dimensional Mo 1.33C MXene with divacancy ordering prepared from parent 3D laminate with in-plane chemical ordering. Nat. Commun. 2017, 8, 14949. [Google Scholar] [CrossRef]
- Xin, Y.; Yu, Y.X. Possibility of bare and functionalized niobium carbide MXenes for electrode materials of supercapacitors and field emitters. Mater. Des. 2017, 130, 512–520. [Google Scholar] [CrossRef]
- He, X.; Bai, Y.; Zhu, C.; Sun, Y.; Li, M.; Barsoum, M.W. General trends in the structural, electronic andelastic properties of the M3AlC2 phases (M = transition metal): A first-principle study. Comput. Mater. Sci. 2010, 49, 691–698. [Google Scholar] [CrossRef]
- Kayali, E.; Vahidmohammadi, A.; Orangi, J.; Beidaghi, M. Controlling the dimensions of 2D MXenes for ultrahigh-rate pseudocapacitive energy storage. ACS Appl. Mater. Interfaces 2018, 10, 25949–25954. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.; Wang, T.; Chen, S.; Liu, Y.; Shao, L. Constructing expanded ion transport channels in flexible MXene film for pseudocapacitive energy storage. Appl. Surf. Sci. 2020, 511, 145627. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, S.; Cai, D.; Cao, J.; Wang, L.; Han, W. Planar supercapacitor with high areal capacitance based on Ti3C2/Polypyrrole composite film. Electrochimica Acta 2020, 330, 135277. [Google Scholar] [CrossRef]
- Shan, Q.; Mu, X.; Alhabeb, M.; Shuck, C.E.; Pang, D.; Zhao, X.; Chu, X.F.; Wei, Y.; Du, F.; Chen, G.; et al. Two-dimensional vanadium carbide (V2C) MXene as electrode for supercapacitors with aqueous electrolytes. Electrochem. Commun. 2018, 96, 103–107. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional, ordered double transitionmetals carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef]
- Halim, J.; Kota, S.; Lukatskaya, M.R.; Naguib, M.; Zhao, M.Q.; Moon, E.J.; Pitock, J.; Nanda, J.; May, S.J.; Gogotsi, Y.; et al. Synthesis and characterization of 2D molybdenum carbide (MXene). Adv. Funct. Mater. 2016, 26, 3118–3127. [Google Scholar] [CrossRef]
- Boota, T.M.; Anasori, B.; Voigt, C.; Zhao, M.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive electrodes produced by oxidant-free polymerization of pyrrole between the layers of 2D titanium carbide (MXene). Adv. Mater. 2015, 28, 1517–1522. [Google Scholar] [CrossRef]
- Liu, P.; Yao, Z.; Ng, V.M.H.; Zhou, J.; Kong, L.B.; Yue, K. Facile synthesis of ultrasmall Fe3O4 nanoparticles on MXenes for high microwave absorption performance. Compos. Part A 2018, 115, 371–382. [Google Scholar] [CrossRef]
- Sobolčiak, P.; Ali, A.; Hassan, M.K.; Helal, M.I.; Tanvir, A.; Popelka, A. 2D Ti3C2Tx (MXene)-reinforced polyvinyl alcohol (PVA) nanofibers with enhanced mechanical and electrical properties. PLoS ONE 2017, 12, e0183705. [Google Scholar] [CrossRef]
- Kunkel, C.; Viñes, F.; Illas, F. Transition metal carbides as novel materials for CO2 capture, storage and activation. Energy Environ. Sci. 2016, 9, 141–144. [Google Scholar] [CrossRef]
- Reuter, K. Modelling and Simulation of Heterogeneous Catalytic Reactions; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2011; Chapter 3. [Google Scholar]
- Morales-García, Á.; Fernández-Fernández, A.; Viñes, F.; Illas, F. CO2 abatement using two-dimensional MXene carbides. J. Mater. Chem. A 2018, 6, 3381–3385. [Google Scholar] [CrossRef]
- Shah, S.; Shah, M.; Shah, A. Evolution in the membrane-based materials and comprehensive review on carbon capture and storage in industries. Emerg. Mater. 2020. [Google Scholar] [CrossRef]
- Singh, G.; Tiburcius, S.; Ruban, S.M.; Shanbhag, D.; Sathish, C.I.; Ramadass, K.; Vinu, A. Pure and strontium carbonate nanoparticles functionalized microporous carbons with high specific surface areas derived from chitosan for CO2 adsorption. Emerg. Mater. 2019, 2, 337–349. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Y.; Li, J.; Wang, Y.; Jiang, G.; Zhao, Z.; Wei, Y. Facile in situ synthesis of graphitic carbon nitride (g-C3N4)-N-TiO2 heterojunction as an efficient photocatalyst for the selective photoreduction of CO2 to CO. Appl. Catal. B Environ. 2014, 158–159, 20–29. [Google Scholar] [CrossRef]
- Peng, Q.; Guo, J.; Zhang, Q.; Xiang, J.; Liu, B.; Zhou, A.; Liu, R.; Tian, Y. Unique Lead Adsorption Behavior of Activated Hydroxyl Group in Two-Dimensional Titanium Carbide. J. Am. Chem. Soc. 2014, 136, 4113–4116. [Google Scholar] [CrossRef]
- Liu, Y.; Kelly, T.G.; Chen, J.G.; Mustain, W.E. Metal Carbides as Alternative Electrocatalysts Supports. ACS Catal. 2013, 3, 1184–1194. [Google Scholar] [CrossRef]
- Chen, W.F.; Wang, C.H.; Sasaki, K.; Marinkovic, N.; Xu, W.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Highly Active and Durable Nanostructured Molybdenum Carbide Electrocatalysts for Hydrogen Production. Energy Environ. Sci. 2013, 6, 943–951. [Google Scholar] [CrossRef]
- Lee, J.S. Metal Carbides. In Encyclopedia of Catalysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Hong, S.; Kyun Rhee, C.; Sohn, Y. Photoelectrochemical hydrogen evolution and CO2 reduction over MoS2/Si and MoSe2/Si nanostructures by combined photoelectrochemical deposition and rapid thermal annealing process. Catalysis 2019, 9, 494–505. [Google Scholar] [CrossRef]
- Avila-Bolivar, B.; Garcia-Cruz, L.; Montiel, V.; Solla-Gullon, J. Electrochemical reduction of CO2 to formate on easily prepared carbon-supported Bi nanoparticles. Molecules 2019, 24, 2032–2046. [Google Scholar] [CrossRef] [PubMed]
- Hiragond, C.B.; Kim, H.; Lee, J.; Sorcar, S.; Erkey, C.; In, S.I. Electrochemical CO2 reduction to CO catalysed by 2D nanostructures. Catalysts 2020, 10, 98. [Google Scholar] [CrossRef]
- Kumaravel, V.; John, B.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5, 486–519. [Google Scholar] [CrossRef]
- Xia, T.; Long, R.; Gao, C.; Xiong, Y. Design of atomically dispersed catalytic sites for photocatalytic CO2 reduction. Nanoscale 2019, 11, 11064–11070. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yin, J.; Zheng, X.; Ait Ahsaine, H.; Zhou, Y.; Dong, C.; Mohammed, O.F.; Takanabe, K.; Bakr, O.M. Compositionally Screened Eutectic Catalytic Coatings on Halide Perovskite Photocathode for Photo-Assisted Selective CO2 Reduction. ACS Energy Lett. 2019, 4, 1279–1286. [Google Scholar] [CrossRef]
- Low, J.; Zhang, L.; Tong, T.; Shen, B.; Yu, J. TiO2/MXene Ti3C2 composite with excellent photocatalytic CO2 reduction activity. J. Catal. 2018, 361, 255–266. [Google Scholar] [CrossRef]
- Zubair, M.; Kim, H.; Razzaq, A.; Grimes, C.A.; In, S.I. Solar spectrum photocatalytic conversion of CO2 to CH4 utilizing TiO2 nanotube arrays embedded with graphene quantum dots. J. CO2 Util. 2018, 26, 70–79. [Google Scholar] [CrossRef]
- Razzaq, A.; Grimes, C.A.; In, S.I. Facile fabrication of a noble metal-free photocatalyst: TiO2 nanotube arrays covered with reduced graphene oxide. Carbon 2016, 98, 537–544. [Google Scholar] [CrossRef]
- Wang, T.; Meng, X.G.; Liu, G.G.; Chang, K.; Li, P.; Kang, Q.; Liu, L.Q.; Li, M.; Ouyang, S.X.; Ye, J.H. In situ synthesis of ordered mesoporous Co-doped TiO2 and its enhanced photocatalytic activity and selectivity for the reduction of CO2. J. Mater. Chem. A 2015, 3, 9491–9501. [Google Scholar] [CrossRef]
- Manzanares, M.; Fabrega, C.; Osso, J.O.; Vega, L.F.; Andreu, T.; Morante, J.R. Engineering the TiO 2 outermost layers using magnesium for carbon dioxide photoreduction. Appl. Catal. B 2014, 150, 57–62. [Google Scholar] [CrossRef]
- Adekoya, D.O.; Tahir, M.; Amin, N.A.S. g-C3N4/(Cu/TiO2) nanocomposite for enhanced photoreduction of CO2 to CH3OH and HCOOH under UV/visible light. J. CO2 Util. 2017, 18, 261–274. [Google Scholar] [CrossRef]
- Shi, H.; Long, S.; Hu, S.; Hou, J.; Ni, W.; Song, C.; Li, K.; Gurzadyan, G.G.; Guo, X. Interfacial charge transfer in 0D/2D defect-rich heterostructures for efficient solar-driven CO2 reduction. Appl. Catal. B Environ. 2019, 245, 760–769. [Google Scholar] [CrossRef]
- Yang, J.; Wen, Z.; Shen, X.; Dai, J.; Li, Y.; Li, Y. A comparative study on the photocatalytic behavior of graphene-TiO2 nanostructures: Effect of TiO2 dimensionality on interfacial charge transfer. Chem. Eng. J. 2018, 334, 907–921. [Google Scholar] [CrossRef]
- Li, N.; Chen, X.; Ong, W.J.; MacFarlane, D.R.; Zhao, X.; Cheetham, A.K.; Sun, C. Understanding of Electrochemical Mechanisms for CO2 Capture and Conversion into Hydrocarbon Fuels in Transition-Metal Carbides (MXenes). ACS Nano 2017, 11, 10825–10833. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Handoko, A.D.; Xiao, J.; Feng, X.; Fan, Y.; Wang, T.; Zhang, Q. Catalytic effect on CO2 electroreduction by hydroxyl-terminated two-dimensional MXenes. ACS Appl. Mater. Interfaces 2019, 11, 36571–36579. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Z.; Yang, J. Transition-metal diboride: A new family of two-dimensional materials designed for selective CO2 electroreduction. J. Phys. Chem. C 2019, 123, 16294–16299. [Google Scholar] [CrossRef]
- Kumar, B.; Brian, J.P.; Atla, V.; Kumari, S.; Bertram, K.A.; White, R.T.; Spurgeon, J.M. New trends in the development of heterogenous catalyst for electrochemical CO2 reduction. Catal. Today 2016, 270, 19–30. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, K.; Jiang, B.; Li, J.; Zeng, M.; Fu, L. Emerging two-dimensional nanomaterials for electrochemical hydrogen evolution. J. Mater. Chem. A 2017, 5, 8187–8208. [Google Scholar] [CrossRef]
- Zhang, S.; Zhuo, H.; Li, S.; Bao, Z.; Deng, S.; Zhuang, G.; Wang, J.G. Effects of surface functionalization of MXene based nanocrystals on hydrogen evolution reaction performance. Catal. Today 2020. [Google Scholar] [CrossRef]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-Dimensional Molybdenum Carbide (MXene) as an Efficient Electrocatalyst for Hydrogen Evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Dai, J.H.; Zhang, Y.M.; Song, Y. Two-Dimensional, Ordered. Double TransitionMetal Carbides (MXenes): A New Family of Promising Catalysts for the Hydrogen Evolution Reaction. J. Phys. Chem. C 2018, 122, 28113–28122. [Google Scholar] [CrossRef]
- Qu, G.; Zhou, Y.; Wu, T.; Zhao, G.; Li, F.; Kang, Y.; Xu, C. Phosphorized MXene-Phase Molybdenum Carbide as an Earth-Abundant Hydrogen Evolution Electrocatalyst. ACS Appl. Energy Mater. 2018, 1, 7206–7212. [Google Scholar] [CrossRef]
- Yuan, W.; Huang, Q.; Yang, X.; Cui, Z.; Zhu, S.; Li, Z.; Du, S.; Qiu, N.; Liang, Y. Two-Dimensional Lamellar Mo2C for Electrochemical Hydrogen Production: Insights into the Origin of Hydrogen Evolution Reaction Activity in Acidic and Alkaline Electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 40500–40508. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.H.; Schäfer, T.; Shahraei, A.; Dürrschnabel, M.; Molina-Luna, L.; Kramm, U.I.; Birkel, C.S. Adding a New Member to the MXene Family: Synthesis, Structure and ElectrocatalyticActivity for the Hydrogen Evolution Reaction of V4C3Tx. ACS Appl. Energy Mater. 2018, 1, 3908–3914. [Google Scholar] [CrossRef]
- Xiong, J.; Li, J.; Shi, J.; Zhang, X.; Suen, N.T.; Liu, Z.; Huang, Y.; Xu, G.; Cai, W.; Lei, X.; et al. In Situ Engineering of Double-Phase Interface in Mo/Mo2C Heteronanosheets for Boosted Hydrogen Evolution Reaction. ACS Energy Lett. 2018, 3, 341–348. [Google Scholar] [CrossRef]
- Handoko, A.D.; Fredrickson, K.D.; Anasori, B.; Convey, K.W.; Johnson, L.R.; Gogotsi, Y.; Vojvodic, A.; Seh, Z.W. Tuning the Basal Plane Functionalization of Two-Dimensional Metal Carbides (MXenes) To Control Hydrogen Evolution Activity. ACS Appl. Energy Mater. 2018, 1, 173–180. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xu, D.; Zhu, Y.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Hierarchical “nanoroll” like MoS2/Ti3C2Tx hybrid with high electrocatalytic hydrogen evolution activity. Appl. Catal. B Environ. 2019, 241, 89–94. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.L.; Liu, R.S.; Han, C.P.; Li, Y.; Gogotsi, Y.; Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S. Current status and future development of catalyst materials and catalyst layers for proton exchange membrane fuel cells: An industrial perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J. Metal-air batteries: Will they be the future electrochemical energy storage device of choice? ACS Energy Lett. 2017, 2, 1370–1377. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.; Niu, J.; Liu, Y.; Bridges, D.; Liu, X.; Hu, A. Recent progress of metal-air batteries—A mini review. Appl. Sci. 2019, 9, 2787. [Google Scholar] [CrossRef]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current progress of pt and pt- based electrocatalysts used for fuel cells. Sustain. Energy Fuels 2020, 4, 15–30. [Google Scholar] [CrossRef]
- Li, K.; Jiao, T.; Xing, R.; Zou, G.; Zhou, J.; Zhang, L.; Peng, Q. Fabrication of tunable hierarchical MXene@AuNPs nanocomposites constructed by self-reduction reactions with enhanced catalytic performances. Sci. China Mater. 2018, 61, 728–736. [Google Scholar] [CrossRef]
- Murata, T.; Kotsuki, K.; Murayama, H.; Tsuji, R.; Morita, Y. Metal-free electrocatalysts for oxygen reduction reaction based on trioxotriangulene. Commun. Chem. 2019, 2, 46. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. NPJ Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, X.; Pei, W.; Liu, N.; Zhao, J. Heterostructures of MXene and N-doped graphene as highly active bifunctional electrocatalysts. Nanoscale 2020, 14, 10876–10883. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Shi, J. Insights into 2D MXenes for versatile biomedical applications: Current advances and challenges ahead. Adv. Sci. 2018, 5, 1800518. [Google Scholar] [CrossRef]
- Hussain, Z.; Ojha, R.; Martin, L.L. Controlling the morphological and redox properties of the CuTCNQ catalyst through solvent engineering. Emerg. Mater. 2019, 2, 35–44. [Google Scholar] [CrossRef]
- Wu, Z.; Ambrožová, N.; Eftekhari, E.; Šihor, M.; Praus, P.; Svoboda, L.; Mamulová, K.K.; Kočí, K. Photocatalytic H2 generation from aqueous ammonia solution using TiO2 nanowires-intercalated reduced graphene oxide composite membrane under low power UV light. Emerg. Mater. 2019, 2, 303–311. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen-and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Du, C.; Huang, H.; Feng, X.; Wu, S.; Song, W. Confining MoS2 nanodots in 3D porous nitrogen-doped graphene with amendable ORR performance. J. Mater. Chem. A 2015, 3, 7616–7622. [Google Scholar] [CrossRef]
- Wang, M.; Ma, Z.; Li, R.; Tang, B.; Bao, X.; Zhang, Z.; Wang, X. Novel flower-like PdAu(Cu) anchoring on a 3D rGO-CNT sandwich-stacked framework for highly efficient methanol and ethanol electro–oxidation. Electrochim. Acta 2017, 227, 330–344. [Google Scholar] [CrossRef]
- Li, R.; Ma, Z.; Zhang, F.; Meng, H.; Wang, M.; Bao, X.; Tang, B.; Wang, X. Facile Cu3P-C hybrid supported strategy to improve Pt nanoparticle electrocatalytic performance toward methanol, ethanol, glycol and formic acid electro–oxidation. Electrochim. Acta 2016, 220, 193–204. [Google Scholar] [CrossRef]
- Liu, J.; Peng, W.; Li, Y.; Fan, X. 2D MXene-Based Materials for Electrocatalysis. Trans. Tianjin Univ. 2020. [Google Scholar] [CrossRef]
- Lei, J.; Zhang, X.; Zhou, Z. Recent advances in MXene: Preparation, properties, and applications. Front. Phys. 2015, 10, 276–286. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, C.; Tian, Y.; Luo, Y.; Yin, X.; Que, W. The effect of in situ nitrogen doping on the oxygen evolution reaction of Mxenes. Nanoscale Adv. 2020, 2, 1187–1194. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, R.F.; Chen, X.M.; Tang, Y.H.; Qiao, S.Z. Fe-N Decorated Hybrids of CNTs Grown on Hierarchically Porous Carbon for High-Performance Oxygen Reduction. Adv. Mater. 2014, 26, 6074–6079. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lai, Y.J.; Song, L.; Zhou, Z.Y.; Liu, J.G.; Wang, Q.; Yang, X.D.; Chen, C.; Shi, W.; Zheng, Y.P.; et al. S-Doping of an Fe/N/C Orr Catalyst for Polymer Electrolyte Membrane Fuel Cells with High Power Density. Angew. Chem. Int. Ed. 2015, 54, 9907–9910. [Google Scholar] [CrossRef]
- Deng, Y.; Dong, Y.; Wang, G.; Sun, K.; Shi, X.; Zheng, L.; Li, X.; Liao, S. Well-Defined ZIF-Derived Fe-N Codoped Carbon Nanoframes as Efficient Oxygen Reduction Catalysts. ACS Appl. Mater. Interfaces 2017, 9, 9699–9709. [Google Scholar] [CrossRef]
- Li, Q.; Chen, W.; Xiao, H.; Gong, Y.; Li, Z.; Zheng, L.; Zheng, X.; Yan, W.; Cheong, W.C.; Shen, R.; et al. Fe Isolated Single Atoms on S. N CodopedCarbon by Copolymer Pyrolysis Strategy for Highly Efficient Oxygen Reduction Reaction. Adv. Mater. 2018, 2, 1800588. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Pei, Z.; Huang, Y.; Liang, G.; Mo, F.; Yang, Q.; Su, J.; Gao, Y.; Zapien, J.A.; et al. Single-Site Active Iron-Based Bifunctional Oxygen Catalyst for a Compressible and Rechargeable Zinc-Air Battery. ACS Nano 2018, 12, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Sfaelou, S.; Qiu, M.; Lützenkirchen-Hecht, D.; Zhuang, X.; Chen, Y.; Yuan, C.; Feng, X.; Scherf, U. Synergetic Contribution of Boron and Fe–Nx Species in Porous Carbons toward Efficient Electrocatalysts for Oxygen Reduction Reaction. ACS Energy Lett. 2018, 3, 252–260. [Google Scholar] [CrossRef]
- Jiang, L.; Duan, J.; Zhu, J.; Chen, S.; Antonietti, M. Iron Clusters-Directed Synthesis of 2D/2D Fe-N-C/MXene Superlattice Like Heterostructure with Enhanced Oxygen Reduction Electrocatalysis. ACS Nano 2020. [Google Scholar] [CrossRef] [PubMed]




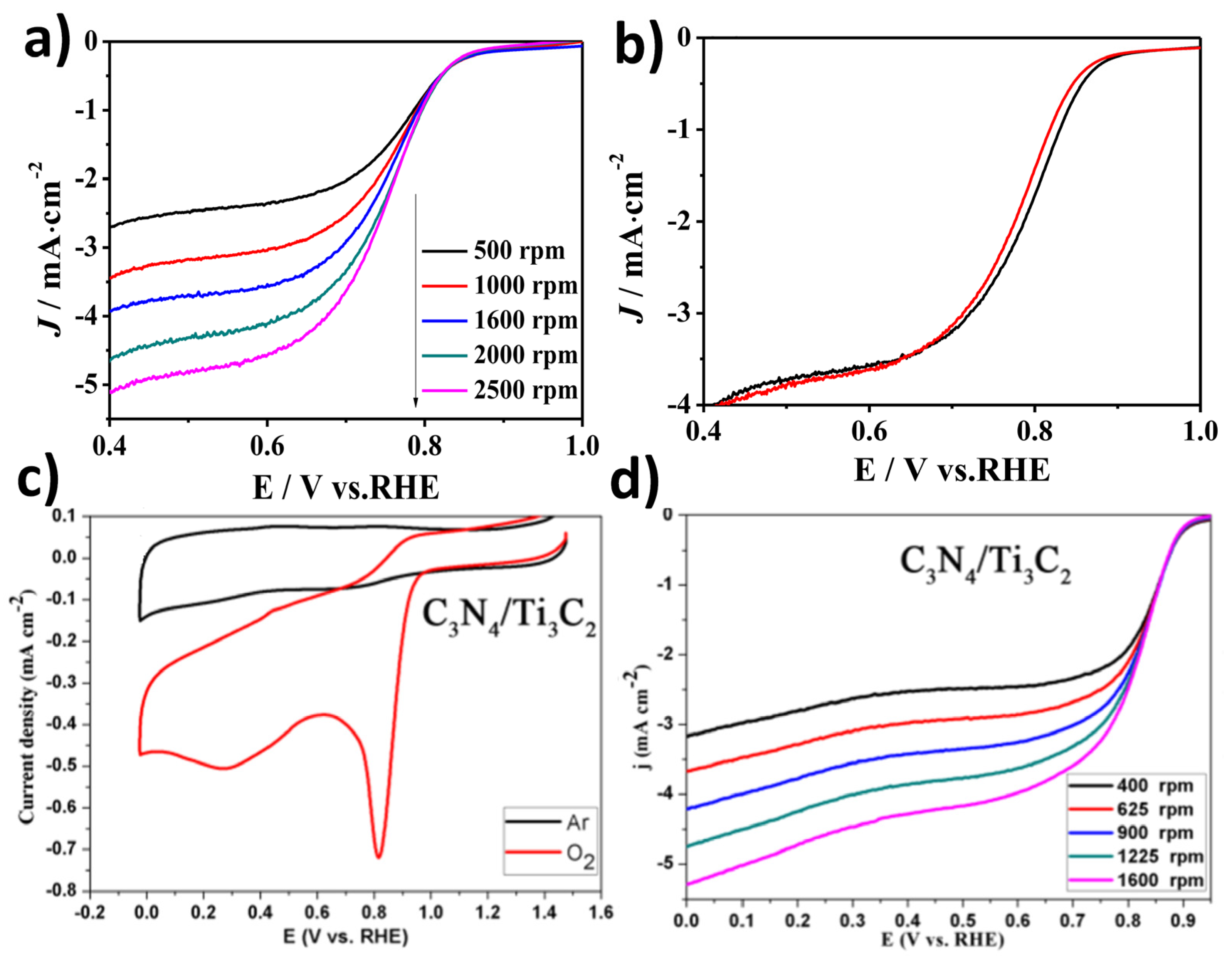
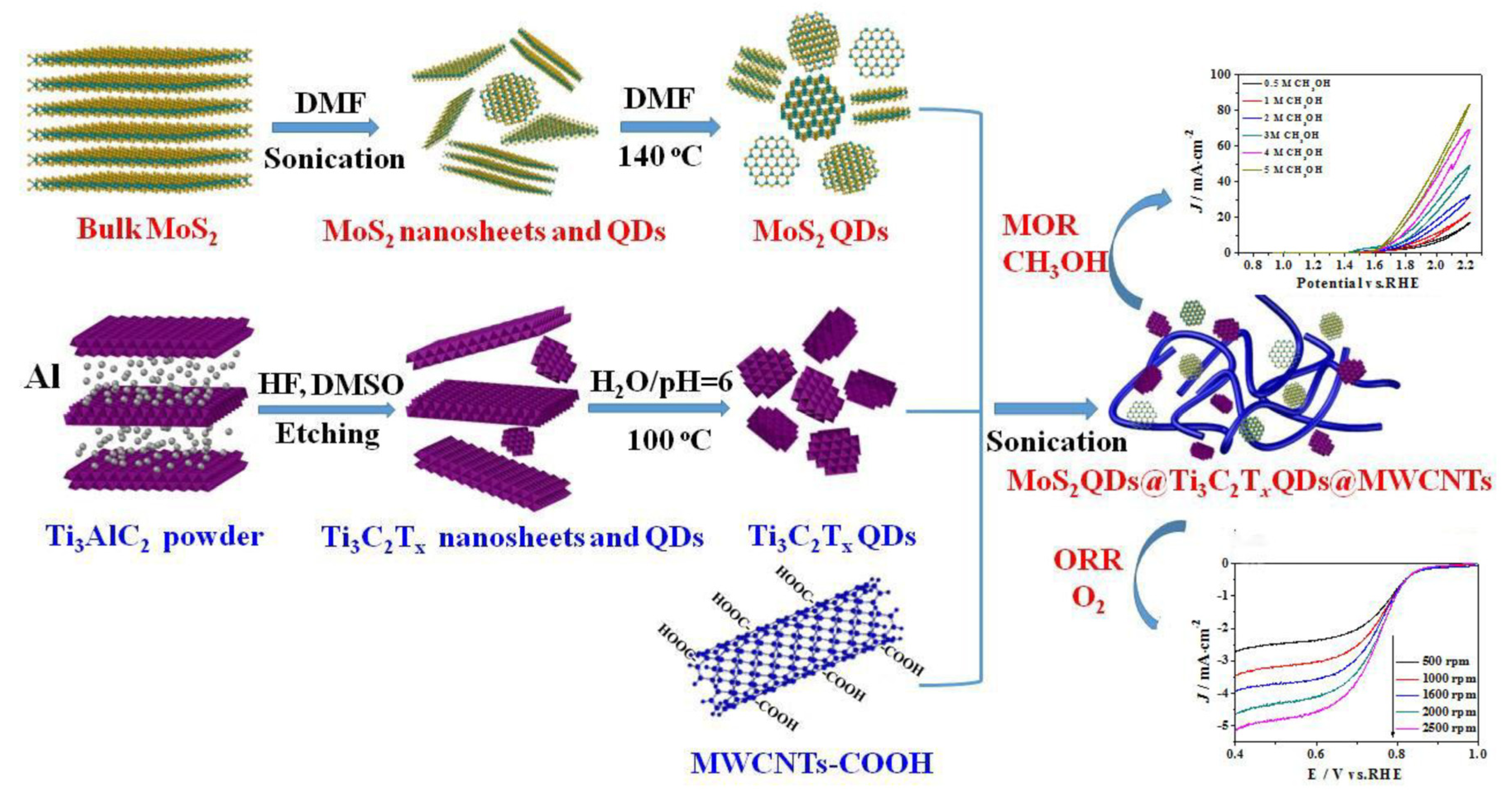
| Sample | Method of Synthesis | Electrical Conductivity (S/cm) | Ref. |
|---|---|---|---|
| Ti3C2 | Hot press sintering | 850 | [21] |
| Ti3C2 @400 °C | 1430 | [21] | |
| Ti3C2 @600 °C | 2410 | [21] | |
| Mo2CTx | Vacuum-assisted filtration method | 1.2 | [23] |
| (Mo2Ti)C2Tx | 1494 | [23] | |
| (Mo2Ti2)C3Tx | 614 | [63] | |
| Ti3C2Tx | 2410 | [63] | |
| Ti3C2Tx (100%) | Hydrothermal | 4556 | [64] |
| 99% MXene/1% rGO | 3326 | [64] | |
| 95% MXene/5% rGO | 2261 | [65] | |
| 90% MXene/10% rGO | 1231 | [65] |
| Materials | Method of Synthesis | Reversible Capacity (mAhg−1) | Rate Capability (mAhg−1) | Cycles | Ref |
|---|---|---|---|---|---|
| Ti2CTx | Exfoliation | 225 | 70 | 200 | [22] |
| Ti2C/TiO2 | Chemical exfoliation | 389 | 280 | 1000 | [24] |
| Ti3C2/CNF | Liquid-phase impregnation | 320 | 97 | 2900 | [25] |
| Ti3C2Tx/CNT | Chemical vapor deposition | 1250 | 500 | 100 | [26] |
| Nb4C3Tx | Spark plasma sintering | 380 | 320 | 1000 | [27] |
| Ti3C2Tx/NiCo2O4 | In-situ growth | 1330 | 1200 | 100 | [28] |
| SnO2/ Ti3C2Tx | Atomic layer deposition | 1041 | 451 | 50 | [29] |
| MoS2@ Ti3C2Tx | Acid etching | 843 | 132 | 200 | [30] |
| V2CTx | Ball-milling | 288 | 125 | 150 | [86] |
| Nb2CTx | Ball-milling | 250 | 110 | 150 | [86] |
| Nb2CTx/CNT | Amine-assisted delamination process | 420 | 370 | 100 | [88] |
| Sn(IV)@ Ti3C2 | Facile liquid-phase immersion process | 635 | 544 | 200 | [89] |
| Ti3C2Tx/Ag | Direct reduction | 310 | 260 | 5000 | [90] |
| Materials | Synthesis Route | Volumetric Capacitance (F/cm3) | Scan Rate (mV/s) | Ref |
|---|---|---|---|---|
| 90 wt% Ti3C2Tx/10 wt% PVA | HF etching | 528 | 2 | [31] |
| Ti3C2Tx | Ball milling | 900 | 2 | [38] |
| 88 wt% Mxene/GO fiber | Liquid crystal-assisted fiber spinning approach | 341 | 2 | [43] |
| Mxene/rGO-90 fiber (containing 90 w/w% of Mxene) | Wet spinning assembly | 586.4 | 2 | [44] |
| Mxene/rGO-50 fiber (containing 50 w/w% of Mxene) | 276 | 2 | [44] | |
| EGMX (1:9) | Solution process | 33 | 2 | [45] |
| 2:1:1.1:2 MO2TiC2 | HF etching | 413 | 2 | [107] |
| 2D MO2TiCx | HCL-LiF | 196 | 2 | [108] |
| Ti3AlC2/Polypyrrole (2:1) | HCL-LiF | 416 | 5 | [109] |
| Materials | Dielectric Constant | Dielectric Loss | Ref. |
|---|---|---|---|
| MXene/P (VDF-TrFE-CTFE) | 47 | 0.2 | [37] |
| 4% MXene/P (VDF-TrFE-CTFE) | 159 | 0.22 | [37] |
| MXene/P (VDF-TrFE) | 18 | 0.006 | [37] |
| 4.3% MXene/P (VDF-TrFE) | 80 | 0.06 | [40] |
| MXene/PVP | 2.5 | 0.01 | [40] |
| 4% MXene/PVP | 16.4 | 0.03 | [40] |
| MXene/P (VDF-TrFE-CFE) | 55 | 0.06 | [41] |
| 4% MXene/P (VDF-TrFE-CFE) | 317 | 0.17 | [41] |
| Materials | Light Source | Catalytic Performance | Ref. |
|---|---|---|---|
| Sulfur doped g-C3N4/Pt | 300 W Xenon lamp | CH3OH: 0.04 µmolh−1 | [49] |
| g-C3N4/N-TiO2 nanosheets | 300 W Xenon lamp | CO: 14.73 µmolg−1 CH4: 03.94 µmolg−1 | [117] |
| TiO2/Ti3C2 | 300 W Xenon lamp | CH4 = 0.22 µmolh−1 | [128] |
| Graphene QDs—TiO2 nanotube arrays (TNT) | 100 W Xenon lamp | CH4: 1.98 ppmcm−2h−1 | [129] |
| TNT-rGO | 100 W Xenon lamp | CH4: 5.67 ppmcm−2h−1 | [130] |
| Ordered mesoporous co-doped TiO2 | 300 W Xenon lamp | CO: 0.19 µmolh−1 | [131] |
| TiO2/Mg | 300 W Xenon lamp | CH4: 0.15 µmolg−1 | [132] |
| Cu modified g-C3N4 sheets with TiO2 nanoparticles | 500 W Xe lamp | CH3OH: 614 µmolg−1 HCOOH: 6709 µmolg−1 | [133] |
| 2-D-g-C3N4 with o-D-TiO2-x nanoparticles | 300 W Xenon lamp | CO: 388.9 µmolg−1 | [134] |
| Graphene—TiO2 nanostructures (nanoparticles, nanotubes, nanosheets) | 300 W Xenon lamp | CO: 75.8 µmolg−1h−1 CH4: 12.3 µmolg−1h−1 | [135] |
| Materials | Over Potential at 10 mA cm–2geo | Media (Aqueous Solution) | Reference |
|---|---|---|---|
| Mo2CTx | 300 mV | 0.5 M H2SO4 | [142] |
| P–Mo2CTx | 186 mV | 0.5 M H2SO4 | [142] |
| Mo2CTX MXene | 283 mV | 0.5 M H2SO4 | [142] |
| MoS2 | 280 mV | 0.5 M H2SO4 | [142] |
| Ti2CTx | 609 mV | 0.5 M H2SO4 | [143] |
| Mo2CTx | 189 mV | 0.5 M H2SO4 | [143,148] |
| L-Mo2C (lamellar structure β-Mo2C) | 170 mV | 0.5 M H2SO4 | [145] |
| L-Mo2C (lamellar structure β-Mo2C) | 95.8 mV | 1 M KOH | [145] |
| Ti3C2 nanofibers | 169 mV | 0.5 M H2SO4 | [149] |
| Ti3C2 flakes | 385 mV | 0.5 M H2SO4 | [149] |
| MoS2/Ti3C2Tx | 152 mV | 0.5 M H2SO4 | [149] |
| Catalyst | Load Mass (mg/cm2) | Electrolyte | Half-Wave Potential | Ref |
|---|---|---|---|---|
| FeNx embedded in 2D porous nitrogen doped carbon | 0.14 | 0.1 M KOH | 0.86 V | [46] |
| B, Fe doped porous carbon | 0.6 | 0.1 M KOH | 0.838 V | [50] |
| Fe isolated single atoms on S, N co-doped carbon | 0.51 | 0.1 M KOH | 0.896 V | [51] |
| Fe nanoparticles | 0.1 | 0.1 M KOH | 0.6 V | [65] |
| MXene | 0.1 | 0.1 M KOH | 0.75 V | [65] |
| Fe-N-C/MXene | 0.1 | 0.1 M KOH | 0.84 V | [65] |
| Fe-N co-doped carbon nanotubes | 0.1 | 0.1 M KOH | 0.83 V | [163] |
| S-doped Fe-N-C | 0.6 | 0.1 M H2SO4 | 0.836V | [164] |
| Fe-N-co-doped carbon frame | 0.5 | 0.1 M KOH | 0.7 V | [165] |
| Fe-N-co-doped carbon frame | 0.5 | 0.1 M HClO4 | 0.77 V | [165] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kannan, K.; Sadasivuni, K.K.; Abdullah, A.M.; Kumar, B. Current Trends in MXene-Based Nanomaterials for Energy Storage and Conversion System: A Mini Review. Catalysts 2020, 10, 495. https://doi.org/10.3390/catal10050495
Kannan K, Sadasivuni KK, Abdullah AM, Kumar B. Current Trends in MXene-Based Nanomaterials for Energy Storage and Conversion System: A Mini Review. Catalysts. 2020; 10(5):495. https://doi.org/10.3390/catal10050495
Chicago/Turabian StyleKannan, Karthik, Kishor Kumar Sadasivuni, Aboubakr M. Abdullah, and Bijandra Kumar. 2020. "Current Trends in MXene-Based Nanomaterials for Energy Storage and Conversion System: A Mini Review" Catalysts 10, no. 5: 495. https://doi.org/10.3390/catal10050495
APA StyleKannan, K., Sadasivuni, K. K., Abdullah, A. M., & Kumar, B. (2020). Current Trends in MXene-Based Nanomaterials for Energy Storage and Conversion System: A Mini Review. Catalysts, 10(5), 495. https://doi.org/10.3390/catal10050495









