Catalytic Pyrolysis of Tetra Pak over Acidic Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Potential Use of TPs as Pyrolysis Feedstock
2.2. Catalysts
2.3. TG Analysis
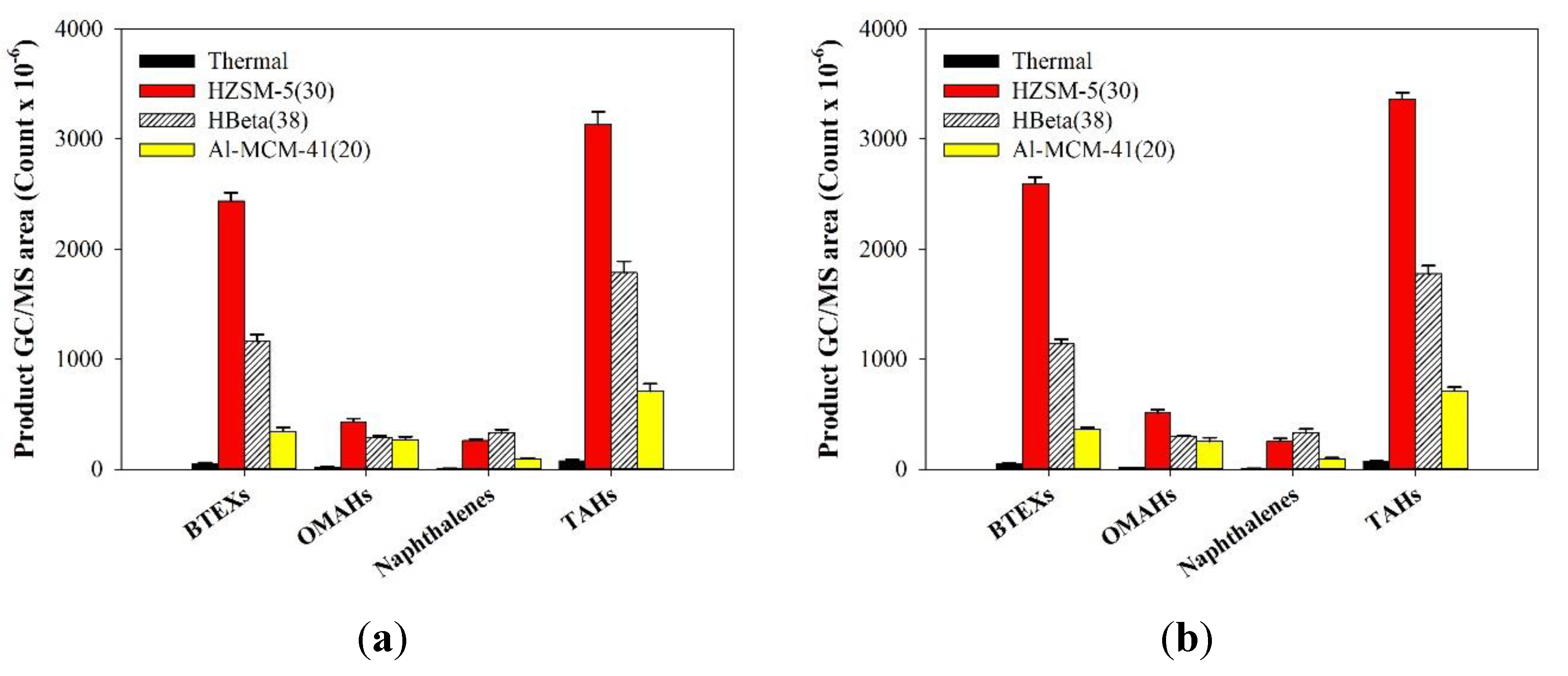
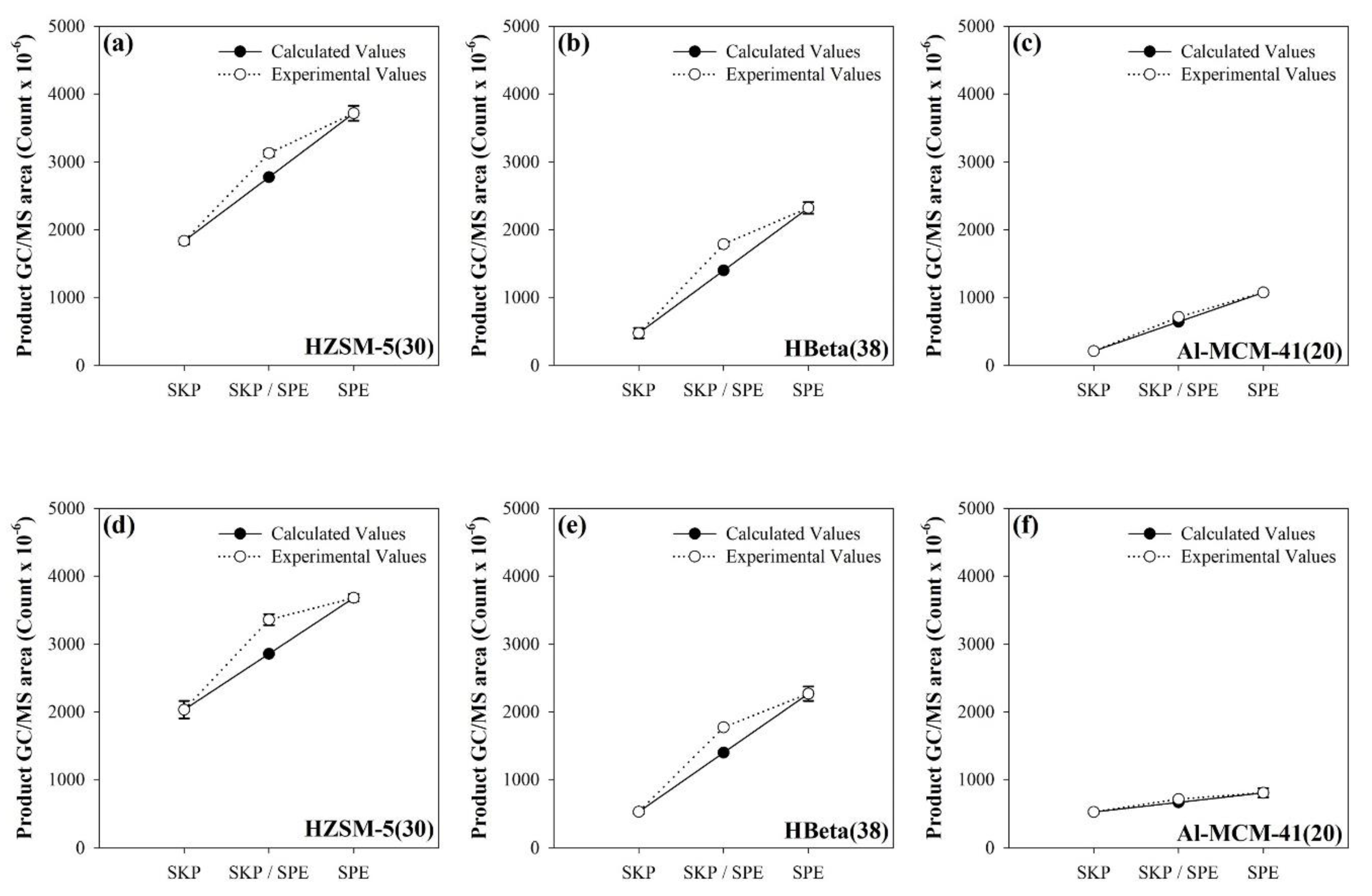
2.4. TMR-GC/MS Measurement
3. Materials and Methods
3.1. TP and Their Separation
3.2. Catalysts
3.3. TG Analysis
3.4. TMR-GC/MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Da Cruz, N.F.; Ferreira, S.; Cabral, M.; Simoes, P.; Marques, R.C. Packaging waste recycling in Europe: Is the industry paying for it? Waste Manag. 2014, 34, 298–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Adams, M.; Walker, T.R. Are exports of recyclables from developed to developing countries waste pollution transfer or part of the global circular economy? Resour. Conserv. Recycl. 2018, 136, 22–23. [Google Scholar] [CrossRef]
- Baggio, P.; Baratieri, M.; Gasparella, A.; Longo, G.A. Energy and environmental analysis of an innovative system based on municipal solid waste (MSW) pyrolysis and combined cycle. Appl. Thermal Eng. 2008, 28, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Velghe, I.; Carleer, R.; Yperman, J.; Schreurs, S. Study of the pyrolysis of municipal solid waste for the production of valuable products. J. Anal. Appl. Pyrolysis 2011, 92, 366–375. [Google Scholar] [CrossRef]
- Kim, S.; Ko, D.; Mun, J.; Kim, T.H.; Kim, J. Techno-economic evaluation of gas separation processes for long-term operation of CO2 injected enhanced coalbed methane (ECBM). Korean J. Chem. Eng. 2018, 35, 941–955. [Google Scholar] [CrossRef]
- Matsakas, L.; Gao, Q.; Jansson, S.; Rova, U.; Christakopoulos, P. Green conversion of municipal solid wastes into fuels and chemicals. Electron. J. Biotechnol. 2017, 26, 69–83. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Cepeliogullar, O.; Putun, A.E. Thermal and kinetic behaviors of biomass and plastic wastes in co-pyrolysis. Energy Convers. Manag. 2013, 75, 263–270. [Google Scholar] [CrossRef]
- Ozsin, G.; Putun, A.E. Co-pyrolytic behaviors of biomass and polystyrene: Kinetics, thermodynamics and evolved gas analysis. Korean J. Chem. Eng. 2018, 35, 428–437. [Google Scholar] [CrossRef]
- Europe, P. An Analysis of European Plastics Production, Demand and Waste Data. Plast. Fact 2018. [Google Scholar]
- Korkmaz, A.; Yanik, J.; Brebu, M.; Vasile, C. Pyrolysis of the tetra pak. Waste Manag. 2009, 29, 2836–2841. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.J.; Ping, Z.P.; Shi, F.X. Discussion on recovery and reuse of Tetra pak package. J. Agr. Eng. Res. 2007, 79, 221–223. [Google Scholar]
- Xie, M.H.; Li, L.; Huang, Z.C.; Zhu, X.M.; Zhang, J.F.; Sun, T.C. Life cycle assessment of environ mental impacts of Al-PE-Pa laminated packaging and waste treatments. Res. Environ. Sci. 2009, 22, 1299–1304. [Google Scholar]
- Bertoluci, G.; Leroy, Y.; Olsson, A. Exploring the environmental impacts of olive packaging solutions for the European food market. J. Clean. Prod. 2014, 64, 234–243. [Google Scholar] [CrossRef]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of polymer-based multilayer packaging: A review. Recycling 2018, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Gente, V.; Marca, F.L.; Lucci, F.; Massacci, P. Electrical separation of plastics coming from special waste. Waste Manag. 2003, 23, 951–958. [Google Scholar] [CrossRef]
- Lopes, C.M.A.; Felisberti, M.I. Composite of low-density polyethylene and aluminum obtained from the recycling of postconsumer aseptic packaging. J. Appl. Polym. Sci. 2006, 101, 3183–3191. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, D.; Li, L.; Yang, Z.; Li, Z. Research on the effect of separation reagent concentration and reaction temperature on Al-PE wet separation technics. Chin. J. Environ. Eng. 2010, 4, 665–670. [Google Scholar]
- Kulkarni, A.K.; Daneshvarhosseini, S.; Yoshida, H. Effective recovery of pure aluminum from waste composite laminates by sub- and super-critical water. J. Supercrit. Fluids 2011, 55, 992–997. [Google Scholar] [CrossRef]
- Zhang, S.F.; Mei, X.X.; Zhang, L.L. Research progress of separations technology of aluminum-plastic in aseptic composite packaging. China Pulp Pap. 2012, 31, 65–68. [Google Scholar]
- Yan, D.; Peng, Z.; Liu, Y.; Li, L.; Huang, Q.; Xie, M.; Wang, Q. Optimizing and developing a continuous separation system for the wet process separation of aluminum and polyethylene in aseptic composite packaging waste. Waste Manag. 2015, 35, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Ackermann, P.W. Method of Recovering Individual Component Parts from Packaging Material Waste. U.S. Patent 5421526 A, 6 June 1995. [Google Scholar]
- Zhang, S.F.; Zhang, L.L.; Luo, K.; Sun, Z.X.; Mei, X.X. Separation properties of aluminium-plastic laminates in post-consumer Tetra Pak with mixed organic solvent. Waste Manag. Res. 2014, 32, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Chang, H.S. Pyrolysis of tetra pack in municipal solid waste. J. Chem. Technol. Biotechnol. 2001, 76, 779–792. [Google Scholar]
- Ebadi, M.; Farsi, M.; Narchin, P.; Madhoushi, M. The effect of beverage storage packets (Tetra PakTM) waste on mechanical properties of wood-plastic composites. J. Thermoplast. Compos. Mater. 2016, 29, 1601–1610. [Google Scholar] [CrossRef]
- Lokahita, B.; Aziz, M.; Yoshikawa, K.; Takahashi, F. Energy and resource recovery from Tetra Pak waste using hydrothermal treatment. Appl. Energy 2017, 207, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Singh, R.K. Recovery of hydrocarbon liquid from waste high density polyethylene by thermal pyrolysis. Braz. J. Chem. Eng. 2011, 28, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Singh, R.K. Thermolysis of high-density polyethylene to petroleum products. J. Petrol. Eng. 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Ismail, I.M.I.; Nizami, A.S. Effect of plastic waste types on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 2017, 119, 239–252. [Google Scholar] [CrossRef]
- Braz, C.E.M.; Crnkovic, P.C.G.M. Physical-Chemical characterization of biomass samples for application in pyrolysis process. Chem. Eng. Trans. 2014, 37, 523–528. [Google Scholar]
- Viana, H.F.S.; Rodrigues, A.M.; Godina, R.; Matias, J.C.O.; Nunes, L.J.R. Evaluation of the physical, chemical and thermal properties of Portuguese maritime pine biomass. Sustainability 2018, 10, 2877. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Kim, Y.M.; Jae, J.; Watanabe, C.; Kim, S.; Jung, S.C.; Park, Y.K. Pyrolysis and catalytic upgrading of Citrus unshiu peel. Bioresour. Technol. 2015, 194, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Pyra, K.; Tarach, K.A.; Janiszewska, E.; Majda, D.; Góra-Marek, K. Evaluation of the textural parameters of zeolite beta in LDPE catalytic degradation: Thermogravimetric analysis coupled with FTIR operando studies. Molecules 2020, 25, 916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krans, N.A.; Weber, J.L.; van den Bosch, W.; Zecević, J.; de Jongh, P.E.; de Jong, K.P. Influence of promotion on the growth of anchored colloidal iron oxide nanoparticles during synthesis gas conversion. ACS Catal. 2020, 10, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Tian, H.; Hu, Z.; Yin, Y.; Chen, D.; Yan, X. Characteristics and kinetics analyses of different genus biomass pyrolysis. Korean J. Chem. Eng. 2018, 35, 511–517. [Google Scholar] [CrossRef]
- Xu, F.; Zhong, L.; Xu, Y.; Feng, S.; Zhang, C.; Zhang, F.; Zhang, G. Highly efficient flame-retardant kraft paper. J. Mater. Sci. 2019, 54, 1884–1897. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jae, J.; Kim, B.S.; Hong, Y.; Jung, S.C.; Park, Y.K. Catalytic co-pyrolysis of torrefied yellow poplar and high-density polyethylene using microporous HZSM-5 and mesoporous Al-MCM-41 catalysts. Energy Convers. Manag. 2017, 149, 966–973. [Google Scholar] [CrossRef]
- Yuan, H.; Fan, H.; Shan, R.; He, M.; Gu, J.; Chen, Y. Study of synergistic effects during co-pyrolysis of cellulose and high-density polyethylene at various ratios. Energy Convers. Manag. 2018, 157, 517–526. [Google Scholar] [CrossRef]
- Haydary, J.; Susa, D.; Dudas, J. Pyrolysis of aseptic packages (tetrapak) in a laboratory screw type reactor and secondary thermal/catalytic tar decomposition. Waste Manag. 2013, 33, 1136–1141. [Google Scholar] [CrossRef]
- Grieco, E.M.; Baldi, G. Pyrolysis of polyethylene mixed with paper and wood: Interaction effects on tar, char and gas yields. Waste Manag. 2012, 32, 833–839. [Google Scholar]
- Zhou, Z.; Chen, X.; Wang, Y.; Liu, C.; Ma, H.; Zhou, C.; Qi, F.; Yang, J. Online photoionization mass spectrometric evaluation of catalytic co-pyrolysis of cellulose and polyethylene over HZSM-5. Bioresour. Technol. 2019, 275, 130–137. [Google Scholar] [CrossRef]
- Park, Y.K.; Siddiqui, M.Z.; Kang, Y.; Watanabe, A.; Lee, H.W.; Jeong, S.J.; Kim, S.; Kim, Y.M. Increased aromatics formation by the use of high-density polyethylene on the catalytic pyrolysis of mandarin peel over HY and HZSM-5. Catalysts 2018, 8, 656. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.K.; Jung, J.; Ryu, S.; Lee, H.W.; Siddiqui, M.Z.; Jae, J.; Watanabe, A.; Kim, Y.M. Catalytic co-pyrolysis of yellow poplar wood and polyethylene terephthalate over two stage calcium oxide-ZSM-5. Appl. Energy 2019, 250, 1706–1718. [Google Scholar]
- Auxilio, A.R.; Choo, W.L.; Kohli, I.; Srivatsa, S.H.; Bhattacharya, S. An experimental study on thermo-catalytic pyrolysis of plastic waste using a continuous pyrolyser. Waste Manag. 2017, 67, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Ma, X.; Li, Y.; Chen, P.; Liu, Y.; Lin, X.; Lei, H.; Ruan, R. Production of aromatic hydrocarbons by catalytic pyrolysis of microalgae with zeolites: Catalyst screening in a pyroprobe. Bioresour. Technol. 2013, 139, 397–401. [Google Scholar] [CrossRef]
- Marcilla, A.; Gomez-Siurana, A.; Valdes, F. Catalytic pyrolysis of LDPE over H-beta and HZSM-5 zeolites in dynamic conditions. Study of the evolution of the process. J. Anal. Appl. Pyrolysis 2007, 79, 433–442. [Google Scholar] [CrossRef]
- Cardona, S.C.; Corma, A. Tertiary recycling of polypropylene by catalytic cracking in a semibatch stirred reactor: Use of spent equilibrium FCC commercial catalyst. Appl. Catal. B Environ. 2000, 25, 151–162. [Google Scholar] [CrossRef]
- Park, Y.K.; Jung, J.S.; Jae, J.; Hong, S.B.; Watanabe, A.; Kim, Y.M. Catalytic fast pyrolysis of wood plastic composite over microporous zeolites. Chem. Eng. J. 2019, 377, 119742. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Ding, H.; Zhu, X. Two-step pyrolysis of corncob for value-added chemicals and high-quality bio-oil: Effects of alkali and alkaline earth metals. Waste Manag. 2019, 87, 709–718. [Google Scholar] [CrossRef]
- Hujuri, U.; Ghoshal, A.K.; Gumma, S. Temperature-dependent pyrolytic product evolution profile for low-density polyethylene from gas chromatographic study. Waste Manag. 2010, 30, 814–820. [Google Scholar] [CrossRef]
- Gracida-Alvarez, U.R.; Mitchell, M.K.; Sacramento-Rivero, J.C.; Shonnard, D.R. Effect of temperature and vapor residence time on the micropyrolysis products of waste high density polyethylene. Ind. Eng. Chem. Res. 2018, 57, 1912–1923. [Google Scholar] [CrossRef]
- Fukushima, M.; Shioya, M.; Wakai, K.; Ibe, H. Toward maximizing the recycling rate in a Sapporo waste plastics liquefaction plant. J. Mater. Cycles Waste Manag. 2009, 11, 11–18. [Google Scholar] [CrossRef]
- Mastral, F.J.; Esperanza, E.; Garcia, P.; Juste, M. Pyrolysis of high-density polyethylene in a fluidised bed reactor. Influence of the temperature and residence time. J. Anal. Appl. Pyrolysis 2002, 63, 1–15. [Google Scholar] [CrossRef]
- Xue, Y.; Kelkar, A.; Bai, X. Catalytic co-pyrolysis of biomass and polyethylene in a tandem micropyrolyzer. Fuel 2016, 166, 227–236. [Google Scholar] [CrossRef]
- Jae, J.; Tompsett, G.A.; Foster, A.J.; Hammond, K.D.; Auerbach, S.M.; Lobo, R.F.; Huber, G.W. Investigation into the shape selectivity of zeolite catalysts for biomass conversion. J. Catal. 2011, 279, 257–268. [Google Scholar] [CrossRef]
- Chang, Y.T.; Huber, G.W. Chemistry of furan conversion into aromatics and olefins over HZSM-5: A model biomass conversion reaction. ACS Catal. 2011, 1, 611–628. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Bao, Z.; Xia, S.; Lu, Q.; Walters, K.B. Catalytic pyrolysis of biomass and polymer wastes. Catalysts 2018, 8, 659. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Johnston, P.A.; Brown, R.C. Comparison of in-situ and ex-situ catalytic pyrolysis in a micro-reactor system. Bioresour. Technol. 2014, 173, 124–131. [Google Scholar] [CrossRef]
- Gamliel, D.P.; Du, S.; Bollas, G.M.; Valla, J.A. Investigation of in situ and ex situ catalytic pyrolysis of miscanthus × giganteus using a PyGC-MS microsystem and comparison with a bench-scale spouted-bed reactor. Bioresour. Technol. 2015, 191, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Lee, H.W.; Lee, S.-H.; Kim, S.-S.; Park, S.H.; Jeon, J.-K.; Kim, S.; Park, Y.-K. Pyrolysis properties and kinetics of mandarin peel. Korean J. Chem. Eng. 2011, 28, 2012–2016. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, S.; Han, T.U.; Park, Y.-K.; Watanabe, C. Pyrolysis reaction characteristics of Korean pine (Pinus koraiensis) nut shell. J. Anal. Appl. Pyrolysis 2014, 110, 435–441. [Google Scholar] [CrossRef]
- Ryoo, R.; Kim, J.M. Structural order in MCM-41 controlled by shifting silicate polymerization equilibrium. J. Chem. Soc. Chem. Commun. 1995, 711–712. [Google Scholar] [CrossRef]





| Sample | TP-1 | TP-2 | |
|---|---|---|---|
| Proximate Analysis (wt. %) a | Water | 1.1 | 1.5 |
| Volatiles | 80.7 | 80.3 | |
| Fixed carbon | 8.1 | 7.2 | |
| Ash | 10.1 | 11.0 | |
| Sum | 100.0 | 100.0 | |
| Ultimate Analysis (wt. %) a | C | 45.6 | 47.6 |
| H | 7.0 | 7.3 | |
| O b | 47.3 | 45.0 | |
| N | 0.1 | 0.1 | |
| S | 0.0 | 0.0 | |
| Sum | 100.0 | 100.0 | |
| HHV (kcal/kg) | 3759 | 3712 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, M.Z.; Han, T.U.; Park, Y.-K.; Kim, Y.-M.; Kim, S. Catalytic Pyrolysis of Tetra Pak over Acidic Catalysts. Catalysts 2020, 10, 602. https://doi.org/10.3390/catal10060602
Siddiqui MZ, Han TU, Park Y-K, Kim Y-M, Kim S. Catalytic Pyrolysis of Tetra Pak over Acidic Catalysts. Catalysts. 2020; 10(6):602. https://doi.org/10.3390/catal10060602
Chicago/Turabian StyleSiddiqui, Muhammad Zain, Tae Uk Han, Young-Kwon Park, Young-Min Kim, and Seungdo Kim. 2020. "Catalytic Pyrolysis of Tetra Pak over Acidic Catalysts" Catalysts 10, no. 6: 602. https://doi.org/10.3390/catal10060602








