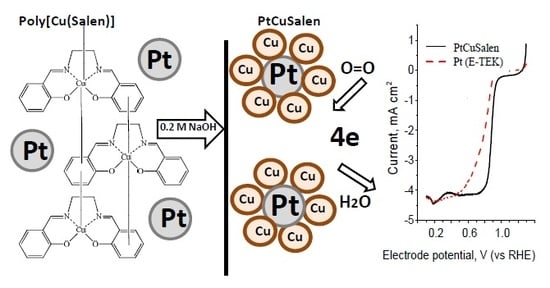
Bimetallic Cu/Pt Oxygen Reduction Reaction Catalyst for Fuel Cells Cathode Materials
Abstract
:1. Introduction
2. Results and Discussion
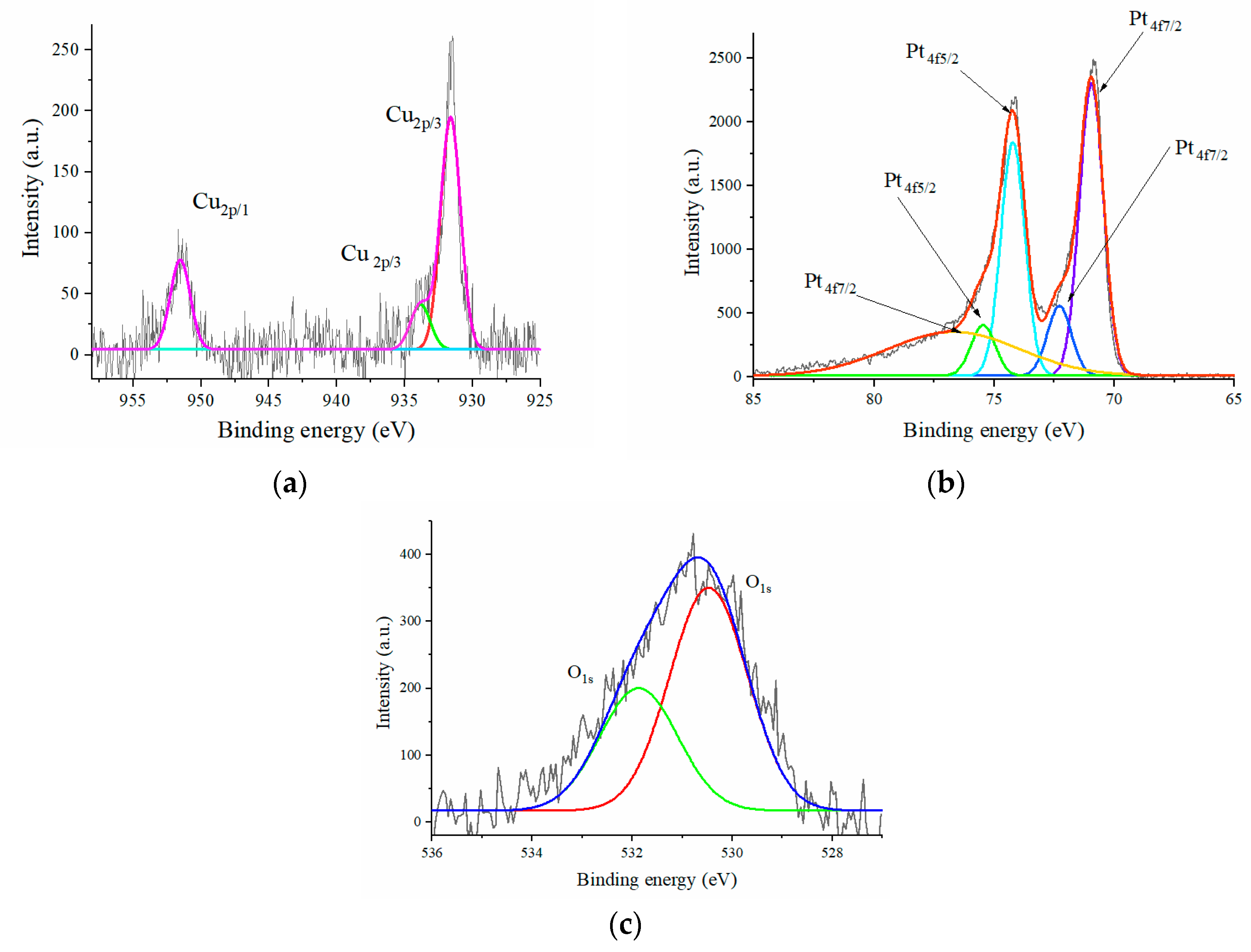
2.1. Catalyst Synthesis and Characterization
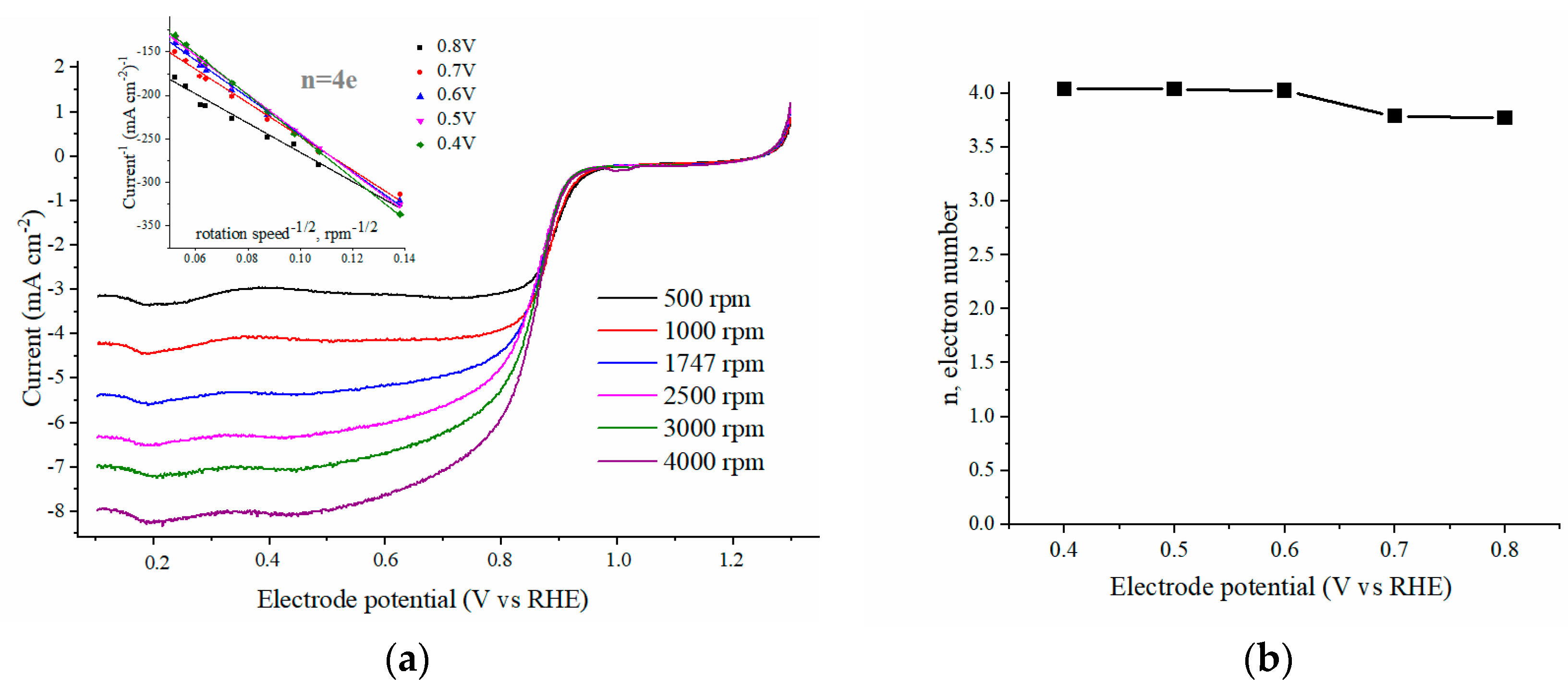
2.2. Kinetics of the ORR
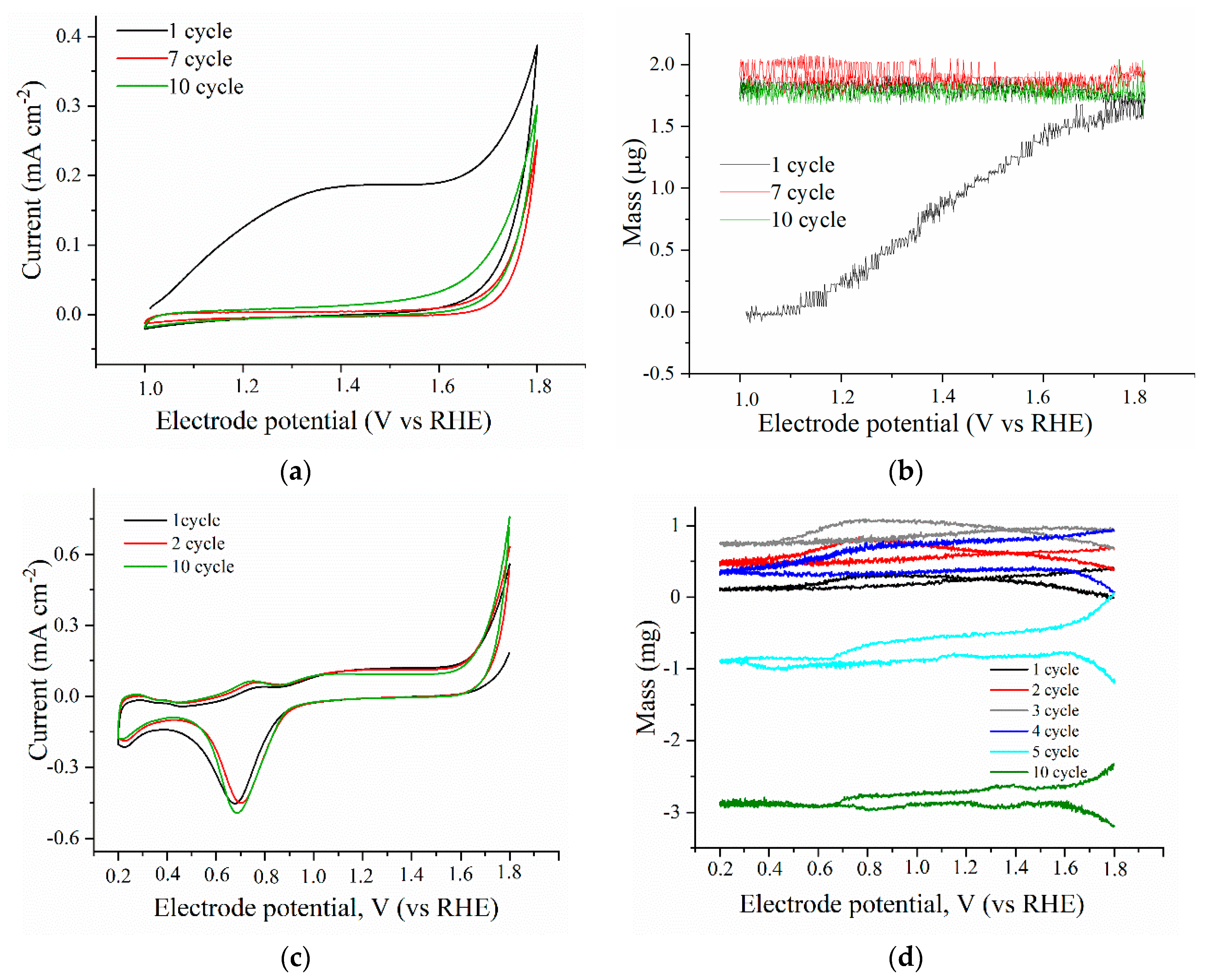
2.3. Stability of the Mixed Catalyst
2.4. Stability of the Mixed Catalyst in the Presence of Methanol in the Electrolyte
3. Materials and Methods
3.1. General Remarks
3.2. Catalyst Preparation
3.3. Electrochemical Measurements
3.3.1. Cyclic Voltammetry
3.3.2. Electrochemical Quartz Microbalance
3.4. Stability
3.4.1. Study of a Catalyst Stability
3.4.2. Study of the Tolerance of the Catalyst to the Presence of Methanol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xia, Z.; Fang, J.; Zhang, X.; Fan, L.; Barlow, A.J.; Lin, T.; Wang, S.; Wallace, G.G.; Sun, G.; Wang, X. Pt nanoparticles embedded metal-organic framework nanosheets: A synergistic strategy towards bifunctional oxygen electrocatalysis. Appl. Catal. B Environ. 2019, 245, 389–398. [Google Scholar] [CrossRef]
- Meng, Z.; Cai, S.; Wang, R.; Tang, H.; Song, S.; Tsiakaras, P. Bimetallic−organic framework-derived hierarchically porous Co-Zn-N-C as efficient catalyst for acidic oxygen reduction reaction. Appl. Catal. B Environ. 2019, 120–127. [Google Scholar] [CrossRef]
- Surya, K.; Michael, M.S.; Prabaharan, S.R.S. A review on advancement in non-noble metal based oxides as bifunctional catalysts for rechargeable non-aqueous Li/air battery. Solid State Ion. 2018, 317, 89–96. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, J.; Huang, T.; Sun, M. Recent advance on polyaniline or polypyrrole-derived electrocatalysts for oxygen reduction reaction. Polymers 2018, 10, 1397. [Google Scholar] [CrossRef] [Green Version]
- Escudero-Escribano, M.; Jensen, K.D.; Jensen, A.W. Recent advances in bimetallic electrocatalysts for oxygen reduction: Design principles, structure-function relations and active phase elucidation. Curr. Opin. Electrochem. 2018, 8, 135–146. [Google Scholar] [CrossRef]
- McNeary, W.W.; Zaccarine, S.F.; Lai, A.; Linico, A.E.; Pylypenko, S.; Weimer, A.W. Improved durability and activity of Pt/C catalysts through atomic layer deposition of tungsten nitride and subsequent thermal treatment. Appl. Catal. B Environ. 2019, 254, 587–593. [Google Scholar] [CrossRef]
- Zhuang, S.; Nunna, B.B.; Mandal, D.; Lee, E.S. A review of nitrogen-doped graphene catalysts for proton exchange membrane fuel cells-synthesis, characterization, and improvement. Nano-Struct. Nano-Objects 2018, 15, 140–152. [Google Scholar] [CrossRef]
- Jia, N.; Weng, Q.; Shi, Y.; Shi, X.; Chen, X.; Chen, P.; An, Z.; Chen, Y. N-doped carbon nanocages: Bifunctional electrocatalysts for the oxygen reduction and evolution reactions. Nano Res. 2018, 11, 1905–1916. [Google Scholar] [CrossRef]
- Jackson, A.; Strickler, A.; Higgins, D.; Jaramillo, T.F. Engineering Ru@Pt core-shell catalysts for enhanced electrochemical oxygen reduction mass activity and stability. Nanomaterials 2018, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Sha, J.; Fei, H.; Liu, M.; Yazdi, S.; Zhang, J.; Zhong, Q.; Zou, X.; Zhao, N.; Yu, H.; et al. Single-Atomic Ruthenium Catalytic Sites on Nitrogen-Doped Graphene for Oxygen Reduction Reaction in Acidic Medium. ACS Nano 2017, 11, 6930–6941. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Gao, F.; Mu, S.; Yu, S.; Zhao, Y. Investigation of oxygen reduction reaction and methanol tolerance on the carbon supported Pt-Pd catalysts. Russ. J. Electrochem. 2015, 51, 345–352. [Google Scholar] [CrossRef]
- Heider, E.A.; Jacob, T.; Kibler, L.A. Platinum overlayers on PtxRu1−x(111) electrodes: Tailoring the ORR activity by lateral strain and ligand effects. J. Electroanal. Chem. 2018, 819, 289–295. [Google Scholar] [CrossRef]
- Yang, G.; Sun, Y.; Lv, P.; Zhen, F.; Cao, X.; Chen, X.; Wang, Z.; Yuan, Z.; Kong, X. Preparation of Pt–Ru/C as an oxygen-reduction electrocatalyst in microbial fuel cells for wastewater treatment. Catalysts 2016, 6, 150. [Google Scholar] [CrossRef] [Green Version]
- Lambert, T.N.; Vigil, J.A.; White, S.E.; Davis, D.J.; Limmer, S.J.; Burton, P.D.; Coker, E.N.; Beechem, T.E.; Brumbach, M.T. Electrodeposited NixCo3-xO4 nanostructured films as bifunctional oxygen electrocatalysts. Chem. Commun. 2015, 51, 9511–9514. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [Green Version]
- Prabu, M.; Ketpang, K.; Shanmugam, S. Hierarchical nanostructured NiCo2O4 as an efficient bifunctional non-precious metal catalyst for rechargeable zinc–air batteries. Nanoscale 2014, 6, 3173–3181. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Z.; Ma, C.; Xu, F.; Giroux, M.; Chi, M.; Greeley, J.; Wang, C. Migration of cobalt species within mixed platinum-cobalt oxide bifunctional electrocatalysts in alkaline electrolytes. J. Elchem. Soc. 2019, 166, F3093–F3097. [Google Scholar] [CrossRef]
- Kepeniene, V.; Stagniunaite, R.; Tamašauskaite-Tamašiunaite, L.; Pakštas, V.; Norkus, E. PtCoNb2O5/graphene as electrocatalyst towards oxygen reduction reaction in alkaline and acidic media. Chemija 2018, 29, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Jiang, Z.; Gong, W.; Shen, P.K. Facile Fabrication of Radial PtCo Nanodendrites for Enhanced Methanol Oxidation Electrocatalysis. ACS Appl. Nano Mater. 2018, 1, 5019–5026. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Li, Y.; Zhou, H.; Duan, X.; Huang, Y. Synthesis of PtPd Bimetal Nanocrystals with Controllable Shape, Composition, and Their Tunable Catalytic Properties. Nano Lett. 2012, 12, 4265–4270. [Google Scholar] [CrossRef]
- Qu, X.; Cao, Z.; Zhang, B.; Tian, X.; Zhu, F.; Zhang, Z.; Jiang, Y.; Sun, S. One-pot synthesis of single-crystalline PtPb nanodendrites with enhanced activity for electrooxidation of formic acid. Chem. Commun. 2016, 52, 4493–4496. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, I.A.; Wadas, A.; Zoladek, S.; Skunik-Nuckowska, M.; Miecznikowski, K.; Negro, E.; Noto, V.D.; Zlotorowicz, A.; Zelenay, P.; Kulesza, P.J. Activation of reduced-graphene-oxide supported Pt nanoparticles by aligning with WO3-nanowires toward oxygen reduction in acid medium: Diagnosis with rotating-ring-disk voltammetry and double-potential-step chronocoulometry. J. Electrochem. Soc. 2018, 165, J3384–J3391. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, E.; Chen, Y.; Li, Y.; Chiu, C.-Y.; Xu, Y.; Lin, Z.; Duan, X.; Huang, Y. A Facile Strategy to Pt3Ni Nanocrystals with Highly Porous Features as an Enhanced Oxygen Reduction Reaction Catalyst. Adv. Mater. 2013, 25, 2974–2979. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.; Baik, H.; Choi, D.S.; Cheon, J.Y.; Kim, B.; Kim, H.; Kwon, S.J.; Joo, S.H.; Jung, Y.; Lee, K. Skeletal Octahedral Nanoframe with Cartesian Coordinates via Geometrically Precise Nanoscale Phase Segregation in a Pt@Ni Core–Shell Nanocrystal. ACS Nano 2015, 9, 2856–2867. [Google Scholar] [CrossRef]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Z.; Zhang, Z.; Niu, W.; Li, C.; Yang, N.; Chen, B.; Zhang, H. Two-Dimensional Metal Nanomaterials: Synthesis, Properties, and Applications. Chem. Rev. 2018, 118, 6409–6455. [Google Scholar] [CrossRef] [PubMed]
- Rameshkumar, P.; Ramaraj, R. Electrodeposited gold nanostructures at Nafion-poly (o-phenylenediamine) modified electrode and its electrocatalytic application. J. Electroanal. Chem. 2015, 741, 64–70. [Google Scholar] [CrossRef]
- Yavuz, E.; Ozdokur, K.V.; Cakar, I.; Kocak, S.; Ertas, F.N. Electrochemical Preparation, Characterization of Molybdenum-Oxide/Platinum Binary Catalysts and Its Application to Oxygen Reduction Reaction in Weakly Acidic Medium. Electrochim. Acta 2015, 151, 72–80. [Google Scholar] [CrossRef]
- Yin, X.; Chung, H.T.; Martinez, U.; Lin, L.; Artyushkova, K.; Zelenay, P. PGM-free ORR catalysts designed by templating Pani-type polymers containing functional groups with high affinity to iron. J. Electrochem. Soc. 2019, 166, F3240–F3245. [Google Scholar] [CrossRef]
- Martinez, U.; Holby, E.F.; Babu, S.K.; Artyushkova, K.; Lin, L.; Choudhury, S.; Purdy, G.M.; Zelenay, P. Experimental and theoretical trends of PGM-free electrocatalysts for the oxygen reduction reaction with different transition metals. J. Electrochem. Soc. 2019, 166, F3136–F3142. [Google Scholar] [CrossRef]
- Smirnova, E.A.; Karushev, M.P.; Timonov, A.M.; Alekseeva, E.V.; Levin, O.V.; Malev, V.V. New functional materials based on conductive polymer—Metal complexes modified with metallic nanoelectrodes. Russ. Chem. Bull. 2015, 64, 1919–1925. [Google Scholar] [CrossRef]
- Novozhilova, M.V.; Smirnova, E.A.; Karushev, M.P.; Timonov, A.M.; Malev, V.V.; Levin, O.V. Synthesis and study of catalysts of electrochemical oxygen reduction reaction based on polymer complexes of nickel and cobalt with Schiff bases. Russ. J. Electrochem. 2016, 52, 1183–1190. [Google Scholar] [CrossRef]
- Kuznetsov, N.; Yang, P.; Gorislov, G.; Zhukov, Y.; Bocharov, V.; Malev, V.; Levin, O. Electrochemical transformations of polymers formed from nickel (II) complexes with salen-type ligands in aqueous alkaline electrolytes. Electrochim. Acta 2018, 271, 190–202. [Google Scholar] [CrossRef]
- Lu, X.Y.; Du, L.; Wang, D.; Yang, P.X.; Liu, L.L.; Zhang, J.Q.; An, M.Z.; Levin, O.; Wang, J.P.; Ge, L.P. Highly Dispersed Cu-N-X Moieties Embedded in Graphene: A Promising Electrocatalyst towards the Oxygen Reduction Reaction. ChemElectroChem 2018, 5, 3323–3329. [Google Scholar] [CrossRef]
- Alekseeva, E.; Ershov, V.; Konev, A.; Levin, O. Dependence of Stability of the Polymerizesd Nickel Complexes with Schiff Bases on the Structure of the Ligand Diimine Bridge. ECS Trans. 2018, 87, 167–177. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Gasteiger, H.A.; Stäb, G.D.; Urban, P.M.; Kolb, D.M.; Behm, R.J. Characterization of high-surface-area electrocatalysts using a rotating disk electrode configuration. J. Elchem. Soc. 1998, 145, 2354–2358. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Alekseeva, E.V.; Chepurnaya, I.A.; Malev, V.V.; Timonov, A.M.; Levin, O.V. Polymeric nickel complexes with salen-type ligands for modification of supercapacitor electrodes: Impedance studies of charge transfer and storage properties. Electrochim. Acta 2017, 225, 378–391. [Google Scholar] [CrossRef]
- Shagisultanova, G.A.; Shchukarev, A.V.; Semenistaya, T.V. Possibilities of X-ray photoelectron spectroscopy in studying the structure and properties of polymers based on transition metal complexes with Schiff bases. Russ. J. Inorg. Chem. 2005, 50, 912–924. [Google Scholar]
- Gómez, L.E.; Sollier, B.M.; Lacoste, A.M.; Miró, E.E.; Boix, A.V. Hydrogen purification for fuel cells through CO preferential oxidation using PtCu/Al2O3 structured catalysts. J. Environ. Chem. Eng. 2019, 7, 103376. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Cook, M.G. X-ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]
- Fierro, J.L.G.; Palacios, J.M.; Tomas, F. An analytical SEM and XPS study of platinum–rhodium gauzes used in high pressure ammonia burners. Surf. Interface Anal. 1988, 13, 25–32. [Google Scholar] [CrossRef]
- Videla, A.H.A.M.; Esfahani, R.A.M.; Peter, I.; Specchia, S. Influence of the preparation method on Pt 3 Cu/C electrocatalysts for the oxygen reduction reaction. Electrochim. Acta 2015, 177, 51–56. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, Y. An Effective Pt-Cu/SiO2 Catalyst for the Selective Hydrogenation of Cinnamaldehyde. J. Chem. 2018, 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.D.; Willis, J.; McLellan, G.D.; Webb, G.; Keegan, M.B.T.; Moyes, R.B.; Simpson, S.; Wells, P.B.; Whyman, R. Supported Metal Catalysts: Preparation, Characterization, and Function: I. Preparation and Physical Characterization of Platinum Catalysts. J. Catal. 1993, 139, 191–206. [Google Scholar] [CrossRef]
- Fu, W.; Han, C.; Li, D.; Chen, W.; Ji, J.; Qian, G.; Yuan, W.; Duan, X.; Zhou, X. Polyoxometalates-engineered hydrogen generation rate and durability of Pt/CNT catalysts from ammonia borane. J. Energy Chem. 2020, 41, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Papa, F.; Negrila, C.; Dobrescu, G.; Miyazaki, A.; Balint, I. Preparation, characterization and catalytic behavior of Pt-Cu nanoparticles in methane combustion. J. Nat. Gas Chem. 2011, 20, 537–542. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, H.; Liu, S.; Zhang, B.; Zhong, H.; Su, D.S. Facile synthesis of supported Pt–Cu nanoparticles with surface enriched Pt as highly active cathode catalyst for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2012, 37, 17978–17983. [Google Scholar] [CrossRef]
- Geboes, B.; Mintsouli, I.; Wouters, B.; Georgieva, J.; Kakaroglou, A.; Sotiropoulos, S.; Valova, E.; Armyanov, S.; Hubin, A.; Breugelmans, T. Surface and electrochemical characterisation of a Pt-Cu/C nano-structured electrocatalyst, prepared by galvanic displacement. Appl. Catal. B Environ. 2014, 150–151, 249–256. [Google Scholar] [CrossRef]
- Chen, G.; Yang, X.; Xie, Z.; Zhao, F.; Zhou, Z.; Yuan, Q. Hollow PtCu octahedral nanoalloys: Efficient bifunctional electrocatalysts towards oxygen reduction reaction and methanol oxidation reaction by regulating near-surface composition. J. Colloid Interface Sci. 2020, 562, 244–251. [Google Scholar] [CrossRef]
- Mayrhofer, K.J.J.; Strmcnik, D.; Blizanac, B.B.; Stamenkovic, V.; Arenz, M.; Markovic, N.M. Measurement of oxygen reduction activities via the rotating disc electrode method: From Pt model surfaces to carbon-supported high surface area catalysts. Electrochim. Acta 2008, 53, 3181–3188. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Jong, Z.; Qu, W.; Geng, D.; Sun, X.; Wang, H. Nitrogen-doped carbon nanotubes with high activity for oxygen reduction in alkaline media. Int. J. Hydrog. Energy 2011, 36, 2258–2265. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, P.; Yuan, N.; Jiang, Q.; Zhang, Y.; Tang, J. Recent Advances and Prospects of Metal-Based Catalysts for Oxygen Reduction Reaction. Energy Technol. 2019, 8. [Google Scholar] [CrossRef]
- Flores-Rojas, E.; Guerrero-Gutierrez, O.X.; Solorza-Feria, O. Versatile synthesis of CuPt nanocatalysts for oxygen reduction reaction. Mater. Lett. 2019, 239, 98–101. [Google Scholar] [CrossRef]
- Illathvalappil, R.; Dhavale, V.M.; Bhange, S.N.; Kurungot, S. Nitrogen-doped graphene anchored with mixed growth patterns of CuPt alloy nanoparticles as a highly efficient and durable electrocatalyst for the oxygen reduction reaction in an alkaline medium. Nanoscale 2017, 9, 9009–9017. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, X.; Bai, C.; Chen, Y.; Wang, L.; Zheng, M.; Dong, Q.; Peng, D.L. Effect of component distribution and nanoporosity in CuPt nanotubes on electrocatalysis of the oxygen reduction reaction. ChemSusChem 2015, 8, 486–494. [Google Scholar] [CrossRef]
- Tong, J.; Li, Y.; Bo, L.; Wang, W.; Li, T.; Zhang, Q. Core-Shell Fe3O4@NCS-Mn Derived from Chitosan-Schiff Based Mn Complex with Enhanced Catalytic Activity for Oxygen Reduction Reaction. Catalysts 2019, 9, 692. [Google Scholar] [CrossRef] [Green Version]
- Audebert, P.; Sadki, S.; Miomandre, F.; Clavier, G.; Vernieres, M.C.; Saoud, M.; Hapiot, P. Synthesis of new substituted tetrazines: Electrochemical and spectroscopic properties. New J. Chem. 2004, 28, 387–392. [Google Scholar] [CrossRef]
- Qin, Y.; Chao, L.; He, J.J.; Liu, Y.; Chu, F.; Cao, J.; Kong, Y.; Tao, Y. Pt nanoparticle and Fe,N-codoped 3D graphene as synergistic electrocatalyst for oxygen reduction reaction. J. Power Sources 2016, 335, 31–37. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, Z.; Chen, B.; Wei, C.; Zhao, J.; Chen, J.; Zhang, X.; Lai, Z.; Fan, Z.; Tan, C.; et al. One-Pot Synthesis of Highly Anisotropic Five-Fold-Twinned PtCu Nanoframes Used as a Bifunctional Electrocatalyst for Oxygen Reduction and Methanol Oxidation. Adv. Mater. 2016, 28, 8712–8717. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.X.; Sun, Z.J.; Jiang, D.C.; Yang, S.F.; Du, P.W. An iron porphyrin-based conjugated network wrapped around carbon nanotubes as a noble-metal-free electrocatalyst for efficient oxygen reduction reaction. Inorg. Chem. Front. 2016, 3, 821–827. [Google Scholar] [CrossRef]
- Maillard, F.; Martin, M.; Gloaguen, F.; Léger, J.M. Oxygen electroreduction on carbon-supported platinum catalysts. Particle-size effect on the tolerance to methanol competition. Electrochim. Acta 2002, 47, 3431–3440. [Google Scholar] [CrossRef]
- Markovic, N. Surface science studies of model fuel cell electrocatalysts. Surf. Sci. Rep. 2002, 45, 117–229. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Marković, N.; Ross, P.N.; Cairns, E.J. Electro-oxidation of small organic molecules on well-characterized Pt Ru alloys. Electrochim. Acta 1994, 39, 1825–1832. [Google Scholar] [CrossRef]
- Lamy, C.; Lima, A.; LeRhun, V.; Delime, F.; Coutanceau, C.; Léger, J.-M. Recent advances in the development of direct alcohol fuel cells (DAFC). J. Power Sources 2002, 105, 283–296. [Google Scholar] [CrossRef]
- Gasteiger, H.A. Temperature-Dependent Methanol Electro-Oxidation on Well-Characterized Pt-Ru Alloys. J. Electrochem. Soc. 1994, 141, 1795. [Google Scholar] [CrossRef]
- Holm, R.H.; Everett, G.W., Jr.; Chakravorty, A. Metal Complexes of Schiff Bases and β-Ketoamines. Prog. Inorg. Chem. 1966. [Google Scholar] [CrossRef]
- Xing, W.; Yin, M.; Lv, Q.; Hu, Y.; Liu, C.; Zhang, J. 1-Oxygen Solubility, Diffusion Coefficient, and Solution Viscosity. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Xing, W., Yin, G., Zhang, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–31. [Google Scholar] [CrossRef]
- Sauerbray, G. The use of quartz oscillators for weighing thin layers and for microweighing. Z. Phys. 1959, 155, 206. [Google Scholar]


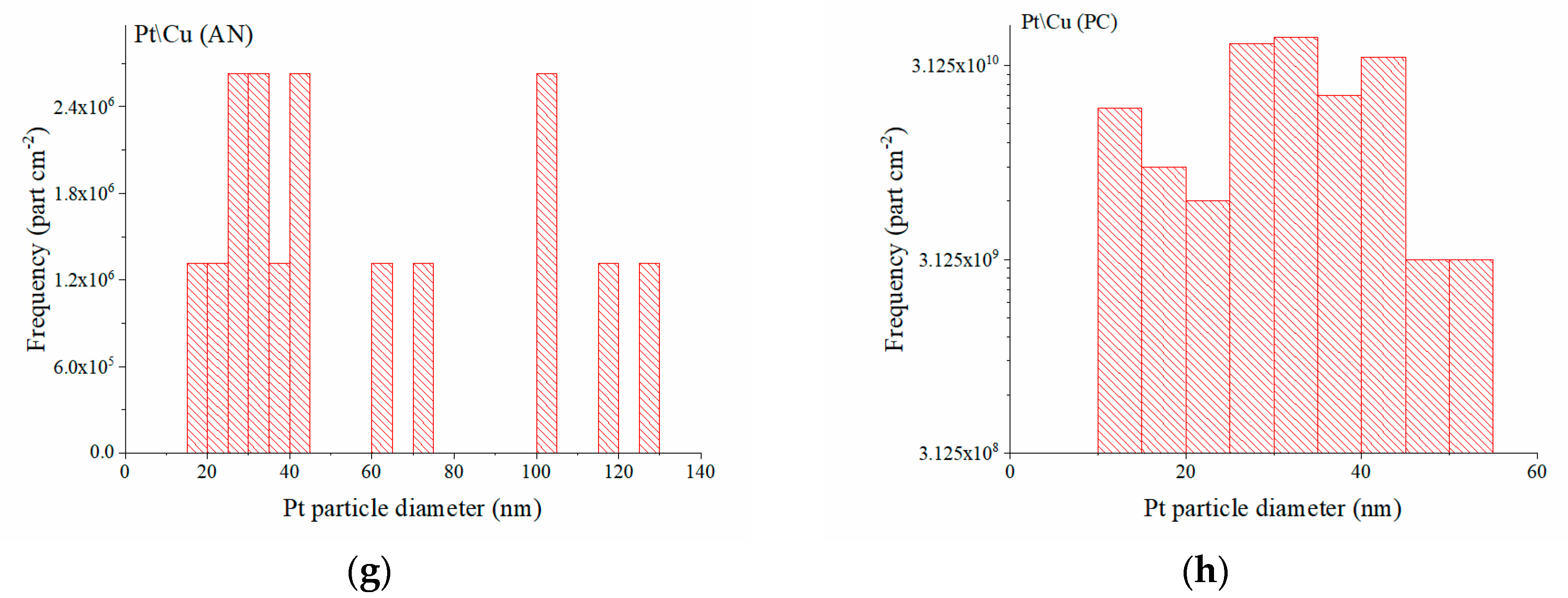



| Sample Number, Solvent | Pt Deposition Charge, Q, mC | Pt Loading, g | Pt Loading, g cm−2 | E1/2, V |
|---|---|---|---|---|
| 1, acetonitrile | 73 | 3.69 × 10−5 | 5.3 × 10−4 | 0.87 |
| 2, acetonitrile | 19 | 9.70 × 10−6 | 1.4 × 10−4 | 0.85 |
| 3, acetonitrile | 7 | 3.38 × 10−6 | 4.83 × 10−5 | 0.82 |
| 4, propylene carbonate | 1.1 | 5.6 × 10−7 | 8.0 × 10−6 | 0.89 |
| E-TEK (prepared according to [36]) | 1.97 × 10−6 | 2.8 × 10−5 | 0.78 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alekseeva, E.; Stelmashuk, T.; Danilov, S.; Yang, P.; Levin, O. Bimetallic Cu/Pt Oxygen Reduction Reaction Catalyst for Fuel Cells Cathode Materials. Catalysts 2020, 10, 667. https://doi.org/10.3390/catal10060667
Alekseeva E, Stelmashuk T, Danilov S, Yang P, Levin O. Bimetallic Cu/Pt Oxygen Reduction Reaction Catalyst for Fuel Cells Cathode Materials. Catalysts. 2020; 10(6):667. https://doi.org/10.3390/catal10060667
Chicago/Turabian StyleAlekseeva, Elena, Tatyana Stelmashuk, Stepan Danilov, Peixia Yang, and Oleg Levin. 2020. "Bimetallic Cu/Pt Oxygen Reduction Reaction Catalyst for Fuel Cells Cathode Materials" Catalysts 10, no. 6: 667. https://doi.org/10.3390/catal10060667