The Impact of Reduction Temperature and Nanoparticles Size on the Catalytic Activity of Cobalt-Containing BEA Zeolite in Fischer–Tropsch Synthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Textural Properties of C-Co5.0SiBEA and C-Co5.0AlBEA Samples
2.2. Prove of Co Incorporation in the HAlBEA and SiBEA Zeolite Structure
2.3. The Reducibility of Co Present in C-Co5.0SiBEA and C-Co5.0AlBEA
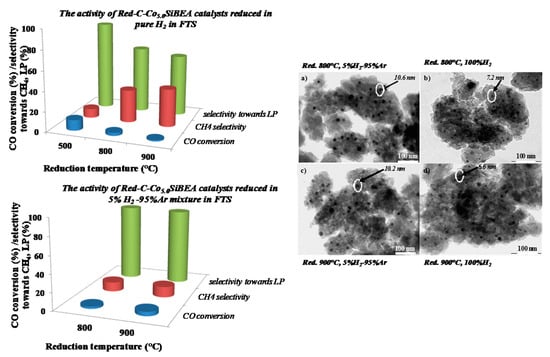
2.4. The Cobalt Nanoparticles Distribution in Red-C-Co5.0SiBEA after Reduction in Different Conditions
2.5. The Acidity of C-CoxSiBEA and C-CoxAlBEA
2.6. The Catalytic Activity of Red-C-Co5.0AlBEA and Red-C-Co5.0SiBEA
3. Materials and Methods
3.1. Samples Preparation
3.2. Samples Characterization
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chalupka, K.; Casale, S.; Żurawicz, E.; Rynkowski, J.; Dzwigaj, S. The remarkable effect of the preparation procedure on the catalytic activity of CoBEA zeolites in the Fischer–Tropsch synthesis. Microporous Mesoporous Mater. 2015, 211, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Tsakoumis, N.E.; Rønning, M.; Borg, Ø.; Rytter, E.; Holmen, A. Deactivation of cobalt based Fischer–Tropsch catalysts: A review. Catal. Today 2010, 154, 162–182. [Google Scholar] [CrossRef]
- Weststrate, C.; Van De Loosdrecht, J.; Niemantsverdriet, J.W. Spectroscopic insights into cobalt-catalyzed Fischer-Tropsch synthesis: A review of the carbon monoxide interaction with single crystalline surfaces of cobalt. J. Catal. 2016, 342, 1–16. [Google Scholar] [CrossRef]
- Concepción, P.; López, C.; Martínez, A.; Puntes, V. Characterization and catalytic properties of cobalt supported on delaminated ITQ-6 and ITQ-2 zeolites for the Fischer–Tropsch synthesis reaction. J. Catal. 2004, 228, 321–332. [Google Scholar] [CrossRef]
- Pour, A.N.; Zamani, Y.; Tavasoli, A.; Shahri, S.M.K.; Taheri, S.A. Study on products distribution of iron and iron–zeolite catalysts in Fischer–Tropsch synthesis. Fuel 2008, 87, 2004–2012. [Google Scholar] [CrossRef]
- Martínez, A.; Lopez, C. The influence of ZSM-5 zeolite composition and crystal size on the in situ conversion of Fischer–Tropsch products over hybrid catalysts. Appl. Catal. A Gen. 2005, 294, 251–259. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, Y.; Zhang, Q.; Wan, H. Preparation of metallic cobalt inside NaY zeolite with high catalytic activity in Fischer–Tropsch synthesis. Catal. Commun. 2003, 4, 253–258. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Li, X.-H.; Asami, K.; Fujimoto, K. Iso-paraffins synthesis from modified Fischer–Tropsch reaction—Insights into Pd/beta and Pt/beta catalysts. Catal. Today 2005, 104, 41–47. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Meng, M.; Yoneyama, Y.; Tsubaki, N. One-step synthesis of H–β zeolite-enwrapped Co/Al2O3 Fischer–Tropsch catalyst with high spatial selectivity. J. Catal. 2009, 265, 26–34. [Google Scholar] [CrossRef]
- Bessell, S. Investigation of bifunctional zeolite supported cobalt Fischer-Tropsch catalysts. Appl. Catal. A: Gen. 1995, 126, 235–244. [Google Scholar] [CrossRef]
- Sadek, R.; Chalupka, K.; Mierczynski, P.; Rynkowski, J.; Gurgul, J.; Dzwigaj, S. Cobalt Based Catalysts Supported on Two Kinds of Beta Zeolite for Application in Fischer-Tropsch Synthesis. Catal. 2019, 9, 497. [Google Scholar] [CrossRef] [Green Version]
- Roberge, D.M.; Hausmann, H.; Holderich, W.F. Dealumination of zeolite beta by acid leaching: A new insight with two-dimensional multi-quantum and cross polarization 27Al MAS NMR. Phys. Chem. Chem. Phys. 2002, 4, 3128–3135. [Google Scholar] [CrossRef]
- Bernasconi, S.; Van Bokhoven, J.A.; Krumeich, F.; Pirngruber, G.D.; Prins, R. Formation of mesopores in zeolite beta by steaming: A secondary pore channel system in the plane. Microporous Mesoporous Mater. 2003, 66, 21–26. [Google Scholar] [CrossRef]
- Lenarda, M.; Da Ros, M.; Casagrande, M.; Storaro, L.; Ganzerla, R. Post-synthetic thermal and chemical treatments of H-BEA zeolite: Effects on the catalytic activity. Inorganica Chim. Acta 2003, 349, 195–202. [Google Scholar] [CrossRef]
- Marques, J.; Gener, I.; Ayrault, P.; Bordado, J.; Lopes, J.; Ribeiro, F.R.; Guisnet, M. Infrared spectroscopic study of the acid properties of dealuminated BEA zeolites. Microporous Mesoporous Mater. 2003, 60, 251–262. [Google Scholar] [CrossRef]
- Omegna, A.; Vasic, M.; Van Bokhoven, J.A.; Pirngruber, G.D.; Prins, R. Dealumination and realumination of microcrystalline zeolite beta: An XRD, FTIR and quantitative multinuclear (MQ) MAS NMR study. Phys. Chem. Chem. Phys. 2004, 6, 447. [Google Scholar] [CrossRef]
- Oumi, Y.; Mizuno, R.; Azuma, K.; Nawata, S.; Fukushima, T.; Uozumi, T.; Sano, T. Riversibility of dealumination-realumination process of BEA zeolite. Microporous Mesoporous Mater. 2001, 49, 103–109. [Google Scholar] [CrossRef]
- Hajjar, R.; Millot, Y.; Man, P.P.; Che, M.; Dzwigaj, S. Two Kinds of Framework Al Sites Studied in BEA Zeolite by X-ray Diffraction, Fourier Transform Infrared Spectroscopy, NMR Techniques, and V Probe. J. Phys. Chem. C 2008, 112, 20167–20175. [Google Scholar] [CrossRef]
- Janas, J.; Machej, T.; Gurgul, J.; Socha, R.; Che, M.; Dzwigaj, S. Effect of Co content on the catalytic activity of CoSiBEA zeolite in the selective catalytic reduction of NO with ethanol: Nature of the cobalt species. Appl. Catal. B Environ. 2007, 75, 239–248. [Google Scholar] [CrossRef]
- Janas, J.; Shishido, T.; Che, M.; Dzwigaj, S. Role of tetrahedral Co(II) sites of CoSiBEA zeolite in the selective catalytic reduction of NO: XRD, UV–vis, XAS and catalysis study. Appl. Catal. B Environ. 2009, 89, 196–203. [Google Scholar] [CrossRef]
- Camiloti, A.; Jahn, S.; Velasco, N.; Moura, L.; Cardoso, D. Acidity of Beta zeolite determined by TPD of ammonia and ethylbenzene disproportionation. Appl. Catal. A Gen. 1999, 182, 107–113. [Google Scholar] [CrossRef]
- Camblor, M.; Corma, A.; Pérez-Pariente, J. Synthesis of titanoaluminosilicates isomorphous to zeolite Beta, active as oxidation catalysts. Zeolites 1993, 13, 82–87. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Che, M. Incorporation of Co(II) in Dealuminated BEA Zeolite at Lattice Tetrahedral Sites Evidenced by XRD, FTIR, Diffuse Reflectance UV−Vis, EPR, and TPR. J. Phys. Chem. B 2006, 110, 12490–12493. [Google Scholar] [CrossRef] [PubMed]
- Chalupka, K.; Maniukiewicz, W.; Mierczynski, P.; Maniecki, T.; Rynkowski, J.; Dzwigaj, S. The catalytic activity of Fe-containing SiBEA zeolites in Fischer–Tropsch synthesis. Catal. Today 2015, 257, 117–121. [Google Scholar] [CrossRef]
- Xiao, F.-S.; Wang, L.; Yin, C.; Lin, K.; Di, Y.; Li, J.; Xu, R.; Su, D.S.; Schlögl, R.; Yokoi, T.; et al. Catalytic Properties of Hierarchical Mesoporous Zeolites Templated with a Mixture of Small Organic Ammonium Salts and Mesoscale Cationic Polymers. Angew. Chem. Int. Ed. 2006, 45, 3090–3093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savost’Yanov, A.P.; Narochnyi, G.B.; Yakovenko, R.E.; Saliev, A.N.; Sulima, S.I.; Zubkov, I.; Nekroenko, S.V.; Mitchenko, S. Synthesis of Low-Pour-Point Diesel Fuel in the Presence of a Composite Cobalt-Containing Catalyst. Pet. Chem. 2017, 57, 1186–1189. [Google Scholar] [CrossRef]
- Sadek, R.; Chalupka, K.; Mierczynski, P.; Rynkowski, J.; Millot, Y.; Valentin, L.; Casale, S.; Dzwigaj, S. Fischer-Tropsch reaction on Co-containing microporous and mesoporous Beta zeolite catalysts: The effect of porous size and acidity. Catal. Today 2019. [Google Scholar] [CrossRef]
- Emeis, C. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, Y.-J.; Karandikar, P.R.; Jun, K.-W.; Ha, K.-S.; Park, H.-G. Fischer–Tropsch catalysts deposited with size-controlled Co3O4 nanocrystals: Effect of Co particle size on catalytic activity and stability. Appl. Catal. A: Gen. 2012, 411, 15–23. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, J.; Wang, Y. Development of Novel Catalysts for Fischer-Tropsch Synthesis: Tuning the Product Selectivity. ChemCatChem 2010, 2, 1030–1058. [Google Scholar] [CrossRef]
- Li, H.; Hou, B.; Wang, J.; Qin, C.; Zhong, M.; Huang, X.; Jia, L.; Li, D. Direct conversion of syngas to isoparaffins over hierarchical beta zeolite supported cobalt catalyst for Fischer-Tropsch synthesis. Mol. Catal. 2018, 459, 106–112. [Google Scholar] [CrossRef]











| Sample | SSA (m2 g−1) | PV (cm3 g−1) |
|---|---|---|
| HAlBEA | 610 | 0.22 |
| C-Co5.0AlBEA | 535 | 0.22 |
| SiBEA | 600 | 0.21 |
| C-Co5.0SiBEA | 496 | 0.21 |
| Sample | C-Co5.0AlBEA | C-Co5.0SiBEA |
|---|---|---|
| Ions: | The Normalized Ions Intensities | |
| Al+/total Ion | 3.3 × 10−2 | 1.9 × 10−3 |
| Si+/total Ion | 0.11 | 0.2 |
| Al+/Si+ | 0.32 | 9.7 × 10−3 |
| SiOAl+/total Ion | 4.7 × 10−4 | - |
| CoAlO2H+/total Ion | 2.7 × 10−4 | - |
| CoAl2O4H+/total Ion | 1.1 × 10−4 | - |
| CoSiO+/total Ion | 1.7 × 10−3 | 9.0 × 10−4 |
| CoSiOH+/total Ion | 3.3 × 10−4 | 1.7 × 10−4 |
| CoSiO2H+/total Ion | 7.8 × 10−4 | 3.9 × 10−4 |
| Sample | Amount of NH3 Adsorbed (μmol g−1) |
|---|---|
| C-Co5.0AlBEA | 3000 |
| Red-C-Co5.0AlBEA | 32 |
| C-Co5.0SiBEA | 1740 |
| Red-C-Co5.0SiBEA | 13 |
| Sample | B Acidic Sites (μmol g−1) a | L Acidic Sites (μmol g−1) |
|---|---|---|
| HAlBEA | 353 | 158 |
| C-Co5.0HAlBEA | 85 | 439 |
| SiBEA | 0 | 0 |
| C-Co5.0SiBEA | 0 | 259 |
| Zeolite Catalysts | Red-C-Co5.0AlBEA Red. 500 °C, 100% H2 | Red-C-Co5.0SiBEA Red. 500°C, 100% H2 | Red-C-Co5.0SiBEA Red. 800 Red. 800°C 5% H2-95% Ar | Red-C-Co5.0SiBEA Red. 800 °C Red. 800 °C 100% H2 | Red-C-Co5.0SiBEA Red. 900 °C 5% H2-95% Ar | Red-C-Co5.0SiBEA Red. 900 °C, 100% H2 |
|---|---|---|---|---|---|---|
| Reaction time (h) | 19 | 20 | 16 | 20 | 22 | 20 |
| CO conversion (%) | 0.64 | 10.72 | 2.56 | 3.12 | 4.88 | 1.3 |
| Selectivity to CH4 (%) | 0.61 | 9.30 | 10.89 | 33.05 | 12.27 | 38.09 |
| Selectivity to CO2 (%) | 0 | 0 | 0 | 0 | 0.09 | 0 |
| Selectivity to LP (%) | 0 | 90.70 | 89.11 | 66.95 | 87.64 | 61.91 |
| Iso-/n-alkanes ratio | - | 0.25 (C7–C16) | - | 0.39 (C10–C25) | 0.47 (C11–C18) | 0.95 (C10–C16) |
| Olefins/n-alkanes ratio | - | 0.03 | - | - | - | - |
| Average nanoparticles size (nm) | 27.2 | 6.3 | 10.6 | 7.2 | 10.2 | 6.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalupka, K.A.; Grams, J.; Mierczynski, P.; Szynkowska, M.I.; Rynkowski, J.; Onfroy, T.; Casale, S.; Dzwigaj, S. The Impact of Reduction Temperature and Nanoparticles Size on the Catalytic Activity of Cobalt-Containing BEA Zeolite in Fischer–Tropsch Synthesis. Catalysts 2020, 10, 553. https://doi.org/10.3390/catal10050553
Chalupka KA, Grams J, Mierczynski P, Szynkowska MI, Rynkowski J, Onfroy T, Casale S, Dzwigaj S. The Impact of Reduction Temperature and Nanoparticles Size on the Catalytic Activity of Cobalt-Containing BEA Zeolite in Fischer–Tropsch Synthesis. Catalysts. 2020; 10(5):553. https://doi.org/10.3390/catal10050553
Chicago/Turabian StyleChalupka, Karolina A., Jacek Grams, Pawel Mierczynski, Malgorzata I. Szynkowska, Jacek Rynkowski, Thomas Onfroy, Sandra Casale, and Stanislaw Dzwigaj. 2020. "The Impact of Reduction Temperature and Nanoparticles Size on the Catalytic Activity of Cobalt-Containing BEA Zeolite in Fischer–Tropsch Synthesis" Catalysts 10, no. 5: 553. https://doi.org/10.3390/catal10050553
APA StyleChalupka, K. A., Grams, J., Mierczynski, P., Szynkowska, M. I., Rynkowski, J., Onfroy, T., Casale, S., & Dzwigaj, S. (2020). The Impact of Reduction Temperature and Nanoparticles Size on the Catalytic Activity of Cobalt-Containing BEA Zeolite in Fischer–Tropsch Synthesis. Catalysts, 10(5), 553. https://doi.org/10.3390/catal10050553