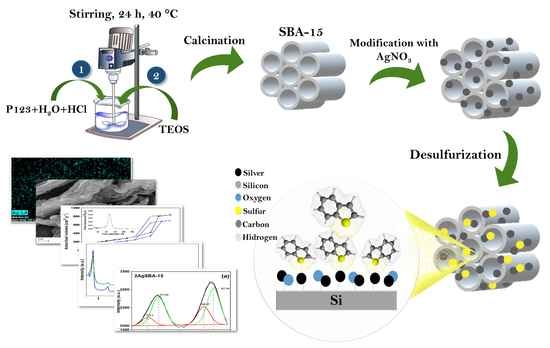
Effective Interactions of Ag Nanoparticles on the Surface of SBA-15 in Performing Deep Desulfurization of Real Diesel Fuel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preliminary Adsorption Data
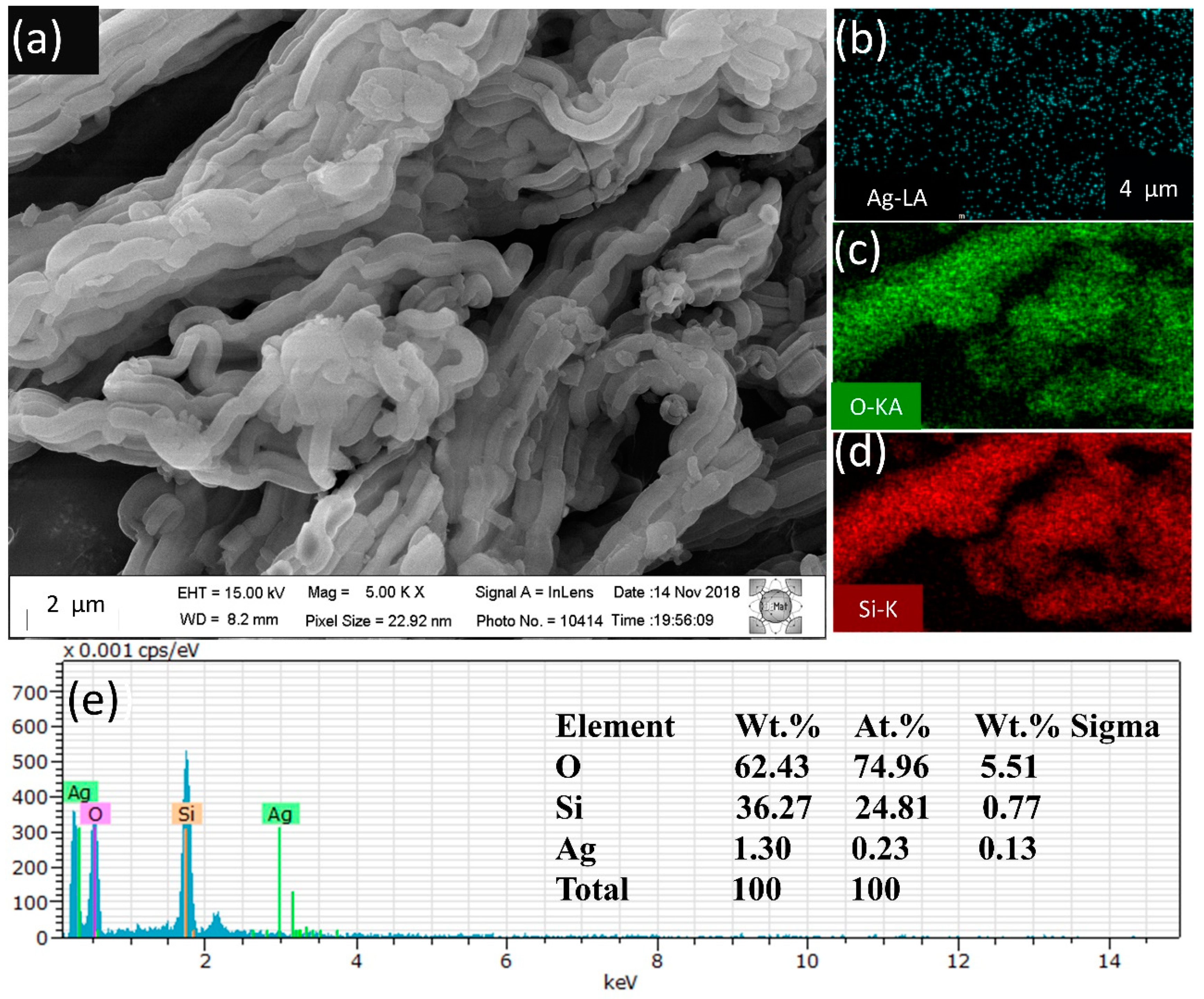
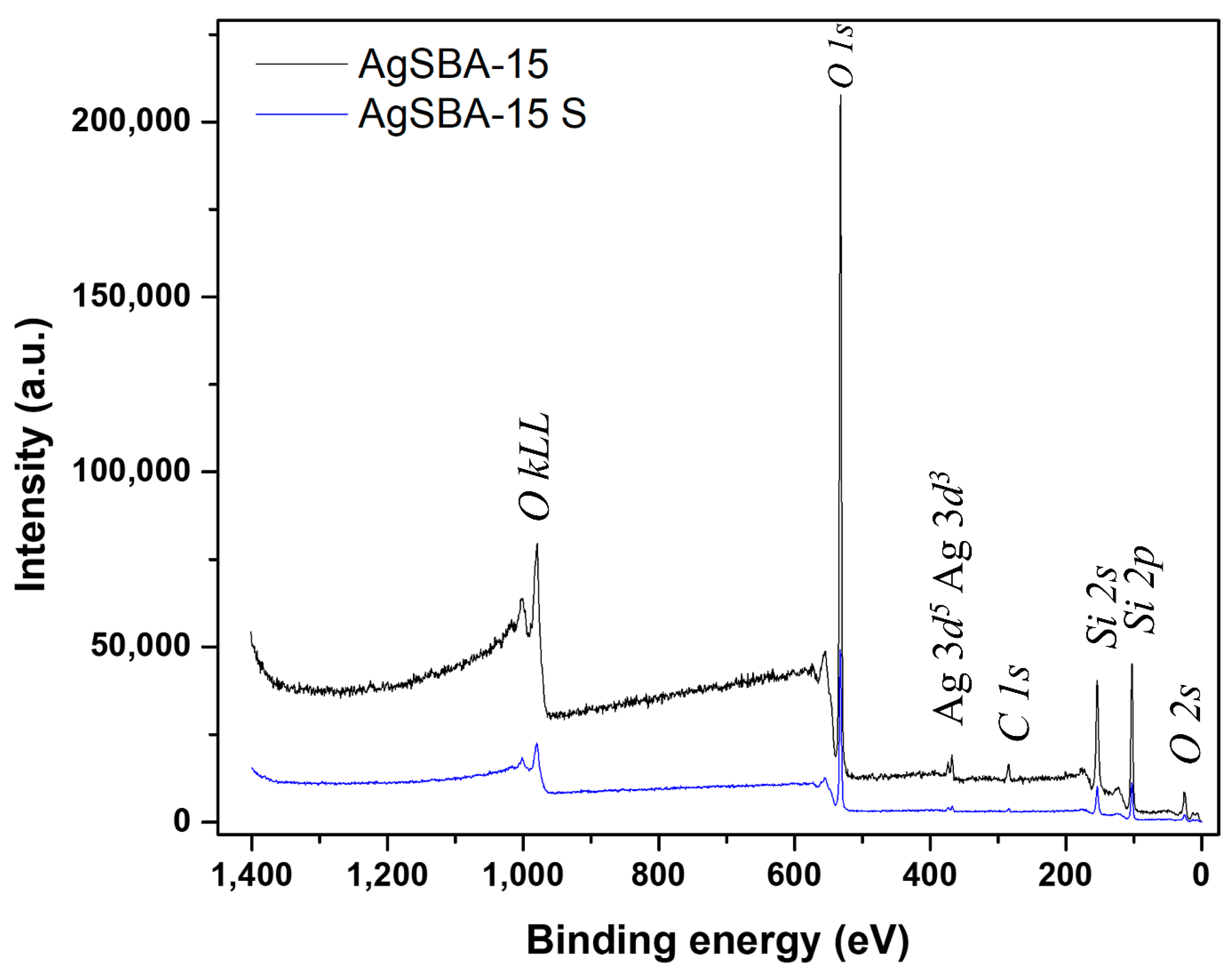
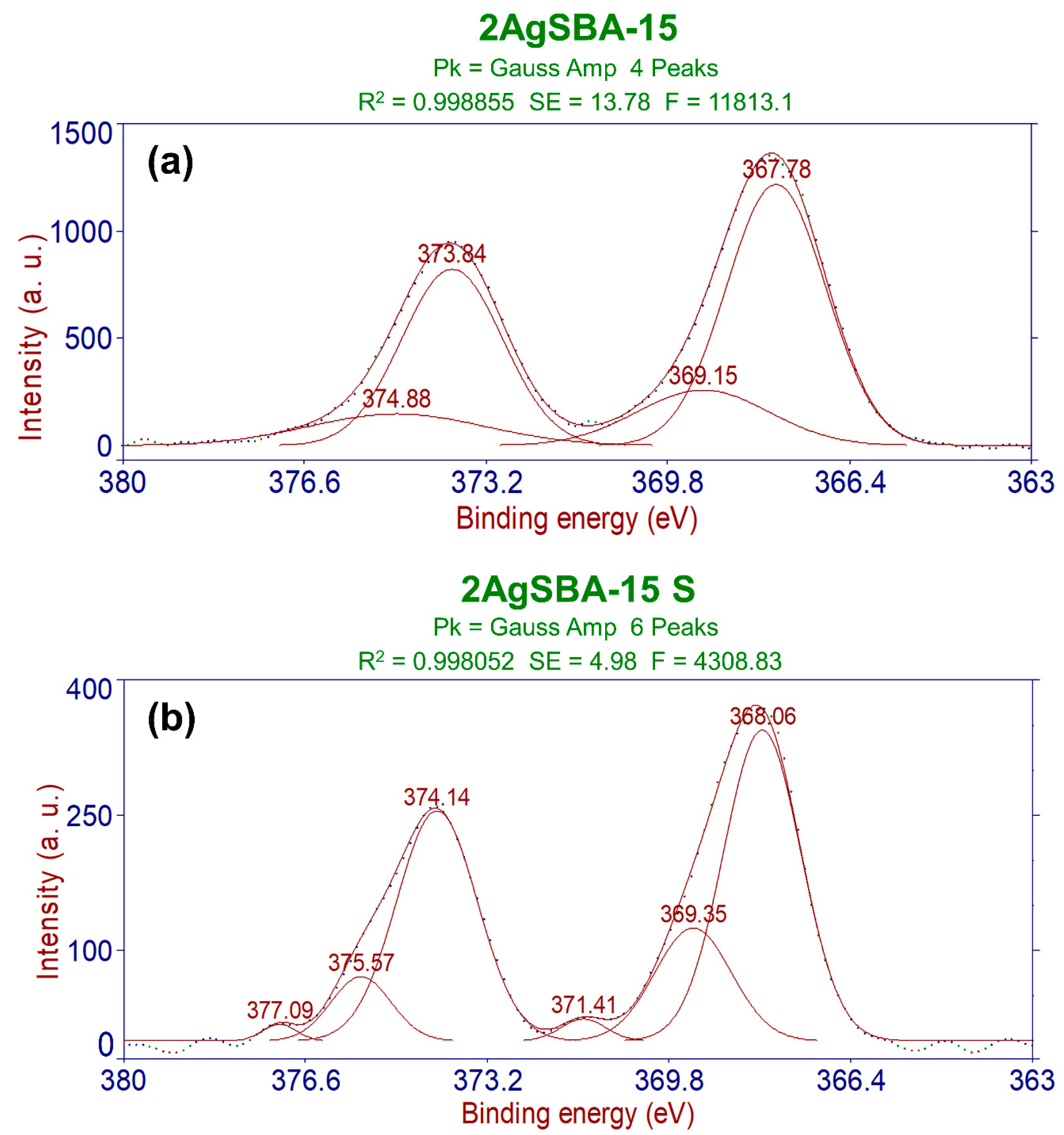
2.2. Characterization of the Materials
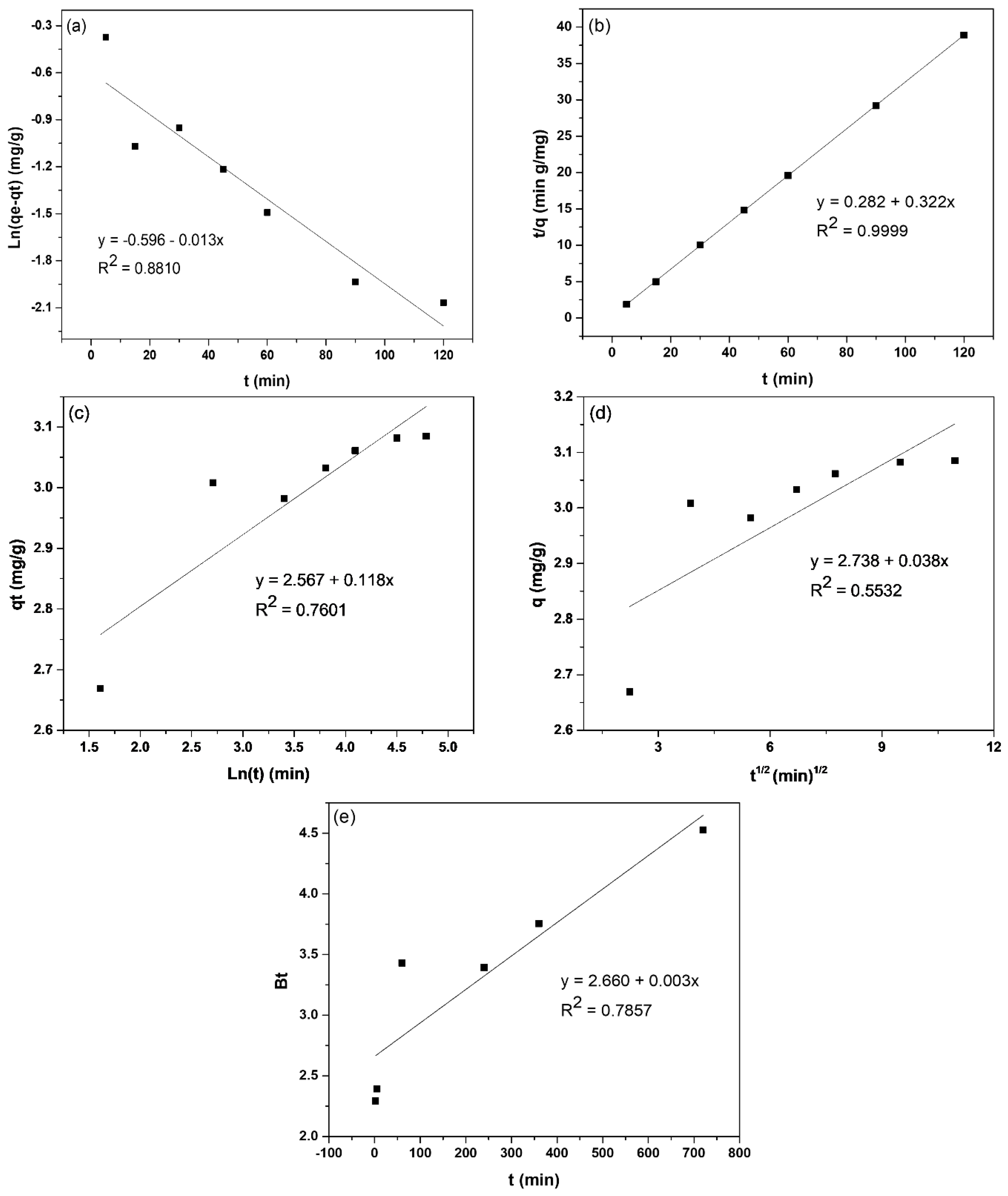
2.3. Adsorption Kinetics
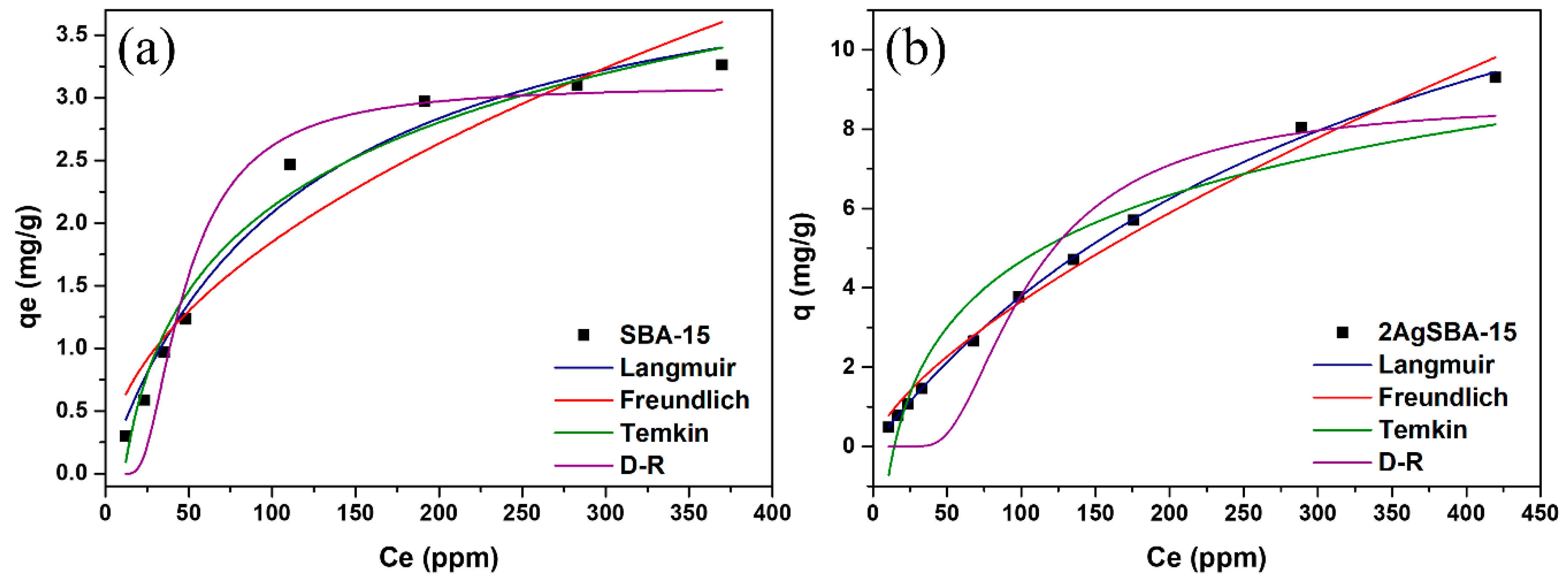
2.4. Adsorption Equilibrium Isotherms
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of SBA-15 Material
3.3. Adsorbents’ Characterization
3.4. Preliminary Adsorption Tests
3.5. Adsorption Study
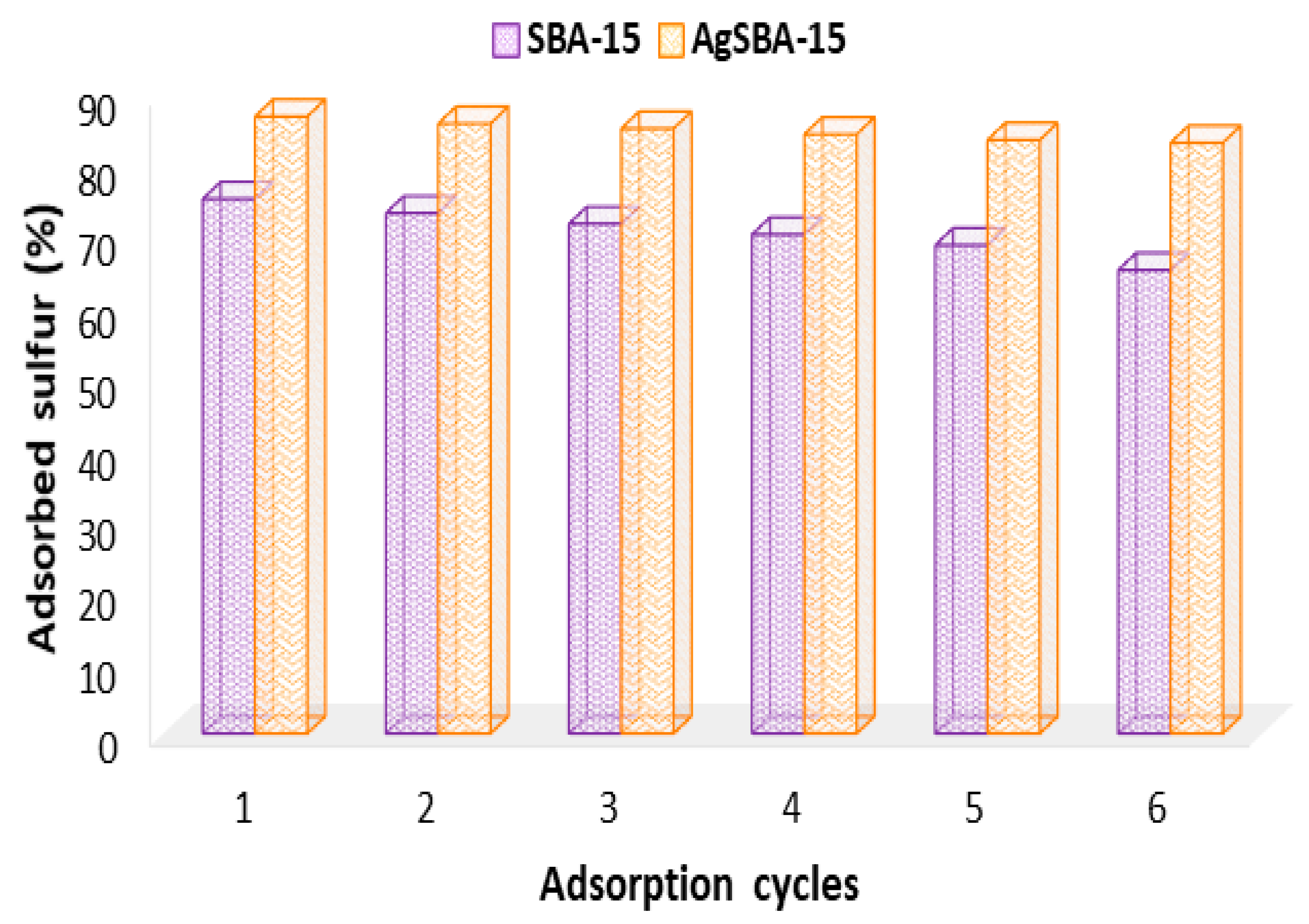
3.6. Recycling Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morselli, D.; Campagnolo, L.; Prato, M.; Papadopoulou, E.L.; Scarpellini, A.; Athanassiou, A.; Fragouli, D. Ceria/Gold Nanoparticles in Situ Synthesized on Polymeric Membranes with Enhanced Photocatalytic and Radical Scavenging Activity. ACS Appl. Nano Mater. 2018, 1, 5601–5611. [Google Scholar] [CrossRef]
- Campagnolo, L.; Lauciello, S.; Athanassiou, A.; Fragouli, D. Au/ZnO Hybrid Nanostructures on Electrospun Polymeric Mats for Improved Photocatalytic Degradation of Organic Pollutants. Water 2019, 11, 1787. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Maldonado, A.J.; Yang, F.H.; Qi, G.; Yang, R.T. Desulfurization of transportation fuels by π-complexation sorbents: Cu (I)-, Ni (II)-, and Zn (II)-Zeolites. Appl. Catal. B Enrivon. 2005, 56, 111–126. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.; Zhen, L.; Ikram, M.; Ullah, R.; Etim, U.J.; Ahmad, A. Ammonia assisted functionalization of cuprous oxide within confined spaces of SBA-15 for adsorptive desulfurization. Chem. Eng. J. 2018, 339, 557–565. [Google Scholar] [CrossRef]
- Aslam, S.; Subhan, F.; Yan, Z.; Xing, W.; Zeng, J.; Liu, Y.; Ikram, M.; Rehman, S.; Ullah, R. Rapid functionalization of as-synthesized KIT-6 with nickel species occluded with template for adsorptive desulfurization. Microporous Mesoporous Mater. 2015, 214, 54–63. [Google Scholar] [CrossRef]
- Ahmadi, M.; Anvaripour, B.; Khosravi-NiKou, M.R.; Mohammadian, M. Selective denitrogenation of model fuel through iron and chromium modified microporous materials (MSU-S). J. Environ. Chem. Eng. 2017, 5, 846–860. [Google Scholar] [CrossRef]
- Janus, R.; Wardrzyk, M.; Natkanski, P.; Cool, P.; Kustrowski, P. Dynamic adsorption–desorption of methyl ethyl ketone on MCM-41 and SBA-15 decorated with thermally activated polymers. J. Ind. Eng. Chem. 2019, 71, 465–480. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1988, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Sales, R.F.; Moura, H.O.M.A.; Câmara, A.B.F.; Rodríguez-Castellón, E.; Silva, J.A.B.; Pergher, S.B.C.; Campos, L.M.A.; Urbina, M.M.; Bicudo, T.C.; Carvalho, L.S. Assessment of Ag Nanoparticles Interaction over Low-Cost Mesoporous Silica in Deep Desulfurization of Diesel. Catalysts 2019, 9, 651. [Google Scholar] [CrossRef] [Green Version]
- Sareen, S.; Mutreja, V.; Pal, B.; Singh, S. Homogeneous dispersion of Au nanoparticles into mesoporous SBA-15 exhibiting improved catalytic for nitroaromatic reduction. Microporous Mesoporous Mater. 2015, 202, 219–225. [Google Scholar] [CrossRef]
- Sikarwar, P.; Kumar, U.K.A.; Gosu, V.; Subbaramaiah, V. Catalytic oxidativedesulfurization of DBT using green catalyst (Mo/MCM-41) derived from coal fly ash. J. Environ. Chem. Eng. 2018, 6, 1736–1744. [Google Scholar] [CrossRef]
- Liao, J.-J.; Bao, W.-R.; Chang, L.-P. An approach to study the desulfurization mechanism and the competitive behavior from aromatics: A case study on CeY zeolite. Fuel Process. Technol. 2015, 140, 104–112. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, B.; Ding, Y.; Wang, L.; Xie, F.; Cai, W.; Liu, S.; Zhou, J. New composite aerogel-like adsorbents for thiophene based on π-complexation. Sep. Purif. Technol. 2018, 192, 46–54. [Google Scholar] [CrossRef]
- Hussain, A.H.M.S.; Tatarchuk, B.J. Mechanism of hydrocarbon fuel desulfurization using Ag/TiO2–Al2O3 adsorbent. Fuel Process. Technol. 2014, 126, 233–242. [Google Scholar] [CrossRef]
- Souza, M.J.B.; Pedrosa, A.M.G.; Cecilia, J.A.; Gil-Mora, A.M.; Rodríguez-Castellón, E. Hydrodesulfurization of dibenzothiophene over PtMo/MCM-48 catalysts. Catalysis. Communications 2015, 69, 217–222. [Google Scholar]
- Shan, J.-H.; Chen, L.; Sun, L.-B.; Liu, X.-Q. Adsorptive Removal of Thiophene by Cu-Modified Mesoporous Silica MCM-48 Derived from Direct Synthesis. Energy Fuels 2011, 25, 3093–3099. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.; Ikram, M.; Rehman, S. Enhanced desulfurization characteristics of Cu-KIT-6 for thiophene. Microporous Mesoporous Mater. 2014, 199, 108–116. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Veer, A.; Shirsath, S.R.; Sonawane, S.H. Ultrasound assisted preparation, characterization and adsorption study of ternary chitosan-ZnO-TiO2nanocomposite: Advantage over conventional method. Ultrason. Sonochemistry 2019, 52, 120–130. [Google Scholar] [CrossRef]
- Prajapati, Y.N.; Verma, N. Adsorptive desulfurization of diesel oil using nickel nanoparticle-doped activated carbon beads with/without carbon nanofibers: Effects of adsorbate size and adsorbent texture. Fuel 2017, 189, 186–194. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structure. J. Am. Chem. Soc. 1988, 120, 6024–6036. [Google Scholar] [CrossRef]
- Wang, X.; Ji, G.; Song, C.; Zhang, H.; Gao, C. Surface hydroxylation of SBA-15 via alkaline for efficient amidoxime-functionalization and enhanced uranium adsorption. Sepatation Publ. Technol. 2019, 209, 623–635. [Google Scholar] [CrossRef]
- Socci, J.; Osatiashtiani, A.; Kyriakou, G.; Bridgwater, T. The catalytic cracking of sterically challenging plastic feedstocks over high acid density Al-SBA-15 catalysts. Appl. Catal. A Gen. 2019, 570, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Sales, R.F.; Moura, H.O.M.A.; Silva, S.R.B.; Souza, M.A.F.; Campos, L.M.A.; Rodríguez-Castellón, E.; Carvalho, L.S. Experimental and theoretical study of adsorptive interactions in diesel fuel desulfurization over Ag/MCM-41 adsorbent. Adsorption 2020, 26, 189–201. [Google Scholar] [CrossRef]
- Shen, S.; Chen, J.; Koodali, R.T.; Hu, Y.; Xiao, Q.; Zhou, J.; Wang, X.; Guo, L. Activation of MCM-41 mesoporous silica by transition-metal incorporation for photocatalytic hydrogen production. Appl. Catal. B Environ. 2014, 150, 138–146. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Borcuch, A.; Michalik, M.; Rutkowska, M.; Gil, B.; Sojka, Z.; Indyka, P.; Chmielarz, L. MCM-41 modified with transition metals by template ion-exchange method as catalysts for selective catalytic oxidation of ammonia to dinitrogen. Microporous Mesoporous Mater. 2017, 240, 9–21. [Google Scholar] [CrossRef]
- Chotirach, M.; Tungasmita, S.; Tungasmita, D.N.; Tantayanon, S. Titanium nitride promoted Ni-based SBA-15 catalyst for dry reforming of methane. Int. J. Hydrogen Energy 2018, 43, 21322–21332. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Imbrahim, A.A.; Atia, H.; Fakeeha, A.H.; Armbruster, U.; Abasaeed, A.E.; Frusteri, F. Evaluation of Co-Ni/Sc-SBA-15 as a novel coke resistant catalyst for syngas production via CO2reformingofmethane. Appl. Catal. A Gen. 2018, 567, 102–111. [Google Scholar] [CrossRef]
- Valles, V.A.; Sa- ngasaeng, Y.; Martínez, M.L.; Jongpatiwut, S.; Beltramone, A.R. HDT of model diesel feed over Ir-modified Zr-SBA-15 catalysts. Fuel 2019, 240, 138–152. [Google Scholar] [CrossRef]
- Chiang, H.-L.; Wu, T.-N.; Zeng, L.-X. Carbon material formation and residue characteristics of SBA-15 and nickel impregnated SBA-15 as exemplified bya cetone decomposition. Microporous Mesoporous Mater. 2019, 279, 286–292. [Google Scholar] [CrossRef]
- Sanches, S.G.; Flores, J.H.; Silva, M.I.P. Ti dispersion on SBA-15 porous host to enhance photocatytic hydrogen production. J. Mol. Struct. 2018, 1170, 9–17. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Wang, Y.; Xia, C.; Hong, E.; Bai, L.; Li, T.; Wang, B. Study on the controlled synthesis and photocatalytic performance of rare earth Nd deposited on mesoporous TiO2 photocatalysts. Sci. Total Environ. 2019, 652, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.; Chong, C.C.; Teh, D.-V.N.V.; Ainirazali, N.; Triwahyono, S.; Jalil, A.A.; Setiabudi, H.D. Promising hydrothermal technique for efficient CO2 methanation over Ni/SBA-15. Int. J. Hydrogen Energy 2019, 44, 20792–20804. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, D.H.; Kang, Y.S. High temperature proton exchange membranes based on triazoles attached onto SBA-15 type mesoporous silica. J. Membr. Sci. 2010, 357, 1–5. [Google Scholar] [CrossRef]
- Shahriary, M.; Veisi, H.; Hekmati, M.; Hemmati, S. In situ green synthesis of Ag nanoparticles on herbal tea extract (Stachys lavandulifolia)-modified magnetic iron oxide nanoparticles as antibacterial agent and their 4-nitrophenol catalytic reduction activity. Mater. Sci. Eng. C 2018, 90, 57–66. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, D.K.; Cha, H.G.; Kim, C.W.; Kang, Y.S. Synthesis and Characterization of Antibacterial Ag-SiO2 Nanocomposite. J. Phys. Chem. 2007, 111, 3629–3635. [Google Scholar] [CrossRef]
- Misran, H.; Salim, M.A.; Ramesh, S. Effect of Ag nanoparticles seeding on the properties of silica spheres. Ceram. Int. 2018, 44, 5901–5908. [Google Scholar] [CrossRef]
- Dutov, V.V.; Mamontov, G.V.; Zaikovskii, V.I.; Vodyankina, O.V. The effect of support pretreatment on activity of Ag/SiO2 catalysts in low-temperature CO oxidation. Catal. Today 2016, 278, 150–156. [Google Scholar] [CrossRef]
- Calderon, S.; Galindo, R.E.; Benito, N.; Palacio, C.; Cavaleiro, A.; Carvalho, S. Ag+ release inhibition from ZrCN–Ag coatings by surface agglomeration mechanism: Structural characterization. J. Phys. D Appl. Phys. 2013, 46, 325303. [Google Scholar] [CrossRef]
- Losurdo, M.; Bergmair, I.; Dastmalchi, B.; Kim, T.-H.; Giangregroio, M.M.; Jiao, W.; Bianco, G.V.; Brown, A.S.; Hingerl, K.; Bruno, G. Graphene as an Electron Shuttle for Silver Deoxidation: Removing a Key Barrier to Plasmonics and Metamaterials for SERS in the Visible. Adv. Funct. Mater. 2014, 24, 1864–1878. [Google Scholar] [CrossRef]
- Hu, L.; Yuan, H.; Zou, L.; Chen, F.; Hu, X. Adsorption and visible light-driven photocatalytic degradation of Rhodamine B in aqueous solutions by Ag@AgBr/SBA-15. Appl. Surf. Sci. Vol. 2015, 355, 706–715. [Google Scholar] [CrossRef]
- Tang, H.; Li, W.; Zhang, T.; Li, Q.; Xing, J.; Liu, H. Improvement in diesel desulfurization capacity by equilibrium isotherms analysis. Sep. Purif. Technol. 2011, 78, 352–356. [Google Scholar] [CrossRef]
- Grzelak, A.; Jarón, T.; Mazej, Z.; Michalowski, T.; Szarek, P.; Grochala, W. Anomalous chemical shifts in X-ray photoelectron spectra of sulfur-containing compounds of silver (I) and (II). J. Electron Spectrosc. Relat. Phenom. 2015, 202, 38–45. [Google Scholar] [CrossRef]
- Khan Rao, R.A.; Khatoon, A. Aluminate treated Casuarina equisetifolia leaves as potential adsorbent for sequestering Cu(II), Pb(II) and Ni(II) from aqueous solution. J. Clean. Prod. 2017, 165, 1280–1295. [Google Scholar] [CrossRef]
- Berger, A.H.; Bhown, A.S. Comparing Physisorption and Chemisorption Solid Sorbents for use Separating CO2 from Flue Gas using Temperature Swing Adsorption. Energy Procedia 2011, 4, 562–576. [Google Scholar] [CrossRef] [Green Version]
- Saleh, T.A.; Sulaiman, K.O.; AL-Hammadi, S.A.; Dafalla, H.; Danmaliki, G.I. Adsorptive desulfurization of thiophene, benzothiophene and dibenzothiophene over activated carbon manganese oxide nanocomposite: With column system evaluation. J. Clean. Prod. 2017, 154, 401–412. [Google Scholar] [CrossRef]
- Habibi, A.; Belaroui, L.S.; Bengueddach, A.; Galindo, A.L.; Díaz, C.I.S.; Peña, A. Adsorption of metronidazole and spiramycin by an Algerian palygorskite. Effect of modification with tin. Microporous Mesoporous Mater. 2018, 268, 293–302. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Silva, M.G.C.; Vieira, M.G.A. Equilibrium and kinetic studies of caffeine adsorption from aqueous solutions on thermally modified Verde-lodo bentonite. Appl. Clay SCI 2019, 168, 366–373. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Choi, A.E.S.; Roces, S.; Dugos, N.; Arcega, A.; Wan, M. Adsorptive removal of dibenzothiophene sulfone from fuel oil using clay material adsorbents. J. Clean. Prod. 2017, 161, 267–276. [Google Scholar] [CrossRef]
- Srivastav, A.; Srivastava, V.C. Adsorptive desulfurization by activated alumina. J. Hazard. Mater. 2009, 170, 1133–1140. [Google Scholar] [CrossRef]
- Tang, K.; Hong, X.; Zhao, Y.H.; Wang, Y.G. Adsorption Desulfurization on a Nanocrystalline NaY Zeolite Synthesized Using Carbon Nanotube Templated Growth. Pet. Sci. Technol. 2011, 29, 779–787. [Google Scholar] [CrossRef]
- Moosavi, S.E.; Dastgheib, S.A.; Karimzadeh, R. Adsorption of Thiophenic Compounds from Model Diesel Fuel Using Copper and Nickel Impregnated Activated Carbons. Energies 2012, 5, 4233–4250. [Google Scholar] [CrossRef]
- Ren, X.; Miao, G.; Xiao, Z.; Ye, F.; Li, Z.; Wang, H.; Xiao, J. Catalytic adsorptive desulfurization of model diesel fuel using TiO2/SBA-15 under mild condition. Fuel 2016, 174, 118–125. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, T.; Yao, R.; Zeng, Y.; Zhang, L.; Jian, P. Adsorptive desulfurization of fuels with Cu(I)/SBA-15 via low-temperature reduction. Microporous Mesoporous Mater. 2017, 251, 69–76. [Google Scholar] [CrossRef]
- Danmaliki, G.; Saleh, T.A. Effects of bimetallic Ce/Fe nanoparticles on the desulfurization of thiophenes using activated carbon. Chem. Eng. J. 2017, 307, 914–927. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.; Liu, Z.; Etim, U.J.; Wadood, A.; Ullah, R. Confinement of mesopores within ZSM-5 and functionalization with Ni NPs for deep desulfurization. Chem. Eng. J. 2018, 354, 706–715. [Google Scholar] [CrossRef]
- Câmara, A.B.F.; Sales, R.V.; Bertolino, L.C.; Furlanetto, R.P.P.; Rodríguez-Castellón, E.; Carvalho, L.S. Novel application for palygorskite clay mineral: A kinetic and thermodynamic assessment of diesel fuel desulfurization. Adsorption 2020, 26, 267–282. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Ko, C.H.; Kim, S.H.; Kim, J.-N. Removal of Refractory Sulfur Compounds in Diesel Using Activated Carbon with Controlled Porosity. Energy Fuels 2009, 23, 2537–2543. [Google Scholar] [CrossRef]
- Bu, j.; Loh, G.; Gwie, C.G.; Dewiyanti, S.; Tasrif, M.; Borgna, A. Desulfurization of diesel fuels by selective adsorption on activated carbons: Competitive adsorption of polycyclic aromatic sulfur heterocycles and polycyclic aromatic hydrocarbons. Chem. Eng. J. 2011, 166, 166,207–217. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Akmal, Z.M. Direct decomposition of methane over Pd promoted Ni/SBA-15catalysts. Appl. Surf. Sci. 2015, 353, 127–136. [Google Scholar] [CrossRef]
- Bergaoui, M.; Nakhli, A.; Benguerba, Y.; Khalfaoui, M.; Erto, A.; Soetaredj, F.E.; Ismadji, S.; Ernst, B. Novel insights into the adsorption mechanism of methylene blue onto organo-bentonite: Adsorption isotherms modeling and molecular simulation. J. Mol. Liq. 2018, 272, 697–707. [Google Scholar] [CrossRef]
- Liu, Y.; Ying, D.; Sanguansri, L.; Augustin, M.A. Comparison of the adsorption behavior of catechin onto cellulose and pectin. Food Chem. 2019, 271, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AlChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Demiral, H.; Güngör, C. Adsorption of copper (II) from aqueous solutions on activated carbon prepared from grape bagasse. J. Clean. Prod. 2016, 124, 103–113. [Google Scholar] [CrossRef]
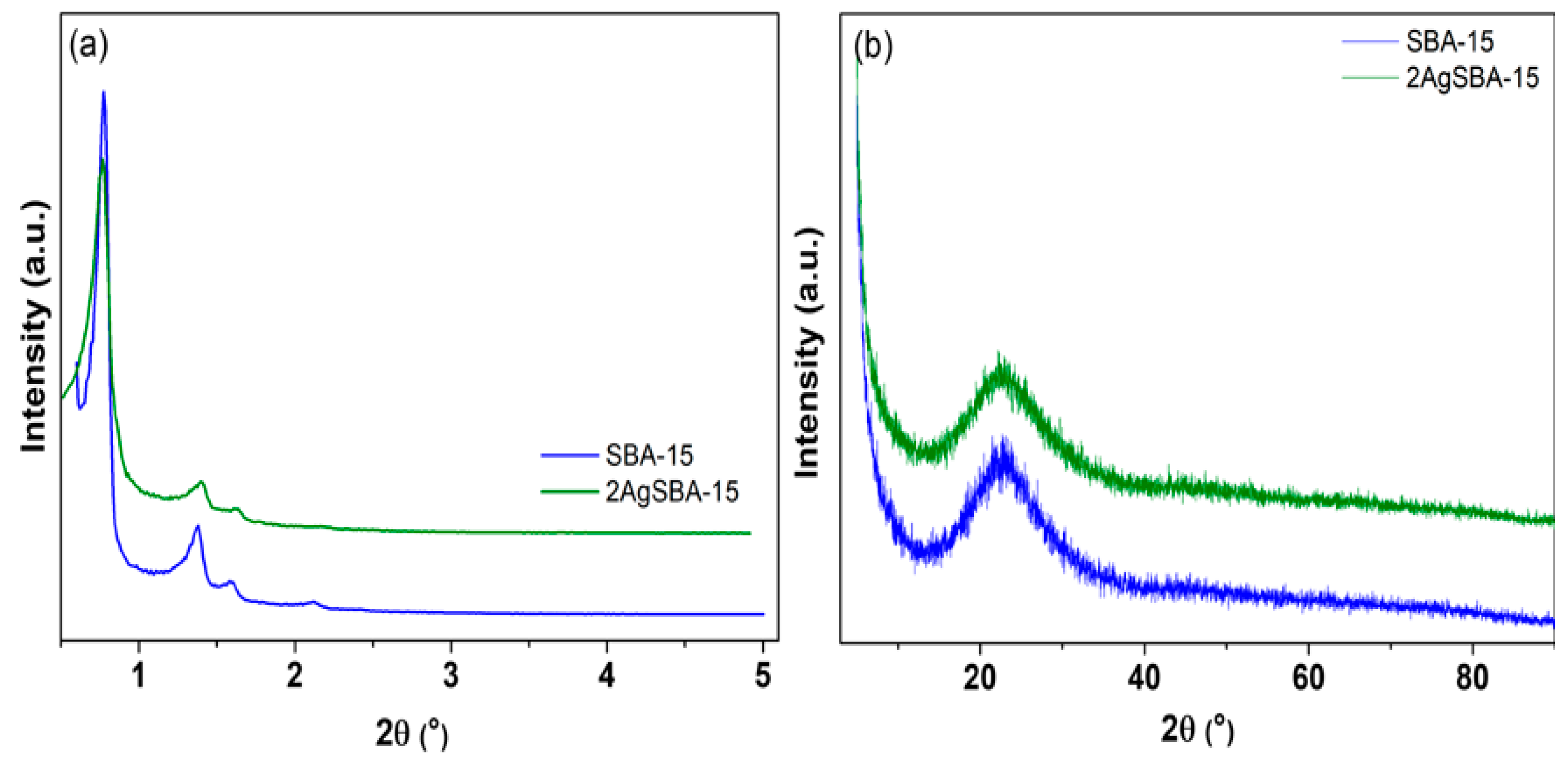
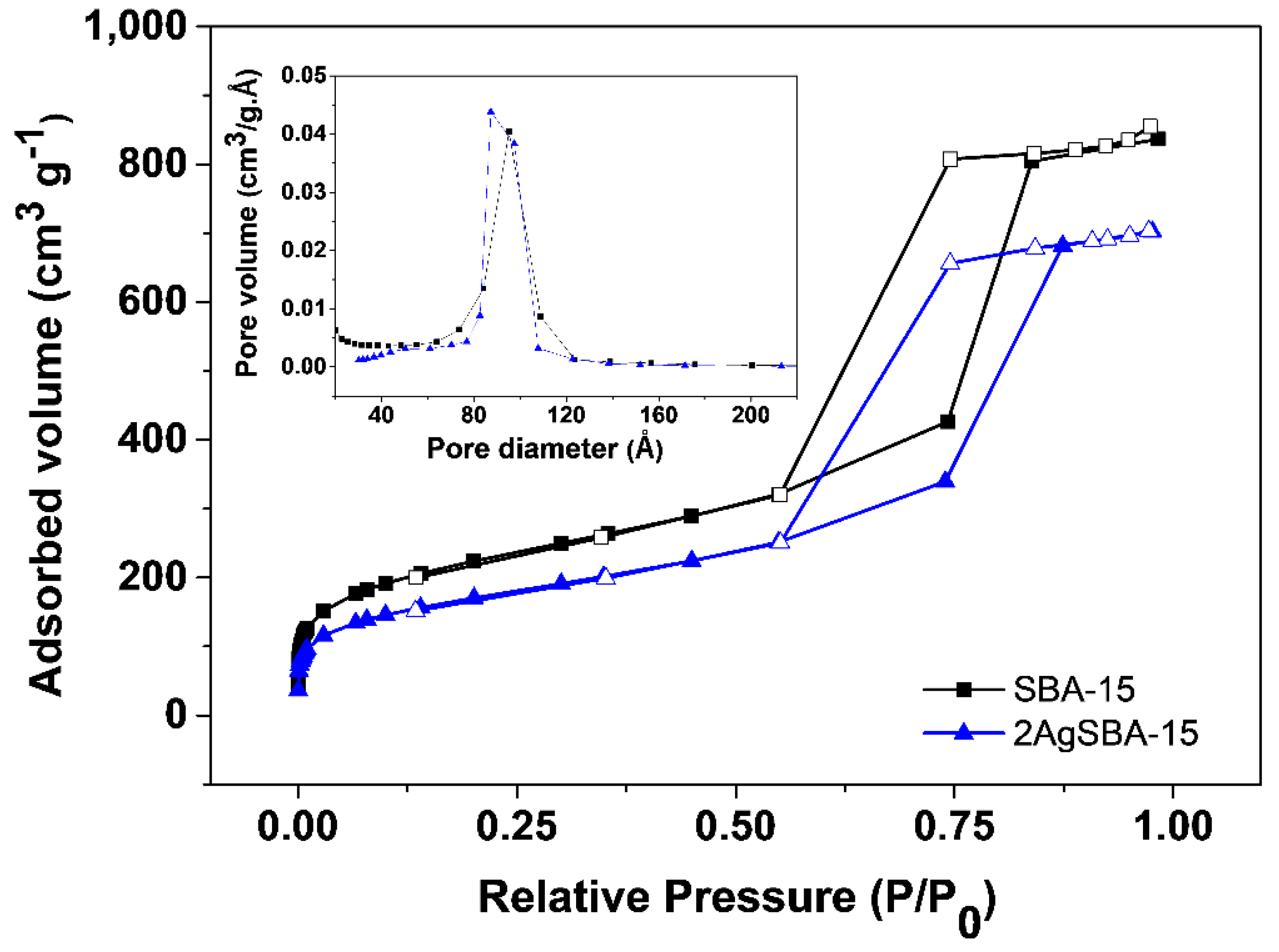
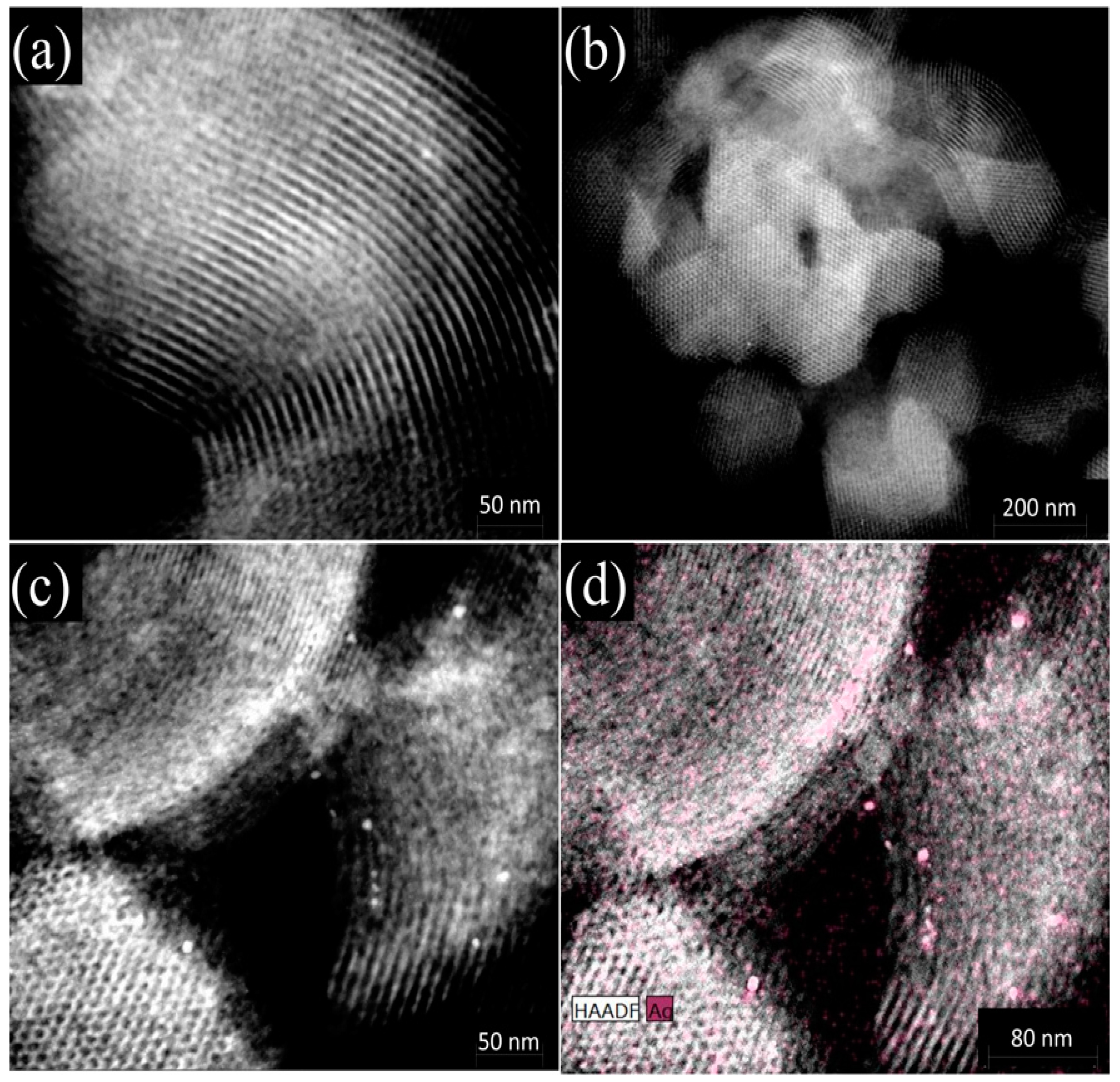

| Adsorbent | SBET 1 (m2/g) | VP 2 (cm3/g) | Maximum Pore Diameter (Å) |
|---|---|---|---|
| SBA-15 | 757 | 1.32 | 95 |
| 2AgSBA-15 | 576 | 1.10 | 87 |
| Model or Parameter | Adsorbent | |
|---|---|---|
| SBA-15 | 2AgSBA-15 | |
| qexp | 3.085 | 5.221 |
| Pseudo-first-order model | ||
| qe (mg/g) | 0.253 | 0.273 |
| k1 (min−1) | 0.029 | 0.005 |
| R2 | 0.8810 | 0.8904 |
| Pseudo-second-order model | ||
| qe (mg/g) | 3.115 | 5.219 |
| k2 (g/(mg min)) | 0.365 | 0.107 |
| R2 | 0.9999 | 0.9999 |
| Intraparticle diffusion | ||
| kid (mg/(g min)) | 0.037 | 0.008 |
| C (mg/g) | 2.7438 | 4.973 |
| R2 | 0.5532 | 0.606 |
| Elovich | ||
| α (mg/(g min)) | 2.37 × 1010 | 57.33 |
| β (g/mg) | 0.118 | 20.47 |
| R2 | 0.7601 | 0.8402 |
| Boyd | ||
| B 1 | 0.002 | 0.005 |
| R2 | 0.7857 | 0.9034 |
| Isotherm | Parameter | Adsorbent | |
|---|---|---|---|
| SBA-15 | 2AgSBA-15 | ||
| Langmuir | KL (L/mg) | 0.0024 | 0.0023 |
| qm (mg/g) | 10.62 | 20.30 | |
| R2 | 0.9909 | 0.9994 | |
| Freundlich | KF | 0.0696 | 0.079 |
| n | 1.39 | 1.22 | |
| R2 | 0.9412 | 0.9909 | |
| Temkin | α | 0.0900 | 0.069 |
| β | 0.96 | 2.406 | |
| R2 | 0.9787 | 0.9169 | |
| D-R | qs | 10.29 | 4.087 |
| β | 5 × 10−7 | 5.318 × 10−5 | |
| E | 1000 | 96.964 | |
| R2 | 0.5066 | 0.6242 | |
| Adsorbent | Feedstock | Conditions | qm a (mg/ g) | Reference |
|---|---|---|---|---|
| Alumina | DBT | 25 °C | 21.02 | [50] |
| NaY Zeolite | DBT; 4,6-DMDBT | room temperature | 6.7; 1.7 | [51] |
| Activated carbons | DBT | room temperature, Cu+ | 19.0 | [52] |
| TiO2/SBA-15 | DBT | 35 °C | 19.3; 12.7; 7.2 | [53] |
| Cu(I)/SBA-15(30) | Tiophene | 30 °C, 6.27% de Cu (wt%) | 21.85 | [54] |
| 10Cu/SBA-15 | Tiophene | room temperature | 14.33 | [4] |
| AC/Ce/Fe | Tiophene, BT, DBT | 0.33; 2.48; 3.70 | [55] | |
| MMZ; 5Ni/MMZ; 10Ni/MMZ; UR.10Ni/MMZ; 15Ni/MMZ | Tiophene | room temperature | 16.96; 21.37; 23.98; 9.095; 10.87 | [56] |
| MCM-41 | Diesel fuel | 25 °C | 3.06 | [9] |
| AgNO3 or Ag2O/MCM-41 | Diesel fuel | 25 °C, 2% Ag | 31.25; 13.95 | |
| Ni(NO3)2 or NiO/MCM-41 | Diesel fuel | 25 °C, 2% Ni | 8.97; 5.38 | [9] |
| MCM-41 or AgMCM-41 | Diesel fuel | 25 °C, 2% Ag | 7.25; 15.41 | [23] |
| Palygorskite | Diesel fuel | 45 °C | 6.25 | [57] |
| SBA-15 | Diesel fuel | 25 °C | 10.62 | This study |
| 2AgSBA-15 | Diesel fuel | 25 °C, 2% Ag | 20.30 | This study |
| Adsorption Capacity Equation | ||||
|---|---|---|---|---|
| Equation | Parameter | Unity | ||
| Adsorption capacity | 1qe, 2V, 3Ci, 4Ce and 5MAD | mg/g, L, mg/L, mg/L and g | (1) | |
| Equations and parameters of the kinetic models | ||||
| Equation | Parameter | Unity | ||
| Pseudo-first-order | 1qe, 6qt, 7t and 8k1 | mg/g, mg/g, min and min−1 | (2) | |
| Pseudo-second-order | 7t, 6qt, 9k2 and 1qe | min, mg/g, mg/g min and mg/g | (3) | |
| Intraparticle diffusion | 6qt, 10kid and 7t | mg/g, mg/g min1/2 and min | (4) | |
| Elovich | 6qt, 11α, 12β and 7t | mg/g, mg/g min, g/mg, and min | (5) | |
| Boyd | 13Bt and 14F | - | (6) | |
| Equations and parameters of the equilibrium models | ||||
| Equation | Parameter | Unity | ||
| Langmuir model | 4Ce, 1qe, 15qmax and 16KL | g/L, mg/g, mg/g and L/g | (7) | |
| 16KL and 17C0 | L/g and mg/L | (8) | ||
| Freundlich model | 1qe,4Ce, 18KF and 19n | mg/g, g/L, L/g and Const. | (9) | |
| Temkin model | 1qe, 20β, 21Kt and 4Ce | mg/g, J/mol, L/g and g/L | (10) | |
| D-R | 24ε, 22R, 23T, 4Ce, 1qe, 25qs, 26β and 27E | J/mol, J/mol K, K, g/L, mg/g, mg/g, mol2/kJ2 and kJ/mol | (11) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Longe, C.; Viana Sales, R.; Figueira Câmara, A.B.; Oliveira Medeiros de Araújo Moura, H.; Rodríguez-Castellón, E.; Berenice Castellã Pergher, S.; Aguilera Campos, L.M.; Montoya Urbina, M.; Santos de Carvalho, L. Effective Interactions of Ag Nanoparticles on the Surface of SBA-15 in Performing Deep Desulfurization of Real Diesel Fuel. Catalysts 2020, 10, 593. https://doi.org/10.3390/catal10050593
de Longe C, Viana Sales R, Figueira Câmara AB, Oliveira Medeiros de Araújo Moura H, Rodríguez-Castellón E, Berenice Castellã Pergher S, Aguilera Campos LM, Montoya Urbina M, Santos de Carvalho L. Effective Interactions of Ag Nanoparticles on the Surface of SBA-15 in Performing Deep Desulfurization of Real Diesel Fuel. Catalysts. 2020; 10(5):593. https://doi.org/10.3390/catal10050593
Chicago/Turabian Stylede Longe, Clenildo, Rafael Viana Sales, Anne Beatriz Figueira Câmara, Heloise Oliveira Medeiros de Araújo Moura, Enrique Rodríguez-Castellón, Sibele Berenice Castellã Pergher, Leila Maria Aguilera Campos, Maritza Montoya Urbina, and Luciene Santos de Carvalho. 2020. "Effective Interactions of Ag Nanoparticles on the Surface of SBA-15 in Performing Deep Desulfurization of Real Diesel Fuel" Catalysts 10, no. 5: 593. https://doi.org/10.3390/catal10050593