Multi-Scale Analysis of Integrated C1 (CH4 and CO2) Utilization Catalytic Processes: Impacts of Catalysts Characteristics up to Industrial-Scale Process Flowsheeting, Part I: Experimental Analysis of Catalytic Low-Pressure CO2 to Methanol Conversion
Abstract
1. Introduction
2. State-of-the-Art and Literature Review
3. Results and Discussion
3.1. Catalyst Characterizations
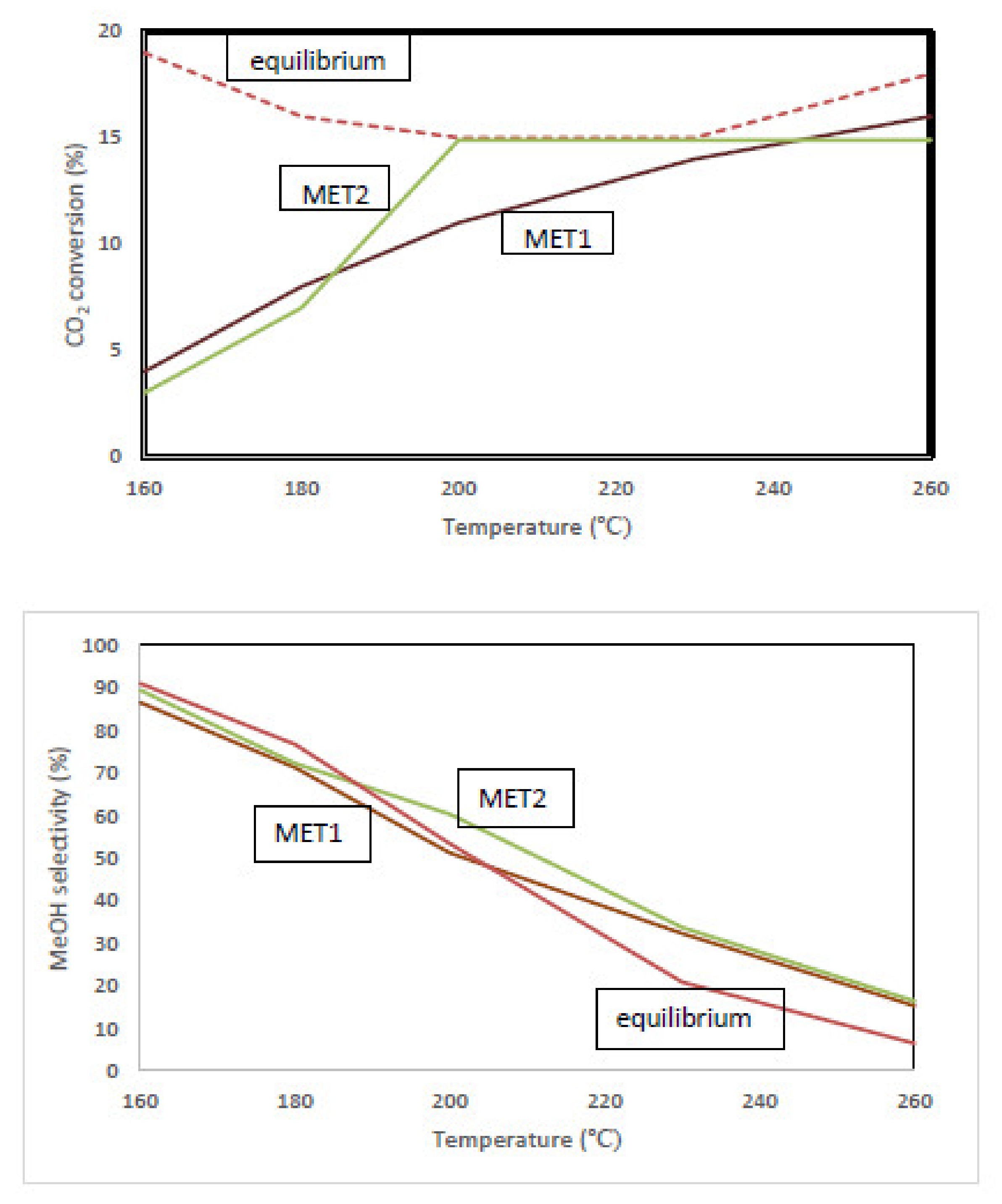
3.2. Catalytic Performance
3.3. Equilibrium-Limited Achievable Performance
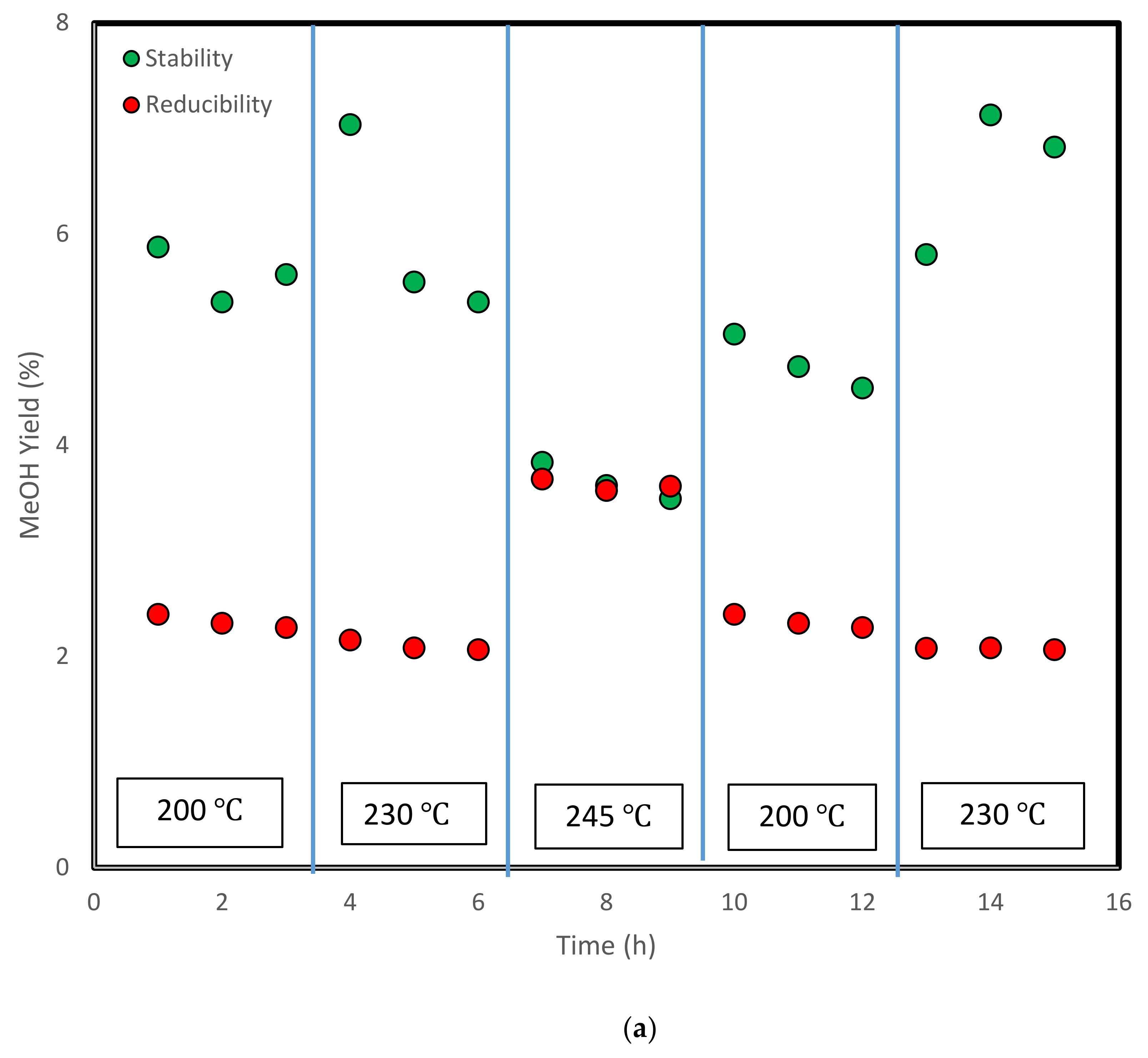
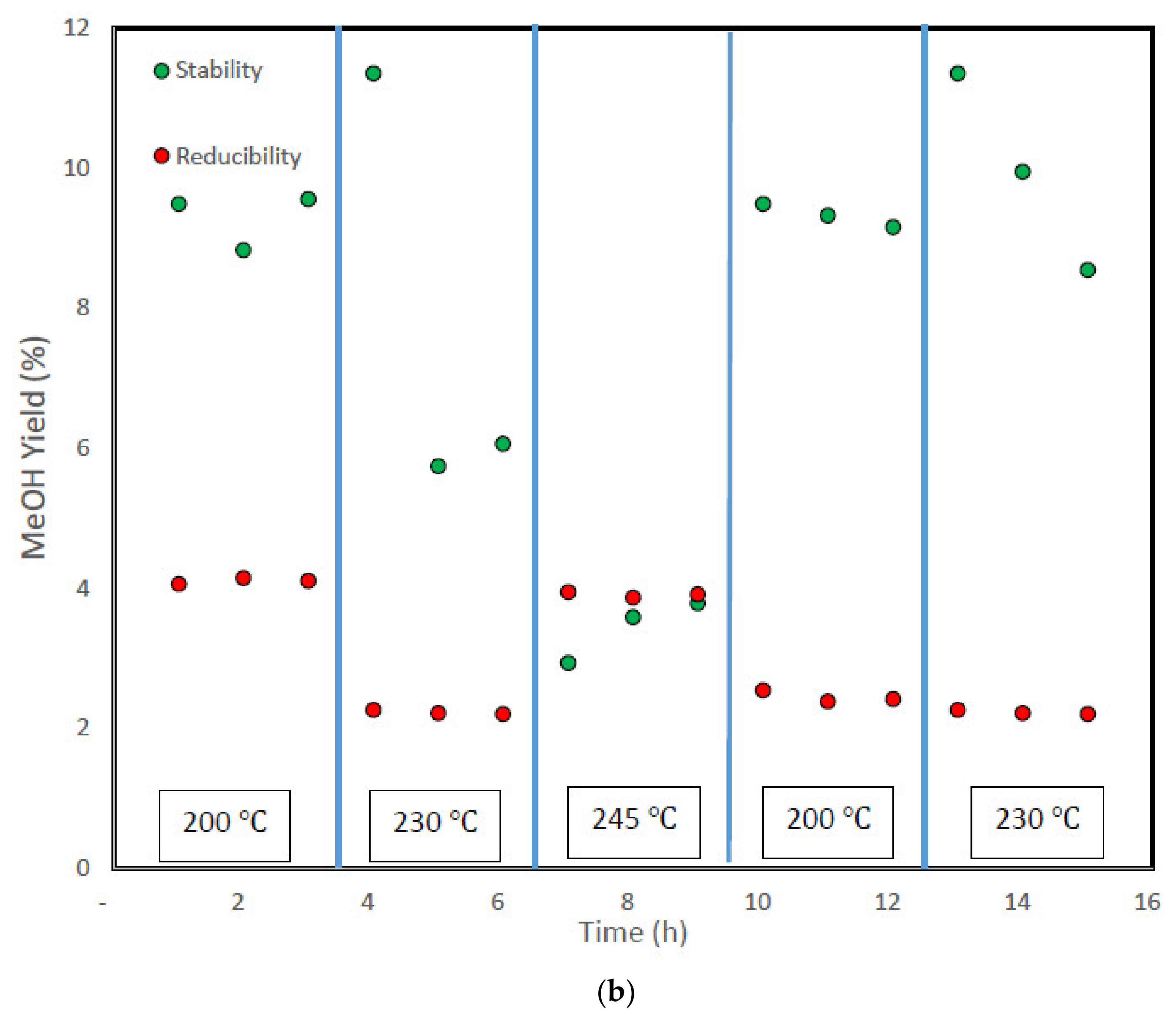
3.4. Stability Test and Analysis
4. Material and Methods
4.1. Selected Catalysts and Synthesis Methods
4.1.1. Co-Precipitation Method for Preparation of Cu/ZnO/Al2O3 Catalyst
4.1.2. Hydrolysis Method for Preparation of Cu/ZnO/Al2O3 Catalyst
4.1.3. Coprecipitation Method for Preparation of Cu/ZnO/Al2O3 Catalyst
4.1.4. Gel-Coprecipitation Method for Preparation of Cu/ZnO/Al2O3 Catalyst
4.1.5. Conventional Carbonate Coprecipitation Method for Preparation of Cu/ZnO/Al2O3 Catalyst
4.1.6. Citrate and Impregnation Method for Preparation of Cu/ZnO Catalyst
4.2. Catalyst Characterization
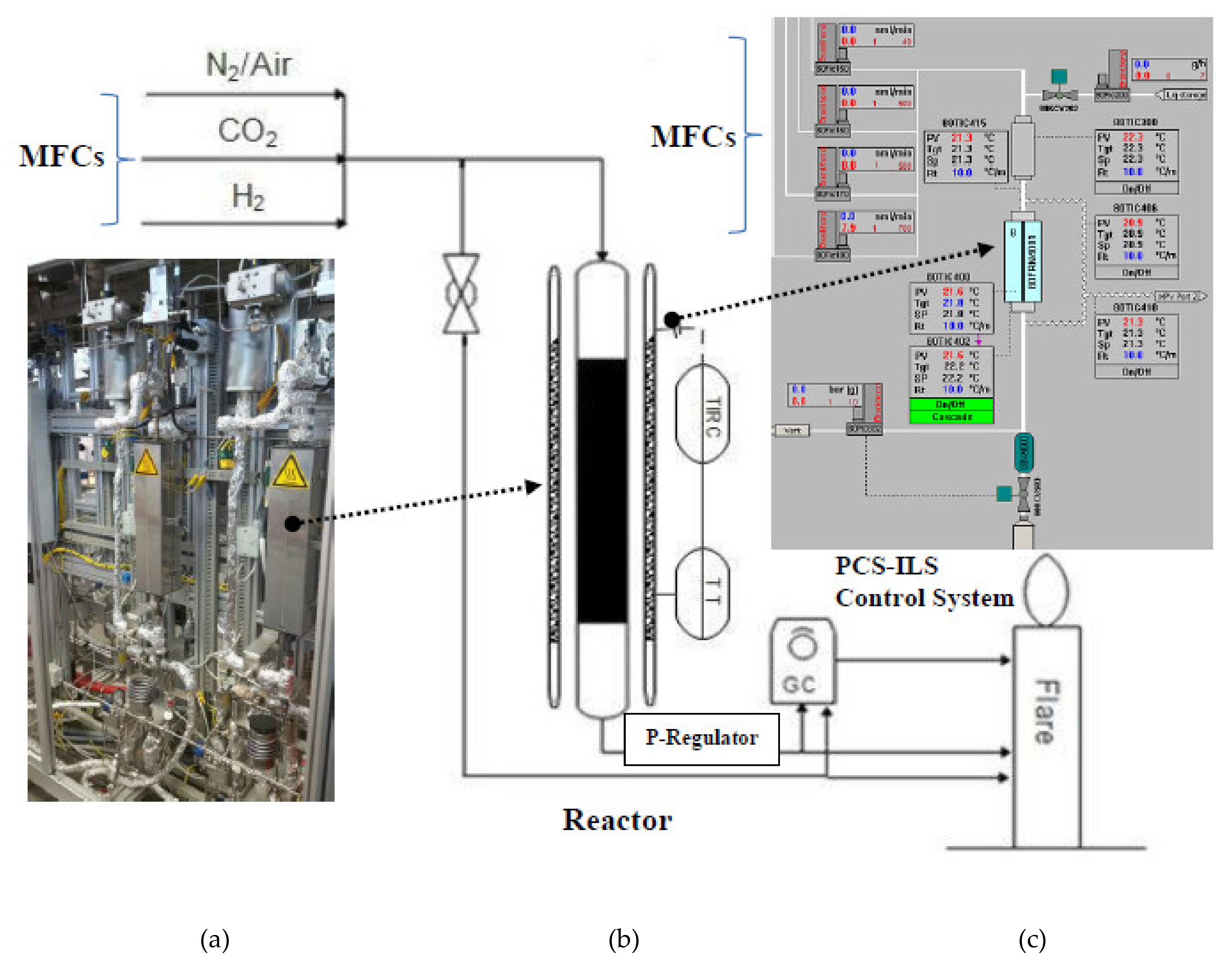
5. Experimentation for Catalyst Testing
Experimental Setup
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BET | Measuring the specific surface based on Brunauer–Emmett–Teller theory |
| BJH | Barrett–Joyner–Halenda |
| Cat | Catalyst |
| CCSU | Carbon capture separation utilization |
| Dilu. | Dilution |
| DME | Dimethyl ether (Methoxymethane) |
| EDX | Energy-dispersive X-ray spectroscopy |
| FESEM | Field emission scanning electron microscopy |
| Gas | Gas phase |
| GC | Gas chromatography |
| In | Inlet stream |
| MeOH (CH3OH) | Methanol |
| MET | Methanol catalysts prepared with different methods |
| Out | Outlet stream |
| PC-ILS | Process control system—integrated lab solution |
| RWGS | Reverse water gas shift |
| UniCat | “Unifying Concepts in Catalysis” (a research group in Berlin) |
| XRD | X-ray diffraction |
Nomenclature
| A | Ambient | - |
| Cb | catalytic bed | - |
| D | Diameter or equivalent diameter | nm |
| F | Molar flow rate | mol/min |
| GHSV | Gas hourly space velocity | L/h |
| P | Pressure | bar |
| Q | Total flow rate | Nml/min |
| S (Selectivity) | Portion of the whole consumed carbon dioxide which appears in the (desired) products | - |
| T | Temperature | °C |
| V | Volume | ml |
| X (CO2 Conversion) | Portion of the inlet carbon dioxide converted to the desired and undesired products | - |
| X | Mole fraction | - |
| Y (Yield) | Amount of the converted carbon dioxide appears in each product per whole total amount of the inlet carbon dioxide | - |
| ΔHR | Reaction enthalpy | kJ/mol |
| Ρ | Density | kg/m³ |
References
- Artz, J.; Müller, T.E.; Thenert, K. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Cuellar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Benchaita, T. Greenhouse Gas Emissions from New Petrochemical Plants, Background Information Paper for the Elaboration of Technical Notes and Guidelines for IDB Projects; Technical Note, No. IDB-TN-562; Inter-American Development Bank, Environmental Safeguards Unit: Washington, DC, USA, 2013. [Google Scholar]
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic carbon dioxide hydrogenation to methanol: A review of recent studies. Chem. Eng. Res. Des. 2014, 92, 2557–2567. [Google Scholar] [CrossRef]
- Ali, K.A.; Abdullah, A.Z.; Mohamed, A.R. Recent development in catalytic technologies for methanol synthesis from renewable sources: A critical review. Renew. Sustain. Energy Rev. 2015, 44, 508–518. [Google Scholar] [CrossRef]
- Iaquaniello, G.; Centi, G.; Salladini, A.; Palo, E. Methanol Economy: Environment, Demand, and Marketing with a Focus on the Waste-to-Methanol Process (Chapter 22). In Methanol Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 595–612. [Google Scholar]
- Díez-Ramírez, J.; Díaz, J.A.; Sánchez, P.; Dorado, F. Optimization of the Pd/Cu ratio in Pd-Cu-Zn/SiC catalysts for the CO2 hydrogenation to methanol at atmospheric pressure. J. CO₂ Util. 2017, 22, 71–80. [Google Scholar] [CrossRef]
- Kourtelesis, M.; Kousi, K.; Kondarides, D.I. CO2 Hydrogenation to Methanol over La2O3-Promoted CuO/ZnO/Al2O3 Catalysts: A Kinetic and Mechanistic Study. Catalysts 2020, 10, 183. [Google Scholar] [CrossRef]
- Masgutova, V.A.; Potemkin, D.I.; Kurochkin, A.V.; Snytnikov, P.V.; Amosov, Y.I.; Kirillov, V.A.; Sobyanin, V.A. Synthesis of Methanol from Oil- and Gas-Field Flare Gases at the Same Pressure of the Syngas Generation and Methanol Synthesis Steps. Theor. Found. Chem. Eng. 2018, 52, 935–942. [Google Scholar] [CrossRef]
- Godini, H.R.; Gili, A.; Görke, O.; Simon, U.; Hou, K.; Wozny, G. Performance analysis of a porous packed-bed membrane reactor for Oxidative Coupling of Methane: Structural and operational characteristics. Energy Fuels 2014, 28, 877–890. [Google Scholar] [CrossRef]
- Parishan, S.; Littlewood, P.; Arinchtein, A.; Fleischer, V.; Schomäcker, R. Chemical looping as a reactor concept for the oxidative coupling of methane over the MnxOy-Na2WO4/SiO2 catalyst: Benefits and limitation. Catal. Today 2018, 311, 40–47. [Google Scholar] [CrossRef]
- Saito, M. R&D activities in Japan on methanol synthesis from CO2 and H2. Catal. Surv. Jpn. 1998, 2, 175–184. [Google Scholar]
- Dang, S.; Yang, H.; Gao, P.; Wang, H.; Li, X.; Wei, W.; Sun, Y. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation. Catal. Today 2019, 330, 61–75. [Google Scholar] [CrossRef]
- Gao, P.; Li, F.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Sun, Y. Influence of modifier (Mn, La, Ce, Zr and Y) on the performance of Cu/Zn/Al catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol. Appl. Catal. Gen. 2013, 468, 442–452. [Google Scholar] [CrossRef]
- Arena, F.; Mezzatesta, G.; Zafarana, G.; Trunfio, G.; Frusteri, F.; Spadaro, L. Effects of oxide carriers on surface functionality and process performance of the Cu–ZnO system in the synthesis of methanol via CO2 hydrogenation. J. Catal. 2013, 300, 141–151. [Google Scholar] [CrossRef]
- Xu, Z.; Qian, Z.; Mao, L.; Tanabe, K.; Hattori, H. Methanol Synthesis from CO2 and H2 over CuO-ZnO Catalysts Combined with Metal Oxides under 13 atm Pressure. Bull. Chem. Soc. Jpn. 1991, 64, 1658–1663. [Google Scholar] [CrossRef]
- Arakawa, H.; Sayama, K. Methanol Synthesis from CO2 and H2 over Supported Copper-Zinc Oxide Catalyst. Significant Influence of Support on Methanol Formation. Stud. Surf. Sci. Catal. 1993, 75, 2777–2780. [Google Scholar]
- Ramaroson, E.; Kieffer, R.; Kiennemenn, A. Reaction of CO-H2 and CO2–H2 on Copper-zinc catalysts promoted by metal oxides of group III and IV. Appl. Catal. 1982, 4, 281–286. [Google Scholar] [CrossRef]
- Gallucci, F.; Paturzo, L.; Basile, A. An experimental study of CO2 hydrogenation into methanol involving a zeolite membrane reactor. Chem. Eng. Process. 2004, 43, 1029–1036. [Google Scholar] [CrossRef]
- Jingfa, D.; Qi, S.; Yulong, Z.; Songying, C.; Dong, W. A novel process for preparation of Cu/ZnO/Al2O3 ultrafine catalyst for methanol synthesis from CO2 + H2: Comparison of various preparation methods. Appl. Catal. Gen. 1996, 139, 75–85. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiza, P. The role of copper particle size in low pressure methanol synthesis via CO2 hydrogenation over Cu/ZnO catalysts. Catal. Sci. Technol. 2015, 5, 869–881. [Google Scholar] [CrossRef]
- Karelovic, A.; Bargibant, A.; Fernández, C.; Ruiz, P. Effect of the structural and morphological properties of Cu/ZnO catalysts prepared by citrate method on their activity toward methanol synthesis from CO2 and H2 under mild reaction conditions. Catal. Today 2012, 197, 109–118. [Google Scholar] [CrossRef]
- Schumann, J.; Lunkenbein, T.; Tarasov, A.; Thomas, N.; Schlögl, R.; Behrens, M. Synthesis and Characterisation of a Highly Active Cu/ZnO:Al Catalyst. ChemCatChem 2014, 6, 2889–2897. [Google Scholar] [CrossRef]
- Lunkenbein, T.; Schumann, J.; Behrens, M.; Schlögl, R.; Willinger, M.G. Formation of a ZnO Overlayer in Industrial Cu/ZnO/Al2O3 Catalysts Induced by Strong Metal–Support Interaction. Angew. Chem. Int. Ed. 2015, 54, 4544–4548. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Zander, S.; Kurr, P.; Jacobsen, N.; Senker, J.; Koch, G.; Ressler, T.; Fischer, R.W.; Schlögl, R. Performance Improvement of Nanocatalysts by Promoter-Induced Defects in the Support Material: Methanol Synthesis over Cu/ZnO:Al. J. Am. Chem. Soc. 2013, 135, 6061–6068. [Google Scholar] [CrossRef]
- Gao, P.; Yang, H.; Zhang, L.; Zhang, C.; Zhong, L.; Wang, H.; Wei, W.; Sun, Y. Fluorinated Cu/Zn/Al/Zr hydrotalcites derived nanocatalysts for CO2 hydrogenation to methanol. J. CO2 Util. 2016, 16, 32–41. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, H.; Gao, P.; Zhu, H.; Zhong, L.; Wang, H.; Wei, W.; Sun, Y. Preparation and CO2 hydrogenation catalytic properties of alumina microsphere supported Cu-based catalyst by deposition-precipitation method. J. CO2 Util. 2017, 17, 263–272. [Google Scholar] [CrossRef]
- Graciani, J.; Mudiyanselage, K.; Xu, F.; Baber, A.E.; Evans, J.; Senanayake, S.D.; Stacchiola, D.J.; Liu, P.; Hrbek, J.; Fernández Sanz, J.; et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 2014, 345, 546–550. [Google Scholar] [CrossRef]
- Martin, O.; Martin, A.J.; Mondelli, C.; Mitchell, S.; Segawa, T.F.; Hauert, R.; Drouilly, C.; Curulla-Ferre, D.; Perez-Ramirez, J. Indium Oxide as a Superior Catalyst for Methanol Synthesis by CO2 Hydrogenation. Angew. Chem. Int. Ed. 2016, 55, 6261–6265. [Google Scholar] [CrossRef]
- Rungtaweevoranit, B.; Baek, J.; Araujo, J.R.; Archanjo, B.S.; Choi, K.M.; Yaghi, O.M.; Somorjai, G.A. Copper Nanocrystals Encapsulated in Zr-based Metal–Organic Frameworks for Highly Selective CO2 Hydrogenation to Methanol. Nano Lett. 2016, 16, 7645–7649. [Google Scholar] [CrossRef]
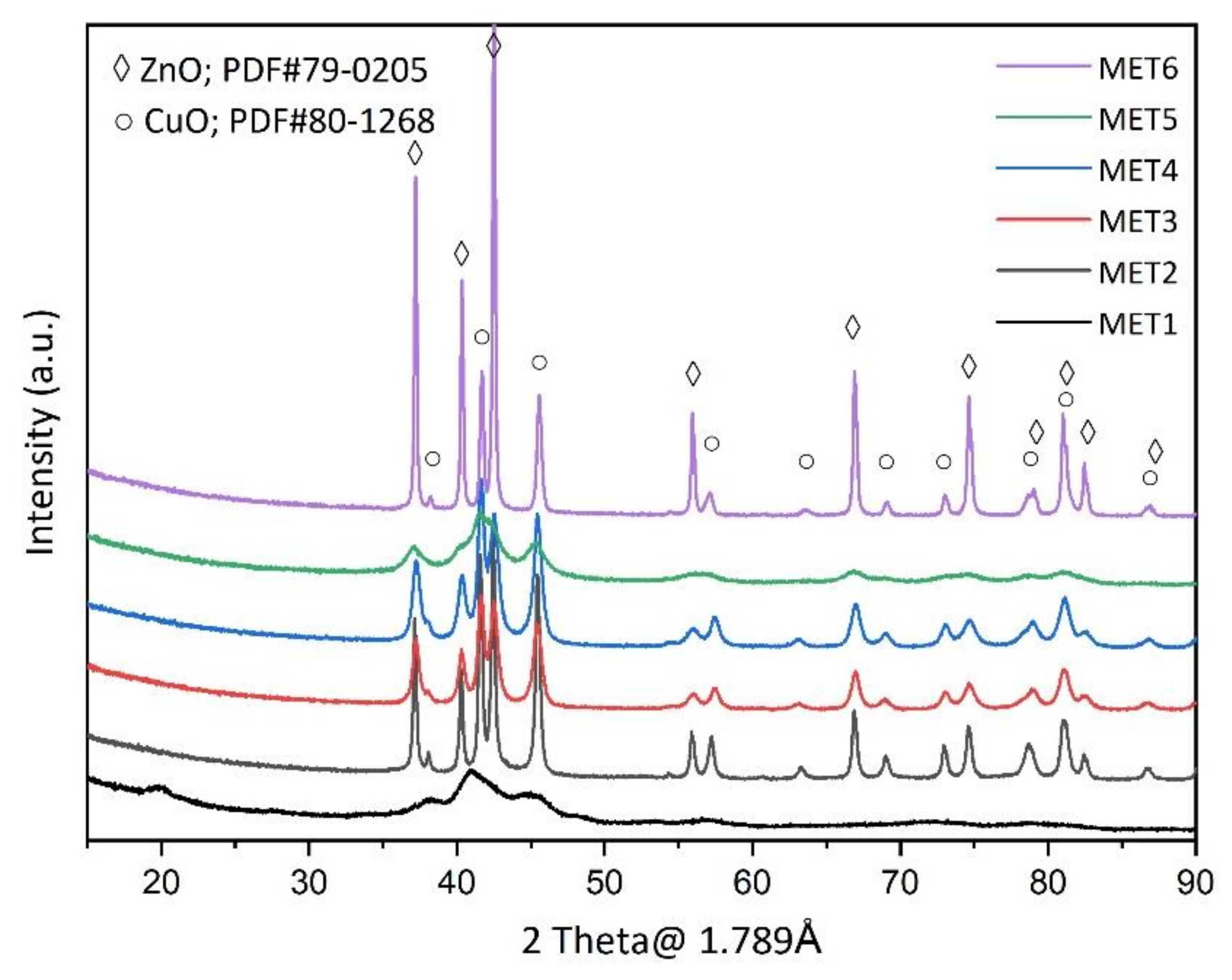
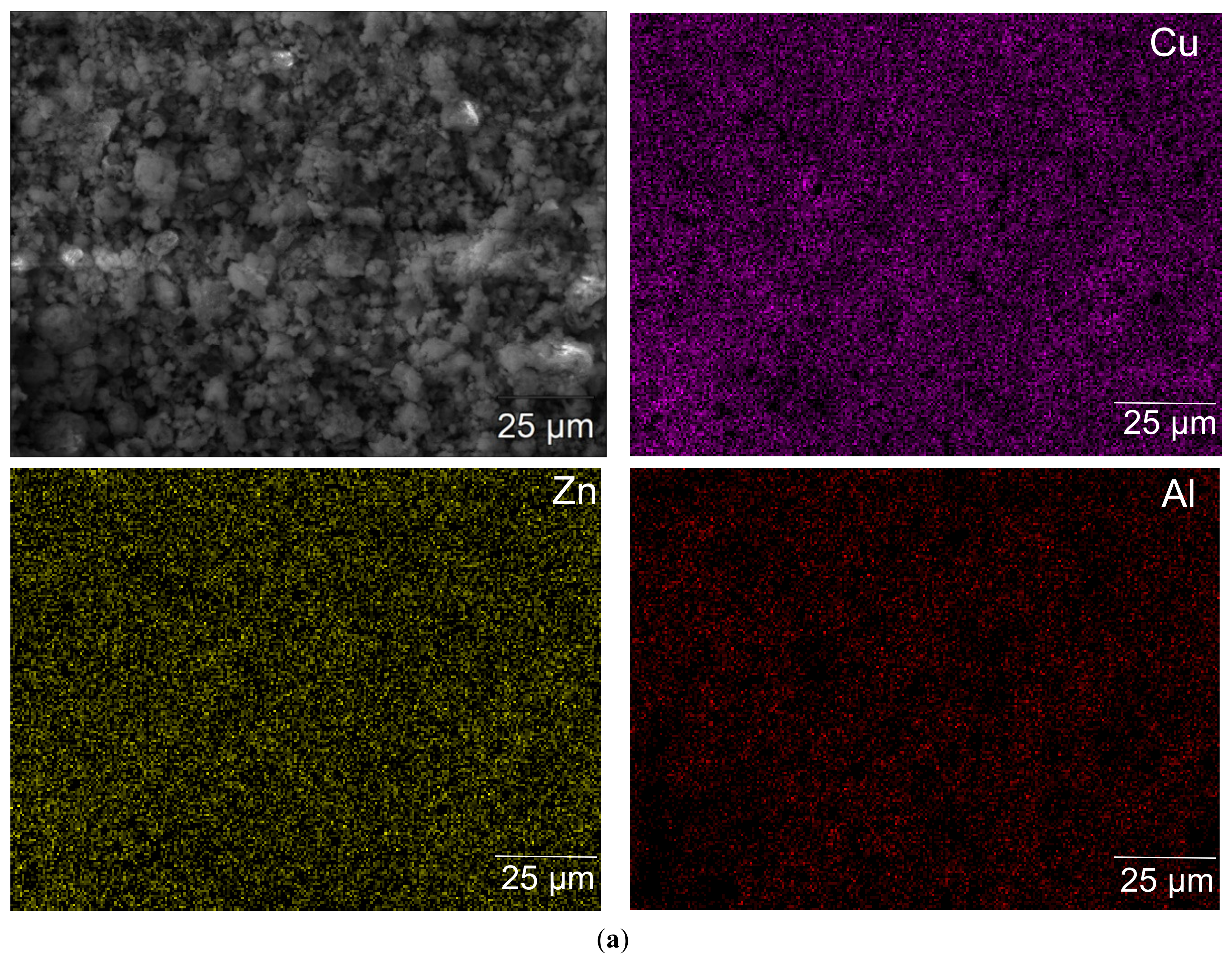

| Catalyst | SBET (m2 g−1) | Mean Pore Size (nm) |
|---|---|---|
| MET1 | 79 | 7 |
| MET2 | 45 | 6 |
| MET3 | 19 | 5.5 |
| MET4 | 22 | 5.5 |
| MET5 | 52 | 5 |
| MET6 | 11 | 6.5 |
| MET7 | 28 | 6 |
| H2/CO2 | 3 | 6 | 9 | 3 | 6 | 9 | 3 | 6 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| T(°C) | Coprecipitation method Cu/ZnO/Al2O3 (MET1) | ||||||||
| 200 | 7.9 | 14.5 | 18.6 | 66.1 | 77.6 | 77.6 | 5.2 | 11.3 | 14.4 |
| 230 | 16.9 | 25.4 | 31 | 33.7 | 38.8 | 43.7 | 5.7 | 9.9 | 13.5 |
| 260 | 17.1 | 24.7 | 29.3 | 0 | 0.2 | 0 | 0.0 | 0.0 | 0.0 |
| T(°C) | Hydrolysis method-Cu/ZnO/Al2O3 (MET2) | ||||||||
| 200 | 9.6 | 14.7 | 18.4 | 74.3 | 79.4 | 82.5 | 7.1 | 11.7 | 15.2 |
| 230 | 15.7 | 21.4 | 25.9 | 43.2 | 52.7 | 52.8 | 6.8 | 11.3 | 13.7 |
| 260 | 19 | 23.8 | - | 13.4 | 17.4 | - | 2.5 | 4.1 | - |
| T(°C) | Coprecipitation method-Cu/ZnO/Al2O3 (MET3) | ||||||||
| 200 | 8 | 11.9 | 16.8 | 80.3 | 76.8 | 79.2 | 6.4 | 9.1 | 13.3 |
| 230 | 15.2 | 22.8 | 29.6 | 39 | 46.5 | 44.7 | 5.9 | 10.6 | 13.2 |
| 260 | 20.6 | 26.9 | 34.3 | 18.8 | 15.1 | 14.9 | 3.9 | 4.1 | 5.1 |
| T(°C) | Gel coprecipitation method-Cu/ZnO/Al2O3 (MET4) | ||||||||
| 200 | 8.8 | 13.4 | 18.6 | 74 | 75.6 | 78.6 | 6.5 | 10.1 | 14.6 |
| 230 | 16.1 | 24.8 | 31.2 | 37.8 | 41.9 | 45.1 | 6.1 | 10.4 | 14.1 |
| 260 | 19.8 | 27.5 | 34.2 | 10.6 | 12.5 | 16.1 | 2.1 | 3.4 | 5.5 |
| T(°C) | Carbonate coprecipitation method-Cu/ZnO/Al2O3 (MET5) | ||||||||
| 200 | 11 | 18 | 24.7 | 66.2 | 73.8 | 73.3 | 7.3 | 13.3 | 18.1 |
| 230 | 17.2 | 24.8 | 33.6 | 33.6 | 42.2 | 38.9 | 5.8 | 10.5 | 13.1 |
| 260 | 20.8 | 30.2 | 37.5 | 11.6 | 13.3 | 16.8 | 2.4 | 4.0 | 6.3 |
| T(°C) | Citric and impregnation method-Cu/ZnO (MET6) | ||||||||
| 200 | 3.3 | 4.9 | 4.4 | 97.4 | 98 | 100 | 3.2 | 4.8 | 4.4 |
| 230 | 4.4 | 7.1 | 15.3 | 78.8 | 80 | 63.2 | 3.5 | 5.7 | 9.7 |
| 260 | 14.5 | 20.6 | 22.6 | 21.8 | 26.4 | 14.6 | 3.2 | 5.4 | 3.3 |
| T(°C) | Impregnation method-Cu/YAG (MET7) | ||||||||
| 200 | 1.4 | 2.7 | 3.7 | 96.9 | 98.3 | 87.2 | 1.4 | 2.7 | 3.2 |
| 230 | 3.7 | 6.3 | 8.7 | 64.9 | 68.2 | 69.7 | 2.4 | 4.3 | 6.1 |
| 260 | 8.8 | 14.2 | 18.3 | 33.6 | 37.4 | 40.3 | 3.0 | 5.3 | 7.4 |
| Cat | T(°C) | S MeOH | Y MeOH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H2/CO2 | 3 | 6 | 9 | 3 | 6 | 9 | 3 | 6 | 9 | |
| MET1 | 200 | 11 | 17.1 | 21.1 | 51.2 | 65.4 | 69.2 | 5.6 | 11.2 | 14.5 |
| 230 | 18.5 | 25.5 | 31.9 | 32.3 | 38.7 | 39.1 | 6 | 9.8 | 12.5 | |
| MET2 | 200 | 15.4 | 25 | 31.1 | 60.3 | 66.1 | 67.9 | 9.3 | 16.5 | 21.1 |
| 230 | 17.5 | 25.8 | 32.7 | 33.7 | 41 | 47.3 | 5.9 | 10.6 | 15.5 | |
| MET3 | 200 | 14.6 | 21.5 | 28.2 | 54.5 | 63.4 | 65.6 | 7.9 | 13.6 | 18.5 |
| 230 | 18.2 | 26 | 31.5 | 33.4 | 39.2 | 45.1 | 6.1 | 10.2 | 14.2 | |
| MET4 | 200 | 14.7 | 22.5 | 29.1 | 53.4 | 60.7 | 63.4 | 7.8 | 13.6 | 18.4 |
| 230 | 16.5 | 24.6 | 30.8 | 26.9 | 32.7 | 39.7 | 4.4 | 8 | 12.2 | |
| MET5 | 200 | 5.6 | 8.7 | 11.5 | 75.9 | 79.7 | 80.5 | 4.2 | 6.9 | 9.3 |
| 230 | 11 | 16.4 | 20.7 | 36.8 | 41 | 43 | 4 | 6.7 | 8.9 | |
| Used Basic Material | CAS-Number | Supplier |
|---|---|---|
| Copper II nitrate trihydrate | 10031-43-3 | Sigma-Aldrich (Darmstadt, Germany) |
| Zinc nitrate hexahydrate | 10196-18-6 | Sigma-Aldrich(Taufkirchen, Germany) |
| Citric acid anhydrous | 77-92-9 | Sigma-Aldrich (Taufkirchen, Germany) |
| Oxalic acid | 144-62-7 | Sigma-Aldrich (Taufkirchen, Germany) |
| Aluminium nitrate | 7784-27-2 | Sigma-Aldrich (Darmstadt, Germany) |
| Sodium carbonate | 497-19-8 | Sigma-Aldrich (Taufkirchen, Germany) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godini, H.R.; Khadivi, M.; Azadi, M.; Görke, O.; Jazayeri, S.M.; Thum, L.; Schomäcker, R.; Wozny, G.; Repke, J.-U. Multi-Scale Analysis of Integrated C1 (CH4 and CO2) Utilization Catalytic Processes: Impacts of Catalysts Characteristics up to Industrial-Scale Process Flowsheeting, Part I: Experimental Analysis of Catalytic Low-Pressure CO2 to Methanol Conversion. Catalysts 2020, 10, 505. https://doi.org/10.3390/catal10050505
Godini HR, Khadivi M, Azadi M, Görke O, Jazayeri SM, Thum L, Schomäcker R, Wozny G, Repke J-U. Multi-Scale Analysis of Integrated C1 (CH4 and CO2) Utilization Catalytic Processes: Impacts of Catalysts Characteristics up to Industrial-Scale Process Flowsheeting, Part I: Experimental Analysis of Catalytic Low-Pressure CO2 to Methanol Conversion. Catalysts. 2020; 10(5):505. https://doi.org/10.3390/catal10050505
Chicago/Turabian StyleGodini, Hamid Reza, Mohammadali Khadivi, Mohammadreza Azadi, Oliver Görke, Seyed Mahdi Jazayeri, Lukas Thum, Reinhard Schomäcker, Günter Wozny, and Jens-Uwe Repke. 2020. "Multi-Scale Analysis of Integrated C1 (CH4 and CO2) Utilization Catalytic Processes: Impacts of Catalysts Characteristics up to Industrial-Scale Process Flowsheeting, Part I: Experimental Analysis of Catalytic Low-Pressure CO2 to Methanol Conversion" Catalysts 10, no. 5: 505. https://doi.org/10.3390/catal10050505
APA StyleGodini, H. R., Khadivi, M., Azadi, M., Görke, O., Jazayeri, S. M., Thum, L., Schomäcker, R., Wozny, G., & Repke, J.-U. (2020). Multi-Scale Analysis of Integrated C1 (CH4 and CO2) Utilization Catalytic Processes: Impacts of Catalysts Characteristics up to Industrial-Scale Process Flowsheeting, Part I: Experimental Analysis of Catalytic Low-Pressure CO2 to Methanol Conversion. Catalysts, 10(5), 505. https://doi.org/10.3390/catal10050505





