Synergistic Interaction of Cerium and Barium-New Insight into the Promotion Effect in Cobalt Systems for Ammonia Synthesis
Abstract
1. Introduction
2. Results
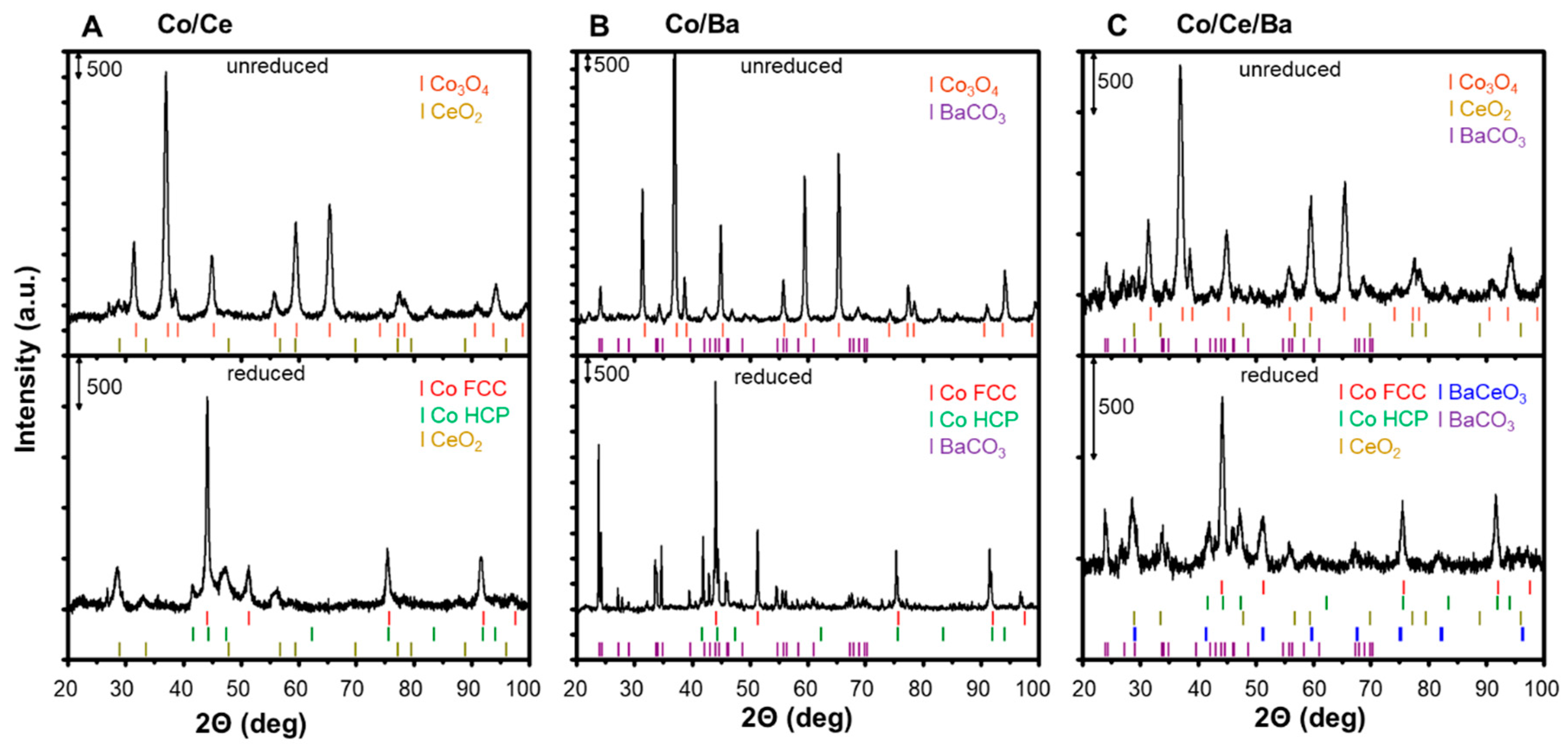
2.1. X-ray Powder Diffraction (XRPD) Measurements
2.2. X-ray Photoelectron Spectroscopy (XPS) Studies
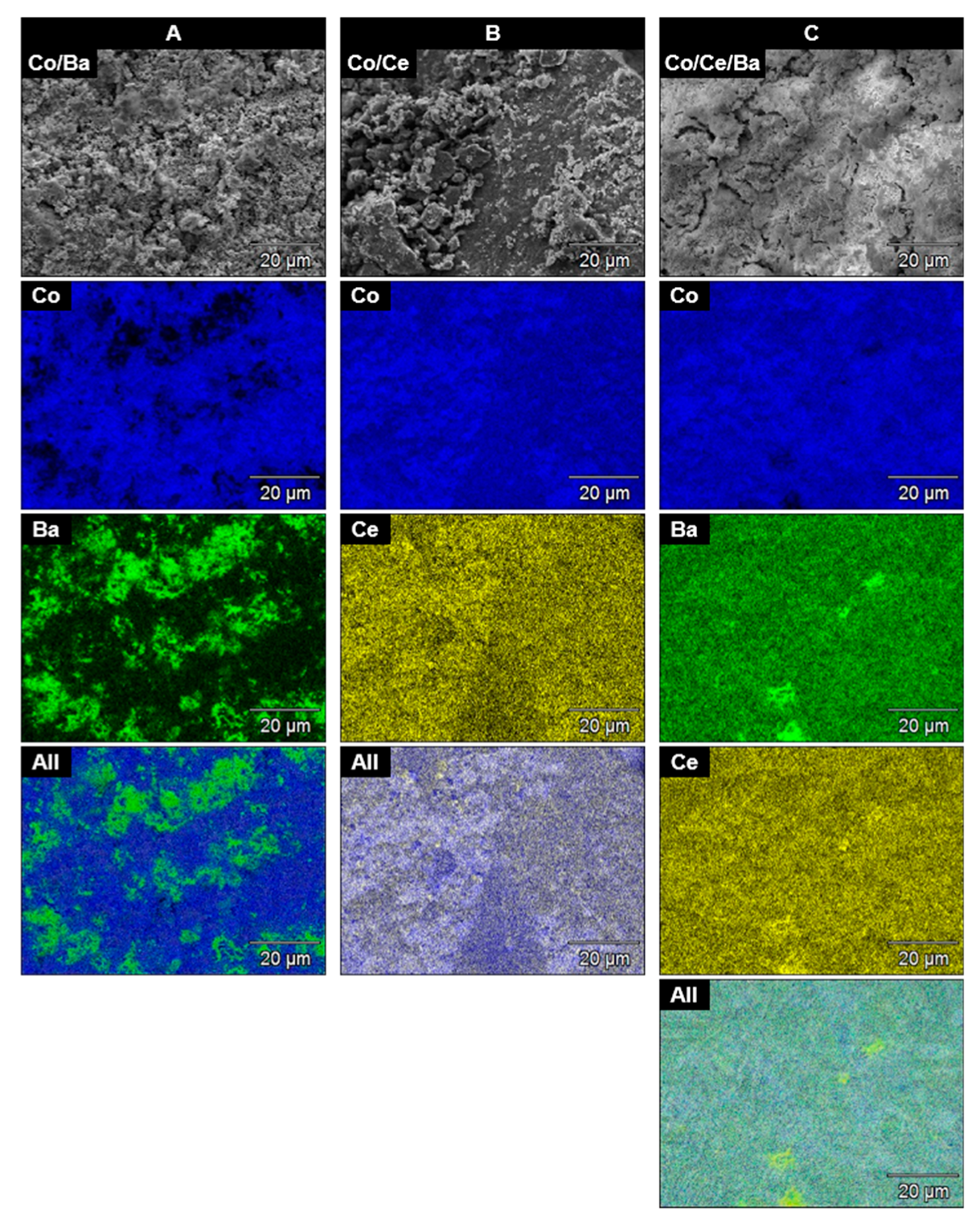
2.3. Scanning Electron Microscopy Coupled with Energy Dispersive X-ray Spectroscopy (SEM-EDS)
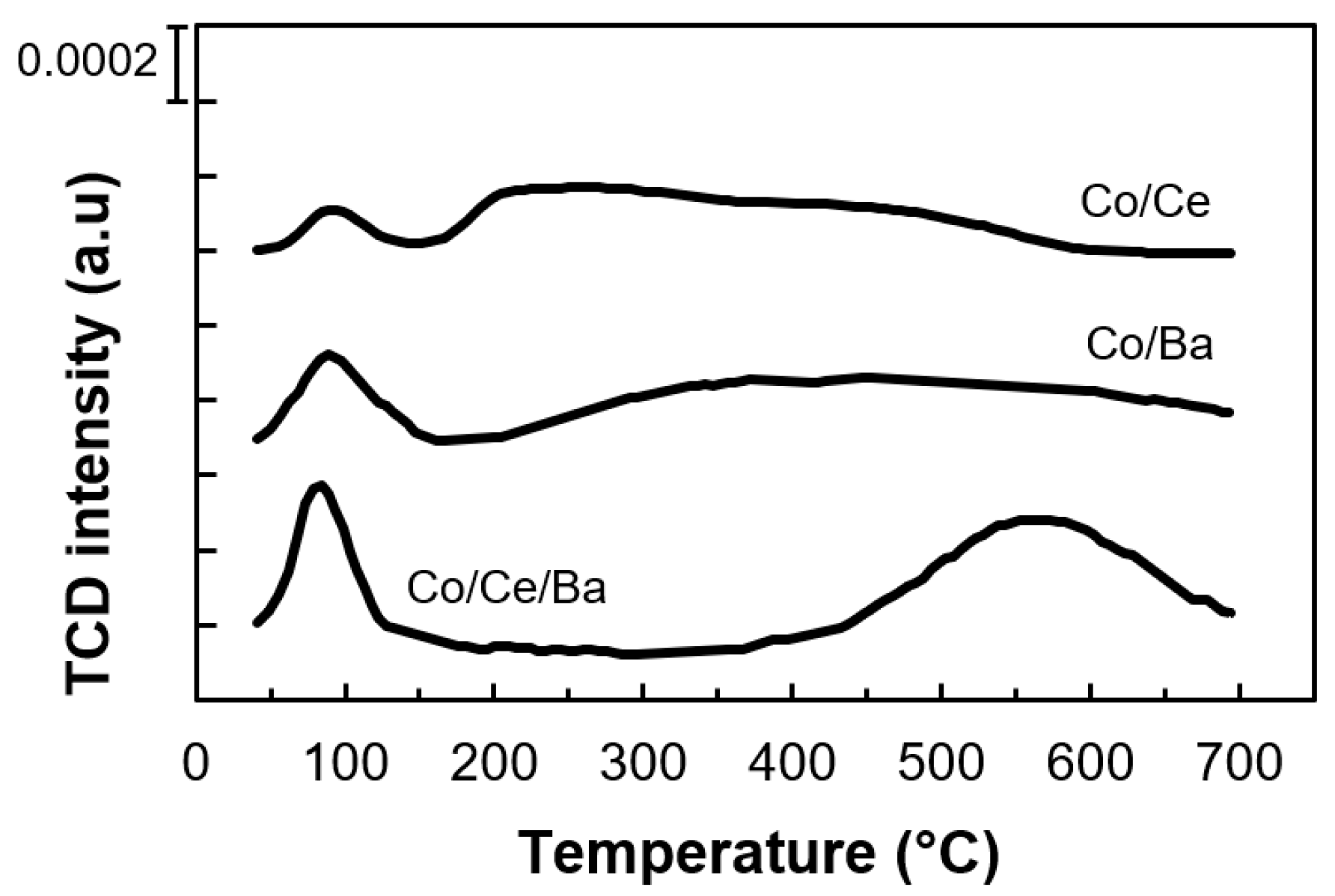
2.4. Temperature-Programmed Carbon Dioxide Desorption (CO2-TPD)
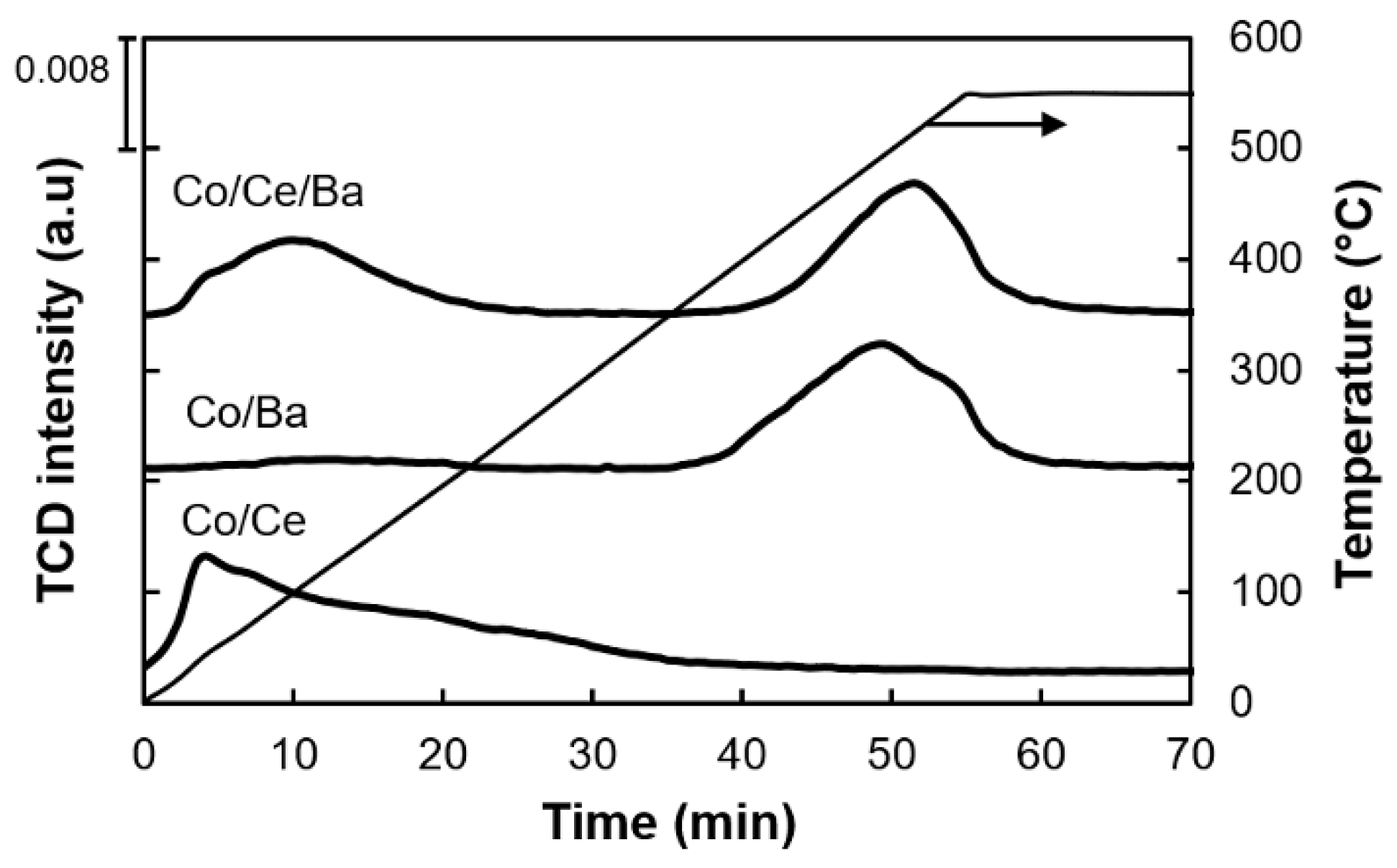
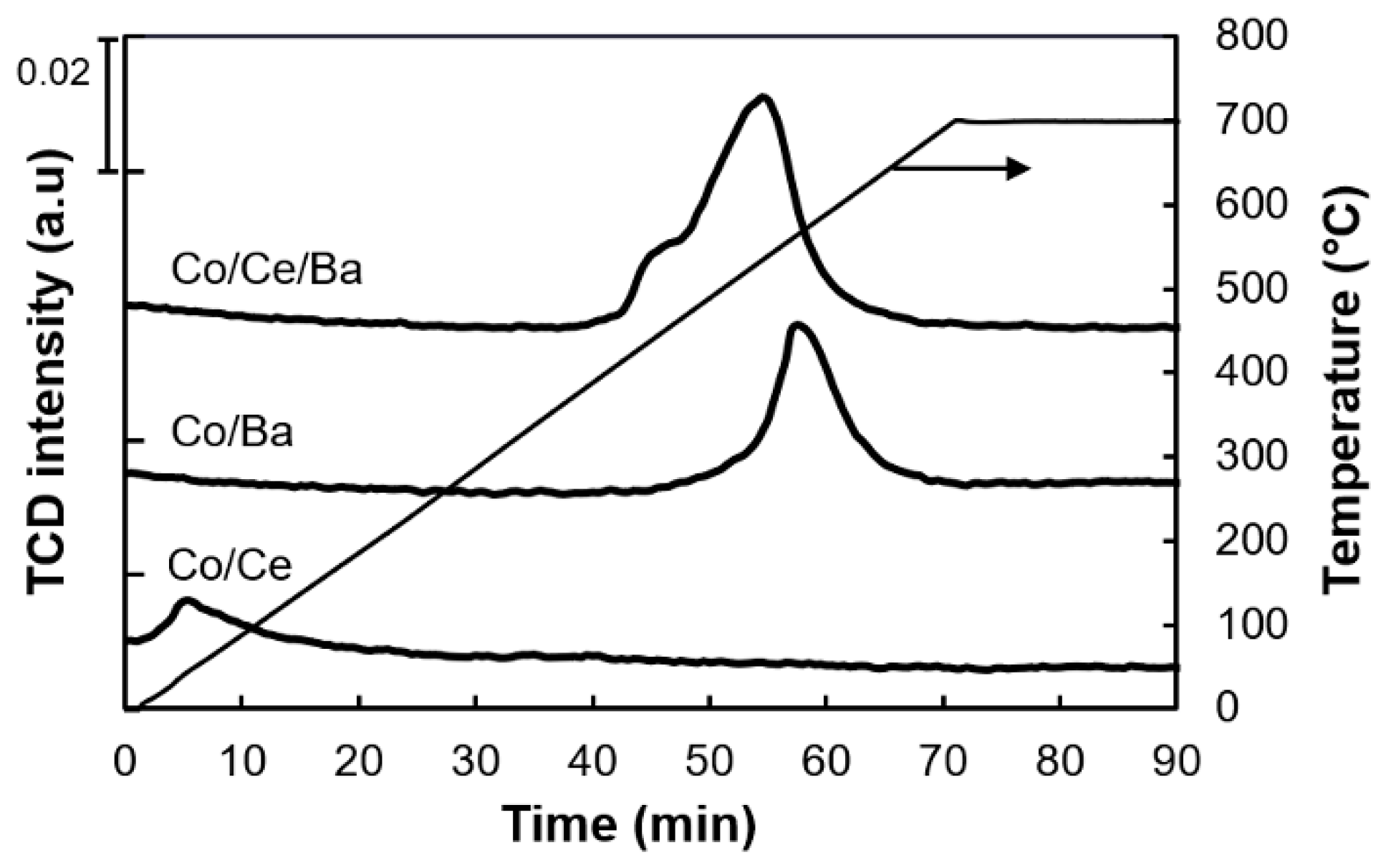
2.5. Temperature Programmed (TP) Measurements
3. Discussion
4. Materials and Methods
4.1. Catalysts Preparation
4.2. Characterization Techniques
- Temperature-programmed carbon dioxide desorption (CO2-TPD)–adsorption of carbon dioxide was carried out at 40 °C for 2 h. Subsequently, the system was purged with He (40 °C) for 1 h. Then, temperature was raised to 700 °C with a rate of 10 °C min−1 in He and the concentration of desorbing CO2 in the outlet gas was monitored.
- Temperature-programmed hydrogen desorption (H2-TPD)–hydrogen adsorption was carried out at 150 °C for 15 min, during cooling the sample to 0 °C and at 0 °C for 15 min. Subsequently, the system was flushed with Ar (0 °C) for 1 h to remove weakly adsorbed hydrogen. Next, in Ar stream temperature was raised to 550 °C with a rate of 10 °C min−1 and kept for 25 min, while monitoring the concentration of desorbing H2 in the outlet gas.
- Temperature-programmed nitrogen desorption (N2-TPD) and surface reaction of preadsorbed nitrogen with hydrogen (Nads + H2 TPSR)–adsorption of nitrogen was carried out at 200 °C for 14 h and during cooling the sample to 0 °C. Next, it was purged with He (0 °C) for 1 h to remove weakly adsorbed nitrogen. Then, temperature was raised to 700 °C with a rate of 10 °C min−1 in He (N2-TPD) or H2 stream (Nads + H2 TPSR) and kept for 25 min. The concentration of desorbing molecules in the outlet gas was monitored – nitrogen or ammonia, respectively. It was assumed that only ammonia, as a product, was observed during Nads + H2 TPSR measurements. To prove this state, an additional experiment was conducted, according to the same procedure as the main measurement, but the reactor outlet gas was cooled in a trap maintained at −86 °C. When gas was flowing through the cold trap, TCD readings indicated no products present in the stream. It was therefore concluded that the ammonia, as the only product of Nads + H2 TPSR, was entirely caught in a cold trap.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomson, S.J. Promotion in heterogeneous catalysis: Retrospect and prospect. J. Chem. Soc. Faraday Trans. 1987, 83, 1893–1914. [Google Scholar] [CrossRef]
- Kiskinova, M. Poisoning and promotion in catalysis based on surface science concepts and experiments. Stud. Surf. Sci. Catal. 1991, 70, 1–345. [Google Scholar] [CrossRef]
- Hutchings, G.J. Promotion in heterogeneous catalysis: A topic requiring a new approach? Catal. Lett. 2001, 75, 1–12. [Google Scholar] [CrossRef]
- Richardson, J.T. Principles of Catalysts Development; Springer Science+Business Media: New York, NY, USA, 1989. [Google Scholar]
- Ryczkowski, J.; Borowiecki, T. Modyfikatory katalizatorów heterogenicznych. Przem. Chem. 2016, 82, 763–765. [Google Scholar]
- Wang, X.; Li, L.; Zhang, T.; Lin, B.; Ni, J.; Au, C.T.; Jiang, L. Strong metal-support interactions of Co-based catalysts facilitated by dopamine for highly efficient ammonia synthesis: In situ XPS and XAFS spectroscopy coupled with TPD studies. Chem. Commun. 2019, 55, 474–477. [Google Scholar] [CrossRef]
- Gao, W.; Wang, P.; Guo, J.; Chang, F.; He, T.; Wang, Q.; Wu, G.; Chen, P. Barium Hydride-Mediated Nitrogen Transfer and Hydrogenation for Ammonia Synthesis: A Case Study of Cobalt. ACS Catal. 2017, 7, 3654–3661. [Google Scholar] [CrossRef]
- Wang, X.; Peng, X.; Chen, W.; Liu, G.; Zheng, A.; Zheng, L.; Ni, J.; Au, C.; Jiang, L. Insight into dynamic and steady-state active sites for nitrogen activation to ammonia by cobalt-based catalyst. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ronduda, H.; Zybert, M.; Patkowski, W.; Tarka, A.; Jodłowski, P.; Kępiński, L.; Sarnecki, A.; Moszyński, D.; Raróg-Pilecka, W. Tuning the catalytic performance of Co/Mg-La system for ammonia synthesis via the active phase precursor introduction method. Appl. Catal. A Gen. 2020, 598, 117553. [Google Scholar] [CrossRef]
- Lin, B.; Liu, Y.; Heng, L.; Ni, J.; Lin, J.; Jiang, L. Effect of barium and potassium promoter on Co/CeO2 catalysts in ammonia synthesis. J. Rare Earths 2018, 36, 703–707. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Karolewska, M.; Truszkiewicz, E.; Iwanek, E.; Mierzwa, B. Cobalt catalyst doped with cerium and barium obtained by Co-precipitation method for ammonia synthesis process. Catal. Lett. 2011, 141, 678–684. [Google Scholar] [CrossRef]
- Karolewska, M.; Truszkiewicz, E.; Mierzwa, B.; Kępiński, L.; Raróg-Pilecka, W. Ammonia synthesis over cobalt catalysts doped with cerium and barium. Effect of the ceria loading. Appl. Catal. A Gen. 2012, 445–446, 280–286. [Google Scholar] [CrossRef]
- Karolewska, M.; Truszkiewicz, E.; Wściseł, M.; Mierzwa, B.; Kępiński, L.; Raróg-Pilecka, W. Ammonia synthesis over a Ba and Ce-promoted carbon-supported cobalt catalyst. Effect of the cerium addition and preparation procedure. J. Catal. 2013, 303, 130–134. [Google Scholar] [CrossRef]
- Zybert, M.; Wyszyńska, M.; Tarka, A.; Patkowski, W.; Ronduda, H.; Mierzwa, B.; Kępiński, L.; Sarnecki, A.; Moszyński, D.; Raróg-Pilecka, W. Surface enrichment phenomenon in the Ba-doped cobalt catalyst for ammonia synthesis. Vacuum 2019, 168, 108831. [Google Scholar] [CrossRef]
- Hwang, S.M.; Han, S.J.; Min, J.E.; Park, H.G.; Jun, K.W.; Kim, S.K. Mechanistic insights into Cu and K promoted Fe-catalyzed production of liquid hydrocarbons via CO2 hydrogenation. J. CO2 Util. 2019, 34, 522–532. [Google Scholar] [CrossRef]
- Xie, J.; Paalanen, P.P.; van Deelen, T.W.; Weckhuysen, B.M.; Louwerse, M.J.; de Jong, K.P. Promoted cobalt metal catalysts suitable for the production of lower olefins from natural gas. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Torres Galvis, H.M.; Koeken, A.C.J.; Bitter, J.H.; Davidian, T.; Ruitenbeek, M.; Dugulan, A.I.; De Jong, K.P. Effects of sodium and sulfur on catalytic performance of supported iron catalysts for the Fischer-Tropsch synthesis of lower olefins. J. Catal. 2013, 303, 22–30. [Google Scholar] [CrossRef]
- Zafari, R.; Abdouss, M.; Zamani, Y.; Tavasoli, A. An Efficient Catalyst for Light Olefins Production from CO Hydrogenation: Synergistic Effect of Zn and Ce Promoters on Performance of Co–Mn/SiO2 Catalyst. Catal. Lett. 2017, 147, 2475–2486. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Chang, Q.; Wang, L.; Lv, B.; Xu, J.; Xiang, H.; Yang, Y.; Li, Y. Enhanced Fischer-Tropsch synthesis performances of Fe/h-BN catalysts by Cu and Mn. Catal. Today 2020, 343, 91–100. [Google Scholar] [CrossRef]
- Gao, J.; Mo, X.; Chien, A.C.Y.; Torres, W.; Goodwin, J.G. CO hydrogenation on lanthana and vanadia doubly promoted Rh/SiO2 catalysts. J. Catal. 2009, 262, 119–126. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, J.; Qin, L.; Wang, J.; Zhu, Q.; Li, X.; Gong, M.; Chen, Y. Kerosene cracking over supported monolithic Pt catalysts: Effects of SrO and BaO promoters. Chin. J. Catal. 2013, 34, 1139–1147. [Google Scholar] [CrossRef]
- Rambeau, G.; Jorti, A.; Amariglio, H. Catalytic activity of a cobalt powder in NH3 synthesis in relation with the allotropic transformation of the metal. J. Catal. 1985, 94, 155–165. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Chen, P.P.; Liu, J.X.; Su, H.Y.; Li, W.X. Influence of Cobalt Crystal Structures on Activation of Nitrogen Molecule: A First-Principles Study. J. Phys. Chem. C 2019, 123, 10956–10966. [Google Scholar] [CrossRef]
- Lin, B.; Qi, Y.; Wei, K.; Lin, J. Effect of pretreatment on ceria-supported cobalt catalyst for ammonia synthesis. RSC Adv. 2014, 4, 38093–38102. [Google Scholar] [CrossRef]
- Lin, B.; Liu, Y.; Heng, L.; Ni, J.; Lin, J.; Jiang, L. Effect of ceria morphology on the catalytic activity of Co/CeO2 catalyst for ammonia synthesis. Catal. Commun. 2017, 101, 15–19. [Google Scholar] [CrossRef]
- Lee, Y.L.; Jha, A.; Jang, W.J.; Shim, J.O.; Jeon, K.W.; Na, H.S.; Kim, H.M.; Lee, D.W.; Yoo, S.Y.; Jeon, B.H.; et al. Optimization of Cobalt Loading in Co–CeO2 Catalyst for the High Temperature Water–Gas Shift Reaction. Top. Catal. 2017, 60, 721–726. [Google Scholar] [CrossRef]
- Lee, Y.L.; Jha, A.; Jang, W.J.; Shim, J.O.; Rode, C.V.; Jeon, B.H.; Bae, J.W.; Roh, H.S. Effect of alkali and alkaline earth metal on Co/CeO2 catalyst for the water-gas shift reaction of waste derived synthesis gas. Appl. Catal. A Gen. 2018, 551, 63–70. [Google Scholar] [CrossRef]
- Hansen, T.W.; Wagner, J.B.; Hansen, P.L.; Dahl, S.; Topsøe, H.; Jacobsen, C.J.H. Atomic-resolution in situ transmission electron microscopy of a promoter of a heterogeneous catalyst. Science 2001, 294, 1508–1510. [Google Scholar] [CrossRef]
- Rossetti, I.; Pernicone, N.; Forni, L. Promoters effect in Ru/C ammonia synthesis catalyst. Appl. Catal. A Gen. 2001, 208, 271–278. [Google Scholar] [CrossRef]
- Sheng Zeng, H.; Inazu, K.; Aika, K.I. The working state of the barium promoter in ammonia synthesis over an active-carbon-supported ruthenium catalyst using barium nitrate as the promoter precursor. J. Catal. 2002, 211, 33–41. [Google Scholar] [CrossRef]
- Truszkiewicz, E.; Raróg-Pilecka, W.; Schmidt-Szałowski, K.; Jodzis, S.; Wilczkowska, E.; Łomot, D.; Kaszkur, Z.; Karpiński, Z.; Kowalczyk, Z. Barium-promoted Ru/carbon catalyst for ammonia synthesis: State of the system when operating. J. Catal. 2009, 265, 181–190. [Google Scholar] [CrossRef]
- Zhong, Z.H.; Aika, K.I. The effect of hydrogen treatment of active carbon on Ru catalysts for ammonia synthesis. J. Catal. 1998, 173, 535–539. [Google Scholar] [CrossRef]
- Bielawa, H.; Hinrichsen, O.; Birkner, A.; Muhler, M. The Ammonia-Synthesis Catalyst of the Next Generation: Barium-Promoted Oxide-Supported Ruthenium. Angew. Chem. Int. Ed. Engl. 2001, 40, 1061–1063. [Google Scholar] [CrossRef]
- Freels, M.; Liaw, P.K.; Jiang, L.; Klarstrom, D.L. Advanced Structural Materials: Properties, Design Optimization, and Applications. In Advanced Structural Materials: Properties, Design Optimization, and Applications; Soboyejo, W.O., Srivatsan, T.S., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 187–224. [Google Scholar]
- Lin, S.S.Y.; Kim, D.H.; Ha, S.Y. Metallic phases of cobalt-based catalysts in ethanol steam reforming: The effect of cerium oxide. Appl. Catal. A Gen. 2009, 355, 69–77. [Google Scholar] [CrossRef]
- Ducreux, O.; Rebours, B.; Lynch, J.; Roy-Auberger, M.; Bazin, D. Microstructure of Supported Cobalt Fischer-Tropsch Catalysts. Oil Gas Sci. Technol. Rev. IFP 2009, 64, 49–62. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Cherepanova, S.V.; Tsybulya, S.V. In situ XRD investigation of Co3O4 reduction. Z. Krist. Suppl. 2009, 50, 192–198. [Google Scholar]
- Nichols, M.L.; Lafferty, R.H. Decomposition Temperatures of Some Analytical Precipitates Barium Carbonate. Ind. Eng. Chem. Anal. Ed. 1942, 14, 481–485. [Google Scholar] [CrossRef]
- Tarka, A.; Zybert, M.; Kindler, Z.; Szmurło, J.; Mierzwa, B.; Raróg-Pilecka, W. Effect of precipitating agent on the properties of cobalt catalysts promoted with cerium and barium for NH3 synthesis obtained by co-precipitation. Appl. Catal. A Gen. 2017, 532, 19–25. [Google Scholar] [CrossRef]
- Sarnecki, A.; Adamski, P.; Albrecht, A.; Komorowska, A.; Nadziejko, M.; Moszyński, D. XPS study of cobalt-ceria catalysts for ammonia synthesis—The reduction process. Vacuum 2018, 155, 434–438. [Google Scholar] [CrossRef]
- Menon, P.G.; Prasada Rao, T.S.R. Surface Enrichment in Catalysts. Catal. Rev. Sci. Eng. 1979, 20, 97–120. [Google Scholar] [CrossRef]
- Aika, K.; Hori, H.; Ozaki, A. Activation of nitrogen by alkali metal promoted transition metal I. Ammonia synthesis over ruthenium promoted by alkali metal. J. Catal. 1972, 27, 424–431. [Google Scholar] [CrossRef]
- Aika, K.; Shimazaki, K.; Hattori, Y.; Ohya, A.; Ohshima, S.; Shirota, K.; Ozaki, A. Support and promoter effect of ruthenium catalyst. I. Characterization of alkali-promoted ruthenium/alumina catalysts for ammonia synthesis. J. Catal. 1985, 92, 296–304. [Google Scholar] [CrossRef]
- Aika, K.; Ohya, A.; Ozaki, A.; Inoue, Y.; Yasumori, I. Support and promoter effect of ruthenium catalyst. II. Ruthenium/alkaline earth catalyst for activation of dinitrogen. J. Catal. 1985, 92, 305–311. [Google Scholar] [CrossRef]
- Ertl, G.; Lee, S.B.; Weiss, M. Adsorption of nitrogen on potassium promoted Fe (111) and (100) surfaces. Surf. Sci. 1982, 114, 527–545. [Google Scholar] [CrossRef]
- Strongin, D.R.; Somorjai, G.A. The effects of potassium on ammonia synthesis over iron single-crystal surfaces. J. Catal. 1988, 109, 51–60. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhang, W.Q.; Xia, C.G.; Xiong, X.M.; Mu, X.Y.; Hu, B. Low temperature ruthenium catalyst for ammonia synthesis supported on BaCeO3 nanocrystals. Catal. Commun. 2010, 11, 867–870. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, J.; Wang, R.; Wei, K. Ammonia synthesis over ruthenium catalyst supported on perovskite type BaTiO3. Catal. Commun. 2013, 32, 11–14. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, B.; Lin, J. Highly effective perovskite-type BaZrO3 supported Ru catalyst for ammonia synthesis. Appl. Catal. A Gen. 2013, 458, 130–136. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Li, J. Highly Effective Ru/BaCeO3 Catalysts on Supports with Strong Basic Sites for Ammonia Synthesis. Chem. An Asian J. 2019, 14, 2815–2821. [Google Scholar] [CrossRef]
- Ahmadi, S.; Kaghazchi, P. On the origin of high activity of hcp metals for ammonia synthesis. Phys. Chem. Chem. Phys. 2016, 18, 5291–5298. [Google Scholar] [CrossRef]
- Rambeau, G.; Amariglio, H. Ammonia synthesis on ruthenium powder from 100 to 500 °C and hydrogenation of preadsorbed nitrogen down to −70 °C. J. Catal. 1981, 72, 1–11. [Google Scholar] [CrossRef]
- Lin, B.; Wang, R.; Lin, J.; Ni, J.; Wei, K. Sm-promoted alumina supported Ru catalysts for ammonia synthesis: Effect of the preparation method and Sm promoter. Catal. Commun. 2011, 12, 553–558. [Google Scholar] [CrossRef]
- Wang, X.; Ni, J.; Lin, B.; Wang, R.; Lin, J.; Wei, K. Highly efficient Ru/MgO-CeO2 catalyst for ammonia synthesis. Catal. Commun. 2010, 12, 251–254. [Google Scholar] [CrossRef]
- León-Reina, L.; Garciá-Maté, M.; Álvarez-Pinazo, G.; Santacruz, I.; Vallcorba, O.; De La Torre, A.G.; Aranda, M.A.G. Accuracy in Rietveld quantitative phase analysis: A comparative study of strictly monochromatic Mo and Cu radiations. J. Appl. Crystallogr. 2016, 49, 722–735. [Google Scholar] [CrossRef] [PubMed]


| Catalyst Symbol | Ce Content (mmol gCo−1) | Ba Content (mmol gCo−1) | Active Phase Surface (m2 gCo−1) a | Productivity (gNH3 gCo−1 h−1) b | TOF (s−1) c |
|---|---|---|---|---|---|
| Co | - | - | - | - | 0.0004 fcc 0.0008 hcp d |
| Co/Ce | 1.0 | - | 8.8 | 0.39 | 0.023 |
| Co/Ba | - | 1.4 | 4.1 | 1.20 | 0.103 |
| Co/Ce/Ba | 1.0 | 1.4 | 9.8 | 4.54 | 0.238 |
| Catalyst Symbol | Co fcc | Co hcp | CeO2 | BaCO3 | BaCeO3 | Rwp | Rp | GOF |
|---|---|---|---|---|---|---|---|---|
| (wt%) | (%) | (%) | ||||||
| Co/Ce | 45 | 43 a | 12 | - | - | 6.29 | 4.37 | 3.25 |
| Co/Ba | 53 | - | - | 47 | - | 6.57 | 3.84 | 2.92 |
| Co/Ce/Ba | 31 | 46 | - | 15 | 8 | 6.00 | 4.63 | 1.70 |
| Catalyst Symbol | Content of | Co a | Ce b | Ba c | C d | O e |
|---|---|---|---|---|---|---|
| (at%) | ||||||
| Co/Ce | unreduced | 33 | 6 | - | 13 | 48 |
| reduced | 69 | 16 | - | - | 15 | |
| Co/Ba | unreduced | 19 | - | 5 | 9 | 67 |
| reduced | 6 | - | 31 | 9 | 54 | |
| Co/Ce/Ba | unreduced | 20 | 5 | 5 | 16 | 54 |
| reduced | 6 | 1 | 29 | 21 | 43 |
| Catalyst Symbol | Element Content (wt.%) | ||
|---|---|---|---|
| Co | Ce | Ba | |
| Co/Ce | 76.8 | 9.3 | - |
| Co/Ba | 84.1 | - | 13.7 |
| Co/Ce/Ba | 77.3 | 9.0 | 11.3 |
| Catalyst Symbol | CO2 Uptake (μmolCO2 g−1) | Basicity (μmolCO2 Surface Atom Co−1) |
|---|---|---|
| Co/Ce | 54.4 | 0.8 |
| Co/Ba | 7.9 | 1.3 |
| Co/Ce/Ba | 8.4 | 1.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarka, A.; Patkowski, W.; Zybert, M.; Ronduda, H.; Wieciński, P.; Adamski, P.; Sarnecki, A.; Moszyński, D.; Raróg-Pilecka, W. Synergistic Interaction of Cerium and Barium-New Insight into the Promotion Effect in Cobalt Systems for Ammonia Synthesis. Catalysts 2020, 10, 658. https://doi.org/10.3390/catal10060658
Tarka A, Patkowski W, Zybert M, Ronduda H, Wieciński P, Adamski P, Sarnecki A, Moszyński D, Raróg-Pilecka W. Synergistic Interaction of Cerium and Barium-New Insight into the Promotion Effect in Cobalt Systems for Ammonia Synthesis. Catalysts. 2020; 10(6):658. https://doi.org/10.3390/catal10060658
Chicago/Turabian StyleTarka, Aleksandra, Wojciech Patkowski, Magdalena Zybert, Hubert Ronduda, Piotr Wieciński, Paweł Adamski, Adam Sarnecki, Dariusz Moszyński, and Wioletta Raróg-Pilecka. 2020. "Synergistic Interaction of Cerium and Barium-New Insight into the Promotion Effect in Cobalt Systems for Ammonia Synthesis" Catalysts 10, no. 6: 658. https://doi.org/10.3390/catal10060658
APA StyleTarka, A., Patkowski, W., Zybert, M., Ronduda, H., Wieciński, P., Adamski, P., Sarnecki, A., Moszyński, D., & Raróg-Pilecka, W. (2020). Synergistic Interaction of Cerium and Barium-New Insight into the Promotion Effect in Cobalt Systems for Ammonia Synthesis. Catalysts, 10(6), 658. https://doi.org/10.3390/catal10060658








