High Stabilization of Enzymes Immobilized on Rigid Hydrophobic Glyoxyl-Supports: Generation of Hydrophilic Environments on Support Surfaces
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison of the Physical Properties of the Different Glyoxyl-Activated Supports
2.2. Effect of the Support Surface on the Activity of the Enzymes
2.3. Effect of the Support on the Thermal Stability of the Enzymes
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Protein Production
3.2.2. Enzymatic Assays
3.2.3. Support Preparations
3.2.4. Protein Immobilization
3.2.5. Thermal Inactivation Assays
3.2.6. Estimation of the Lysine Residues Involved in the Immobilization of the PGA
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Basso, A.; Hesseler, M.; Serban, S. Hydrophobic microenvironment optimization for efficient immobilization of lipases on octadecyl functionalised resins. Tetrahedron 2016, 72, 7323–7328. [Google Scholar] [CrossRef]
- Neto, W.; Schürmann, M.; Panella, L.; Vogel, A.; Woodley, J.M. Immobilisation of ω-transaminase for industrial application: Screening and characterisation of commercial ready to use enzyme carriers. J. Mol. Catal. B Enzym. 2015, 117, 54–61. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Peirce, S.; Torrestiana-Sanchez, B.; Yates, M.; Rosales-Quintero, A.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Evaluation of different commercial hydrophobic supports for the immobilization of lipases: Tuning their stability, activity and specificity. Rsc Adv. 2016, 6, 100281–100294. [Google Scholar] [CrossRef]
- Romero-Fernández, M.; Moreno-Perez, S.; Orrego, A.H.; Martins de Oliveira, S.; Santamaría, R.I.; Díaz, M.; Guisan, J.M.; Rocha-Martin, J. Designing continuous flow reaction of xylan hydrolysis for xylooligosaccharides production in packed-bed reactors using xylanase immobilized on methacrylic polymer-based supports. Bioresour. Technol. 2018, 266, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.; Majumdar, S.; Weiss, G.A. Continuous flow biocatalysis. Chem. Soc. Rev. 2018, 47, 5891–5918. [Google Scholar] [CrossRef]
- Tamborini, L.; Fernandes, P.; Paradisi, F.; Molinari, F. Flow bioreactors as complementary tools for biocatalytic process intensification. Trends Biotechnol. 2018, 36, 73–88. [Google Scholar] [CrossRef]
- Abreu Silveira, E.; Moreno-Perez, S.; Basso, A.; Serban, S.; Pestana Mamede, R.; Tardioli, P.W.; Sanchez Farinas, C.; Rocha-Martin, J.; Fernandez-Lorente, G.; Guisan, J.M. Modulation of the regioselectivity of Thermomyces lanuginosus lipase via biocatalyst engineering for the Ethanolysis of oil in fully anhydrous medium. Bmc Biotechnol. 2017, 17, 88. [Google Scholar] [CrossRef] [Green Version]
- Abaházi, E.; Lestál, D.; Boros, Z.; Poppe, L. Tailoring the spacer arm for covalent immobilization of Candida antarctica Lipase B—Thermal stabilization by bisepoxide-activated aminoalkyl resins in continuous-flow reactors. Molecules 2016, 21, 767. [Google Scholar] [CrossRef] [Green Version]
- Abreu Silveira, E.; Moreno-Perez, S.; Basso, A.; Serban, S.; Pestana-Mamede, R.; Tardioli, P.W.; Farinas, C.S.; Castejon, N.; Fernandez-Lorente, G.; Rocha-Martin, J.; et al. Biocatalyst engineering of Thermomyces lanuginosus lipase adsorbed on hydrophobic supports: Modulation of enzyme properties for ethanolysis of oil in solvent-free systems. J. Biotechnol. 2019, 289, 126–134. [Google Scholar] [CrossRef]
- Serra, I.; Benucci, I.; Robescu, M.S.; Lombardelli, C.; Esti, M.; Calvio, C.; Pregnolato, M.; Terreni, M.; Bavaro, T. Developing a novel enzyme immobilization process by activation of epoxy carriers with glucosamine for pharmaceutical and food applications. Catalysts 2019, 9, 843. [Google Scholar] [CrossRef] [Green Version]
- Woodley, J.M. New opportunities for biocatalysis: Making pharmaceutical processes greener. Trends Biotechnol. 2008, 26, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Meyer, A.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Eş, I.; Vieira, J.D.G.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Lopez-Gallego, F.; Bolivar, J.M.; Rocha-Martin, J.; Moreno-Perez, S.; Guisán, J.M. Immobilization of proteins on highly activated glyoxyl supports: Dramatic increase of the enzyme stability via multipoint immobilization on pre-existing carriers. Curr. Org. Chem. 2015, 19, 1719–1731. [Google Scholar] [CrossRef] [Green Version]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishna, M.; Grimaldi, J.; Belfort, G.; Kumar, S.K. Stability of proteins inside a hydrophobic cavity. Langmuir 2013, 29, 8922–8928. [Google Scholar] [CrossRef]
- Richter, A.G.; Kuzmenko, I. Using in situ X-ray reflectivity to study protein adsorption on hydrophilic and hydrophobic surfaces: Benefits and limitations. Langmuir 2013, 29, 5167–5180. [Google Scholar] [CrossRef]
- Anand, G.; Sharma, S.; Dutta, A.K.; Kumar, S.K.; Belfort, G. Conformational Transitions of Adsorbed Proteins on Surfaces of Varying Polarity. Langmuir 2010, 26, 10803–10811. [Google Scholar] [CrossRef]
- Caillou, S.; Gerin, P.A.; Nonckreman, C.J.; Fleith, S.; Dupont-Gillain, C.C.; Landoulsi, J.; Pancera, S.M.; Genet, M.J.; Rouxhet, P.G. Enzymes at solid surfaces: Nature of the interfaces and physico-chemical processes. Electrochim. Acta 2008, 54, 116–122. [Google Scholar] [CrossRef]
- Moskovitz, Y.; Srebnik, S. Conformational changes of globular proteins upon adsorption on a hydrophobic surface. Phys. Chem. Chem. Phys. 2014, 16, 11698–11707. [Google Scholar] [CrossRef] [Green Version]
- Orrego, A.H.; Romero-Fernández, M.; Millán-Linares, M.; Yust, M.; Guisán, J.; Rocha-Martin, J. Stabilization of enzymes by multipoint covalent attachment on aldehyde-supports: 2-Picoline borane as an alternative reducing agent. Catalysts 2018, 8, 333. [Google Scholar] [CrossRef] [Green Version]
- Cristina, M.-R. Reacciones de química fina catalizadas por derivados estabilizados de Penicilina G Acilasa. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 1993. [Google Scholar]
- Romero, O.; Guisán, J.M.; Illanes, A.; Wilson, L. Reactivation of penicillin acylase biocatalysts: Effect of the intensity of enzyme–support attachment and enzyme load. J. Mol. Catal. B Enzym. 2012, 74, 224–229. [Google Scholar] [CrossRef]
- Pedroche, J.; del Mar Yust, M.; Mateo, C.; Fernández-Lafuente, R.; Girón-Calle, J.; Alaiz, M.; Vioque, J.; Guisán, J.M.; Millán, F. Effect of the support and experimental conditions in the intensity of the multipoint covalent attachment of proteins on glyoxyl-agarose supports: Correlation between enzyme–support linkages and thermal stability. Enzym. Microb. Technol. 2007, 40, 1160–1166. [Google Scholar] [CrossRef]
- Guisán, J.M. Aldehyde-agarose gels as activated supports for immobilization-stabilization of enzymes. Enzym. Microb. Technol. 1988, 10, 375–382. [Google Scholar] [CrossRef]
- López-Gallego, F.; Fernandez-Lorente, G.; Rocha-Martin, J.; Bolivar, J.M.; Mateo, C.; Guisan, J.M. Stabilization of enzymes by multipoint covalent immobilization on supports activated with glyoxyl groups. Methods Mol. Biol. 2013, 1051, 59–71. [Google Scholar]
- Blanco, R.M.; Calvete, J.J.; Guisán, J. Immobilization-stabilization of enzymes; variables that control the intensity of the trypsin (amine)-agarose (aldehyde) multipoint attachment. Enzym. Microb. Technol. 1989, 11, 353–359. [Google Scholar] [CrossRef]
- Orrego, A.H.; Trobo-Maseda, L.; Rocha-Martin, J.; Guisan, J.M. Immobilization-stabilization of a complex multimeric sucrose synthase from Nitrosomonas europaea. Synthesis of UDP-glucose. Enzym. Microb. Technol. 2017, 105, 51–58. [Google Scholar] [CrossRef]
- Alvaro, G.; Fernandez-Lafuente, R.; Blanco, R.M.; Guisán, J.M. Immobilization-stabilization of Penicillin G acylase from Escherichia coli. Appl. Biochem. Biotechnol. 1990, 26, 181–195. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Continuous flow reactors: A perspective. Green Chem. 2012, 14, 38–54. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; Vega, D.; Bolivar, J.M.; Hidalgo, A.; Berenguer, J.; Guisán, J.M.; López-Gallego, F. Characterization and further stabilization of a new anti-prelog specific alcohol dehydrogenase from Thermus thermophilus HB27 for asymmetric reduction of carbonyl compounds. Bioresour. Technol. 2012, 103, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Abian, O.; Grazú, V.; Hermoso, J.; González, R.; García, J.L.; Fernández-Lafuente, R.; Guisán, J.M. Stabilization of penicillin G acylase from Escherichia coli: Site-directed mutagenesis of the protein surface to increase multipoint covalent attachment. Appl. Environ. Microbiol. 2004, 70, 1249–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
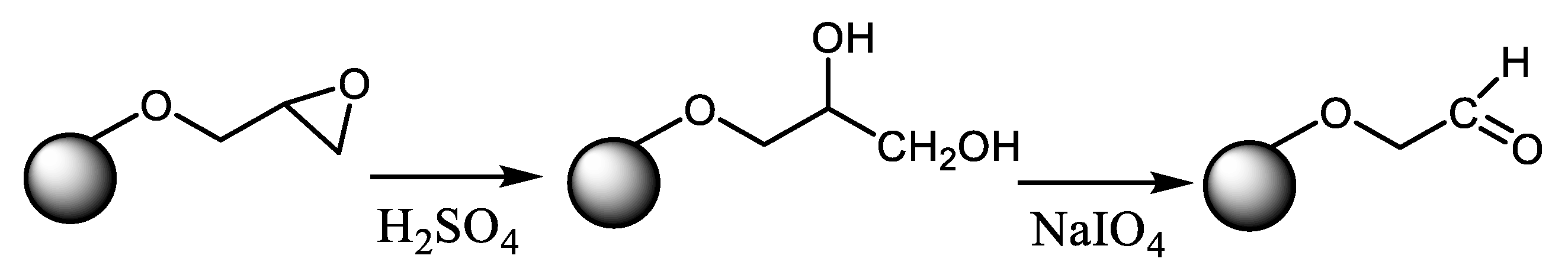
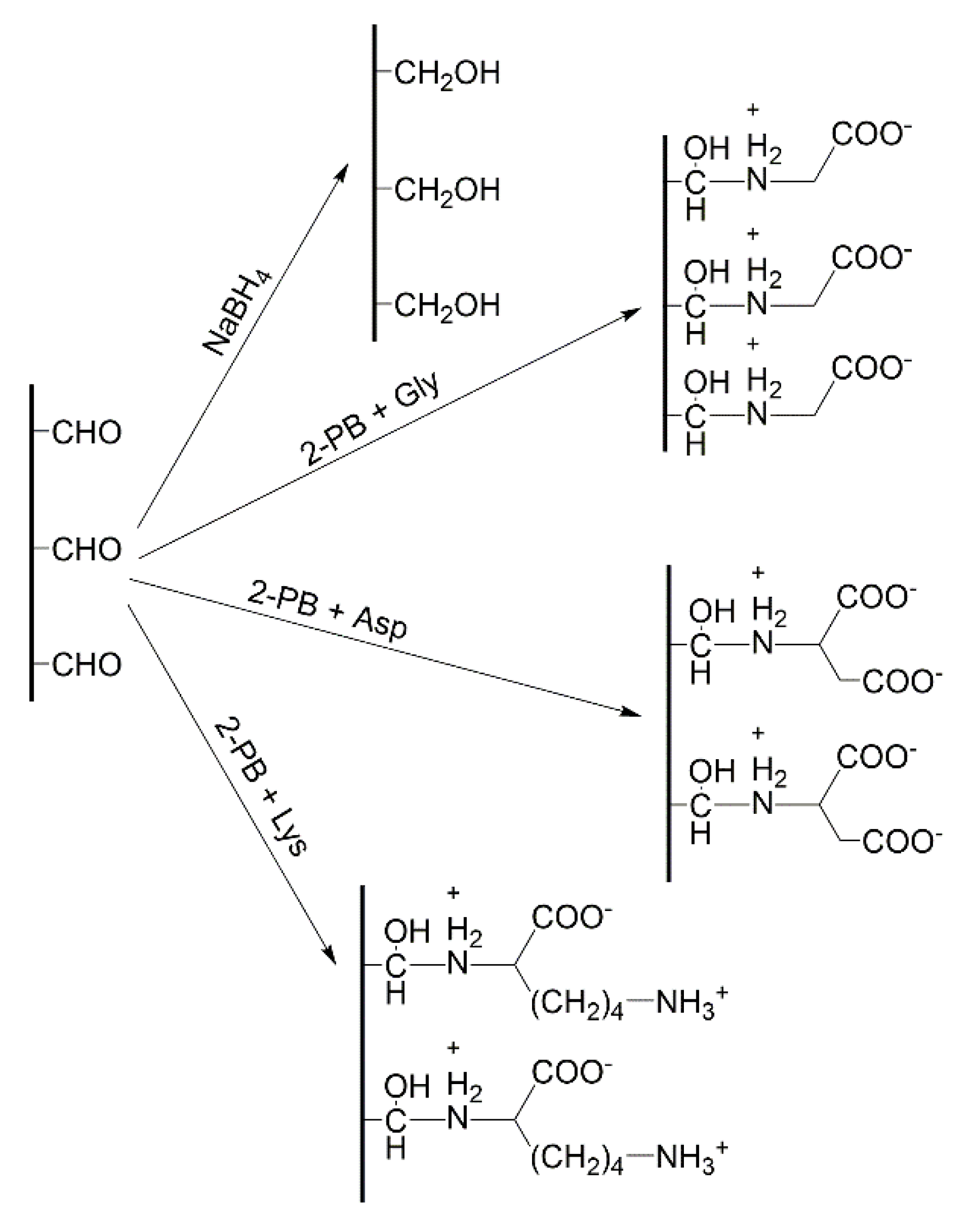
| Support | BET Area (m2/g) 1 | Aldehyde Density (Molecules of CHO/ 1000Å2) 2 | Number of Immobilized Lysine Residues 3 | Reference |
|---|---|---|---|---|
| Agarose 6BCL (AG) | 36 | 16.7 | 8 | [24] |
| Relizyme EP403/S (ReG) | 69 | 8.7 | 5 | [4] |
| Relisorb HG-400/SS (RbG) | 62 | 9.7 | 5 | This work |
| Enzyme | Preparation 1 | Immobilization Yield 2 (%) | Expressed Activity before Reducing Step 3 (%) | Expressed Activity after Reducing Step 3 (%) |
|---|---|---|---|---|
| PGA | AG-B | 100 | 40 | 40 |
| AG-G | 45 | |||
| AG-L | 43 | |||
| AG-A | 21 | |||
| ReG-B | 98 | 23 | 23 | |
| ReG-G | 24 | |||
| ReG-L | 24 | |||
| ReG-A | 10 | |||
| RbG-B | 100 | 37 | 37 | |
| RbG-G | 42 | |||
| RbG-L | 47 | |||
| RbG-A | 11 |
| Enzyme | Preparation 1 | Immobilization Yield 2 (%) | Expressed Activity before Reducing Step 3 (%) | Expressed Activity after Reducing Step 3 (%) |
|---|---|---|---|---|
| ADH2 | AG-B | 100 | 75 | 75 |
| AG-G | 79 | |||
| AG-L | 83 | |||
| AG-A | 75 | |||
| ReG-B | 97 | 21 | 20 | |
| ReG-G | 25 | |||
| ReG-L | 27 | |||
| ReG-A | 21 | |||
| RbG-B | 98 | 52 | 52 | |
| RbG-G | 52 | |||
| RbG-L | 54 | |||
| RbG-A | 50 |
| Enzyme | PGA | ADH2 | ||
|---|---|---|---|---|
| Preparation 1 | Half-Life 2 (h) at 60 °C pH 7.0 | Stabilization Factor 3 | Half-Life 2 (h) at 80 °C pH 7.0 | Stabilization Factor 3 |
| AG-B | 124 | 1.0 | 16 | 1.0 |
| AG-G | 7.4 | 0.06 | 58 | 3.6 |
| AG-L | 0.9 | 0.01 | 106 | 6.6 |
| AG-A | 29 | 0.2 | 29 | 1.8 |
| ReG-B | 1.1 | 1.0 | 4.4 | 1.0 |
| ReG-G | 1.5 | 1.3 | 22 | 5.0 |
| ReG-L | 1.5 | 1.4 | 175 | 40 |
| ReG-A | 16 | 14 | 12 | 2.8 |
| RbG-B | 0.6 | 1.0 | 6.7 | 1.0 |
| RbG-G | 0.4 | 0.7 | 7.3 | 1.1 |
| RbG-L | 0.7 | 1.2 | 36 | 5.4 |
| RbG-A | 169 | 281 | 1.8 | 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
H. Orrego, A.; Romero-Fernández, M.; Millán-Linares, M.d.C.; Pedroche, J.; Guisán, J.M.; Rocha-Martin, J. High Stabilization of Enzymes Immobilized on Rigid Hydrophobic Glyoxyl-Supports: Generation of Hydrophilic Environments on Support Surfaces. Catalysts 2020, 10, 676. https://doi.org/10.3390/catal10060676
H. Orrego A, Romero-Fernández M, Millán-Linares MdC, Pedroche J, Guisán JM, Rocha-Martin J. High Stabilization of Enzymes Immobilized on Rigid Hydrophobic Glyoxyl-Supports: Generation of Hydrophilic Environments on Support Surfaces. Catalysts. 2020; 10(6):676. https://doi.org/10.3390/catal10060676
Chicago/Turabian StyleH. Orrego, Alejandro, María Romero-Fernández, María del Carmen Millán-Linares, Justo Pedroche, José M. Guisán, and Javier Rocha-Martin. 2020. "High Stabilization of Enzymes Immobilized on Rigid Hydrophobic Glyoxyl-Supports: Generation of Hydrophilic Environments on Support Surfaces" Catalysts 10, no. 6: 676. https://doi.org/10.3390/catal10060676
APA StyleH. Orrego, A., Romero-Fernández, M., Millán-Linares, M. d. C., Pedroche, J., Guisán, J. M., & Rocha-Martin, J. (2020). High Stabilization of Enzymes Immobilized on Rigid Hydrophobic Glyoxyl-Supports: Generation of Hydrophilic Environments on Support Surfaces. Catalysts, 10(6), 676. https://doi.org/10.3390/catal10060676








