Tandem Catalysis: Synthesis of Nitrogen-Containing Heterocycles
Abstract
:1. Introduction
2. Taxonomy
2.1. One-Pot Synthesis of Biologically Active Molecules
2.2. Processes That Are Not Tandem Catalyses
3. Tandem Catalysis
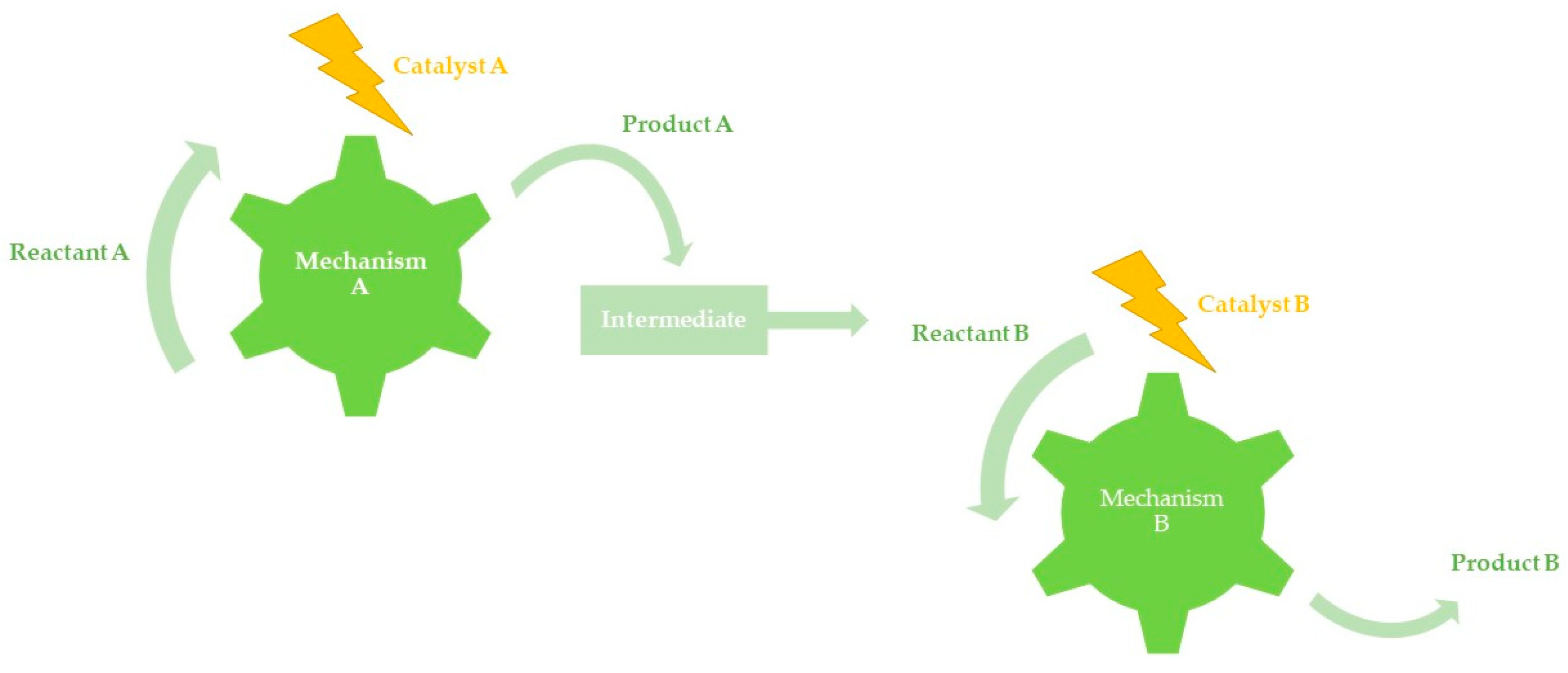
3.1. Orthogonal Tandem Catalysis
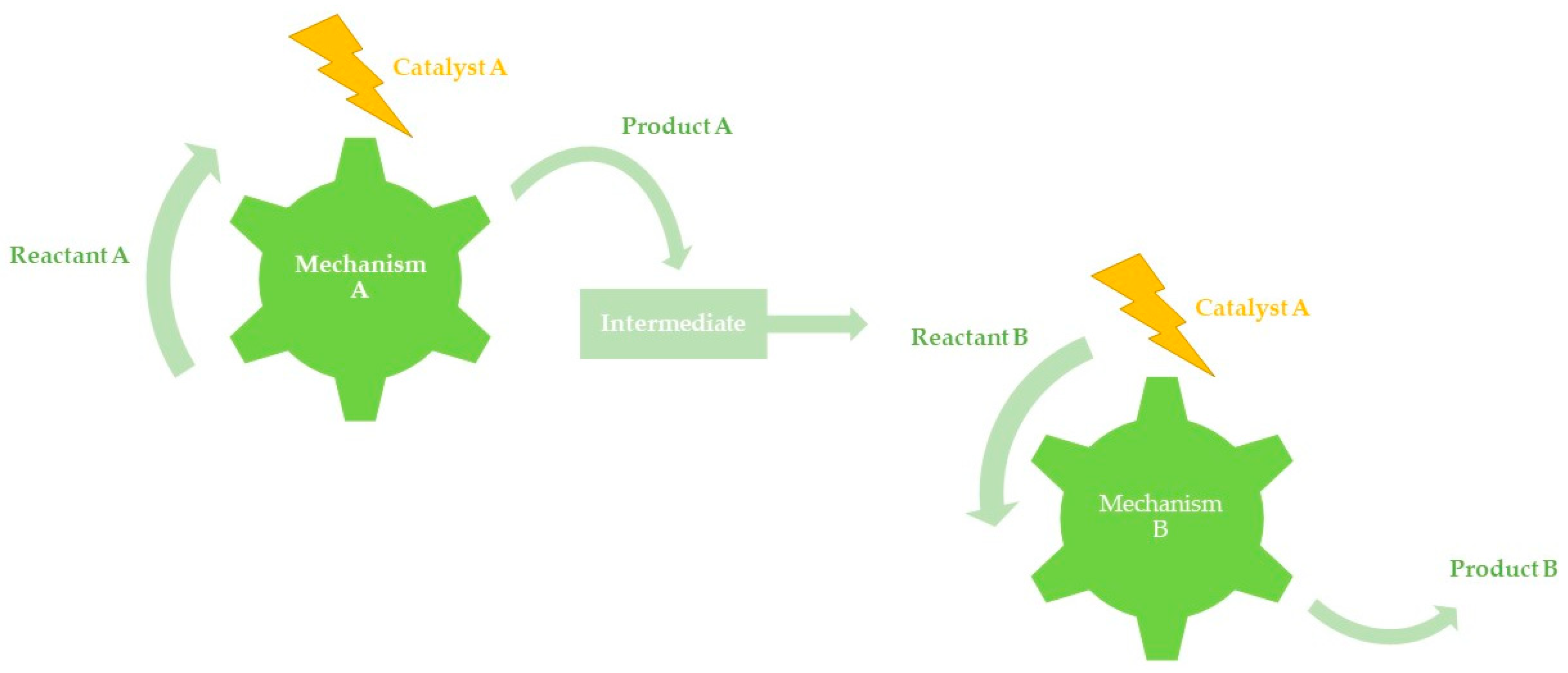
3.2. Auto-Tandem Catalysis
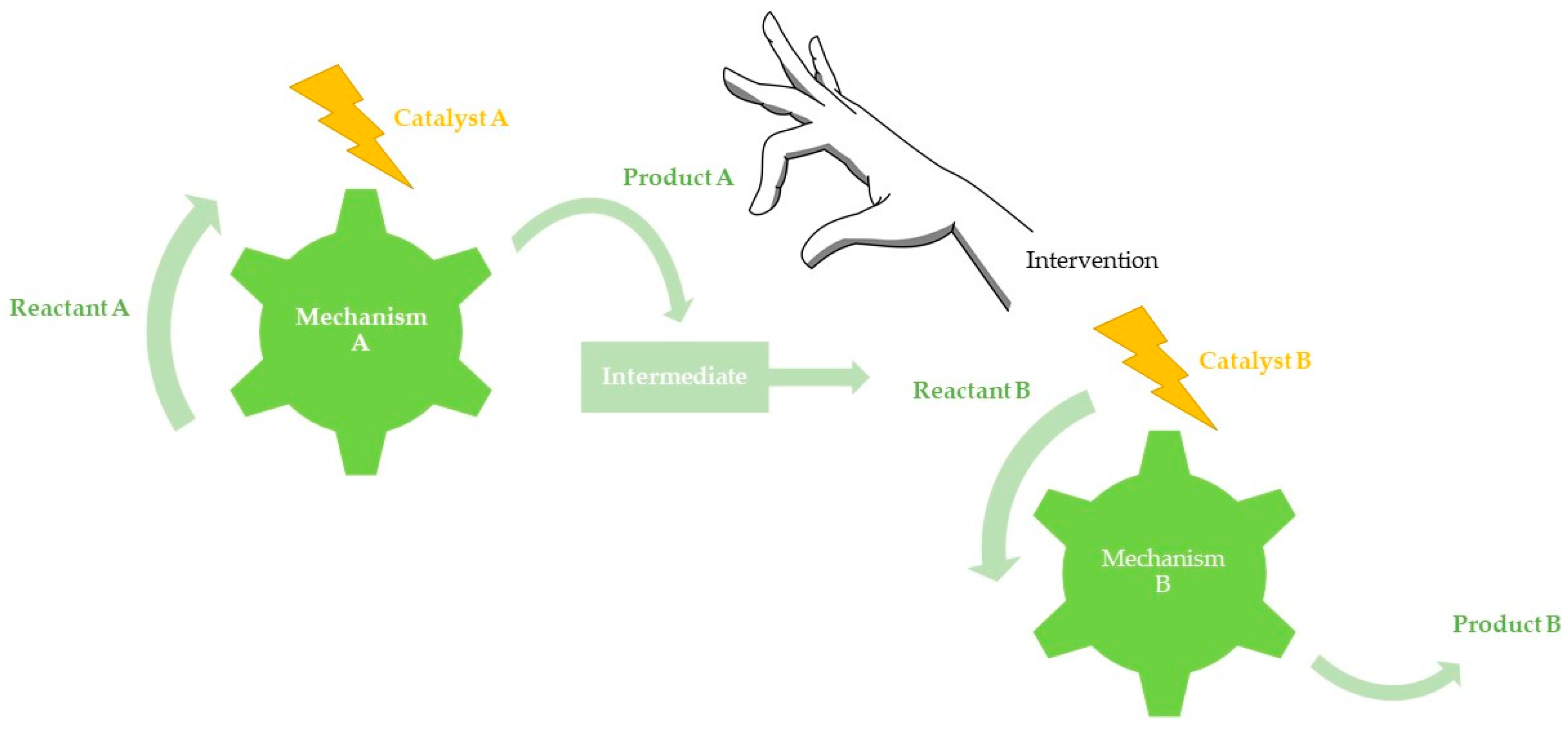
3.3. Assisted Tandem Catalysis
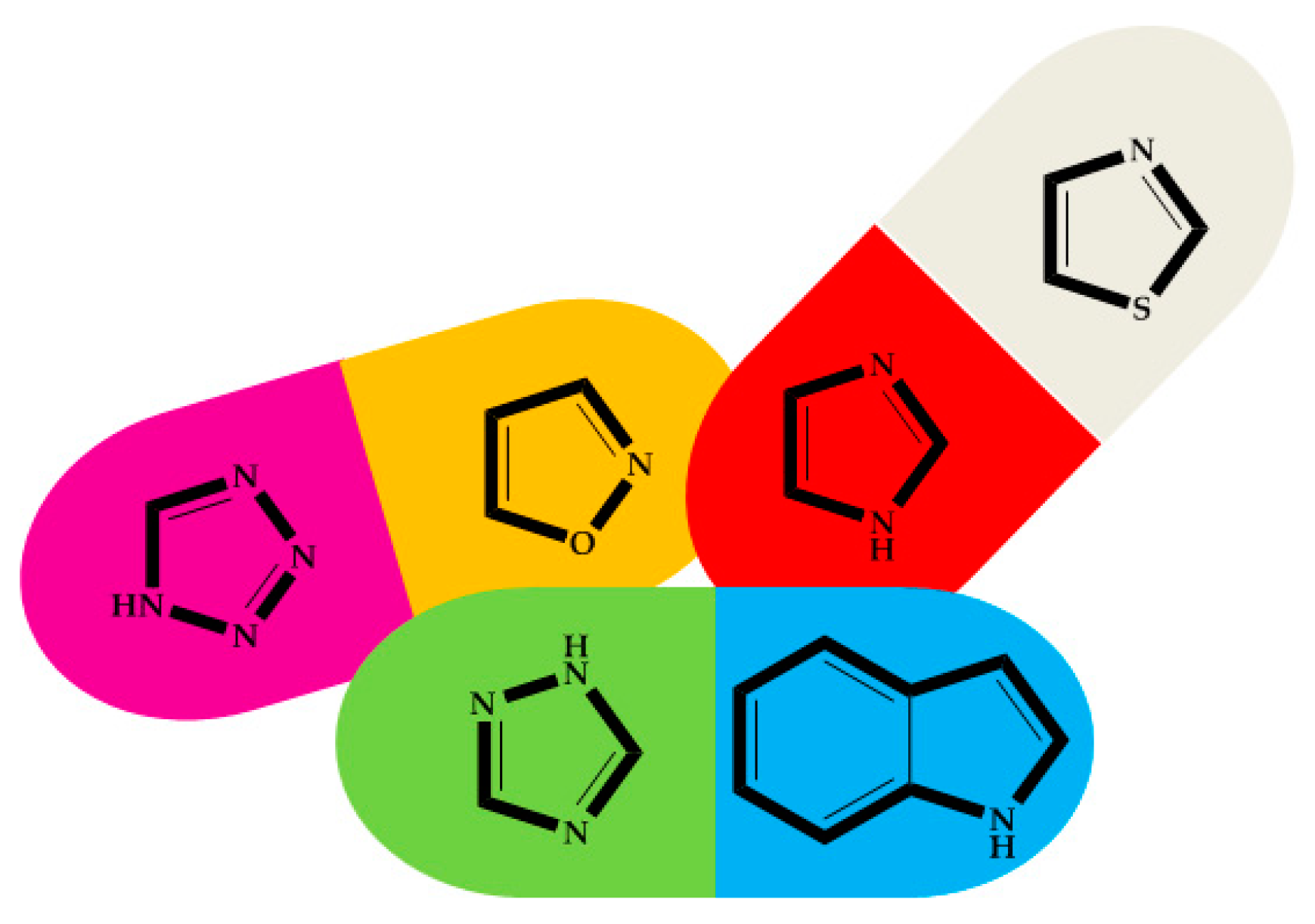
4. Review of Tandem Reactions Generating the Formation of Five-Membered Aromatic Nitrogen Heterocycles
4.1. Scope of Review
4.2. Five-Membered Aromatic Nitrogen Heterocycles
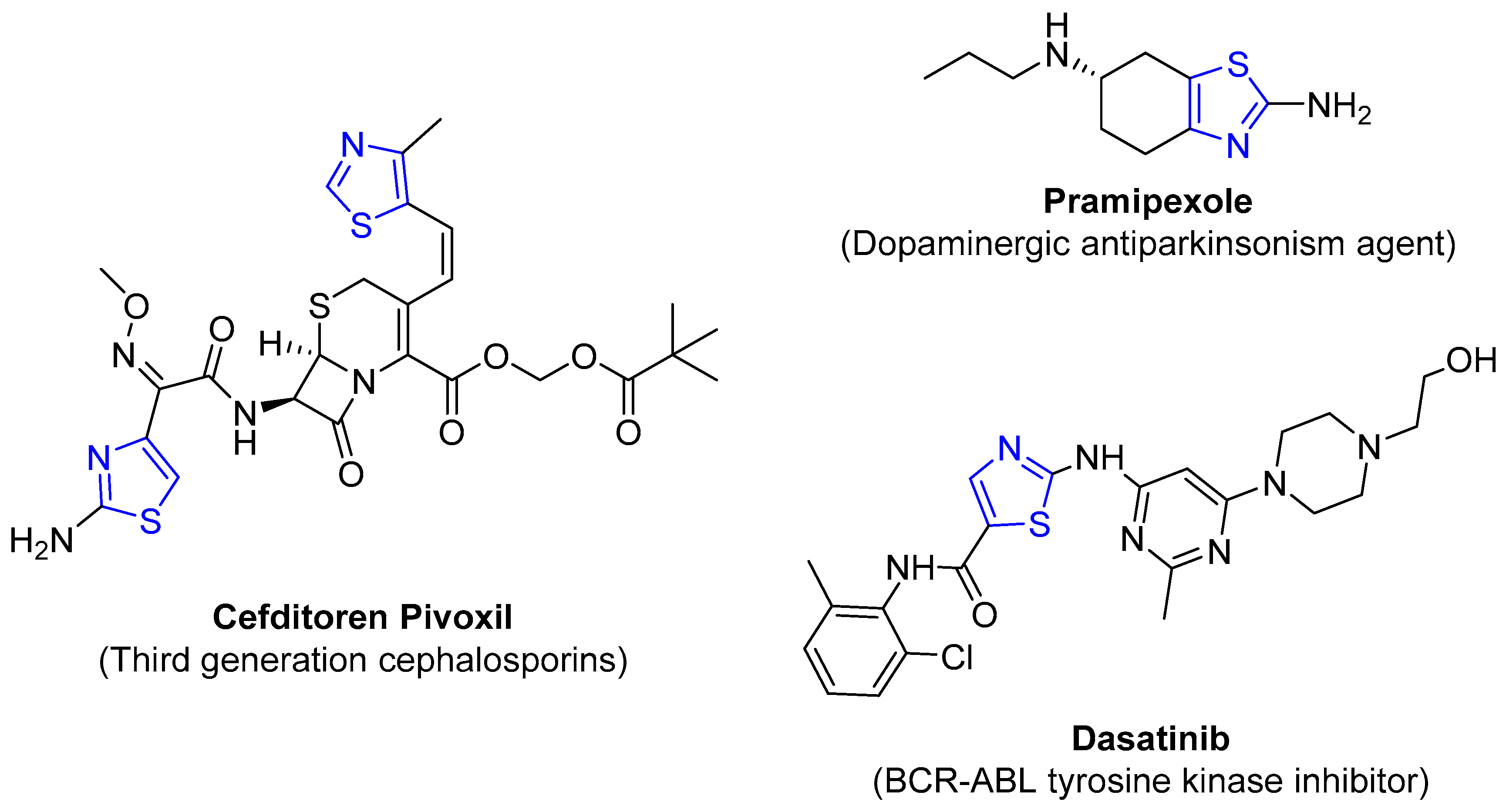
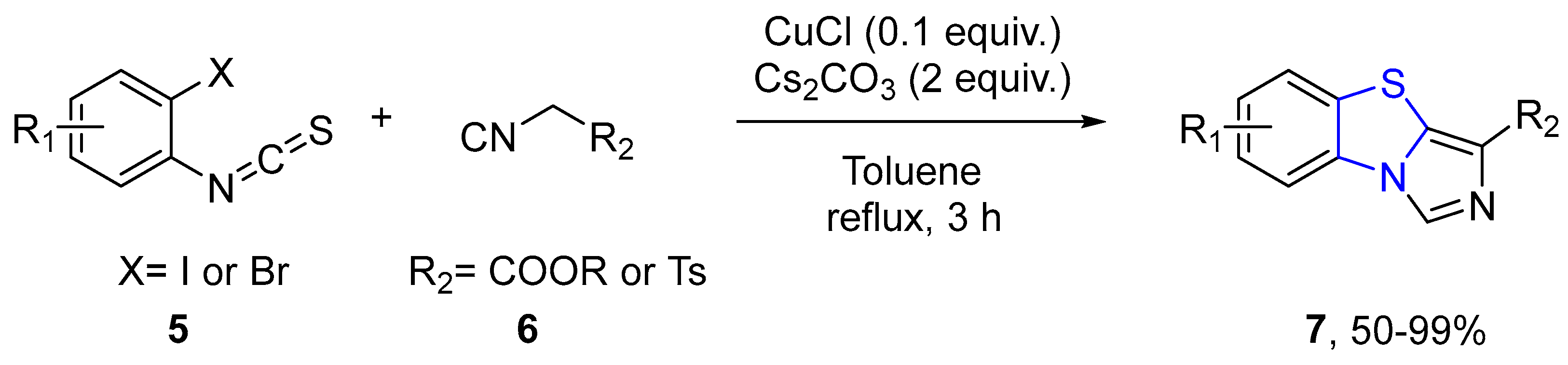
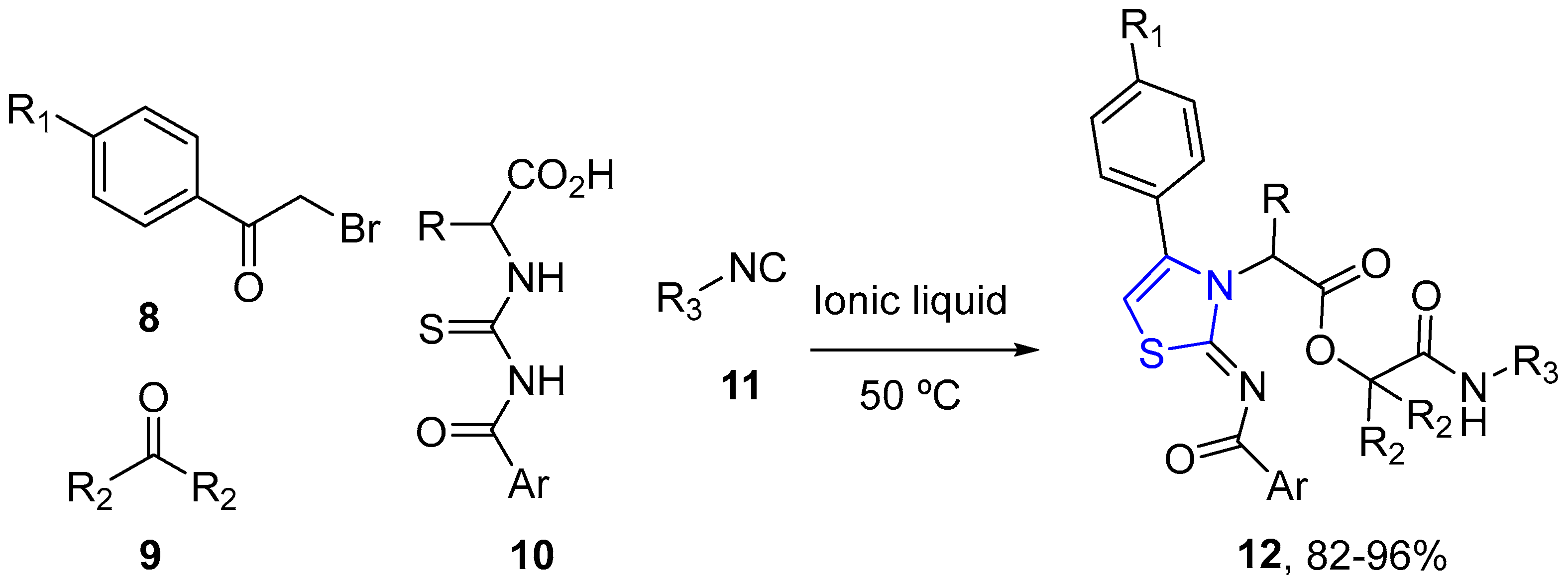
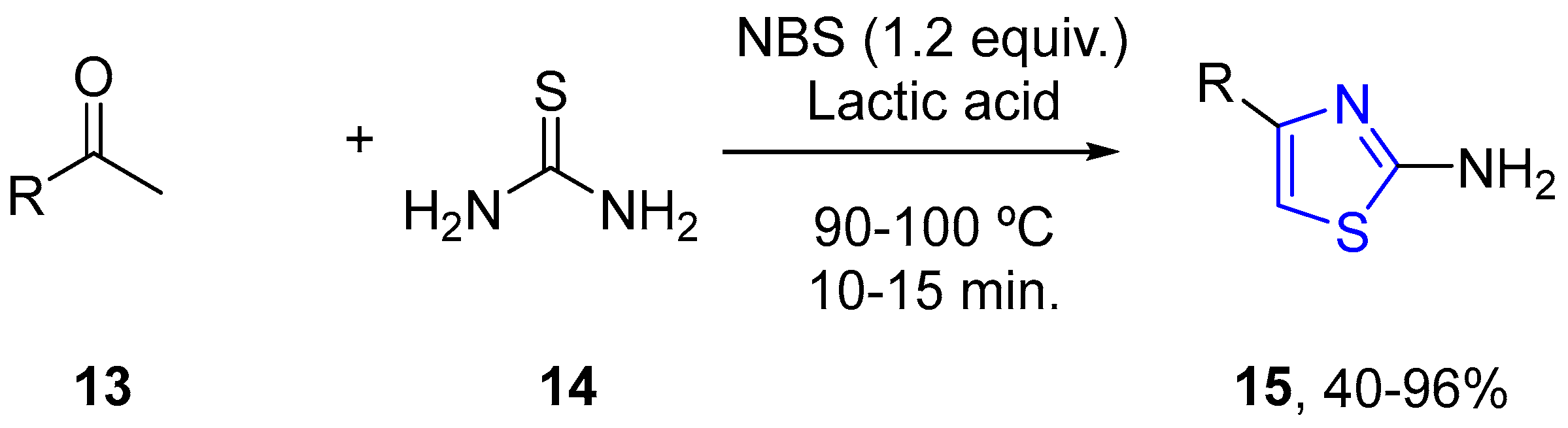
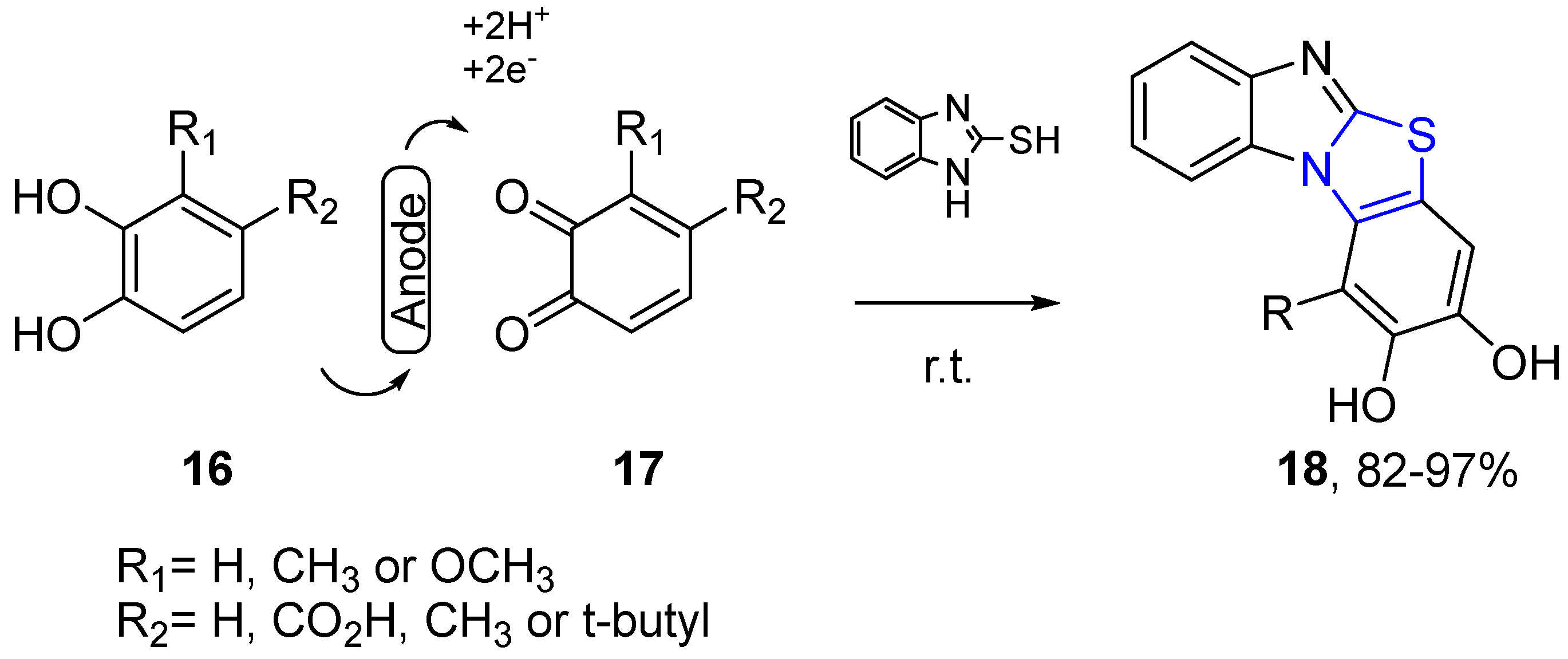
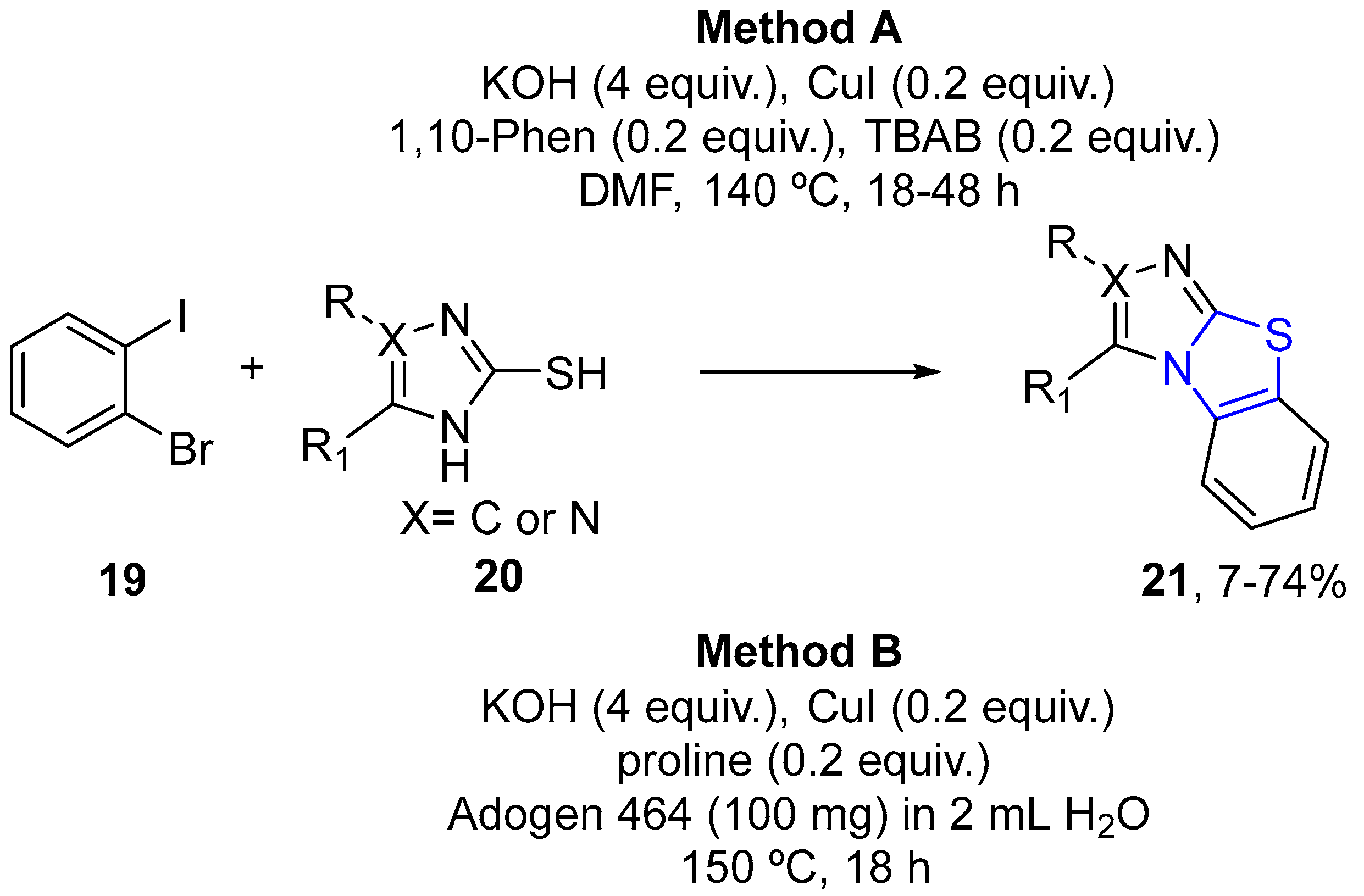
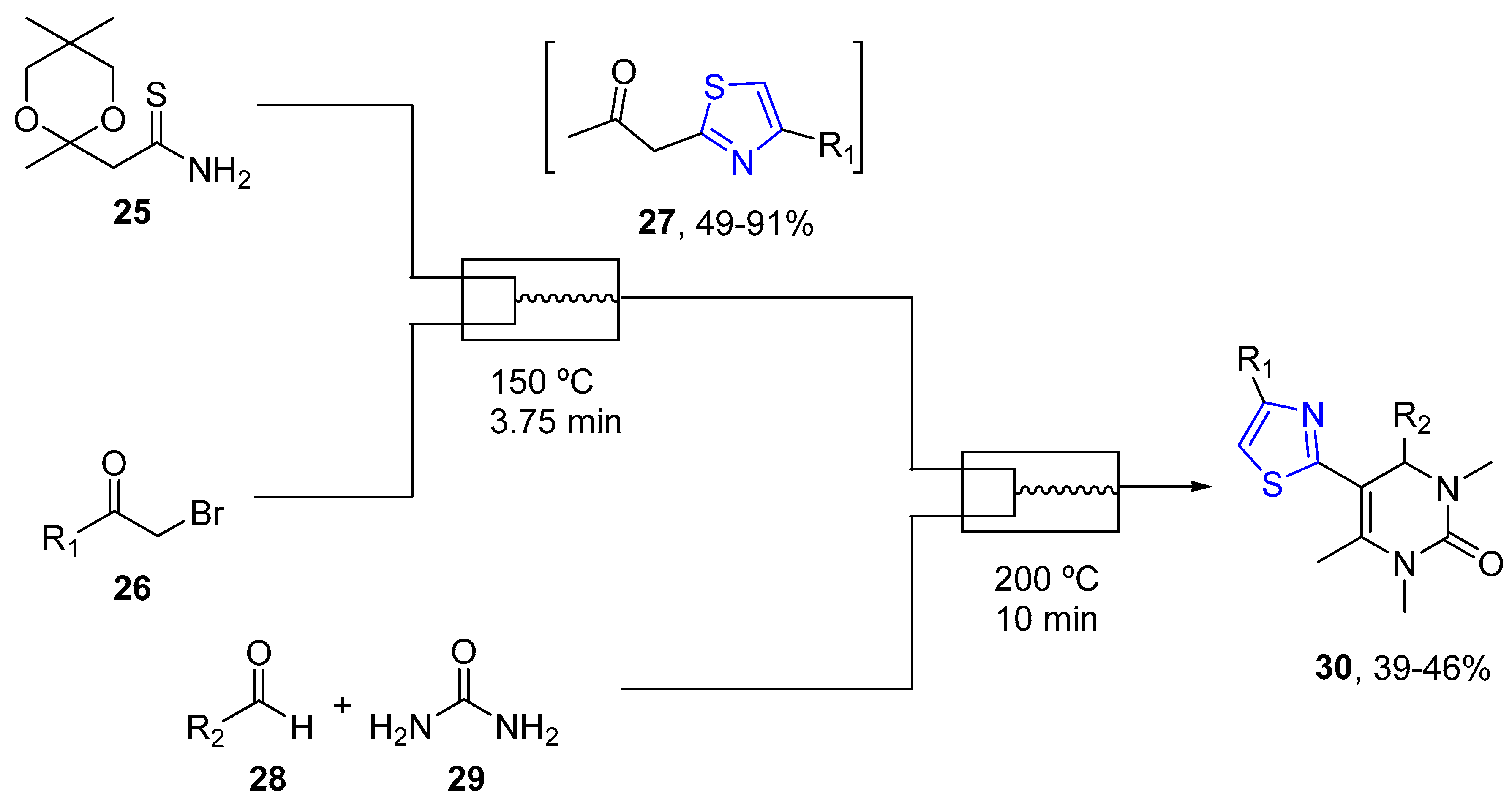
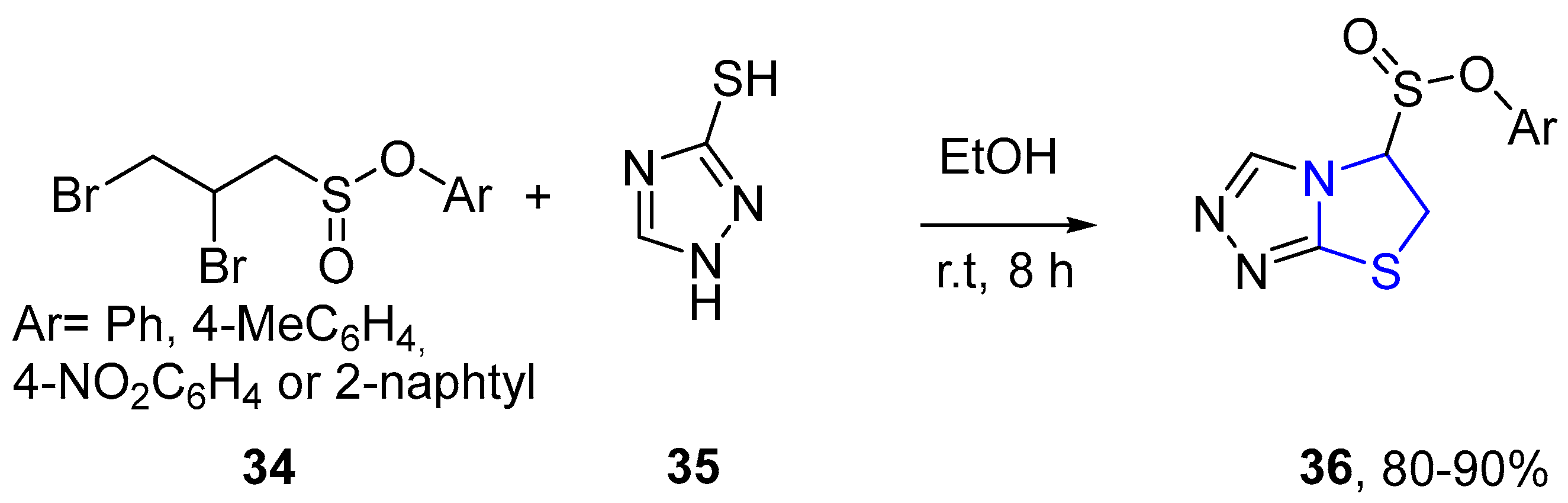
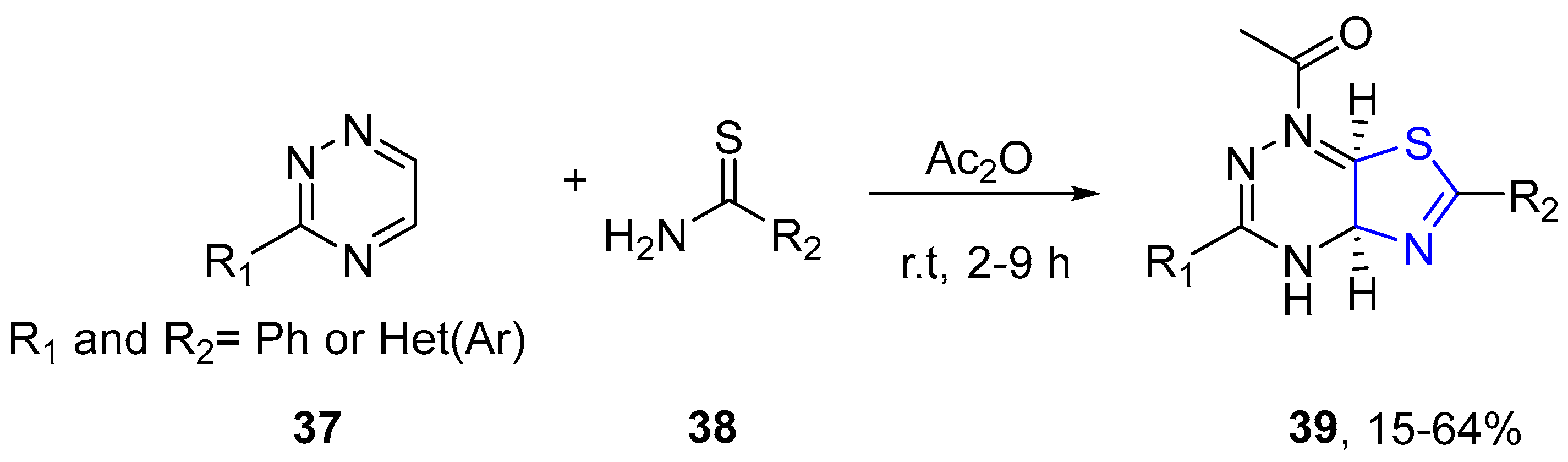
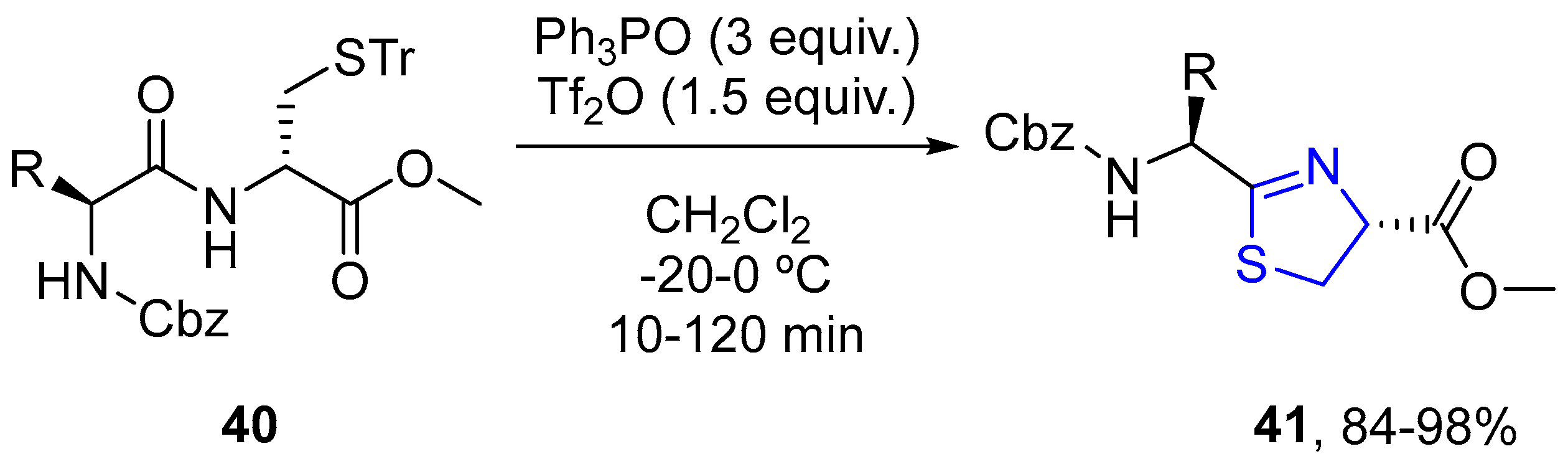
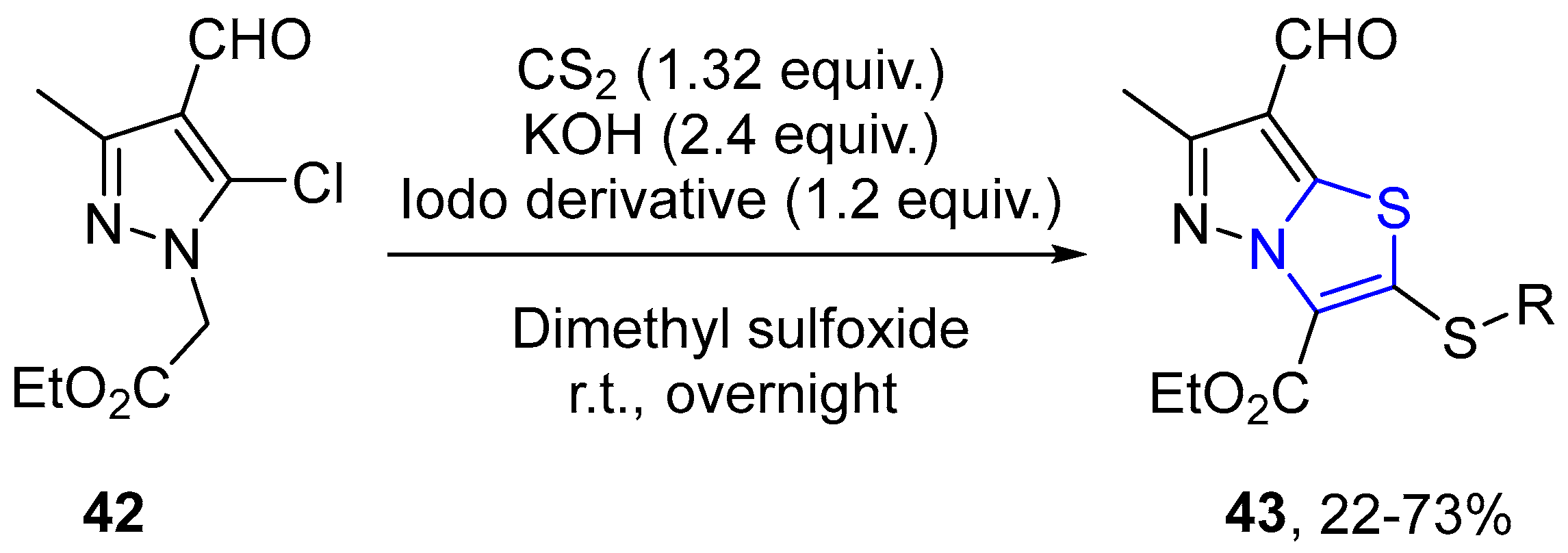
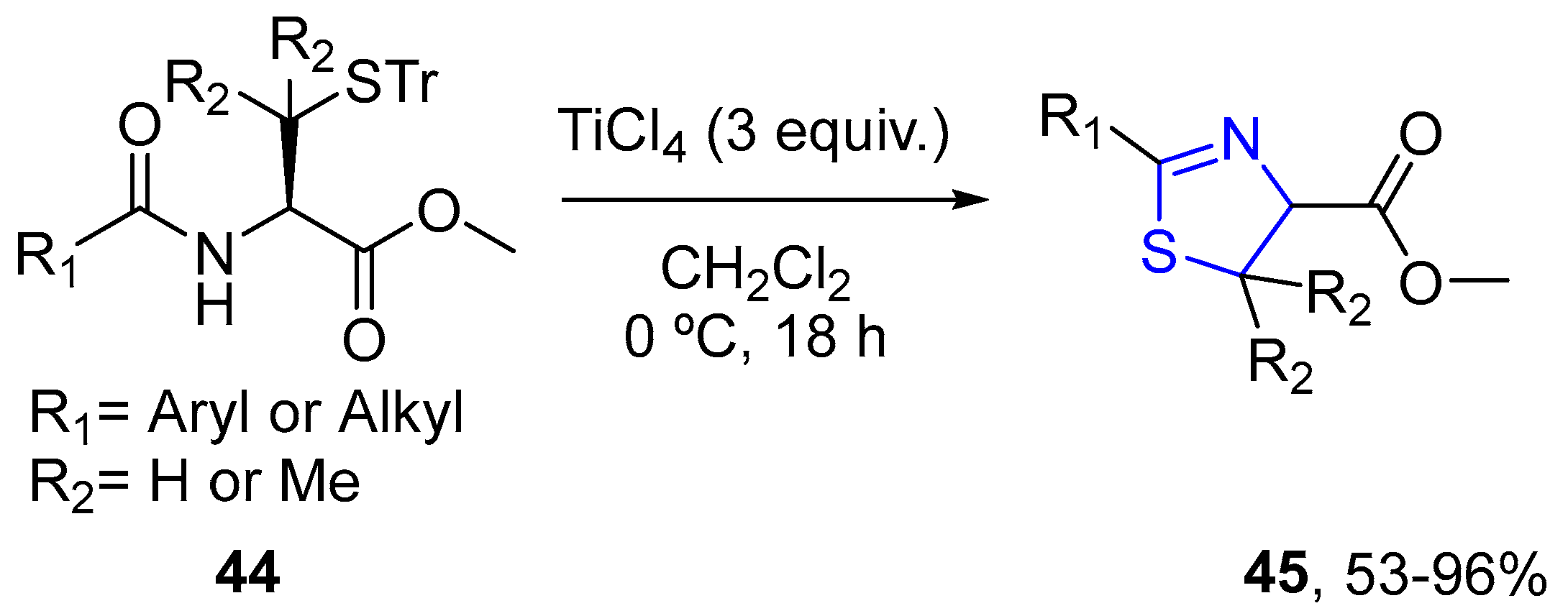
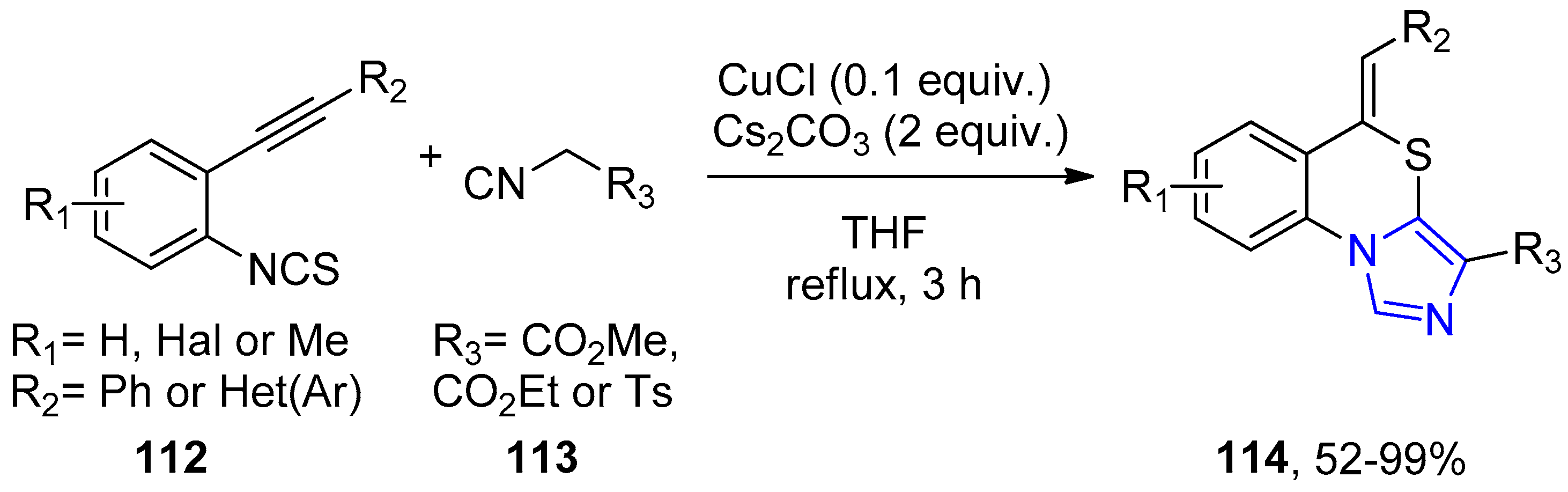
4.2.1. Thiazole
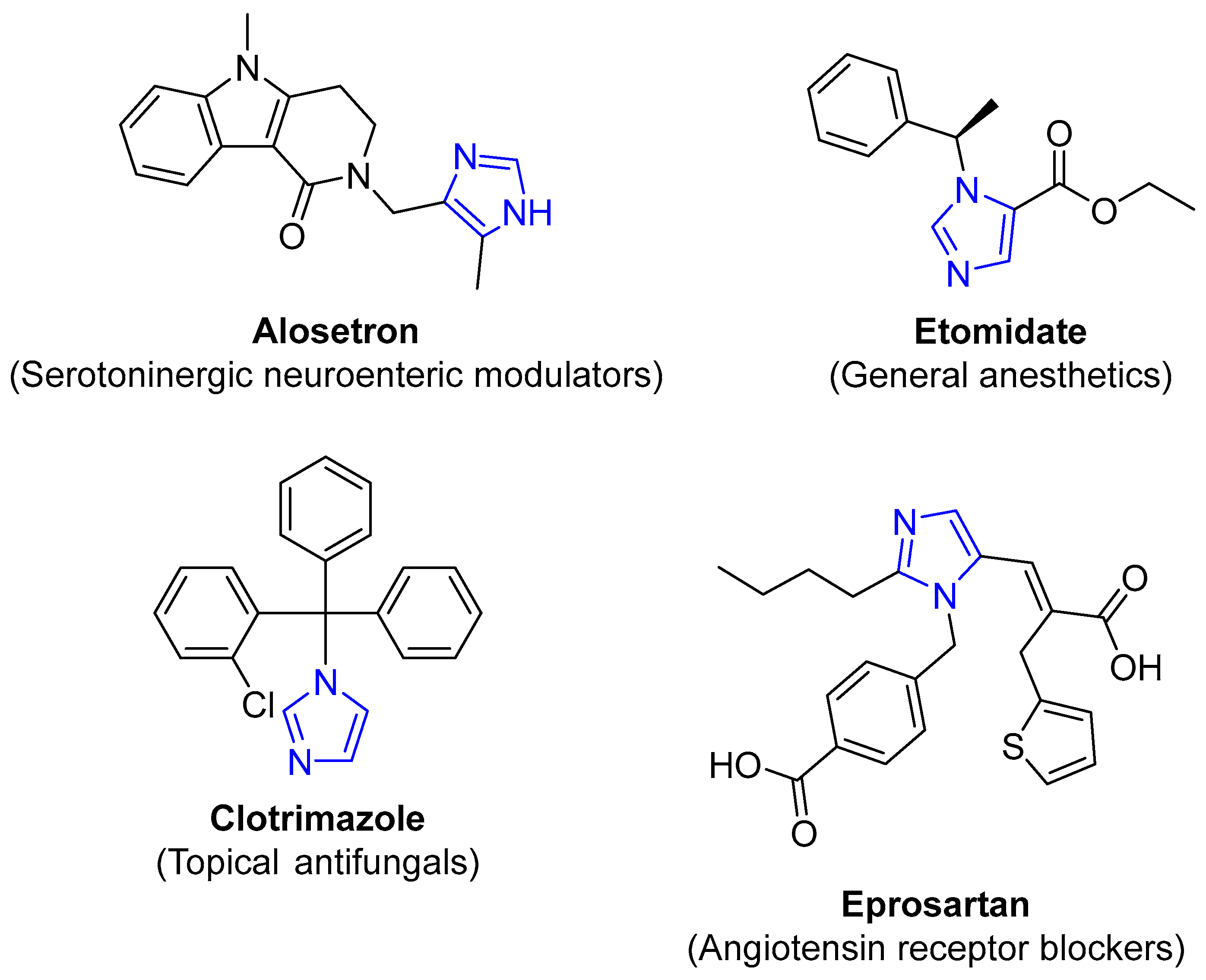
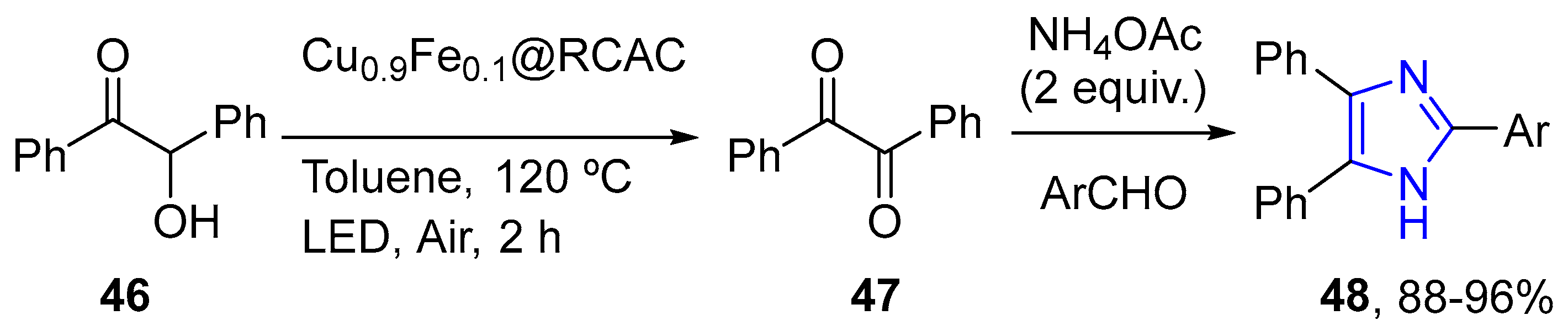
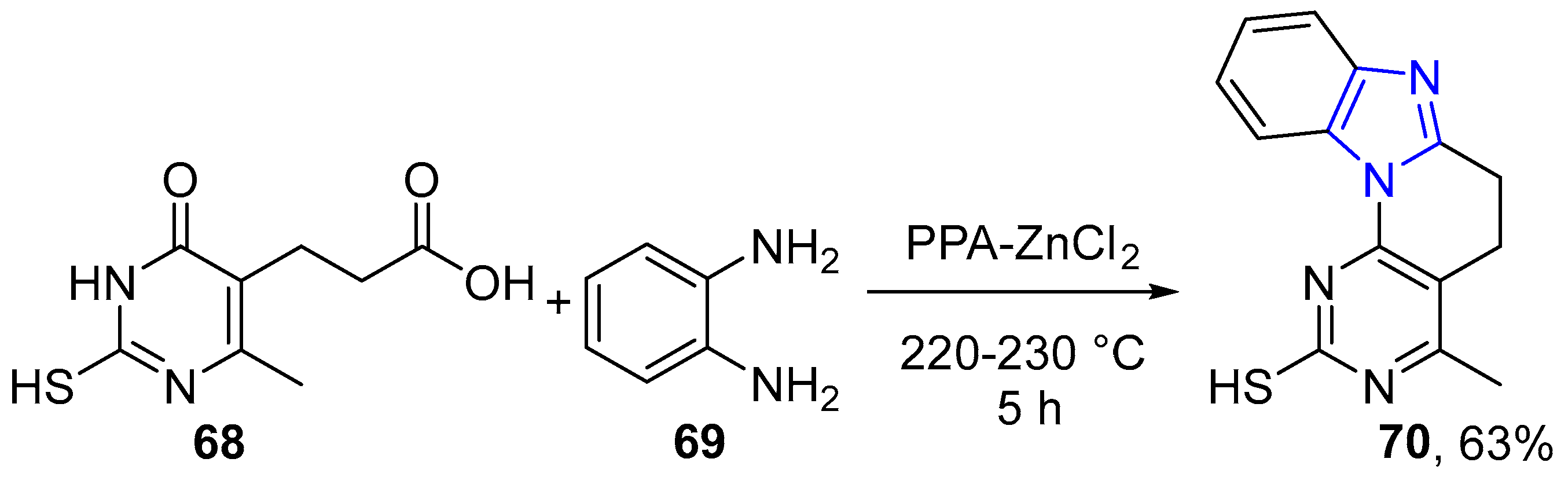
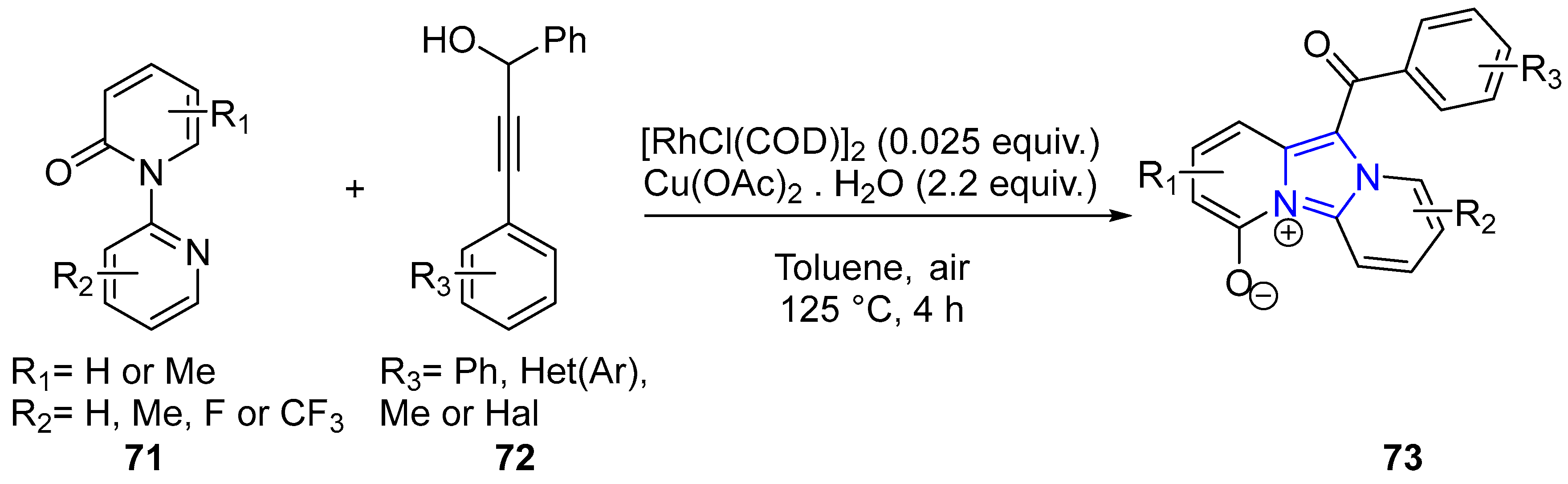
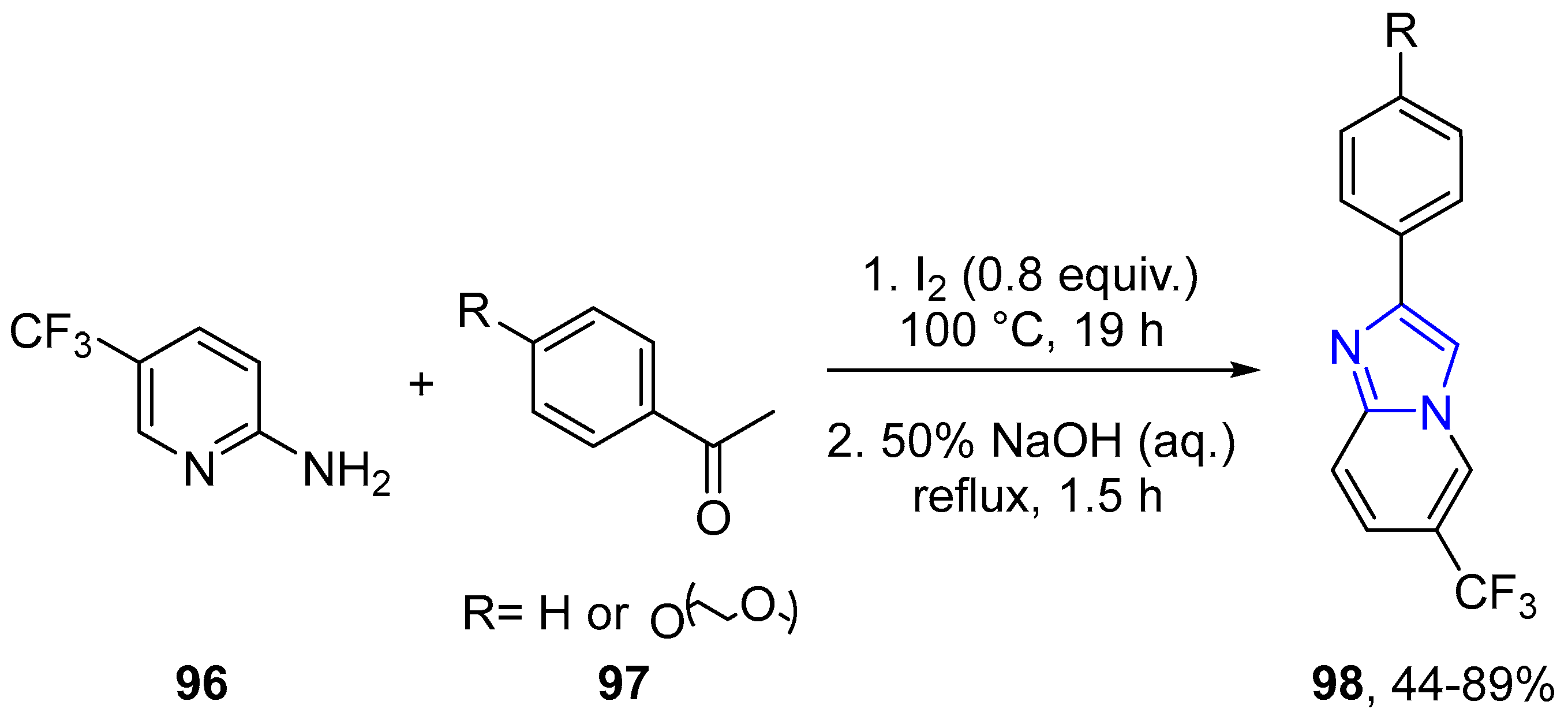
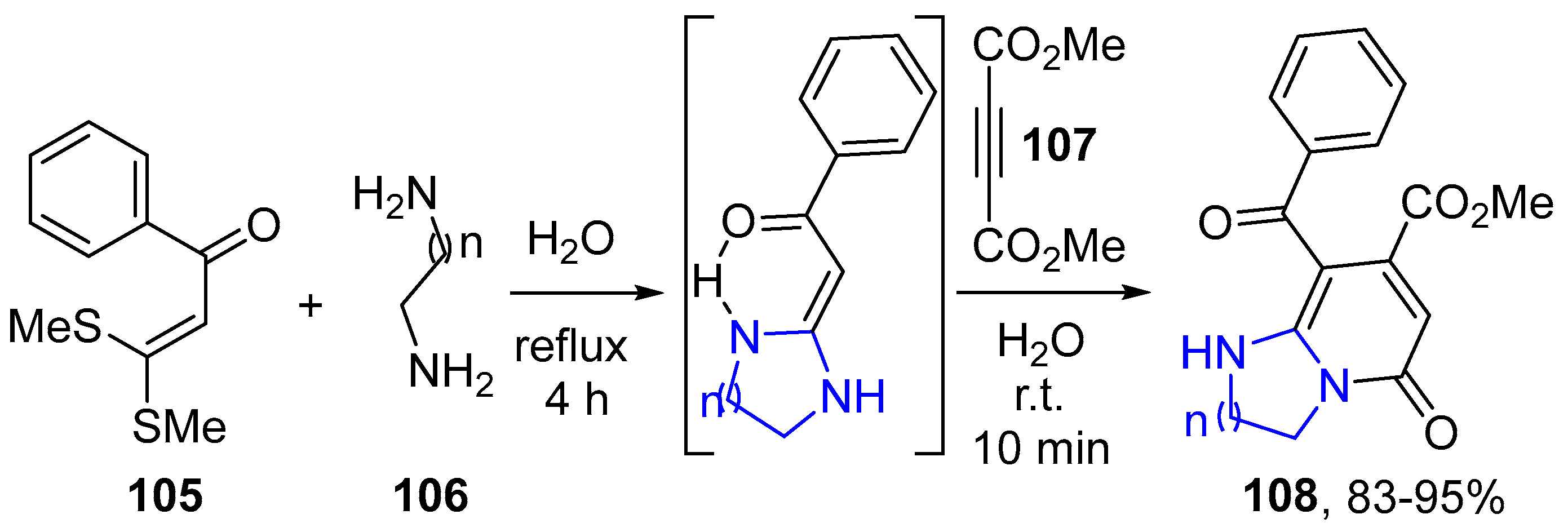
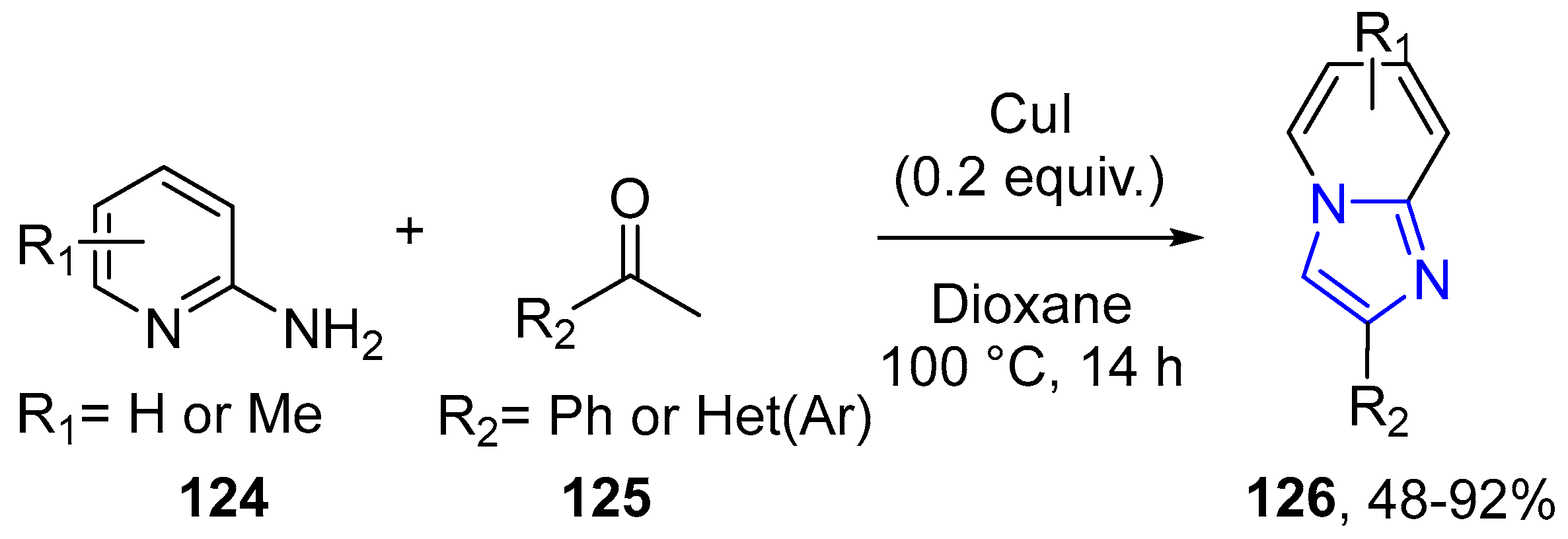
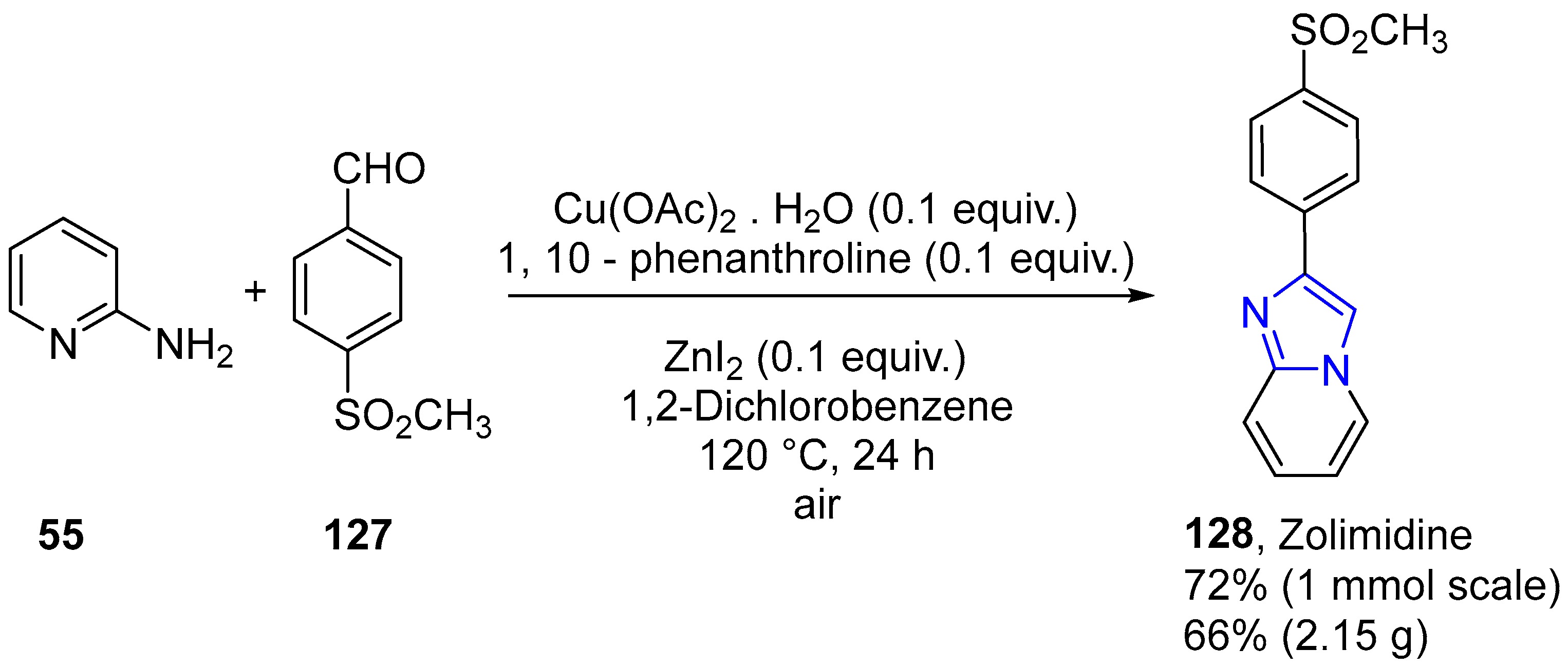
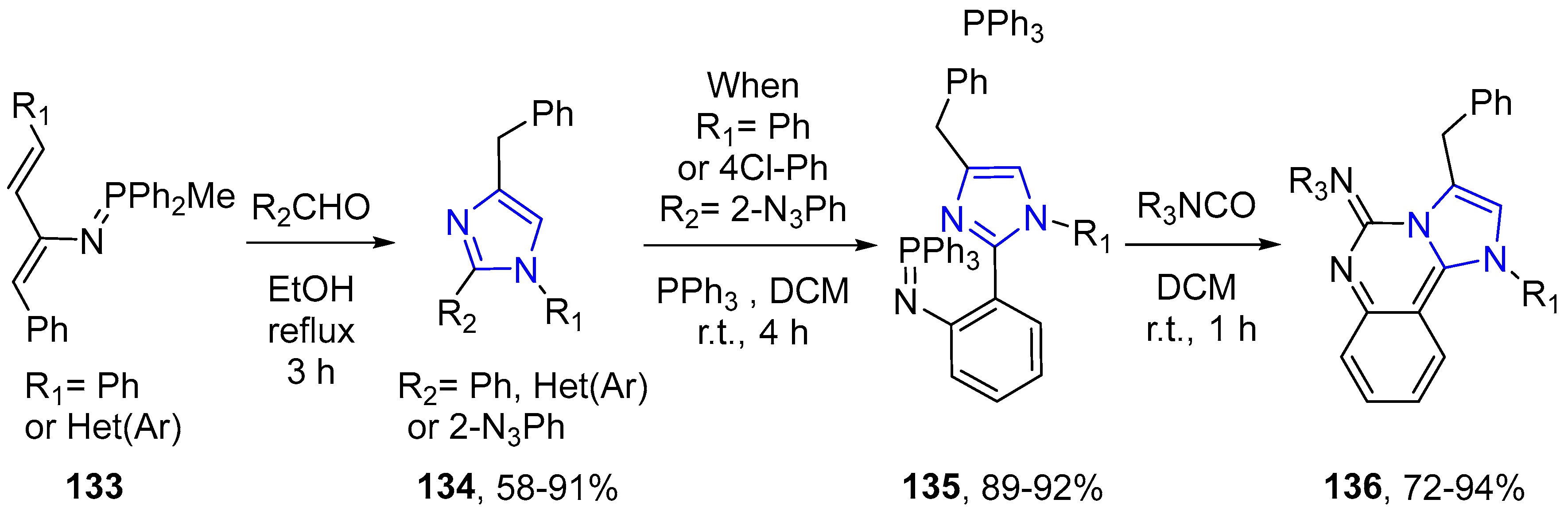
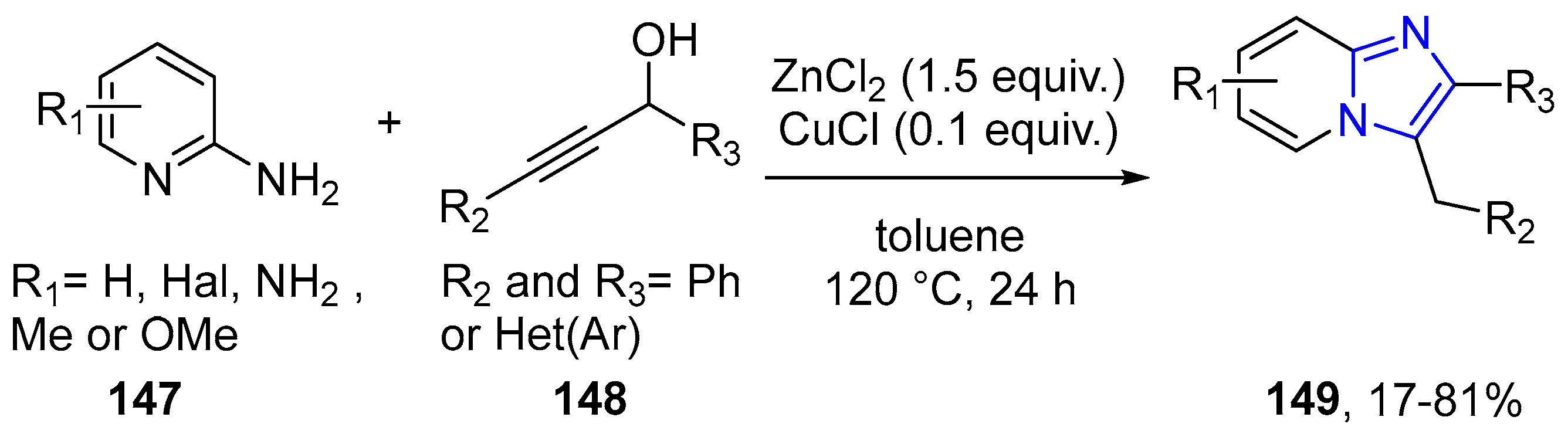
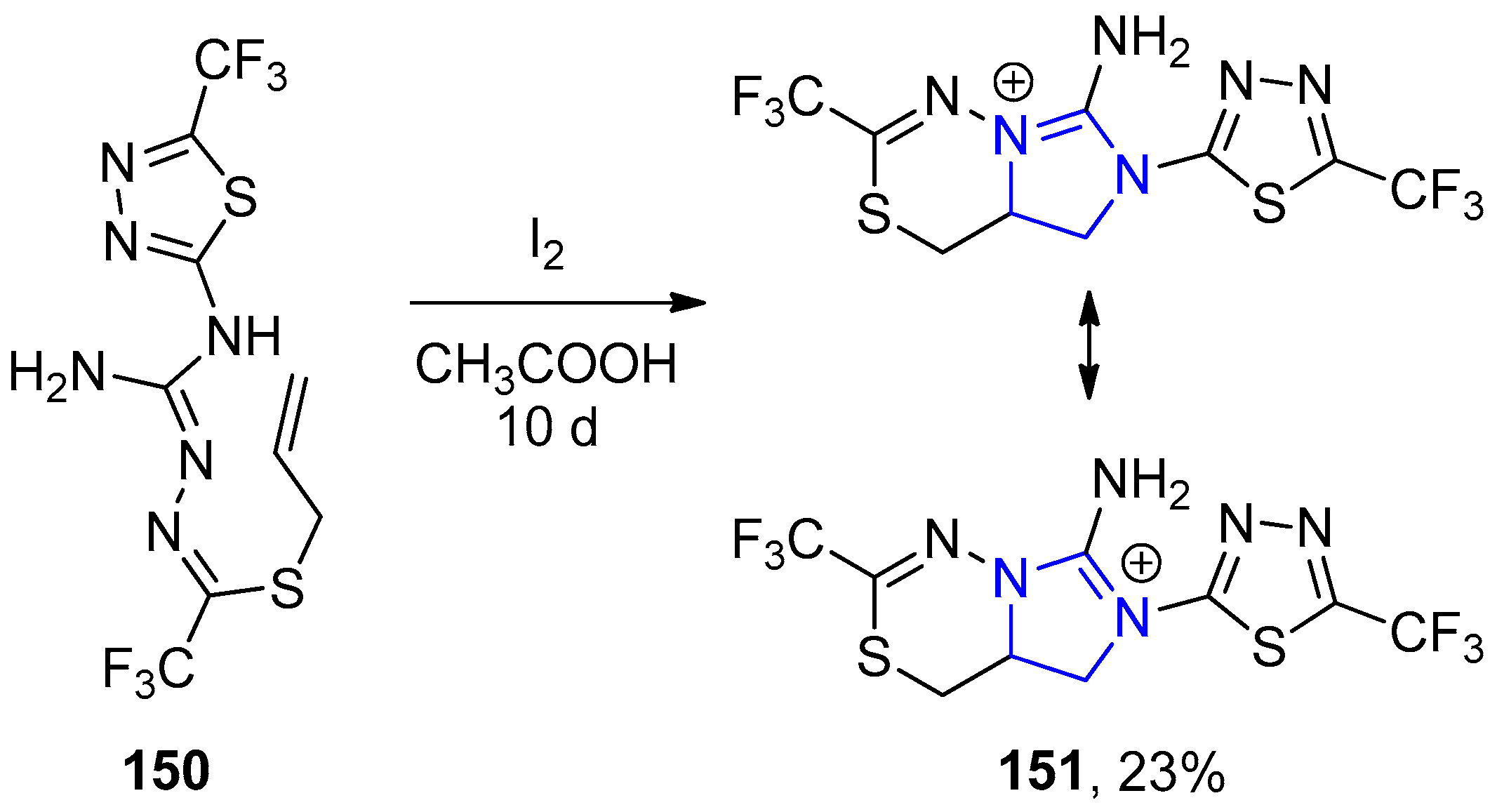
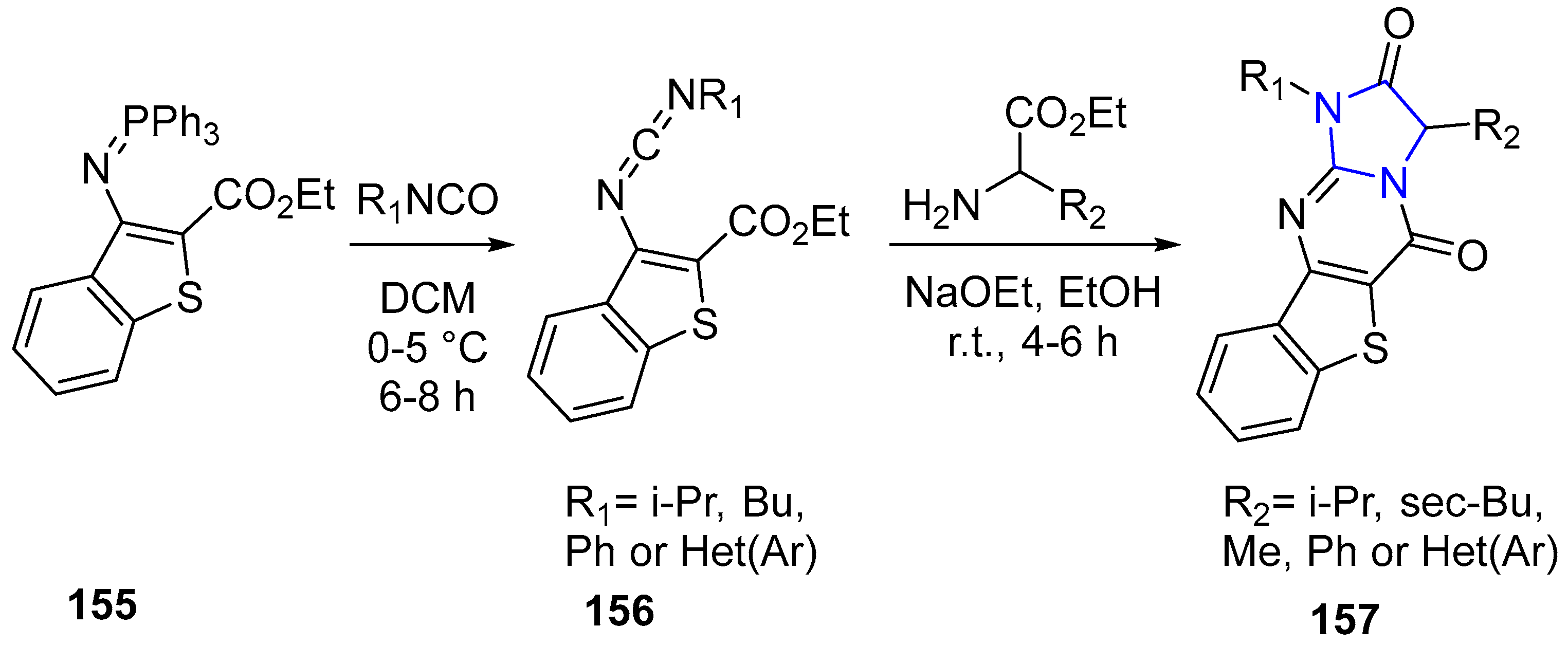
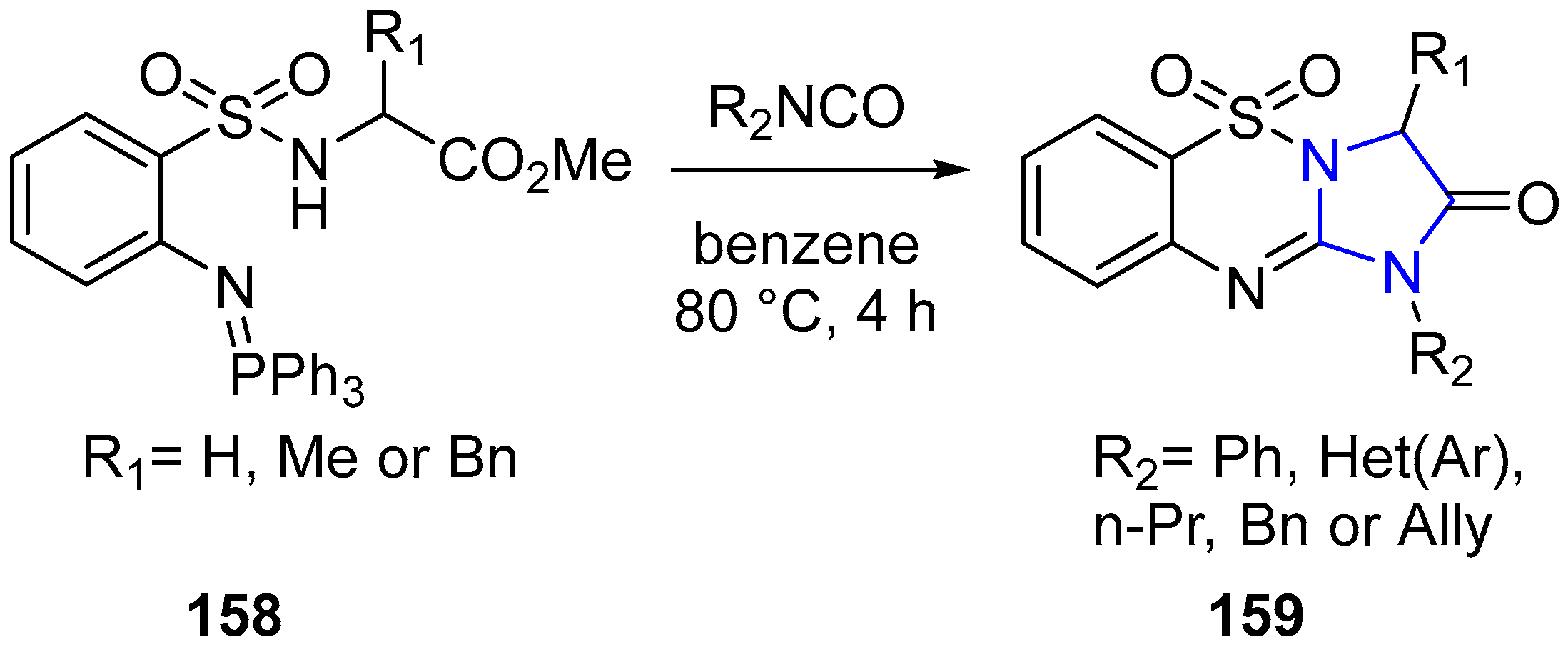
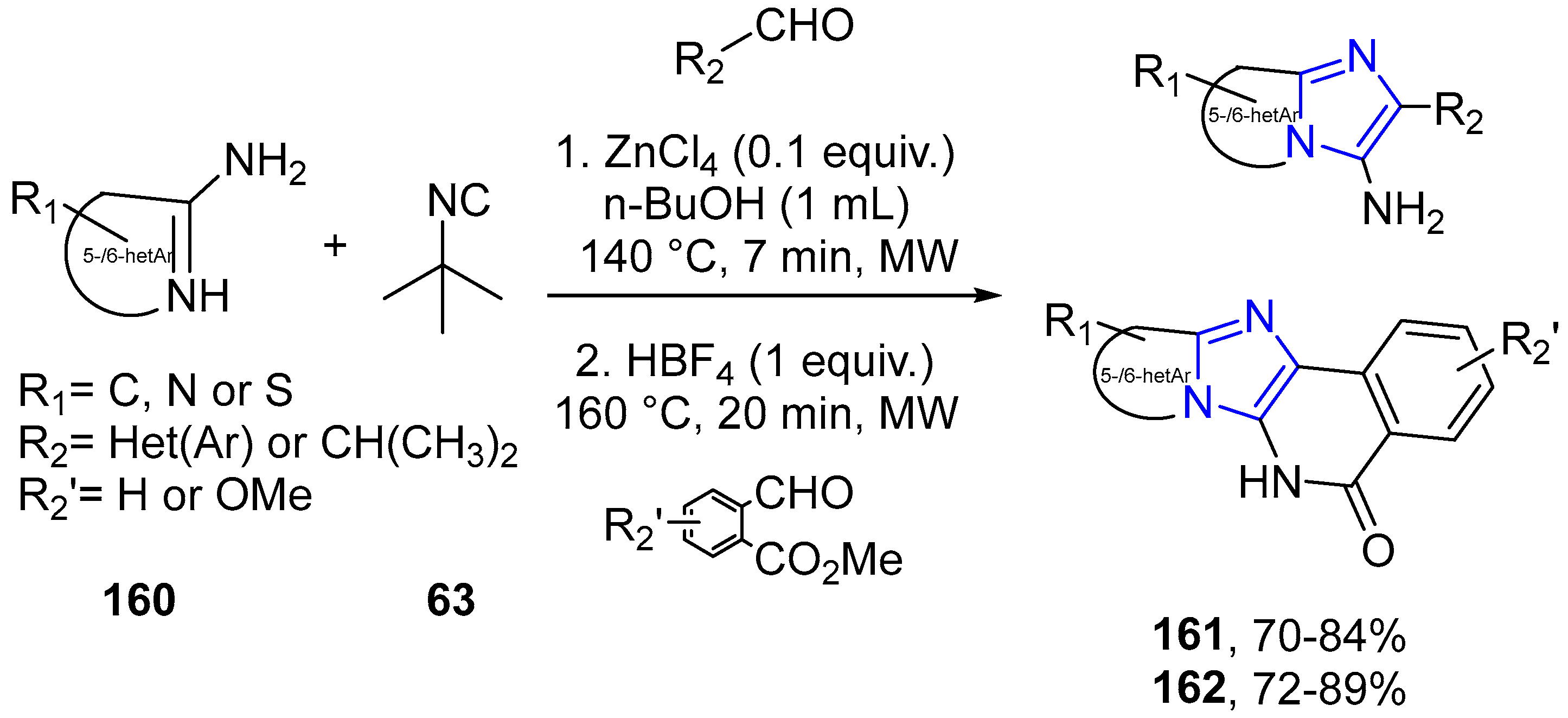
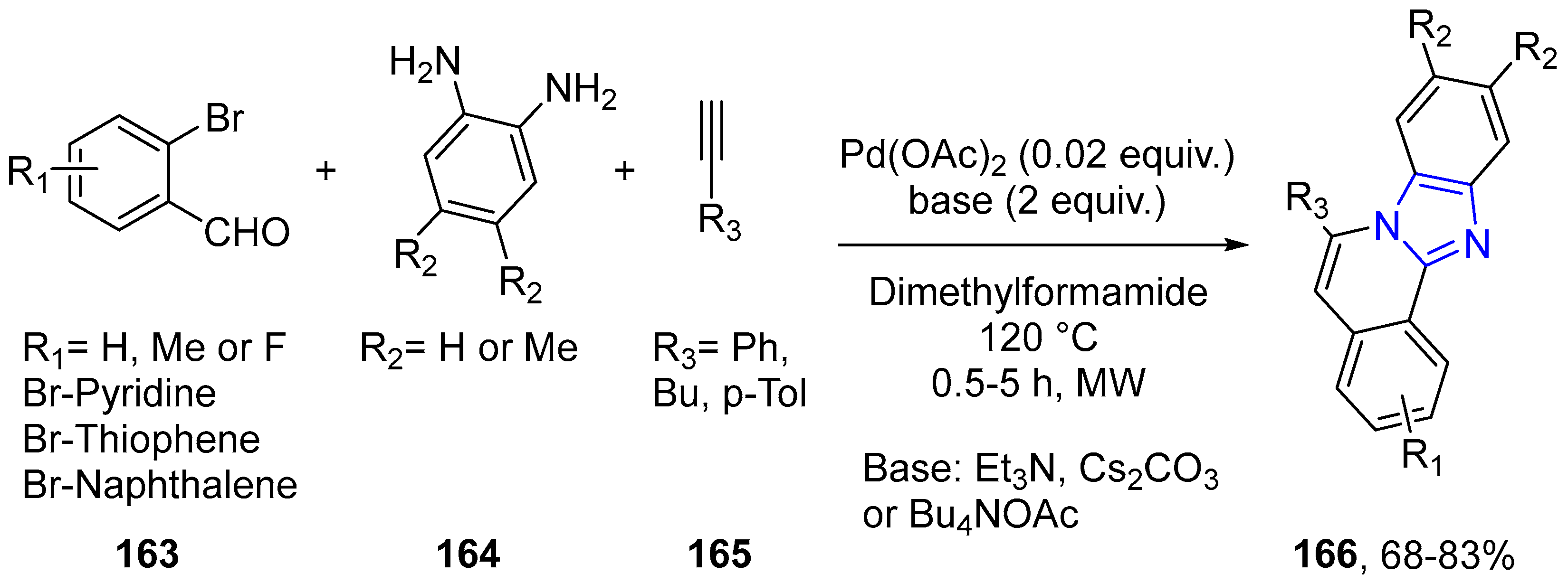
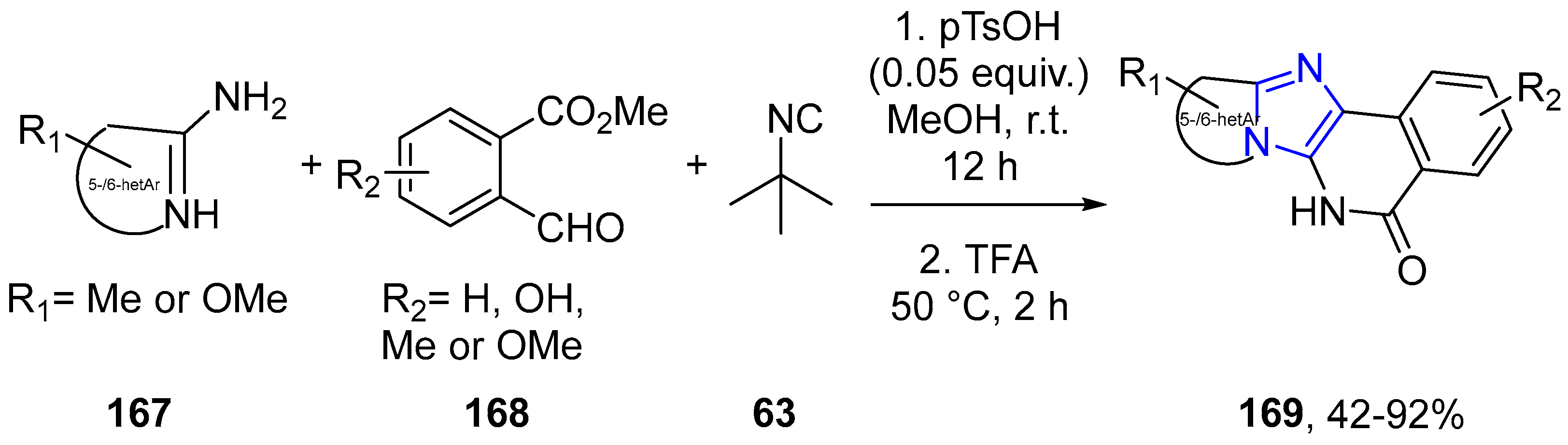
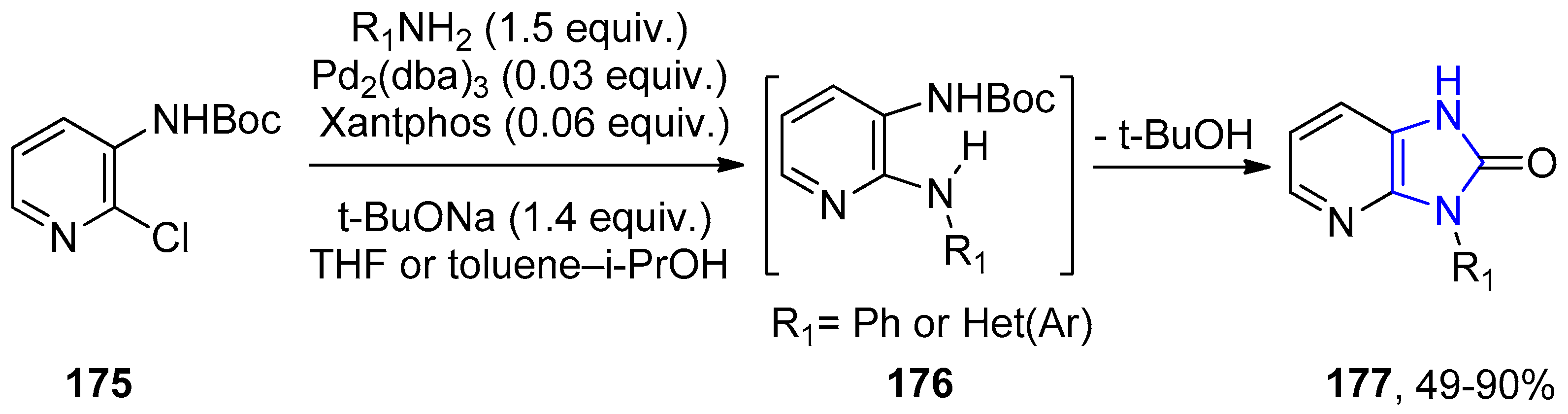
4.2.2. Imidazole
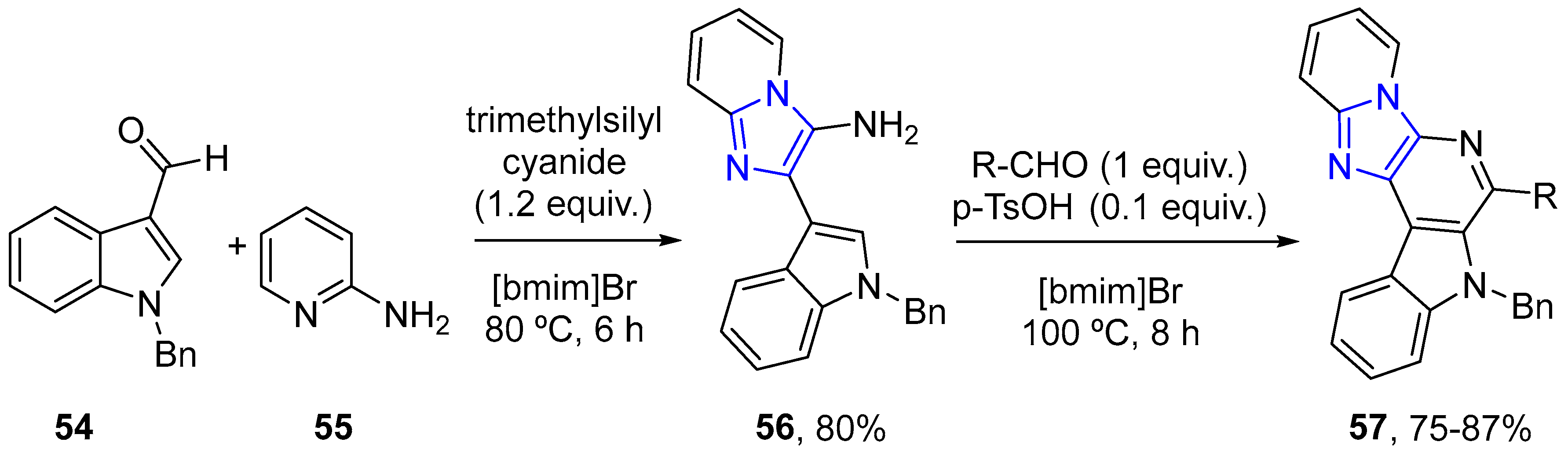
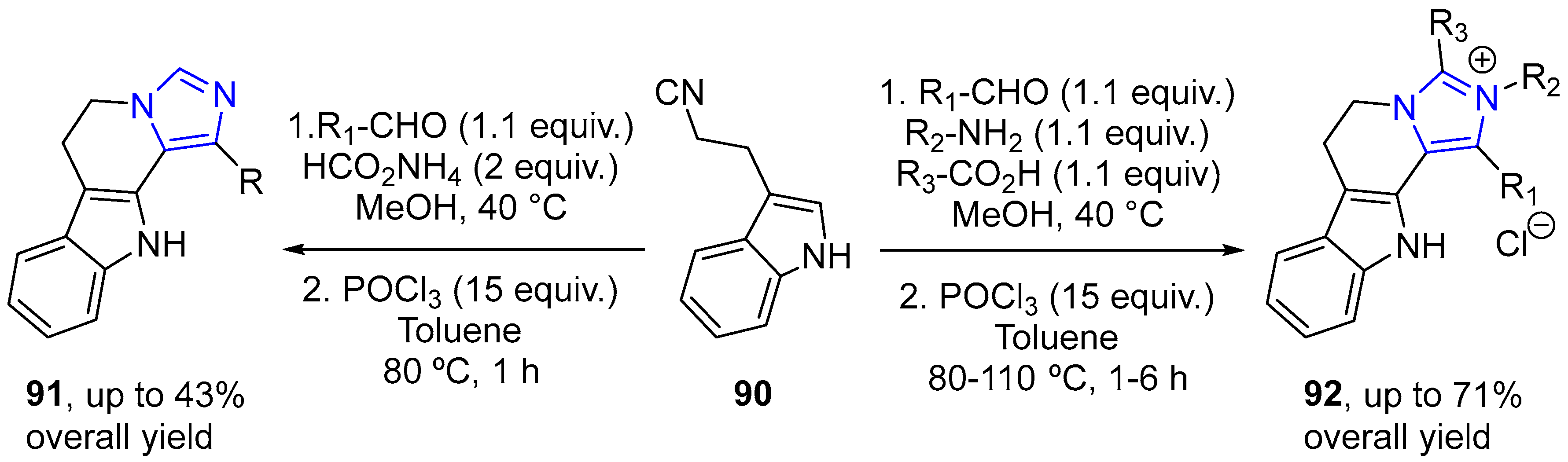
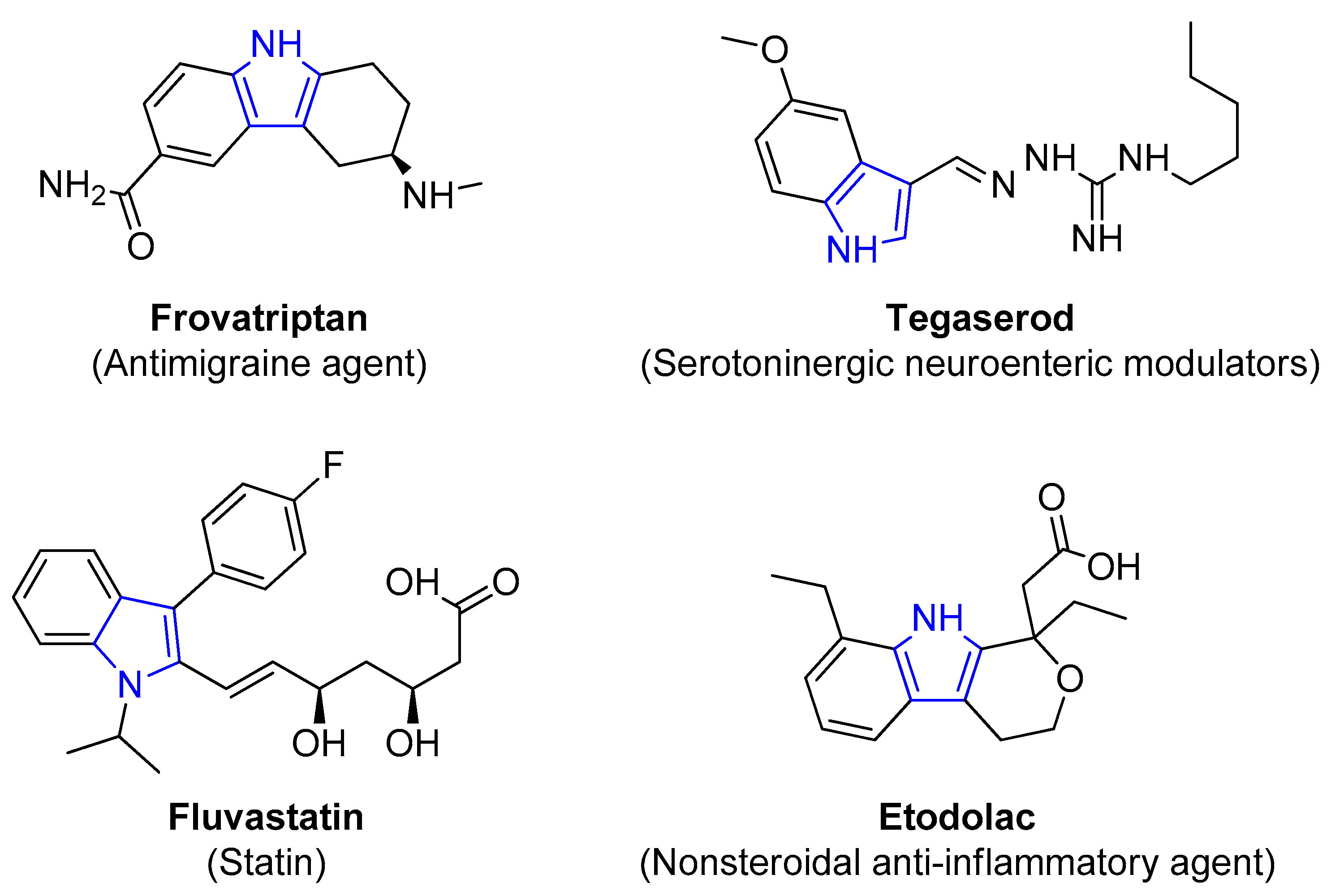
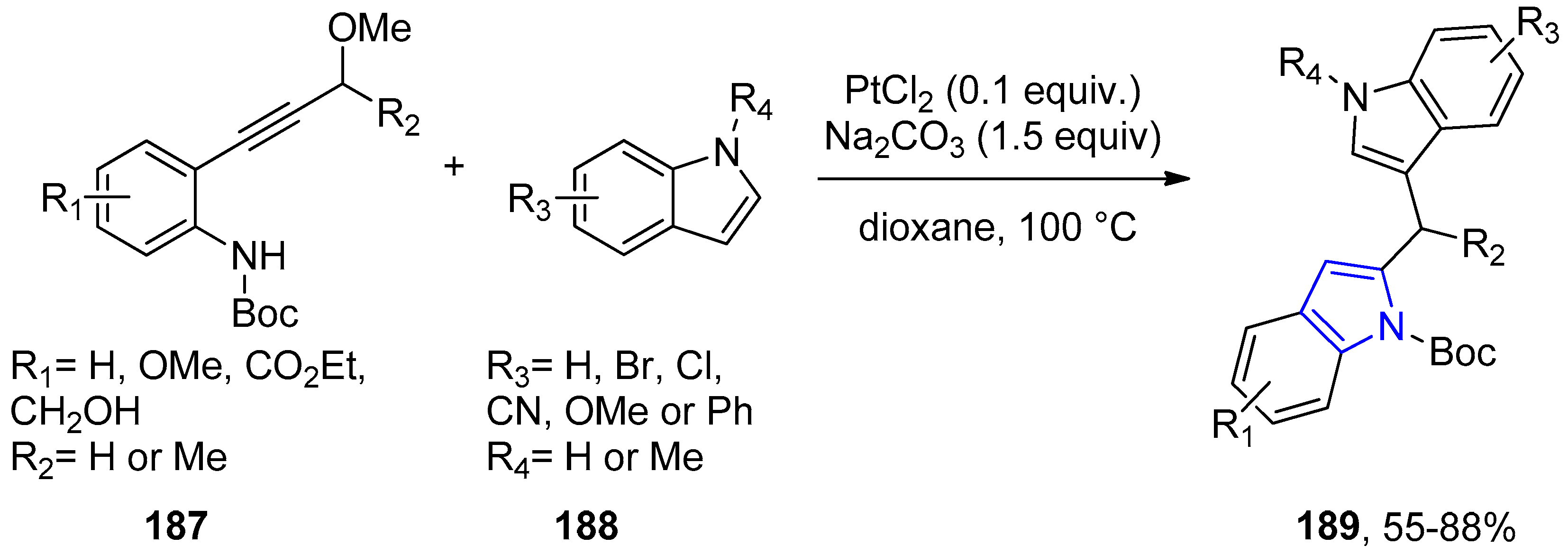
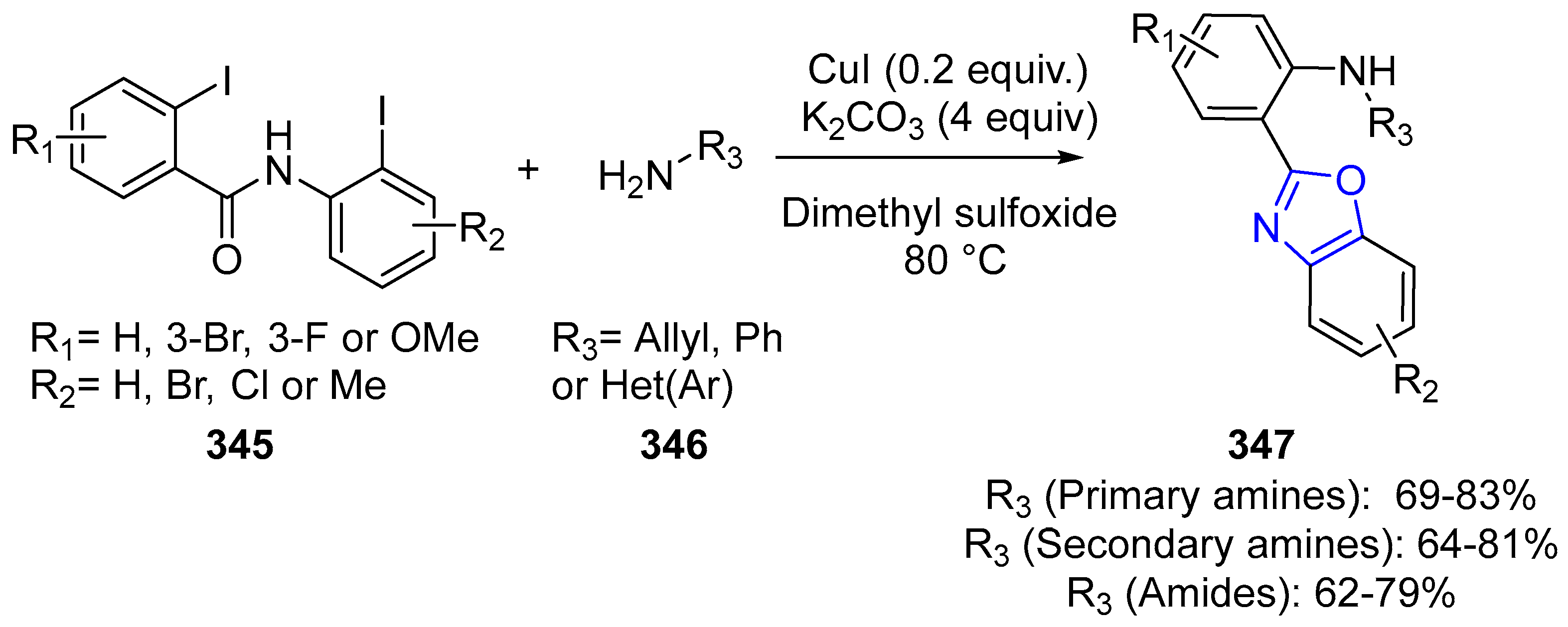
4.2.3. Indole
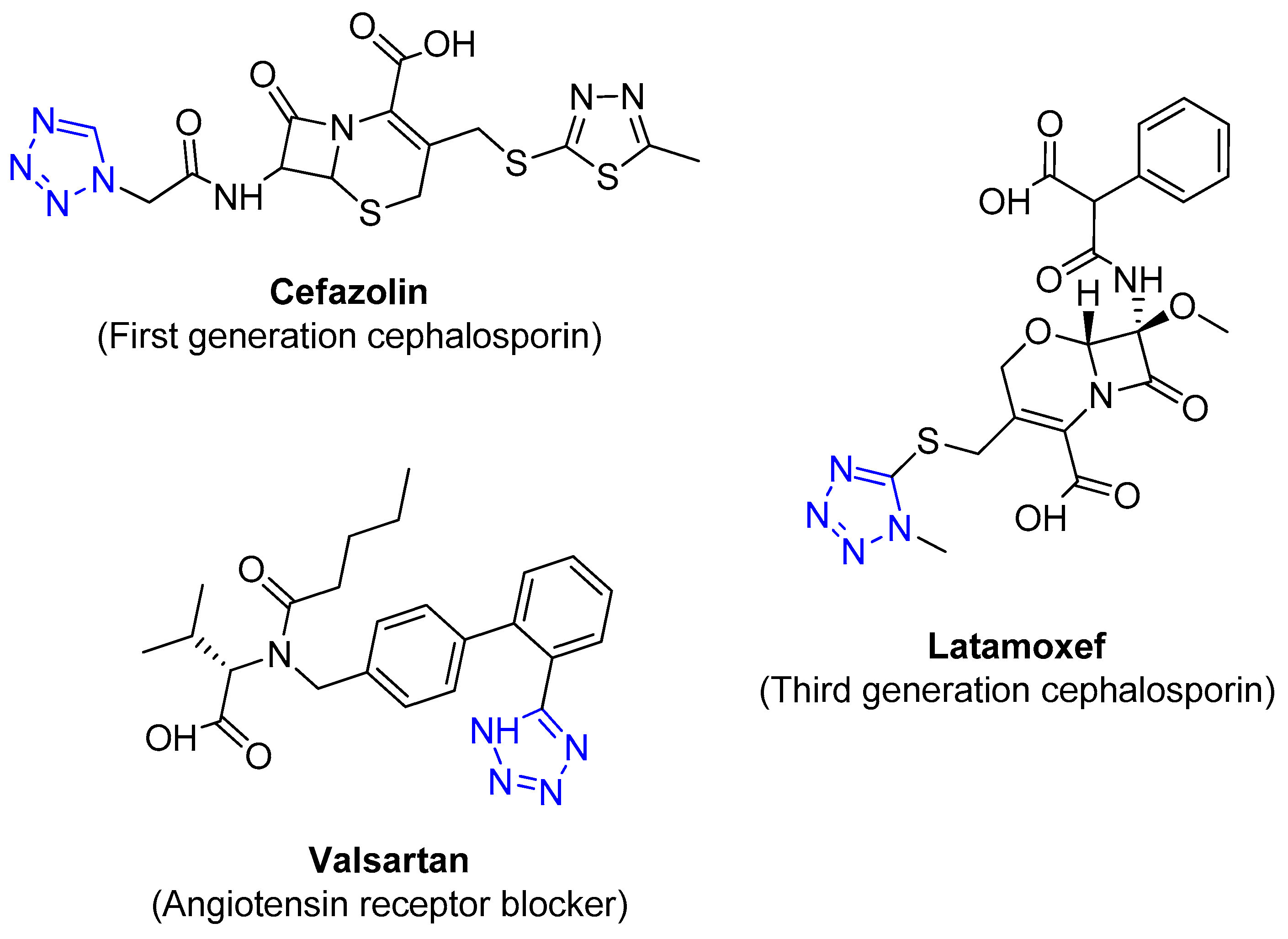
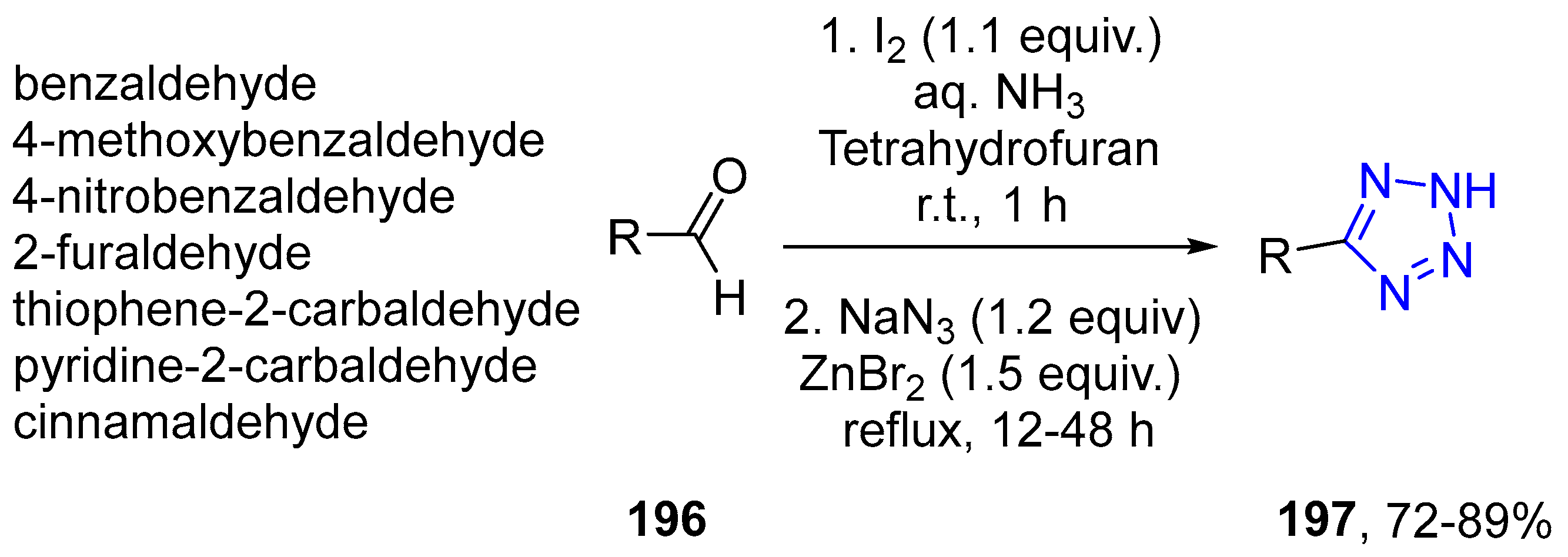
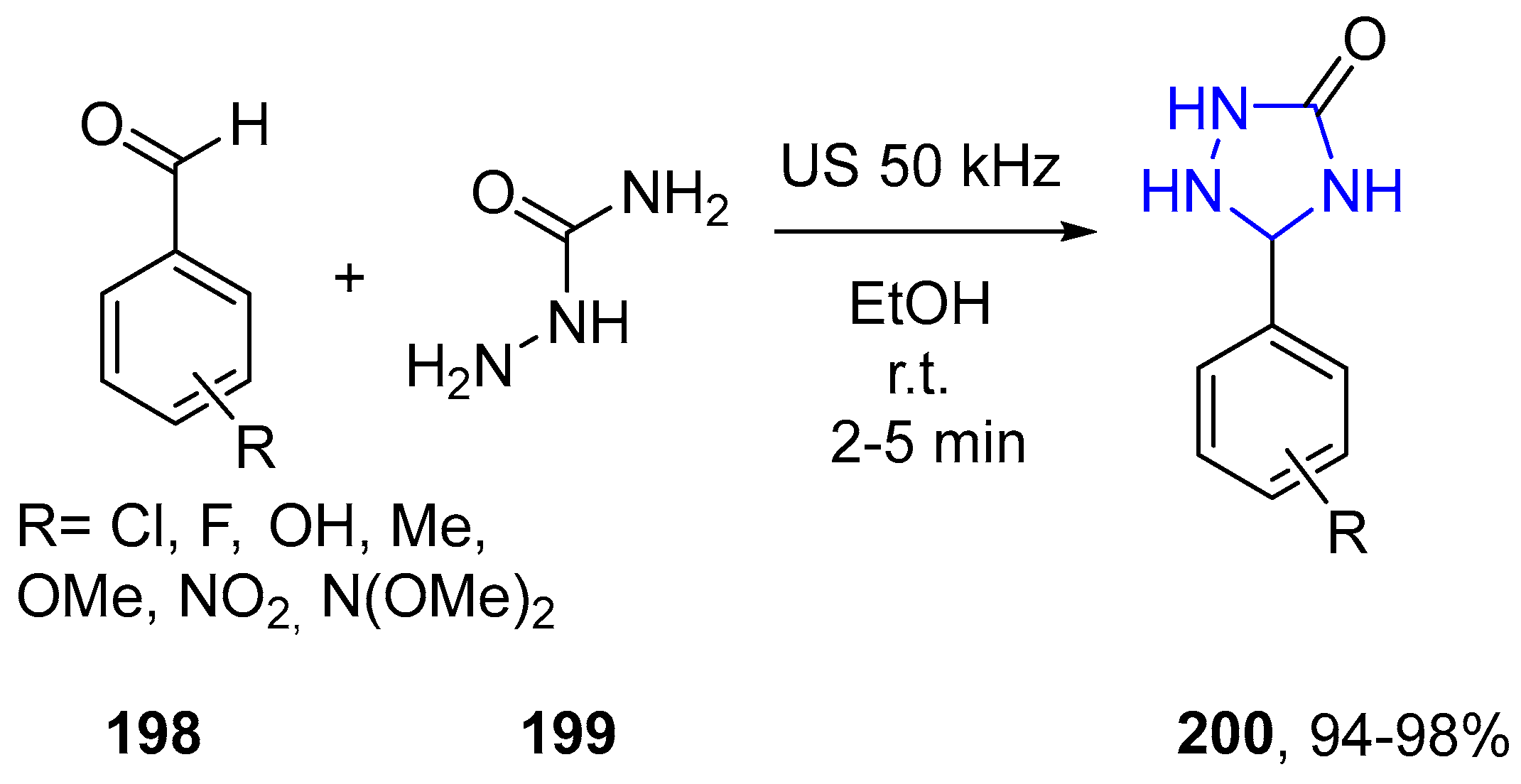
4.2.4. Tetrazole
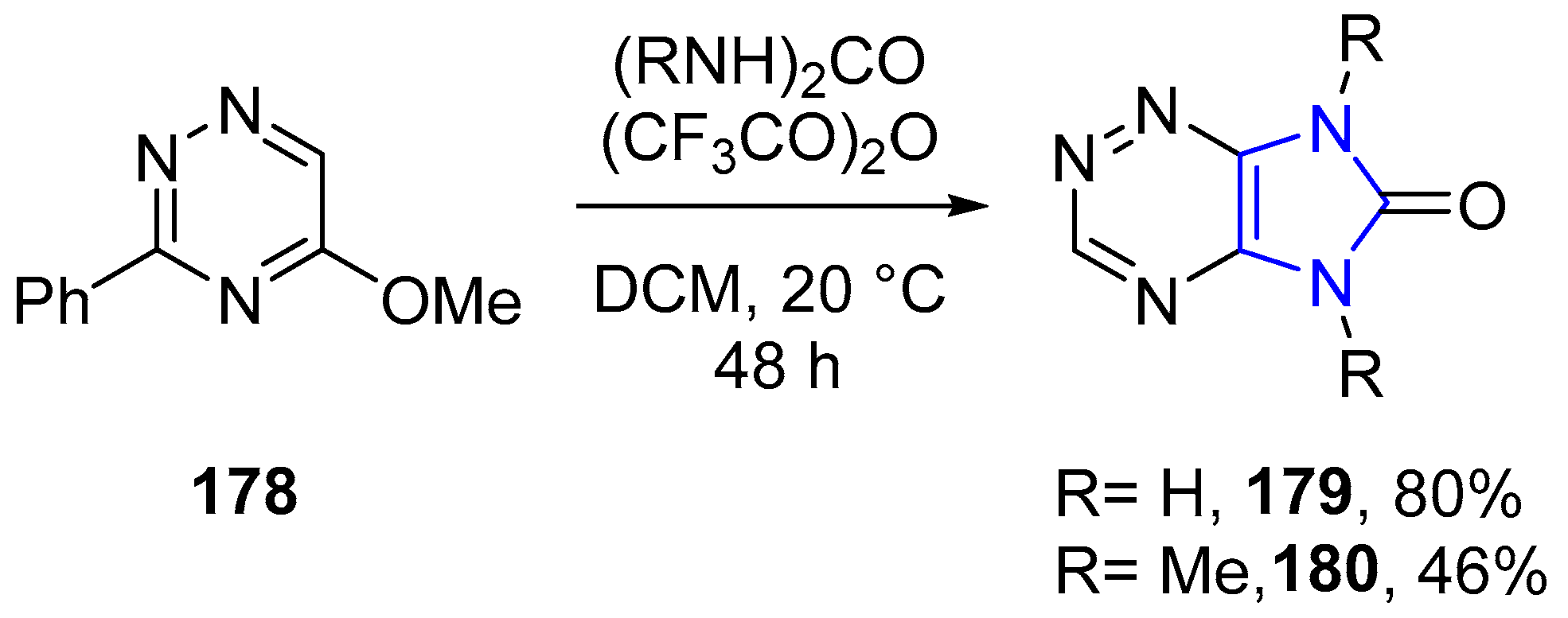
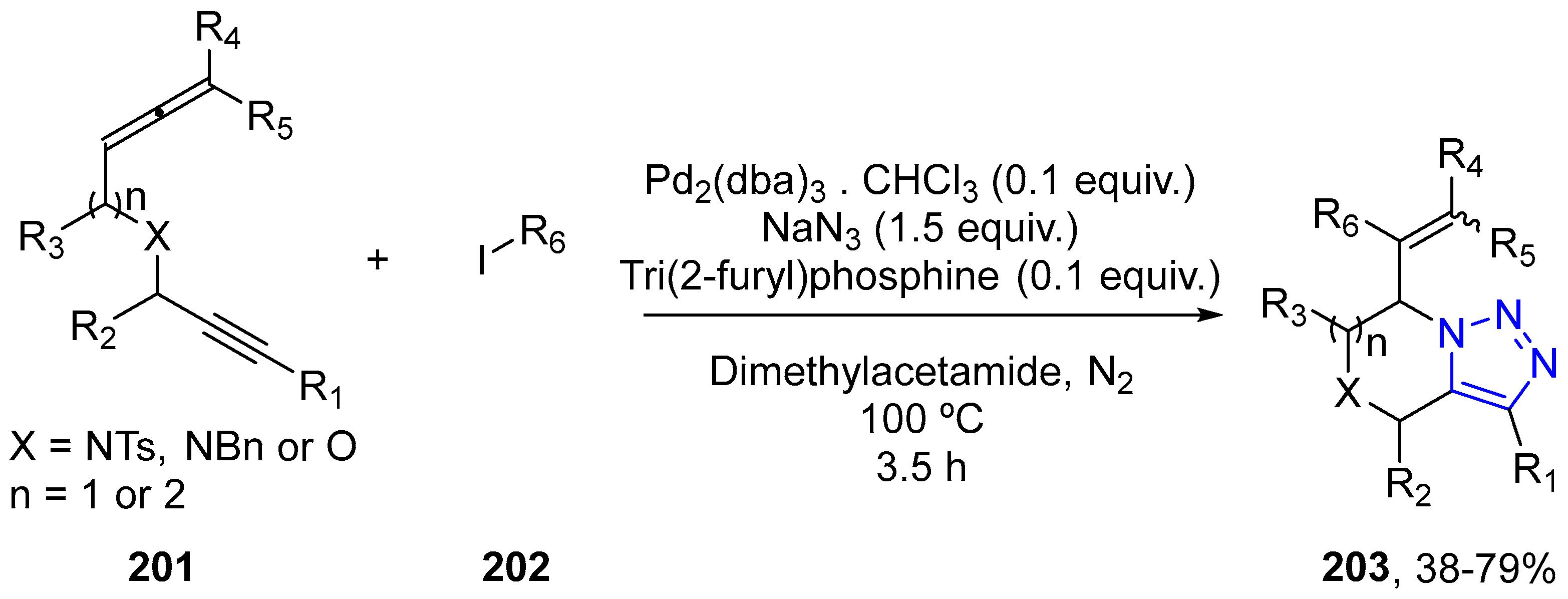
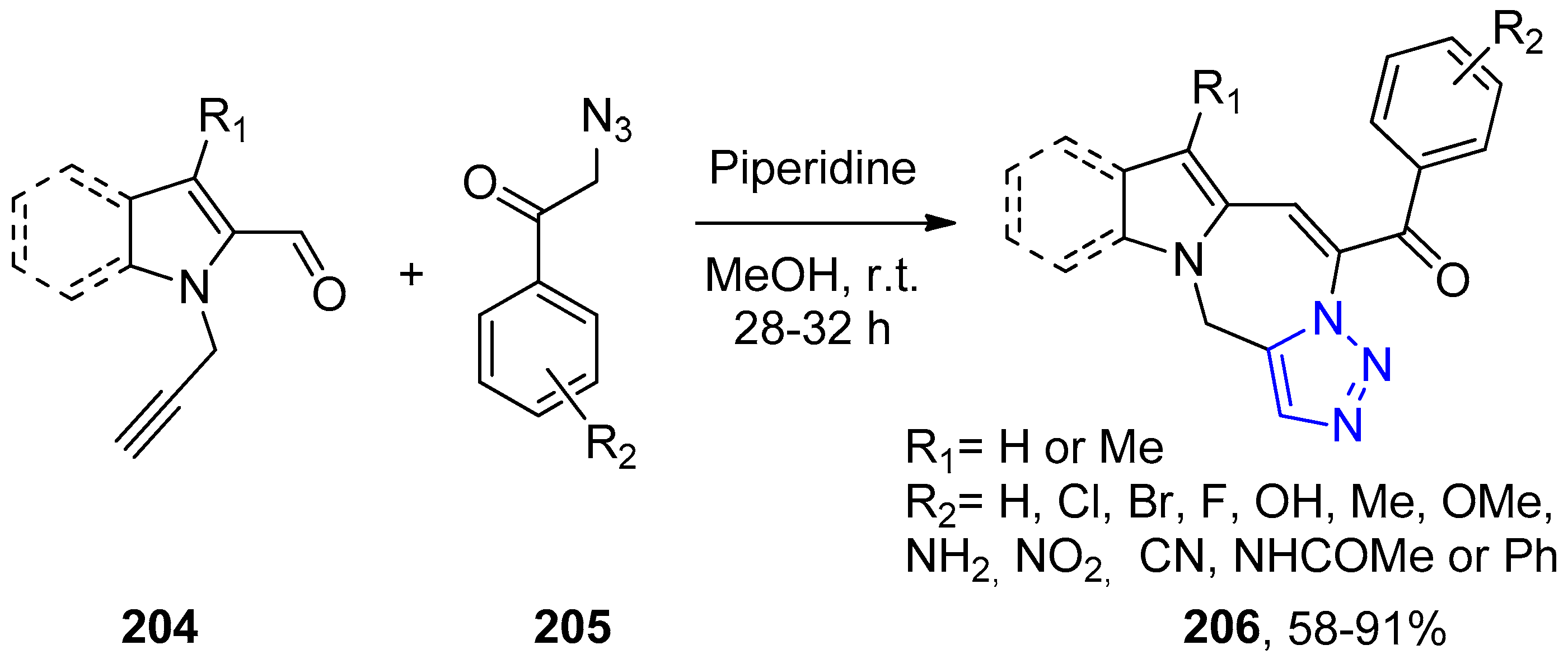
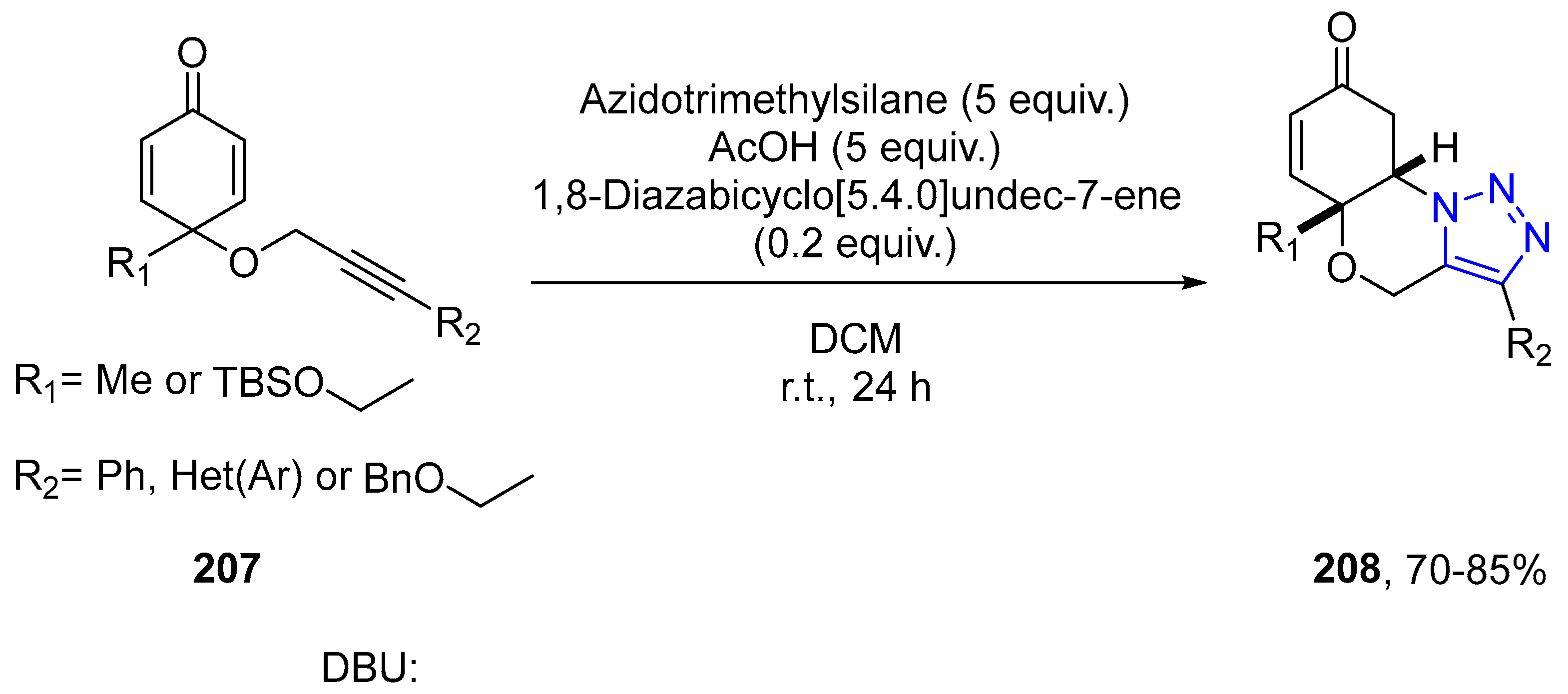
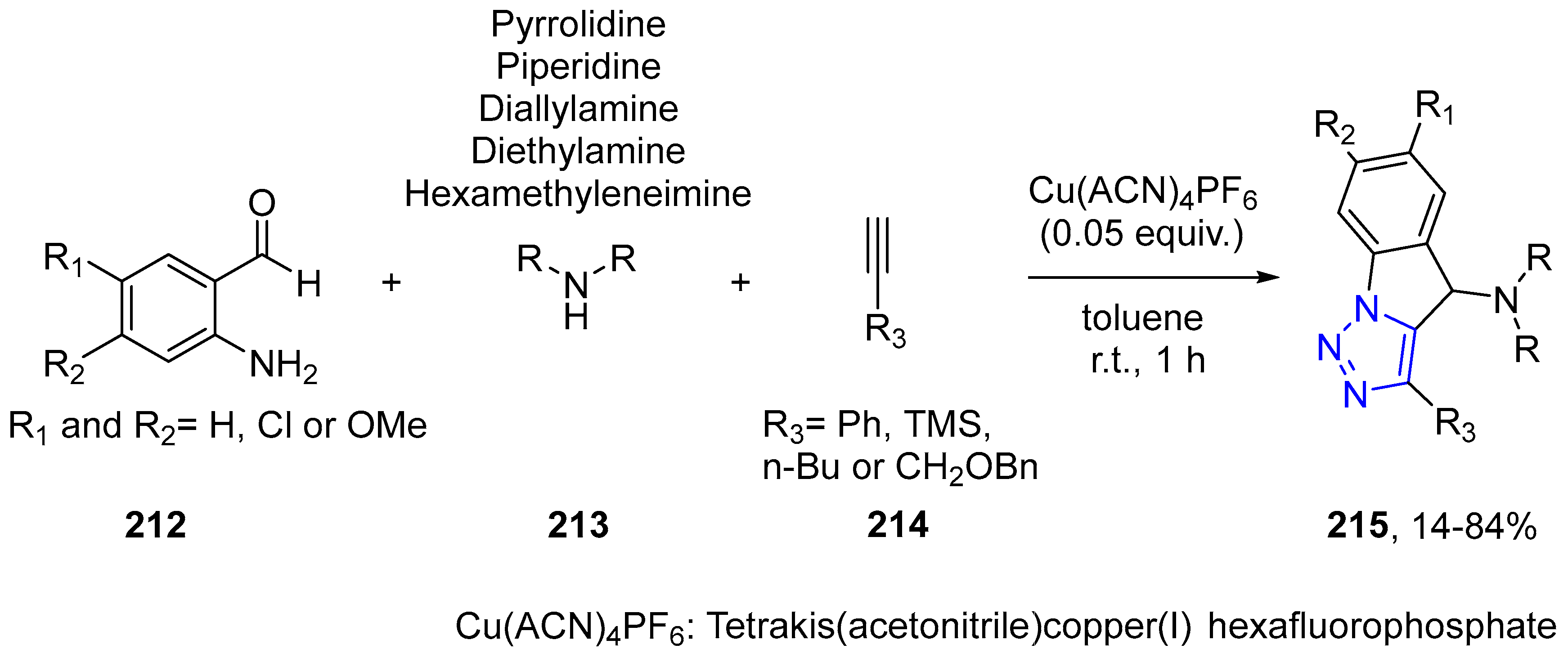
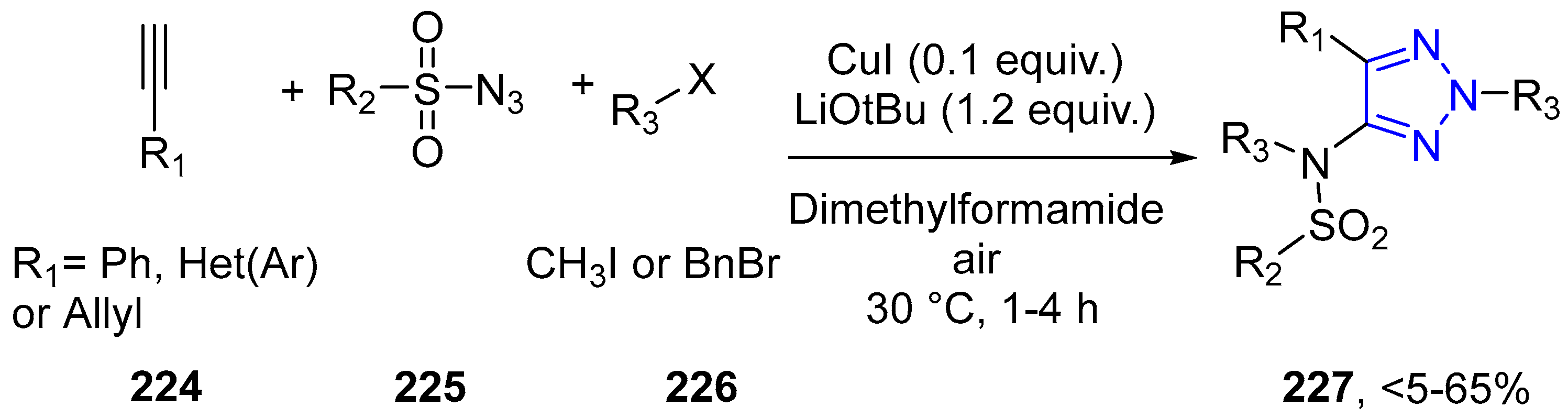
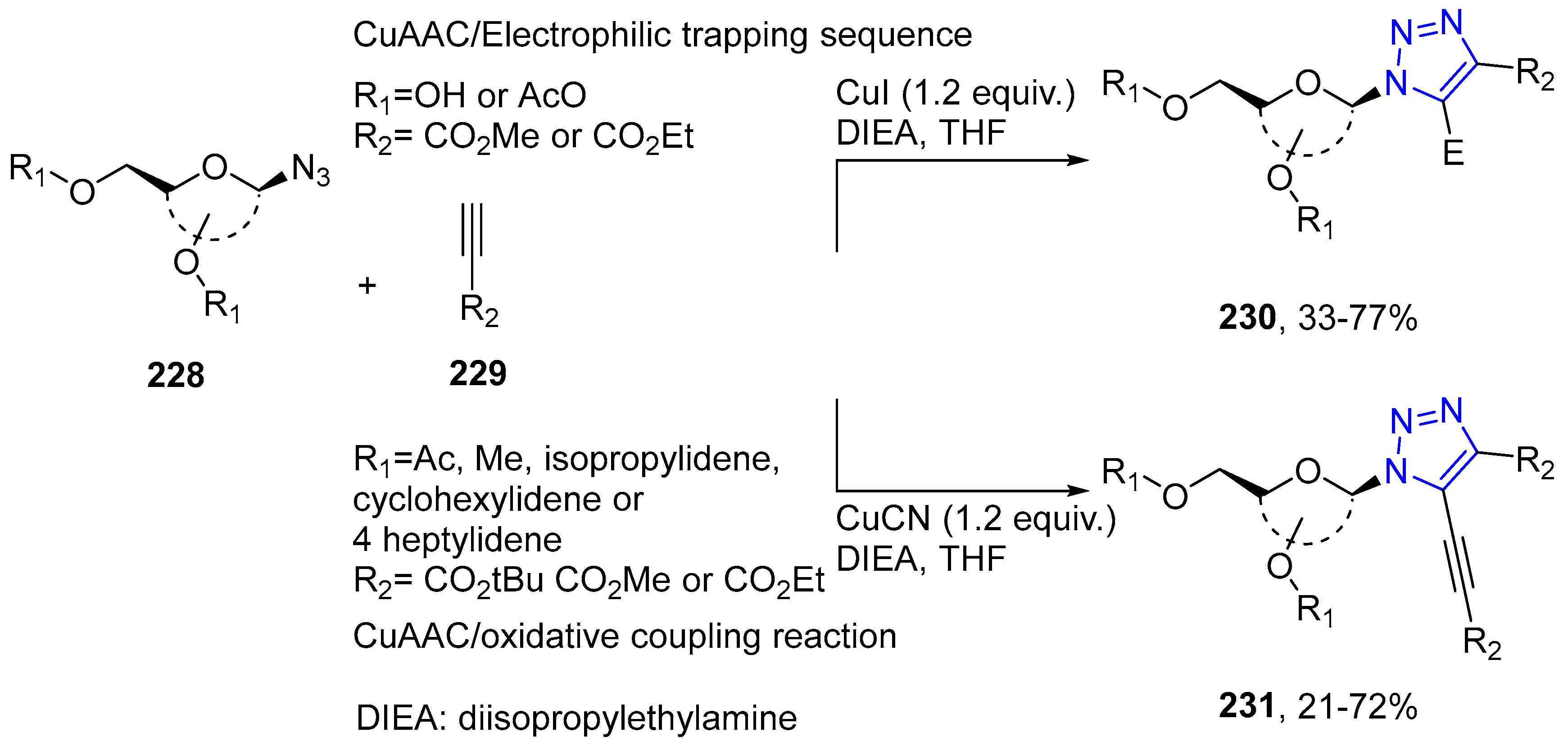
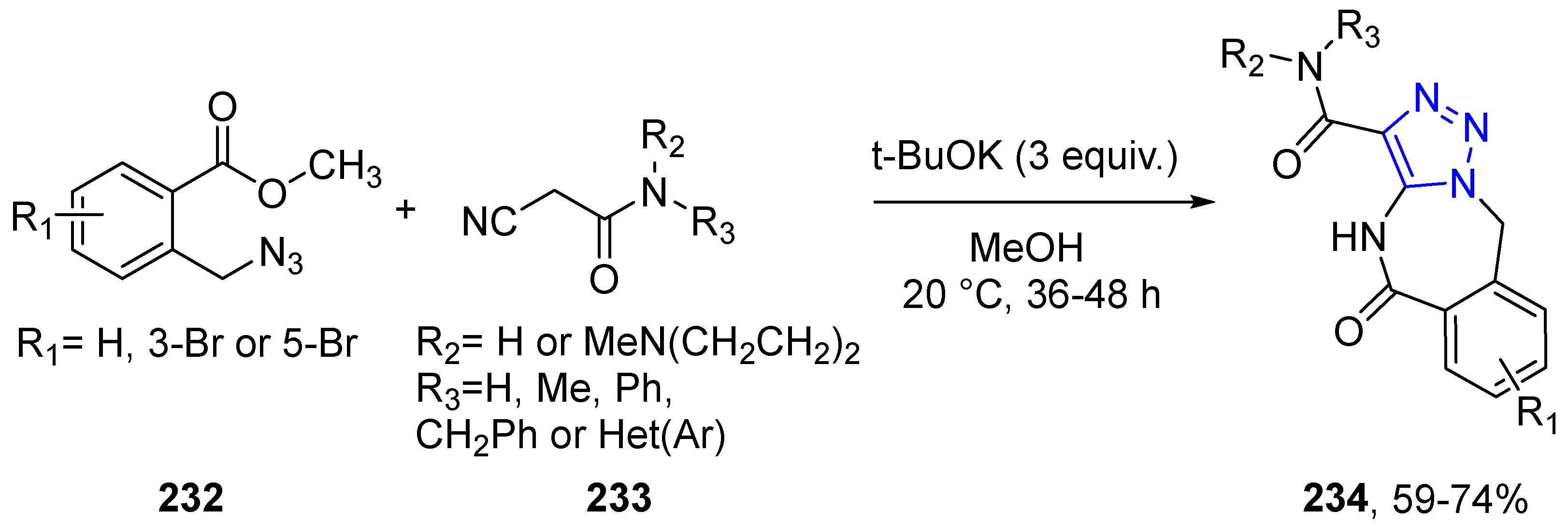
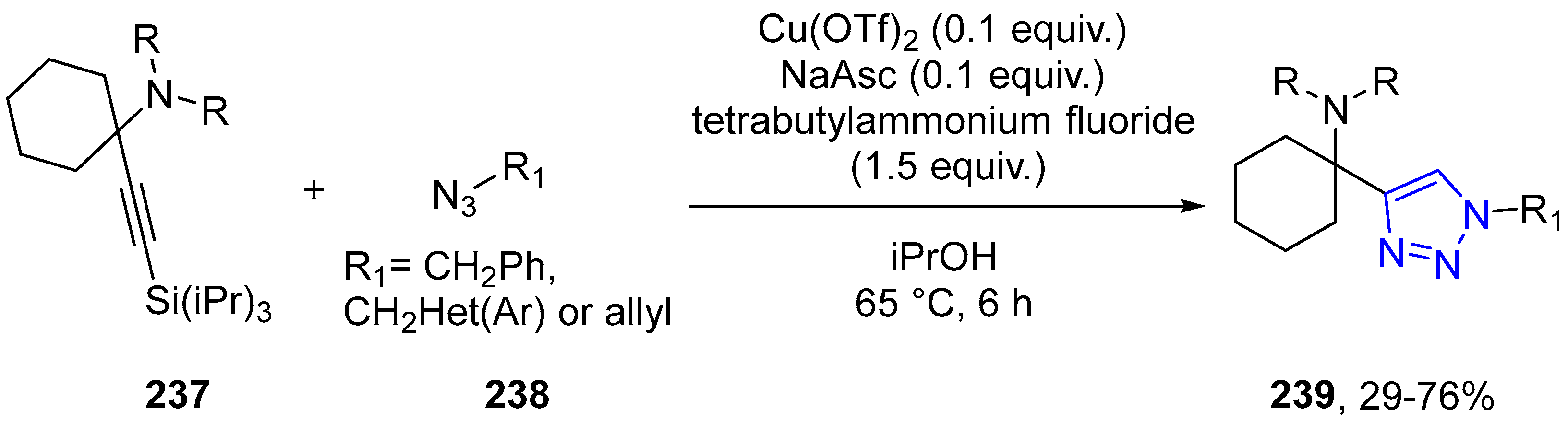
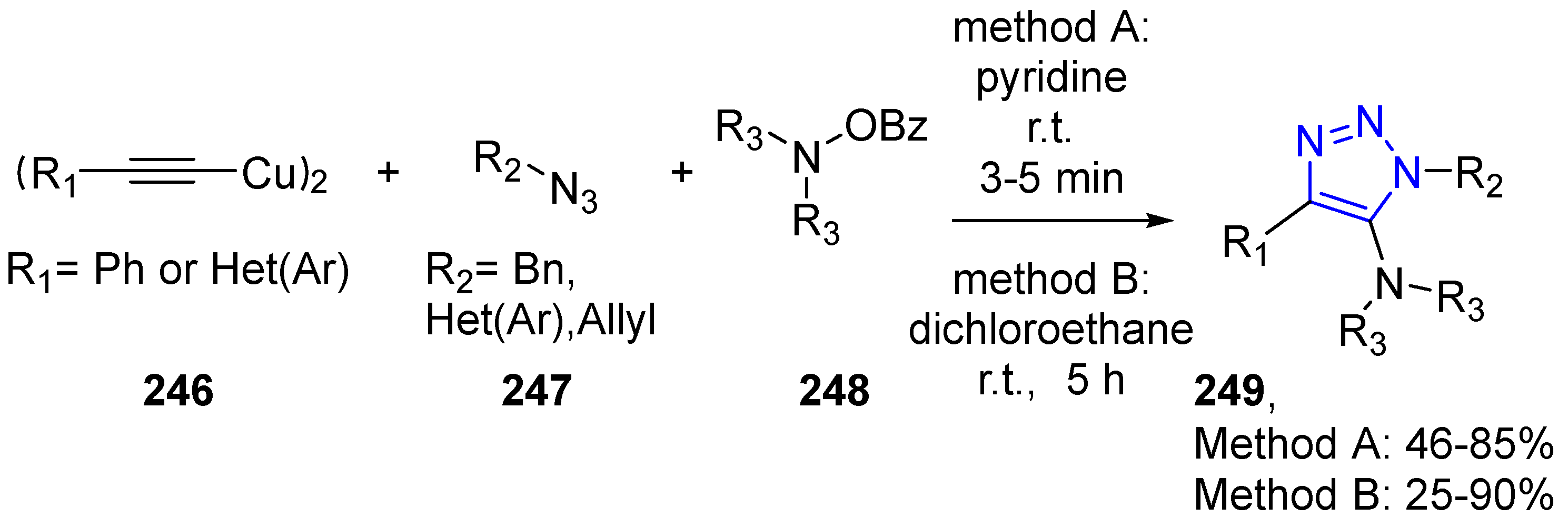
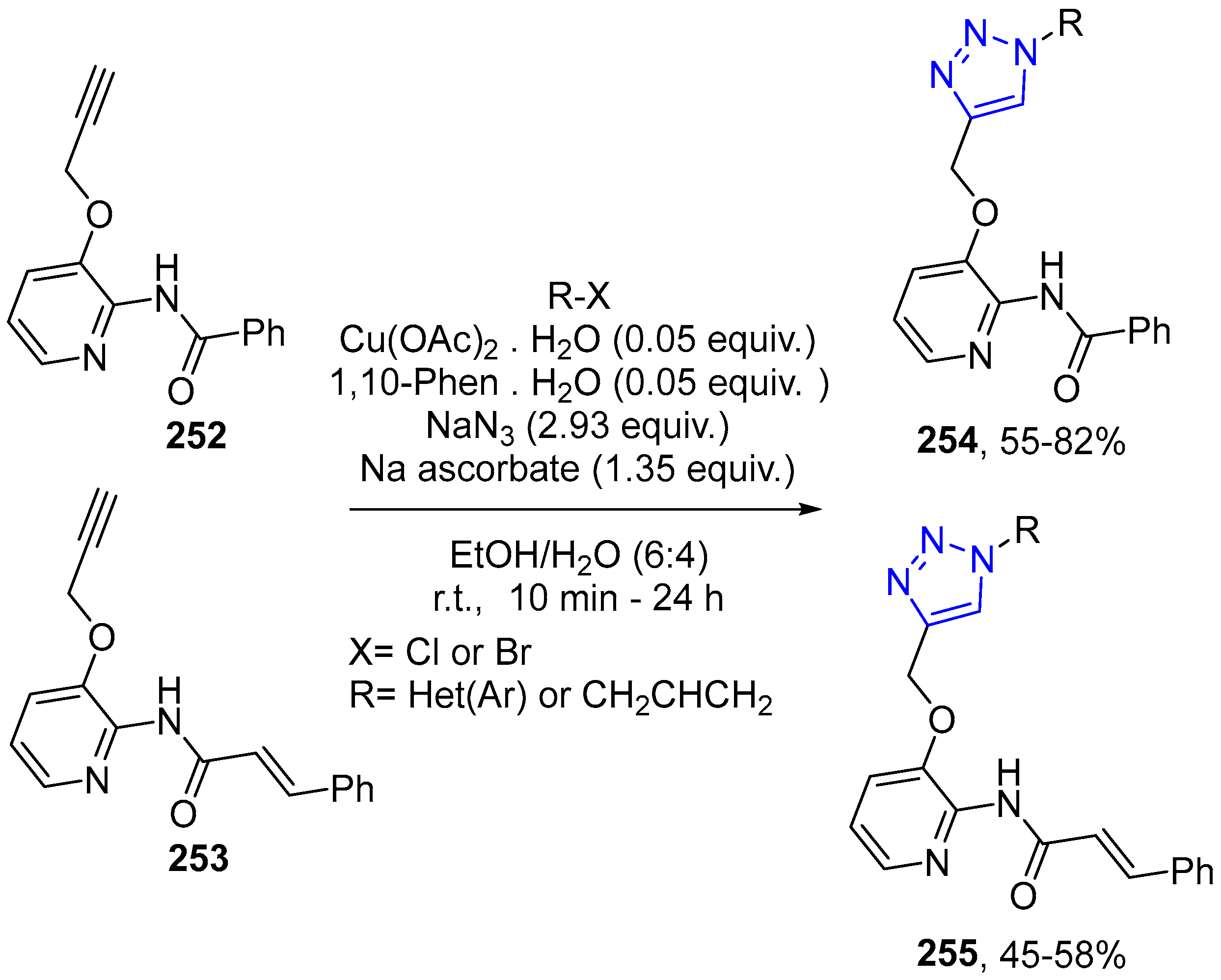
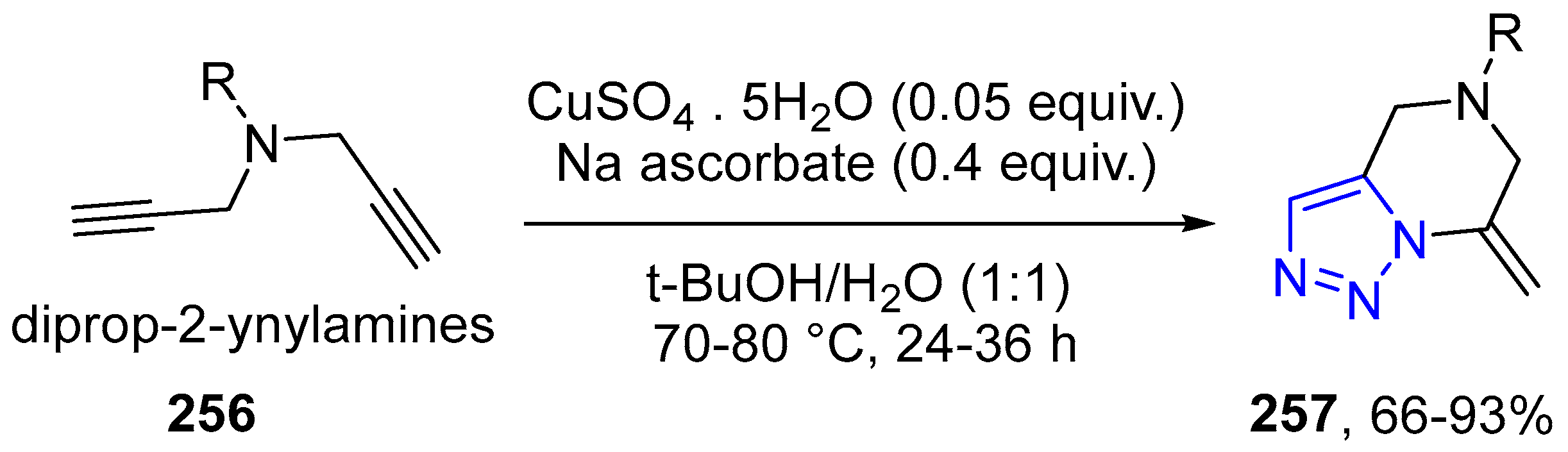
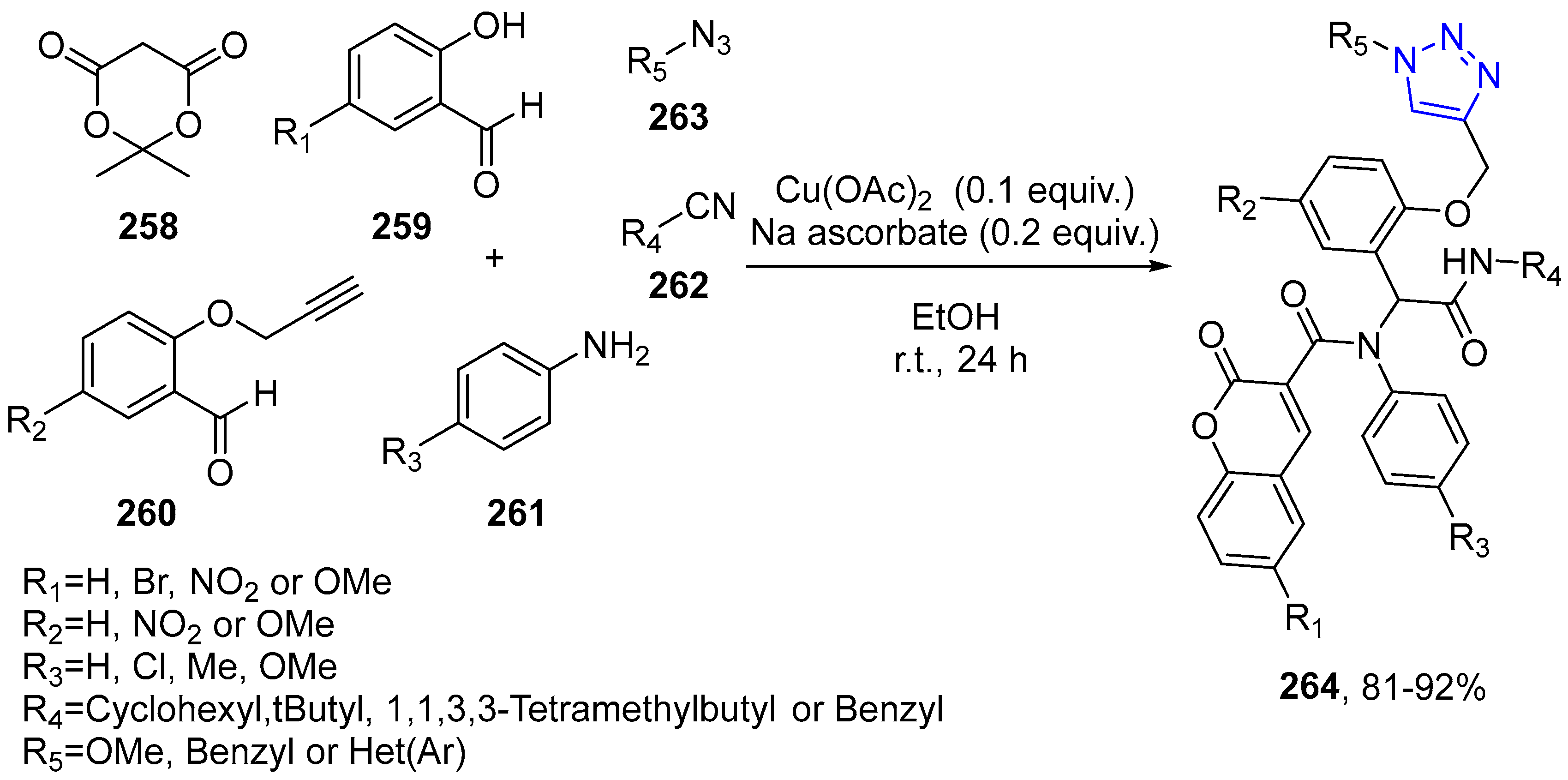
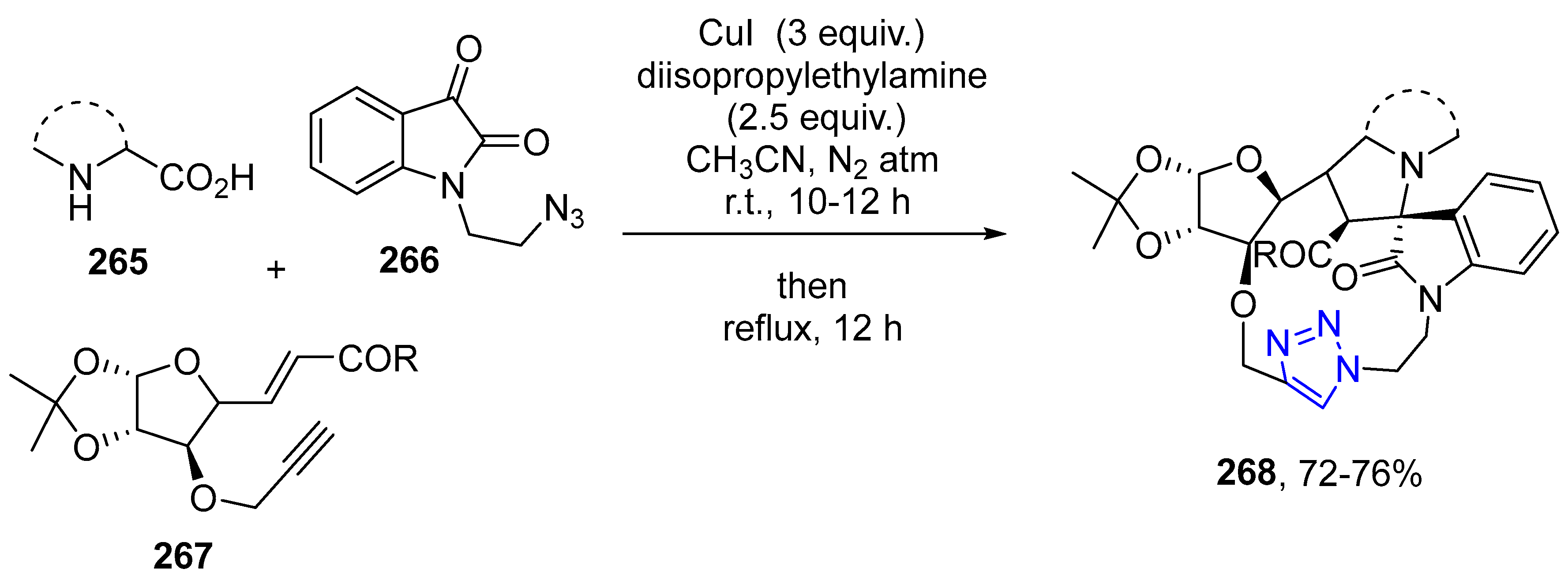
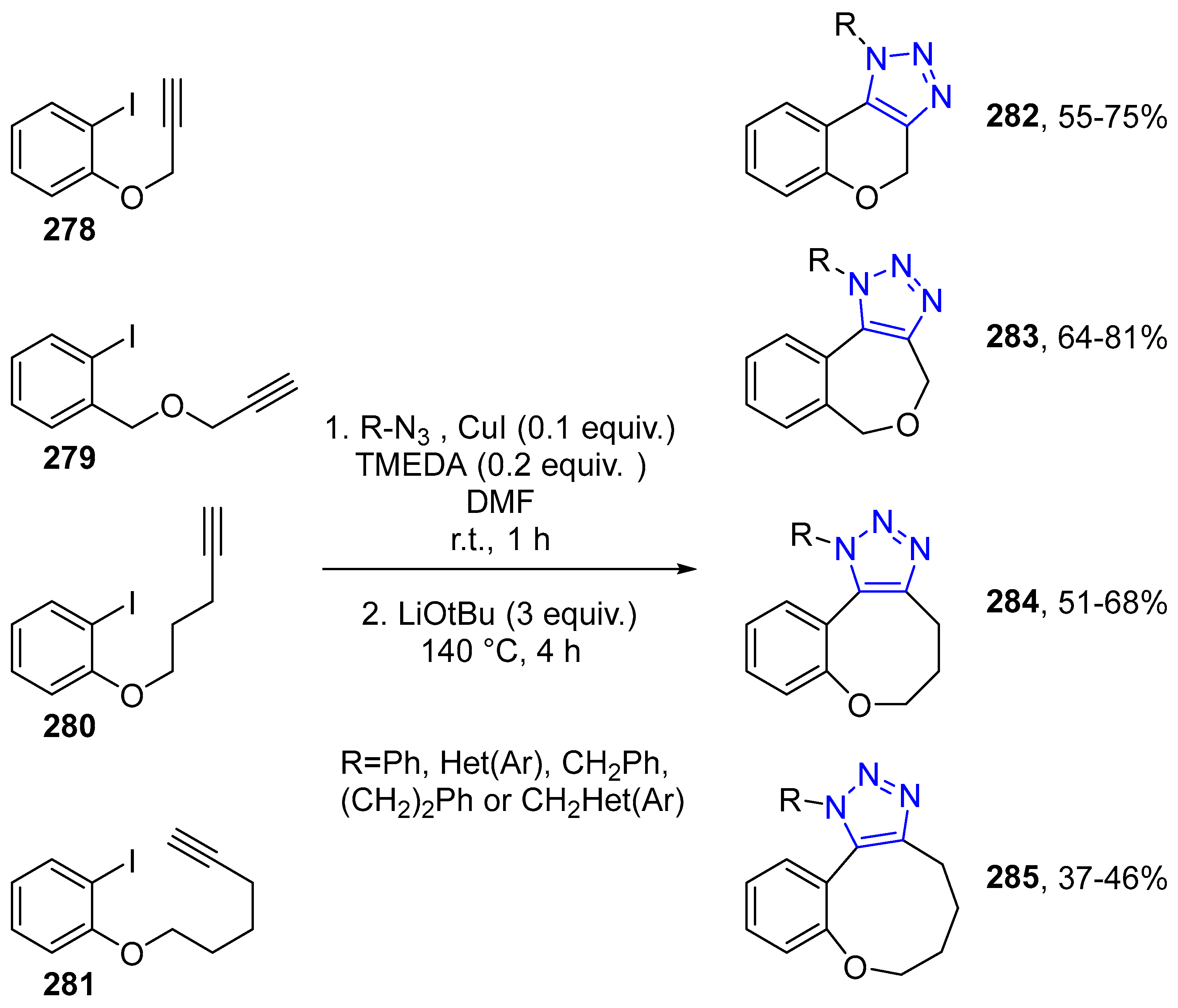
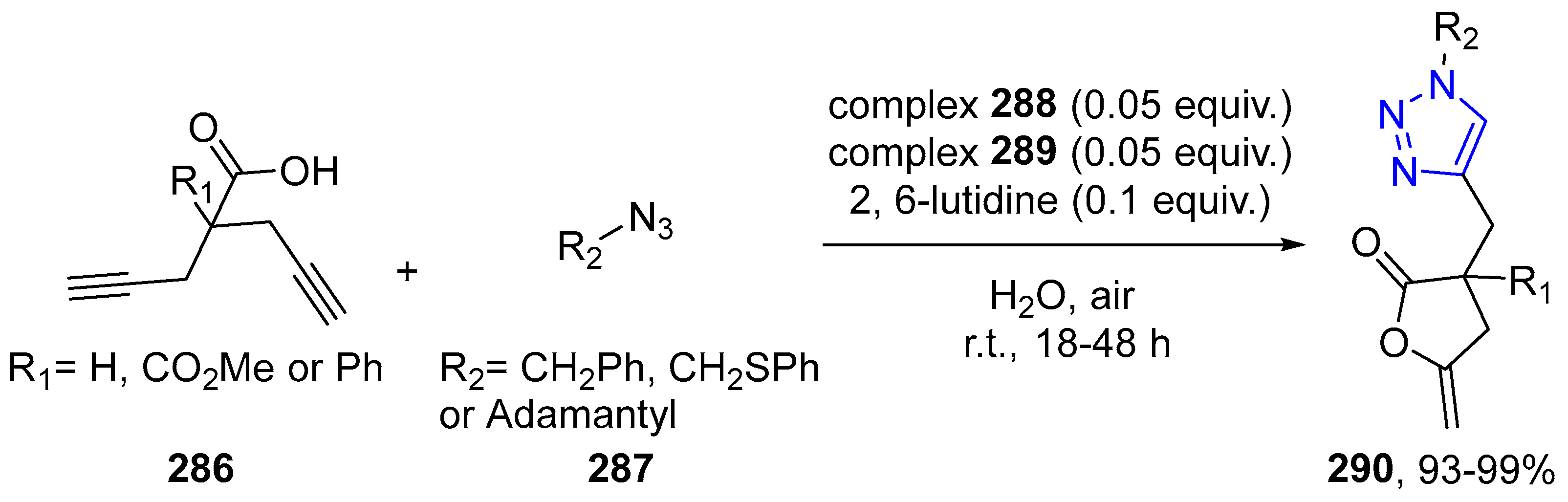
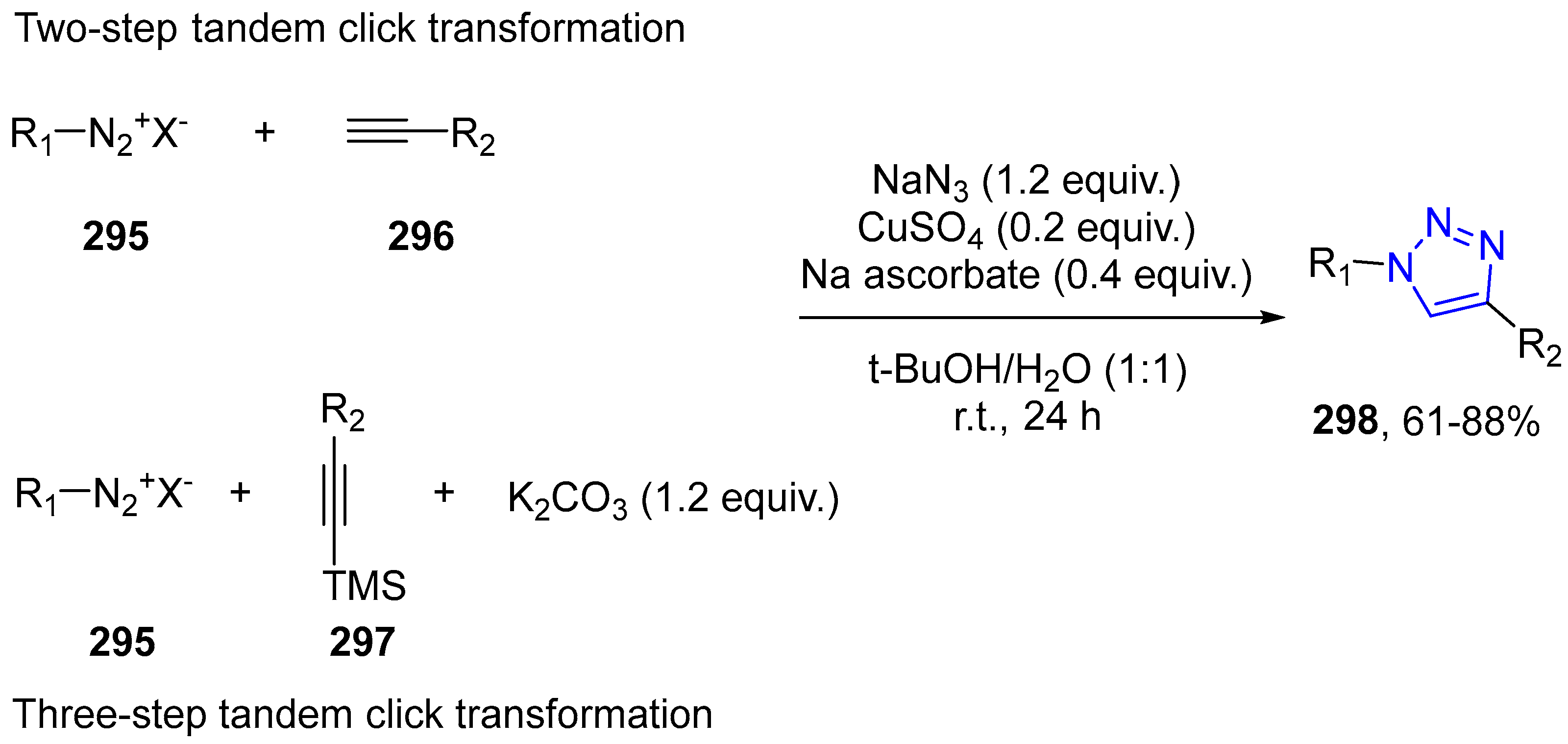
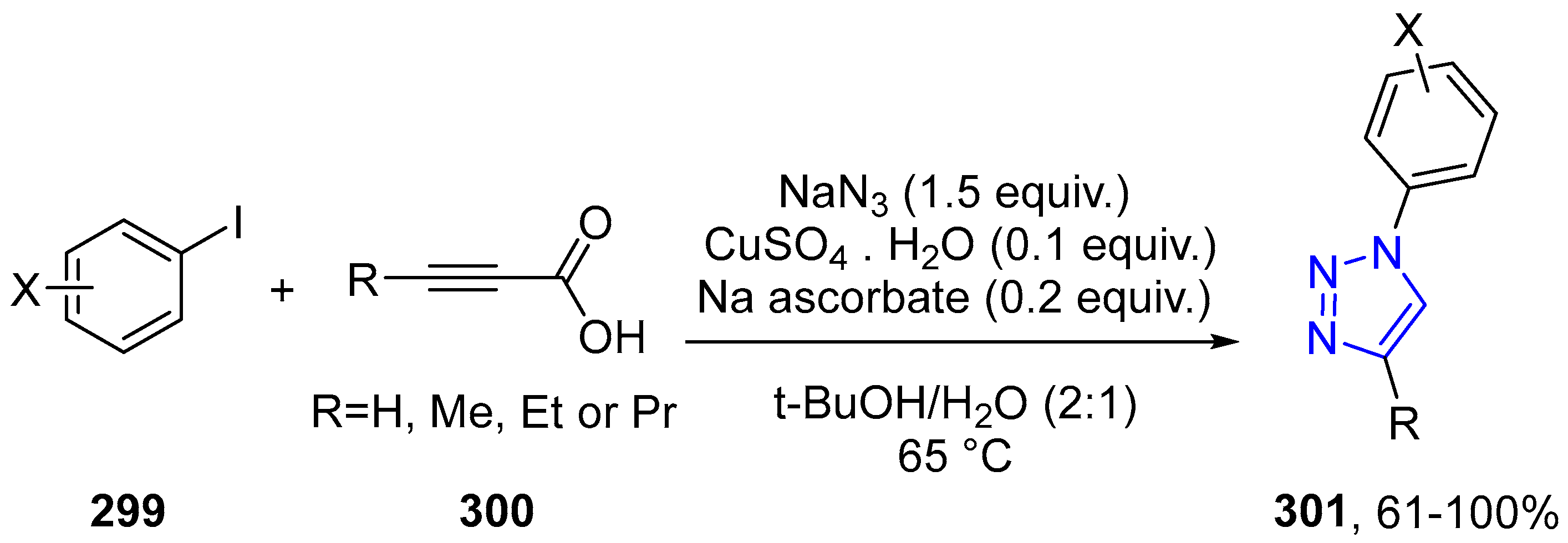
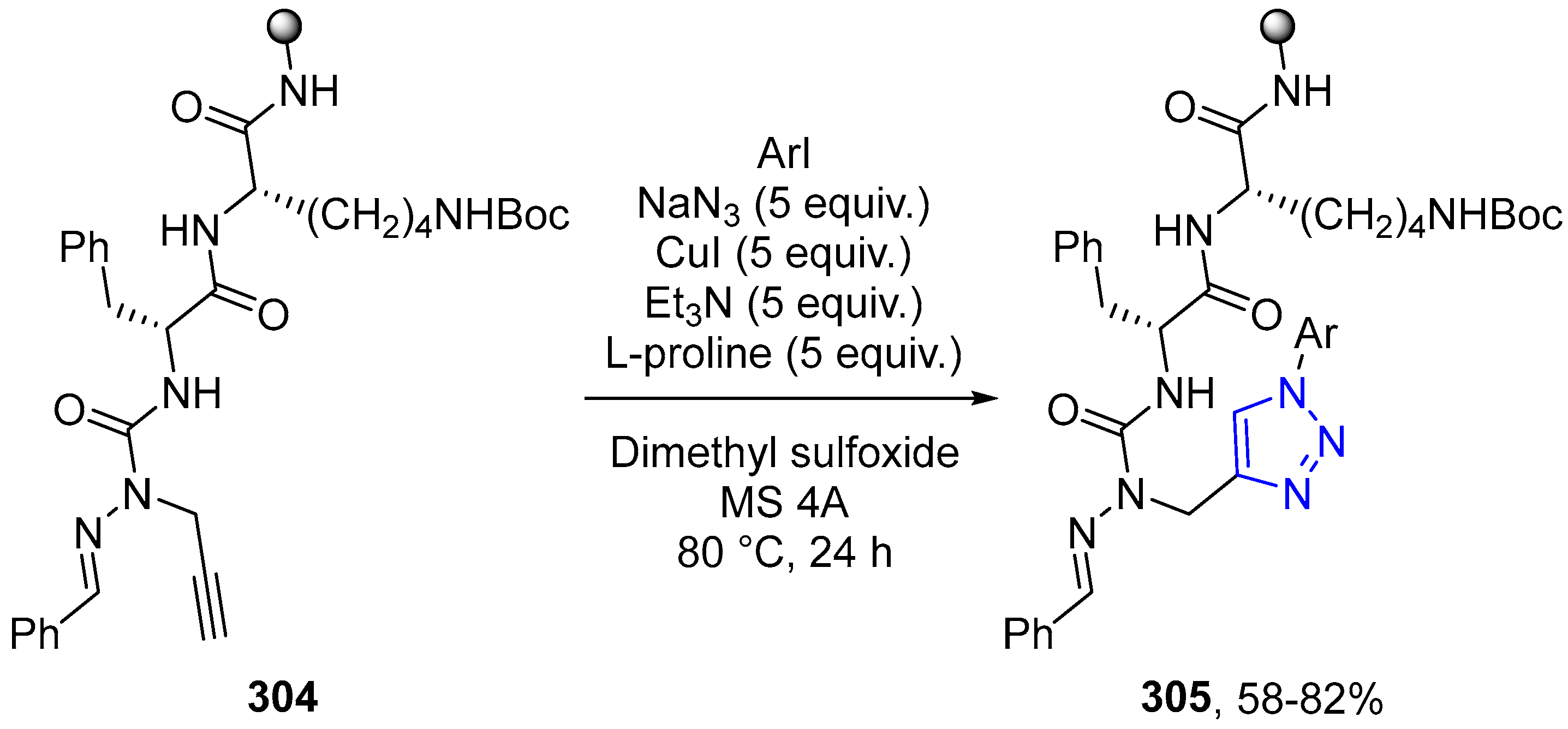
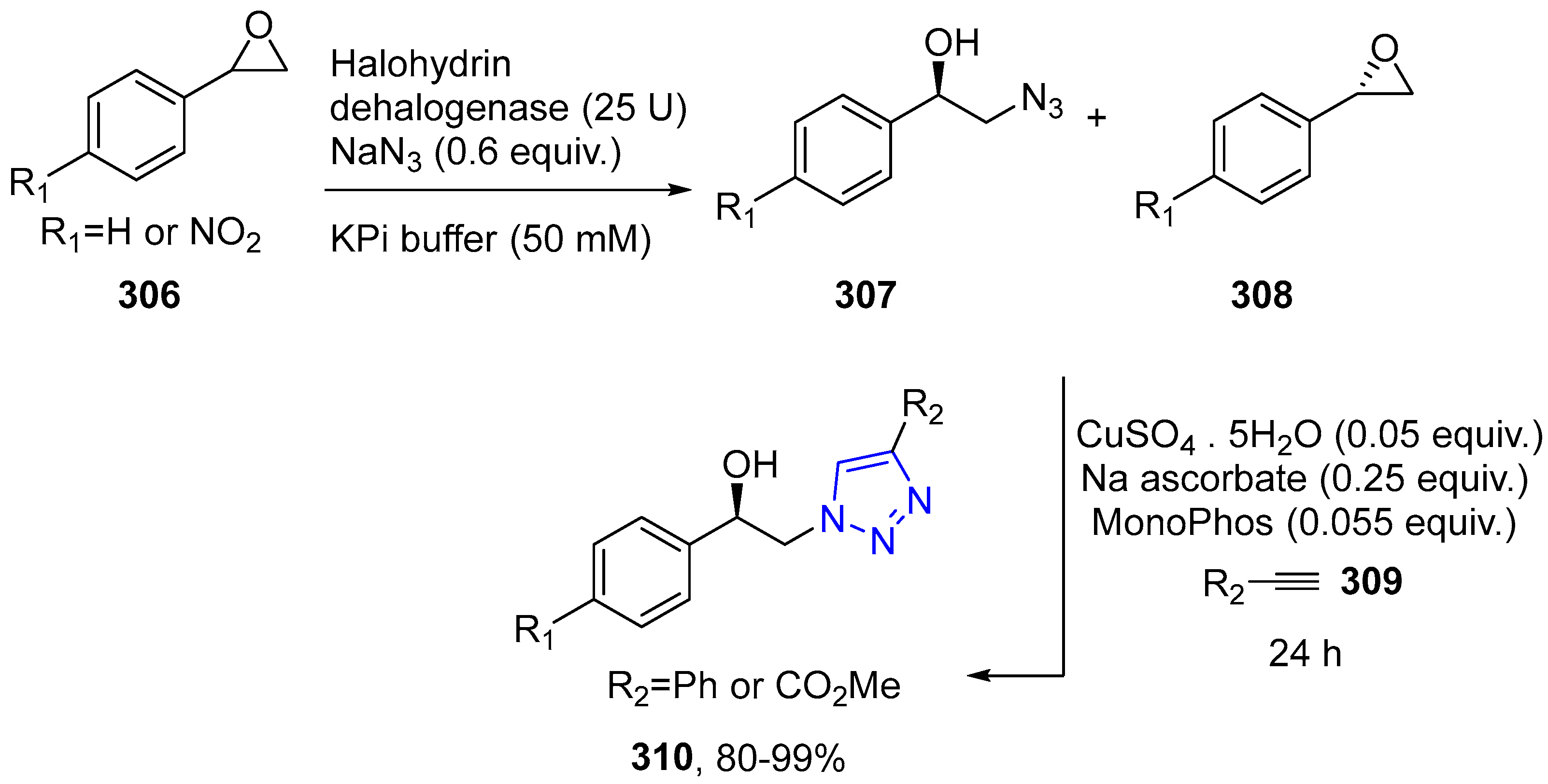
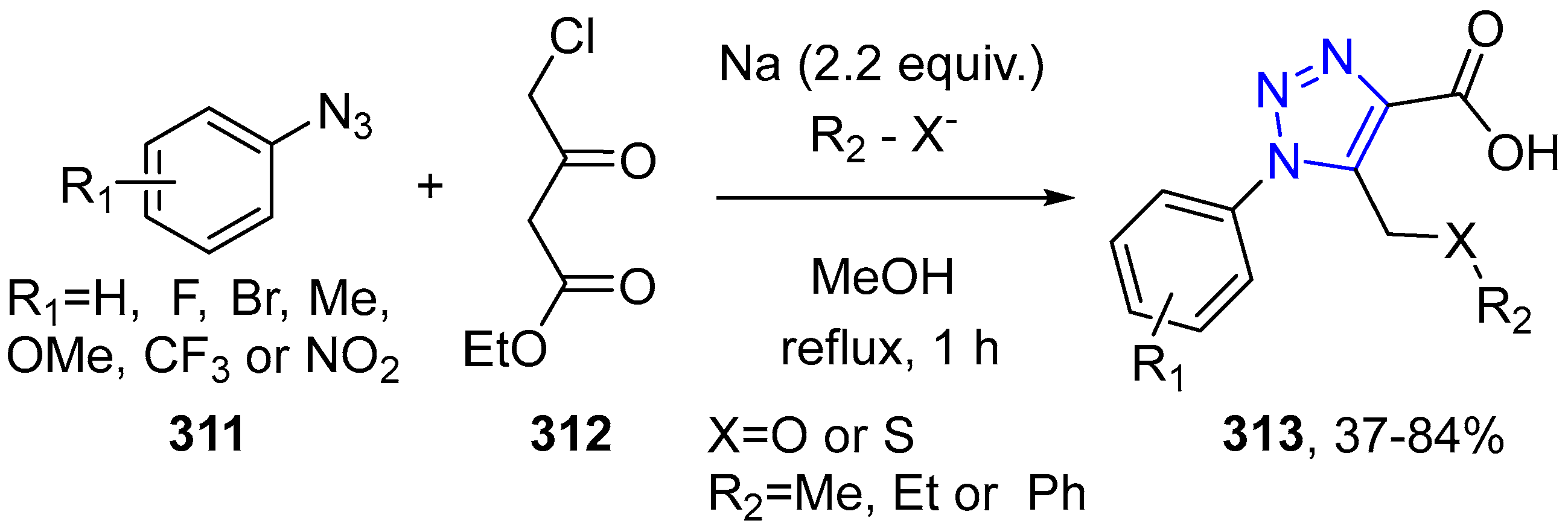
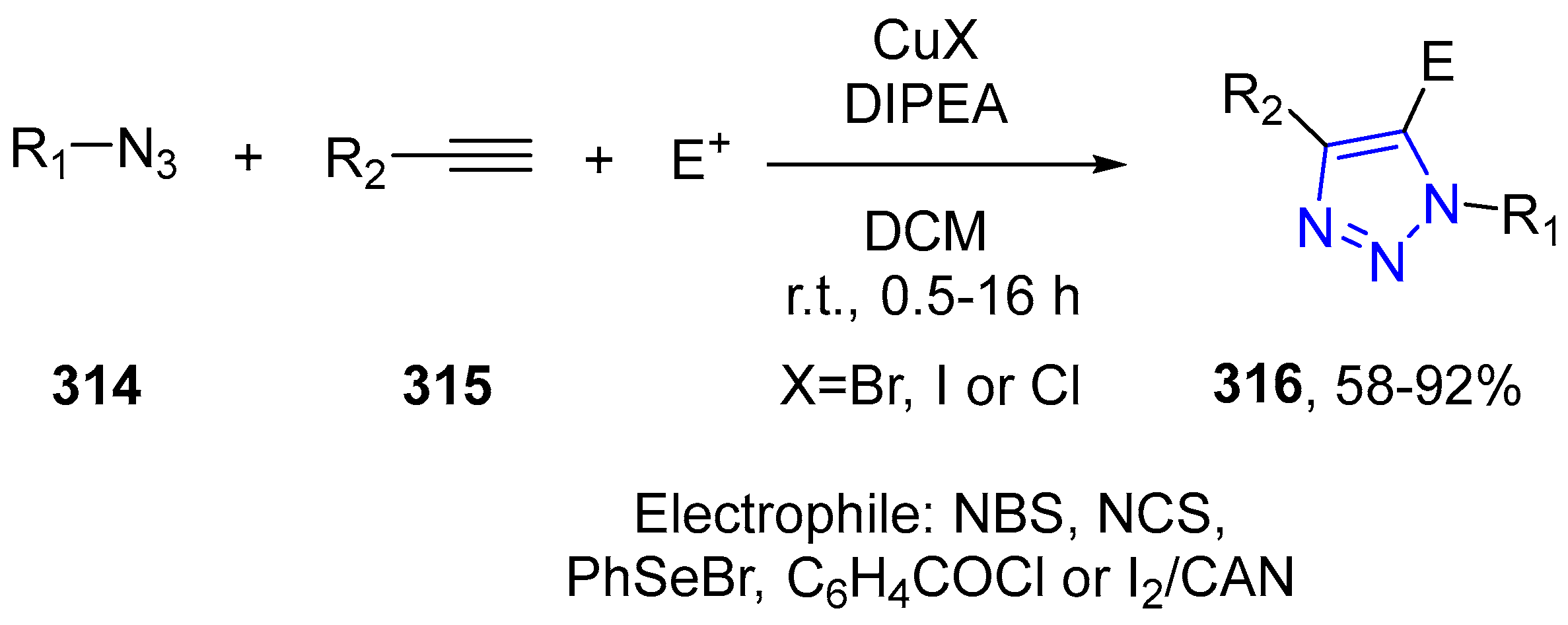
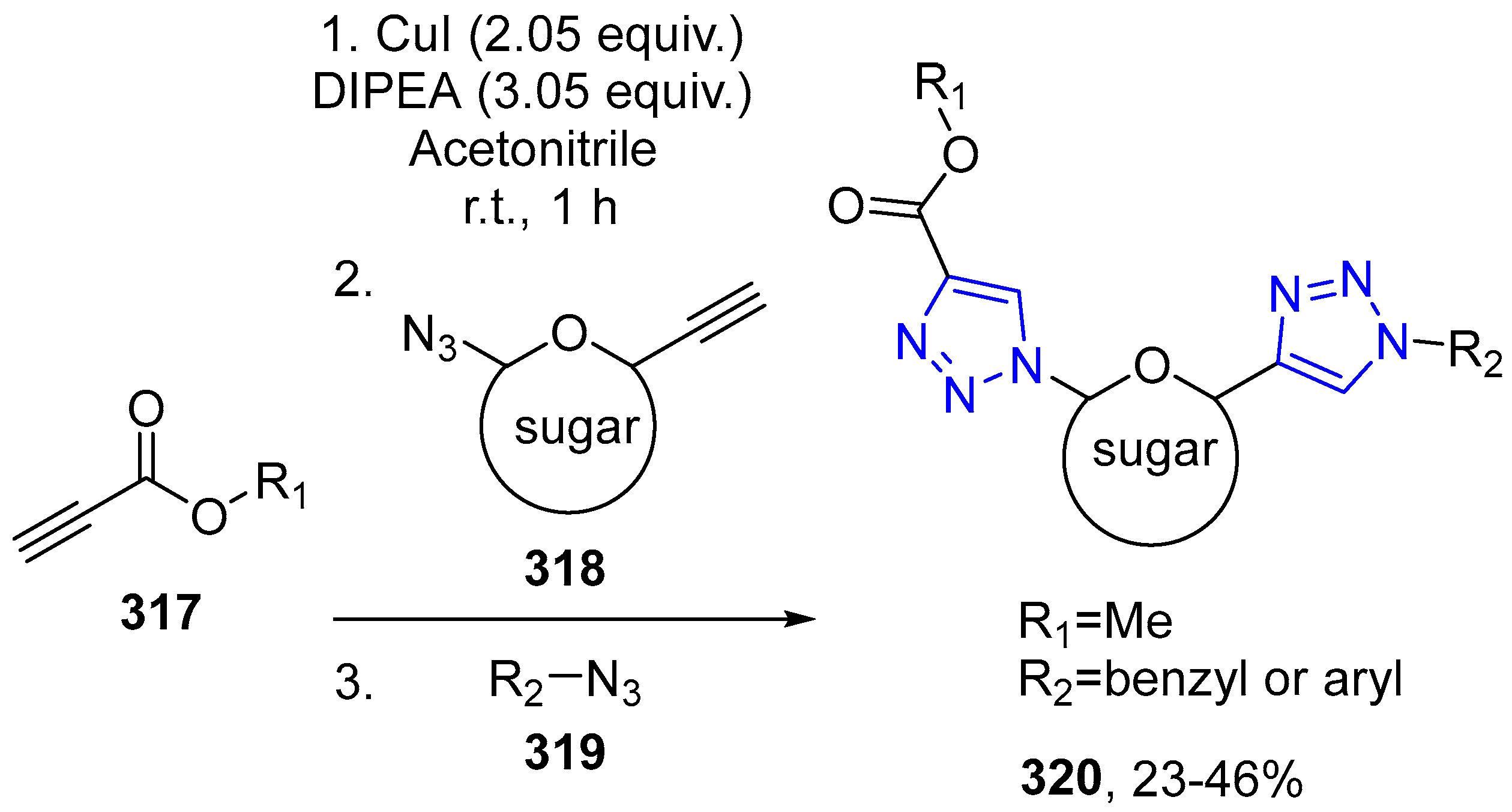
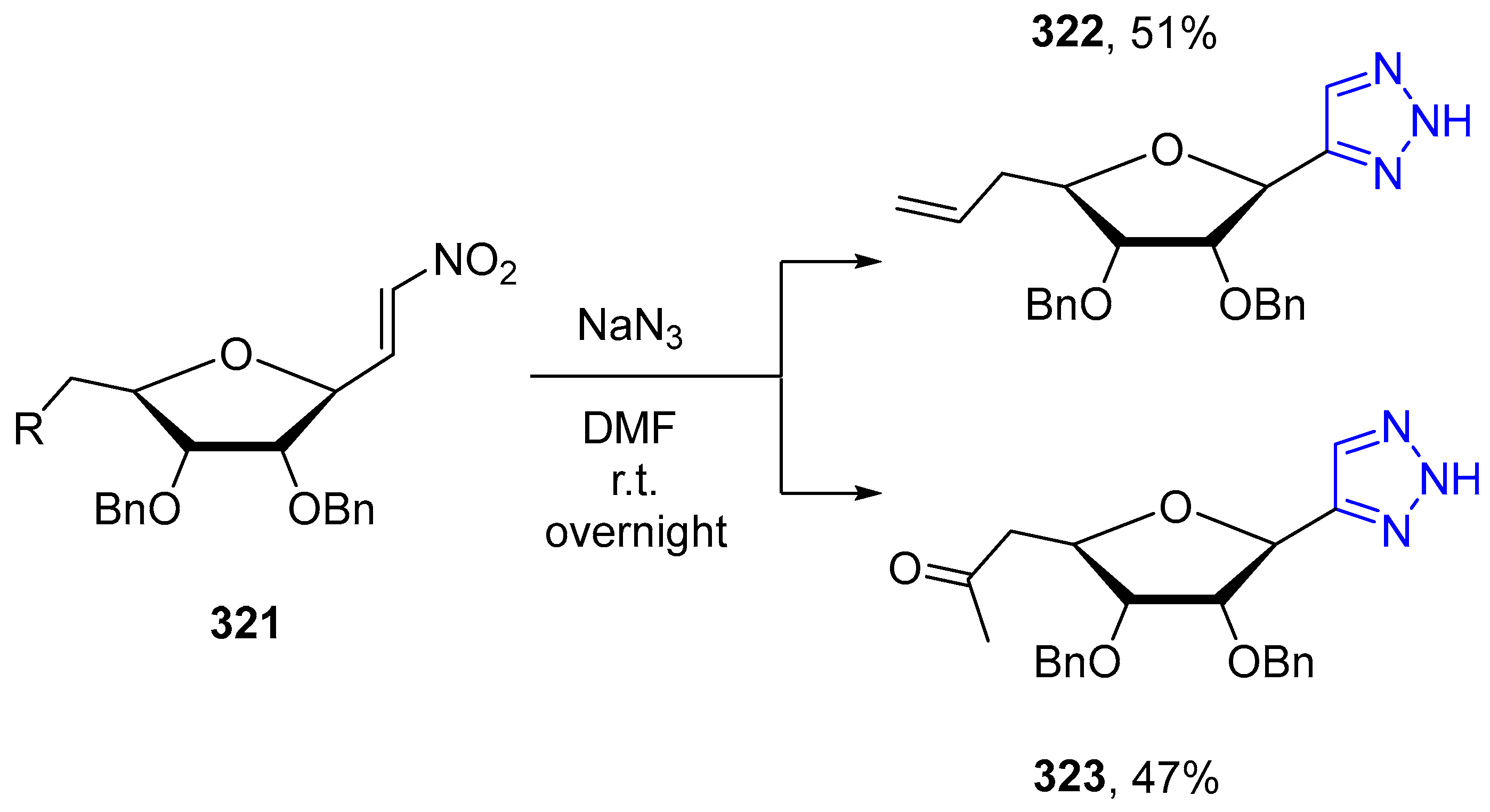
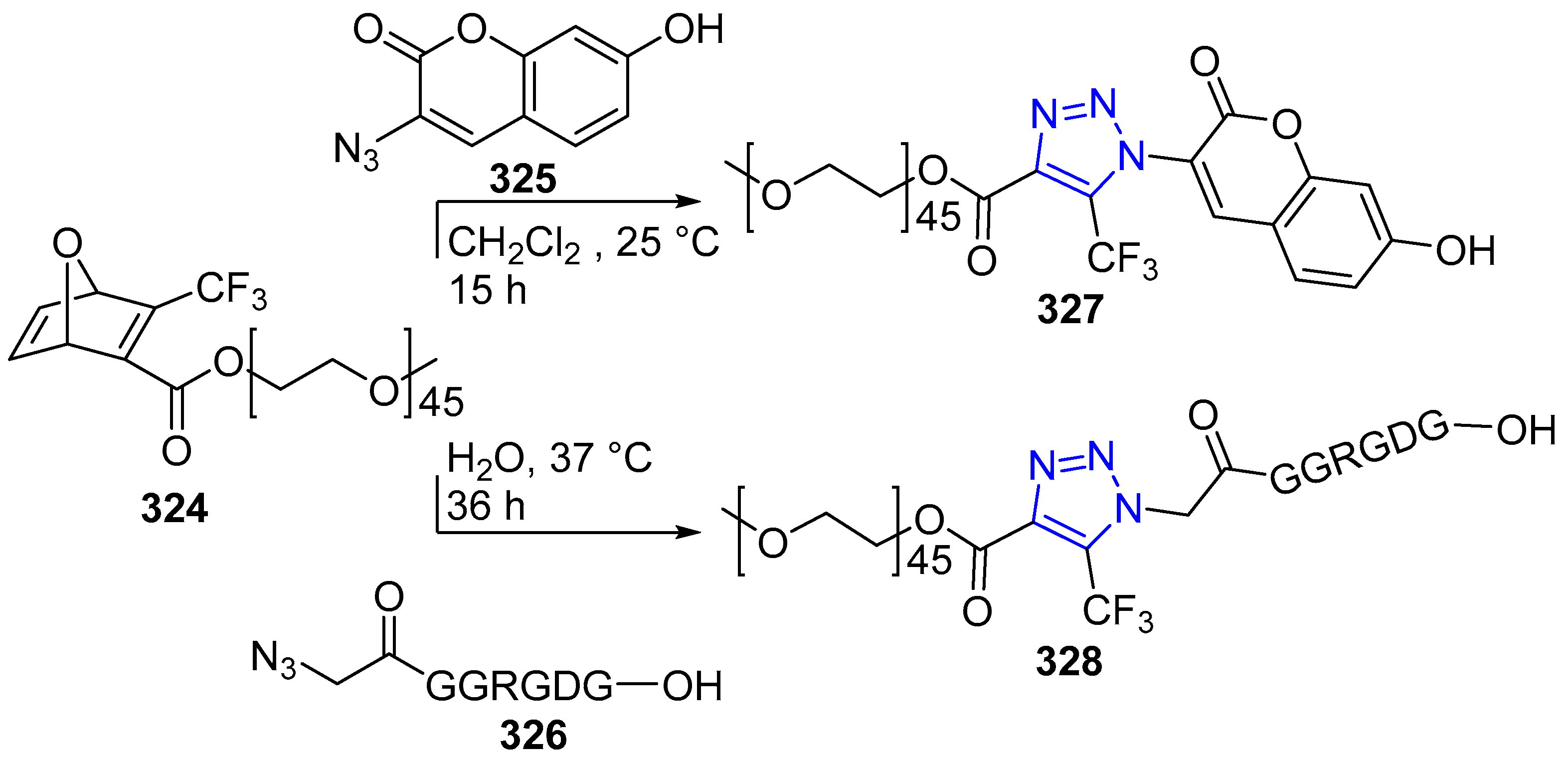
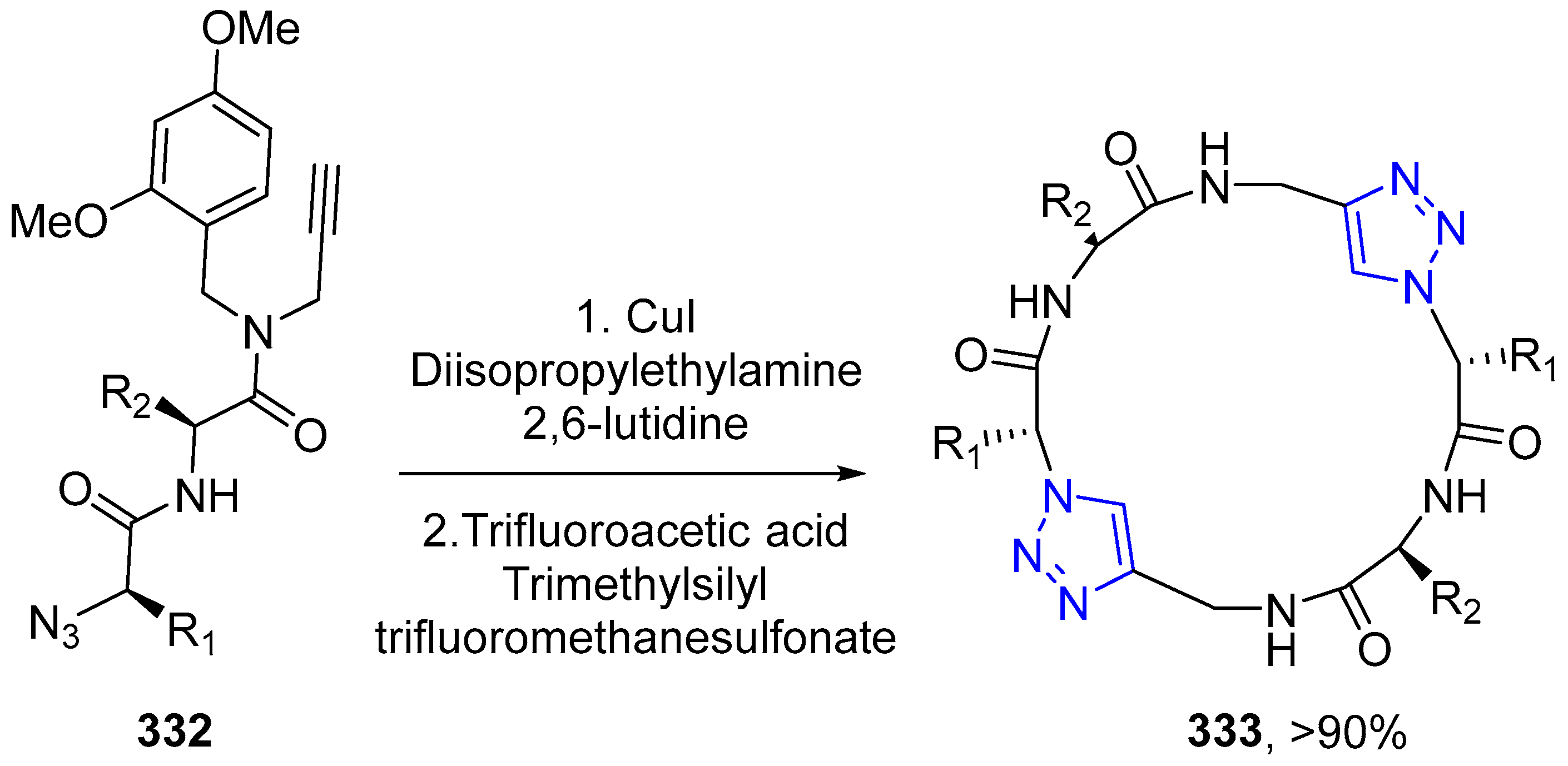
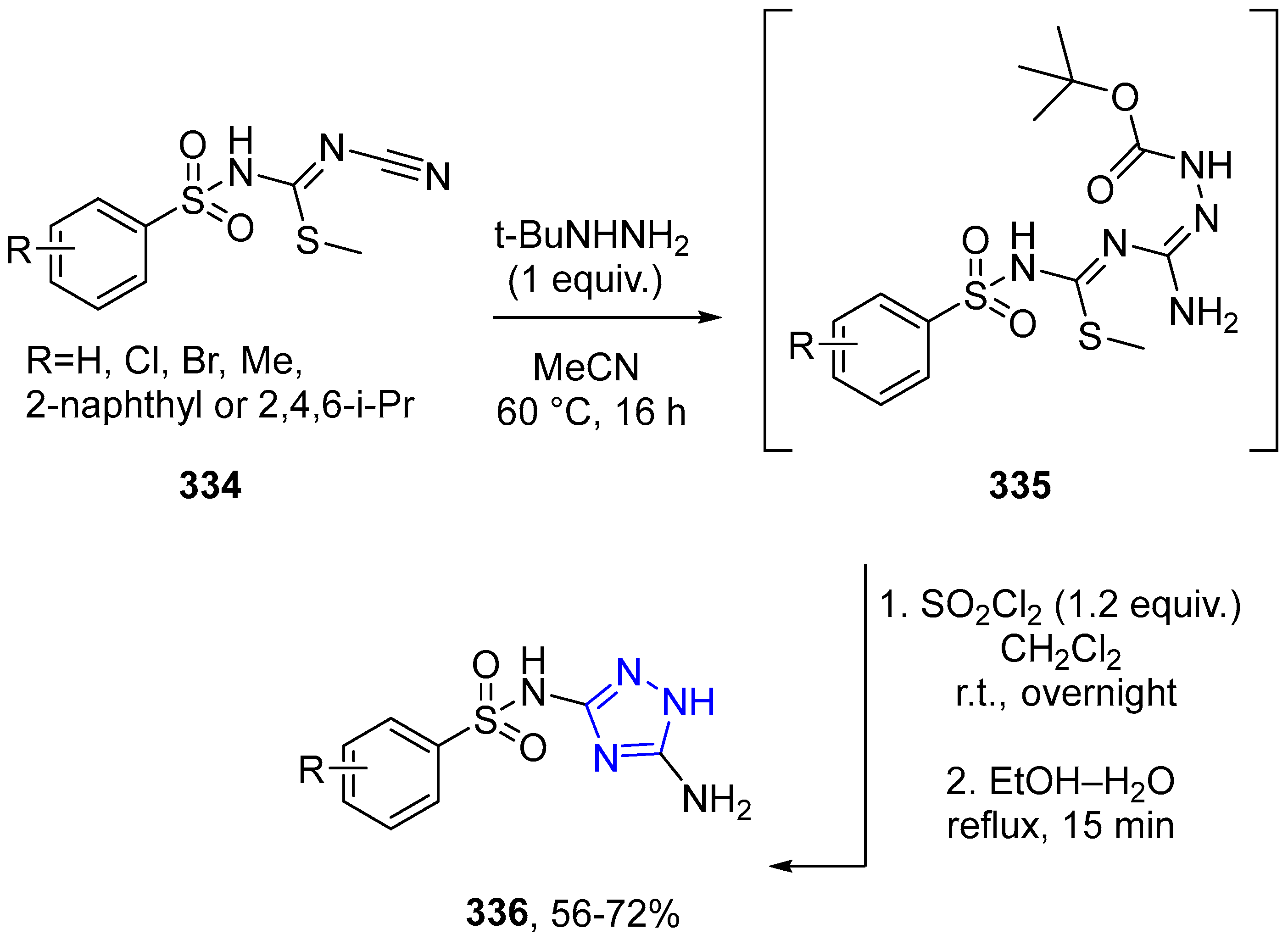
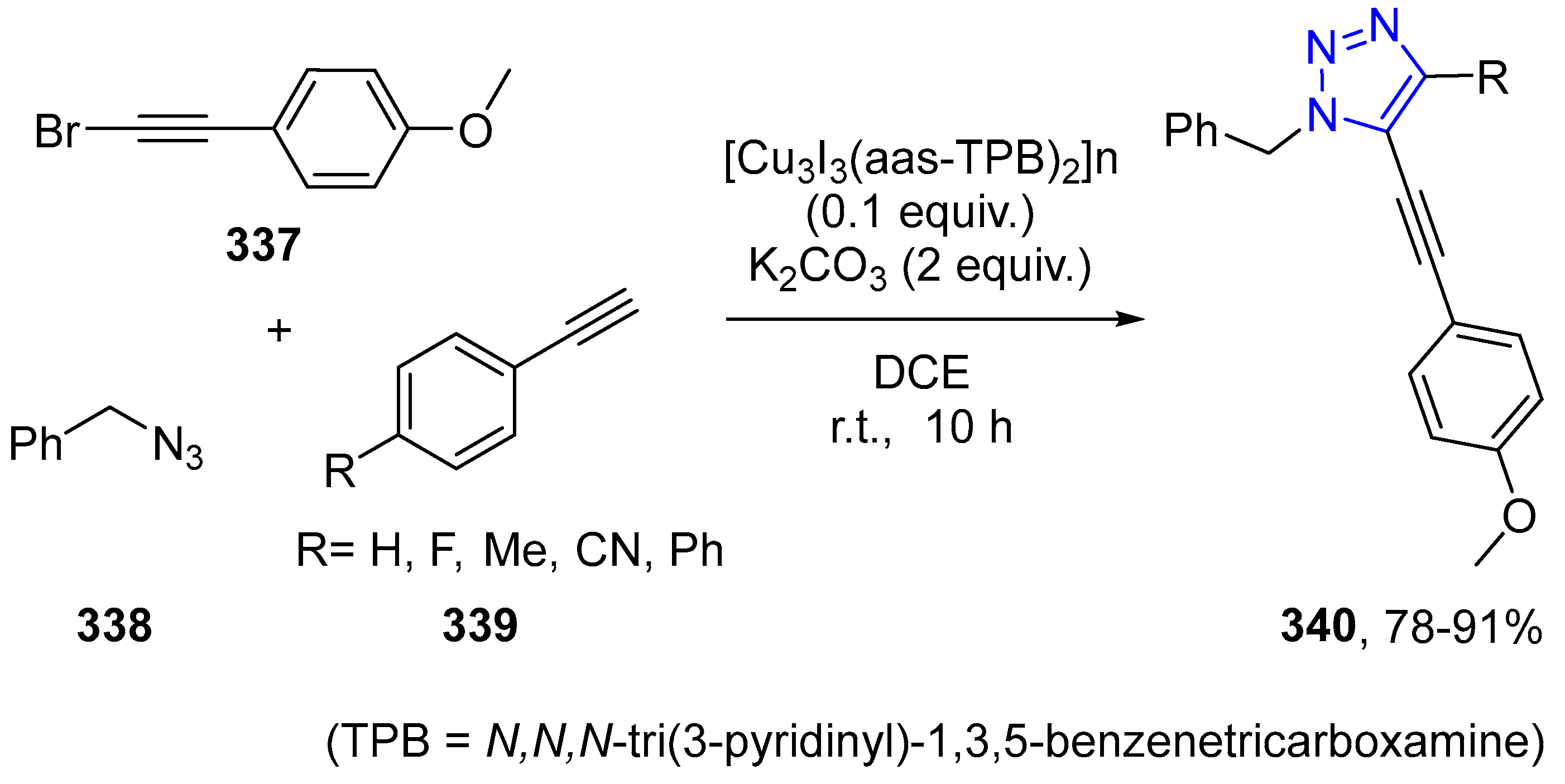
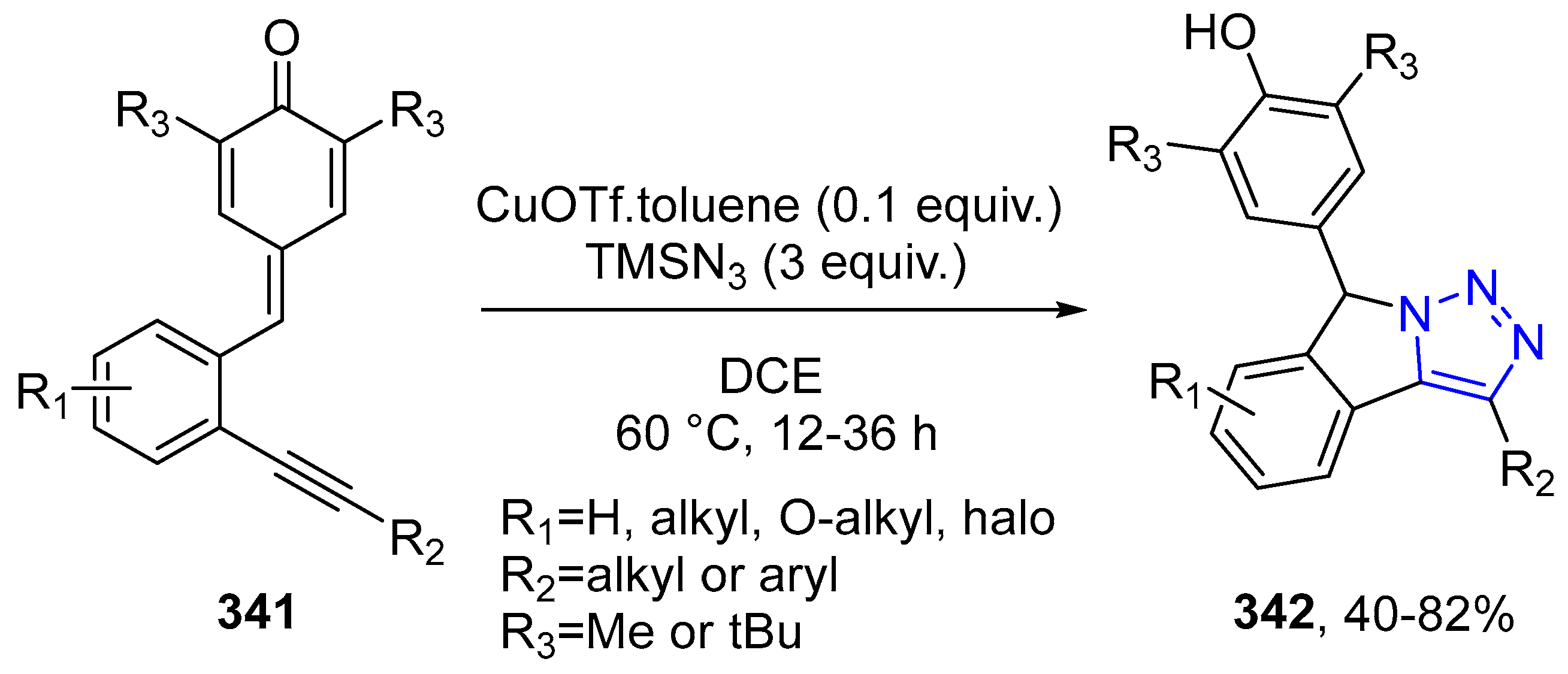
4.2.5. Triazole
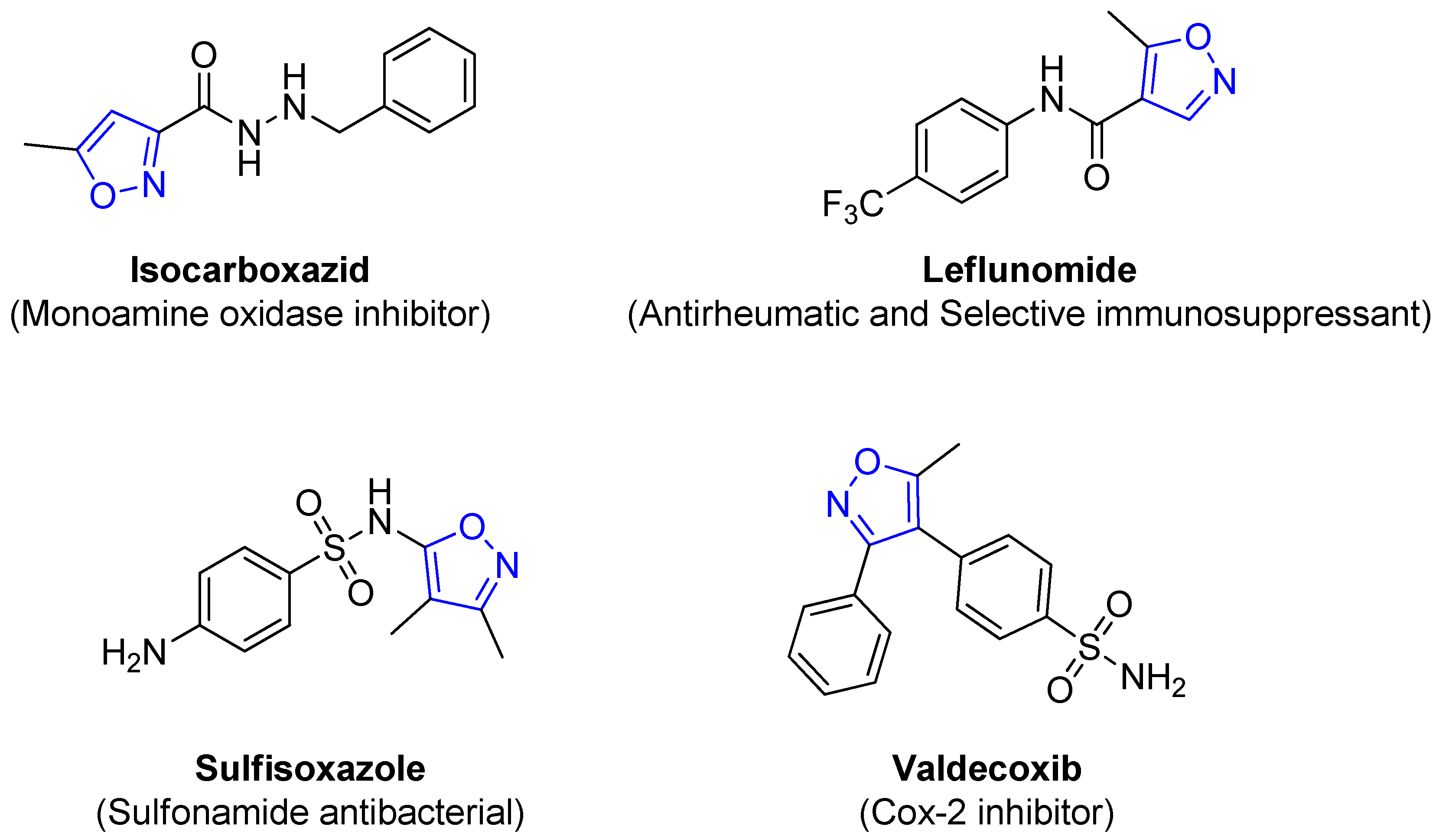
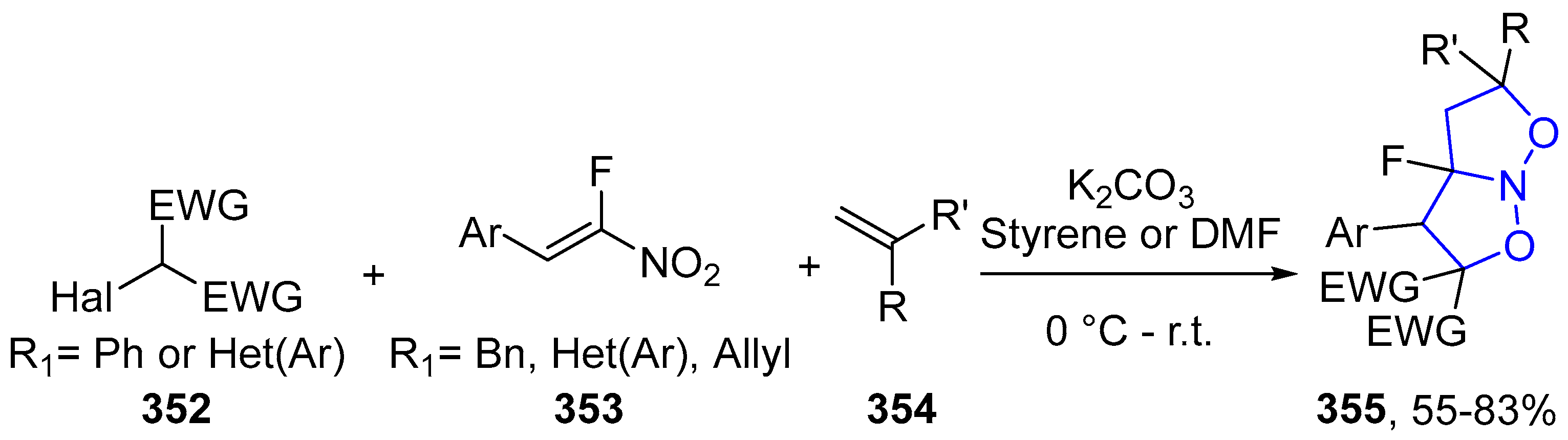
4.2.6. Isoxazole
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Heterogeneous Catalysts for the One-Pot Synthesis of Chemicals and Fine Chemicals. Chem. Rev. 2011, 111, 1072–1133. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.J.; Corma, A.; Iborra, S. Homogeneous and heterogeneous catalysts for multicomponent reactions. RSC Adv. 2012, 2, 16–58. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef] [Green Version]
- Fogg, D.E.; dos Santos, E.N. Tandem catalysis: A taxonomy and illustrative review. Coord. Chem. Rev. 2004, 248, 2365–2379. [Google Scholar] [CrossRef]
- Abou-Shehada, S.; Williams, J.M.J. Separated tandem catalysis: It’s about time. Nat. Chem. 2014, 6, 12–13. [Google Scholar] [CrossRef]
- Lohr, T.L.; Marks, T.J. Orthogonal tandem catalysis. Nat. Chem. 2015, 7, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Molina, D.; Martín-Castro, A.M. Tandem Sequences Involving Michael Additions and Sigmatropic Rearrangements. Synthesis 2016, 48, 3459–3469. [Google Scholar] [CrossRef]
- Sears, J.E.; Boger, D.L. Tandem Intramolecular Diels–Alder/1,3-Dipolar Cycloaddition Cascade of 1,3,4-Oxadiazoles: Initial Scope and Applications. Acc. Chem. Res. 2016, 49, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Kou, K.G.M.; Dong, V.M. Tandem rhodium catalysis: Exploiting sulfoxides for asymmetric transition-metal catalysis. Org. Biomol. Chem. 2015, 13, 5844–5847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Climent, M.J.; Corma, A.; Iborra, S.; Sabater, M.J. Heterogeneous Catalysis for Tandem Reactions. ACS Catal. 2014, 4, 870–891. [Google Scholar] [CrossRef]
- Pellissier, H. Recent Developments in Enantioselective Multicatalysed Tandem Reactions. Tetrahedron 2013, 69, 7171–7210. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, C.M. Tandem catalysis: A new approach to polymers. Chem. Soc. Rev. 2013, 42, 9392–9402. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.; Vorholt, A.J.; Ostrowskia, K.A.; Seidensticker, T. Towards resource efficient chemistry: Tandem reactions with renewables. Green Chem. 2014, 16, 982–1006. [Google Scholar] [CrossRef]
- Foster, R.A.A.; Willis, M.C. Tandem inverse-electron-demand hetero-/retro-Diels–Alder reactions for aromatic nitrogen heterocycle synthesis. Chem. Soc. Rev. 2013, 42, 63–76. [Google Scholar] [CrossRef]
- Albert, M.; Fensterbank, L.; Lacôte, E.; Malacria, M. Tandem Radical Reactions. In Radicals in Synthesis II, Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2006; Volume 264, pp. 1–62. ISBN 978-3-540-31326-7. [Google Scholar]
- Wasilke, J.-C.; Obrey, S.J.; Baker, R.T.; Bazan, G.C. Concurrent Tandem Catalysis. Chem. Rev. 2005, 105, 1001–1020. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Montagnon, T.; Snyder, S.A. Tandem reactions, cascade sequences, and biomimetic strategies in total synthesis. Chem. Commun. 2003, 5, 551–564. [Google Scholar] [CrossRef]
- Schmalz, H.-G.; Geis, O. Reactions of Acylpalladium Derivatives with Oxygen, Nitrogen, and Other Group 15, 16, and 17 Atom Nucleophiles: Tandem and Cascade Processes Terminated by Carbonylative Esterification, Amidation, and Related Reactions. In Handbook of Organopalladium Chemistry for Organic Synthesis; Negishi, E.-I., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2002; Volume 6, pp. 2377–2397. ISBN 9780471212461. [Google Scholar]
- Camp, J.E. Auto-Tandem Catalysis: Activation of Multiple, Mechanistically Distinct Process by a Single Catalyst. Eur. J. Org. Chem. 2017, 2017, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Baumann, M.; Baxendale, I.R.; Ley, S.V.; Nikbin, N. An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. Beilstein J. Org. Chem. 2011, 7, 442–495. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.; Singh, S.; Tufail, F.; Jaiswal, D.; Singh, J.; Singh, J. Glycerol Micellar Catalysis: An Efficient Multicomponent-Tandem Green Synthetic Approach to Biologically Important 2,4-Disubstituted Thiazole Derivatives. ChemistrySelect 2018, 3, 11634–11642. [Google Scholar] [CrossRef]
- Hao, W.; Sang, X.; Jiang, J.; Cai, M. Copper(I)-catalyzed cascade reaction of 2-haloaryl isothiocyanates with isocyanides: A strategy to construct benzo[d]imidazo[5,1-b]thiazoles. Tetrahedron Lett. 2016, 57, 1511–1514. [Google Scholar] [CrossRef]
- Shahvelayati, A.S.; Ashouri, A.; Delbari, A.S. Ionic Liquid as a Green Media for the One-Pot Synthesis of New α- Thiazolodepsipeptide Derivatives via a Four-Component Reaction. Lett. Org. Chem. 2016, 13, 100–106. [Google Scholar] [CrossRef]
- Bodireddy, M.R.; Mohinuddin, P.M.K.; Gundala, T.R.; Reddy, N.C.G. Lactic acid-mediated tandem one-pot synthesis of 2-aminothiazole derivatives: A rapid, scalable, and sustainable process. Cogent Chem. 2016, 2, 1154237. [Google Scholar] [CrossRef]
- Khodaei, M.M.; Alizadeh, A.; Kanjouri, T. An Efficient, One-Pot, Green Synthesis of Tetracyclic Imidazo[2,1-b]Thiazoles via Electrochemically Induced Tandem Heteroannulation Reactions. J. Heterocycl. Chem. 2013, 50, 23–28. [Google Scholar] [CrossRef]
- Beresneva, T.; Popelis, J.; Abele, E. Novel Cu-catalyzed methods for the synthesis of fused thiazoles using S,N-diarylation reaction. Chem. Heterocycl. Compd. 2013, 49, 345–347. [Google Scholar] [CrossRef]
- Madhav, B.; Murthy, S.N.; Kumar, B.S.P.A.; Ramesh, K.; Nageswar, Y.V.D. A tandem one-pot aqueous phase synthesis of thiazoles/selenazoles. Tetrahedron Lett. 2012, 53, 3835–3838. [Google Scholar] [CrossRef]
- Pagano, N.; Heil, M.L.; Cosford, N.D.P. Automated Multistep Continuous Flow Synthesis of 2-(1H-Indol-3-yl)thiazole Derivatives. Synthesis 2012, 44, 2537–2546. [Google Scholar] [CrossRef]
- Kwak, S.H.; Lee, G.-H.; Gong, Y.-D. Synthesis of N-Substituted-2-Aminothiazolo[4,5-b]pyrazines by Tandem Reaction of o-Aminohalopyrazines with Isothiocyanates. Bull. Korean Chem. Soc. 2012, 33, 4271–4274. [Google Scholar] [CrossRef] [Green Version]
- Shklyarenko, A.A.; Nasledov, D.G.; Yakovlev, V.V. (2,3-Dibromopropylsulfonyl)arenes in S,N-Tandem Heterocyclizations. New Synthesis of Triazolothiazolidines. Russ. J. Org. Chem. 2005, 41, 627–628. [Google Scholar] [CrossRef]
- Mochulskaya, N.N.; Andreiko, A.A.; Kodess, M.I.; Vasileva, E.B.; Filyakova, V.I.; Gubaidullin, A.T.; Litvinov, I.A.; Sinyashin, O.G.; Aleksandrov, G.G.; Charushin, V.N. Annelation of the thiazole ring to 1,2,4-triazines by tandem AN—AN or SN H—SN H reactions. Russ. Chem. Bull. 2004, 53, 1279–1289. [Google Scholar] [CrossRef]
- You, S.-L.; Razavi, H.; Kelly, J.W. A Biomimetic Synthesis of Thiazolines Using Hexaphenyloxodiphosphonium Trifluoromethanesulfonate. Angew. Chem. Int. Ed. 2003, 42, 83–85. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, J.; Li, Z. A Novel Method for the Synthesis of Pyrazolo[5,1-b]Thiazole. Synth. Commun. 2000, 30, 763–769. [Google Scholar] [CrossRef]
- Raman, P.; Razavi, H.; Kelly, J.W. Titanium(IV)-Mediated Tandem Deprotection−Cyclodehydration of Protected Cysteine N-Amides: Biomimetic Syntheses of Thiazoline- and Thiazole-Containing Heterocycles. Org. Lett. 2000, 2, 3289–3292. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Patel, G.; Banerjee, S. Visible Light-Emitting Diode Light-Driven Cu0.9Fe0.1@RCAC- Catalyzed Highly Selective Aerobic Oxidation of Alcohols and Oxidative Azo-Coupling of Anilines: Tandem One Pot Oxidation− Condensation to Imidazoles and Imines. ACS Omega 2019, 4, 22445–22455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, E.; Shao, J.; Tang, P.; Shu, K.; Chen, W.; Yu, Y. Catalyst-free three-component sequencing for efficient assembly of [1,3]oxazine N-fused imidazole-2-thiones. Green Chem. 2018, 20, 3696–3699. [Google Scholar] [CrossRef]
- Kumar, S.; Ho, P.-H.; Barve, I.J.; Sun, C.-M. Enantiospecific Synthesis of Imidazoquinazolin-2-ones via Base-Catalyzed Tandem Cyclization. ChemistrySelect 2017, 2, 8917–8921. [Google Scholar] [CrossRef]
- Yan, L.; Wang, D.-L.; Qian, J.-H. A Facile Synthesis of Novel Pyrido[2′,1′:2,3]Imidazo[4,5-c]β-Carbolines. Heterocycles 2017, 94, 334–341. [Google Scholar] [CrossRef]
- Wang, F.-J.; Xu, H.; Xin, M.; Zhang, Z. Ι₂-mediated amination/cyclization of ketones with 2-aminopyridines under high-speed ball milling: Solvent- and metal-free synthesis of 2,3-substituted imidazo[1,2-a]pyridines and zolimidine. Mol. Divers. 2016, 20, 659–666. [Google Scholar] [CrossRef]
- Devi, N.; Singh, D.; Sunkaria, R.K.; Malakar, C.C.; Mehra, S.; Rawal, R.K.; Singh, V. In(OTf) 3-HBF4 Assisted Multicomponent Approach for One-Pot Synthesis of Pyrazolopyridinone Fused Imidazopyridines. ChemistrySelect 2016, 1, 4696–4703. [Google Scholar] [CrossRef]
- An, L.; Sun, X.; Lv, M.; Zhou, J.; Zhu, F.; Zhong, H. Metal-free oxidative coupling of aminopyridines with nitroolefins to imidazo[1,2-a]pyridine in the presence of I2–TBHP–pyridine. Z. Nat. B 2016, 71, 141–147. [Google Scholar] [CrossRef]
- Harutyunyan, A.A. One-stage synthesis of condensed pyrimidines by reaction of substituted 3-(pyrimidin-5-yl)propanoic acids with ortho-diamines: Extension of limits. Russ. J. Org. Chem. 2016, 52, 235–239. [Google Scholar] [CrossRef]
- Li, T.; Wang, Z.; Xu, K.; Liu, W.; Zhang, X.; Mao, W.; Guo, Y.; Ge, X.; Pan, F. Rhodium-Catalyzed/Copper-Mediated Tandem C(sp2)–H Alkynylation and Annulation: Synthesis of 11-Acylated Imidazo[1,2-a:3,4-a′]dipyridin-5-ium-4-olates from 2H-[1,2′-Bipyridin]-2-ones and Propargyl Alcohols. Org. Lett. 2016, 18, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
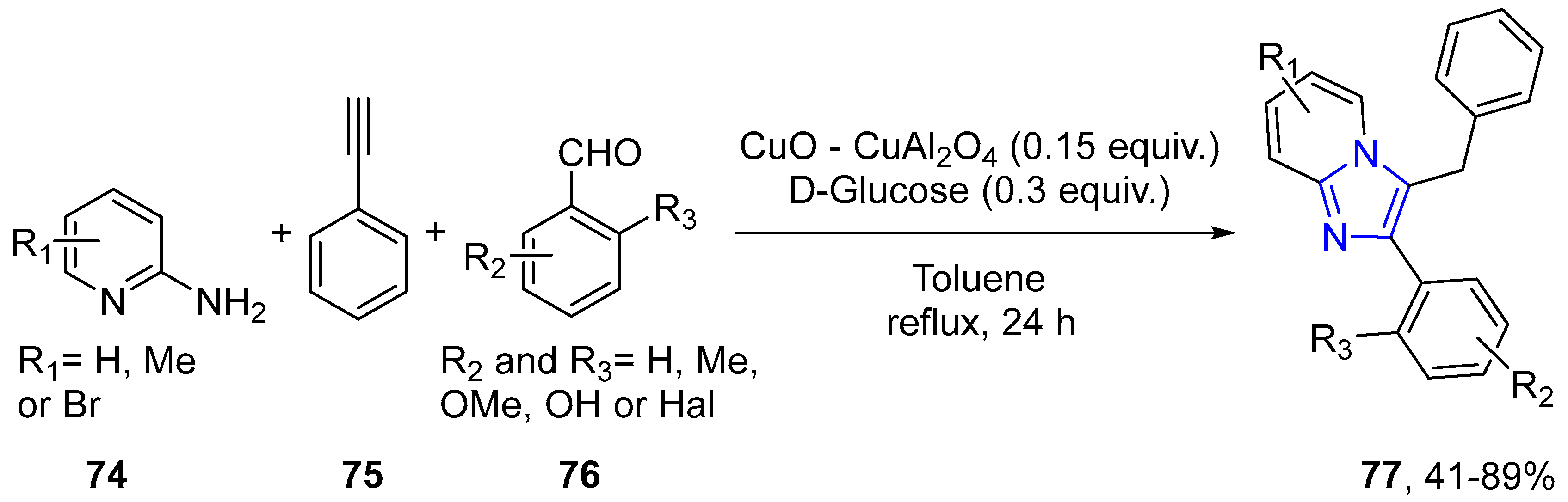
- Balijapalli, U.; Iyer, S.K. CuO-CuAl2O4 and D-glucose catalyzed synthesis of a family of excited state intramolecular proton transfer imidazo[1,2-a]pyridine analogues and their optical properties. Dyes Pigments 2015, 121, 88–98. [Google Scholar] [CrossRef]
- Cai, Q.; Liu, M.-C.; Mao, B.-M.; Xie, X.; Jia, F.-C.; Zhu, Y.-P.; Wu, A.-X. Direct one-pot synthesis of zolimidine pharmaceutical drug and imidazo[1,2-a]pyridine derivatives via I2/CuO-promoted tandem strategy. Chin. Chem. Lett. 2015, 26, 881–884. [Google Scholar] [CrossRef]
- Milišiūnaitė, V.; Arbačiauskienė, E.; Bieliauskas, A.; Vilkauskaitė, G.; Šačkus, A.; Holzer, W. Synthesis of pyrazolo[4′,3′:3,4]pyrido[1,2-a]benzimidazoles and related new ring systems by tandem cyclisation of vic-alkynylpyrazole-4-carbaldehydes with (het) aryl-1,2-diamines and investigation of their optical properties. Tetrahedron 2015, 71, 3385–3395. [Google Scholar] [CrossRef]
- Stasyuk, A.J.; Banasiewicz, M.; Ventura, B.; Cyranski, M.K.; Gryko, D.T. Benzo[a]imidazo[5,1,2-cd]indolizines—A new class of molecules displaying excited state intramolecular proton transfer. New J. Chem. 2014, 38, 189–197. [Google Scholar] [CrossRef]
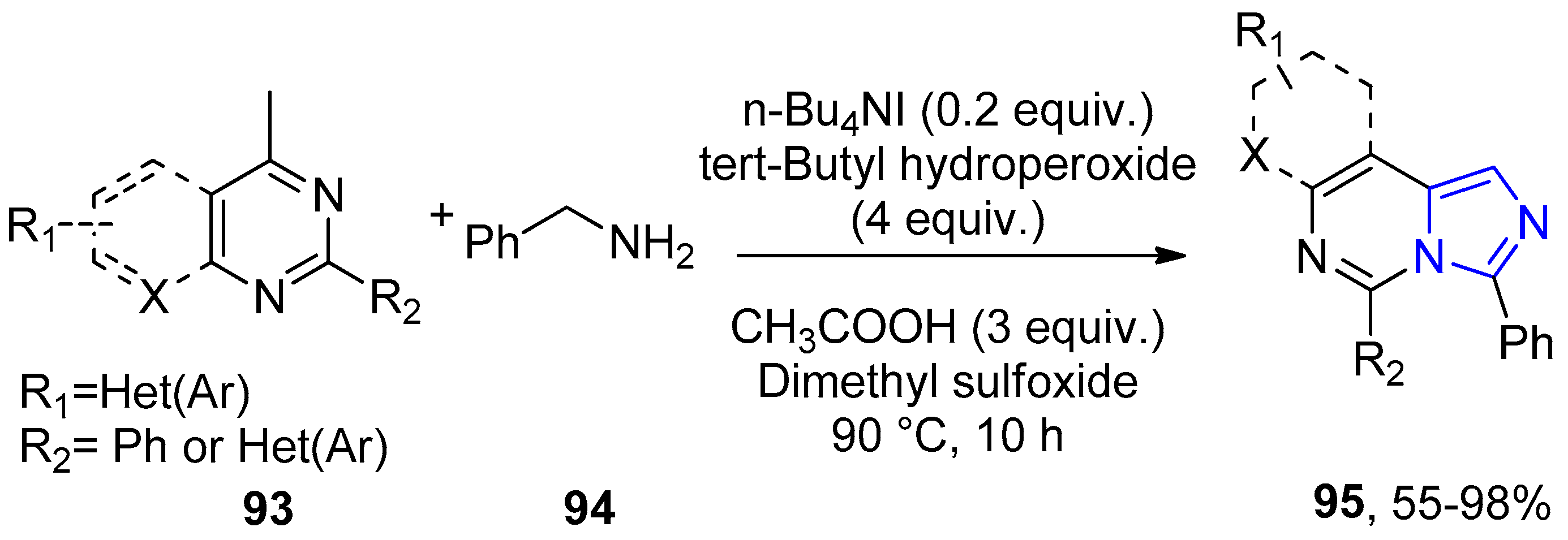
- Stasyuk, A.J.; Banasiewicz, M.; Cyrański, M.K.; Gryko, D.T. Imidazo[1,2-a]pyridines Susceptible to Excited State Intramolecular Proton Transfer: One-Pot Synthesis via an Ortoleva–King Reaction. J. Org. Chem. 2012, 77, 5552–5558. [Google Scholar] [CrossRef]
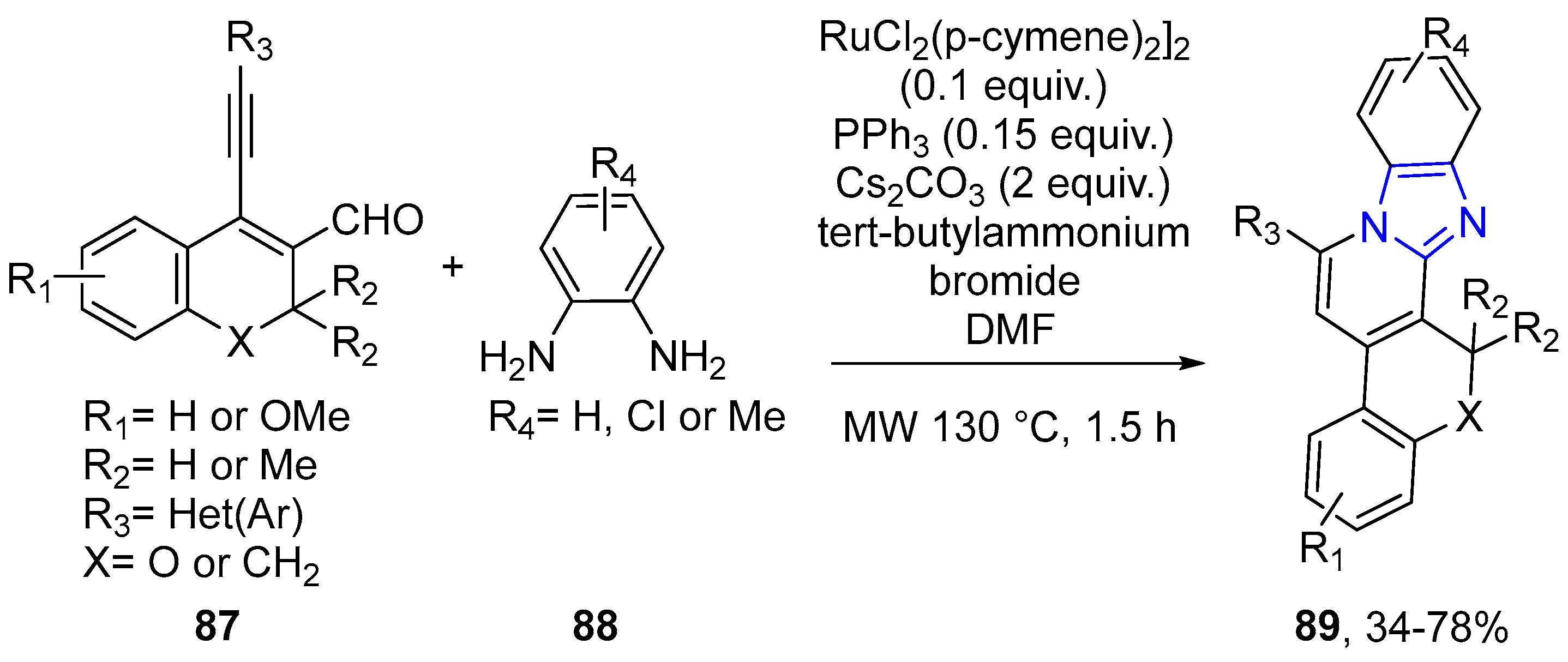
- Manna, S.K.; Panda, G. Microwave assisted [RuCl2(p-cymene)2]2 catalyzed regioselective endo-tandem cyclization involving imine and alkyne activation: An approach to benzo[4,5]imidazo[2,1-a]pyridine scaffold. RSC Adv. 2014, 4, 21032–21041. [Google Scholar] [CrossRef]
- Silvani, A.; Lesma, G.; Crippa, S.; Vece, V. Multicomponent access to novel dihydroimidazo[1′,5′:1,2]pyrido[3,4-b]indol-2-ium salts and indoles by means of Ugi/Bischler–Napieralski/heterocyclization two step strategy. Tetrahedron 2014, 70, 3994–4001. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, T.; Shen, Q.; Li, J.-X. n-Bu4NI-catalyzed selective dual amination of sp3 C–H bonds: Oxidative domino synthesis of imidazo[1,5-c]quinazolines on a gram-scale. Chem. Commun. 2014, 50, 4302–4304. [Google Scholar] [CrossRef] [PubMed]
- Ciuciu, A.I.; Firmansyah, D.; Hugues, V.; Blanchard-Desce, M.; Gryko, D.T.; Flamigni, L. Non-classical donor–acceptor–donor chromophores. A strategy for high two-photon brightness. J. Mater. Chem. C 2014, 2, 4552–4565. [Google Scholar] [CrossRef]
- Ventosa-Andrés, P.; Hradilová, L.; Krchňák, V. Privileged Structures as Peptide Backbone Constraints: Polymer-Supported Stereoselective Synthesis of Benzimidazolinopiperazinone Peptides. ACS Comb. Sci. 2014, 16, 359–366. [Google Scholar] [CrossRef]
- Cankarova, N.; Krchňák, V. Polymer-Supported Stereoselective Synthesis of Benzimidazolinopiperazinones. J. Org. Chem. 2012, 77, 5687–5695. [Google Scholar] [CrossRef] [PubMed]
- Chanu, L.G.; Singh, T.P.; Jang, Y.J.; Yoon, Y.-J.; Singh, O.M.; Lee, S.-G. Synthesis of Imidazo[1,2-a]pyridines and Pyrido[1,2-a]pyrimidines in Water and their SNAr Cyclizations. Bull. Korean Chem. Soc. 2014, 35, 994–1000. [Google Scholar] [CrossRef] [Green Version]
- Cherney, E.; Macor, J.; Papanagapolous, C.; Hunt, D.A. Tandem cyclization reactions of electron rich arylethylamino acid amides. An entry to the dihydroimidazoisoquinolin-3(2H)-one ring system. Tetrahedron Lett. 2014, 55, 4837–4839. [Google Scholar] [CrossRef]
- Hao, W.; Zeng, J.; Cai, M. The copper(i)-catalyzed tandem reaction of o-alkynylphenyl isothiocyanates with isocyanides: A rapid synthesis of 5H-benzo[d]imidazo[5,1-b][1,3]thiazines. Chem. Commun. 2014, 50, 11686–11689. [Google Scholar] [CrossRef]
- Hao, W.; Jiang, Y.; Cai, M. Synthesis of Indolyl Imidazole Derivatives via Base-Promoted Tandem Reaction of N-[2-(1-Alkynyl)phenyl]carbodiimides with Isocyanides. J. Org. Chem. 2014, 79, 3634–3640. [Google Scholar] [CrossRef]
- Santra, S.; Bagdi, A.K.; Majee, A.; Hajra, A. Iron (III)-Catalyzed Cascade Reaction between Nitroolefins and 2-Aminopyridines: Synthesis of Imidazo[1,2-a]pyridines and Easy Access towards Zolimidine. Adv. Synth. Catal. 2013, 355, 1065–1070. [Google Scholar] [CrossRef]
- Ge, W.; Zhu, X.; Wei, Y. Aerobic Multicomponent Tandem Synthesis of 3-Sulfenylimidazo[1,2-a]pyridines from Ketones, 2-Aminopyridines, and Disulfides. Eur. J. Org. Chem. 2013, 27, 6015–6020. [Google Scholar] [CrossRef]
- Pericherla, K.; Kaswan, P.; Khedar, P.; Khungar, B.; Parang, K.; Kumar, A. Copper catalyzed tandem oxidative C-H amination/cyclizations: Direct access to imidazo[1,2-a]pyridines. RSC Adv. 2013, 3, 18923–18930. [Google Scholar] [CrossRef]
- Bagdi, A.K.; Rahman, M.; Santra, S.; Majee, A.; Hajra, A. Copper-Catalyzed Synthesis of Imidazo[1,2-a]pyridines through Tandem Imine Formation-Oxidative Cyclization under Ambient Air: One-Step Synthesis of Zolimidine on a Gram-Scale. Adv. Synth. Catal. 2013, 355, 1741–1747. [Google Scholar] [CrossRef]
- Ramesha, A.B.; Raghavendra, G.M.; Nandeesh, K.N.; Rangappa, K.S.; Mantelingu, K. Tandem approach for the synthesis of imidazo[1,2-a]pyridines from alcohols. Tetrahedron Lett. 2013, 54, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Nie, Y.-B.; Duan, Z.; Ding, M.-W. New efficient synthesis of 1,2,4-trisubstituted imidazoles and imidazo[1,2-c]quinazolines by a tandem aza-Wittig/electrocyclic ring-closure process. Tetrahedron 2012, 68, 965–971. [Google Scholar] [CrossRef]
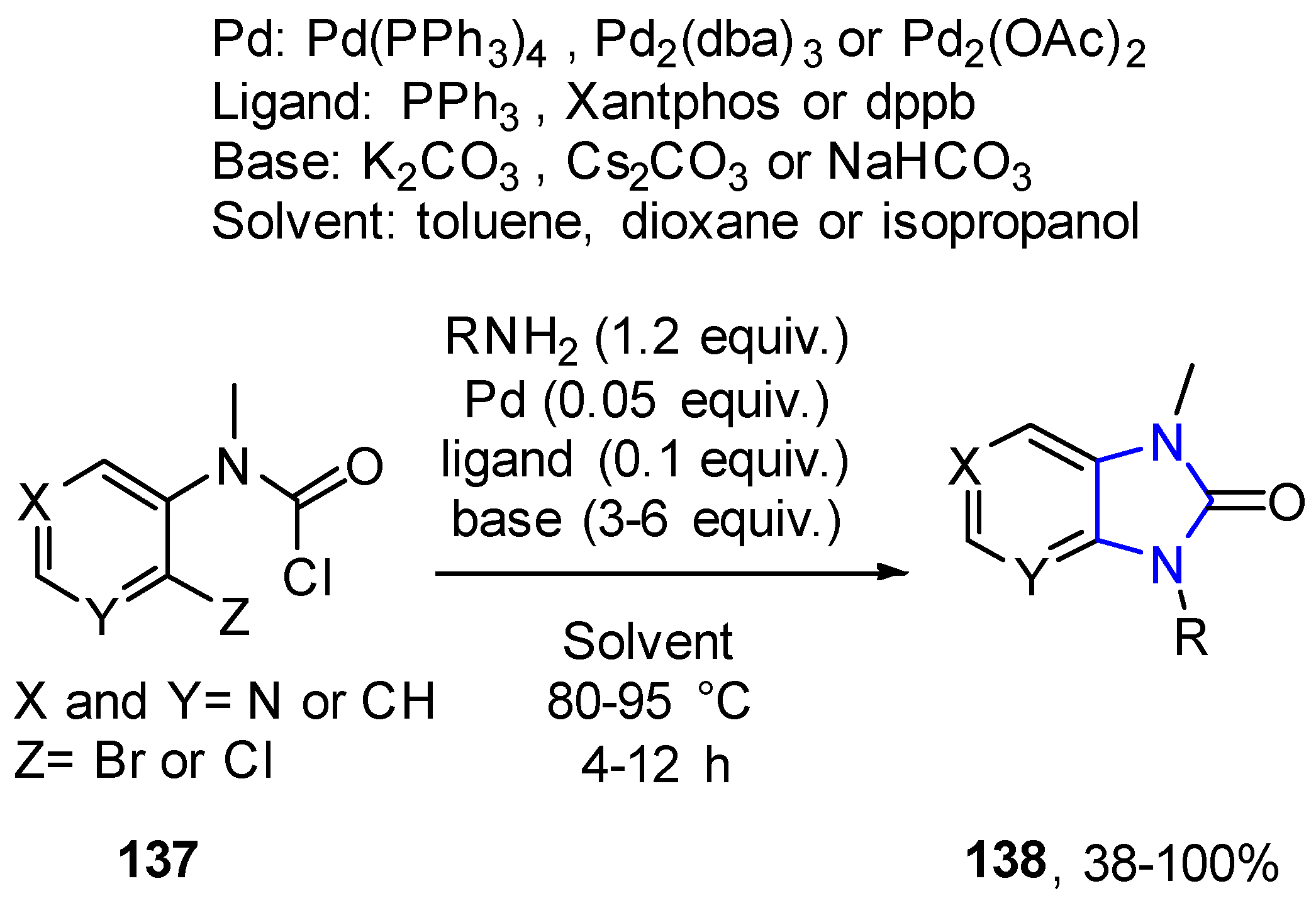
- Lach, F.; Koza, P. Practical Way to Imidazo[4,5-b] and [4,5-c]Pyridine-2-ones via Cascade Ureidation/Palladium-Catalyzed Cyclization. ACS Comb. Sci. 2012, 14, 491–495. [Google Scholar] [CrossRef]
- Liu, G.; Cong, X.; He, J.; Luo, S.; Wu, D.; Lan, J. Regioselective Synthesis of 2- and 3-Substituted Imidazo[1,2-a]pyridines. J. Chem. Res. 2012, 36, 687–690. [Google Scholar] [CrossRef]
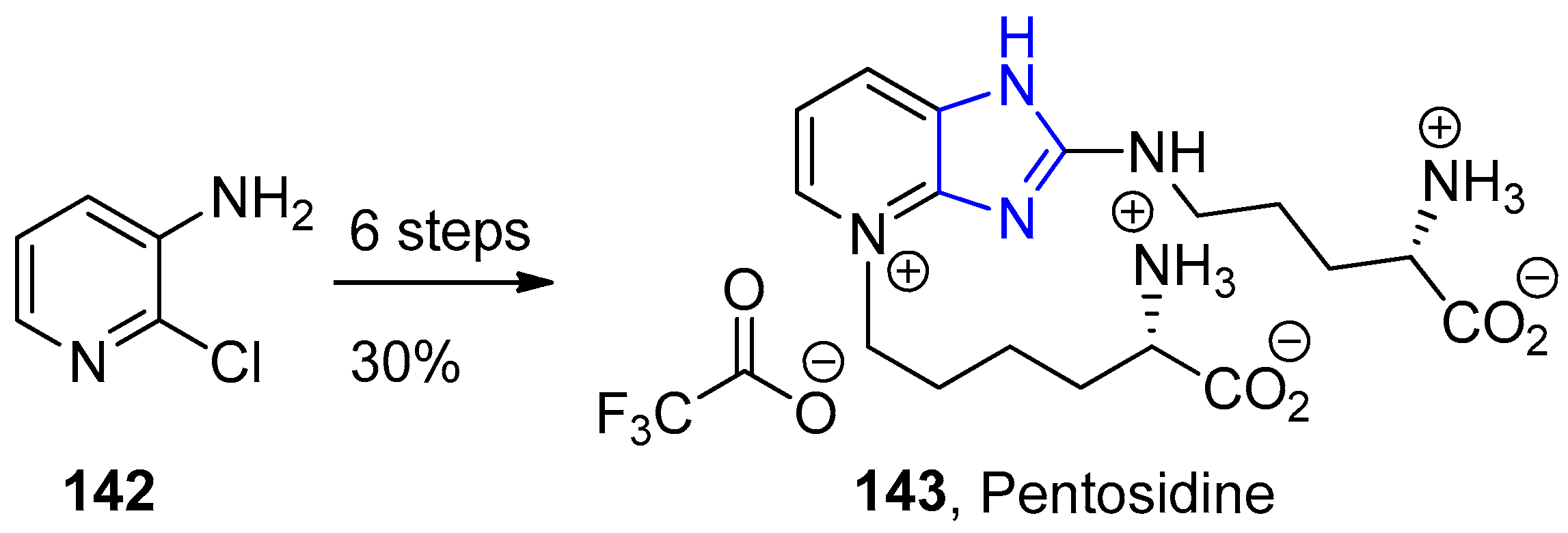
- Rosenberg, A.J.; Clark, D.A. Total Synthesis of Pentosidine. Org. Lett. 2012, 14, 4678–4681. [Google Scholar] [CrossRef]
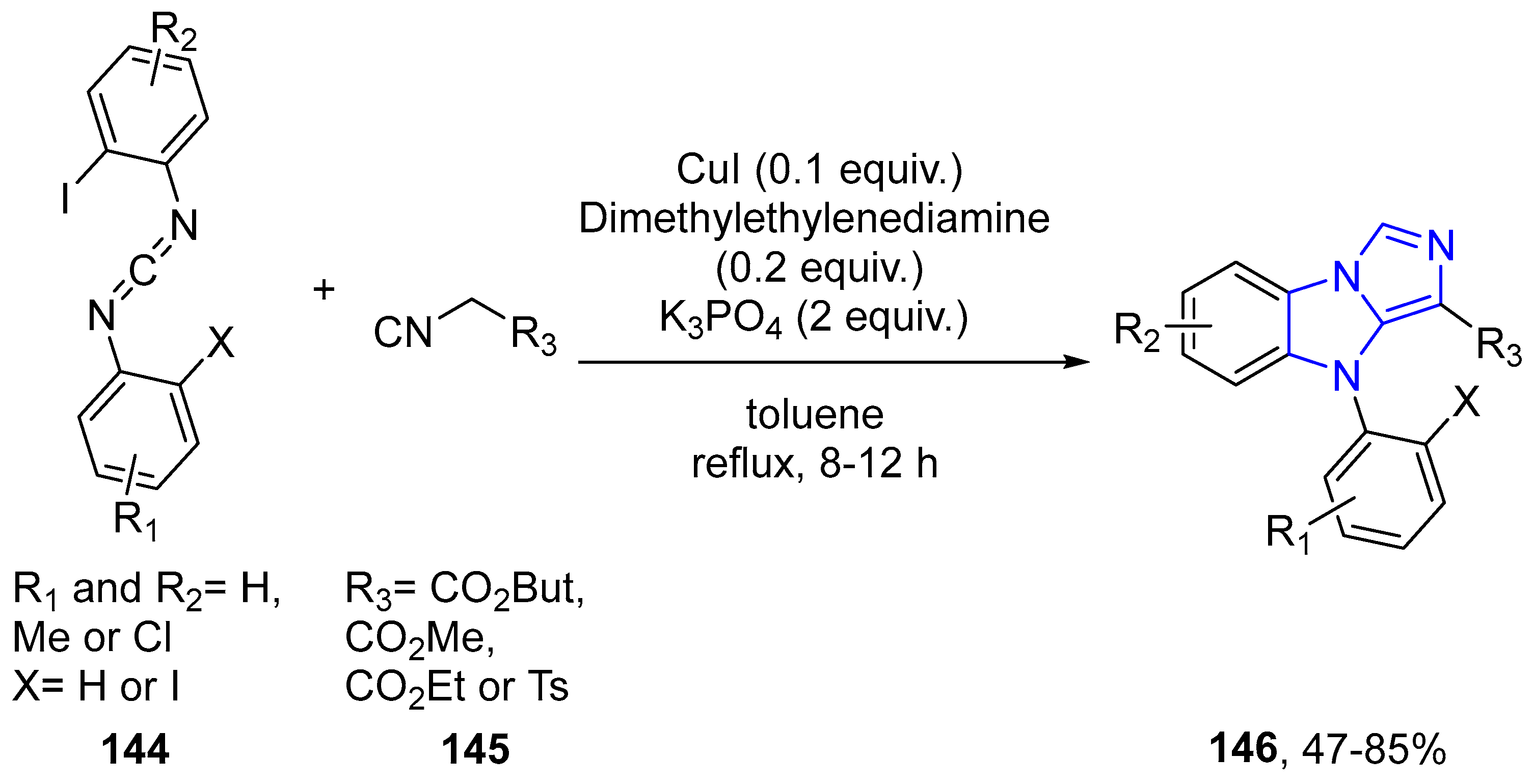
- Qiu, G.; Wu, J. Generation of benzoimidazo[1,5-a]imidazoles via a copper-catalyzed tandem reaction of carbodiimide and isocyanoacetate. Chem. Commun. 2012, 48, 6046–6048. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Deng, C.-L.; Lei, X.; Lin, G.-Q. Tandem Amination/Cycloisomerization of Aryl Propargylic Alcohols with 2-Aminopyridines as an Expedient Route to Imidazo[1,2-a]pyridines. Eur. J. Org. Chem. 2011, 36, 7308–7316. [Google Scholar] [CrossRef]
- Kim, D.G.; Il’inykh, E.S.; Slepuhin, P.A. Tandem heterocyclization in the synthesis of novel imidazo[1,5-d][1,3,4]thiadiazine. Chem. Heterocycl. Compd. 2011, 46, 1418–1419. [Google Scholar] [CrossRef]
- Ouyang, H.-C.; Tang, R.-Y.; Zhong, P.; Zhang, X.-G.; Li, J.-H. CuI/I2-Promoted Electrophilic Tandem Cyclization of 2-Ethynylbenzaldehydes with ortho-Benzenediamines: Synthesis of Iodoisoquinoline-Fused Benzimidazoles. J. Org. Chem. 2011, 76, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Z.; Wu, J.; Cao, M.-H.; Ding, M.-W. Efficient synthesis of benzothieno[3,2-d]-imidazo[1,2-a]pyrimidine-2,5-(1H, 3H)-diones via a tandem aza-wittig/heterocumulene-mediated annulation. J. Heterocycl. Chem. 2010, 47, 68–71. [Google Scholar] [CrossRef]
- Hirota, S.; Sakai, T.; Kitamura, N.; Kubokawa, K.; Kutsumura, N.; Otani, T.; Saito, T. Synthesis of nitrogen heterocycle-fused 1,2,4-benzothiadiazine-1,1-dioxide, quinazolinone, and pyrrolidinone derivatives with a guanidine joint via sequential aza-Wittig reaction/intramolecular NH-addition cyclization/nucleophilic substitution ring closure methodology, using functionalyzed carbodiimides as key intermediates. Tetrahedron 2010, 66, 653–662. [Google Scholar] [CrossRef]
- Guchhait, S.K.; Madaan, C. Towards molecular diversity: Dealkylation of tert-butyl amine in Ugi-type multicomponent reaction product establishes tert-butyl isocyanide as a useful convertible isonitrile. Org. Biomol. Chem. 2010, 8, 3631–3634. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Sakurai, K.; Ishikura, M.; Takeda, K.; Yanada, R. One-pot concise syntheses of benzimidazo[2,1-a]isoquinolines by a microwave-accelerated tandem process. Tetrahedron Lett. 2009, 50, 4167–4169. [Google Scholar] [CrossRef]
- Che, C.; Xiang, J.; Wang, G.-X.; Fathi, R.; Quan, J.-M.; Yang, Z. One-Pot Synthesis of Quinoline-Based Tetracycles by a Tandem Three-Component Reaction. J. Comb. Chem. 2007, 9, 982–989. [Google Scholar] [CrossRef]
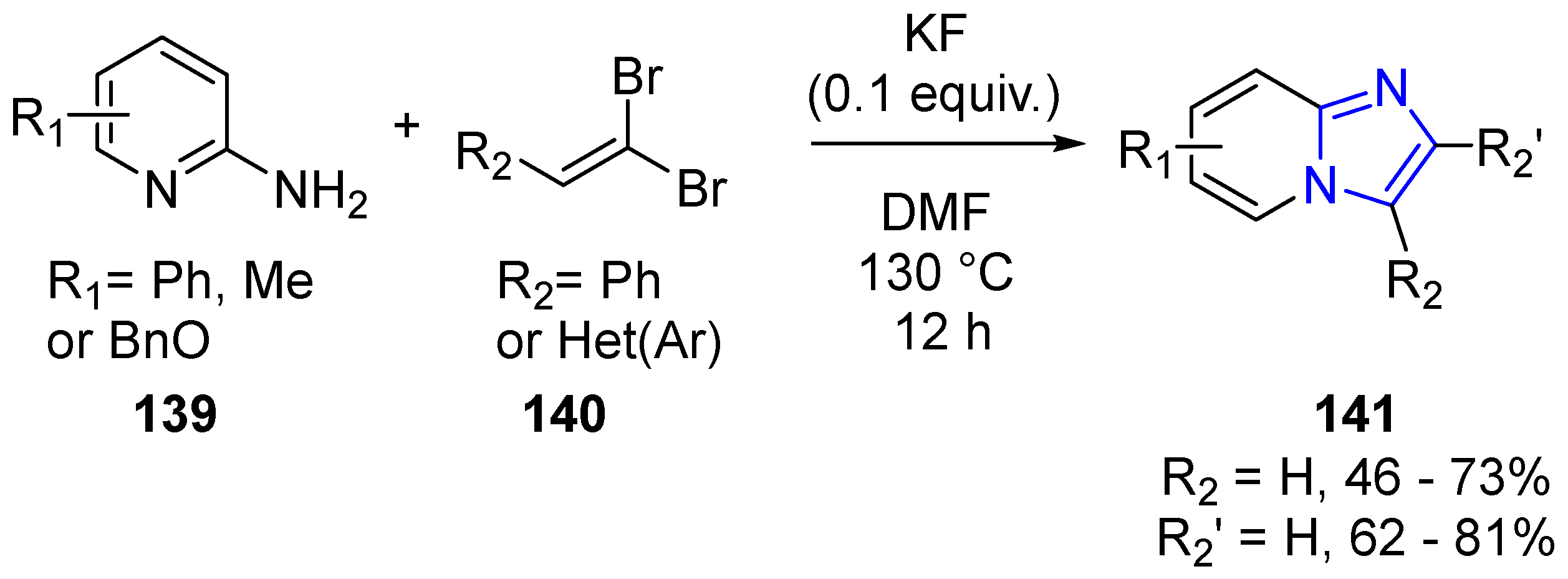
- Loones, K.T.J.; Maes, B.U.W.; Dommisse, R.A. Synthesis of pyrido[2′,1′:2,3]imidazo[4,5-b]quinoline and pyrido[1′,2′:1,2]imidazo[4,5-b]quinoline and their benzo and aza analogs via tandem catalysis. Tetrahedron 2007, 63, 8954–8961. [Google Scholar] [CrossRef]
- Scott, J.P. Two-Step Synthesis of 3-Aryl-1,3-Dihydro-2H-imidazo[4,5-b]pyridin-2-ones. Synlett 2006, 13, 2083–2086. [Google Scholar] [CrossRef]
- Beresnev, D.G.; Rusinov, G.L.; Chupakhin, O.N.; Neunhoeffer, H. Interaction of 5-methoxy-1,2,4-triazines with ureas as a new route to 6-azapurines. Mendeleev Commun. 2000, 10, 58–59. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Wang, H.-Y.; Winston-McPherson, G.N.; Geng, H.-M.J.; Guzeic, I.A.; Tang, W. Copper-catalyzed tandem annulation/arylation for the synthesis of diindolylmethanes from propargylic alcohols. Chem. Commun. 2014, 50, 12293–12296. [Google Scholar] [CrossRef] [Green Version]
- Shu, D.; Song, W.; Li, X.; Tang, W. Rhodium- and Platinum-Catalyzed[4+3]Cycloaddition with Concomitant Indole Annulation: Synthesis of Cyclohepta[b]indoles. Angew. Chem. Int. Ed. 2013, 52, 3237–3240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, D.; Winston-McPherson, G.N.; Song, W.; Tang, W. Platinum-Catalyzed Tandem Indole Annulation/Arylation for the Synthesis of Diindolylmethanes and Indolo[3,2-b]carbazoles. Org. Lett. 2013, 15, 4162–4165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
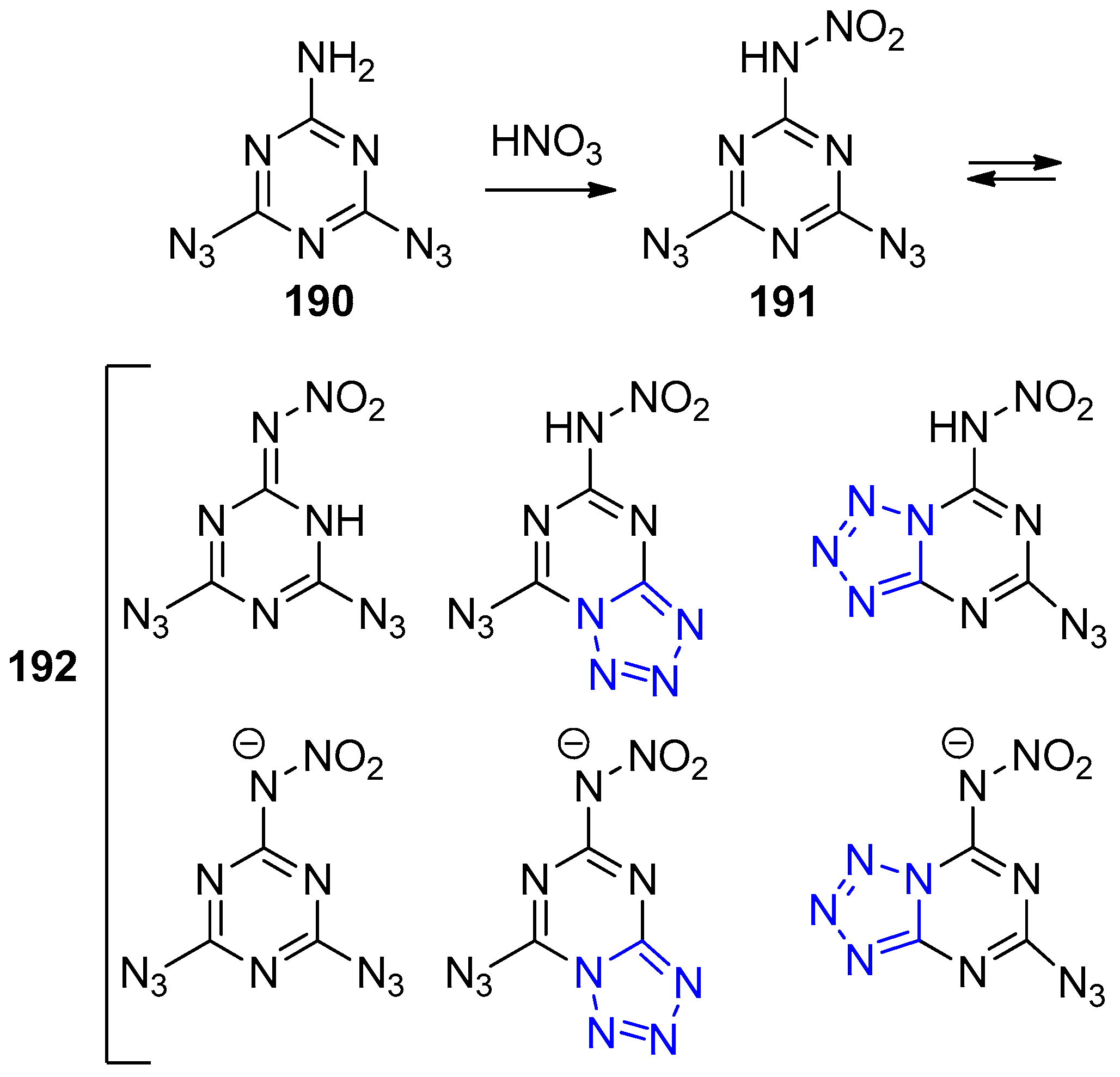
- Chapyshev, S.V.; Ushakov, E.N. Tandem deprotonation/azide–tetrazole tautomerization of 4,6-diazido-N-nitro-1,3,5-triazin-2-amine in dimethylsulfoxide solutions: A theoretical study. Phys. Chem. Chem. Phys. 2015, 17, 17296–17300. [Google Scholar] [CrossRef] [PubMed]
- Ek, F.; Manner, S.; Wistrand, L.-G.; Frejd, T. Synthesis of Fused Tetrazole Derivatives via a Tandem Cycloaddition and N-Allylation Reaction and Parallel Synthesis of Fused Tetrazole Amines. J. Org. Chem. 2004, 69, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Shie, J.-J.; Fang, J.-M. Direct Conversion of Aldehydes to Amides, Tetrazoles, and Triazines in Aqueous Media by One-Pot Tandem Reactions. J. Org. Chem. 2003, 68, 1158–1160. [Google Scholar] [CrossRef]
- Venigalla, L.S.; Maddila, S.; Jonnalagadda, S.B. Facile, efficient, catalyst-free, ultrasound-assisted one-pot green synthesis of triazole derivatives. J. Iran. Chem. Soc. 2020. [Google Scholar] [CrossRef]
- Duan, X.; Huang, X.; Fu, C.; Ma, S. Palladium-Catalyzed Highly Selective Three-Component Tandem Reaction to Bicyclic 1,2,3-Triazole Derivatives. Adv. Synth. Catal. 2019, 362, 627–647. [Google Scholar] [CrossRef]
- Gour, J.; Gatadi, S.; Pooladanda, V.; Ghouse, S.M.; Malasala, S.; Madhavi, Y.V.; Godugu, C.; Nanduri, S. Facile synthesis of 1,2,3-triazole-fused Indolo- and Pyrrolo[1,4]diazepines, DNA-binding and evaluation of their anticancer activity. Bioorg. Chem. 2019, 93, 103306. [Google Scholar] [CrossRef]
- Donikela, S.; Mainkar, P.S.; Nayani, K.; Chandrasekhar, S. Metal Free Domino β-Azidation/[3+2]Cycloaddition Reaction for the Synthesis of 1,2,3-Triazole-Fused Dihydrobenzoxazinones. J. Org. Chem. 2019, 84, 10546–10553. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, H.; Han, H.; Song, J.; Liu, Y.; Guo, W.; Sun, Z.; Chu, W. A one-pot method for synthesis of reduced graphene oxide- supported Cu–Cu2O and catalytic application in tandem reaction of halides and sodium azide with terminal alkynes. Appl. Organomet. Chem. 2018, 32, e4301. [Google Scholar] [CrossRef]
- Boobalan, R.; Chen, C.; Lee, G.-H. Copper (I)-mediated tandem A3 coupling/[3+2] cycloaddition chemoselective synthesis of 4H-[1,2,3]triazolo[1,5-a]indoles. Catal. Commun. 2018, 107, 33–38. [Google Scholar] [CrossRef]
- Nandwana, N.K.; Shinde, V.N.; Saini, H.K.; Kumar, A. Copper-Catalyzed One-Pot Tandem Reaction for the Synthesis of Imidazo[1,2-c][1,2,3]triazolo[1,5-a]quinazolines. Eur. J. Org. Chem. 2017, 43, 6445–6449. [Google Scholar] [CrossRef]
- Hosseini, H.G.; Doustkhah, E.; Kirillova, M.V.; Rostamnia, S.; Mahmoudi, G.; Kirillov, A.M. Combining ethylenediamine and ionic liquid functionalities within SBA-15: A promising catalytic pair for tandem Cu–AAC reaction. Appl. Catal. A 2017, 548, 96–102. [Google Scholar] [CrossRef]
- Zheng, X.; Wan, Y.; Ling, F.; Ma, C. Copper-Catalyzed Tandem Reaction of Terminal Alkynes and Sulfonyl Azides for the Assembly of Substituted Aminotriazoles. Org. Lett. 2017, 19, 3859–3862. [Google Scholar] [CrossRef]
- Amdouni, H.; Robert, G.; Driowya, M.; Furstoss, N.; Métier, C.; Dubois, A.; Dufies, M.; Zerhouni, M.; Orange, F.; Lacas-Gervais, S.; et al. In Vitro and in Vivo Evaluation of Fully Substituted (5-(3-Ethoxy-3-oxopropynyl)-4-(ethoxycarbonyl)-1,2,3-triazolyl-glycosides as Original Nucleoside Analogues to Circumvent Resistance in Myeloid Malignancies. J. Med. Chem. 2017, 60, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Yakovenko, G.G.; Yagodkina, M.S.; Bol’but, A.V.; Shishkina, S.V.; Vovk, M.V. Synthesis of new triazolo[1,5-b][2,4]benzodiazepines via tandem cyclization of o-(azidomethyl)benzoates with cyanoacetamides. Monatsh. Chem. 2017, 148, 1035–1041. [Google Scholar] [CrossRef]
- Phanindrudu, M.; Tiwari, D.K.; Aravilli, V.K.; Bhardwaj, K.C.; Sabapathi, G.; Likhar, P.R.; Tiwari, D.K. Magnetically Recoverable Cu0/Fe3O4-Catalysed One-Pot Tandem Synthesis of Sulfur-Containing Triazoles from Alkynes and Azide: DMSO Acts as an Alkylating Agent. Eur. J. Org. Chem. 2016, 27, 4629–4634. [Google Scholar] [CrossRef]
- Palchak, Z.L.; Nguyen, P.T.; Larsen, C.H. Synthesis of alpha-tetrasubstituted triazoles by copper-catalyzed silyl deprotection/azide cycloaddition. Beilstein J. Org. Chem. 2015, 11, 1425–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakshit, M.; Kar, G.K.; Chakrabarty, M. A new general synthesis of annulated 1,2,3-triazoles using tandem Sonogashira-CuAAC reaction. Monatsh. Chem. 2015, 146, 1681–1688. [Google Scholar] [CrossRef]
- Wen, L.-R.; Li, S.-L.; Zhang, J.; Li, M. Convenient synthesis of benzo[4,5]thiazolo[2,3-c][1,2,4]triazoles with 1 mol% CuCl2·2H2O as catalyst in water. Green Chem. 2015, 17, 1581–1588. [Google Scholar] [CrossRef]
- Wang, B.; Liu, N.; Chen, W.; Huang, D.; Wang, X.; Hu, Y. Minutes Synthesis of 1,4,5-Trisubstituted 5-Dialkylamino-1,2,3-triazoles by 1-Copper(I)-Alkyne Controlled Tandem Process. Adv. Synth. Catal. 2015, 357, 401–407. [Google Scholar] [CrossRef]
- Ning, Y.; Wu, N.; Yu, H.; Liao, P.; Li, X.; Bi, X. Silver-Catalyzed Tandem Hydroazidation/Alkyne–Azide Cycloaddition of Diynes with TMS-N3: An Easy Access to 1,5-Fused 1,2,3-Triazole Frameworks. Org. Lett. 2015, 17, 2198–2201. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Luxami, V.; Paul, K. Synthesis of new 2,3-disubstituted pyridines containing a 1,2,3-triazole in the side-chain via one-pot copper-catalyzed azide-alkyne cycloaddition. Arch. Org. Chem. 2015, 7, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Roy, B.; Mondal, D.; Hatai, J.; Bandyopadhyay, S. A highly efficient tandem [3+2] “click” cycloaddition/6-exo-cyclization strategy for the construction of triazole fused pyrazines. RSC Adv. 2014, 4, 56952–56956. [Google Scholar] [CrossRef] [Green Version]
- Shaabani, S.; Shaabani, A.; Ng, S.W. One-Pot Synthesis of Coumarin-3-carboxamides Containing a Triazole Ring via an Isocyanide-Based Six-Component Reaction. ACS Comb. Sci. 2014, 16, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, R.; Purushothaman, S.; Raghunathan, R. Rapid assembly of heterocycle grafted macrocycles via tandem one-pot double 1,3-dipolar cycloaddition reaction. Org. Biomol. Chem. 2014, 12, 9375–9383. [Google Scholar] [CrossRef] [PubMed]
- Das, U.K.; Jena, R.K.; Bhattacharjee, M. Synthesis, structure and catalytic properties of [Ru(dppp)2(CH3CN)Cl][BPh4] and isolation of catalytically active [Ru(dppp)2Cl][BPh4]: Ruthenium catalysed alkyne homocoupling and tandem alkyne–azide cycloaddition. RSC Adv. 2014, 4, 21964–21970. [Google Scholar] [CrossRef]
- Gomes, A.T.P.C.; Martins, P.R.C.; Rocha, D.R.; Neves, M.G.P.M.S.; Ferreira, V.F.; Silva, A.M.S.; Cavaleiro, J.A.S.; da Silva, F.C. Consecutive Tandem Cycloaddition between Nitriles and Azides; Synthesis of 5-Amino-1H-[1,2,3]-triazoles. Synlett 2013, 24, 41–44. [Google Scholar] [CrossRef]
- Niu, T.-F.; Cai, C.; Yi, L. A One-Pot Tandem Ugi Multicomponent Reaction (MCR)/Click Reaction as an Efficient Protecting-Group-Free Route to 1H-1,2,3-Triazole-Modified α-Amino Acid Derivatives and Peptidomimetics. Helv. Chim. Acta 2012, 95, 87–99. [Google Scholar] [CrossRef]
- Reddy, M.N.; Swamy, K.C.K. Facile Construction of [6,6]-, [6,7]-, [6,8]-, and [6,9]Ring-Fused Triazole Frameworks by Copper-Catalyzed, Tandem, One-Pot, Click and Intramolecular Arylation Reactions: Elaboration to Fused Pentacyclic Derivatives. Eur. J. Org. Chem. 2012, 10, 2013–2022. [Google Scholar] [CrossRef]
- García-Álvarez, J.; Díez, J.; Vidal, C. Pd(ii)-catalyzed cycloisomerisation of γ-alkynoic acids and one-pot tandem cycloisomerisation/CuAAC reactions in water. Green Chem. 2012, 14, 3190–3196. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, F.; Qin, D.; Cai, T.; Ding, K.; Cai, Q. Synthesis of [1,2,3]Triazolo[1,5-a]quinoxalin-4(5H)-ones through Copper-Catalyzed Tandem Reactions of N-(2-Haloaryl)propiolamides with Sodium Azide. Org. Lett. 2012, 14, 1262–1265. [Google Scholar] [CrossRef]
- Barange, D.K.; Tu, Y.-C.; Kavala, V.; Kuo, C.-W.; Yao, C.-F. One-Pot Synthesis of Triazolothiadiazepine1,1-Dioxide Derivatives via Copper-Catalyzed Tandem[3+2]Cycloaddition/N-Arylation. Adv. Synth. Catal. 2011, 353, 41–48. [Google Scholar] [CrossRef]
- Fletcher, J.T.; Reilly, J.E. Fast dye salts provide fast access to azidoarene synthons in multi-step one-pot tandem click transformations. Tetrahedron Lett. 2011, 52, 5512–5515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolarovic, A.; Schnürch, M.; Mihovilovic, M.D. Tandem Catalysis: From Alkynoic Acids and Aryl Iodides to 1,2,3-Triazoles in One Pot. J. Org. Chem. 2011, 76, 2613–2618. [Google Scholar] [CrossRef] [PubMed]
- Gulevskayaa, A.V.; Danga, S.V.; Tyaglivya, A.S.; Pozharskiia, A.F.; Kazhevab, O.N.; Chekhlovb, A.N.; Dyachenko, O.A. A novel tandem cyclization of condensed 2,3-dialkynylpyrazines into[1,2,3]triazolo[1′,5′;1,2]pyrido[3,4-b]pyrazines promoted by sodium azide. Tetrahedron 2010, 66, 146–151. [Google Scholar] [CrossRef]
- Proulx, C.; Lubell, W.D. Aza-1,2,3-triazole-3-alanine Synthesis via Copper-Catalyzed 1,3-Dipolar Cycloaddition on Aza-progargylglycine. J. Org. Chem. 2010, 75, 5385–5387. [Google Scholar] [CrossRef]
- Campbell-Verduyn, L.S.; Szymański, W.; Postema, C.P.; Dierckx, R.A.; Elsinga, P.H.; Janssen, D.B.; Feringa, B.L. One pot ‘click’ reactions: Tandem enantioselective biocatalytic epoxide ring opening and [3+2]azide alkyne cycloaddition. Chem. Commun. 2010, 46, 898–900. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Matiychuk, V.S.; Obushak, M.D. Synthesis of 1-(R-Phenyl)-5-(R-Methyl)-1H-1,2,3-triazole-4-carboxylic Acids by One-Pot Tandem Reaction. Synth. Commun. 2010, 40, 1932–1938. [Google Scholar] [CrossRef]
- Malnuit, V.; Duca, M.; Manout, A.; Bougrin, K.; Benhida, R. Tandem Azide-Alkyne 1,3-Dipolar Cycloaddition/Electrophilic Addition: A Concise Three-Component Route to 4,5-Disubstituted Triazolyl-Nucleosides. Synlett 2009, 13, 2123–2128. [Google Scholar] [CrossRef]
- Kaliappan, K.P.; Kalanidhi, P.; Mahapatra, S. ‘Click’ Chemistry on Sugar-Derived Alkynes: A Tandem ‘Click-Click’ Approach to Bistriazoles. Synlett 2009, 13, 2162–2166. [Google Scholar] [CrossRef]
- Zou, W.; Bhasin, M.; Vembaiyan, K.; Williams, D.T. Triazole-fused sugars from nitroalkene-containing C-glycosides by a tandem 1,3-dipolar cycloaddition and intramolecular Michael addition. Carbohydr. Res. 2009, 344, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- van Berkel, S.S.; Dirks, A.T.J.; Debets, M.F.; van Delft, F.L.; Cornelissen, J.J.L.M.; Nolte, R.J.M.; Rutjes, F.P.J.T. Metal-Free Triazole Formation as a Tool for Bioconjugation. ChemBioChem 2007, 8, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Marco, C.N.D.; Kuduk, S.D. Synthesis of Fused[5,5]-1,2,4-Triazoles via Tandem Thioimidate Cyclopropane Rearrangement–Cyclization. Synth. Commun. 2006, 36, 3377–3386. [Google Scholar] [CrossRef]
- van Maarseveen, J.H.; Horne, W.S.; Ghadiri, M.R. Efficient Route to C2 Symmetric Heterocyclic Backbone Modified Cyclic Peptides. Org. Lett. 2005, 7, 4503–4506. [Google Scholar] [CrossRef]
- Chibale, K.; Dauvergne, J.; Wyatt, P.G. A Novel and Efficient Regiospecific Preparation of Arenesulfonamide Derivatives of 3,5-Diamino-1,2,4-triazole. Synthesis 2002, 2, 185–190. [Google Scholar] [CrossRef]
- Guo, X.; Huang, C.; Yang, H.; Shao, Z.; Gao, K.; Qin, N.; Li, G.; Wu, J.; Hou, H. Cu (i) coordination polymers (CPs) as tandem catalysts for three-component sequential click/alkynylation cycloaddition reaction with regiocontrol. Dalton Trans. 2018, 47, 16895–16901. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.S.; Pankhade, Y.A.; Anand, R.V. Tandem One-Pot Approach to Access 1,2,3-Triazole-fused Isoindolines through Cu-Catalyzed 1,6-Conjugate Addition of Me3SiN3 to p-Quinone Methides followed by Intramolecular Click Cycloaddition. J. Org. Chem. 2018, 83, 8596–8606. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Chen, X.; Zhang, X.; Guan, W.; Li, Y. A Tandem Ring-Opening/Michael Addition/Ring-Closure Sequence for the Regiospecific Synthesis of 5-Hydroxy-4,5-dihydroisoxazoles. Asian J. Org. Chem. 2019, 8, 1479–1483. [Google Scholar] [CrossRef]
- Kavala, V.; Janreddy, D.; Raihan, M.J.; Kuo, C.-W.; Ramesh, C.; Yao, C.-F. One-Pot Tandem Synthesis of 2-Arylbenzoxazole Derivatives via Copper-Catalyzed C-N and C-O Bond Formation. Adv. Synth. Catal. 2012, 354, 2229–2240. [Google Scholar] [CrossRef]
- Wei, W.; Tang, Y.; Zhou, Y.; Deng, G.; Liu, Z.; Wu, J.; Li, Y.; Zhang, J.; Xu, S. Recycling Catalyst as Reactant: A Sustainable Strategy to Improve Atom Efficiency of Organocatalytic Tandem Reactions. Org. Lett. 2018, 20, 6559–6563. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Singh, A.S.; Agrahari, A.K.; Singh, S.K.; Singh, M.; Tiwari, V.K. Synthesis of Benz-Fused Azoles via C-Heteroatom Coupling Reactions Catalyzed by Cu (I) in the Presence of Glycosyltriazole Ligands. ACS Comb. Sci. 2019, 21, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Motornov, V.A.; Tabolin, A.A.; Novikov, R.A.; Nelyubina, Y.V.; Nenajdenko, V.G.; Ioffe, S.L. Fluoronitroalkenes in tandem[4+1]/[3+2]-cycloaddition: One-pot three-component assembly of fluorinated bicyclic nitroso acetals. Org. Chem. Front. 2018, 5, 2588–2594. [Google Scholar] [CrossRef]

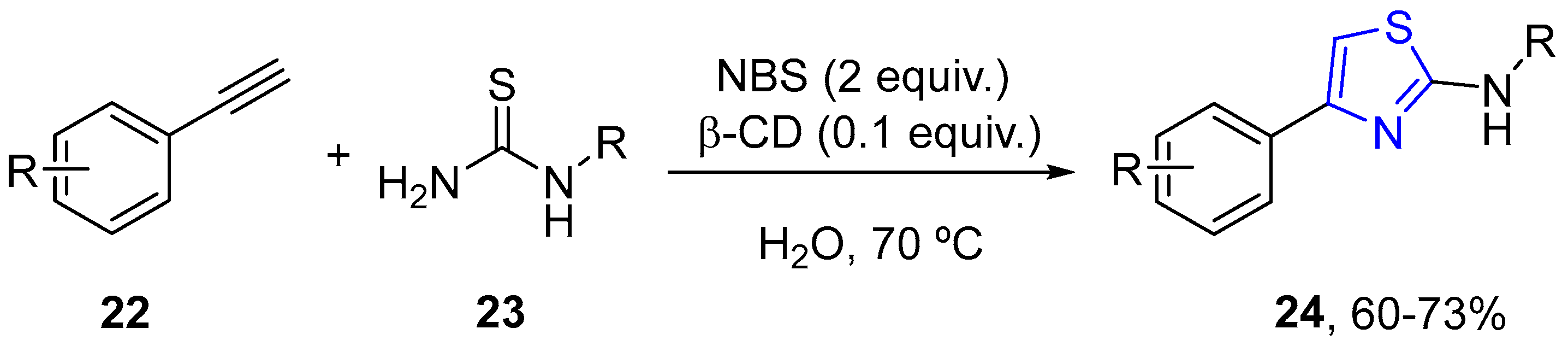


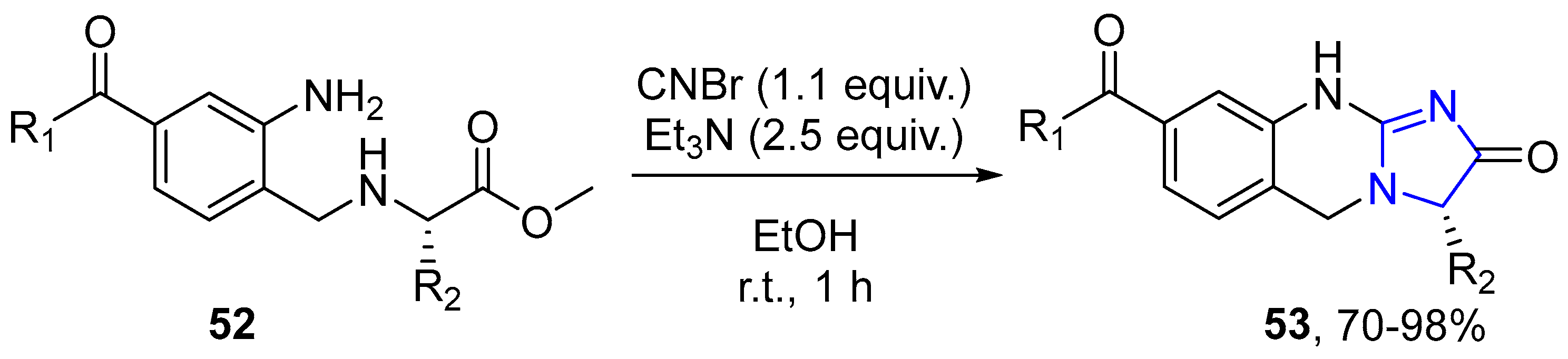
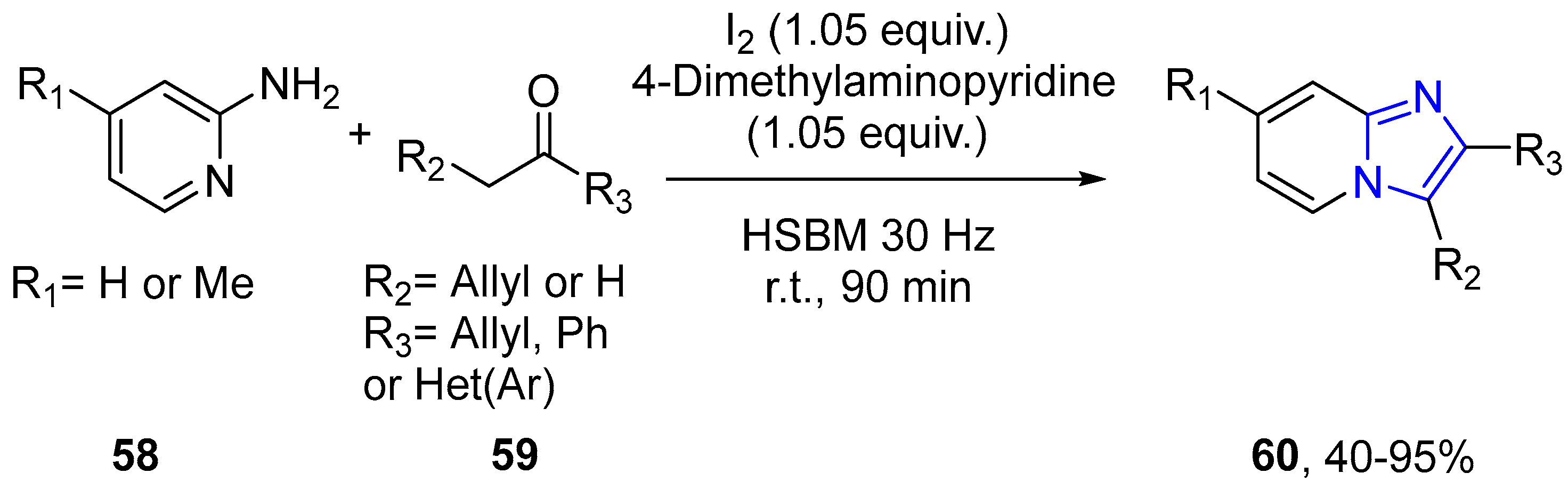









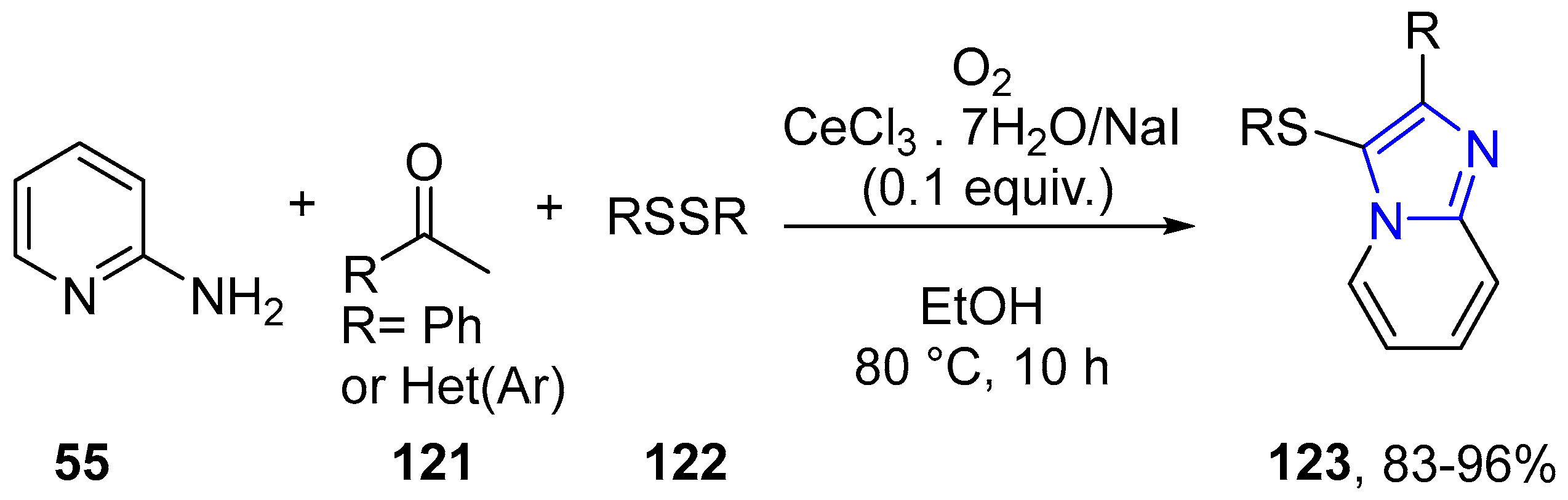
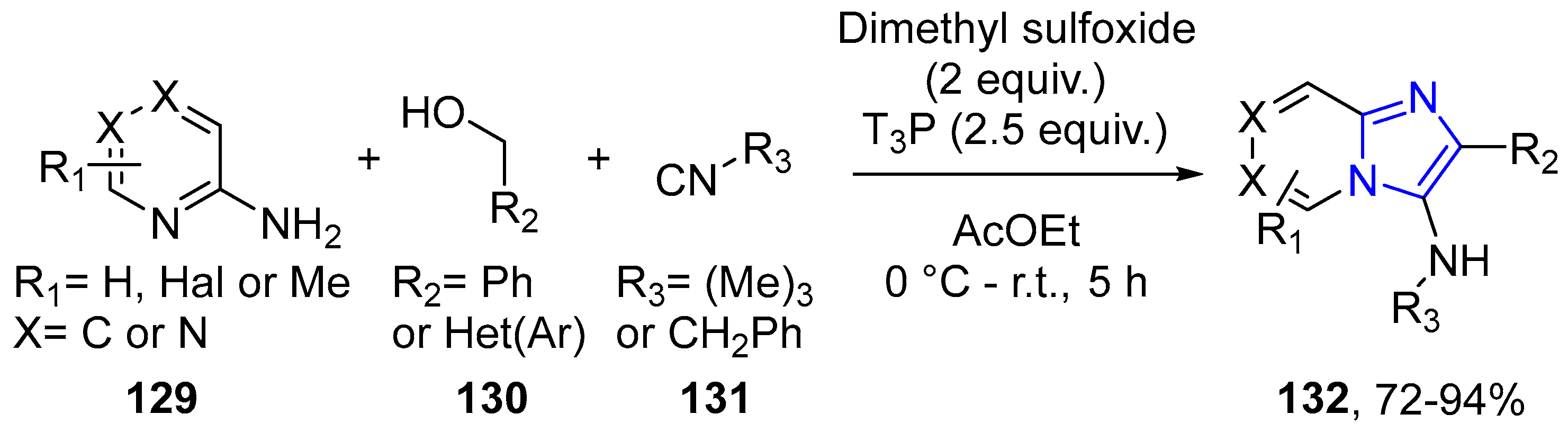






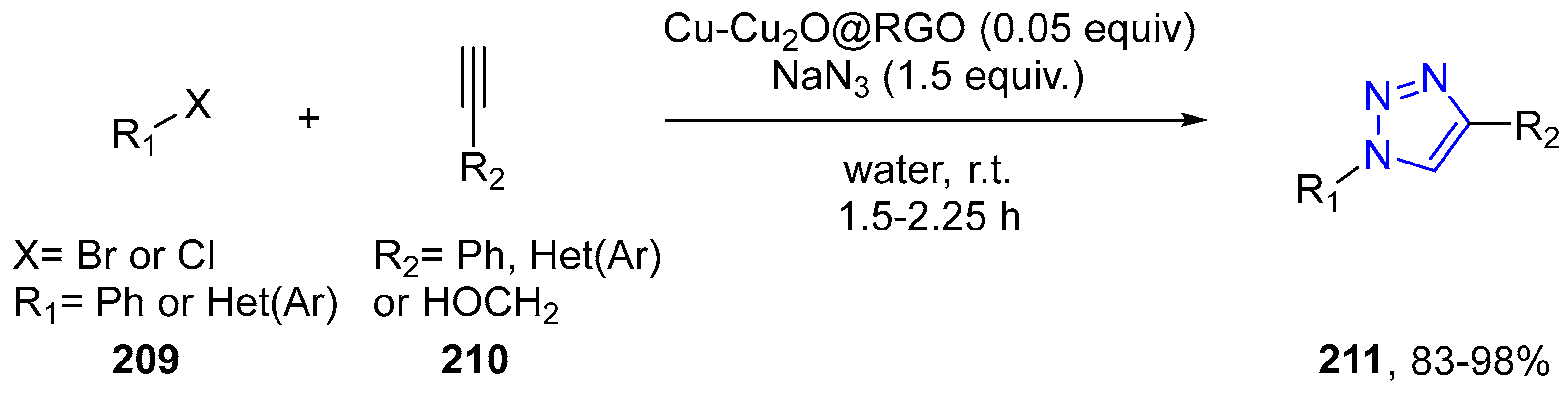
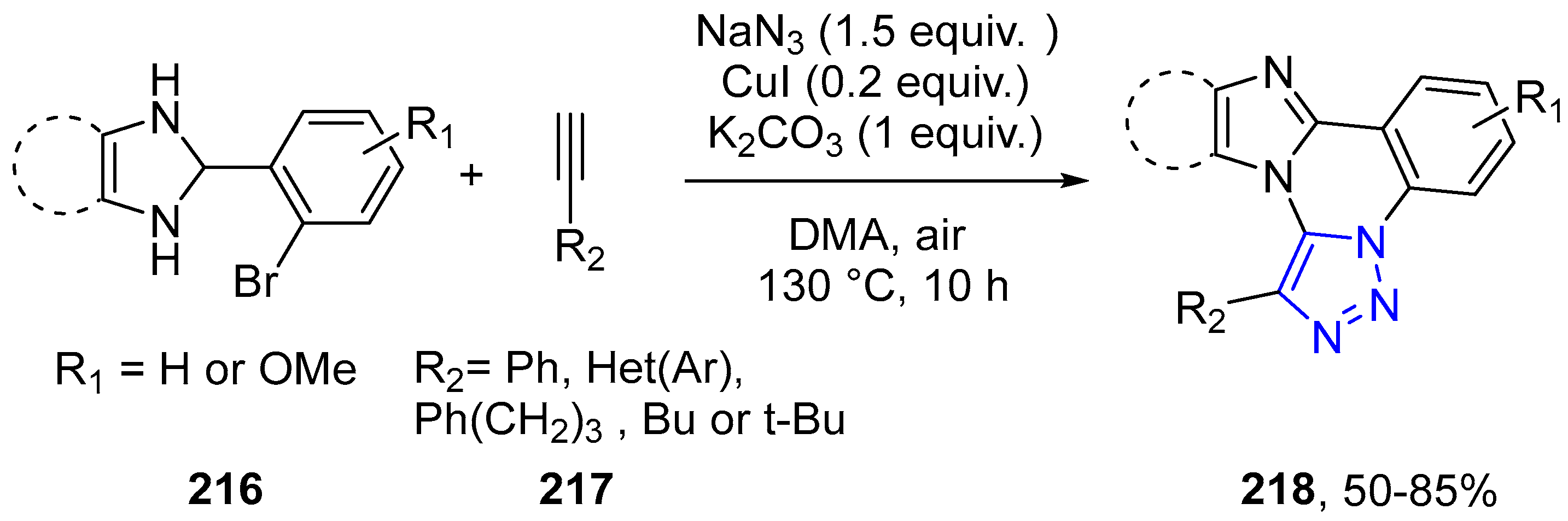
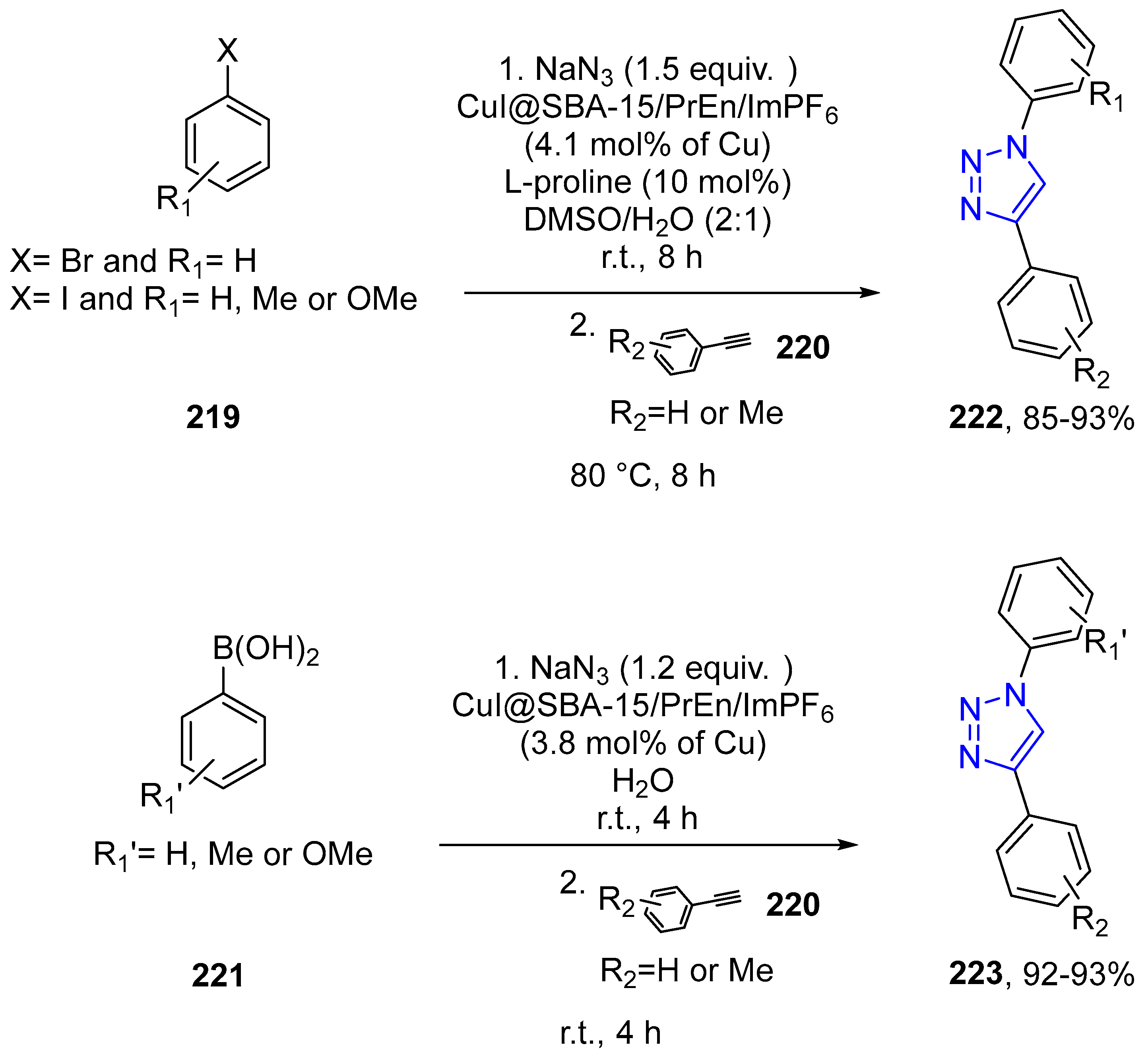
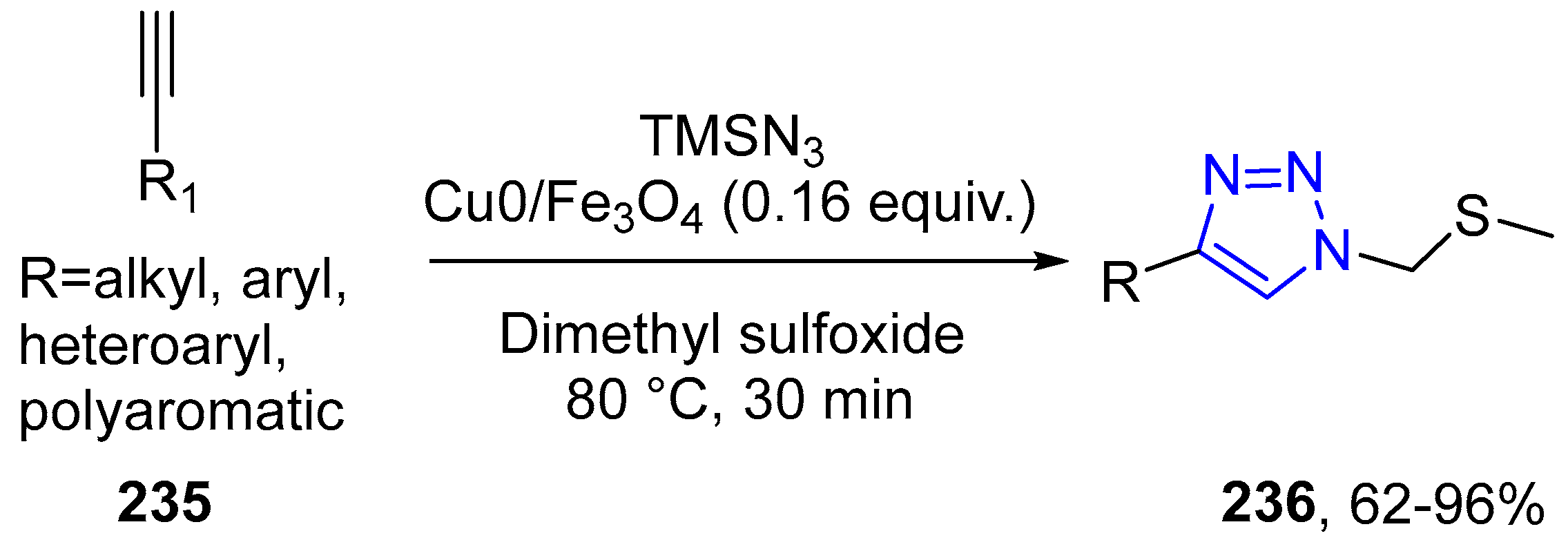


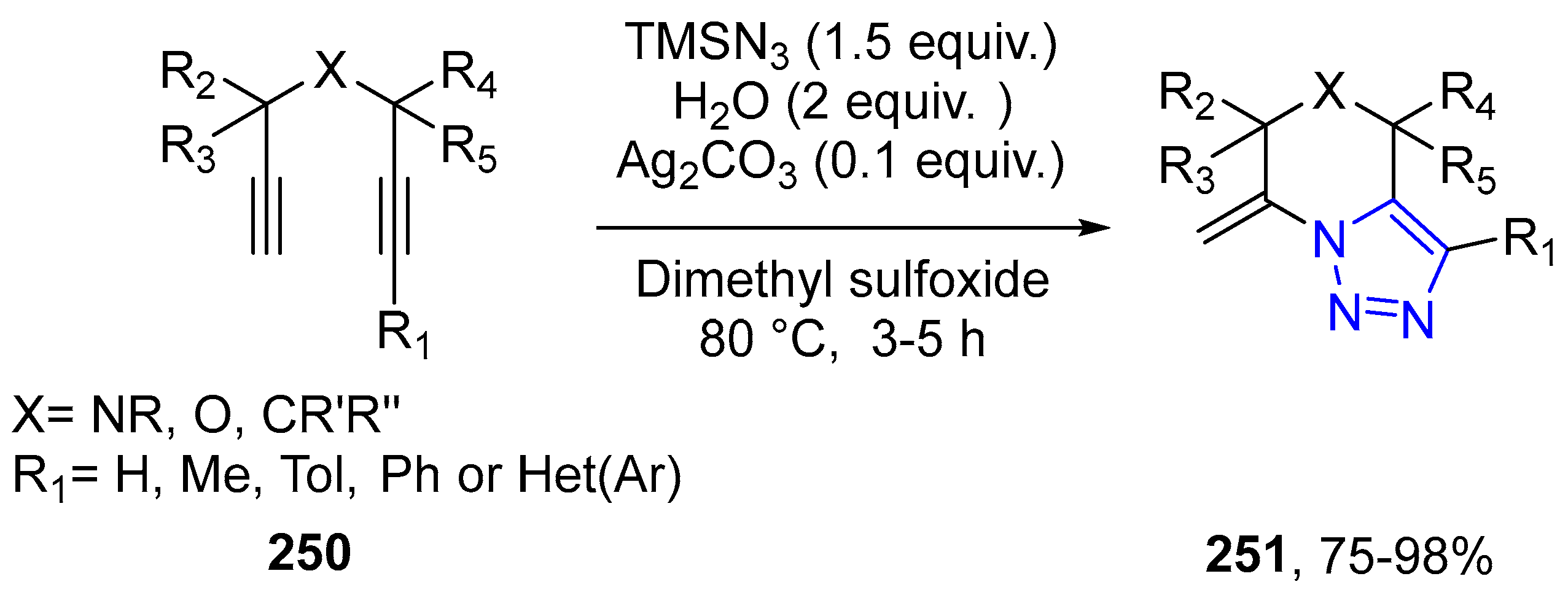
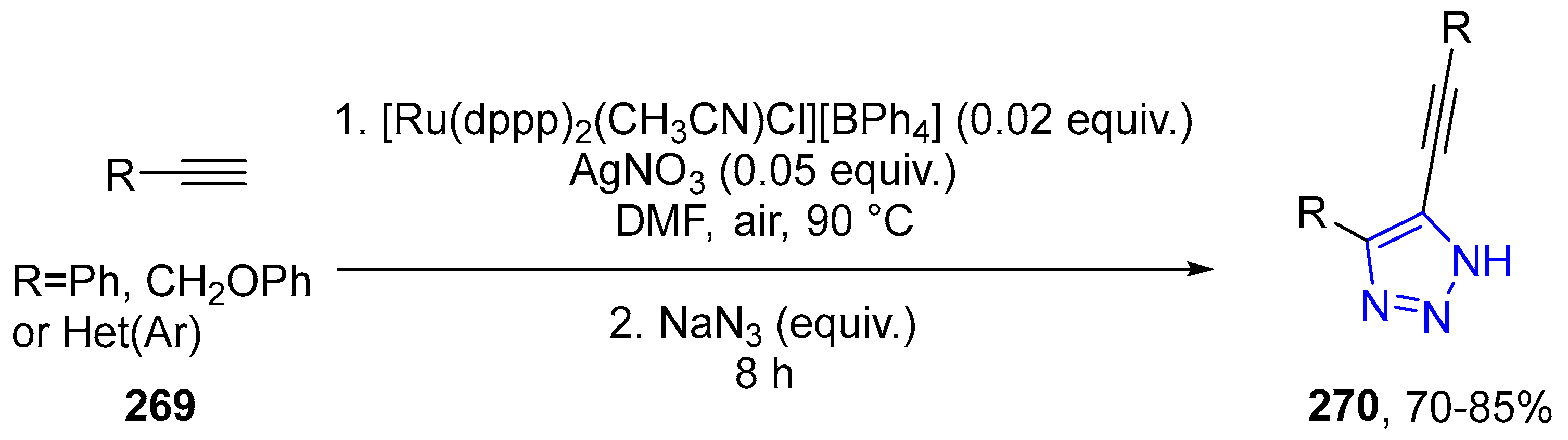
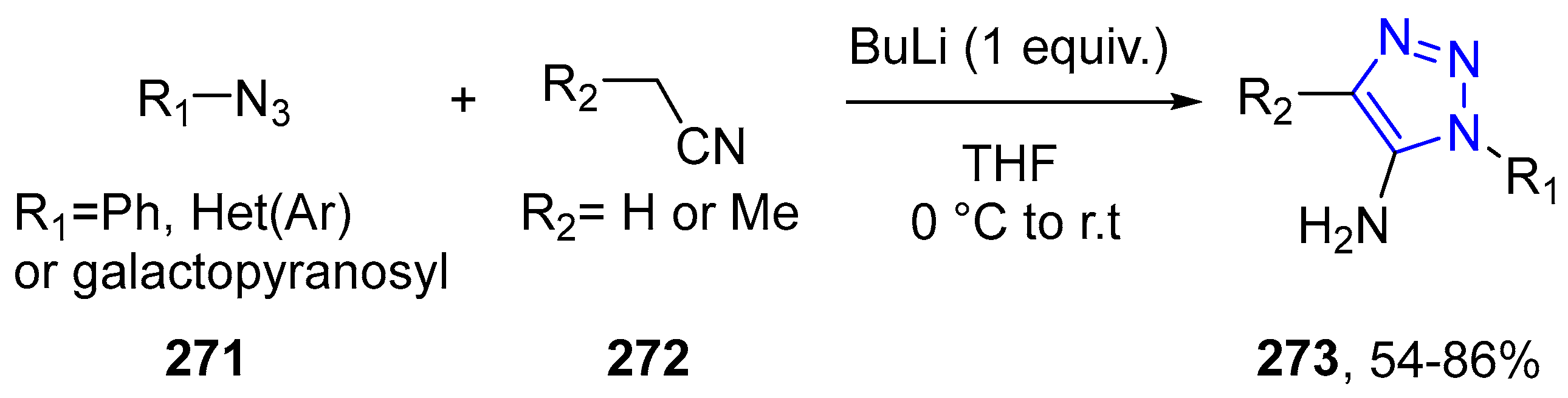


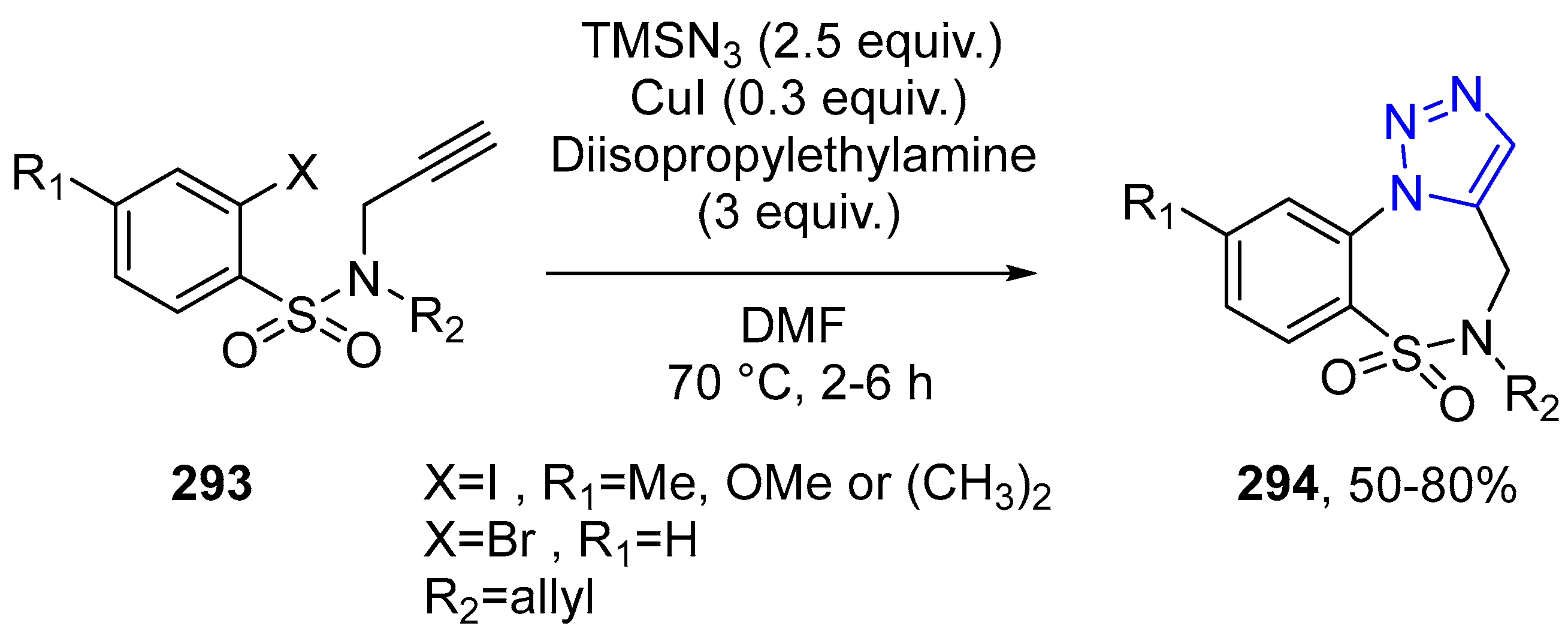
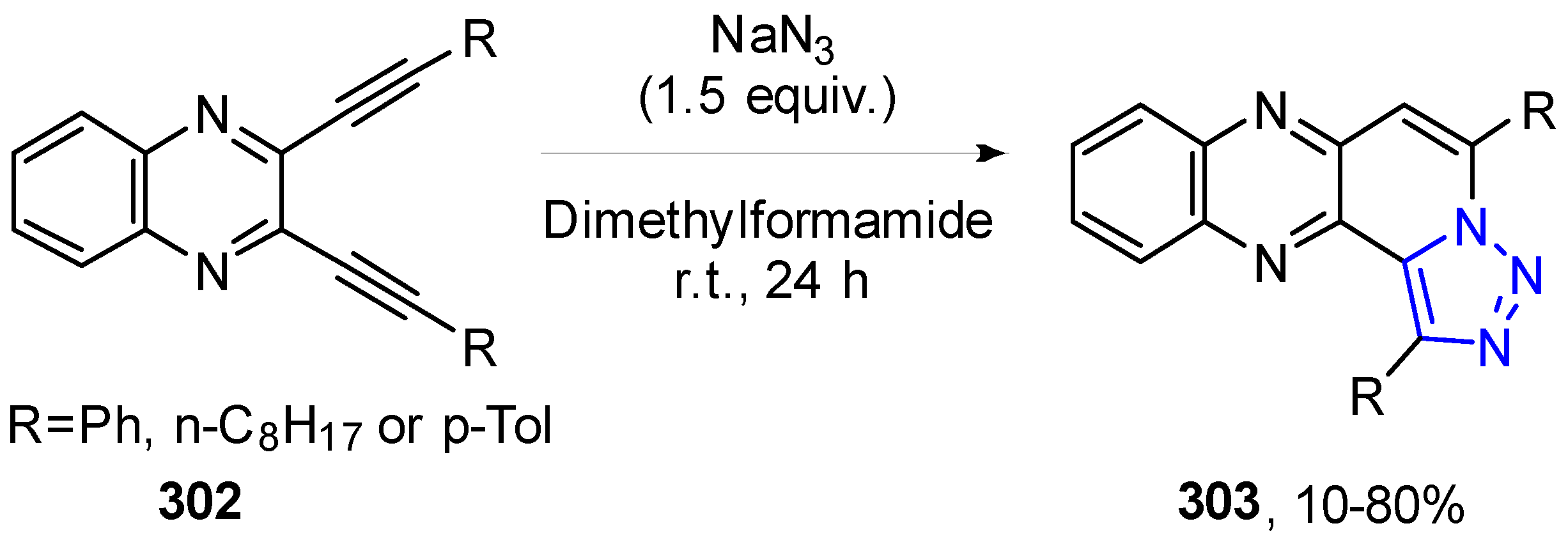
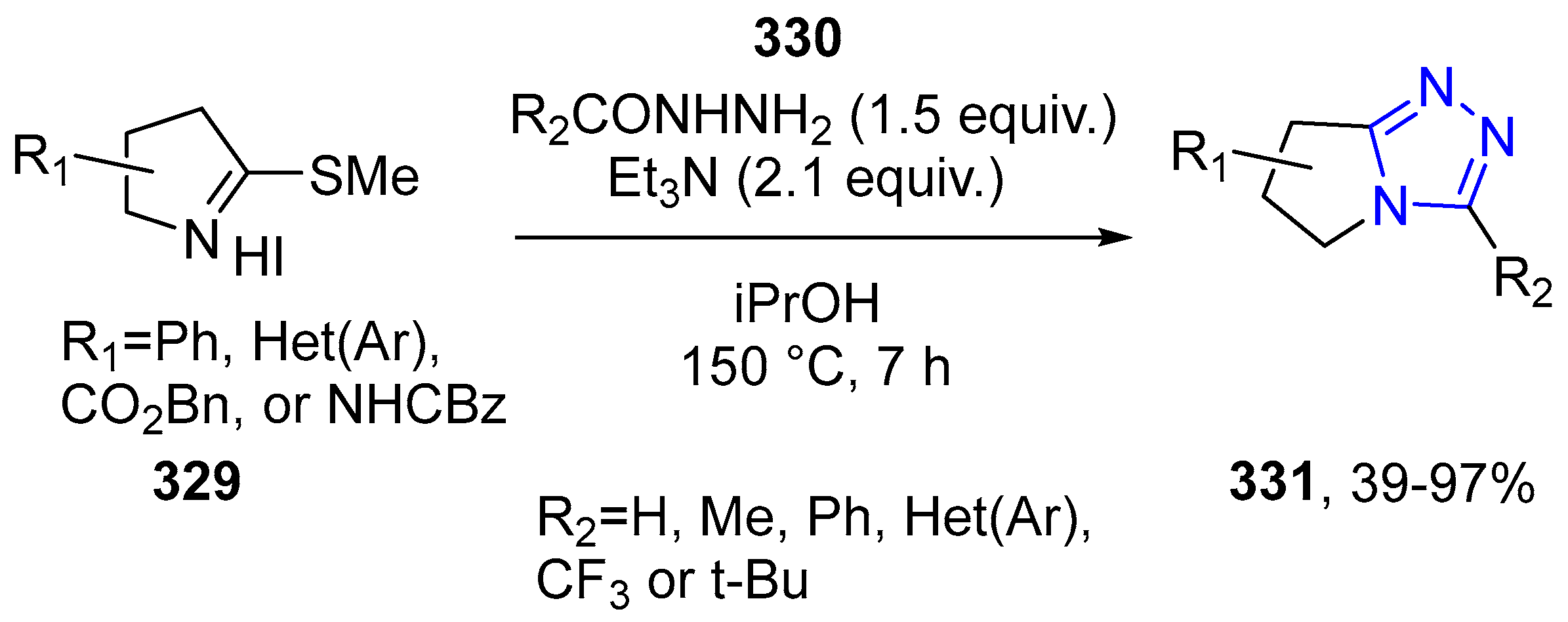
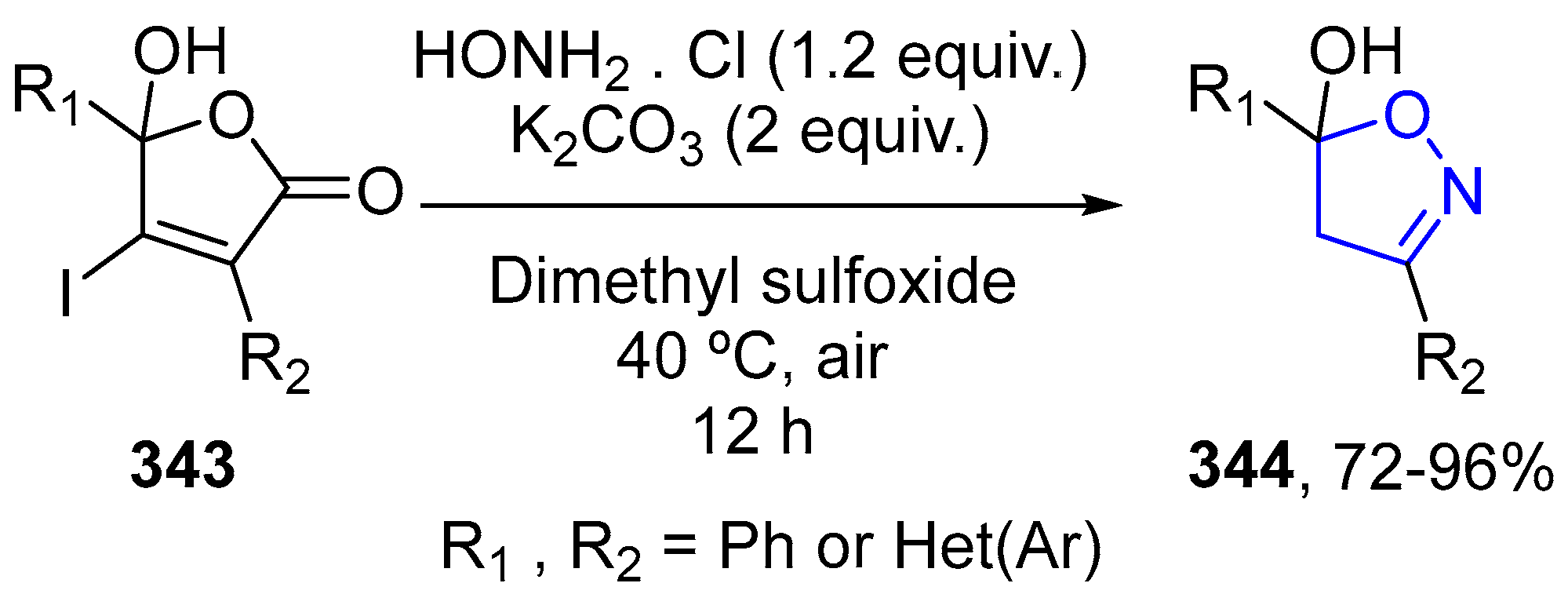


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, J.F.; Berteina-Raboin, S. Tandem Catalysis: Synthesis of Nitrogen-Containing Heterocycles. Catalysts 2020, 10, 631. https://doi.org/10.3390/catal10060631
Campos JF, Berteina-Raboin S. Tandem Catalysis: Synthesis of Nitrogen-Containing Heterocycles. Catalysts. 2020; 10(6):631. https://doi.org/10.3390/catal10060631
Chicago/Turabian StyleCampos, Joana F., and Sabine Berteina-Raboin. 2020. "Tandem Catalysis: Synthesis of Nitrogen-Containing Heterocycles" Catalysts 10, no. 6: 631. https://doi.org/10.3390/catal10060631





