Facile Synthesis of Amorphous C3N4ZnxOy (x, y = 0.32–1.10) with High Photocatalytic Efficiency for Antibiotic Degradation
Abstract
1. Introduction
2. Results and Discussion
2.1. Element Analysis
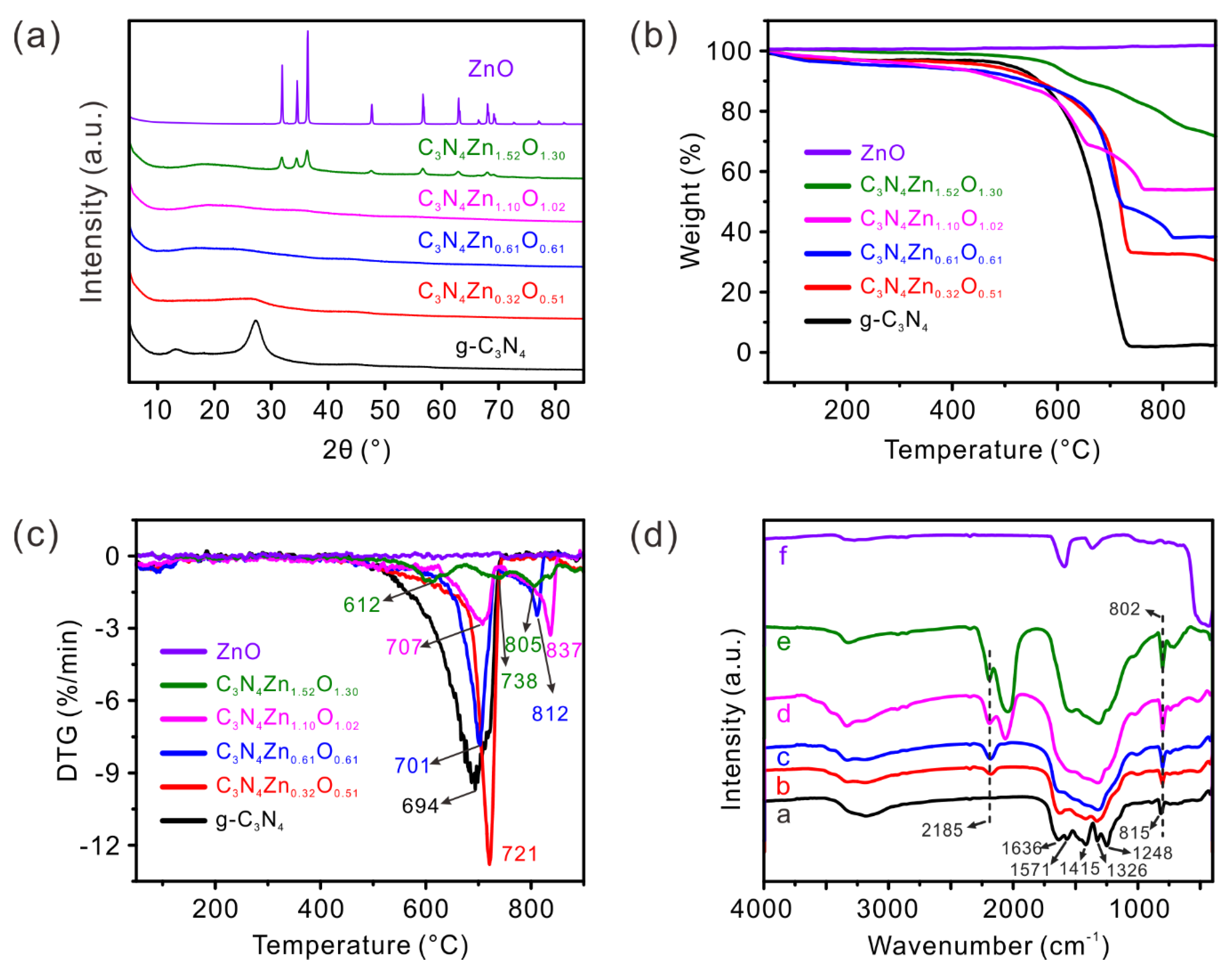
2.2. X-Ray Diffraction
2.3. Thermogravimetric Analysis
2.4. FTIR Spectra
2.5. SEM Images
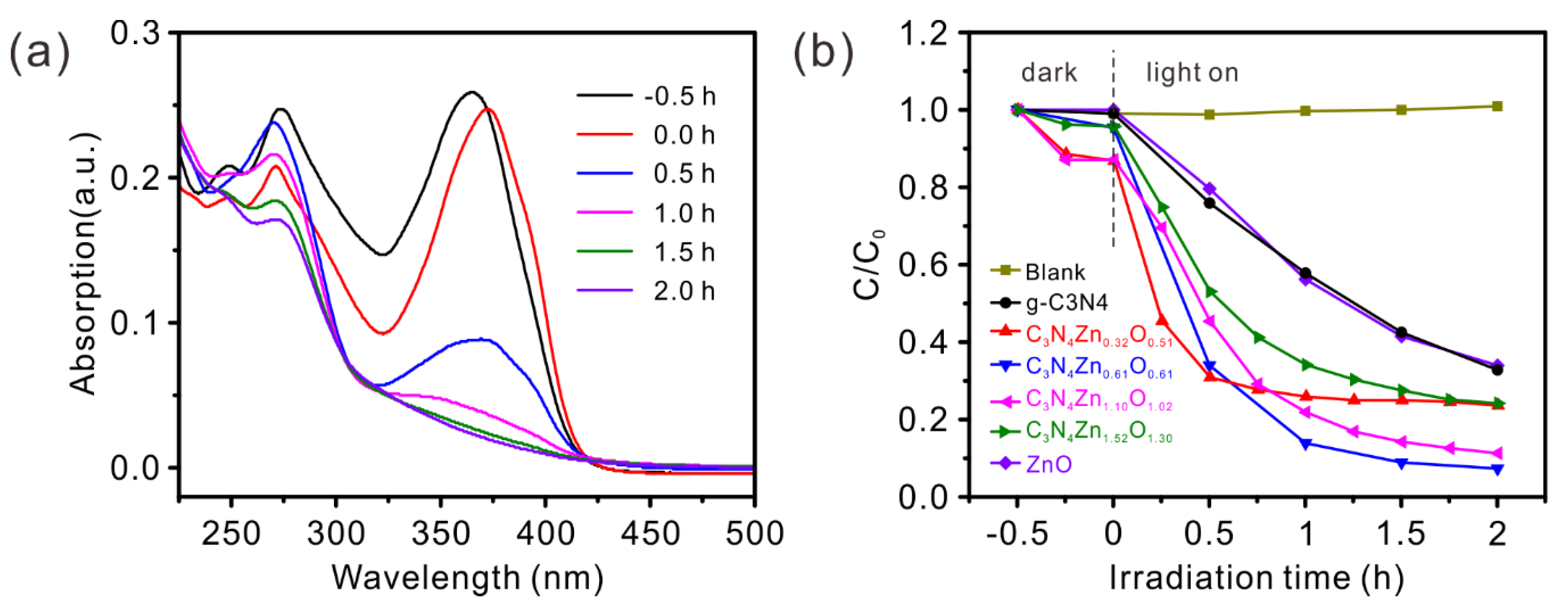
2.6. Optical Band Gap Measurement
2.7. Photoluminescence and Photocurrent Measurements
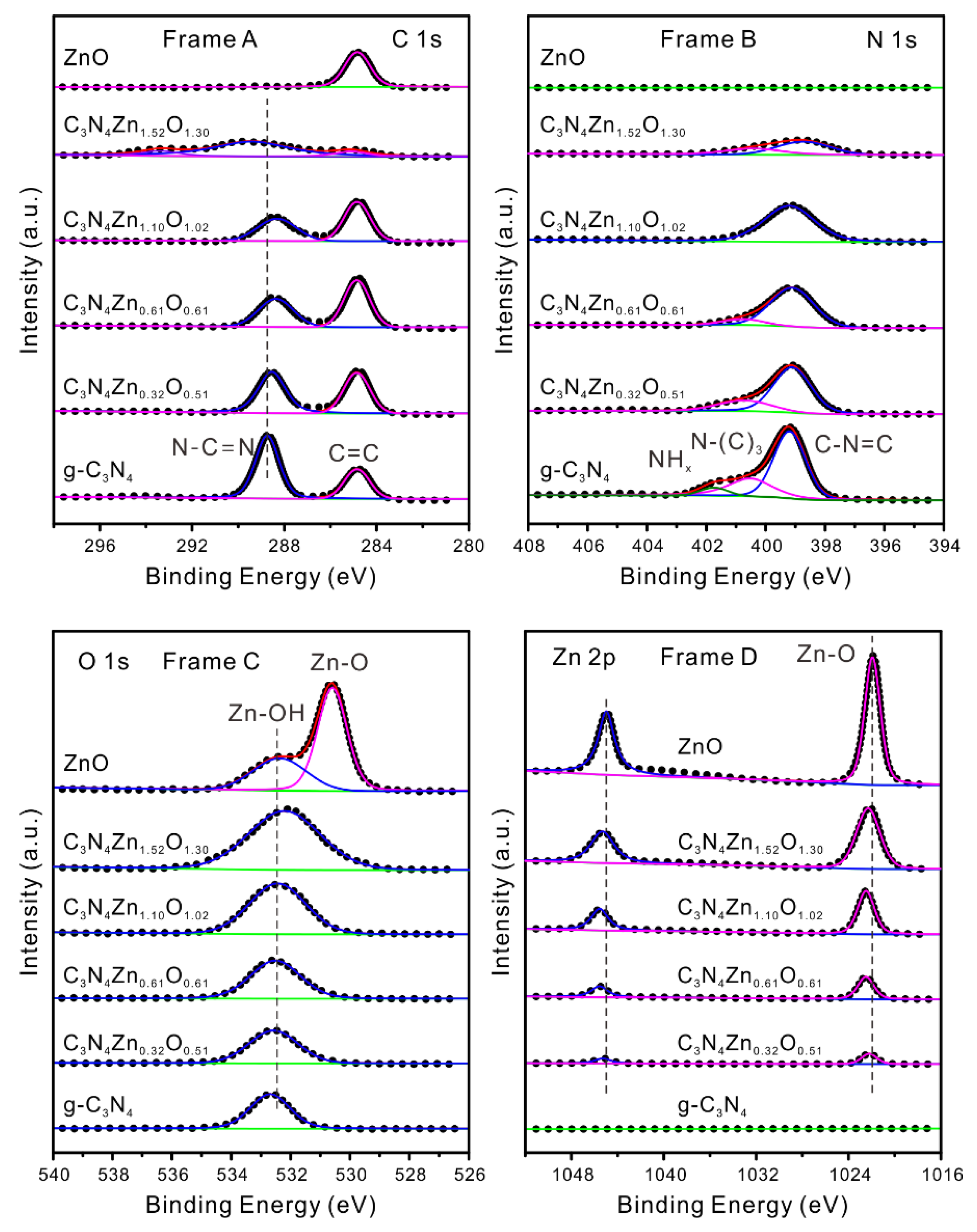
2.8. X-Ray Photoelectron Spectroscopy (XPS)
2.9. 1H and 13C NMR Spectra
2.10. Photodegradation of Tetracycline
3. Materials and Methods
3.1. Sample Preparation
3.2. Material Characterization
3.3. Photocatalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Leong, S.; Li, D.; Hapgood, K.; Zhang, X.; Wang, H. Ni(OH)2 decorated rutile TiO2 for efficient removal of tetracycline from wastewater. Appl. Catal. B Environ. 2016, 198, 224–233. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, M.; Yuan, X.; Si, M.; Jiang, L.; Wu, Z.; Wang, H.; Zeng, G. Nitrogen doped carbon quantum dots mediated silver phosphate/bismuth vanadate Z-scheme photocatalyst for enhanced antibiotic degradation. J. Colloid Interface Sci. 2018, 529, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hailili, R.; Wang, Z.-Q.; Li, Y.; Wang, Y.; Sharma, V.K.; Gong, X.-Q.; Wang, C. Oxygen vacancies induced visible-light photocatalytic activities of CaCu3Ti4O12 with controllable morphologies for antibiotic degradation. Appl. Catal. B Environ. 2018, 221, 422–432. [Google Scholar] [CrossRef]
- Pan, X.; Lv, N.; Li, C.; Ning, J.; Wang, T.; Wang, R.; Zhou, M.; Zhu, G. Impact of nano zero valent iron on tetracycline degradation and microbial community succession during anaerobic digestion. Chem. Eng. J. 2019, 359, 662–671. [Google Scholar] [CrossRef]
- Wang, W.; Tadé, M.O.; Shao, Z. Research progress of perovskite materials in photocatalysis- and photovoltaics-related energy conversion and environmental treatment. Chem. Soc. Rev. 2015, 44, 5371–5408. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Qian, F.; Zhou, C.; Zhang, S.; Chen, J. Preparation of magnetic porous carbon from waste hydrochar by simultaneous activation and magnetization for tetracycline removal. Bioresour. Technol. 2014, 154, 209–214. [Google Scholar] [CrossRef]
- Kakavandi, B.; Takdastan, A.; Jaafarzadeh, N.; Azizi, M.; Mirzaei, A.; Azari, A.; Jaafarzadeh, N. Application of Fe3O4@C catalyzing heterogeneous UV-Fenton system for tetracycline removal with a focus on optimization by a response surface method. J. Photochem. Photobiol. A Chem. 2016, 314, 178–188. [Google Scholar] [CrossRef]
- Gómez-Pacheco, C.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Peñalver, J.J.L. Tetracycline removal from waters by integrated technologies based on ozonation and biodegradation. Chem. Eng. J. 2011, 178, 115–121. [Google Scholar] [CrossRef]
- Da Silva, L.F.; M’Peko, J.-C.; Andrés, J.; Beltran, A.; Gracia, L.; Bernardi, M.I.B.; Mesquita, A.; Antonelli, E.; Moreira, M.L.; Mastelaro, V.R. Insight into the Effects of Fe Addition on the Local Structure and Electronic Properties of SrTiO3. J. Phys. Chem. C 2014, 118, 4930–4940. [Google Scholar] [CrossRef]
- Liu, X.; Lv, P.; Yao, G.; Ma, C.; Huo, P.; Yan, Y. Microwave-assisted synthesis of selective degradation photocatalyst by surface molecular imprinting method for the degradation of tetracycline onto Cl-TiO2. Chem. Eng. J. 2013, 217, 398–406. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.; Shao, S.; Lai, M.; Lu, C. Compact and uniform TiO2@g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B Environ. 2017, 217, 57–64. [Google Scholar] [CrossRef]
- Liu, W.; Liu, H.; Dang, L.; Zhang, X.; Wu, X.; Yang, B.; Li, Z.; Zhang, X.; Lei, L.; Jin, S. Amorphous Cobalt-Iron Hydroxide Nanosheet Electrocatalyst for Efficient Electrochemical and Photo-Electrochemical Oxygen Evolution. Adv. Funct. Mater. 2017, 27, 1603904. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In Situ Formation of an Oxygen-Evolving Catalyst in Neutral Water Containing Phosphate and Co2+. Science 2008, 321, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Trudel, S.; Berlinguette, C.P. Water Oxidation Catalysis: Electrocatalytic Response to Metal Stoichiometry in Amorphous Metal Oxide Films Containing Iron, Cobalt, and Nickel. J. Am. Chem. Soc. 2013, 135, 11580–11586. [Google Scholar] [CrossRef] [PubMed]
- Indra, A.; Menezes, P.W.; Sahraie, N.R.; Bergmann, A.; Das, C.; Tallarida, M.; Schmeißer, D.; Strasser, P.; Driess, M. Unification of Catalytic Water Oxidation and Oxygen Reduction Reactions: Amorphous Beat Crystalline Cobalt Iron Oxides. J. Am. Chem. Soc. 2014, 136, 17530–17536. [Google Scholar] [CrossRef]
- Kornienko, N.; Resasco, J.; Becknell, N.; Jiang, C.-M.; Liu, Y.-S.; Nie, K.; Sun, X.; Guo, J.; Leone, S.R.; Yang, P. Operando Spectroscopic Analysis of an Amorphous Cobalt Sulfide Hydrogen Evolution Electrocatalyst. J. Am. Chem. Soc. 2015, 137, 7448–7455. [Google Scholar] [CrossRef]
- Tran, P.D.; Tran, T.V.; Orio, M.; Torelli, S.; Truong, Q.D.; Nayuki, K.; Sasaki, Y.; Chiam, S.Y.; Yi, R.; Honma, I.; et al. Coordination polymer structure and revisited hydrogen evolution catalytic mechanism for amorphous molybdenum sulfide. Nat. Mater. 2016, 15, 640–646. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Jia, X.; Xu, B.; Lin, H.; Yang, H.; Song, L.; Wang, X. Amorphous nickel-cobalt complexes hybridized with 1T-phase molybdenum disulfide via hydrazine-induced phase transformation for water splitting. Nat. Commun. 2017, 8, 15377. [Google Scholar] [CrossRef]
- Lee, S.C.; Benck, J.D.; Tsai, C.; Park, J.; Koh, A.L.; Abild-Pedersen, F.; Jaramillo, T.; Sinclair, R. Chemical and Phase Evolution of Amorphous Molybdenum Sulfide Catalysts for Electrochemical Hydrogen Production. ACS Nano 2015, 10, 624–632. [Google Scholar] [CrossRef]
- Staszak-Jirkovsky, J.; Malliakas, C.D.; Lopes, P.P.; Danilovic, N.; Kota, S.S.; Chang, K.-C.; Genorio, B.; Strmcnik, D.; Stamenkovic, V.R.; Kanatzidis, M.G.; et al. Design of active and stable Co–Mo–Sx chalcogels as pH-universal catalysts for the hydrogen evolution reaction. Nat. Mater. 2015, 15, 197–203. [Google Scholar] [CrossRef]
- Farrow, C.L.; Bediako, D.K.; Surendranath, Y.; Nocera, D.G.; Billinge, S.J.L. Intermediate-Range Structure of Self-Assembled Cobalt-Based Oxygen-Evolving Catalyst. J. Am. Chem. Soc. 2013, 135, 6403–6406. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Piccinin, S.; Laio, A.; Fabris, S. Atomistic Structure of Cobalt-Phosphate Nanoparticles for Catalytic Water Oxidation. ACS Nano 2012, 6, 10497–10504. [Google Scholar] [CrossRef]
- Salvatore, D.A.; Dettelbach, K.E.; Hudkins, J.R.; Berlinguette, C.P. Near-infrared–driven decomposition of metal precursors yields amorphous electrocatalytic films. Sci. Adv. 2015, 1, e1400215. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Zhang, Z.; Sedach, P.A.; Siu, M.K.J.; Trudel, S.; Berlinguette, C.P. Photochemical Route for Accessing Amorphous Metal Oxide Materials for Water Oxidation Catalysis. Science 2013, 340, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lambert, F.; Policar, C.; Mavré, F.; Limoges, B. Fast magnetically driven electrodeposition of amorphous metal oxide water oxidation catalysts from carbon-coated metallic nanoparticles. J. Mater. Chem. A 2015, 3, 16190–16197. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Y.; Yin, L.-C.; Kang, X.; Liu, G.; Cheng, H.-M. An Amorphous Carbon Nitride Photocatalyst with Greatly Extended Visible-Light-Responsive Range for Photocatalytic Hydrogen Generation. Adv. Mater. 2015, 27, 4572–4577. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Z.; Tapping, P.C.; Kee, T.W.; Smernik, R.; Spooner, N.; Moffatt, J.; Tang, Y.; Davey, K.; Qiao, S. A Benchmark Quantum Yield for Water Photoreduction on Amorphous Carbon Nitride. Adv. Funct. Mater. 2017, 27, 1702384. [Google Scholar] [CrossRef]
- Zhang, R.; Li, P.; Wang, F.; Ye, L.; Gaur, A.; Huang, Z.; Zhao, Z.; Bai, Y.; Zhou, Y. Atomically dispersed Mo atoms on amorphous g-C3N4 promotes visible-light absorption and charge carriers transfer. Appl. Catal. B Environ. 2019, 250, 273–279. [Google Scholar] [CrossRef]
- Han, Q.; Cheng, Z.; Wang, B.; Zhang, H.-M.; Qu, L. Significant Enhancement of Visible-Light-Driven Hydrogen Evolution by Structure Regulation of Carbon Nitrides. ACS Nano 2018, 12, 5221–5227. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, P.; Chao, Y.; Lin, F.; Lai, J.; Li, H.; Guo, S. Face-to-face engineering of ultrathin Pd nanosheets on amorphous carbon nitride for efficient photocatalytic hydrogen production. Sci. China Mater. 2018, 62, 351–358. [Google Scholar] [CrossRef]
- Zou, M.; Feng, L.; Pervaiz, E.; Ganeshraja, A.S.; Gao, T.; Jiang, H.; Yang, M. An Amorphous Mn, N-Codoped TiO2 Microspheres Photocatalyst Induced by High Defects with Greatly Extended Visible-Light-Responsive Range for Photocatalytic Degradation. Nano Adv. 2017, 2, 36–44. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Zhao, H.; Wang, G.; Xiang, L.; Xu, J.; Komarneni, S. Oxygen defects-mediated Z-scheme charge separation in g-C3N4/ZnO photocatalysts for enhanced visible-light degradation of 4-chlorophenol and hydrogen evolution. Appl. Catal. B Environ. 2017, 206, 406–416. [Google Scholar] [CrossRef]
- Yue, B.; Li, Q.; Iwaï, H.; Kako, T.; Ye, J. Hydrogen production using zinc-doped carbon nitride catalyst irradiated with visible light. Sci. Technol. Adv. Mater. 2011, 12, 34401. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.-D.; Chang, V.W.-C.; Hu, Z.-T.; Goei, R.; Lim, T.-T. Enhancing the catalytic activity of g-C3N4 through Me doping (Me = Cu, Co and Fe) for selective sulfathiazole degradation via redox-based advanced oxidation process. Chem. Eng. J. 2017, 323, 260–269. [Google Scholar] [CrossRef]
- Li, B.; Si, Y.; Zhou, B.-X.; Fang, Q.; Li, Y.-Y.; Huang, W.-Q.; Hu, W.; Pan, A.; Fan, X.; Huang, G.-F. Doping-Induced Hydrogen-Bond Engineering in Polymeric Carbon Nitride To Significantly Boost the Photocatalytic H2 Evolution Performance. ACS Appl. Mater. Interfaces 2019, 11, 17341–17349. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Aksoy, S.; Caglar, Y.; Ilican, S.; Caglar, M. Sol–gel derived Li–Mg co-doped ZnO films: Preparation and characterization via XRD, XPS, FESEM. J. Alloy. Compd. 2012, 512, 171–178. [Google Scholar] [CrossRef]
- Abdei-Wahab, M.S.; Jilani, A.; Yahia, I.; Alghamdi, A. Enhanced the photocatalytic activity of Ni-doped ZnO thin films: Morphological, optical and XPS analysis. Superlattices Microstruct. 2016, 94, 108–118. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Yu, Y.; Pei, Z.; Bai, X.; Sun, C.; Huang, R.; Wen, L. X-ray photoelectron spectroscopy and auger electron spectroscopy studies of Al-doped ZnO films. Appl. Surf. Sci. 2000, 158, 134–140. [Google Scholar] [CrossRef]
- Jeong, Y.; Bae, C.; Kim, D.; Song, K.; Woo, K.; Shin, H.; Cao, G.; Moon, J. Bias-Stress-Stable Solution-Processed Oxide Thin Film Transistors. ACS Appl. Mater. Interfaces 2010, 2, 611–615. [Google Scholar] [CrossRef]
- Yu, W.; Chen, J.; Shang, T.; Chen, L.; Gu, L.; Peng, T. Direct Z-scheme g-C3N4/WO3 photocatalyst with atomically defined junction for H2 production. Appl. Catal. B Environ. 2017, 219, 693–704. [Google Scholar] [CrossRef]
- Sun, J.-X.; Yuan, Y.; Qiu, L.-G.; Jiang, X.; Shena, Y.; Shena, Y.; Zhu, J. Fabrication of composite photocatalyst g-C3N4–ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans. 2012, 41, 6756–6763. [Google Scholar] [CrossRef]
- Wang, X.L.; Fang, W.Q.; Wang, H.F.; Zhang, H.; Zhao, H.; Yao, Y.; Yang, H.G. Surface hydrogen bonding can enhance photocatalytic H2 evolution efficiency. J. Mater. Chem. A 2013, 1, 14089. [Google Scholar] [CrossRef]
- Cao, J.; Lai, L.; Lai, B.; Yao, G.; Chen, X.; Song, L. Degradation of tetracycline by peroxymonosulfate activated with zero-valent iron: Performance, intermediates, toxicity and mechanism. Chem. Eng. J. 2019, 364, 45–56. [Google Scholar] [CrossRef]
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; Lv, W.; Liu, G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Panneri, S.; Ganguly, P.; Nair, H.; Mohamed, A.A.P.; Warrier, K.G.; Hareesh, U.N.S. Copyrolysed C3N4-Ag/ZnO Ternary Heterostructure Systems for Enhanced Adsorption and Photocatalytic Degradation of Tetracycline. Eur. J. Inorg. Chem. 2016, 2016, 5068–5076. [Google Scholar] [CrossRef]
- Rasheed, H.U.; Lv, X.; Wei, W.; Yaseen, W.; Ullah, N.; Xie, J.; Zhu, W. Synthesis and studies of ZnO doped with g-C3N4 nanocomposites for the degradation of tetracycline hydrochloride under the visible light irradiation. J. Environ. Chem. Eng. 2019, 7, 103152. [Google Scholar] [CrossRef]
- Hao, R.; Xiao, X.; Zuo, X.; Nan, J.; Zhang, W. Efficient adsorption and visible-light photocatalytic degradation of tetracycline hydrochloride using mesoporous BiOI microspheres. J. Hazard. Mater. 2012, 209, 137–145. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Zhang, H.; Zong, R.; Zhu, Y. Photocorrosion Inhibition and Photoactivity Enhancement for Zinc Oxide via Hybridization with Monolayer Polyaniline. J. Phys. Chem. C 2009, 113, 4605–4611. [Google Scholar] [CrossRef]
- Fu, H.; Xu, T.; Zhu, S.; Zhu, Y. Photocorrosion Inhibition and Enhancement of Photocatalytic Activity for ZnO via Hybridization with C60. Environ. Sci. Technol. 2008, 42, 8064–8069. [Google Scholar] [CrossRef] [PubMed]
- Neppolian, B. Solar/UV-induced photocatalytic degradation of three commercial textile dyes. J. Hazard. Mater. 2002, 89, 303–317. [Google Scholar] [CrossRef]
- Wang, X.L.; Yang, H.G. Facile fabrication of high-yield graphitic carbon nitride with a large surface area using bifunctional urea for enhanced photocatalytic performance. Appl. Catal. B Environ. 2017, 205, 624–630. [Google Scholar] [CrossRef]




| Sample | H (wt %) | C (wt %) | N (wt %) | O (wt %) | Zn (wt %) | Atom Ratio (C:N:O:Zn) | Specific Surface Area (m2/g) |
|---|---|---|---|---|---|---|---|
| g-C3N4 | 1.94 | 34.60 | 59.86 | 4.23 | 0 | 2.70:4:0.25:0.00 | 89.8 |
| C3N4Zn0.32O0.51 | 1.35 | 27.41 | 46.82 | 6.83 | 17.60 | 2.73:4:0.51:0.32 | 75.5 |
| C3N4Zn0.61O0.61 | 0.98 | 23.21 | 40.24 | 7.06 | 28.52 | 2.69:4:0.61:0.61 | 45.9 |
| C3N4Zn1.10O1.02 | 0.80 | 17.69 | 31.72 | 9.28 | 40.52 | 2.60:4:1.02:1.10 | 42.7 |
| C3N4Zn1.52O1.30 | 0.55 | 16.07 | 26.53 | 9.86 | 47.00 | 2.83:4:1.30:1.52 | 41.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Dong, J.X.; Gao, G.L.; Wang, X.L.; Yao, Y.-F. Facile Synthesis of Amorphous C3N4ZnxOy (x, y = 0.32–1.10) with High Photocatalytic Efficiency for Antibiotic Degradation. Catalysts 2020, 10, 514. https://doi.org/10.3390/catal10050514
Zhang R, Dong JX, Gao GL, Wang XL, Yao Y-F. Facile Synthesis of Amorphous C3N4ZnxOy (x, y = 0.32–1.10) with High Photocatalytic Efficiency for Antibiotic Degradation. Catalysts. 2020; 10(5):514. https://doi.org/10.3390/catal10050514
Chicago/Turabian StyleZhang, Ran, Jing Xian Dong, Guo Liang Gao, Xue Lu Wang, and Ye-Feng Yao. 2020. "Facile Synthesis of Amorphous C3N4ZnxOy (x, y = 0.32–1.10) with High Photocatalytic Efficiency for Antibiotic Degradation" Catalysts 10, no. 5: 514. https://doi.org/10.3390/catal10050514
APA StyleZhang, R., Dong, J. X., Gao, G. L., Wang, X. L., & Yao, Y.-F. (2020). Facile Synthesis of Amorphous C3N4ZnxOy (x, y = 0.32–1.10) with High Photocatalytic Efficiency for Antibiotic Degradation. Catalysts, 10(5), 514. https://doi.org/10.3390/catal10050514





