Morphological and Optical Properties of Cobalt Ion-Modified ZnO Nanowires
Abstract
1. Introduction
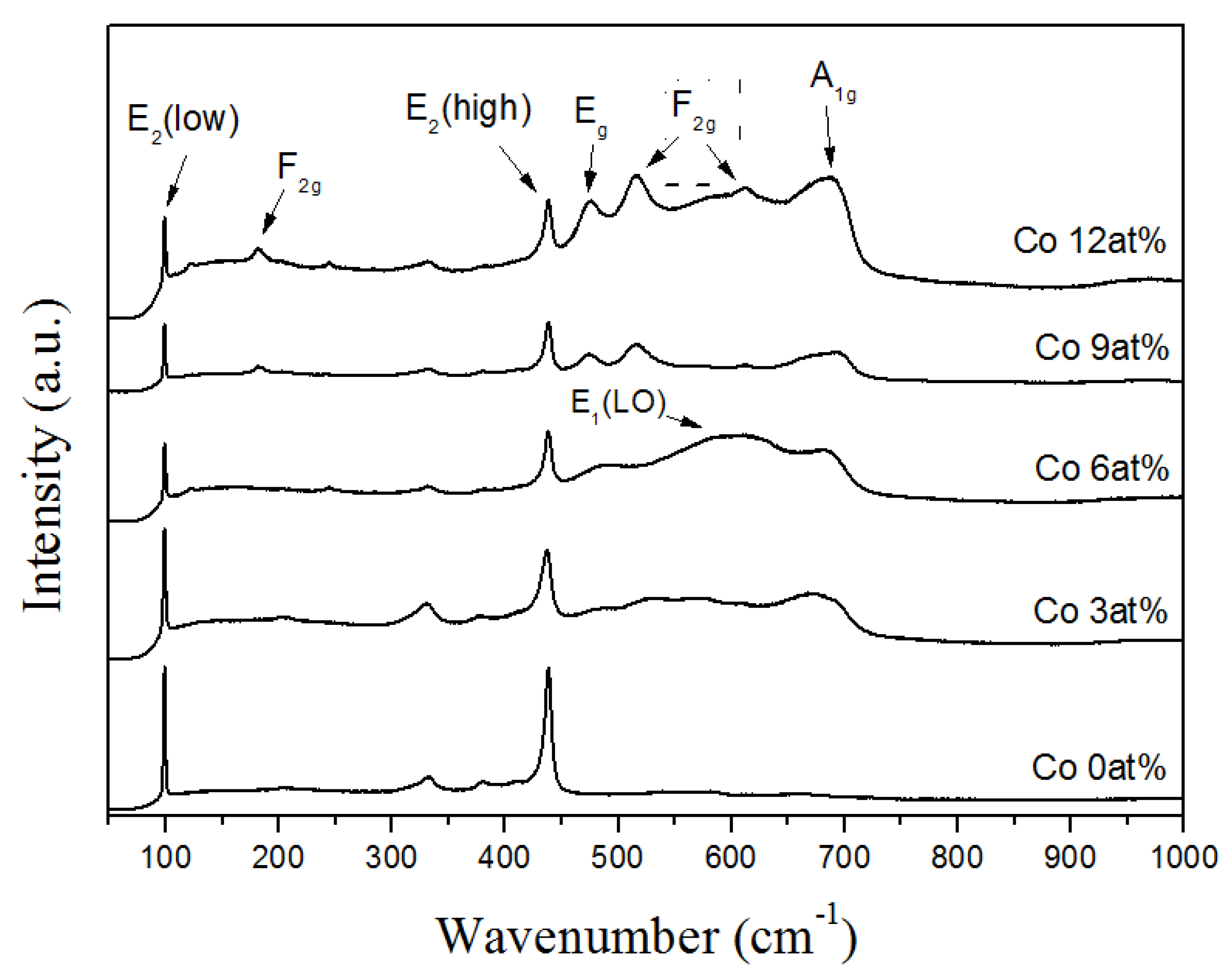
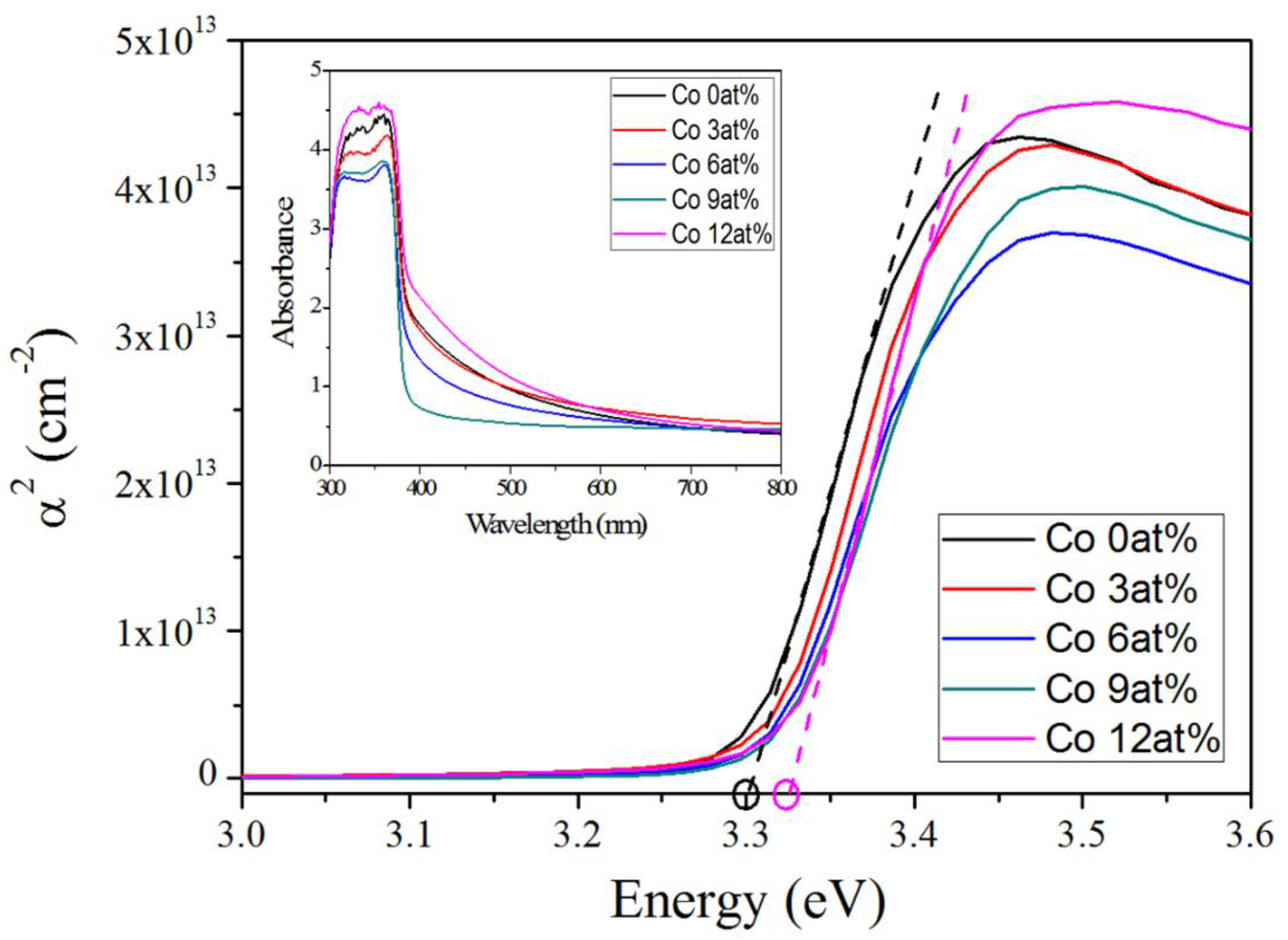
2. Results and Discussion
3. Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, L.; Guo, Y.; Cui, X.Y.; Zheng, R.K.; Ohtani, K.; Kong, C.; Ceguerra, A.V.; Moody, M.P.; Ye, J.D.; Tan, H.H.; et al. Magnetism of Co-doped ZnO epitaxially grown on a ZnO substrate. Phys. Rev. B 2012, 85, 174430. [Google Scholar] [CrossRef]
- De Carvalho, H.B.; deGodoy, M.P.F.; Paes, R.W.D.; Mir, M.; OrtizdeZevallos, A.; Iikawa, F.; Brasil, M.J.S.P.; Chitta, V.A.; Ferraz, W.B.; Boselli, M.A.; et al. Absence of ferromagnetic order in high quality bulk Co-doped ZnO samples. J. Appl. Phys. 2010, 108, 033914. [Google Scholar] [CrossRef]
- Pearton, S.J.; Abernathy, C.R.; Thaler, G.T.; Frazier, R.M.; Norton, D.P.; Ren, F.; Park, Y.D.; Zavada, J.M.; Buyanova, I.A.; Chen, W.M.; et al. Wide bandgap GaN-based semiconductors for spintronics. J. Phys. Condens. Matter 2004, 16, R209. [Google Scholar] [CrossRef]
- Sonoda, S.; Tanaka, I.; Ikeno, H.; Yamamoto, T.; Oba, F.; Araki, T.; Yamamoto, Y.; Suga, K.; Nanishi, Y.; Akasaka, Y.; et al. Coexistence of Mn2+ and Mn3+ in ferromagnetic GaMnN. J. Phys. Condens. Matter 2006, 18, 4615. [Google Scholar] [CrossRef]
- Sutka, A.; Kaambre, T.; Parna, R.; Juhnevica, I.; Maiorov, M.; Joost, U.; Kisand, V. Co doped ZnO nanowires as visible light photocatalysts. Solid State Sci. 2016, 56, 54–62. [Google Scholar] [CrossRef]
- Nair, M.G.; Nirmala, M.; Rekha, K.; Anukaliani, A. Structural, optical, photo catalytic and antibacterial activity of ZnO and Co doped ZnO nanoparticles. Mater. Lett. 2011, 65, 1797–1800. [Google Scholar] [CrossRef]
- Kuriakose, S.; Satpati, B. Mohapatra Enhanced photocatalytic activity of Co doped ZnO nanodisks and nanorods prepared by a facile wet chemical method. Phys. Chem. Chem. Phys. 2014, 16, 12741–12749. [Google Scholar] [CrossRef]
- Víctor-Román, S.; García-Bordejé, E.; Hernández-Ferrer, J.; González-Domínguez, J.M.; Ansón-Casaos, A.; Silva, A.M.T.; Maser, W.K.; Benito, A.M. Controlling the surface chemistry of graphene oxide: Key towards efficient ZnO-GO photocatalysts. Catal. Today 2019, in press. [Google Scholar]
- Liu, T.; Xu, H.R.; Chin, W.S.; Yong, Z.H.; Wee, A.T.S. Local Structural Evolution of Co-Doped ZnO Nanoparticles upon Calcination Studied by in Situ Quick-Scan XAFS. J. Phys. Chem. C 2008, 112, 3489–3495. [Google Scholar] [CrossRef]
- Phan, T.L.; Vincent, R.; Cherns, D.; Yu, N.H.D.S.C. Enhancement of multiple-phonon resonant Raman scattering in Co-doped ZnO nanorods. Appl. Phys. Lett. 2008, 93, 082110. [Google Scholar] [CrossRef]
- Liang, W.J.; Yuhas, B.D.; Yang, P.D. Magnetotransport in Co-Doped ZnO Nanowires. Nano Lett. 2009, 9, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Pung, S.Y.; Choy, K.L.; Hou, X. Tip-growth mode and base-growth mode of Au-catalyzed zinc oxide nanowires using chemical vapor deposition technique. J. Cryst. Growth 2010, 312, 2049–2055. [Google Scholar] [CrossRef]
- Chen, S.J.; Wang, G.R.; Liu, Y.C. The structure and photoluminescence properties of ZnO nanobelts prepared by a thermal evaporation process. J. Lumin. 2009, 129, 340–343. [Google Scholar] [CrossRef]
- Fabbiyola, S.; Kennedy, L.J.; Aruldoss, U.; Bououdina, M.; Dakhel, A.A.; Vijaya, J.J. Synthesis of Co-doped ZnO nanoparticles via co-precipitation: Structural, optical and magnetic properties. Powder Technol. 2015, 286, 757–765. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, Y.; Wang, D.; Wang, L.; Xie, T.; Jiang, T. A high performance cobalt-doped ZnO visible light photocatalyst and its photogenerated charge transfer properties. Nano Res. 2011, 4, 1144–1152. [Google Scholar] [CrossRef]
- Caglar, Y. Sol–gel derived nanostructure undoped and cobalt doped ZnO: Structural, optical and electrical studies. J. Alloys Compd. 2013, 560, 181–188. [Google Scholar] [CrossRef]
- Wang, A.; Zhong, Z.; Lu, C.; Lv, L.; Wang, X.; Zhang, B. Study on field-emission characteristics of electrodeposited Co-doped ZnO thin films. Phys. B Condens. Matter. 2011, 406, 1049–1052. [Google Scholar] [CrossRef]
- Wang, L.L.; Lin, B.Z.; Zhou, L.; Shang, Y.X.; Panin, G.N.; Fu, D.J. Nitrogen-doped ZnO nanorods prepared by hydrothermal diffusion. Mater. Lett. 2012, 85, 171–174. [Google Scholar] [CrossRef]
- Guria, A.K.; Pradhan, N. Doped or Not Doped: Ionic Impurities for Influencing the Phase and Growth of Semiconductor Nanocrystals. Chem. Mater. 2016, 28, 5224–5237. [Google Scholar] [CrossRef]
- Zou, C.; Liang, F.; Xue, S. Synthesis and oxygen vacancy related NO2 gas sensing properties of ZnO:Co nanorods arrays gown by a hydrothermal method. Appl. Surf. Sci. 2015, 353, 1061–1069. [Google Scholar] [CrossRef]
- He, X.; Yang, H.; Chen, Z.; Lia, S.S.Y. Effect of Co-doping content on hydrothermal derived ZnO array films. Phys. B Condens. Matter 2012, 407, 2895–2899. [Google Scholar] [CrossRef]
- Liu, J.; Lee, S.I.; Lee, K.M.; Ahn, Y.H.; Park, J.Y.; Koh, K.H. Bending and bundling of metal-free vertically aligned ZnO nanowires due to electrostatic interaction. Nanotechnology 2008, 19, 185607. [Google Scholar] [CrossRef] [PubMed]
- Erwin, S.C.; Zu, L.; Haftel, M.I.; Efros, A.L.; Kennedy, T.A.; Norris, D.J. Doping semiconductor nanocrystals. Nature 2005, 436, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Yuhas, B.D.; Zitoun, D.O.; Pauzauskie, P.J.; He, R.; Yang, P.D. Transition-Metal Doped Zinc Oxide Nanowires. Angew. Chem. Int. Ed. 2006, 45, 420–423. [Google Scholar] [CrossRef]
- Djerdj, I.; Garnweitner, G.; Arcon, D.; Pregeli, M.; Jaqlicic, Z.; Niedegrberger, M. Diluted magnetic semiconductors: Mn/Co-doped ZnO nanorods as case study. J. Mater. Chem. 2008, 18, 5208–5217. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, W.J.; Nahm, C.W.; Kim, J.M.; Byun, S.J.; Hwang, T.H.; Lee, B.K.; Jang, Y.I.; Lee, S.G.; Lee, H.M.; et al. Nanostructural analysis of ZnO:Al thin films for carrier-transport mechanisms. Curr. Appl. Phys. 2013, 13, 775–778. [Google Scholar]
- Yilmaz, M.; Caldiran, Z.; Deniz, A.R.; Aydogan, S.; Gunturkun, R.; Turut, A. Preparation and characterization of sol–gel-derived n-ZnO thin film for Schottky diode application. Appl. Phys. A 2015, 119, 547–552. [Google Scholar] [CrossRef]
- Samanta, K.; Bhattacharya, P.; Katiyar, R.S.; Iwamoto, W.; Pagliuso, P.G.; Rettori, C. Raman scattering studies in dilute magnetic semiconductor Zn1−xCoxO. Phys. Rev. B 2006, 73, 245213. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.N.; Vegilov, I.V. The Raman spectra of Co3O4. J. Phys. C Solid State Phys. 1988, L199, 21. [Google Scholar] [CrossRef]
- Alaria, J.; Bieber, H.; Colis, S.; Schmerber, G.; Dinia, A. Absence of ferromagnetism in Al-doped Zn0.9Co0.10O diluted magnetic semiconductors. Appl. Phys. Lett. 2006, 88, 112503. [Google Scholar] [CrossRef]
- Ansón-Casaos, A.; Sampaio, M.J.; Jarauta-Córdoba, C.; Martínez, M.T.; Silva, C.G.; Faria, J.L.; Silva, A.M.T. Evaluation of sol–gel TiO2 photocatalysts modified with carbon or boron compounds and crystallized in nitrogen or air atmospheres. Chem. Eng. J. 2015, 277, 11–20. [Google Scholar] [CrossRef]
- Hadzic, B.; Romcevic, N.; Romcevic, M.; Kuryliszyn-Kudelska, I.; Dobrowolski, W.; Trajic, J.; Timotijevic, D.; Narkiewicz, U.; Sibera, D. Surface optical phonons in ZnO(Co) nanoparticles: Raman study. J. Alloys Compd. 2012, 540, 49–56. [Google Scholar] [CrossRef]
- Lu, J.G.; Ye, Z.Z.; Wang, L.; Huang, J.Y.; Zhao, B.H. Structural, electrical and optical properties of N-doped ZnO films synthesized by SS-CVD. Mater. Sci. Semicond. Process. 2002, 5, 491–496. [Google Scholar] [CrossRef]
- Tauc, J. Amorphous and Liquid Semiconductors; Plenum: London, UK, 1974. [Google Scholar]
- David, E.A.; Mott, N.F. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 903. [Google Scholar]
- Tan, S.T.; Chen, B.J.; Fan, W.J.; Kwok, H.S.; Zhang, X.H.; Chua, S.J.; Sun, X.W. Blueshift of optical band gap in ZnO thin films grown by metal-organic chemical-vapor deposition. J. Appl. Phys. 2005, 98, 013505. [Google Scholar] [CrossRef]
- Guha, S.; Ghosh, K.; Keeth, J.G.; Ogale, S.B.; Shinde, S.R.; Simpson, J.R.; Drew, H.D.; Venkatesan, T. Temperature-dependent optical studies of Ti1−xCoxO2. Appl. Phys. Lett. 2003, 83, 3296. [Google Scholar] [CrossRef]
- Ali, R.N.; Naz, H.; Li, J.; Zhu, X.; Liu, P.; Xiang, B. Band gap engineering of transition metal (Ni/Co) codoped in zinc oxide (ZnO) nanoparticles. J. Alloys Compd. 2018, 744, 90–95. [Google Scholar] [CrossRef]
- Hammad, T.M.; Salem, J.K.; Harrison, R.G. Structure, optical properties and synthesis of Co-doped ZnO superstructures. Appl. Nanosci. 2013, 2013 3, 133–139. [Google Scholar] [CrossRef]
- Kong, Y.C.; Yu, D.P.; Zhang, B.; Fang, W.; Feng, S.Q. Ultraviolet-emitting ZnO nanowires synthesized by a physical vapor deposition approach. Appl. Phys. Lett. 2001, 78, 407. [Google Scholar] [CrossRef]
- Pal, B.; Giri, P.K. High temperature ferromagnetism and optical properties of Co doped ZnO nanoparticles. J. Appl. Phys. 2010, 108, 084322. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Cao, H.; Chang, R.P.H. Growth mechanism and properties of ZnO nanorods synthesized by plasma-enhanced chemical vapor deposition. J. Appl. Phys. 2004, 95, 3141. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.K.; Voigt, J.A.; Gnade, B.E. Mechanisms behind green photoluminescence in ZnO phosphor powders. J. Appl. Phys. 1996, 79, 7983. [Google Scholar] [CrossRef]




| Samples | Concentrations of Co Precursor | Nanowire Shapes | 2Theta (degrees) | d002 1 (Å) | Omega FWHM 2 (degrees) |
|---|---|---|---|---|---|
| A | 0 at% | Vertical | 34.488 | 2.5586 | 8.02 |
| B | 3 at% | Vertical | 34.471 | 2.5602 | 8.22 |
| C | 6 at% | Vertical | 34.462 | 2.5610 | 8.28 |
| D | 9 at% | Bundle | 34.391 | 2.5656 | 8.65 |
| E | 12 at% | Bundle | 34.345 | 2.5690 | 8.97 |
| Samples | Co Precursor Concentrations | Band Gap 1 (eV) | Peak Position at UV Band (nm) | Peak Position at Visible Band (nm) | Intensity Ratio 2 |
|---|---|---|---|---|---|
| A | 0 at % | 3.310 | 386.1 | ~503 | ~12.8 |
| B | 3 at % | 3.312 | 384.9 | ~497 | ~1.1 |
| C | 6 at % | 3.313 | 382.1 | ~565 | ~0.7 |
| D | 9 at % | 3.318 | 381.4 | ~571 | ~0.4 |
| E | 12 at % | 3.327 | 381.1 | ~578 | ~0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.C.; Lee, D.K.; Sohn, S.H. Morphological and Optical Properties of Cobalt Ion-Modified ZnO Nanowires. Catalysts 2020, 10, 614. https://doi.org/10.3390/catal10060614
Choi SC, Lee DK, Sohn SH. Morphological and Optical Properties of Cobalt Ion-Modified ZnO Nanowires. Catalysts. 2020; 10(6):614. https://doi.org/10.3390/catal10060614
Chicago/Turabian StyleChoi, Seok Cheol, Do Kyung Lee, and Sang Ho Sohn. 2020. "Morphological and Optical Properties of Cobalt Ion-Modified ZnO Nanowires" Catalysts 10, no. 6: 614. https://doi.org/10.3390/catal10060614
APA StyleChoi, S. C., Lee, D. K., & Sohn, S. H. (2020). Morphological and Optical Properties of Cobalt Ion-Modified ZnO Nanowires. Catalysts, 10(6), 614. https://doi.org/10.3390/catal10060614





