Enhanced Photoelectrochemical Water Splitting at Hematite Photoanodes by Effect of a NiFe-Oxide co-Catalyst
Abstract
:1. Introduction
- Expensive electrode materials and components;
- Difficulty in separating pure H2 from water vapour in the output stream;
- Too low solar to hydrogen (STH) efficiency;
- Use of corrosive electrolytes limiting the durability of the PEC.
2. Results
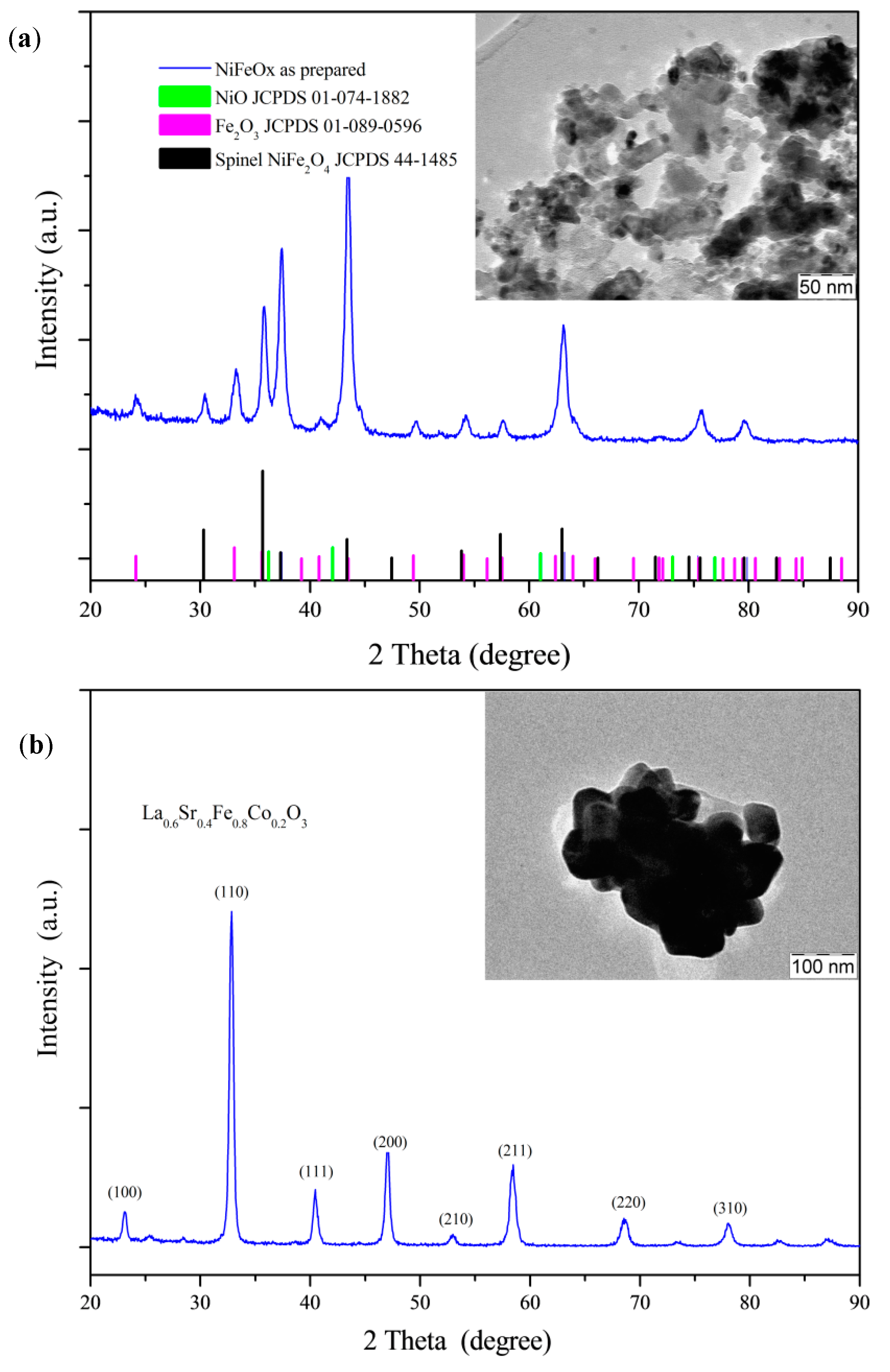
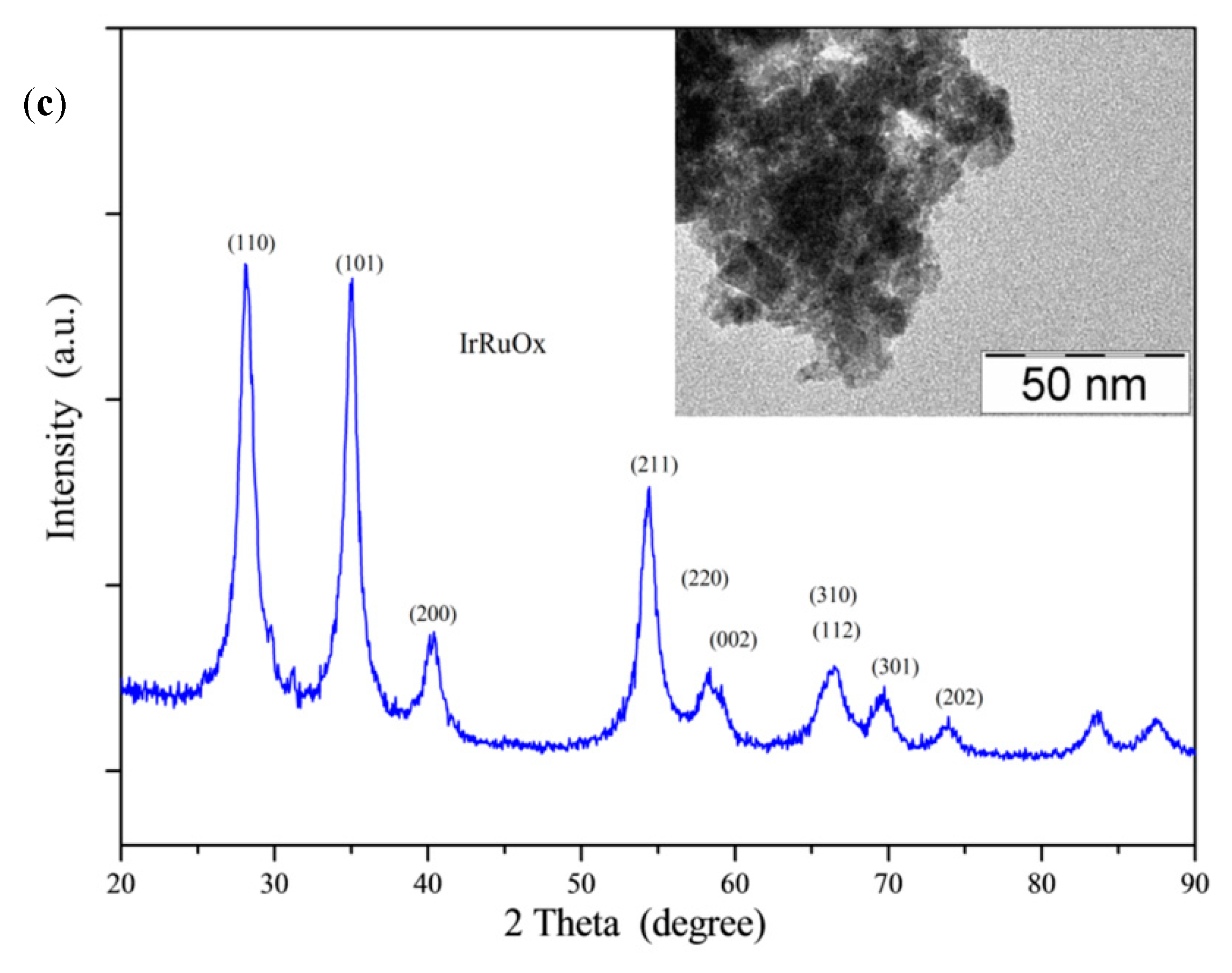
2.1. Physicochemical Characterization
XRD Analysis of co-Catalysts
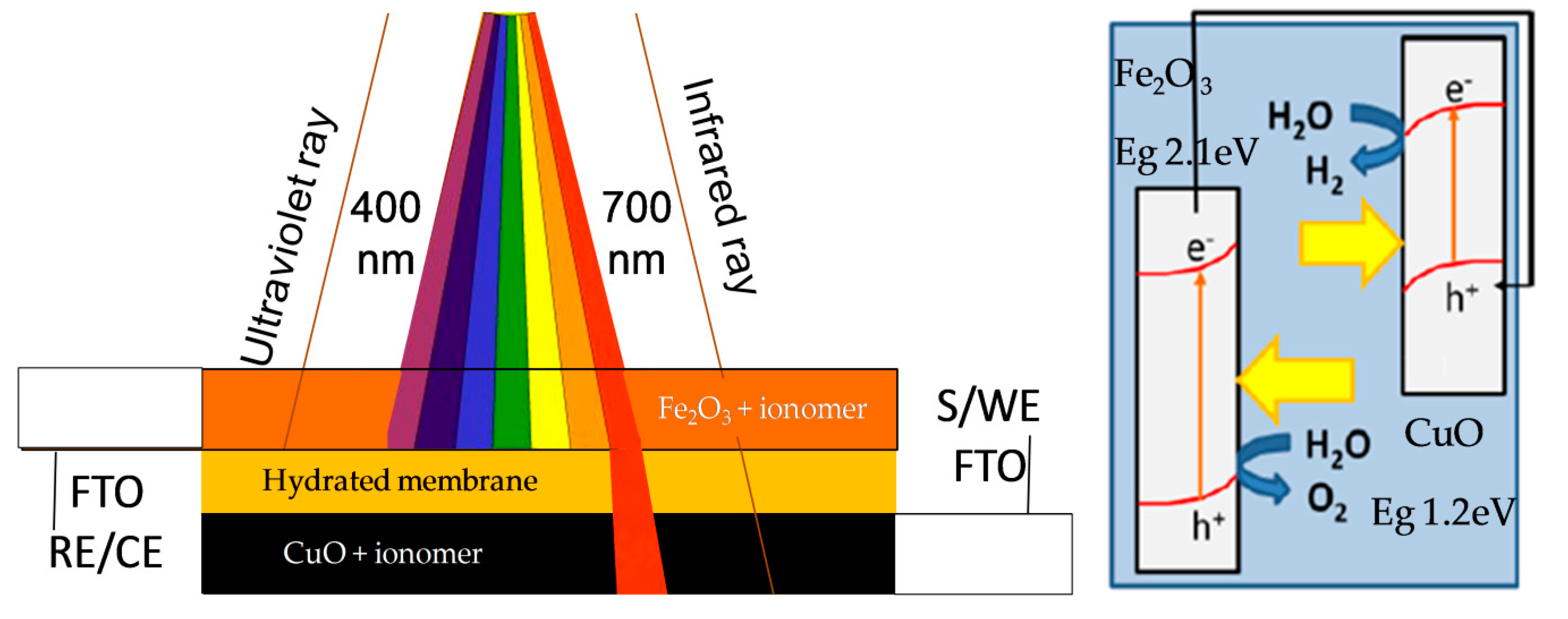
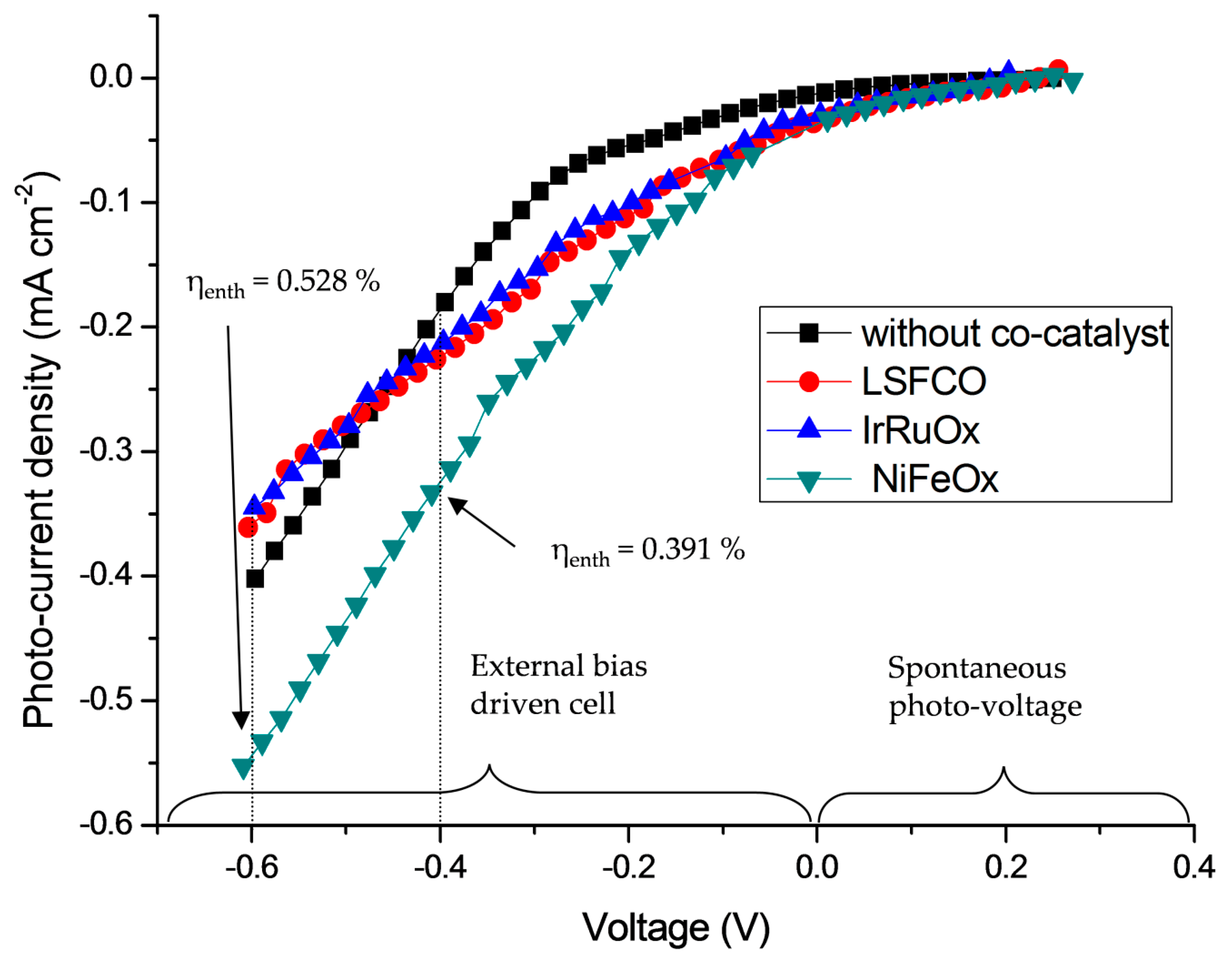
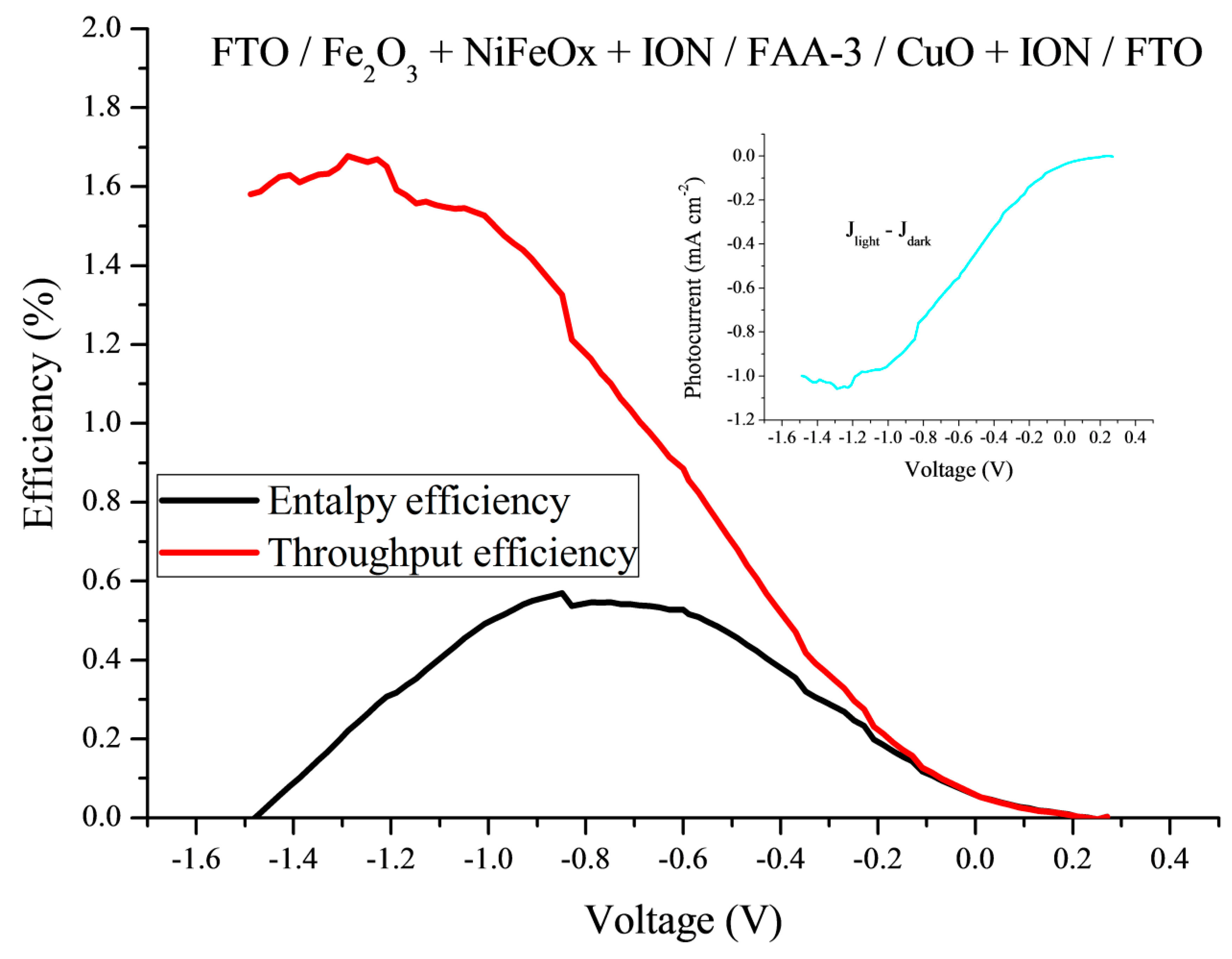
2.2. Photoelectrochemical Characterization
2.3. Morphological Characterisation
3. Materials and Methods
3.1. Synthesis of Photoelectrodes
3.1.1. Synthesis of (110) Oriented Hematite Nanorods
3.1.2. Hematite Modification with Tin and Phosphorus
3.1.3. Synthesis of Cupric Oxide
3.1.4. Synthesis of co-Catalysts
3.1.5. Membrane and Ionomer
3.2. Assembly of the Cell
3.3. Physicochemical Characterization
3.4. Electrochemical Tests
3.5. Efficiency of the PEC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gratzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Khaselev, O.; Turner, J.A. A monolithic photovoltaic-photoelectrochemical device for hydrogen production via water splitting. Science 1998, 280, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Bak, T.; Nowotny, J.; Rekas, M.; Sorrell, C.C. Photo-electrochemical hydrogen generation from water using solar energy. Materials-related aspects. Int. J. Hydrogen Energy 2002, 27, 991–1022. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Passalacqua, R.; Perathoner, S.; Centi, G. Semiconductor, molecular and hybrid systems for photoelectrochemical solar fuel production. J. Energy Chem. 2017, 26, 219–240. [Google Scholar] [CrossRef]
- Jing, S.; Yin, D.S.; Lu, J.; Shen, P.K.; Tsiakaras, P. P-doped CNTs encapsulated nickel hybrids with flower-like structure as efficient catalysts for hydrogen evolution reaction. Electrochim. Acta 2019, 298, 6582–6591. [Google Scholar] [CrossRef]
- Grądzka, I.; Gierszewski, M.; Ziółek, M. The effect of chloride anions on charge transfer in dye-sensitized photoanodes for water splitting. Biomimetics 2019, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Sim, Y.; John, J.; Moon, J.; Sim, U. Photo-assisted hydrogen evolution with reduced graphene oxide catalyst on silicon nanowire photocathode. Appl. Sci. 2018, 8, 2046. [Google Scholar] [CrossRef] [Green Version]
- Cots, A.; Bonete, P.; Sebastian, D.; Baglio, V.; Aricò, A.S.; Gomez, R. Toward tandem solar cells for water splitting using polymer electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 25393–25400. [Google Scholar] [CrossRef]
- Prevot, M.S.; Sivula, K. Photoelectrochemical tandem cells for solar water splitting. J. Phys. Chem. C 2013, 117, 17879–17893. [Google Scholar] [CrossRef]
- Chiam, S.Y.; Kumar, M.H.; Bassi, P.S.; Seng, H.L.; Barber, J.; Wong, L.H. Improving the efficiency of hematite nanorods for photoelectrochemical water splitting by doping with manganese. ACS Appl. Mater. Interfaces 2014, 6, 5852–5859. [Google Scholar] [CrossRef]
- Hsu, Y.-K.; Yu, C.-H.; Lin, H.-H.; Chen, Y.-C.; Lin, Y.-G. Template synthesis of copper oxide nanowires for photoelectrochemical hydrogen generation. J. Electroanal. Chem. 2013, 704, 19–23. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar water splitting: Progress using hematite (α-Fe2O3) photoelectrodes. Chemsuschem 2011, 4, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Cots, A.; Bonete, P.; Gomez, R. Improving the stability and efficiency of CuO photocathodes for solar hydrogen production through modification with iron. ACS Appl. Mater. Interfaces 2018, 10, 26348–26356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Lu, J.; Luo, L.; Jing, S.; Abbo, H.S.; Titinchi, S.J.J.; Chen, Z.; Tsiakaras, P.; Yin, S. Enhanced hydrogen evolution activity over microwave-assisted functionalized 3D structured graphene anchoring FeP nanoparticles. Electrochim. Acta 2019, 317, 242–249. [Google Scholar] [CrossRef]
- Vasilaki, E.; Vernardou, D.; Kenanakis, G.; Vamvakaki, M.; Katsarakis, N. TiO2/WO3 photoactive bilayers in the UV–Vis light region. Appl. Phys. A 2017, 123, 231–238. [Google Scholar] [CrossRef]
- Vasilaki, E.; Georgaki, I.; Vernardou, D.; Vamvakaki, M.; Katsarakis, N. Ag-loaded TiO2/reduced graphene oxide nanocomposites for enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2015, 353, 865–872. [Google Scholar] [CrossRef]
- Hernàndez, S.; Saracco, G.; Barbero, G.; Alexe-Ionescu, A.L. Role of the electrode morphology on the optimal thickness of BiVO4 anodes for photoelectrochemical water splitting cells. J. Electroanal. Chem. 2017, 799, 481–486. [Google Scholar] [CrossRef]
- Valerini, D.; Hernàndez, S.; di Benedetto, F.; Russo, N.; Saracco, G. Sputtered WO3 films for water splitting applications. Mater. Sci. Semicond. Proc. 2016, 42, 150–154. [Google Scholar] [CrossRef]
- Peng, B.; Xia, M.; Li, C.; Yue, C.; Diao, P. Network Structured CuWO4/BiVO4/Co-Pi Nanocomposite for Solar Water Splitting. Catalysts 2018, 8, 663. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Jang, J.-W.; Lee, J.S. Key strategies to advance the photoelectrochemical water splitting performance of α-Fe2O3 photoanode. Chemcatchem 2019, 11, 157–179. [Google Scholar] [CrossRef]
- Xi, L.; Lange, K.M. Surface modification of hematite photoanodes for improvement of photoelectrochemical performance. Catalysts 2018, 8, 497. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Zhang, Z. Simultaneous non-metal doping and cocatalyst decoration for efficient photoelectrochemical water splitting on hematite photoanodes. Electrochim. Acta 2018, 282, 48–55. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, C.; Zhang, X. Ti, Zn co-doped hematite photoanode for solar driven photoelectrochemical water oxidation. J. Energy Chem. 2019, 35, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jiang, S.; Song, W.; Zhou, P.; Ji, H.; Ma, W.; Hao, W.; Chen, C.; Zhao, J. Nonmetal P-doped hematite photoanode with enhanced electron mobility and high water oxidation activity. Energy Environ. Sci. 2015, 8, 1231–1236. [Google Scholar] [CrossRef]
- Liu, T.; Chen, W.; Liu, X.; Zhu, J.; Lu, L. Well-dispersed ultrafine nitrogen-doped TiO2 with polyvinylpyrrolidone (PVP) acted as N-source and stabilizer for water splitting. J. Energy Chem. 2016, 25, 1–9. [Google Scholar] [CrossRef]
- Buller, S.; Strunk, J. Nanostructure in energy conversion. J. Energy Chem. 2016, 25, 171–190. [Google Scholar] [CrossRef]
- Le Formal, F.; Sivula, K.; Gratzel, M. The transient photocurrent and photovoltage behavior of a hematite photoanode under working conditions and the influence of surface treatments. J. Phys. Chem. C. 2012, 116, 26707–26720. [Google Scholar] [CrossRef]
- Kim, J.Y.; Magesh, G.; Youn, D.H.; Jang, J.-W.; Kubota, J.; Domen, K.; Domen, K.; Lee, J.S. Single-crystalline, wormlike hematite photoanodes for efficient solar water splitting. Sci. Rep. 2013, 3, 2681–2689. [Google Scholar] [CrossRef] [Green Version]
- Malara, F.; Minguzzi, A.; Marelli, M.; Morandi, S.; Psaro, R.; Santo, V.D.; Naldoni, A. α-Fe2O3/NiOOH: An effective heterostructure for photoelectrochemical water oxidation. ACS Catal. 2015, 5, 5292–5300. [Google Scholar] [CrossRef]
- Zhou, L.; Shao, M.; Wei, M.; Duan, X. Advances in efficient electrocatalysts based on layered double hydroxides and their derivatives. J. Energy Chem. 2017, 26, 1094–1106. [Google Scholar] [CrossRef] [Green Version]
- Kment, Š.; Sivula, K.; Naldoni, A.; Sarmah, S.P.; Kmentovà, H.; Kulkarni, M.; Rambabu, Y.; Schmuki, P.; Zbořil, R. FeO-based nanostructures and nanohybrids for photoelectrochemical water splitting. Prog. Mater. Sci. 2020, 110, 100632. [Google Scholar] [CrossRef]
- Maeda, K.; Higashi, M.; Siritanaratkul, B.; Abe, R.; Domen, K. SrNbO2N as a water-splitting photoanode with a wide visible-light absorption band. J. Am. Chem. Soc. 2011, 133, 12334–12337. [Google Scholar] [CrossRef] [PubMed]
- Surendranath, Y.; Kanan, M.W.; Nocera, D.G. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J. Am. Chem. Soc. 2010, 132, 16501–16509. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Tran, P.D.; Chiam, S.Y.; Bassi, P.S.; Mak, W.F.; Mulmudi, H.K.; Batabyal, S.K.; Barber, J.; Loo, J.S.C.; Wong, L.H. Co3O4-Decorated Hematite Nanorods As an Effective Photoanode for Solar Water Oxidation. Phys. Chem. C 2012, 116, 13884–13889. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the 2017 List of Critical Raw Material for the Eu; Document 52017DC0490; European Commission: Brussels, Belgium, 2017; pp. 1–8. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/DOC/?uri=CELEX:52017DC0490&from=EN (accessed on 1 May 2020).
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalyst in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Wang, D.; Li, R.; Shi, J.Z.J.; Han, J.; Zong, X.; Li, C. Photocatalytic water oxidation on BiVO4 with the electrocatalyst as an oxidation cocatalyst: Essential relations between electrocatalyst and photocatalyst. J. Phys. Chem. C 2012, 116, 5082–5089. [Google Scholar] [CrossRef]
- Ding, C.; Shi, J.; Wang, Z.; Li, C. Photoelectrocatalytic water splitting: Significance of cocatalysts, electrolyte, and interfaces. ACS Catal. 2016, 7, 675–688. [Google Scholar] [CrossRef]
- Li, D.; Shi, J.; Li, C. Transition-metal-based electrocatalysts as cocatalysts for photoelectrochemical water splitting: A mini review. Small 2018, 14, 1704179–1704201. [Google Scholar] [CrossRef]
- Maitani, M.M.; Yamada, T.; Mashiko, H.; Yoshimatsu, K.; Oshima, T.; Ohtomo, A.; Wada, Y. Microwave Effects on Co–Pi cocatalysts deposited on α-Fe2O3 for application to photocatalytic oxygen evolution. ACS Appl. Mater. Interfaces 2017, 9, 10349–10354. [Google Scholar] [CrossRef]
- Duan, S.-F.; Geng, Y.-Y.; Pan, X.-B.; Yao, X.-Q.; Zhao, Y.-X.; Li, X.; Tao, C.-L.; Qin, D.-D. Tubular morphology preservation and doping engineering of Sn/P-codoped hematite for photoelectrochemical water oxidation. Dalton Trans. 2019, 48, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Siracusano, S.; van DiJk, N.; Payne-Johnson, E.; Baglio, V.; Aricò, A.S. Nanosized IrOx and IrRuOx electrocatalysts for the O2 evolution reaction in PEM water electrolysers. Appl. Catal. B 2015, 164, 488–495. [Google Scholar] [CrossRef]
- Siracusano, S.; Trocino, S.; Briguglio, N.; Baglio, V.; Aricò, A.S. Electrochemical impedance spectroscopy as a diagnostic tool in polymer electrolyte membrane electrolysis. Materials 2018, 11, 1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alegre, C.; Modica, E.; Aricò, A.S.; Baglio, V. Bifunctional oxygen electrode based on a perovskite/carbon composite for electrochemical devices. J. Electroanal. Chem. 2018, 808, 412–419. [Google Scholar] [CrossRef]
- Aricò, A.S.; Girolamo, M.; Siracusano, S.; Sebastian, D.; Baglio, V.; Schuster, M. Polymer electrolyte membranes for water photo-electrolysis. Membranes 2017, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Gatto, I.; Stassi, A.; Baglio, V.; Carbone, A.; Passalacqua, E.; Aricò, A.S.; Schuster, M.; Bauer, B. Optimization of perfluorosulphonic ionomer amount in gas diffusion electrodes for PEMFC operation under automotive conditions. Electrochim. Acta 2015, 165, 450–455. [Google Scholar] [CrossRef]
- Bandal, H.; Jadhav, V.H.; Kim, H. Facile synthesis of bicontinuous Ni3Fe alloy for efficient electrocatalytic oxygen evolution reaction. J. Alloy. Compd. 2017, 726, 875–884. [Google Scholar] [CrossRef]
- Gil, G.-J.H.B.-M.; Ryu, C.-H. Preparation of the electrode using NiFe2O4 powder for the alkaline water electrolysis. J. Ind. Eng. Chem. 2017, 48, 242–248. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Gong, M.; Dai, H. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2014, 8, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Ge, X.; Dong, S. A facile conversion of a Ni/Fe coordination polymer to a robust electrocatalyst for the oxygen evolution reaction. RSC Adv. 2017, 7, 32819–32825. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Chen, W.; Chen, G.; Huang, J.; Song, C.; Du, Y.; Li, C.; Ostrikov, K.K. Plasma-heteroatom-doped Ni-V-Fe trimetallic phospho-nitride as high-performance bifunctional electrocatalyst. Appl. Catal. B Environ. 2019, 268, 118440. [Google Scholar] [CrossRef]
- Qin, F.; Zhao, Z.; Alam, K.; Ni, Y.; Hernandez, F.C.R.; Yu, L.; Chen, S.; Ren, Z.; Wang, Z.; Bao, J. Trimetallic NiFeMo for Overall Electrochemical Water Splitting with a Low Cell Voltage. ACS Energy Lett. 2018, 3, 546–554. [Google Scholar] [CrossRef]
- Pebley, A.; Decolvenaere, E.; Pollock, T.; Gordon, M. Oxygen evolution on Fe-doped NiO electrocatalysts deposited: Via microplasma. Nanoscale 2017, 9, 15070–15082. [Google Scholar] [CrossRef]
- Paulraj, A.R.; Kiros, Y.; Göthelid, M.; Johansson, M.B. NiFeOx as a bifunctional electrocatalyst for oxygen reduction (OR) and evolution (OE) reaction in alkaline media. Catalysts 2018, 8, 328. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Zhang, C.; Ai, L. Hierarchical iron nickel oxide architectures derived from metal-organic frameworks as efficient electrocatalysts for oxygen evolution reaction. Electrochim. Acta 2016, 208, 17–24. [Google Scholar] [CrossRef]
- Fang, J.; Hu, L.; Wang, M.; Gan, L.; Chen, C.; Jiang, Y.; Xiao, B.; Lai, Y.; Li, J. NiO-Fe2O3/carbon nanotubes composite as bifunctional electrocatalyst for rechargeable Zn-air batteries. Mater. Lett. 2018, 218, 36–39. [Google Scholar] [CrossRef]
- Dionigi, F.; Strasser, P. NiFe-Based (Oxy)hydroxide catalysts for oxygen evolution reaction in non-acidic electrolytes. Adv. Energy Mater. 2016, 6, 1600621. [Google Scholar] [CrossRef]
- Pérez-Alonso, F.; Adán, C.; Rojas, S.; Peña, M.A.; Jose, F. Ni/Fe electrodes prepared by electrodeposition method over different substrates for oxygen evolution reaction in alkaline medium. Int. J. Hydrogen Energy 2014, 39, 5204–5212. [Google Scholar] [CrossRef]
- Yang, Y.; Dang, L.; Shearer, M.; Sheng, H.; Li, W.; Chen, J.; Xiao, P.; Zhang, Y.; Hamers, R.J.; Jin, S. Highly Active Trimetallic NiFeCr Layered Double Hydroxide Electrocatalysts for Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1703189. [Google Scholar] [CrossRef]
- Friebel, D.; Louie, M.W.; Bajdich, M.; Sanwald, K.E.; Cai, Y.; Wise, A.M.; Cheng, M.J.; Sokaras, D.; Weng, T.C.; Alonso-Mori, R.; et al. Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 2015, 137, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Landon, J.; Demeter, E.; Inoǧlu, N.; Keturakis, C.; Wachs, I.E.; Vasić, R.; Frenkel, A.I.; Kitchin, J.R. Spectroscopic characterization of mixed Fe-Ni oxide electrocatalysts for the oxygen evolution reaction in alkaline electrolytes. ACS Catal. 2012, 2, 1793–1801. [Google Scholar] [CrossRef]
- Fominykh, K.; Chernev, P.; Zaharieva, I.; Sicklinger, J.; Stefanic, G.; Döblinger, M.; Müller, A.; Pokharel, A.; Böcklein, S.; Scheu, C.; et al. Iron-doped nickel oxide nanocrystals as highly efficient electrocatalysts for alkaline water splitting. ACS Nano 2015, 9, 5180–5188. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, D.; Ling, T.; Vasileff, A.; Qiao, S.Z. S-NiFe2O4 ultra-small nanoparticle built nanosheets for efficient water splitting in alkaline and neutral pH. Nano Energy 2017, 40, 264–273. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Moukheiber, E.; Merlo, L.; Aricò, A.S. Performance of a PEM water electrolyser combining an IrRu-oxide anode electrocatalyst and a shortside chain Aquivion membrane. Int. J. Hydrogen Energy 2015, 40, 14430–14435. [Google Scholar] [CrossRef]
- Faro, M.L.; la Rosa, D.; Nicotera, I.; Antonucci, V.; Aricò, A.S. Electrochemical investigation of a propane-fed solid oxide fuel cell based on a composite Ni–perovskite anode catalyst. Appl. Catal. B Environ. 2009, 89, 49–57. [Google Scholar] [CrossRef]
- Junga, J.-Y.; Yua, J.-Y.; Shindea, S.S.; Kima, S.-H.; Kima, D.-H.; Lina, C.; Wehrspohnb, R.B.; Lee, J.-H. Remarkable improvements in the performance and stability of Si photoanodes adopting nanocrystalline NiOx electrocatalyst and stoichiometric SiO2 protection. Appl. Surface Sci. 2019, 493, 1150–1158. [Google Scholar] [CrossRef]
- Zhang, C.; Gong, N.; Ding, C.; Li, Y.; Peng, W.; Zhang, G.; Zhang, F.; Fan, X. Plasma-assisted synthesis of three-dimensional hierarchical NiFeOx/NiFeP electrocatalyst for highly enhanced water oxidation in alkaline media. Int. J. Hydrogen Energy 2019, 44, 26118–26127. [Google Scholar] [CrossRef]
- Tahira, A.; Ibupoto, Z.H.; Willander, M.; Nur, O. Advanced Co3O4–CuO nano-composite based electrocatalyst for efficient hydrogen evolution reaction in alkaline media. Int. J. Hydrogen Energy 2019, 44, 26148–26157. [Google Scholar] [CrossRef]
- Guo, X.; Diao, P.; Xu, D.; Huang, S.; Yang, Y.; Jin, T.; Wu, Q.; Xiang, M.; Zhang, M. CuO/Pd composite photocathodes for photoelectrochemical hydrogen evolution reaction. Int. J. Hydrogen Energy 2014, 39, 7686–7696. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Wu, Q.; Diao, P. Cu2O/CuO Bilayered Composite as a High-Efficiency Photocathode for Photoelectrochemical Hydrogen Evolution Reaction. Sci. Rep. 2016, 6, 35158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubair, M.; Svenum, I.-H.; Rønning, M.; Yang, J. Core-Shell Nanostructures of Graphene-Wrapped CdS Nanoparticles and TiO2 (CdS@G@TiO2): The Role of Graphene in Enhanced Photocatalytic H2 Generation. Catalysts 2020, 10, 358. [Google Scholar] [CrossRef] [Green Version]
- Cots, A.; Cibrev, D.; Bonete, P.; Gomez, R. Hematite nanorod electrodes modified with molybdenum: Photoelectrochemical studies. ChemElectroChem 2017, 4, 585–593. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Song, G.; Kim, C.W.; Kang, Y.S. Facile preparation of p-CuO and p-CuO/n-CuWO4 junction thin films and their photoelectrochemical properties. Electrochim. Acta 2012, 69, 340–344. [Google Scholar] [CrossRef]
- Aricò, A.S.; Gullo, L.R.; la Rosa, D.; Siracusano, S.; Tavares, A.B.L.C.; Xicola, A.S. Solid Oxide Fuel Cell with Cermet Cu/Ni Alloy Anode. WO 2004/049491, 10 June 2004. [Google Scholar]
- Marshall, A.; Børresen, B.; Hagen, G.; Tsypkin, M.; Tunold, R. Preparation and characterisation of nanocrystalline IrxSn1−xO2 electrocatalytic powders. MatChemPhys 2005, 94, 226–232. [Google Scholar] [CrossRef]
- Carbone, A.; Gatto, S.C.Z.I.; Trocino, S.; Aricò, A.S. Assessment of the FAA3-50 polymer electrolyte in combination with a NiMn2O4 anode catalyst for anion exchange membrane water electrolysis. Int. J. Hydrogen Energy 2020, 45, 9285–9292. [Google Scholar] [CrossRef]
- Tsotridis, G.; Pilenga, A. JRC Technical Reports “EU Harmonised Terminology for Low-Temperature Water Electrolysis for Energy-Storage Applications”; Publications Office of the European Union: Brussels, Belgium, 2018; pp. 1–90. [Google Scholar] [CrossRef]
- Chen, Z.; Dinh, H.N.; Miller, E. Photoelectrochemical Water Splitting Standards, Experimental Methods and Protocols; Springer: New York, NY, USA, 2013. [Google Scholar]


| Photoanode | co-Catalysts | Ionomer | Membrane | Photocathode | Ionomer |
|---|---|---|---|---|---|
| FTO/Fe2O3 + P + Sn | LSFCO | FAA3 ION | FAA3-50 | FTO/CuO | FAA3 ION |
| FTO/Fe2O3 + P + Sn | NiFeOx | FAA3 ION | FAA3-50 | FTO/CuO | FAA3 ION |
| FTO/Fe2O3 + P + Sn | IrRuOx | FAA3 ION | FAA3-50 | FTO/CuO | FAA3 ION |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Vecchio, C.; Trocino, S.; Campagna Zignani, S.; Baglio, V.; Carbone, A.; Díez García, M.I.; Contreras, M.; Gómez, R.; Aricò, A.S. Enhanced Photoelectrochemical Water Splitting at Hematite Photoanodes by Effect of a NiFe-Oxide co-Catalyst. Catalysts 2020, 10, 525. https://doi.org/10.3390/catal10050525
Lo Vecchio C, Trocino S, Campagna Zignani S, Baglio V, Carbone A, Díez García MI, Contreras M, Gómez R, Aricò AS. Enhanced Photoelectrochemical Water Splitting at Hematite Photoanodes by Effect of a NiFe-Oxide co-Catalyst. Catalysts. 2020; 10(5):525. https://doi.org/10.3390/catal10050525
Chicago/Turabian StyleLo Vecchio, Carmelo, Stefano Trocino, Sabrina Campagna Zignani, Vincenzo Baglio, Alessandra Carbone, María Isabel Díez García, Maxime Contreras, Roberto Gómez, and Antonino Salvatore Aricò. 2020. "Enhanced Photoelectrochemical Water Splitting at Hematite Photoanodes by Effect of a NiFe-Oxide co-Catalyst" Catalysts 10, no. 5: 525. https://doi.org/10.3390/catal10050525
APA StyleLo Vecchio, C., Trocino, S., Campagna Zignani, S., Baglio, V., Carbone, A., Díez García, M. I., Contreras, M., Gómez, R., & Aricò, A. S. (2020). Enhanced Photoelectrochemical Water Splitting at Hematite Photoanodes by Effect of a NiFe-Oxide co-Catalyst. Catalysts, 10(5), 525. https://doi.org/10.3390/catal10050525











