Critical Issues and Guidelines to Improve the Performance of Photocatalytic Polymeric Membranes
Abstract
1. Introduction
2. Materials in Polymeric Photocatalytic Membranes
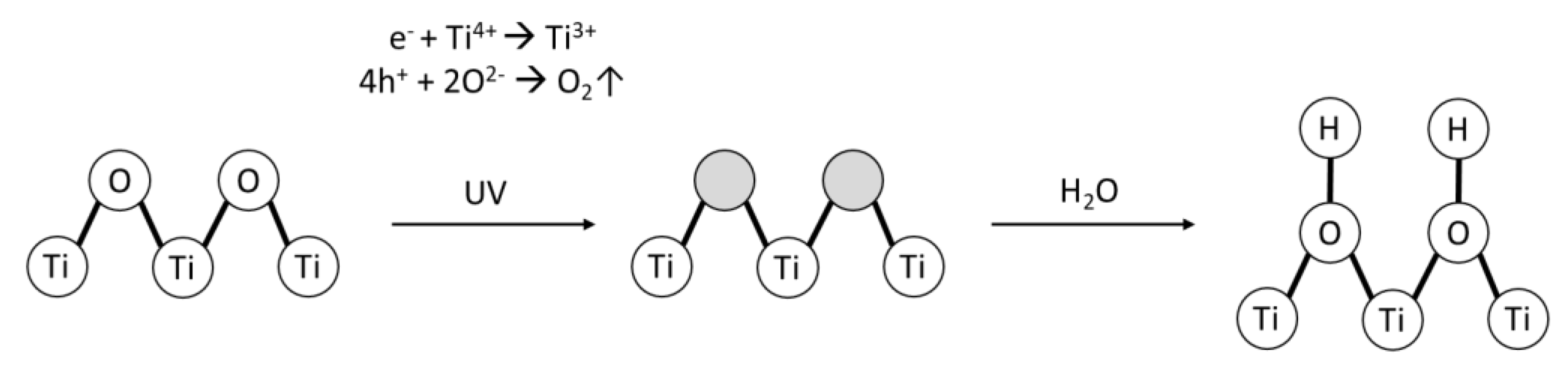
2.1. Nano-Photocatalyst
2.2. Polymer
- Fluorine-based: polyvinylidenefluoride (PVDF), poly(vinylidenefluoride–trifluoroethylene) P(VDF-TrFE), polytetrafluoroethylene (PTFE), and a copolymer of PVDF and hexafluoropropylene (PVDF-HFP)
- Sulfur-based: polysulfone (PSF), and polyethersulfone (PES)
- Nitrogen-based: polyacrylonitrile (PAN), polyethylenimine (PEI), polyamide (PA), and polyamide 6 (PA6)
- Cellulose derivatives: cellulose acetate (CA), and mixed cellulose esters (MCE)
- Other polymer: polycarbonate (PC)
3. Membrane Synthesis Method
3.1. Mixed Matrix Composite Membranes Synthesis Methods
3.1.1. Phase Inversion
3.1.2. Electrospinning
3.2. Thin Film Composite Synthesis Methods
4. Membrane Functionality
4.1. Filtration Performance
4.1.1. Hydraulic Permeability
4.1.2. Antifouling Properties
4.2. Photocatalytic Activity
4.2.1. Comparison between Suspended and Membrane Immobilized Systems
4.2.2. Influence of the Synthesis Method on the Membrane Photocatalytic Performance
4.2.3. Membrane Aging
Polymer Stability
Photocatalyst Detachment or Leaching
5. Guidelines and Recommendations for Researchers
- If a membrane with high filtration and antifouling performance is sought, MMM synthesized by phase inversion is recommended, and particular attention should be paid to the solution rheology to obtain maximum porosity and permeability. The photocatalyst concentration when the rheological change of the polymer solution occurs should be found in the range of 0.2–0.5 wt. % for MMM, albeit each system should be studied in detail. Furthermore, TiO2-based composites of metal or carbon based materials are recommended.
- If high pollutant rejection is desired, either MMM can be produced with techniques to slow down phase inversion or TFCM techniques, as these would reduce the mean pore size and surface porosity. Among TFCM deposition techniques, vacuum filtration is the least recommended method as it produces the lowest performance in the deposition of the photocatalyst and the least photocatalytic activities.
- The influence of the lamp irradiance is crucial on photocatalytic membrane systems, in particular on the effects on membrane aging. In the case of photocatalytic membranes, UV light sources of low power (less than 50 W) or light-emitting diodes (LEDs) are highly recommended to reduce polymer aging and thus, ensuring long-term stability of the membrane.
- Long-term stability is a key issue for process scalability. Therefore, high attention should be paid on this aspect during the membrane viability study. Performing (i) long-term experiments to verify that the membrane maintains its integrity under the experimental conditions, and (ii) reuse experiments to ensure good stability of the photocatalyst is therefore encouraged. It should be considered the effect of elevated heat, UV irradiation and moisture which can lead to polymer degradation.
- The use of analytical techniques such as EDX, XRD, AFM, and SEM or FESEM, before and after the UV exposure, are highly recommended to analyze the aging of the membrane. The quantification of the leaching of nanoparticles from the membrane to the medium should be analyzed with ICP.
- High efforts are being adopted on synthesizing novel and more active photocatalysts. Their physico-chemical characteristics can be notably different from conventional semiconductors such as commercial TiO2. Nanoparticle dispersions in polymeric matrix present different rheology so the membrane processing can suffer significant changes. These changes could play a key role on the improvement of nanoparticles dispersibility on the polymer matrix so far encountered on MMMs.
- When the membrane has a simultaneous filtration and photocatalytic function, experimental reactors should integrate both filtration and photocatalytic degradation. Membrane functionality characterization should consider: (i) pure water flux test to obtain permeability values (L/hm2bar); (ii) photocatalytic degradation comparison with the suspended system to evaluate the change in the photocatalytic activity when the nanoparticles are immobilized, and (iii) pollutant rejection under dark and UV irradiation conditions.
- In the particular case of TFCM synthesis, the influence of the photocatalyst concentration using different coating techniques has not been sufficiently evaluated. There is expected to be an optimal concentration to maintain the balance between the membranes photocatalytic and filtration functions. While, as aforementioned, in MMMs the recommended concentration range is 0.2–0.5 wt. %, in TFMC this evaluation has not yet been assessed and should also be addressed.
- It is noteworthy that most of the works studied in this review analyze the photocatalytic activity using dyes as model organic pollutants. These types of molecules are photosensitive and can be adsorbed on the catalysts and/or on the membranes leading to results that are not representative of other pollutants. Therefore, it is advisable to select other types of organic model compounds, such as, acetic acid, or certain non-biologically degradable organic compounds as those contained in hospital effluents, such as antibiotics, (cautiously) organohalogens, etc., to generalize the obtained conclusions. In this regard, considering the important presence of persistent compounds, antibiotics and disinfectants likely causing bacterial inhibition in the on-site hospital wastewater treatment, the use of photocatalytic membrane reactors for the on-site treatment of hospital wastewaters is envisaged as a promising alternative.
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Secretary-General of the United Nations. The Stockholm Convention on Persistant Organic Pollutants. 2009. Available online: http://chm.pops.int/TheConvention/Overview/TextoftheConvention/tabid/2232/ (accessed on 11 June 2019).
- Dwivedi, A.H.; Pande, U.C. Photochemical Degradation of Halogenated Compounds: A Review. Sci. Rev. Chem. Commun. 2012, 2, 41–65. [Google Scholar]
- Lee, S.Y.; Park, S.J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.-P.; Shi, L.; Cheng, R.; Yuan, D.-H.; Zheng, X.; Shen, Z.-P.; Shi, L.; Cheng, R.; Yuan, D.-H. Photocatalytic Membrane Reactors (PMRs) in Water Treatment: Configurations and Influencing Factors. Catalysts 2017, 7, 224. [Google Scholar] [CrossRef]
- Iglesias, O.; Rivero, M.J.; Urtiaga, A.M.; Ortiz, I. Membrane-based photocatalytic systems for process intensification. Chem. Eng. J. 2016, 305, 136–148. [Google Scholar] [CrossRef]
- Turan, N.B.; Erkan, S.; Engin, G.O.; Bilgili, S. Nanoparticles in the aquatic environment: Usage, properties, transformation and toxicity—A review. Process Saf. Environ. Prot. 2019, 130, 238–249. [Google Scholar] [CrossRef]
- Kumari, P.; Bahadur, N.; Dumée, L.F. Photo-catalytic membrane reactors for the remediation of persistent organic pollutants—A review. Sep. Purif. Technol. 2020, 230, 115878. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Janssens, R.; Mandal, M.K.; Dubey, K.K.; Luis, P. Slurry photocatalytic membrane reactor technology for removal of pharmaceutical compounds from wastewater: Towards cytostatic drug elimination. Sci. Total Environ. 2017, 599–600, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Configurations and Membranes of Photocatalytic Membrane Reactors for Water and Wastewater Treatment. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 208. [Google Scholar]
- Qing, W.; Li, X.; Shao, S.; Shi, X.; Wang, J.; Feng, Y.; Zhang, W.; Zhang, W. Polymeric catalytically active membranes for reaction-separation coupling: A review. J. Memb. Sci. 2019, 583, 118–138. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Memb. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Zeng, G.; Cheng, W.; Hu, J. Photocatalytic membrane in water purification: Is it stepping closer to be driven by visible light? J. Memb. Sci. 2019, 584, 364–392. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.J. An overview of TiO2-based photocatalytic membrane reactors for water and wastewater treatments. J. Ind. Eng. Chem. 2020. [Google Scholar] [CrossRef]
- Méricq, J.-P.; Mendret, J.; Brosillon, S.; Faur, C. High performance PVDF-TiO2 membranes for water treatment. Chem. Eng. Sci. 2015, 123, 283–291. [Google Scholar] [CrossRef]
- Tran, D.T.; Mendret, J.; Méricq, J.P.; Faur, C.; Brosillon, S. Study of permeate flux behavior during photo-filtration using photocatalytic composite membranes. Chem. Eng. Process.-Process Intensif. 2020, 148, 107781. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, Z.; Pan, Y.; Zeng, G.; Shi, H.; Yang, X.; Li, F.; Yang, S.; He, Y. Enhancing the photocatalytic and antibacterial property of polyvinylidene fluoride membrane by blending Ag–TiO2 nanocomposites. J. Mater. Sci. Mater. Electron. 2017, 28, 3865–3874. [Google Scholar] [CrossRef]
- Wang, M.; Qu, F.; Jia, R.; Sun, S.; Li, G.; Liang, H. Preliminary study on the removal of steroidal estrogens using TiO2 -doped PVDF ultrafiltration membranes. Water 2016, 8, 134. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumaran, S.; Jegatheesan, V. Polyvinylidene Fluoride and Titanium Dioxide Ultrafiltration Photocatalytic Membrane: Fabrication, Morphology, and Its Application in Textile Wastewater Treatment. J. Environ. Eng. 2020, 146, 04020053. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, T.; Shi, J.; Teng, K.; Wang, W.; Ma, M.; Li, J.; Qian, X.; Li, C.; Fan, J. Photocatalytic antifouling PVDF ultrafiltration membranes based on synergy of graphene oxide and TiO2 for water treatment. J. Memb. Sci. 2016, 520, 281–293. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Shan, M.; Zhou, B.; Li, Y.; Li, B.; Niu, J.; Qian, X. Synergetic effects of oxidized carbon nanotubes and graphene oxide on fouling control and anti-fouling mechanism of polyvinylidene fluoride ultrafiltration membranes. J. Memb. Sci. 2013, 448, 81–92. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Zhang, Y.; Zhao, S. Comparing the antifouling effects of activated carbon and TiO2 in ultrafiltration membrane development. J. Colloid Interface Sci. 2018, 515, 109–118. [Google Scholar] [CrossRef]
- Song, H.; Shao, J.; He, Y.; Liu, B.; Zhong, X. Natural organic matter removal and flux decline with PEG–TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. J. Memb. Sci. 2012, 405–406, 48–56. [Google Scholar] [CrossRef]
- Song, H.; Shao, J.; Wang, J.; Zhong, X. The removal of natural organic matter with LiCl–TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. Desalination 2014, 344, 412–421. [Google Scholar] [CrossRef]
- Benhabiles, O.; Galiano, F.; Marino, T.; Mahmoudi, H.; Lounici, H.; Figoli, A. Preparation and Characterization of TiO2-PVDF/PMMA Blend Membranes Using an Alternative Non-Toxic Solvent for UF/MF and Photocatalytic Application. Molecules 2019, 24, 724. [Google Scholar] [CrossRef] [PubMed]
- Damodar, R.A.; You, S.-J.; Chou, H.-H. Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes. J. Hazard. Mater. 2009, 172, 1321–1328. [Google Scholar] [CrossRef]
- Mungondori, H.H.; Tichagwa, L.; Katwire, D.M.; Aoyi, O. Preparation of photo-catalytic copolymer grafted asymmetric membranes (N-TiO2-PMAA-g-PVDF/PAN) and their application on the degradation of bentazon in water. Iran. Polym. J. 2016, 25, 135–144. [Google Scholar] [CrossRef]
- Jyothi, M.S.; Nayak, V.; Padaki, M.; Balakrishna, R.G.; Soontarapa, K. Eco-friendly membrane process and product development for complete elimination of chromium toxicity in wastewater. J. Hazard. Mater. 2017, 332, 112–123. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Wang, P.; Zheng, Q.; Li, J. The influence of nano-sized TiO2 fillers on the morphologies and properties of PSF UF membrane. J. Memb. Sci. 2007, 288, 231–238. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, C.; Zhang, G.; Hu, L.; Wang, P. Photocatalytic Fe-doped TiO2/PSF composite UF membranes: Characterization and performance on BPA removal under visible-light irradiation. Chem. Eng. J. 2017, 319, 39–47. [Google Scholar] [CrossRef]
- El-Aassar, A.; Hameed, M.; Isawi, H.; El-Noss, M.; El-Kholy, R.A.; Said, M.M.; Shawky, H.A. Design and fabrication of continuous flow photoreactor using semiconductor oxides for degradation of organic pollutants. J. Water Process Eng. 2019, 32, 100922. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Khumalo, N.; Dlamini, D.; Mamba, B.B. Polysulfone/N,Pd co-doped TiO2 composite membranes for photocatalytic dye degradation. Sep. Purif. Technol. 2018, 191, 122–133. [Google Scholar] [CrossRef]
- Xu, H.; Ding, M.; Chen, W.; Li, Y.; Wang, K. Nitrogen–doped GO/TiO2 nanocomposite ultrafiltration membranes for improved photocatalytic performance. Sep. Purif. Technol. 2018, 195, 70–82. [Google Scholar] [CrossRef]
- Yu, S.; Wang, Y.; Sun, F.; Wang, R.; Zhou, Y. Novel mpg-C3N4/TiO2 nanocomposite photocatalytic membrane reactor for sulfamethoxazole photodegradation. Chem. Eng. J. 2018, 337, 183–192. [Google Scholar] [CrossRef]
- Muhulet, A.; Tuncel, C.; Miculescu, F.; Pandele, A.M.; Bobirica, C.; Orbeci, C.; Bobirica, L.; Palla-Papavlu, A.; Voicu, S.I. Synthesis and characterization of polysulfone–TiO2 decorated MWCNT composite membranes by sonochemical method. Appl. Phys. A Mater. Sci. Process. 2020, 126, 233. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Zhang, R.; Luis, P.; Arsuaga, J.M.; Kim, J.; Van der Bruggen, B. Effect of nanoparticle aggregation at low concentrations of TiO2 on the hydrophilicity, morphology, and fouling resistance of PES–TiO2 membranes. J. Colloid Interface Sci. 2011, 363, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.N.; Pirzaman, A.K.; Aroon, M.A.; Pirbazari, A.E. Photocatalytic degradation of 2,4-dichlorophenol by Co-doped TiO2 (Co/TiO2) nanoparticles and Co/TiO2 containing mixed matrix membranes. J. Water Process Eng. 2017, 17, 124–134. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 2016, 393, 65–78. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.; Zinadini, S.; Feyzi, M.; Bahnemann, D.W. Preparation and characterization of a novel photocatalytic self-cleaning PES nanofiltration membrane by embedding a visible-driven photocatalyst boron doped-TiO2SiO2/CoFe2O4 nanoparticles. Sep. Purif. Technol. 2019, 209, 764–775. [Google Scholar] [CrossRef]
- Salim, N.E.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Yusof, N.; Qtaishat, M.; Matsuura, T.; Aziz, F.; Salleh, W.N.W. Preparation and characterization of hydrophilic surface modifier macromolecule modified poly (ether sulfone) photocatalytic membrane for phenol removal. Chem. Eng. J. 2018, 335, 236–247. [Google Scholar] [CrossRef]
- Salim, N.E.; Nor, N.A.M.; Jaafar, J.; Ismail, A.F.; Qtaishat, M.R.; Matsuura, T.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yusof, N. Effects of hydrophilic surface macromolecule modifier loading on PES/O-g-C3N4 hybrid photocatalytic membrane for phenol removal. Appl. Surf. Sci. 2019, 465, 180–191. [Google Scholar] [CrossRef]
- Sun, T.; Liu, Y.; Shen, L.; Xu, Y.; Li, R.; Huang, L.; Lin, H. Magnetic field assisted arrangement of photocatalytic TiO2 particles on membrane surface to enhance membrane antifouling performance for water treatment. J. Colloid Interface Sci. 2020, 570, 273–285. [Google Scholar] [CrossRef]
- Geng, Z.; Yang, X.; Boo, C.; Zhu, S.; Lu, Y.; Fan, W.; Huo, M.; Elimelech, M.; Yang, X. Self-cleaning anti-fouling hybrid ultrafiltration membranes via side chain grafting of poly(aryl ether sulfone) and titanium dioxide. J. Memb. Sci. 2017, 529, 1–10. [Google Scholar] [CrossRef]
- Rajeswari, A.; Jackcina Stobel Christy, E.; Pius, A. New insight of hybrid membrane to degrade Congo red and Reactive yellow under sunlight. J. Photochem. Photobiol. B Biol. 2018, 179, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, A.; Vismaiya, S.; Pius, A. Preparation, characterization of nano ZnO-blended cellulose acetate-polyurethane membrane for photocatalytic degradation of dyes from water. Chem. Eng. J. 2017, 313, 928–937. [Google Scholar] [CrossRef]
- Martins, P.M.; Ribeiro, J.M.; Teixeira, S.; Petrovykh, D.Y.; Cuniberti, G.; Pereira, L.; Lanceros-Méndez, S. Photocatalytic Microporous Membrane against the Increasing Problem of Water Emerging Pollutants. Materials 2019, 12, 1649. [Google Scholar] [CrossRef] [PubMed]
- Aoudjit, L.; Martins, P.M.; Madjene, F.; Petrovykh, D.Y.; Lanceros-Mendez, S. Photocatalytic reusable membranes for the effective degradation of tartrazine with a solar photoreactor. J. Hazard. Mater. 2018, 344, 408–416. [Google Scholar] [CrossRef]
- Teixeira, S.; Martins, P.M.; Lanceros-Méndez, S.; Kühn, K.; Cuniberti, G. Reusability of photocatalytic TiO2 and ZnO nanoparticles immobilized in poly(vinylidene difluoride)-co-trifluoroethylene. Appl. Surf. Sci. 2016, 384, 497–504. [Google Scholar] [CrossRef]
- Martins, P.M.; Miranda, R.; Marques, J.; Tavares, C.J.; Botelho, G.; Lanceros-Mendez, S. Comparative efficiency of TiO2 nanoparticles in suspension vs. immobilization into P(VDF-TrFE) porous membranes. RSC Adv. 2016, 6, 12708–12716. [Google Scholar] [CrossRef]
- Salazar, H.; Martins, P.M.; Santos, B.; Fernandes, M.M.; Reizabal, A.; Sebastián, V.; Botelho, G.; Tavares, C.J.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Photocatalytic and antimicrobial multifunctional nanocomposite membranes for emerging pollutants water treatment applications. Chemosphere 2020, 250, 126299. [Google Scholar] [CrossRef]
- Melvin Ng, H.K.; Leo, C.P.; Abdullah, A.Z. Selective removal of dyes by molecular imprinted TiO2 nanoparticles in polysulfone ultrafiltration membrane. J. Environ. Chem. Eng. 2017, 5, 3991–3998. [Google Scholar] [CrossRef]
- Paredes, L.; Murgolo, S.; Dzinun, H.; Dzarfan Othman, M.H.; Ismail, A.F.; Carballa, M.; Mascolo, G. Application of immobilized TiO2 on PVDF dual layer hollow fibre membrane to improve the photocatalytic removal of pharmaceuticals in different water matrices. Appl. Catal. B Environ. 2019, 240, 9–18. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; A. Rahman, M.; Jaafar, J.; Adrus, N.; Hashim, N.A. Antifouling behavior and separation performance of immobilized TiO2 in dual layer hollow fiber membranes. Polym. Eng. Sci. 2018, 58, 1636–1643. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Matsuura, T.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Stability study of extruded dual layer hollow fibre membranes in a long operation photocatalysis process. Polym. Test. 2018, 68, 53–60. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Stability study of PVDF/TiO2 dual layer hollow fibre membranes under long-term UV irradiation exposure. J. Water Process Eng. 2017, 15, 78–82. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Photocatalytic degradation of nonylphenol using co-extruded dual-layer hollow fibre membranes incorporated with a different ratio of TiO2/PVDF. React. Funct. Polym. 2016, 99, 80–87. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Performance evaluation of co-extruded microporous dual-layer hollow fiber membranes using a hybrid membrane photoreactor. Desalination 2017, 403, 46–52. [Google Scholar] [CrossRef]
- Galiano, F.; Song, X.; Marino, T.; Boerrigter, M.; Saoncella, O.; Simone, S.; Faccini, M.; Chaumette, C.; Drioli, E.; Figoli, A.; et al. Novel Photocatalytic PVDF/Nano-TiO2 Hollow Fibers for Environmental Remediation. Polymers 2018, 10, 1134. [Google Scholar] [CrossRef]
- Ong, C.S.; Lau, W.J.; Goh, P.S.; Ng, B.C.; Ismail, A.F. Investigation of submerged membrane photocatalytic reactor (sMPR) operating parameters during oily wastewater treatment process. Desalination 2014, 353, 48–56. [Google Scholar] [CrossRef]
- Teow, Y.H.; Ahmad, A.L.; Lim, J.K.; Ooi, B.S. Preparation and characterization of PVDF/TiO2 mixed matrix membrane via in situ colloidal precipitation method. Desalination 2012, 295, 61–69. [Google Scholar] [CrossRef]
- Almeida, N.A.; Martins, P.M.; Teixeira, S.; Lopes da Silva, J.A.; Sencadas, V.; Kühn, K.; Cuniberti, G.; Lanceros-Mendez, S.; Marques, P.A.A.P. TiO2/graphene oxide immobilized in P(VDF-TrFE) electrospun membranes with enhanced visible-light-induced photocatalytic performance. J. Mater. Sci. 2016, 51, 6974–6986. [Google Scholar] [CrossRef]
- Cheng, J.; Pu, H. A facile method to prepare polyvinylidene fluoride composite nanofibers with high photocatalytic activity via nanolayer coextrusion. Eur. Polym. J. 2018, 99, 361–367. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Q.; Xiao, C.; You, Y.; Zhang, C.; Liu, H. Supported Electrospun Ultrafine Fibrous Poly(tetrafluoroethylene)/ZnO Porous Membranes and their Photocatalytic Applications. Chem. Eng. Technol. 2018, 41, 656–662. [Google Scholar] [CrossRef]
- Kang, W.; Ju, J.; He, H.; Li, F.; Tao, L.; Dong, Y.; Cheng, B. Photocatalytic Degradation Performance of TiO2 /PTFE Membrane Catalyst to Methylene Blue. Chem. Lett. 2016, 45, 1440–1443. [Google Scholar] [CrossRef]
- Blanco, M.; Monteserín, C.; Angulo, A.; Pérez-Márquez, A.; Maudes, J.; Murillo, N.; Aranzabe, E.; Ruiz-Rubio, L.; Vilas, J.L. TiO2-Doped Electrospun Nanofibrous Membrane for Photocatalytic Water Treatment. Polymers 2019, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Yar, A.; Haspulat, B.; Üstün, T.; Eskizeybek, V.; Avci, A.; Kamiş, H.; Achour, S. Electrospun TiO2/ZnO/PAN hybrid nanofiber membranes with efficient photocatalytic activity. RSC Adv. 2017, 7, 29806–29814. [Google Scholar] [CrossRef]
- Tissera, N.D.; Wijesena, R.N.; Sandaruwan, C.S.; de Silva, R.M.; de Alwis, A.; de Silva, K.M.M.N. Photocatalytic activity of ZnO nanoparticle encapsulated poly(acrylonitrile) nanofibers. Mater. Chem. Phys. 2018, 204, 195–206. [Google Scholar] [CrossRef]
- Suriyaraj, S.P.; Benasir Begam, M.; Deepika, S.G.; Biji, P.; Selvakumar, R. Photocatalytic removal of nitrate using TiO2/polyacrylonitrile nanofiber membrane synthesized by co-electrospinning process. Water Sci. Technol. Water Supply 2014, 14, 554–560. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, D.; Cho, M.; Lee, S.S.; Zhang, F.; Biswas, P.; Fortner, J.D. In Situ Photocatalytic Synthesis of Ag Nanoparticles (nAg) by Crumpled Graphene Oxide Composite Membranes for Filtration and Disinfection Applications. Environ. Sci. Technol. 2016, 50, 2514–2521. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, Z.; Li, F.; Yang, Y.; Pan, Y.; Peng, Y.; Yang, X.; Zeng, G. A novel photocatalytic membrane decorated with RGO-Ag-TiO2for dye degradation and oil–water emulsion separation. J. Chem. Technol. Biotechnol. 2018, 93, 761–775. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, S.; Quan, X.; Yu, H.; Zhao, H. Integration of microfiltration and visible-light-driven photocatalysis on g-C3N4 nanosheet/reduced graphene oxide membrane for enhanced water treatment. Appl. Catal. B Environ. 2016, 194, 134–140. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Graphene oxide based ultrafiltration membranes for photocatalytic degradation of organic pollutants in salty water. Water Res. 2015, 77, 179–190. [Google Scholar] [CrossRef]
- Xu, C.; Xu, Y.; Zhu, J. Photocatalytic antifouling graphene oxide-mediated hierarchical filtration membranes with potential applications on water purification. ACS Appl. Mater. Interfaces 2014, 6, 16117–16123. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cui, A.; Xu, Y.; Fu, X. Graphene oxide–TiO2 composite filtration membranes and their potential application for water purification. Carbon N. Y. 2013, 62, 465–471. [Google Scholar] [CrossRef]
- Sun, K.; Wang, L.; Wu, C.; Deng, J.; Pan, K. Fabrication of α-Fe2O3 @rGO/PAN Nanofiber Composite Membrane for Photocatalytic Degradation of Organic Dyes. Adv. Mater. Interfaces 2017, 4, 1700845. [Google Scholar] [CrossRef]
- Nair, A.K.; Jagadeesh, J.B. TiO2 nanosheet-graphene oxide based photocatalytic hierarchical membrane for water purification. Surf. Coat. Technol. 2017, 320, 259–262. [Google Scholar] [CrossRef]
- Gao, P.; Liu, Z.; Tai, M.; Sun, D.D.; Ng, W. Multifunctional graphene oxide–TiO2 microsphere hierarchical membrane for clean water production. Appl. Catal. B Environ. 2013, 138–139, 17–25. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Z.; Zhai, D.; Liu, Y.; Liu, Q.; Xue, L.; Gao, C.; Wu, T.; Zhang, Z.; Zhai, D.; et al. Dye Degrading and Fouling-Resistant Membranes Formed by Deposition with Ternary Nanocomposites of N-Doped Graphene/TiO2/Activated Carbon. Membranes 2019, 9, 16. [Google Scholar] [CrossRef]
- Aboamera, N.M.; Mohamed, A.; Salama, A.; Osman, T.A.; Khattab, A. An effective removal of organic dyes using surface functionalized cellulose acetate/graphene oxide composite nanofibers. Cellulose 2018, 25, 4155–4166. [Google Scholar] [CrossRef]
- Mohamed, A.; Osman, T.A.; Toprak, M.S.; Muhammed, M.; Yilmaz, E.; Uheida, A. Visible light photocatalytic reduction of Cr(VI) by surface modified CNT/titanium dioxide composites nanofibers. J. Mol. Catal. A Chem. 2016, 424, 45–53. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, D.; Li, Y.; Qu, J.; Yu, Z.Z. Fabrication of PAN@TiO2/Ag nanofibrous membrane with high visible light response and satisfactory recyclability for dye photocatalytic degradation. Appl. Surf. Sci. 2017, 426, 622–629. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, M.; Mi, B. Membrane surface modification with TiO2–graphene oxide for enhanced photocatalytic performance. J. Memb. Sci. 2014, 455, 349–356. [Google Scholar] [CrossRef]
- Jiang, R.; Wen, W.; Wu, J.M. Titania nanowires coated PEI/P25 membranes for photocatalytic and ultrafiltration applications. New J. Chem. 2018, 42, 3020–3027. [Google Scholar] [CrossRef]
- You, S.-J.; Semblante, G.U.; Lu, S.-C.; Damodar, R.A.; Wei, T.-C. Evaluation of the antifouling and photocatalytic properties of poly(vinylidene fluoride) plasma-grafted poly(acrylic acid) membrane with self-assembled TiO2. J. Hazard. Mater. 2012, 237–238, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Irani, E.; Amoli-Diva, M. Hybrid adsorption–photocatalysis properties of quaternary magneto-plasmonic ZnO/MWCNTs nanocomposite for applying synergistic photocatalytic removal and membrane filtration in industrial wastewater treatment. J. Photochem. Photobiol. A Chem. 2020, 391, 112359. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Son, A.; Seid, M.G.; Shim, S.; Lee, S.H.; Chung, Y.C.; Lee, C.; Lee, J.; Hong, S.W. Photocatalytic applications of paper-like poly(vinylidene fluoride)-titanium dioxide hybrids fabricated using a combination of electrospinning and electrospraying. J. Hazard. Mater. 2015, 285, 267–276. [Google Scholar] [CrossRef]
- Xie, R.; Zhang, L.; Liu, H.; Xu, H.; Zhong, Y.; Sui, X.; Mao, Z. Construction of CQDs-Bi20TiO32/PAN electrospun fiber membranes and their photocatalytic activity for isoproturon degradation under visible light. Mater. Res. Bull. 2017, 94, 7–14. [Google Scholar] [CrossRef]
- Nor, N.A.M.; Jaafar, J.; Ismail, A.F.; Mohamed, M.A.; Rahman, M.A.; Othman, M.H.D.; Lau, W.J.; Yusof, N. Preparation and performance of PVDF-based nanocomposite membrane consisting of TiO2 nanofibers for organic pollutant decomposition in wastewater under UV irradiation. Desalination 2016, 391, 89–97. [Google Scholar] [CrossRef]
- Fischer, K.; Kühnert, M.; Gläser, R.; Schulze, A. Photocatalytic degradation and toxicity evaluation of diclofenac by nanotubular titanium dioxide–PES membrane in a static and continuous setup. RSC Adv. 2015, 5, 16340–16348. [Google Scholar] [CrossRef]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2019, 94, 3049–3063. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2019, 27, 2522–2565. [Google Scholar] [CrossRef]
- Yang, J.; Liu, B.; Zhao, X. A visible-light-active Au-Cu(I)@Na2Ti6O13 nanostructured hybrid pasmonic photocatalytic membrane for acetaldehyde elimination. Chin. J. Catal. 2017, 38, 2048–2055. [Google Scholar] [CrossRef]
- Fang, W.; Xing, M.; Zhang, J. Modifications on reduced titanium dioxide photocatalysts: A review. J. Photochem. Photobiol. C Photochem. Rev. 2017, 32, 21–39. [Google Scholar] [CrossRef]
- Yi, H.; Huang, D.; Qin, L.; Zeng, G.; Lai, C.; Cheng, M.; Ye, S.; Song, B.; Ren, X.; Guo, X. Selective prepared carbon nanomaterials for advanced photocatalytic application in environmental pollutant treatment and hydrogen production. Appl. Catal. B Environ. 2018, 239, 408–424. [Google Scholar] [CrossRef]
- Leary, R.; Westwood, A. Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon 2011, 49, 741–772. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Shandilya, P.; Jeong, D.-Y.; Lim, J.-H.; Singh, P. Review on fabrication of graphitic carbon nitride based efficient nanocomposites for photodegradation of aqueous phase organic pollutants. J. Ind. Eng. Chem. 2018, 67, 28–51. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Taheri, A.H.; Mansourpanah, Y. Coupling TiO2 nanoparticles with UV irradiation for modification of polyethersulfone ultrafiltration membranes. J. Memb. Sci. 2008, 313, 158–169. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Chin, S.S.; Chiang, K.; Fane, A.G. The stability of polymeric membranes in a TiO2 photocatalysis process. J. Memb. Sci. 2006, 275, 202–211. [Google Scholar] [CrossRef]
- Tsehaye, M.T.; Velizarov, S.; Van der Bruggen, B. Stability of polyethersulfone membranes to oxidative agents: A review. Polym. Degrad. Stab. 2018, 157, 15–33. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; Springer Science & Business Media: Twente, The Netherland, 1991; ISBN 0-7923-0978-2. [Google Scholar]
- Aryanti, P.T.P.; Ariono, D.; Hakim, A.N.; Wenten, I.G. Flory-Huggins Based Model to Determine Thermodynamic Property of Polymeric Membrane Solution. J. Phys. 2018, 1090, 012074. [Google Scholar] [CrossRef]
- Mohsenpour, S.; Esmaeilzadeh, F.; Safekordi, A.; Tavakolmoghadam, M.; Rekabdar, F.; Hemmati, M. The role of thermodynamic parameter on membrane morphology based on phase diagram. J. Mol. Liq. 2016, 224, 776–785. [Google Scholar] [CrossRef]
- Bottino, A.; Camera-Roda, G.; Capannelli, G.; Munari, S. The formation of microporous polyvinylidene difluoride membranes by phase separation. J. Memb. Sci. 1991, 57, 1–20. [Google Scholar] [CrossRef]
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid, S.M.S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Mohamed, A.; Yousef, S.; Ali Abdelnaby, M.; Osman, T.A.; Hamawandi, B.; Toprak, M.S.; Muhammed, M.; Uheida, A. Photocatalytic degradation of organic dyes and enhanced mechanical properties of PAN/CNTs composite nanofibers. Sep. Purif. Technol. 2017, 182, 219–223. [Google Scholar] [CrossRef]
- Li, X.; Sotto, A.; Li, J.; Van der Bruggen, B. Progress and perspectives for synthesis of sustainable antifouling composite membranes containing in situ generated nanoparticles. J. Memb. Sci. 2017, 524, 502–528. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds. A review. Catal. Today 2017, 281, 144–164. [Google Scholar] [CrossRef]
- Argurio, P.; Fontananova, E.; Molinari, R.; Drioli, E. Photocatalytic Membranes in Photocatalytic Membrane Reactors. Processes 2018, 6, 162. [Google Scholar] [CrossRef]
- Horovitz, I.; Gitis, V.; Avisar, D.; Mamane, H. Ceramic-based photocatalytic membrane reactors for water treatment—Where to next? Rev. Chem. Eng. 2019. [Google Scholar] [CrossRef]






| Polymer (wt. %) | Solvent | Non-Solvent | Additive (wt. %) | Photocatalyst | wt.% (Polymeric Solution) | wt. %/cm2 (Membrane) | Application (Removed Pollutant) | Author |
|---|---|---|---|---|---|---|---|---|
| PVDF (20) | DMAc | deionized water | PEG (5) | TiO2 | 0–7, 5 * | 0–0.612, 0.478 * | BSA | Méricq, J.P [15] |
| PVDF (20) | DMAc | deionized water | PEG (5) | TiO2 | 4 | 0.30 | (-) | Tran, D. [16] |
| PVDF (19) | DMAc | distilled water | PVP (7) | Ag-TiO2 | 0.01-0.06, 0.06 * | (-) | BSA, E.Coli | Chen, Q. [17] |
| PVDF (18) | DMAc | deionized water | PVP (2) | TiO2 | 0–1, 1 * | 0–0.33, 0.33 * | Estrone, 17β- estradiol | Wang, M. [18] |
| PVDF (16) | DMAc | water: isopropanol (70:30) | (-) | TiO2 | 0–3, 2 * | 0–0.04, 0.027 | RTB | Sakarkar, S. [19] |
| PVDF (15) | DMAc | Water | PVP (1) | GO-TiO2 | 1 | 0.32 | BSA | Xu,Z [20] |
| PVDF (15) | DMAc | distilled water | PVP (1) | GO-OMWCNTs | 1% (carb/pol) | (-) | BSA | Zhang, J. [21] |
| PVDF (14) | DMAc | tap water | (-) | AC-TiO2 | (0–0.5)–(0–0.1) | 0.106 | BSA | Liu, Q. [22] |
| PVDF (12) | DMAc | tap water | PEG (1–5), 2 * | TiO2 | 0.25–2, 0.5 * | 0.042–0.297,0.083 * | NOM, HA | Song, H. [23] |
| PVDF (12) | DMAc | Tap water | LiCl (0–4), 0.5 * | TiO2 | 0–1.5, 0.5 * | 0–0.23, 0.083 * | NOM, HA | Song, H. [24] |
| PVDF+PMMA (12) | TEP | Water | PEG (25) + PEG (5) | TiO2 | 0–0.5, 0.5 * | 0–0.5, 0.5 * | MB | Benhabiles, O. [25] |
| PVDF (10) | NMP | tap water | (-) | TiO2 | 0–4, 2–4 * | 0–0.189, 0.110–0.189 * | E. Coli, RB5, BSA | Damodar, R.A. [26] |
| PMAA-g-PVDF/PAN (-) | DMAc | Ethanol:deionized water (1:3) | (-) | N-TiO2 | 1,3,5 | (-) | Bentazon | Mungondori, H. [27] |
| PSF (20) | NMP | distilled water | (-) | TiO2 | 0–2.43, 1.96 * | 0–0.553, 0.455 * | Cr(VI) | Jyothi, M.S. [28] |
| PSF (18) | DMAc:NMP | Ethanol:water (20:80) | (-) | TiO2 | 0–5, 2 * | (-) | BSA | Yang, Y. [29] |
| PSF (18) | DMAC:NMP (4:1) | deionized water | PEG (8) | Fe-TiO2 | 0–4.5, 3.6 * | 0–0.077 | BPA | Wang, Q. [30] |
| PSF (18) | DMAC | Water | (-) | TiO2-ZnO, TiO2-SiO2 | 0.16 | (-) | MO, phenol | El-Aassar, A. [31] |
| PSF (18) | NMP | deionized water | (-) | N, Pd-TiO2 | 0–1.26, (0–7) | (-) | EY | Kuvarega, A.T. [32] |
| PSF (18) | NMP | Water | PVP (2) | N-rGO-TiO2 | 0.5 | 0.095 | DR 80, DB 15 | Xu, H. [33] |
| PSF (17) | NMP | tap water | PVP (0.5) | mpg-C3N4-TiO2 | 0–1, 1 * | 0–0.653, 0.653 * | SMX | Yu, S. [34] |
| PSF (12) | DMF | distilled water | (-) | CNTs-TiO2 | 1 | (-) | Ampicillin Erithtomycin | Muhulet, A. [35] |
| PES (27 g) | DMF, EtOH (1–4) | distilled water | (-) | TiO2 | 0.1–0.4, 0.1 * | 0.0062–0.024, 0.0062* | HA | Sotto, A. [36] |
| PES (26) | NMP | Water | (-) | Co-TiO2 | 0.5–1, 1 * | 0.065–0.129, 0.129 * | 2-DCP | Hoseini, S. N. [37] |
| PES (21) | DMAc | distilled water | PVP (1) | rGO-TiO2 | 0.05–0.2, 0.1 * | 0.237–0.943, 0.497 * | DY 12, RG 19, RB 21, BSA | Safarpour, M. [38] |
| PES (20) | DMAc | distilled water | PVP (1) | B-TiO2-SiO2/CoFe2O4 | 0–1, 0.5 * | 0–0.497, 0.239 * | DR 16, POME | Zangeneh, H. [39] |
| PES (18) | NMP | tap water | SMM (1) | O-g-C3N4 | 1 | (-) | Phenol | Salim, N. [40] |
| PES (18) | NMP | tap water | SMM (1–5,4 *) | O-g-C3N4 | 1 | (-) | Phenol | Salim, N. [41] |
| PES (15) | DMAc | Water | PVP (5) | mNi-TiO2 | 0–1, 1 * | 0-0.083, 0.083 * | BSA, YEF, SA, HA, MB | Sun, T. [42] |
| PES-F-COOH (20) | DMF | deionized water | PVP (10) | TiO2 | 1–5,5 * | 0.123–0.519, 0.519 * | PAM | Geng, Z. [43] |
| CA-PS (-) | Acetone | distilled water | (-) | ZnO | 0.1 g | (-) | CR, RY 105 | Rajeswari, A. [44] |
| CA-PU (-) | Acetone-chloroform | distilled water | (-) | ZnO | 0.3 | (-) | RR 11, RO 84 | Rajeswari, A. [45] |
| P(VDF-TrFE) (10) | DMF | (-) | (-) | TiO2 | 8 | 0.32 | MB, CIP, IBP | Martins, P.M. [46] |
| P(VDF-TrFE) (10) | DMF | (-) | (-) | TiO2 | 8 | 0.017 | Tartrazine | Aoudjit, L. [47] |
| P(VDF-TrFE) (10) | DMF | (-) | (-) | TiO2, ZnO | 0–15,15 * | 0–3.75, 3.75 | MB | Teixeira, S. [48] |
| P(VDF-TrFE) (10) | DMF | (-) | (-) | TiO2 (NaY) | 0–8, (0–8), 8(8) * | 0–3.70, 3.70 * | MB | Martins, P.M. [49] |
| PVDF-HFP (15) | DMF | (-) | (-) | Ag- TiO2 | 0-10, 10* | 0–0.56, 0.56 * | NOR | Salazar, H. [50] |
| PSF (16) | NMP | distilled water | PVP (2) | TiO2, MIP TiO2, NIP TiO2 | 2 | (-) | MB, MO | Melvin, H.K. [51] |
| PVDF Dul layer HF (18 in, 15 out) | DMAc | tap water | (-) | TiO2 | 0 (in) 3 (out) | (-) | 8 pharmaceutical mixture | Paredes, L. [52] |
| PVDF Dual layer HF (18 in, 15 out) | DMAc | tap water | (-) | TiO2 | 0 (in) 3–15 (out), 3 * | 0 (in) 0.067–0.201 (out), 0.067 * | NOM | Dzinun, H. [53] |
| PVDF Dual layer HF (18 in, 15 out) | DMAc | tap water | (-) | TiO2 | 0 (in) 3–15 (out) | 0 (in) 0.067–0.201 (out) | NP | Dzinun, H. [54] |
| PVDF Dual layer HF (18 in, 15 out) | DMAc | tap water | (-) | TiO2 | 0 (in) 3 (out) | 0 (in) 0.067 (out) | NP | Dzinun, H. [55] |
| PVDF Dual layer HF (18 in, 15 out) | DMAc | tap water | (-) | TiO2 | 0 (in) 0–15 (out), 15 * | 0 (in) 0–0.201 (out), 0.201 * | NP | Dzinun, H. [56] |
| PVDF Dual layer HF (18 in, 15 out) | DMAc | tap water | PEG (5 in, 0 out) | TiO2 | 0 (in) 3 (out) | 0 (in) 0.067 (out) | NP | Dzinun, H. [57] |
| PVDF HF (18–19) | NMP | tap water | PVP (15) PEG | TiO2 | 0.5 | 0.071 | MB | Galiano, F. [58] |
| PVDF HF (18) | DMAc | Water | PVP (5) | TiO2 | 0–4, 2 * | (-) | Oil wastewater | Ong, C.S. [59] |
| PVDF (18) | DMAc, NMP, DMF | Water | (-) | TiO2 | 0.001, 0.01, 0.1 g/L | (-) | HA | Teow, Y.H [60] |
| Polymer (wt. %) | Solvent | Additive | Photocatalyst | wt. % (Polymeric Solution) | Application (Removed Pollutant) | Author |
|---|---|---|---|---|---|---|
| P(VDF-TrFE) (15) | DMF/MEK, 85/15 | (-) | GO-TiO2 | 0–20, 5 * | MB | Almeida, N.A. [61] |
| PVDF (-) | (-) | PEO | TiO2-MWCNTs (20:1) | 0–40 | HA | Chen, J. [62] |
| PTFE: PVA (6:1) | water | PVA | ZnO | 0–30, 20* | RhB | Huang, Y. [63] |
| PTFE (15) | (-) | PVA (1), BA (0.0025) | TiO2 | (-) | MB | Kang, W. [64] |
| PA6 (12) | AA: FA (2:1) | (-) | TiO2 | 25 | E. coli, RBB | Blanco, M. [65] |
| PAN (8) | DMF | (-) | TiO2-ZnO | 2 | MG | Yar, A. [66] |
| PAN (6) | DMF | (-) | ZnO | 0.9 | MO | Tissera, N. D. [67] |
| PAN (7) | DMF | (-) | TiO2 | 3.57 | Nitrate | Suriyaraj, S.P. [68] |
| Synthesis Method | Support Material | Aeff (cm2) | Photocatalyst | Photocatalyst Mass (mg) | Photocatalyst per Membrane Area (mg/cm2) | Application (Removed Pollutant) | Author |
|---|---|---|---|---|---|---|---|
| Vacuum deposition | PES (com) Pretreatment: PAAM | 4.3 | nAg-GO- TiO2 | 3 | 0.697 | E. coli, B. subtilis | Jiang, Y. [69] |
| Vacuum deposition | CA (com) Pretreatment: PEG + GA | 12.56 | Ag-rGO- TiO2 | 2.5–20, 10 * | 0.199–1.59, 0.796 * | MB, RhB, oil water | Chen, Q. [70] |
| Vacuum deposition | CA (com) | 12.56 | rGO-g-C3N4 | 10–100, 25 * | 0.796–7.96, 1.99 * | RhB | Zhao, H. [71] |
| Vacuum deposition | MCE (com) | 1.54 | GO- TiO2 | 10 | 6.49 | DP, MO | Pastrana-Martínez, L [72] |
| Vacuum deposition | PC (com) | (-) | GO- TiO2 | (-) | (-) | DR 80, DB 15 | Xu, C. [73] |
| Vacuum deposition | PC (com) | (-) | GO- TiO2 | (-) | (-) | MO, RhB | Xu, C. [74] |
| Vacuum deposition (Support: Electrospinning) | PAN (8wt. %, DMF) | 12.56 | rGO-α-Fe2O3 | (-) | (-) | MB, MO, RhB, R6G, MG, GV | Sun, K. [75] |
| Filtration | CA (com) | 14.6 | GO-TiO2 | 50–400, 100 * | 3.42–27.4, 6.85 * | CR | Nair, A.K. [76] |
| Filtration | CA (com) | 11.94 | GO-TiO2 | 50–300, 200 * | 4.18–25.12, 16.75 * | RhB, AO7 | Gao, P. [77] |
| Immersion | PSF (com) Pretreatment: PVA (2.5mg) | 17.34 | AC-N-rGO-TiO2 | 10–160, 120 * | 0.57–9.22, 6.92 * | MO | Wu, T. [78] |
| Immersion (Support: Electrospinning) | CA-GO (15−(0–1.5) wt. %, DMF) Pretratment: GA | (-) | NH2-TiO2 | 0.005 g/ml | (-) | MB, IC | Aboamera, N. M. [79] |
| Immersion (Support: Electrospinning) | PAN-CNT (10 wt. %, DMF) Pretratment: GA | (-) | NH2-TiO2 | 40.6 wt. % (CNT-TiO2) NF | (-) | Cr (VI) | Mohamed, A. [80] |
| Immersion - In situ growth (Support: Electrospinning) | PAN (9 wt. %, DMF) Pretreatment: PDA | 20 | Ag-TiO2 | (-) | (-) | MB, Phenol | Shi, Y. [81] |
| Immersion (Layer by Layer (LbL)) | PSF (com) | (-) | GO-TiO2 | (-) | 0.062 | MB | Gao, Y. [82] |
| Immersion (Support: Phase inversion) | PEI/P25 (24/1.23 wt. %, NMP) | 12.56 | TiO2 nw | 250 | 19.9 | RhB | Jiang, R. [83] |
| Immersion (Plasma-grafted) | PVDF-g-PAA (com) | 4.5 | TiO2 | 0.5 1.5 3 (% m/v) | (-) | RB5, BSA | You, S.-J. [84] |
| Immersion (UV-grafted) | PA-g-PAA (com) | 13.4 | Ag-ZnO-Fe3O4-MWCNTs | 8.7 | 0.649 | Amoxicilin | Irani, E. [85] |
| Electrospraying (Support: Electrospinning) | PVDF (18 wt. %, DMF:acetone 60:40) | 45 | TiO2 | 4.5-27, 27* | 0.1–0.6, 0.6* | BPA, 4-CP, CMT | Ramasundaram, S. [86] |
| Coaxial electrospinning | PAN (10, 15 core) DMAc | (-) | CQDs-Bi20-TiO32 | 5, 10, 15 w/v% | (-) | Isoproturon | Xie, R. [87] |
| Hot pressing (Support: Phase inversion) | PVDF (-wt. %, DMAc) | (-) | TiO2 | (-) | (-) | BPA | Nor, N.A.M. [88] |
| Sputtering + Anodization | PES (com) | 17.35 | TiO2 | (-) | (-) | diclofenac | Fischer, K. [89] |
| Neat Membrane | Composite Membrane | |||||||
|---|---|---|---|---|---|---|---|---|
| Polymer (%) | Permeability (L/hm2bar) | Mean Pore Size (nm) | Porosity (%) | Nanoparticles Added (%) | Permeability (L/hm2bar) | Mean Pore Size (nm) | Porosity (%) | Literature |
| PVDF (15) | 150 | 48.1 | 69.6 | TiO2 (1) | 300 | 52.6 | 75.1 | [20] |
| GO (1) | 400 | 55.7 | 78.3 | |||||
| GO-TiO2 (1) | 490 | 65.2 | 83.1 | |||||
| PVDF (14) | 90 | 18.6 | 47.2 | AC (0.5) | 170 | 18 | 56 | [22] |
| TiO2 (0.1) | 280 | 28.2 | 54.3 | |||||
| AC-TiO2 (0.5:0.1) | 255 | 30.6 | 55.4 | |||||
| PSF (18) | 115 | 56.2 | 62.5 | GO (0.5) | 150 | 61.4 | 69.4 | [33] |
| TiO2 (0.5) | 155 | 62.8 | 71.6 | |||||
| rGO-TiO2 (0.5) | 180 | 67.9 | 77.2 | |||||
| N-rGO-TiO2 (0.5) | 230 | 70.5 | 81.8 | |||||
| Photocatalyst/ Polymer | Synthesis Method | Targeted Pollutant | Number of Cycles | Total Irradiation Time (h) | Loss in Degradation Rate (Cycle 1–Last Cycle) | Power of the Lamp | Ref. |
|---|---|---|---|---|---|---|---|
| MMM | |||||||
| TiO2/ PSF | NIPS | Cr (VI) | 4 | - | 0% | Sunlight | [28] |
| N-TiO2/ PMAA-g-PVDF/PAN | NIPS | Bentanzon | 3 | 10 | 0% | UV (5063 lux) | [27] |
| ZnO/ CA-PS | NIPS | CR, RY 105 | 5 | 5 | 45 % | Sunlight | [44] |
| TiO2/ PSF | NIPS | MB, MO | 5 | 7.5 | 0% | UV-C 10 W | [51] |
| TiO2/ P(VDF-TrFE) | EIPS | MB, CIP, IBP | 4 | 20 | 0% | UV-A 48 W | [46] |
| TiO2 or ZnO/ P(VDF-TrFE) | EIPS | MB, Model organic | 3 | 15 | 13 % | UV-A 48 W | [48] |
| Ag-TiO2/ PVDF-HFP | EIPS | NOR | 3 | 15 | 15.6 % | UV-A 8 W | [50] |
| Ag-TiO2/ PVDF-HFP | Electrospinnin | NOR | 3 | 15 | 8.8 % | UV-A 8 W | [50] |
| ZnO/ PAN | Electrospinning | MO | 3 | 30 | 0% | UV-A 40 W UV-B 20 W | [67] |
| ZnO/ PTFE:PVA | Electrospinning | RhB | 5 | 25 | 20 % | UV 500 W | [63] |
| TiO2/ PTFE | Electrospinning | MB | 5 | 7.5 | 45 % | UV 300 W | [64] |
| TiO2/ PA6 | Electrospinning | RBB | 3 | 12 | 0% | UV 6 W | [65] |
| TFCM | |||||||
| TiO2/ PVDF | Electrospraying | BPA, 4-CP, CMT | 10 | 16.7 | 0% | 4 W | [86] |
| GO-TiO2/ CA | Filtration | CR | 3 | - | 10 % | No data | [76] |
| TiO2/ PEI-P25 | Immersion | RhB | 6 | 12 | 10% | UV 18 W | [83] |
| NH2-TiO2/ PAN-CNT | Immersion | Cr (VI) | 5 | 5 | 0% | 125 W (420 nm) | [80] |
| rGO-α-Fe2O3/ PAN | Vacuum deposition | MB, MO, RhB, R6G, MG, GV | 5 | 4.2 | <10% | UV-vis 275 W | [75] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romay, M.; Diban, N.; Rivero, M.J.; Urtiaga, A.; Ortiz, I. Critical Issues and Guidelines to Improve the Performance of Photocatalytic Polymeric Membranes. Catalysts 2020, 10, 570. https://doi.org/10.3390/catal10050570
Romay M, Diban N, Rivero MJ, Urtiaga A, Ortiz I. Critical Issues and Guidelines to Improve the Performance of Photocatalytic Polymeric Membranes. Catalysts. 2020; 10(5):570. https://doi.org/10.3390/catal10050570
Chicago/Turabian StyleRomay, Marta, Nazely Diban, Maria J. Rivero, Ane Urtiaga, and Inmaculada Ortiz. 2020. "Critical Issues and Guidelines to Improve the Performance of Photocatalytic Polymeric Membranes" Catalysts 10, no. 5: 570. https://doi.org/10.3390/catal10050570
APA StyleRomay, M., Diban, N., Rivero, M. J., Urtiaga, A., & Ortiz, I. (2020). Critical Issues and Guidelines to Improve the Performance of Photocatalytic Polymeric Membranes. Catalysts, 10(5), 570. https://doi.org/10.3390/catal10050570






