Abstract
This paper aims to revise research on carbonaceous nanomaterials used in developing sensors. In general, nanomaterials are known to be useful in developing high-performance sensors due to their unique physical and chemical properties. Thus, descriptions were made for various structural features, properties, and manner of functionalization of carbon-based nanomaterials used in electrochemical sensors. Of the commonly used technologies in manufacturing electrochemical sensors, the screen-printing technique was described, highlighting the advantages of this type of device. In addition, an analysis was performed in point of the various applications of carbon-based nanomaterial sensors to detect analytes of interest in different sample types.
1. Introduction
Electrochemical sensors have been extensively developed as a simple, fast, and accessible method of sensitive detection and quantification of a wide variety of analytes [1,2,3]. In particular, electrochemical sensors based on carbonaceous materials are often used due to their high sensitivity, low cost, good stability, and biocompatibility [4,5]. The most common carbon-based electrochemical sensors include electrodes of glassy carbon, graphite, carbon nanoparticles, carbon fibres, carbon microspheres, etc. [6,7,8,9,10]. Carbonaceous nanomaterials such as graphene, graphene oxide, fullerenes, carbon nanohorns, carbon nano-onion, diamond nanoparticles, carbon quantum dots, carbon nanofibers, carbon nanotubes, etc. have been extensively utilized in sensing applications [11].
However, nanomaterials of great importance in sensing were developed and used for developing novel sensors and biosensors with improved performance characteristics and ultrasensitivity [12]. The nanomaterials offer the advantages of high surface-volume ratio and large specific surface, characteristics that are of great importance in sensing [13]. In addition, carbonaceous nanomaterials have improved interfacial adsorption features, higher rate of electron transfer, better electrocatalytic properties, and biocompatibility compared to many materials used in classical electrochemical sensors [14,15]. Furthermore, the carbonaceous nanomaterials immobilized on electrode surface facilitate electron transfer during electrochemical reactions and these favourably interact with both electroactive ionic and covalent species [11,16].
2. Nanomaterials used in Development of Electrochemical Sensors
On 18 October 2011, the European Commission adopted the following definition of a nanomaterial: “A natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1 nm–100 nm. In specific cases and where warranted by concerns for the environment, health, safety or competitiveness the number size distribution threshold of 50% may be replaced by a threshold between 1% to 50%” [17].
The nanomaterials commonly used in electrochemical sensors are mainly carbon-based nanomaterials, metallic nanoparticles, metal oxides nanoparticles, conducting polymers, etc. and a combination of these nanomaterials [5,18,19].
Nanomaterials have unique properties that are of tremendous importance, as compared to traditional materials [20,21]. Generally speaking, the properties of nanomaterials are very different from those of the same materials when they are divided more roughly. As a rule, the properties of nanomaterials change dramatically when particle size is reduced by nanotechnology [22,23]. This is accounted for by the change in volume-surface area ratio. The bigger the area, the more possible is the physical and chemical exchange with the environment [24,25]. The morphology of nanomaterials also plays a considerable role in sensing. The shapes of nanomaterials (nanotubes, nanowires, nanorods) with multiple corners and sharp edges offer superior electrocatalytic activity [26]. Additionally, nanomaterials could acquire magnetic behaviour because of nanometric features [27].
It is well known that nanomaterials have unique electric, electronic, mechanic, and thermal properties, all important in sensing [25,28]. Surface atoms normally have more reactivity than inner atoms and are responsible for features like improving catalytic current. On the other hand, high surface means that more atoms are exposed for interactions, thus increasing sensitivity [16]. The specific morphology of the surface helps in selective detection of target analytes. These features specific to nanomaterials, especially carbonaceous ones, have, as a result, increase the sensitivity and selectivity of specific analytes, a critical point in the case of electrochemical sensors [16].
The surface area of carbonaceous nanomaterials has been studied in numerous papers. In one paper, the authors describe the sensing properties of carbon-based SPEs modified with three types of carbonaceous materials: carbon nanotubes, carbon microfibers, and graphene. The redox system selected for the electrochemical response was K4[Fe(CN)6]/K3[Fe(CN)6]. The electroactive area of SPEs was determined by cyclic voltammetry. The electroactive area of the screen-printed electrodes was much larger than the geometrical electrode surface area, CNT-SPE having the highest value, followed by CNF-SPE and GPH-SPE. The presence of roughness due to nanostructures or defects of such surfaces can lead to an electrode with a real surface area larger than the geometric one. In this paper, the roughness factors (ratio of electroactive surface to geometric surface) were 7.74 (for CNF-SPE), 9.40 (for CNT-SPE), and 7.70 (for GPH-SPE). Higher and better defined peaks were recorded by CNT-SPE due to the larger electroactive area that derived from the introduction of nanostructures as well as the excellent catalytic activity of CNTs [29].
Thangamuthu et al. used SPE-based carbon nanomaterials for bilirubin detection. The authors compared the electrochemical behavior of two screen-printed electrodes: one functionalized with MWCNT and another with graphene oxide (ER-GR-SPE). Following the SEM characterization, it was seen that the empty SPE surface was porous. After functionalization, a clear change of morphology was observed, due to the electrochemical reduction, in both cases. Cyclic voltammetric characterization in the presence of electrochemical probe (Fe3+/Fe2+) showed that graphene modified SPE exhibits higher redox currents than MWCNT-modified SPE. Er-GR-SPE has proven to have a better electrochemical response, which can be explained by improved electron transfer and larger surface area. The Laviron method has shown that the modified sensor (Er-GR-SPE) has electron transfer twice as fast as the MWCNT-SPE. The active area of the SPE was also estimated using the Randles-Sevciks equation. The calculated surface areas are as follows: bare SPE (0.069 cm2)< MWCNT-SPE (0.075 cm2) < Er-GR-SPE (0.09 cm2). The results show that the Er-GR modified SPE has a larger surface area than MWCNT. In addition, the detection limit proved to be more efficient at Er-GR-SPE than at MWCNT-SPE [30].
Nanomaterials also aid in the ready and stable immobilisation of biological receptors in the sensitive layer in order to develop biosensors [31]. Among these, carbonaceous nanomaterials have been successfully used in the development of highly sensitive biosensors, also including enzymes, nucleic acids, deoxyribonucleic acid, living cells, biological tissues, etc. [32,33,34,35]. Carbonaceous nanomaterials have been also used as labels in electrochemical immunosensors and immunoassays. The huge signal enhancement associated with the use of carbonaceous nanomaterial labels represents the basis for highly sensitive electrochemical detection of protein biomarkers or infectious agents [36,37].
Furthermore, the new functionalised nanomaterials are the key components in many chemical and biological sensors, to the purpose of improving the performance of existing devices or bringing a new perspective to analyte identification. The functionalities of nanomaterials are used in different aspects: as sensitive materials, improving the response features of transducers, and as a mobilisation matrix for biological receptors [38].
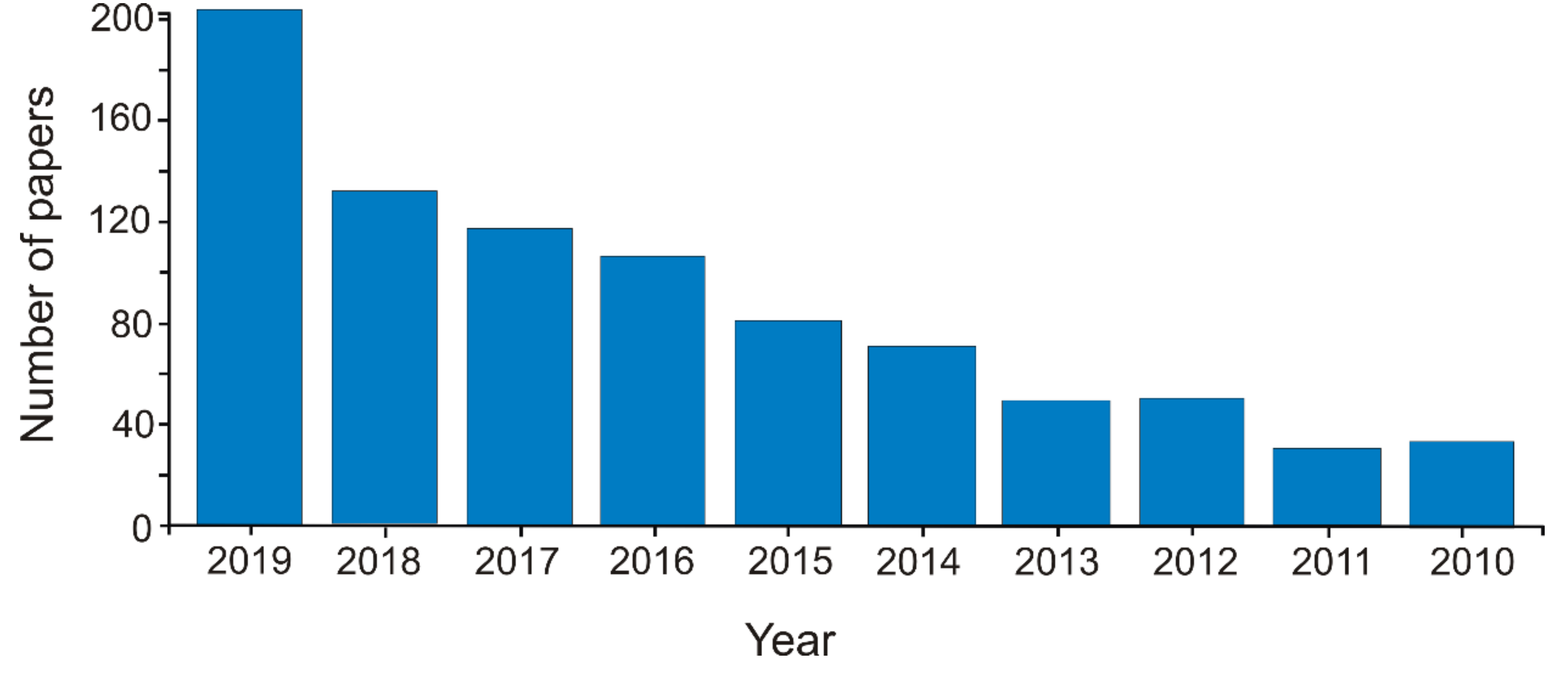
The interest in the field could be highlighted by the exponential increase in the number of papers in this research field. More than 850 papers were published in the last decade in the field of carbonaceous nanomaterials for sensors (Web of Science—Clarivate Analytics, TS = carbon AND nanomaterial AND sensor, May 6, 2020) (Figure 1).
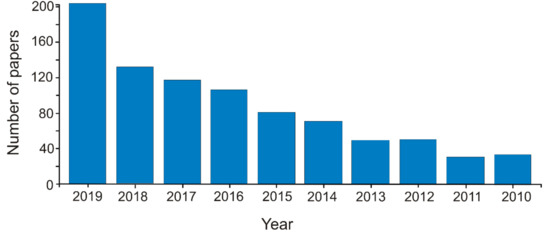
Figure 1.
The number of articles published per year in the last decade on the topic of carbonaceous nanomaterials used for developing sensors.
3. Development of Screen-Printed Based Sensors
Incorporating carbonaceous nanomaterials as a component of the sensitive layer of an electrochemical sensor is carried out by depositing them on a solid support. This process may be achieved by various techniques. One technique often used involves the dispersion of carbonaceous nanomaterials in a solvent. This suspension may be directly added to the electrode surface by casting or drop-and-dry method [39,40]. In some situations, other compounds may be added to improve the solubility of nanomaterials or/and suspension stability [41,42]. Another technique of depositing carbon-based nanomaterials is the preparation of a paste. In this case, carbon nanomaterials are combined with a binder in order to form a paste, which is then deposited [43,44].
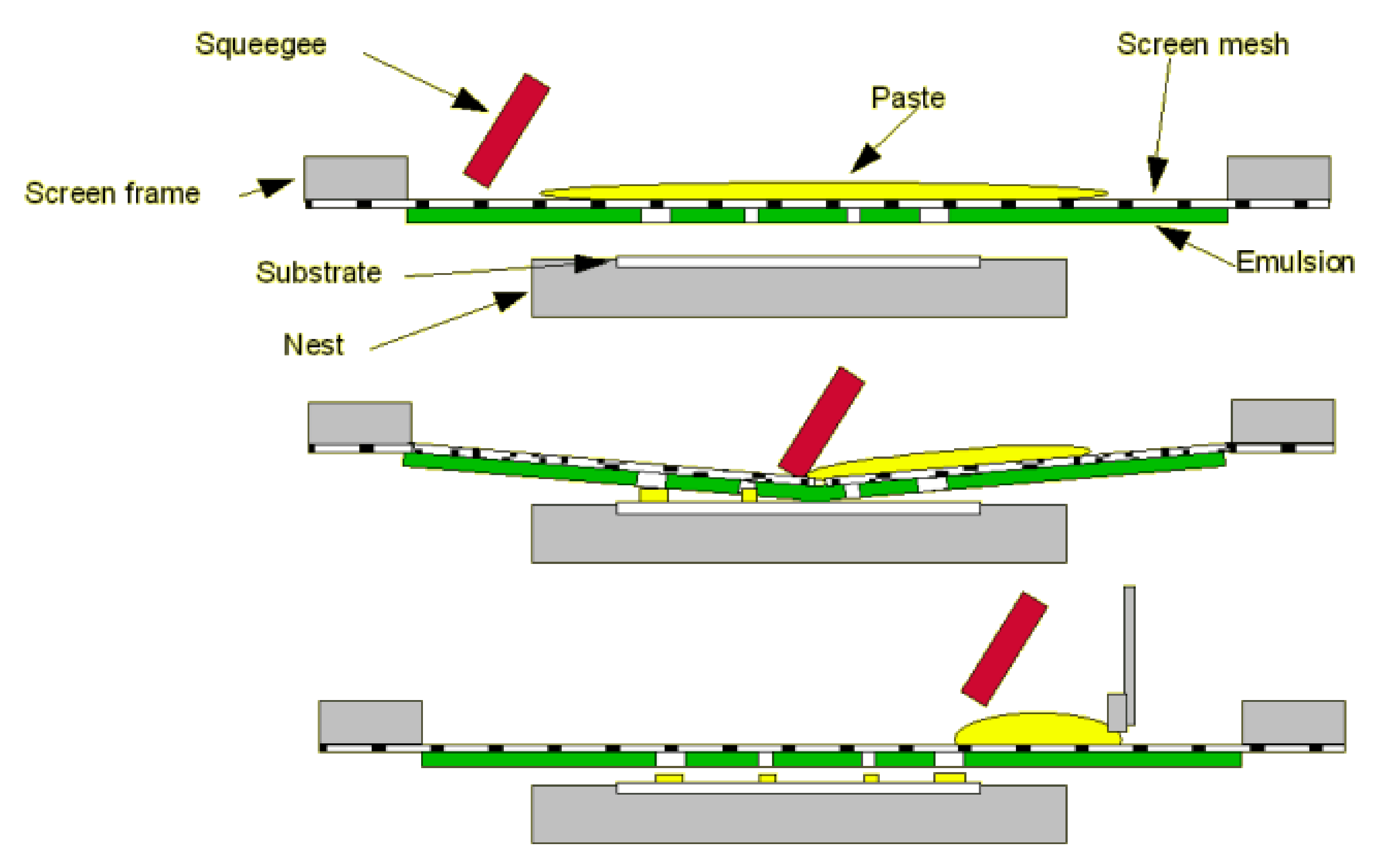
Screen-printing technology is one industrial technique successfully used to develop sensors involving carbonaceous nanomaterials; several sensor devices are already on the market [45,46,47,48]. The technique of screen-printing comprises of several main stages, such as ink manufacture, stencil formation, printing of the layer, and thermal treatment (sinterization) [41,49,50].
Figure 2 shows the complete cycle of the screen-printing process.
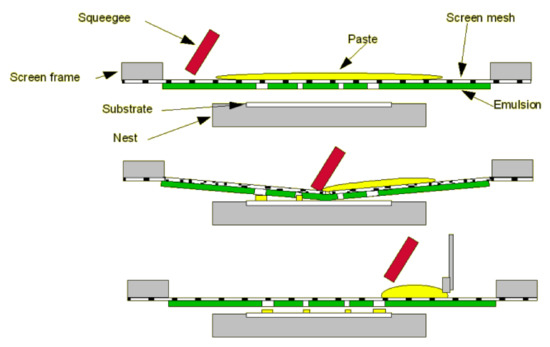
Figure 2.
Basic process of screen-printing technology. Reprinted from [51] with permission of Gwent Group, Sun Chemical Advanced Materials, Sun Chemical, United Kingdom. http://www.gwent.org/gem_screen_printing.html.
The use of screen-printed electrodes (SPE) as electrochemical sensors or transducers for the manufacture of electrochemical sensors acquired notoriety due to their characteristics and the advantages they have as compared to other analytical tools, thus allowing their use in a variety of electrochemical applications in different fields [51,52,53,54,55].
The SPEs made of carbon ink are seen as a good alternative to carbon paste electrodes or glassy carbon electrodes. The SPEs are very attractive, both as electrochemical detectors and transducers used in manufacturing electrochemical sensors [53,56,57,58,59]. That is due to certain useful properties for these applications, such as chemical inertia, use within a wide potential range, low background current due to the crystalline structure of the material, and high signal/noise ratio [60]. Furthermore, due to the small size of SPE, they are portable devices that can be easily handled and transported, allowing tests in situ, online, inline, or real-time, without the necessity to transport the sample to the lab [52,61].
The great variety of screen-printing inks allows the combination of different electrode materials in the same electrochemical device, thus leading to the manufacture of gold, platinum, carbon, silver, or silver/silver chloride on the same support. The great diversity of electrode materials is the greatest advantage of these electrodes, allowing for manufacturing of SPEs based on carbon, metals, metallic oxides, etc., as such or coated in nanostructured films, or even modified by biological compounds in a strictly controlled industrial process [49]. Similarly, the production process of SPEs may also be adapted for various types of supports, so that the nature of the material the electrodes are printed on ranges from plastic polymer materials to ceramic materials or alumina [45].
Of all the possible modifications that may be implemented on the surface of a SPE, the most important is those that lead to nanostructures, as modifications with nanomaterials [60]. Carbon nanotubes, metallic nanowires, and nanoparticles have become predominant in developing sensors, as they facilitate electron transfer reactions, decrease the sensor’s working potential, improve the rate of reaction, increase sensitivity, or in the case of biosensors, ensure extended biocomponent stability [62].
Several applications require immobilisation of biological receptors together with nanostructured compounds to develop electrochemical biosensors [63]. The inclusion of bio-nanomaterials in SPEs may occur according to various alternative strategies. Biological receptors, such as enzymes, nucleic acids, antibodies, or living cells, may be immobilized onto or on the SPE surface in different ways, such as casting, physical adsorption, or electrochemical coating [64,65,66]. Another method is the inclusion of the biological receptor into an ink, which is printed as a final layer on the electrode surface. In this way, a stable and nanostructured bio-nano-composite layer is obtained, which is useful in biosensing [67].
Through such modifications, these electrodes improve their properties and may be used in numerous applications in various fields, such as environmental measurements (to determine heavy metals), foods and agriculture (to detect certain compounds in foods or pesticide in soil), forensic (to determine toxic compounds or drugs), and clinical (to determine biomarkers for celiac disease or prostate cancer) [60,68,69,70,71].
The development of biosensors with a functionalized surface based on nanostructured metal and metal oxides is still challenging. Covalent and non-covalent interactions formed by different methods are used to functionalize nanostructured materials. The introduction of different functional groups improves the attachment of biomolecules through electrostatic interactions or specific bonds. Functionalization increases the stabilization and reduces the agglomeration of metal nanoparticles. Immobilization of biological recognition elements, such as enzymes, protein receptors, cell receptors, etc., on the surface of the transducer develops a bio-sensitive layer, which can more easily capture the target analyte [72]. Therefore, an improved detection performance is obtained.
For example, Ahn et al. reported the fabrication of solution-gated field-effect-transistor (FET) based potassium sensor using iron oxide nanoparticles (Fe2O3 NPs) modified directly grown zinc oxide nanorods (ZnO NRs). The Fe2O3 NPs modification of ZnO NRs provided stability to the nanorod surface and improved surface area for valinomycin immobilization. During sensing measurements, FET sensor showed high sensitivity in the linear range of 0.1 μM to 125 μM, low limit of detection good stability, excellent reproducibility, and favorable selectivity [73].
Another sensor modified with metal particles and biological substance is the one manufactured by Ahmad et al. Their study reported the fabrication of a solution-gated field-effect transistor (FET) sensor based on zinc oxide nanorods (ZnO NRs) modified iron oxide nanoparticles (α-Fe2O3 NPs) grown on a highly conductive sandwich-like seed layer (ZnO seed layer/Ag nanowires/ZnO seed layer). The sandwich-like seed layer and ZnO NRs modification with α-Fe2O3 NPs provide excellent conductivity and prevent possible ZnO NRs surface damage from low pH enzyme immobilization, respectively. The highly conductive solution-gated FET sensor employed calmodulin immobilization on the surface of α-Fe2O3-ZnO NRs for selective detection of calcium ions (Ca2+). The solution-gated FET sensor showed high sensitivity (416.8 µA·cm−2·mM−1) and a wide linear range (0.01–3.0 mM) [74].
Of late, screen-printed sensors have been developed, with multiple working electrodes on the same support, for the simultaneous electrochemical detection of different analytes, like anions, cations, pesticides, phenols, amines, carbohydrates, etc. [75,76,77,78].
Some of the advantages of modifying electrodes with nanomaterials are mentioned in this section. Since nanomaterials have a large superficial area, they facilitate electrochemical processes occurring on the surface of the working electrodes. The use of nanomaterials results in improved electron transfer from the electroactive molecule in the electrode. Nanomaterials may act as catalysts of the electrochemical reactions, which allows the reduction of energy necessary for the reaction to occur and favours process reversibility. Using nanomaterials allows direct contact of the analyte with the electrode surface, thus improving sensitivity [52].
Despite all these advantages, the main problem with these electrodes is the low replicability of the manufacturing process, as differences between lots may be noticed as a result of small modifications/errors during the manufacturing process [79]. At present, the process of SPE industrial manufacturing is quite controlled, but differences are still possible. However, the lack of replication in SPE manufacturing may be improved by implementing a stage of electrochemical pre-treatment of the electrode surface. As a result of the industrial scale production of these relatively cheap electrodes, they may be replaced after each analysis. These devices could be used as disposable electrodes or single use in routine or screening measurements [80,81,82,83]. It is of utmost importance when the analysis focuses on complex samples, which may foul or contaminate the electrode surface after only one electrochemical measurement [84].
4. Chemically Modified Electrodes with Carbonaceous Nanomaterials
The development of sensors using carbonaceous nanomaterials is a wide and provocative research field, and the development of screen-printed sensors based on these materials is of great interest [85,86,87,88]. Numerous carbonaceous nanomaterials, such as fullerene, carbon nanotubes, graphene, carbon nanofibers, carbon quantum dots, etc. are available or could be synthetized in order to develop specific and highly sensitive devices [89,90,91,92]. All these nanomaterials have interesting characteristics, such as high electric conductivity, mechanic resilience, chemical stability, high surface/volume ratio, etc. useful in the electrochemical detection [93]. It is possible for carbon-based nanomaterials to combine with other nanomaterials in order to create composites or nanocomposites whose properties increase synergistically. The synergy of associated nanomaterials may have a positive influence on the sensitivity and selectivity of the modified sensitive surfaces [16].
New architectures of nanomaterials have recently been introduced in order to obtain better results in electrochemical detection. This research has led to the manufacturing of more stable, more reproducible sensors in analyte detection [94,95,96,97].
In the case of carbonaceous nanomaterials, many novel complex structures have been created to increase practical applicability. A case in point is the 3D architecture of GPH. 3D graphene architecture is porous in nature and provides a slight diffusion of the electrolyte containing the analyte. 3D GPH ensures rapid electron and mass transfer. Moreover, loading the catalysts or the enzymes on the 3D GPH is easier and more stable for the manufacturing of highly selective and sensitive sensors [98].
Carbon nanohorns, GPH ribbons, and GPH quantum dots are other kinds of innovative architecture used in developing sensitive and selective electrochemical sensors. Such new materials and complex molecular architectures may improve sensitive properties and provide the possibility to build a sensor with enhanced electrochemical performance characteristics [99].
In most studies, nanomaterials actually act as the middle ground as they are applied on the surface of an electrode made of Au, Pt, glassy carbon, graphite, etc. [98,100]. Assembling nanomaterials into an electrode is a challenging endeavour. It is quite challenging to use nanomaterials, one of the main issues being particle aggregation or agglomeration. Aggregation could compromise the useful characteristics of materials, and the supplementary functionalization of materials may become difficult [101,102]. To improve the replicability of electrochemical sensors, efforts should be taken to homogenise the nanomaterial, in regard to its length and diameter. Another major challenge is integrating the nanomaterial on the electrode surface. In general, if nanoparticles have the tendency to agglomerate on the electrode surface, it will decrease its efficiency and catalytic ability [103]. Nanoparticle functionalization may help reduce aggregation and obtain better dispersion on the electrode surface. In certain cases, the nanomaterial may be used as a platform for the uniform distribution and less agglomeration of the other nanomaterials used on its surface. For instance, reduced GPH oxide may act as a useful sublayer for the distribution of nanostructured nanoparticles of metallic oxides [16].
4.1. Graphene-Modified Electrodes
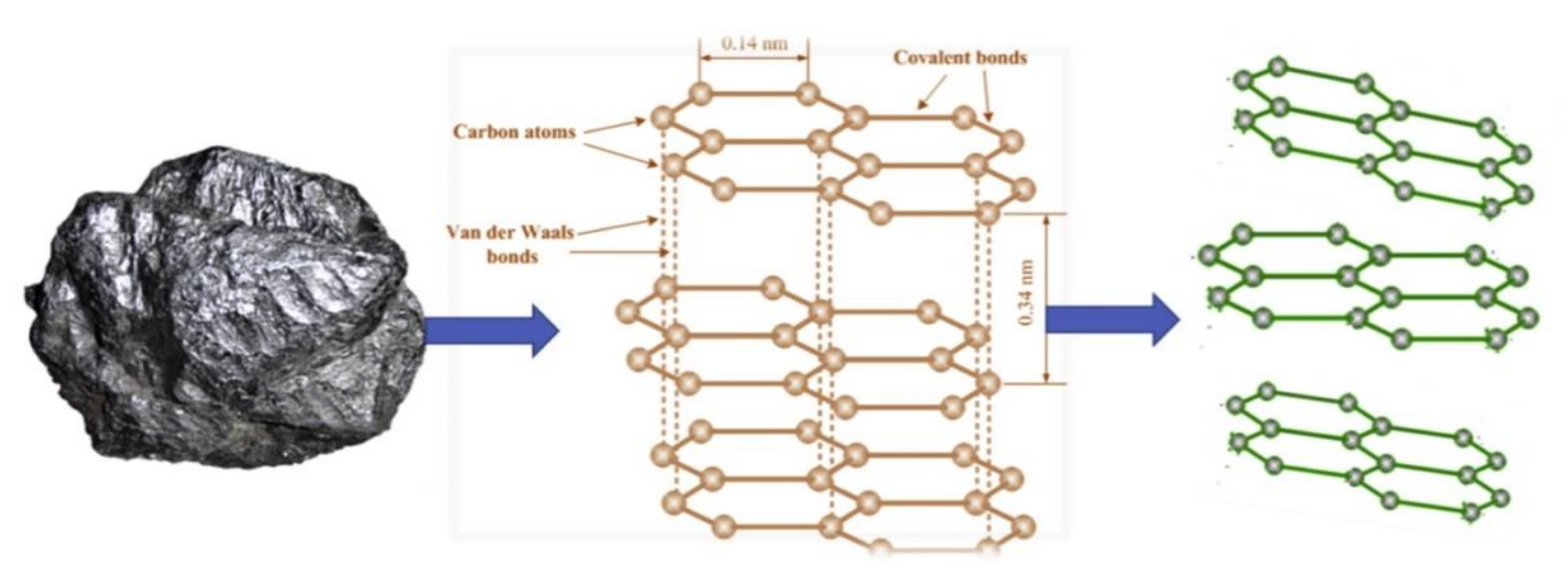
GPH is obtained by different methods such as mechanically exfoliation of graphite, treatment of graphite with oxidative agents, chemical vapour deposition, etc. [104,105,106]. The schematic transformation of graphite on GPH is presented in Figure 3.
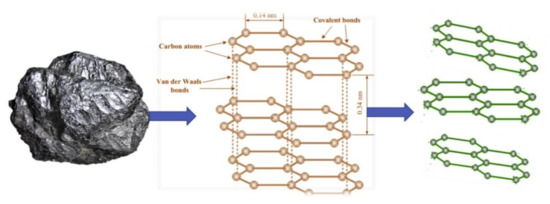
Figure 3.
Schematic transformation of graphite in graphene. Reprinted from [107] with permission of Elsevier.
GPH is an extended 2D carbon network, with a hexagonal structure resembling a honeycomb, with high sensitivity, high selectivity, good stability, low action potential, and excellent electrocatalytic activity [108]. It has numerous interesting properties: very large specific surface, optimal conductivity and transparency, mechanic resilience and excellent flexibility, good thermal and electrical conductivity, and excellent electronic properties [107,109].
The idealised GPH structure is bi-dimensional. It includes a single layer of sp2 hybrid carbon atoms linked by covalent bonds in order to form a plane hexagonal network [110]. However, in practice, the structure is complicated by the difficulty to isolate only one GPH layer in a controlled manner. Usually, this means that what is called GPH in reality includes several layers of GPH, each comprising a different number of atomic layers. Therefore, it is essential that the number of layers should be measured by atomic force microscopy, Raman spectroscopy, or low-energy electronic microscopy (LEEM) in order to accurately determine the number of GPH layers. Another complicating factor for GPH structure is the fact that it is never flat from an atomic point of view, due to its flexibility, which means that the sheets tend to undulate or fold. Large folds tend to occur as a result of processing [111].
To manufacture porous GPH-based materials, several experimental methods were used, by depositing chemical vapours, or casting GPH at very low temperatures [112]. Although the electrical properties of GPH are exceptional, and it is a very good electric conductor, it is very difficult to build three-dimensional GPH materials. However, it was found that GPH particles may form in certain conditions three-dimensional porous structures of remarkable toughness. The 3D models based on GPH have exceptional physical properties, which result from an unusual geometry and less from the material itself [113].
The study of mechanical, electronic, optical, and electrochemical properties of GPH is the most relevant to sensor applications [114,115,116].
Although the mechanical behaviour of GPH was well characterized, the connection between its porous structure and mechanical properties is not yet fully known, and experimental measurements of rigidity and resilience vary widely. Design stages may be guided by mechanical models and new calculation experiments which may improve the mechanical performance of this material. Studies mainly focus on the architecture of the material allowing GPH to form stable 3-D porous materials, implemented at various scales in order to create a set of properties like rigidity, traction, and compression resistance [113].
Many of the electronic properties tend to be very sensitive to the number of GPH layers, as this variable produces important modifications in the structure and superposition of the atomic layers [117,118].
The optical properties of GPH have been granted a lot of attention, especially since Raman spectroscopy and infrared spectroscopy may provide detailed information on its structures under the form of atomic layers. Another interesting optical property of GPH is that the first 4 or 5 layers display a practically constant absorption in the range of visible light [119].
Despite uncertainties about CNT electrochemistry, GPH electrochemistry is even less understood. This is due first and foremost to the fact that few studies have treated its electrochemical properties, and the available studies have dealt with different types of “graphene” [120], making it difficult to understand the general conclusions. In any case, there is evidence that the electrodes modified with GPH and its derivatives may have good electrochemical performance as compared to other electrodes, like glassy carbon, graphite or even carbon modified with CNTs [120]. Furthermore, GPH has significant advantages: numerous reaction sites, suitable surface, efficient biocompatibility, and low production cost, as compared to other types of carbonaceous nanomaterials [121].
Comparing the modified multilayer GPH electrodes with electrodes modified with highly oriented pyrolytic graphite, it was discovered that both electrodes has a one-electron Nernstian behaviour, when are registered by the cyclic voltammograms in [Fe(CN)6]3-/4- solution [122]. The electrodes modified with highly oriented pyrolytic graphite displayed more favourable electrochemical properties, allowing a clear differentiation of the redox peaks in mixtures of electroactive molecules.
Various GPH forms, like GO (GPH oxide), rGO (reduced GPH oxide), GNRs (GPH ribbons less than 50 nm in width) etc. have been obtained by processing CNTs [123,124,125]. GPH could also be functionalized by means of various materials, including metallic or metallic oxides nanoparticles, organic compounds, polymers, and biomolecules, in order to improve the sensing properties [126]. The functionalization of GPH may be readily achieved by various methods like mechanical mixing, hybridization, co-depositing, covalent or non-covalent interaction, etc. [127,128]
In the past decades, many researchers have analysed GPH-based nanomaterials and their electroanalytical applications in order to develop biological, biomedical, or food and environment safety applications [126,129,130].
For instance, it was found that it has excellent electrochemistry for redox reactions of biomolecules [131] or drugs [132] when electrodes incorporated GPH oxide or reduced GPH oxide. In one work, Adhikari et al. developed a sensor using electrochemically reduced GPH oxide (ERGO) for acetaminophen, which proved to be sensitive enough to detect in various pharmaceutical formulations and human body fluids [133]. With very good electrical conductivity and excellent surface, ERG is suitable as a fast, sensitive electrochemical detection platform for acetaminophen. The ERGO-based sensor built in this study has a low detection limit (LOD), 2.13 nM, and a wide linearity range, 5.0 nM–800.0 µM. In addition, this sensor was considered efficient in detecting acetaminophen in the samples of human serum and pharmaceutical products [130,133].
In the same respect, Govindhan et al. designed an electrochemical sensor of β-nicotinamide adenine dinucleotide (NADH) by means of a nanocomposite, gold nanoparticles, and reduced GPH oxide (AuNPs/rGO) without using redox mediators and enzymes. The sensor based on AuNPs/rGO showed excellent electrocatalytic activity in oxidizing NADH in neutral solution, providing an appropriate system for electron transfer and increased electrical conductivity. This sensor had high sensitivity (0.916 µA/µM·cm2) and a large linearity range (50.0 nM–500.0 µM) with a low LOD of 1.13 nM (S/N = 3). Besides, the sensor was tested in detecting NADH in human urine samples, thus showing its suitability for biomedical applications [134].
Three-dimensional porous GPH (3DGN) is seen as a new material useful in immobilisation, suitable to improve electroactivity similar to enzymes in detecting various biomolecules. Tests were conducted on the structural effects of nanomaterials based on metallic oxides, like NiO, Co3O4, Fe3O4, etc. to determine the peroxidase type activity. For example, Wang et al. developed a 3DGN decorated with Fe3O4 nanoparticles for glucose detection with a low LOD of 0.8 μM [135].
A highly sensitive and selective nano-biosensor for rapid, stable, and highly reproducible detection of ascorbic acid (AA) in the presence of dopamine, uric acid, and other interferences by a three-layer sandwich arrangement of nitrogen-doped functionalized graphene (NFG), silver nanoparticles (AgNPs), and nanostructured polyaniline (PANI) nanocomposite was studied. The enhanced AA electrochemical properties of the NFG/AgNPs/PANI electrode is attributed to the superior conductivity of the NFG-PANI and excellent catalytic activity of AgNPs. The critical modification of the AgNPs-grafted NFG-PANI coated on very low-cost fluorine doped tin oxide electrode increased the charge transfer conductivity of the electrode. The nano-biosensor was used to accurately detect AA in vitamin C tablets with recovery of 98%. The sensor demonstrated a low detection limit of 8 µM with a very wide linear detection range of 10–460 µM, good reproducibility and excellent selectivity performance for AA detection [136].
Altun and collaborators present a new amperometric dopamine sensor developed on the basis of modified SPCE with rGO, polyneutral red (PNR), and AuNP. The electrochemical behaviour of dopamine on SPCE/RGO/PNR/AuNP was investigated. The prepared sensor showed a high electrocatalytic effect on the oxidation of dopamine. The limit of detection and sensitivity was 0.17 µM and 13.38 µA·mM−1, respectively. Operational stability studies have shown that the initial amperometric response of the sensor to dopamine decreased by 90.05% on day 60 [137].
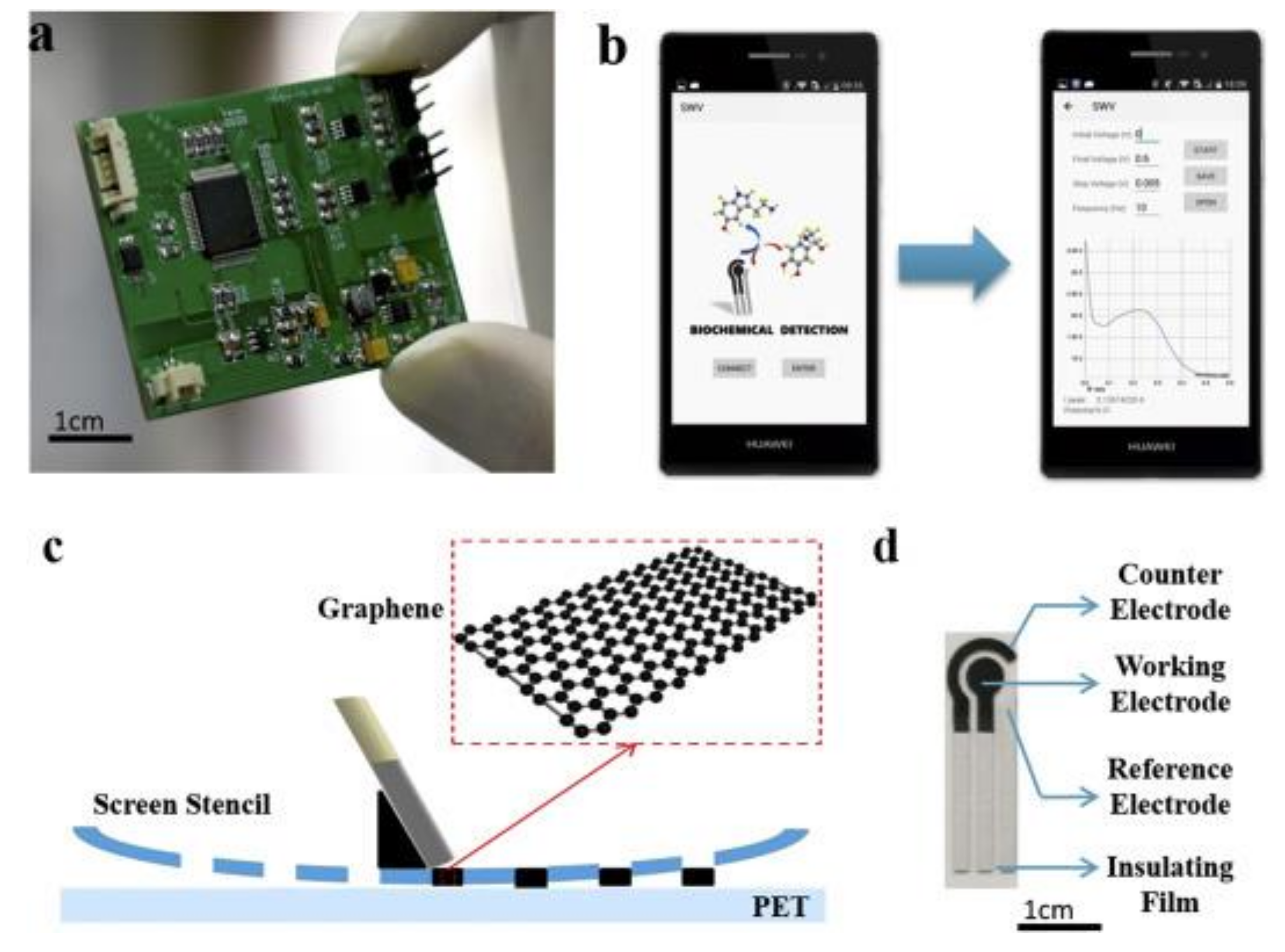
In one paper, a novel sensor based on screen-printed modified GPH electrode, controlled by a smartphone and using square wave voltammetry (SWV) as detection technique was developed [138]. The analytical system consists of an SPE sensor, a coin-sized detector, and a smartphone. Figure 4 depicts the components of the system, the software interface, and the image of SPE.
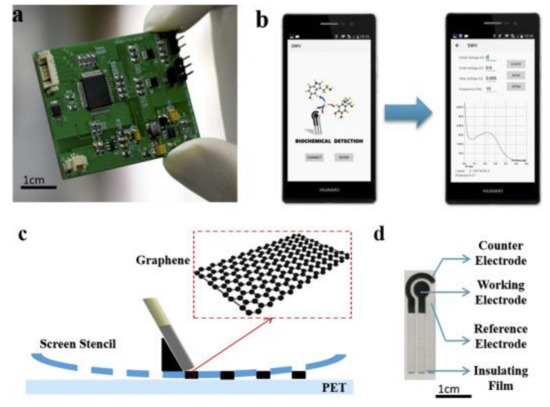
Figure 4.
The images of (a) detector; (b) software interface; (c) scheme of screen-printing process, (d) image of SPE. Reprinted from [138] with permission of Elsevier.
The novel system was successful used for norepinephrine detection, the limit of detection being 0.265 μM. With this system, the sensing of norepinephrine was selective towards the interfering species usually present in body fluids. Therefore, the system could be used in the field of point-of-care testing and screening analysis.
Several electrochemical sensors based on GPH or GPH nanocomposites modified SPE were developed for the detection of several analytes. Some examples of such sensors, analytes, detection techniques, and detection limits (LOD) are presented in Table 1.

Table 1.
Principal novel sensors based on screen-printed electrodes modified with carbonaceous nanomaterials.
4.2. Fullerene-Modified Electrodes
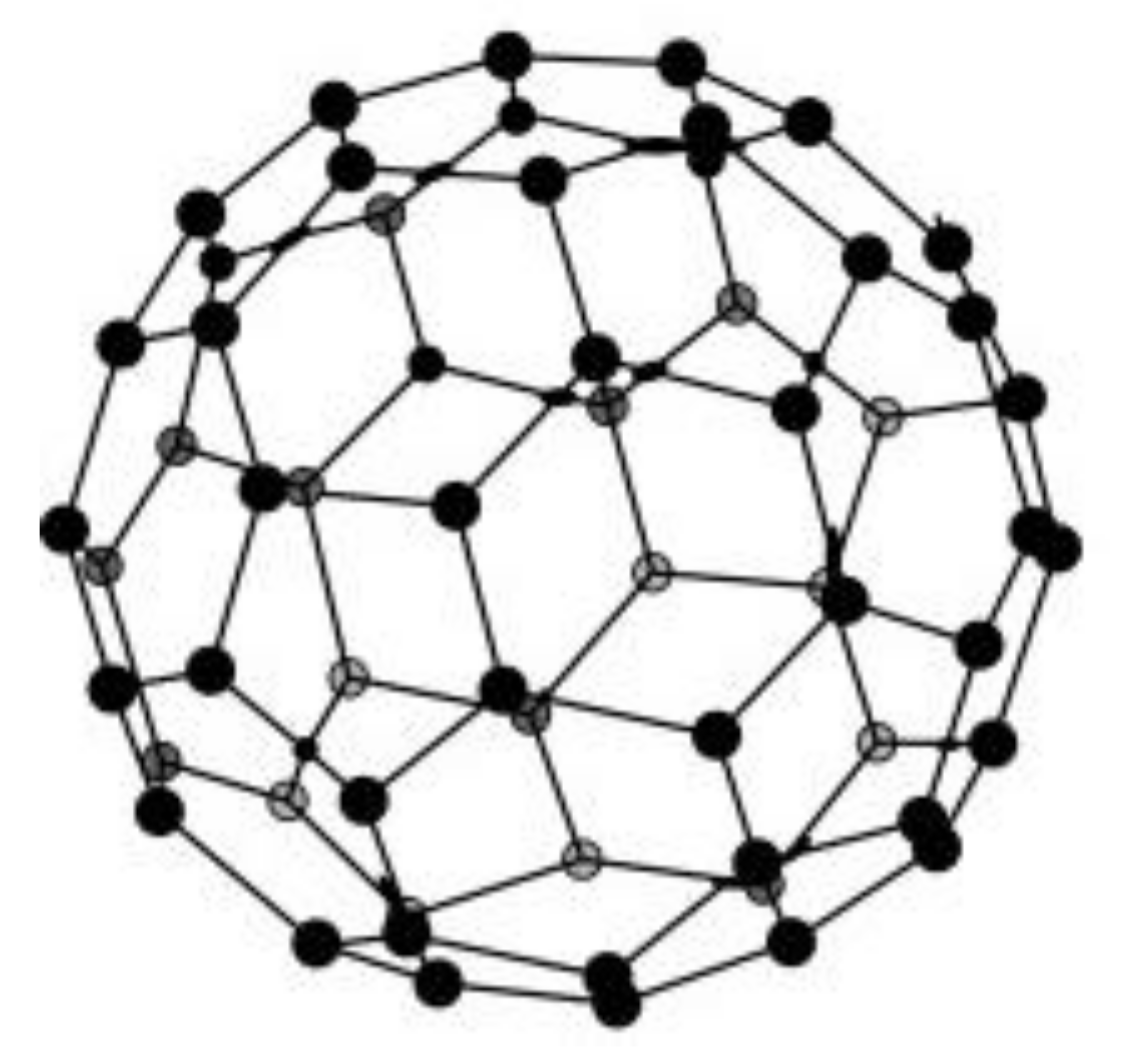
Fullerenes or C60 are a class of carbon-atom compounds that generally display spherical shapes of the geodesic dome type. From the point of view of the chemical bonds between the constituting carbons, fullerenes are structurally related to graphite [16]. This category of compounds is rightly considered, beside amorphous carbon, graphite, and diamond, a distinct allotrope form of carbon.
The main representative of this class is fullerene C60 containing 60 carbons arranged in an icosahedric structure [139]. An icosahedron consists of a polyhedron with 12 pentagonal sides and 20 hexagonal sides; its structure is very similar to a modern football, whose design was inspired by Fuller’s geodesic dome. C60 is the smallest stable fullerene (with non-adjacent pentagonal and alternatively hexagonal sides). Figure 5 presents the schematic soccer-ball structure of fullerene.
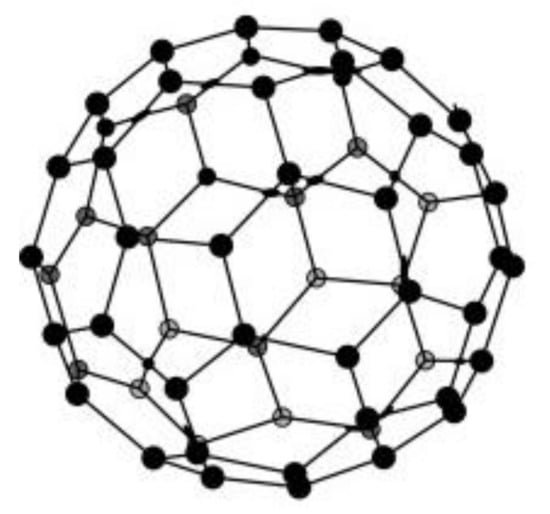
Figure 5.
Spherical structure of fullerene. Reprinted from [140] with permission of Elsevier.
Fullerene (C60) is a 0D material, 0D in whose structure there are rings of 5 and 6 sp2 hybrid carbons and is very commonly used as material for electrodes [139]. It differs from graphite, which has plane sheets of sp2 carbons disposed hexagonally on top of each other in order to create a 3D architecture. A closely related class of compounds, in fact very elongated fullerenes, are carbon nanotubes, discovered after 1991 by the Iijima [141], which will be described below.
Fullerene is electrochemically active as it has a tendency to give and receive electrons [16,139]. Fullerene-based electrochemical sensors are mainly used due to their replicable catalytic properties and their high chemical stability. The electrochemical behaviour of fullerenes may be improved by modifying their structure. For example, the weak electrochemical activity of fullerene in aqueous environment may be improved by electrochemical activation. Further, the sensitivity may be additionally improved by depositing various metallic nanoparticles on the activated fullerene. Even the hydrophilic character of the fullerene may be induced by functionalization with polar groups [142,143].
Black fullerene is a less-known product, with particle size of 40 to 50 nm. It is a fine black powder, with a 300 m2/g surface and a low density of 0.03–0.05 g/cm3. It may be synthesized by evaporating graphite in a helium atmosphere. The electric arch method is considered the most efficient in producing large amounts of fullerene. Some interesting research studies have shown that black fullerene has a good electrochemical activity for acetaminophen and guanine [16,144]. In a recent work, a sensitive SPCE electrochemical sensor based on palladium nanoparticles (PdNPs) decorated fullerene (C60) for dopamine was reported [145]. The C60/PdNPs-SPCE exhibited an improved electrochemical signal to dopamine, higher current, and lower oxidation potential comparing with the unmodified SPCE or modified only with PdNPs or C60.
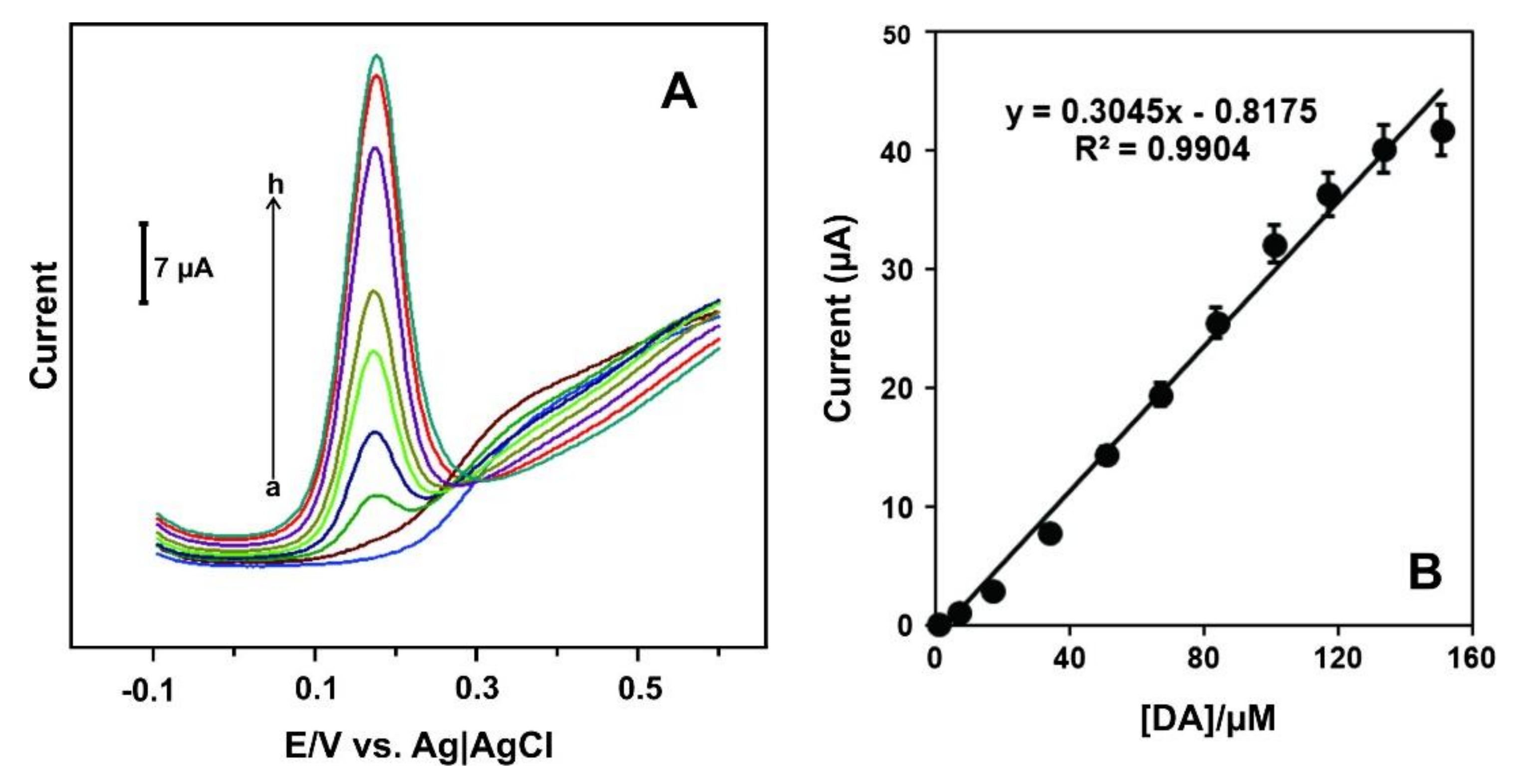
Differential pulse voltammetry (DPV) was the detection technique. Figure 6A shows the DPV curves of the C60/PdNPs-SPCE towards solutions of different concentration of DA. Figure 6B shows the linear fitting between the current and DA concentration (the calibration curve).
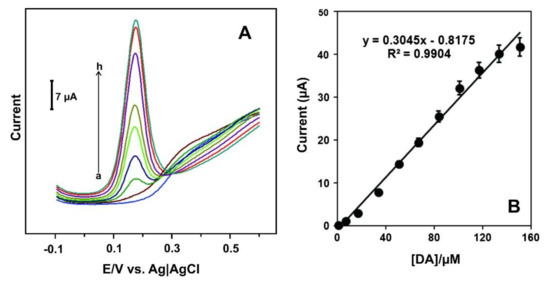
Figure 6.
(A) DPV signals of C60/PdNPs-SPCE in the absence (curve a) and presence of DA at concentrations levels of 0.35 to 150.55 μM (curves b-h); (B) The calibration curve. Reprinted from [145] with permission of Elsevier.
As can be seen, in the absence of dopamine (a), the C60/PdNPs - SPCE did not show any oxidation peak. A well-defined oxidation peak was observed in the presence of DA, the anodic current increased with increasing the dopamine concentrations (b-h) (Figure 6A). The peak current linearly increases when the concentration of dopamine increases in the range of 0.35 to 133.35 μM (Figure 6B) with LOD of 0.056 μM based on S/N = 3 criterion. The sensor shows good recovery of dopamine in injection samples and demonstrates applicability in real samples.
More examples of SPE sensors based on fullerene and their composite materials are included in Table 1.
4.3. Carbon Nanohorns-Modified Electrodes
Carbon nanohorns (CNHs) are conical structures that are usually 30–50 nm in length and 2–5 nm in diameter. The inner cavity of CNHs may be accessed through oxidation. CNHs are similar to short SWCNTs but the diameter of CNHs is considerably bigger than the one of SWCNTs [146]. These are generally arranged in a spherical structure with good porosity, and a large specific surface [147]. They have certain advantages when compared to CNTs. They are high yield products at room temperature and do not contain toxic metallic impurities, which makes them environment-friendly [146].
The oxidation process of CNHs produces an extended functionalization with polar groups with oxygen, which eases the adsorption or immobilization of biocatalysts. For example, a monolayer of cationic tensioactive was immobilized on the carboxyl functionalized CNHs, CNH-COOH, for the electrochemical detection of bisphenol A with good results [148].
If the CNHs are used together with other materials, the sensor sensitivity may improve considerably. Such an example is the sensor for cadmium (II) and lead (II) made of an SPE. The modification of the films of the SPE with functionalized SWCNHs improved sensor sensitivity to cadmium (II) and lead (II) [16,149].
Other sensor applications employed CNHs synthesized by pure graphite ablation. As an active matrix, CNHs are shaped as a horn, made up of GPH sheets, with very good electrical conductivity, and useful in sensor development [150,151,152].
One of the modification methods is electropolymerization, possessing multiple advantages, such as: simplicity, selectivity, sensitivity to analytes, strong adhesion of the polymer film to the electrode surface, ability to provide a larger surface by forming homogeneous membranes, etc. [153]. Specialized literature describes electrodes that are chemically modified by polyimidazole, polyphenylene oxide, polypyrrole, polyaniline, and polyglycine to develop electrochemical sensors [154]. Zhang et al. successfully manufactured a sensor by electrodepositing polyglycine on the carbon electrode modified by carbon nanohorns in order to simultaneously determine uric acid, dopamine, and ascorbic acid, with improved selectivity and sensitivity [155].
Nanocomposites containing CNHs are also used in sensing studies. One relevant paper describes the electrochemical detection of nitenpyram, a nicotinamide pesticide, with an electrode modified with hydroxylated multiwall carbon nanotubes/single-wall carbon nanohorns [156].
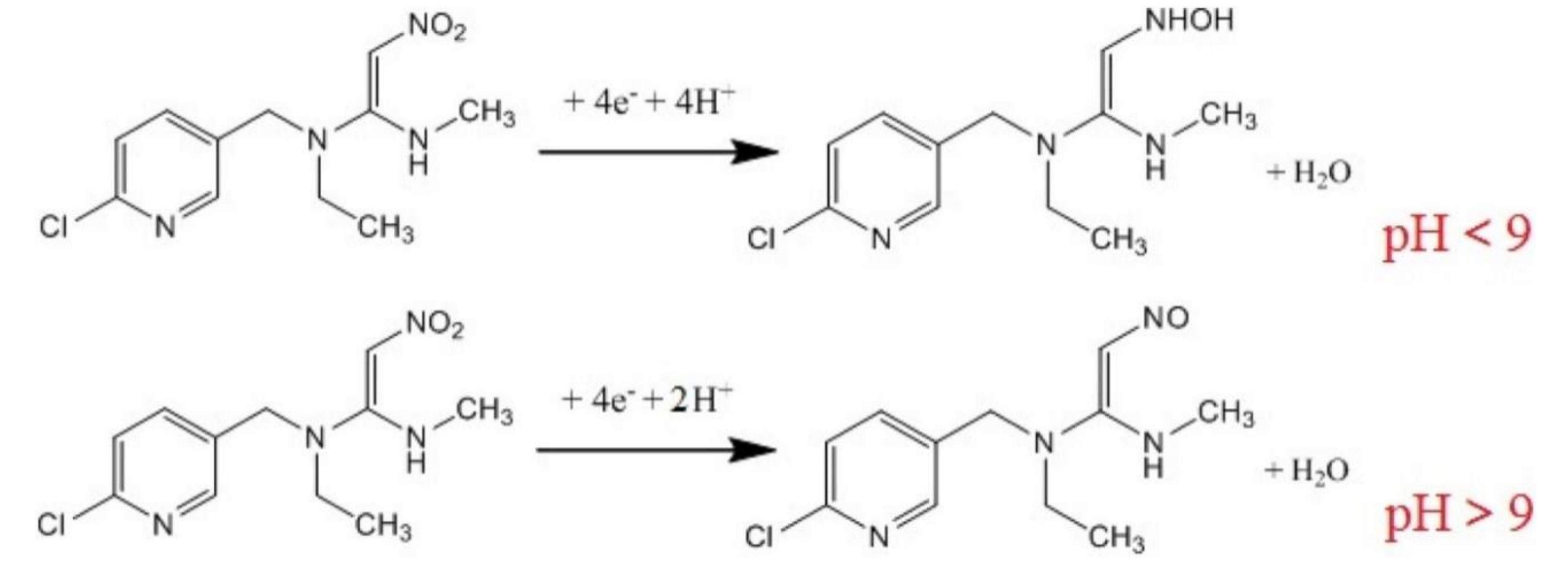
The morphology of the nanohybrid material demonstrates the existence of numerous active sites for nitenpyram, which explains the improved sensitivity of the sensor. The electrochemical behaviour of sensor was investigated by CV and DPV. A reduction peak was observed, and it depends on pH. The scheme of the electrochemical process that takes place at the electrode surface is presented in Figure 7.
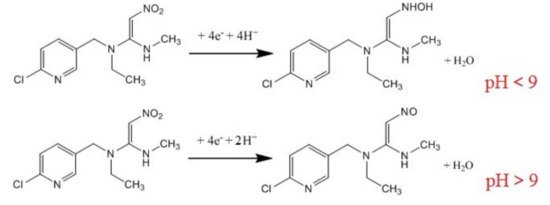
Figure 7.
The scheme of the electrochemical reduction of nitenpyram. Reprinted from [156] with permission of Elsevier.
As can be seen, when the pH was < 9, the nitro group of nitenpyram was reduced to the hydroxylamine group accepting 4H+and 4e−. When the pH was > than 9, the nitro group was reduced to the nitroso group accepting 2H+and 4e−.
The cathodic peak current decreases linearly with nitenpyram in the concentration range of 20–2000 nM, with a LOD of 4.0 nM. The sensor was effective in the detection of nitenpyram in corn samples.
Another sensor based on CNHs-modified SPE, useful for different analytes, were developed. Representative references are included in Table 1.
4.4. Carbon Nanotubes-Modified Electrodes
Carbon nanotubes (CNTs) are one-dimensional cylinder tubes of sp2 hybrid carbon atoms. Their diameter is up to tens of nanometers, and their length may be up to several centimetres [157,158].
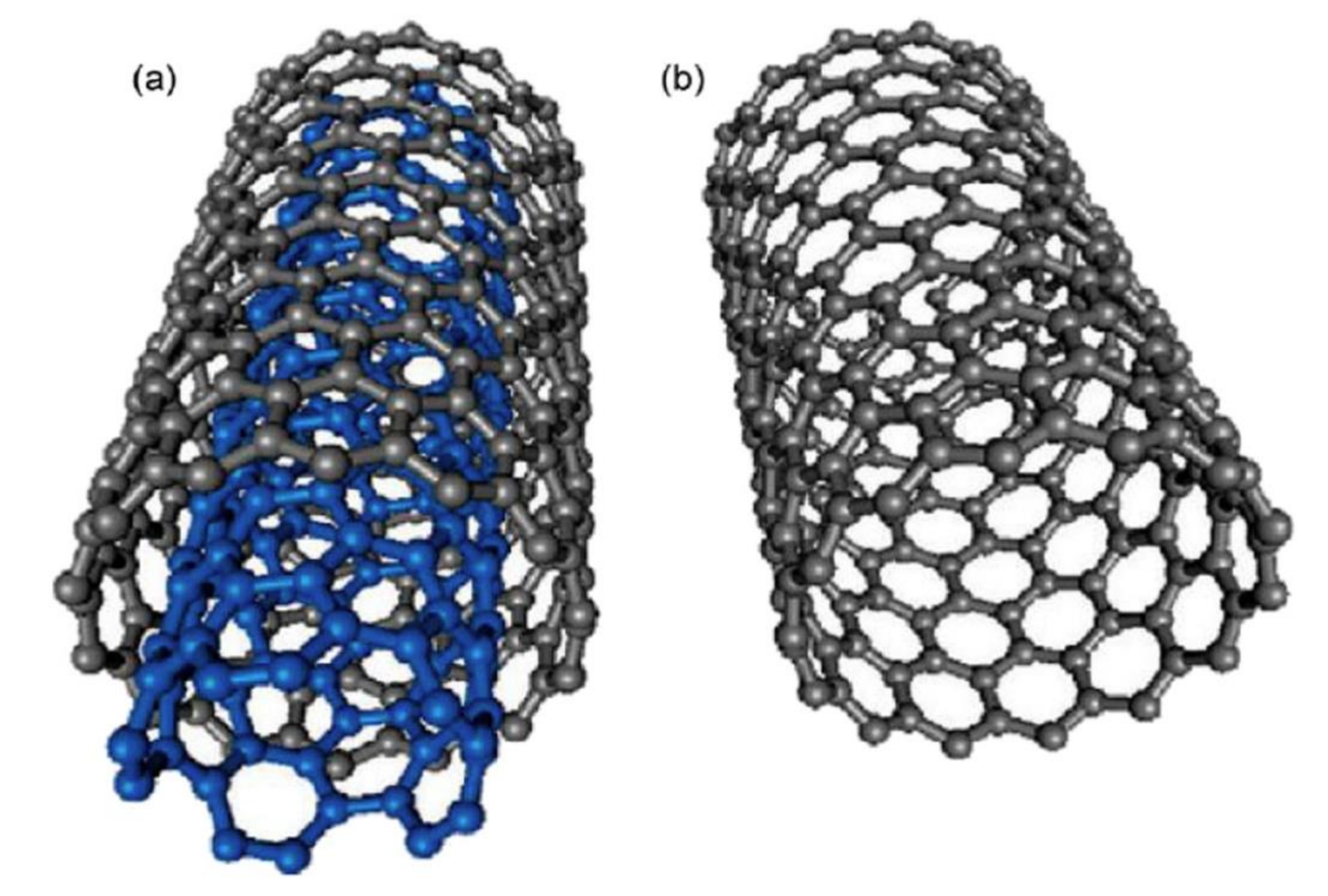
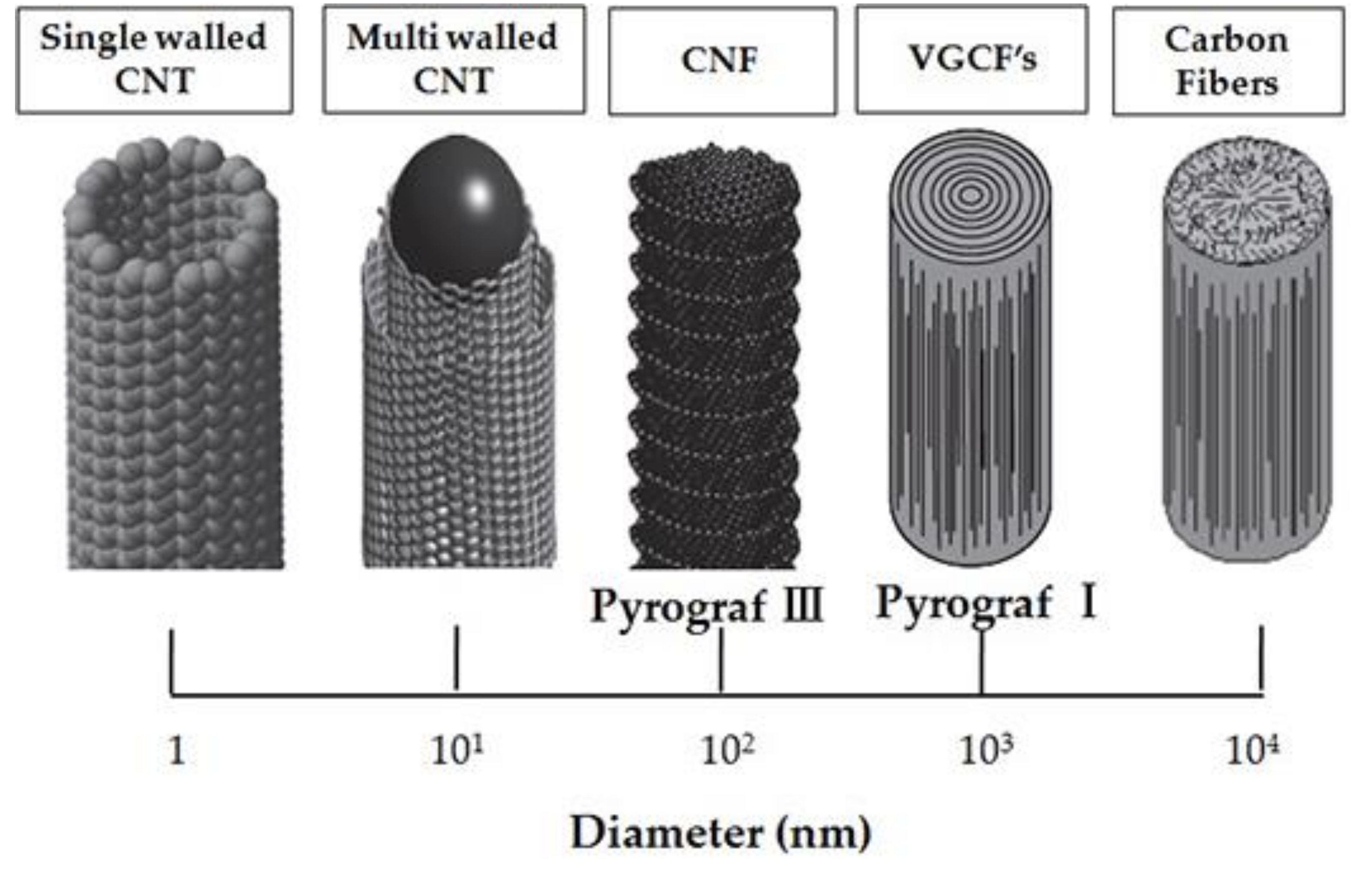
CNTs are classified according to the number of laminated GPH layers, so that there is one layer, or single walled carbon nanotubes (SWCNTs), two layers, or double walled carbon nanotubes (DWCNTs) and several layers, or multi-walled carbon nanotubes (MWCNTs) [159]. SWCNTs have a diameter of 0.4–2 nm and their length is several microns. MWCNTs are made up of coaxial tubes consisting of several laminated sheets, with possible distance between the sheets of about 0.34 nm and a diameter of 2–100 nm (Figure 8). However, these lengths and diameters vary in CNT. CNTs are considered as the biggest anisotropic materials ever produced [160]. In Figure 8, the schematic representation of SWCNTs and SWCNTs is depicted.
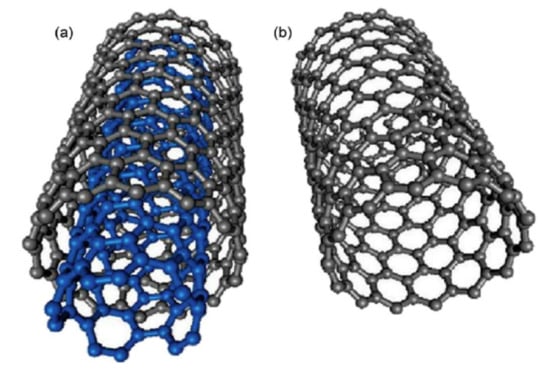
Figure 8.
Schematic representation of (a) MWCNT and (b) SWCNT structures. Reprinted from [161] with permission of Elsevier.
CNTs can be synthesized by various methods, including laser ablation, chemical vapour deposition (CVD), and arc discharge [162,163,164].
CNTs have several remarkable mechanical, thermal, electrical, and adsorption properties, which make them optimal in manufacturing electrochemical sensors and biosensors [165].
Literature shows that CNT-based electrodes have been used on a large scale in both detecting biomolecules and developing sensors by immobilizing biomolecules on the CNT surface. For instance, sensors based on CNTs have been developed for the accurate and sensitive detection of cholesterol. Alagappan et al. reported an electrochemical cholesterol biosensor based on cholesterol oxidase (ChOx) enzyme immobilized on gold nanoparticles—functionalized—multiwalled carbon nanotube (MWCNT)—polypyrrole (PPy) nanocomposite modified electrode. The sensor was fabricated by a two-step approach wherein the Au NPs-f-MWCNT was prepared by wet chemical method followed by electropolymerization of pyrrole. PPy acts as a support matrix to hold ChOx and the presence of Au-f-MWCNT increases the electrical conductivity. The biosensor had a sensitivity and detection limit of 10.12 µA·mM−1·cm−2 and 0.1 × 10−3 M, respectively [166]. Immobilization of biologic receptors on CNTs has been extensively used for the development of enzymatic sensors, biosensors, or imunosensors. The development of immunosensors employing CNTs is also often reported in the literature. One example of immunosensor based on single-walled carbon nanotube is described in the work carried out by Viet et al. In their work, a sensitive electrochemical immunosensor was developed for the detection of hCG, a pregnancy marker, that is based on a single-walled carbon nanotube (SWCNT) modified screen-printed carbon electrode. This electrochemical immunosensor is a sandwich-type immunoassay, where the gold-linked with the second antibody (Au-Mab-hCG) is used as a label. Differential pulse voltammetry was employed to measure the signal current response obtained from dissolved Au-Mab-hCG. The developed electrochemical immunosensor shows the linear range of the hCG concentration from 10 pg/mL to 1000 pg/mL, the limit of detection of 5 pg/mL with the sample volume usage as low as 2 μL [167] Furthermore, Wang et al. investigated the application of CNTs as a label for highly sensitive electrochemical immunoassays. In this study, CNTs were used to load enzyme markers by covalent interaction of their surface. A surface coverage close to 9600 enzyme molecules per 1 μm length of CNT was obtained [168]. SWCNT “forest” amperometric immunosensor devices with antibody-SWCNT bioconjugates for ultrasensitive detection of cancer biomarkers was also reported [169].
However, many times, it is impossible to completely benefit from the characteristics of the nanomaterials modifying the electrode surface. Moreover, electrodes that are usually modified by drop casting have limitations due to the uneven distribution of the nanomaterial on the electrode surface, resulting in electrode fragility [16].
To take advantage of the entire potential of CNTs as modifiers and to face the usual limitations of the drop-and-dry method, a new methodology was introduced to manufacture CNT electrodes involving the use of electrodes based on filtered CNTs [165]. To exploit the full potential of the CNTs as modifiers and cope with usual limitations of the drop casting approach, a new methodology for the CNT electrode fabrication is introduced, which involves the use of filtered CNTs-based electrodes. For instance, García-Carmona et al. [165] built the MWCNTs electrode using a Teflon filter as support for the nanomaterial. It was used to filter the homogeneous suspension of MWCNTs. The Teflon filter is a non-conductive sublayer, and the electrochemical behaviour of the electrode entirely depends on the nanomaterial.
CNTs may be functionalized properly by different methods to manufacture as improved sensor [170]. For instance, the empty cavity of CNTs aids in entrapping the host molecules [171]. However, the insolubility and agglomerating tendency of CNTs limit their use and may affect their unique properties. In such cases, chemical functionalization may be used to adjust CNT properties for the application in question. For example, a better dispersion may be achieved by CNTs’ functionalization [172].
Functionalization may be performed by chemical oxidative processes when CNTs are dispersed in water and organic solvents, by other chemical and physical modifications, or by separating metallic SWNT and semiconductive SWNT [173,174].
Functionalized CNTs may be classified as follows, in covalent bond functionalized CNTs, defect group functionalized CNTs, non-covalent functionalized CNTs, and endohedral functionalized CNTs [14,175].
In covalent functionalized CNTs, chemical functionalization occurs by forming new covalent bonds at the carbon level or functional groups of CNTs. Covalent functionalization generally occurs at one end or another, or at the level of the defects in the side wall. The desired functionalization may be generated first by the CNTs reaction with extremely reactive species, which may be readily substituted by the desired functional group. The disadvantage of the covalent functionalization is that it affects the CNTs’ conjugation system. CNTs contain defects generated by synthesis. In CNTs, about 1–3% carbon is located in defective sites [176,177,178]
The covalent functionalization of CNTs is a promising alternative to extending applications in the biomedical field. According to the placement of functionalized groups within the CNTs, there are two strategies used in the covalent functionalization for optimal interaction with biomolecules. One is functionalization of the groups found in CNT defects and the second is functionalization of side walls. Covalent-modified CNTs have proven to be the ideal host to immobilise certain molecules to manufacture selective sensors and biosensors for some analytes of importance in bioanalysis [179,180].
The noncovalent method is a strategy used to enhance CNTs’ solubility. This method allows the conjugation of CNTs with various molecules without affecting the electronic structure and its properties. This method exploits the interactions between the CNTs surface and the hydrophobic domains of the amphiphilic molecules [181,182].
Noncovalent functionalization is extremely important in ensuring CNT biocompatibility, and thus extending the applications of these nanomaterials to the biomedical field [183,184,185]. Usually this method used to modify the CNT surface involves hydrophobic or π-π interactions, and thus no alterations of the conjugated electronic structure may occur. The noncovalent method presupposes the coating of CNTs with a variety of functionalization agents, like (anionic, cationic, non-ionic) surfactants, polymers, aromatic polynuclear compounds, and various biomolecules [98].
A noncovalent functionalization of CNT with applications in the biomedical field should meet the following requirements:
- Molecules covering the CNT surface should be biocompatible and nontoxic
- The top layer of CNT should be stable enough in biological solutions characterized by a high salt concentration
- Molecules used in altering the CNT surface should have a very low critical micellar concentration (CMC)
- Molecules covering the CNT surface should contain functional groups available for bioconjugation with various biomolecules so that they to lead to bioconjugated systems with different biological applications.
These requirements were met by the noncovalent functionalization of CNT with polyethylenglycol (PEG)-treated phospholipids [186]. It is well-known that phospholipids are majority components in the cell membrane and that is why their use is approved in the biological field. Phospholipid chains attach to the CNT surface, while the hydrophilic PEG chains are oriented in the aqueous phase. By such a modification, the CNTs become biocompatible, water soluble, and stable in various biological solutions. Another noncovalent biofunctionalization method involves attaching CNT proteins in the presence of surfactants (Triton X-100, Triton X-405) or polymers [175].
The covalent and noncovalent functionalizations are called exohedric functionalizations. The endohedric functionalization of CNTs happens when atoms and small molecules are found inside the CNTs [187,188].
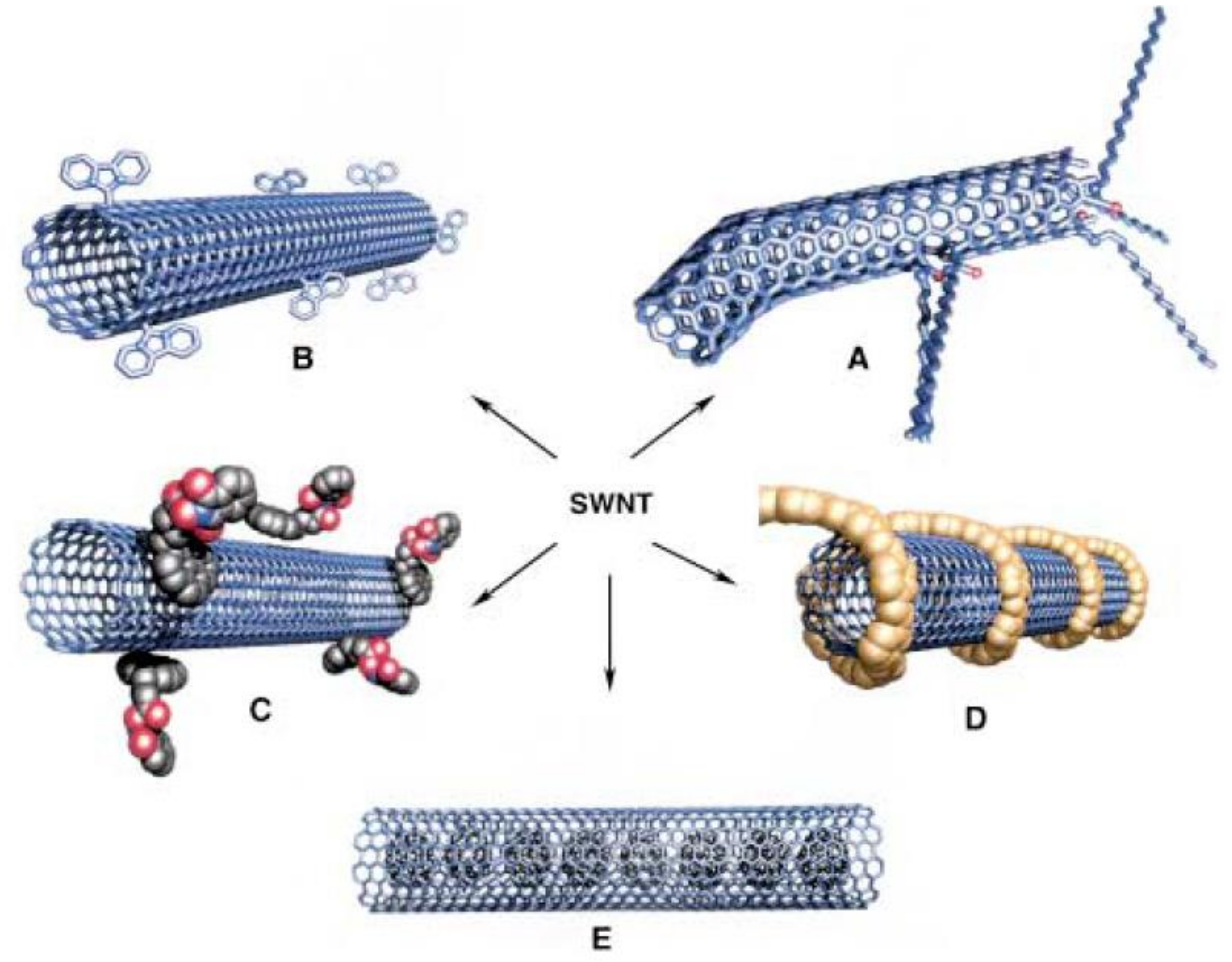
In short, there are numerous means whereby CNTs may be functionalized in order to obtain the desired characteristics. Figure 9 presents the different possibilities for functionalization of SWCNTs.
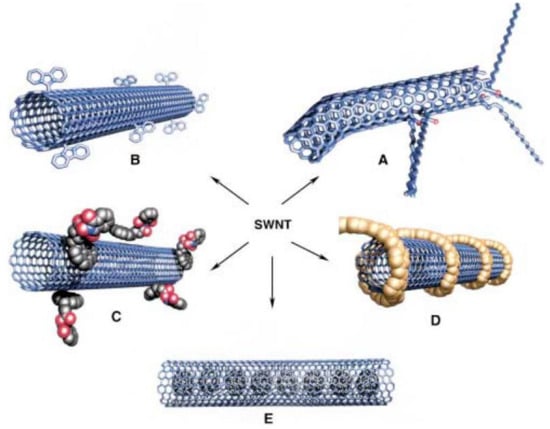
Figure 9.
Possible SWNTs functionalizations: (A) Defect group functionalization, (B) side-wall covalent functionalization, (C) exohedric noncovalent functionalization with tensioactives, (D) exohedric noncovalent functionalization with polymers, and (E) endohedric functionalization with C60. Schematic representation of (a) MWCNT and (b) SWCNT structures. Reprinted from [175] with permission of John Wiley and Sons.
For example, proteins can be immobilized by SWCNTs by covering the following steps. The first stage is the irreversible adsorption of an organic/polymeric compound (1-pirenebutanoic acid succinimidyl ester) on the hydrophobic (graphenic) surface of a CNT. In the next stage, the protein is covalently attached to the functional group (succinimidyl) in the 1-PBA by a nucleophilic substitution of the amine group existing in the protein chain [182,189,190,191].
Functionalized CNTs showed excellent electrocatalytic activity having a central role in sensor selectivity and sensitivity. For example, carboxyl-functionalized CNTs showed considerable enhancement of the electrochemical performance of sensors [192]. In another study, chitosan and cetyltrimethylammonia bromide were used to functionalize CNTs for the non-enzymatic detection of hydroximethanesulfonate. It could be mentioned the material functionalized with chitosan and cetyltrimethylammonia bromide has no catalytic activity for the transformation of hydroximethanesulfonate bromide, therefore showing excellent selectivity [193].
Another interesting feature is that CNTs may be functionalized with other nanomaterials to improve their sensing properties, especially electrocatalytic activity. Numerous sensors have recently been developed based on CNTs functionalized with fullerene. Fullerene is an excellent electron acceptor, and its dispersion on the CNTs facilitates fast electron transfer [194]. Fullerene-functionalized CNTs improved the electrochemical behaviour and sensitivity of the sensor [195]. The behaviour of fullerene-functionalized CNT proved superior in detecting analytes as compared to MWCNTs [16].
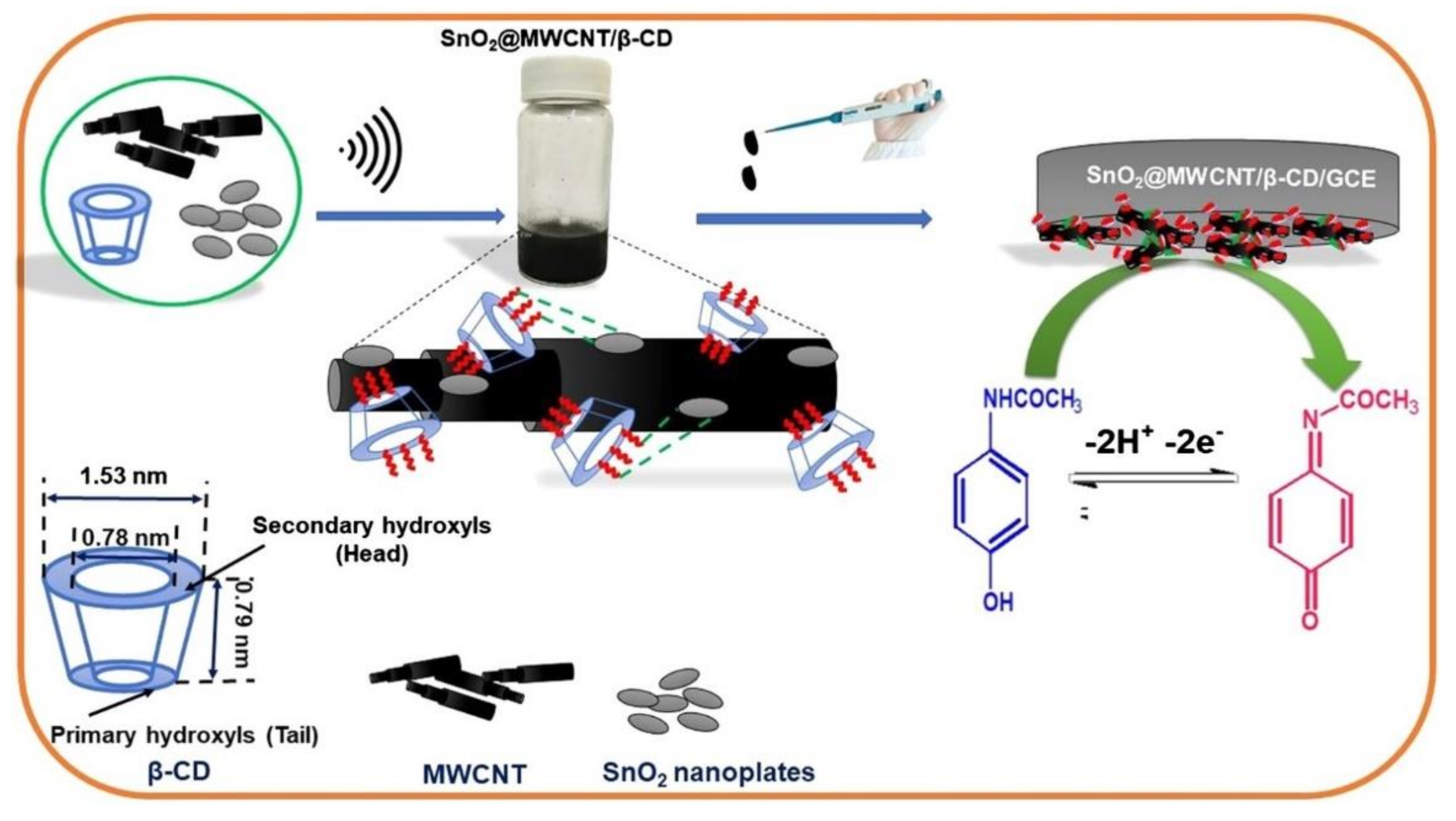
The use of CNTs in developing nanocomposites with applicability in sensing are also reported. One work presented a hybrid nanocomposite sensor based on SnO2, β-CD, and MWCNTs for selective and sensitive detection and quantification of acetaminophen. The presence of β-CD plays a dynamic role in both formation of hybrid nanocomposite and selective sensing. The characterization of hybrid nanocomposite was carried out by FT-IR spectroscopy, high-resolution transmission electron microscopy, and X-ray diffraction analysis. The scheme of the SnO2@MWCNT/β-CD sensor development is presented in Figure 10 [196].
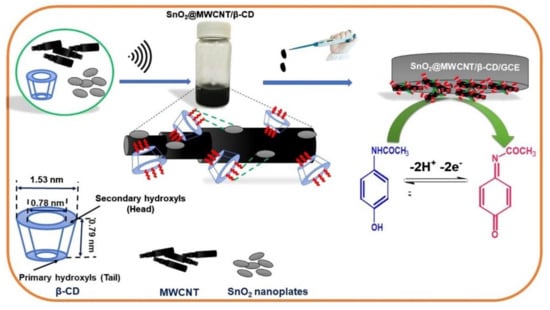
Figure 10.
Scheme of the sensor development: materials employed, techniques for dispersion of the nanomaterials, deposition technique, and detection mechanism. Reprinted from [196] with permission of Elsevier.
Under optimum working condition the sensor shows a linear range of 0.01–340 μM and a low detection limit of 5.8 nM for the voltammetric detection of acetaminophen. The sensor was validated by precise quantification of acetaminophen in tablet and human urine samples.
More sensors based on CNTs/nanocomposite fabricated by screen-printing technique are included in Table 1.
4.5. Carbon and Graphene Quantum Dots-Modified Electrodes
Carbon quantum-dots and graphene quantum dots (GQD) have been recently introduced as superior materials with multiple properties, among which excellent photostability, small size, biocompatibility, adjustable photoluminescence, exceptional multi photon excitation ability, electrochemiluminiscence, easy functionalization with biomolecules, and chemical inertia [197,198,199,200]. These bright carbon nanocrystals provide unprecedented possibilities in bioimagistics and optical detection. Due to their small size and biocompatibility, they may also serve as efficient transporters for drug administration, allowing the simultaneous monitoring of kinetics. Moreover, their unique catalytic and physico-chemical make them ideal for biomedical applications [201].
GQD may be acknowledged as a kind of carbon quantum dots, which usually possess better crystallinity [202]. Despite controversy regarding the origin of luminescence, carbon dots (CDs) and GQDs may lead to manufacturing of low-cost solar cells and organic LEDs (OLEDs) [203] and even improve the performance of supercapacitors [204] and lithium ions batteries [205].
Although GQDs may be considered a type of CDs, CDs have a distinct structure. In general, CDs are quasispherical nanoparticles containing amorphous and crystalline parts [206]. Despite the fact that many researchers have proven the existence of sp2 carbon in crystalline fraction, CDs have lower crystallinity than GQDs [207]. On the other hand, most GQDs are made of GPH, GO [208], and molecules with a specific structure, such as benzene rings [202].
Thus, GQDs usually have GPH lattices inside the dots, resembling the crystalline structure of single or few layered GPH. It is interesting to note that although CDs and GQDs have different basic structures, both may be functionalized with functional groups containing oxygen, like carboxyl and hydroxyl [209]. The presence of these groups on the surface brings considerable contribution to the optical properties of CDs and also of GQDs, also making them water dispersible. On the other hand, heteroatoms, such as nitrogen, sulphur, and other elements, considerably improve luminescence and electrical conductivity [210] by adjusting electronic structures.
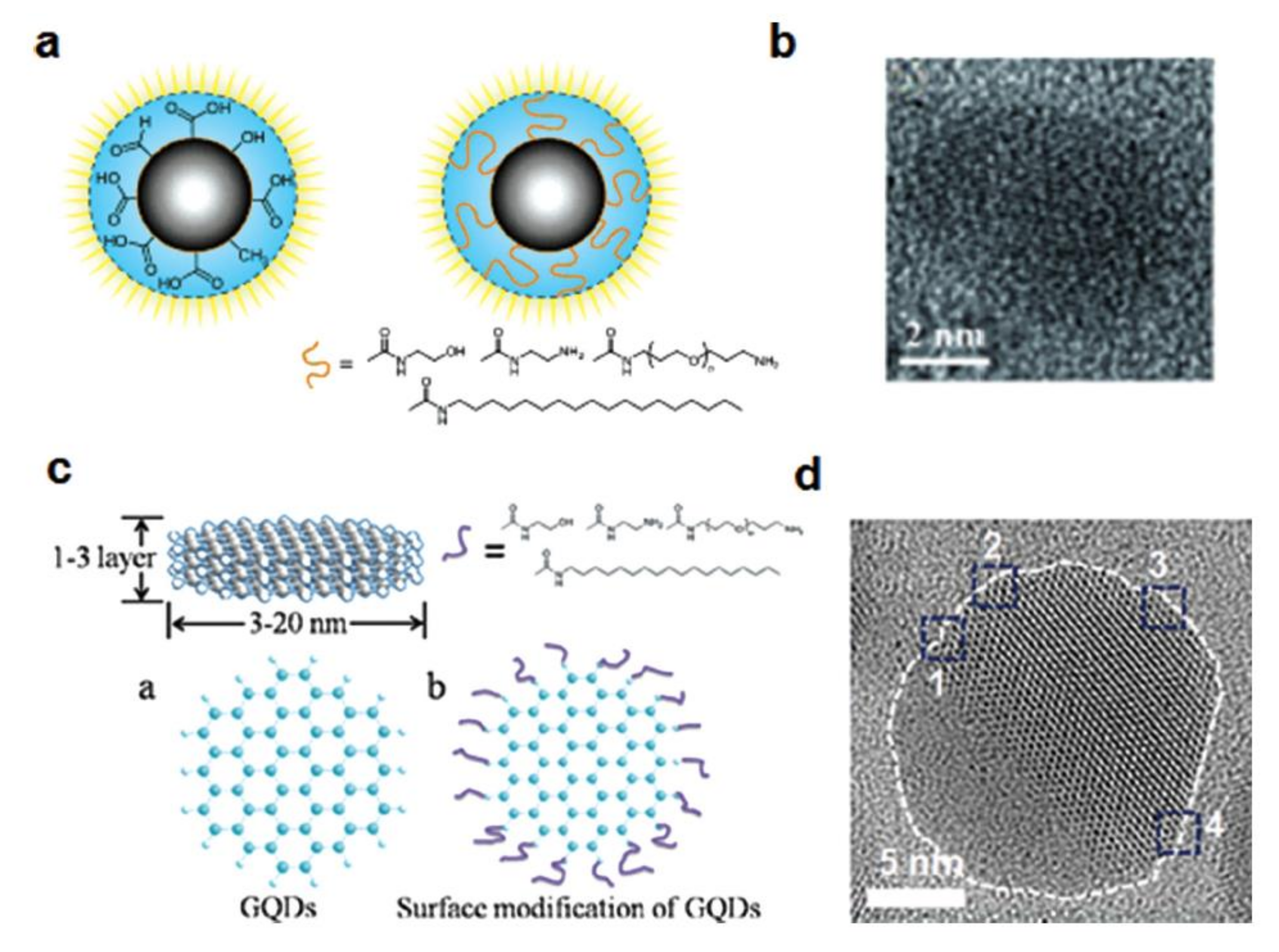
The CDs and GQDs display various colours of photoluminescence, ranging from UV to visible and even proximity of the infrared region [211]. The structural depiction of C-dots and GQDs and its microscopic characterization are presented in Figure 11.
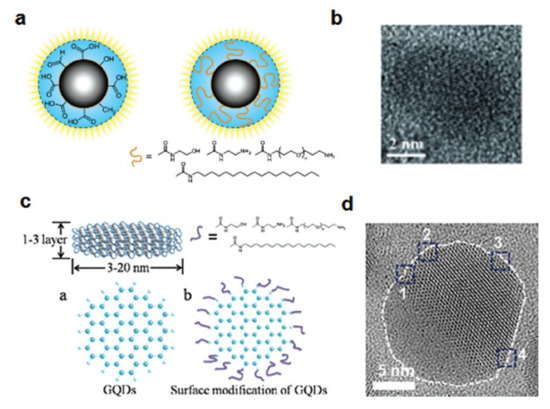
Figure 11.
Structural depiction of (a) C-dots and (c) GQDs. HR-TEM images of (b) C-dots and (d) GQDs showing a combination of zigzag and armchair edges (positions marked as 1–4). Reprinted from [207] with permission of John Wiley and Sons.
In any case, the optical properties of CDs and GQDs are also controversial, like the luminescence mechanism. The debate about the origins of luminescence starts from their behaviour, depending on the excitation, i.e., the emission peak may vary according to the excitation wavelength. Experimental observations as well as the different properties make the origin of luminescence difficult to explain. For example, the desired quantum effect is not always observed or quantified [212], and the exact control of the experimental process cannot be achieved for the moment [213].
The electrochemical characteristics, such as the electron transfer of GQDs and CDs, depend on the complex interaction between the carbon core, the functional groups, and doping heteroatoms [214]. The efficient electron transfer of GQDs is increased by this large specific surface and abundant marginal sites (when the load transfer in some molecules occurs predominantly on the edges). Due to the small size of GQDs, only one electron may be trapped in the well-formed space between a GQD and the surface layer [215]. When there are functional groups containing oxygen, GQD electrochemistry is similar to the one of GO sheets or reduced GO sheets.
The oxygen groups in the base plane affect the electron transfer due to the sp2 conjugation disruption, while oxygen groups on the edges provide the GQDs with catalytic properties, like for H2O2. Heteroatom doping may induce catalytic properties in GQDs (for example, N-doped GQDs to reduce oxygen) [216]. As compared to GQDs, CDs are less attractive for electrochemical applications due to their smaller size, weaker crystallinity, and multi-layered structure [207].
Many types of devices and applications may be developed using CDs and GQDs, due to their special optical and electrical properties. However, research on CDs and GQDs is still in the beginning stages, as compared to other carbonaceous nanomaterials. There are many issues that need solving. For example, the mechanism of photoluminescence has yet to find conclusive evidence or convincing explanations. Several factors, like crystallinity, size, surface functionalization, or the doping influence or optical properties are not yet clear. In addition, it is necessary to find easy synthetic methods to manufacture high-quality CDs and GQDs [213].
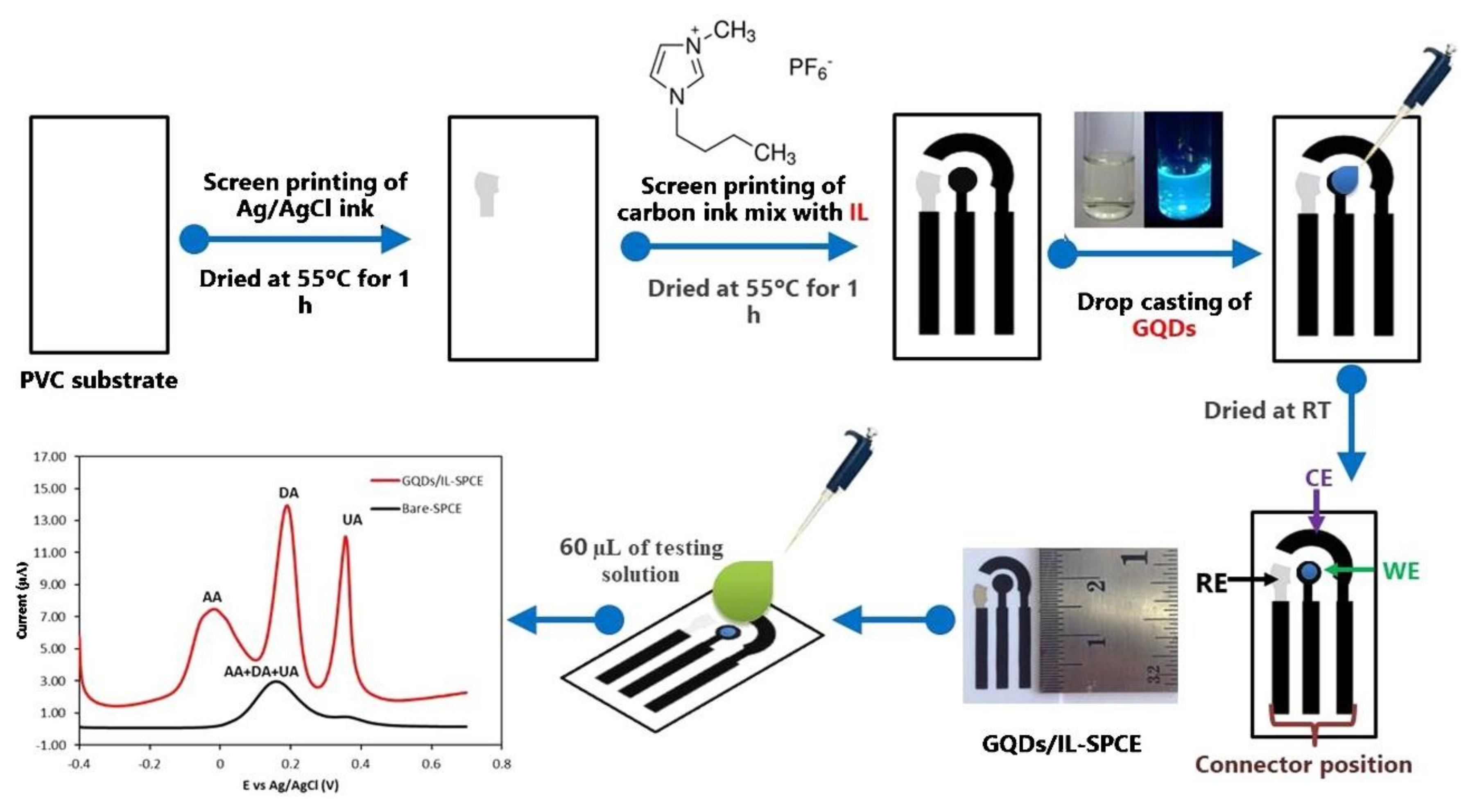
However, GQDs have been employed in the development of electrochemical sensors and useful features have been identified. In a study, GQDs and ionic liquid (IL) modified SPCE (GQDs/IL-SPCE) was developed for the detection of ascorbic acid, dopamine, and uric acid in the mixture solution. The GQDs/IL-SPCE was fabricated in a lab using a screen-printing technique (Figure 12) on polyvinyl chloride substrate. The order of the printed layers was Ag/AgCl and after carbon—IL. Processes of drying and heating were applied, as described in Figure 12. The GQDs were deposited by casting techniques [217].
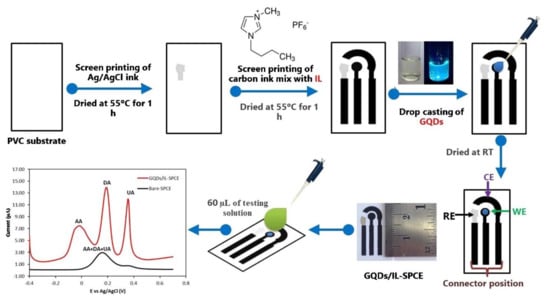
Figure 12.
Schematic diagram of GQDs/IL-SPCE development and DPV detection. Reprinted from [217] with permission of Elsevier.
The GQDs/IL-SPCE demonstrated excellent sensitivity and selectivity for the simultaneous detection of ascorbic acid, dopamine, and uric acid, three well-defined peaks being obtained. The values of LODs were 6.64 μM, 0.06 μM, and 0.03 μM, and the LOLs were 25–400 μM, 0.2–10 μM, and 0.5–20 μM, for ascorbic acid, dopamine, and uric acid. The sensor was useful for the detection of analytes in pharmaceutical products and biological samples.
Some representative references regarding this type of sensor are listed in Table 1.
4.6. Carbon Nanofibers-Modified Electrodes
Carbon nanofibers (CNF) are produced by the catalytic decomposition of hydrocarbonated gases or carbon monoxide in combination with metallic particles including iron, cobalt, nickel, and their alloys at temperatures in the range of 400–1000 °C. CNF manufacturing includes laser ablation, electrospinning, arc discharge, catalytic deposition of chemical vapours and the catalytic deposition of chemical vapours in plasma [218]. Some CNF structures with variations in assembling graphenes along the nanofiber axis are ribbon-type CNFs, plate CNF, and herringbone-type CNFs [219].
Schematic representation of the size and morphology of various structures of CNTs and nanofibers is presented in Figure 13 [220].
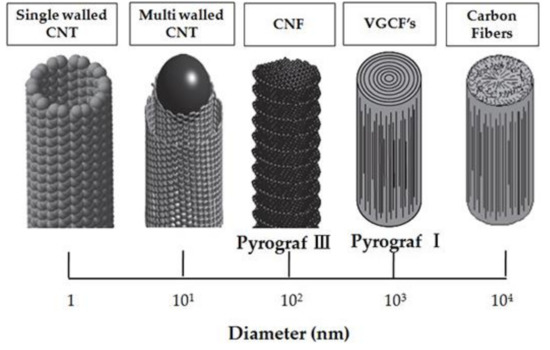
Figure 13.
Schematic representation of the size and morphology of various structures of carbon nanotubes and nanofibers. [220].
As compared to CNTs, CNFs do not have a perfect disposition of atoms and an empty cavity in the centre. In general, CNFs are cylinder-shaped and consist of GPH layers disposed as stacked cones, cups, or plates [221].
Studies have proven that CNFs have an unusual combination of properties, including high electricity and conductivity [90]. These characteristics have stimulated research on optimising the formation of such carbon types. One of the most important characteristics of nanofibers is the presence of a high number of edges, which ensure an adapted surface available for interaction with gases, liquids, or other solids.
Over the past few years, nanofiber-type structures have become more and more attractive due to their numerous advantages, i.e., increased reaction rate and sensor sensitivity [90]. The excellent mechanical and structural characteristics, high porosity and nanofiber interconnectivity [222], as well as the preservation of the activity of the biological elements immobilized on the surface of the nanofibers are features useful in sensing and biosensing [223].
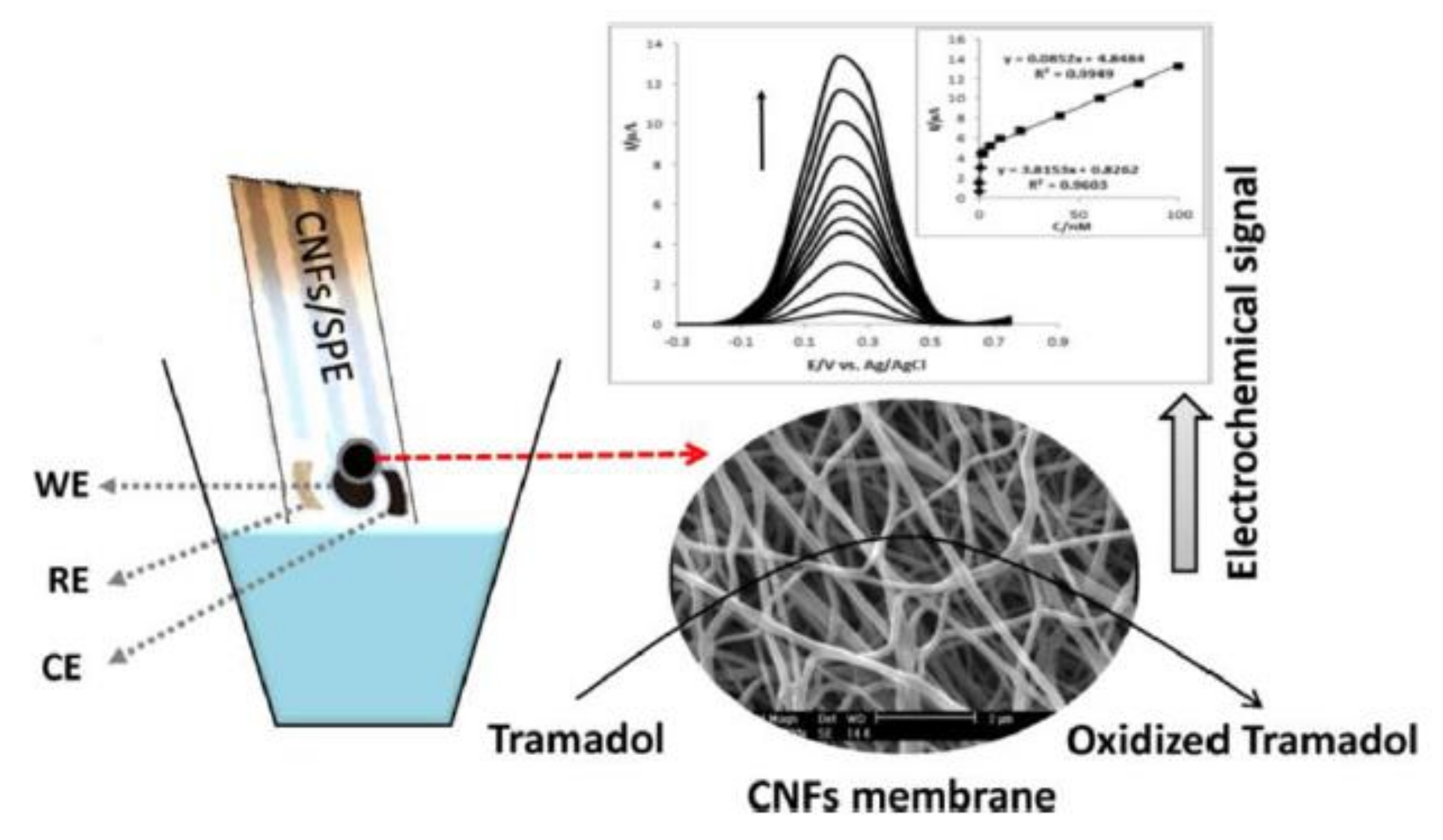
A sensor based on CNFs/SPE for the detection of tramadol has also been developed. After microscopic characterization, the CNFs/SPE was used for the detection of tramadol using CV and SWV techniques. The scheme of the SPE design and detection signals are presented in Figure 14.
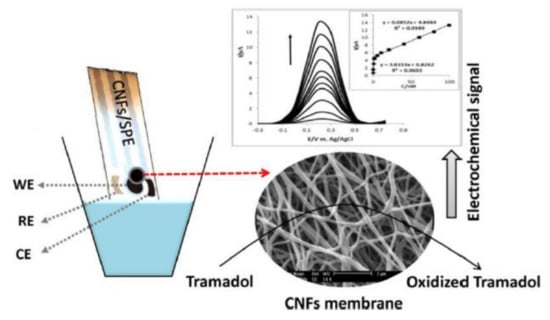
Figure 14.
Schematic representation of SPE, microscopic image, and DPV detection. Reprinted from [90] with the permission of Elsevier.
Using the appropriate supporting electrolyte, optimal pH value, and proper parameters of SWV, a linear range of 0.05–100 nM was obtained. The LOQ and LOD calculated were 0.05 nM and 0.016 nM, respectively. The validation of the sensor was the quantification of tramadol in the urine sample, CNFs/SPE being useful for precise determination of tramadol in real samples.
Table 1 includes a list of SPE sensors based on different carbonaceous materials and its composites recently reported in the literature.
5. Conclusions
The emergence of nanotechnology has proved revolutionary in sensor development and manufacturing, and has opened new vistas in producing sensors with special analytic characteristics and miniaturised sizes. To be more specific, the use of nanomaterials is considered a promising strategy in increasing reaction rate and sensor sensitivity. Recent developments in sensors aim at overcoming the deficiencies of first generation sensors, such as non-specific interaction, size variation and nanomaterial aggregation, as well as stability.
Various types of nanostructured materials, such as nanofibres, nanolayers, and nanoparticles, have been used to develop electrochemical sensors able to detect a wide range of analytes, from glucose to nucleic acids. Due to the nanomaterials used, the analytical results obtained by means of the sensors have been encouraging, with the sensors displaying very good sensitivity, selectivity, stability, and replicability.
The impact of carbonaceous nanomaterials in developing screen-printed based electrochemical sensors is sustained by the superior sensing properties and electrocatalytic abilities. These properties are strongly influenced by the nanostructure features, which improve the electron transfer rate, increase the active surface area, create specific sites for interactions, and have anti-fouling capability.
The authors anticipate that future efforts will be focused on developing sensors and biosensors based on functionalized carbonaceous nanomaterials, which are more appropriate for the immobilization of biological receptors, with important electroanalytical applications in emerging fields of interest such as drug quality, food safety, environment security, and medical analyses. The use of nanotechnologies will permit the development of hybrid sensors and biosensors in flexible substrates and fabrication of portable devices for non-invasive human health monitoring.
Author Contributions
Conceptualization, C.A. and A.V.B.; writing—original draft preparation, A.V.B.; writing—review and editing, C.A. and A.V.B.; supervision C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rajaei, M.; Foroughi, M.M.; Jahani, S.; Shahidi Zandi, M.; Hassani Nadiki, H. Sensitive detection of morphine in the presence of dopamine with La3+ doped fern-like CuO nanoleaves/MWCNTs modified carbon paste electrode. J. Mol. Liq. 2019, 284, 462–472. [Google Scholar] [CrossRef]
- Rubab, M.; Shahbaz, H.M.; Olaimat, A.N.; Oh, D.-H. Biosensors for rapid and sensitive detection of Staphylococcus aureus in food. Biosens. Bioelectron. 2018, 105, 49–57. [Google Scholar] [CrossRef]
- Özcan, B.; Hanbaba, M.A.; Kemal Sezgintürk, M. Ultra-sensitive detection of parathyroid hormone in human serum: A cheap and practical biosensing platform modified by an epoxy ended-silane agent. Int. J. Environ. Anal. Chem. 2020, 100, 393–407. [Google Scholar] [CrossRef]
- Xu, G.; Jarjes, Z.A.; Desprez, V.; Kilmartin, A.; Travas-Sejdic, J. Sensitive; selective; disposable electrochemical dopamine sensor based on PEDOT-modified laser scribed grapheme. Biosens. Bioelectron. 2018, 107, 184–191. [Google Scholar] [CrossRef]
- Beluomini, M.A.; da Silva, J.L.; de Sá, A.C.; Buffon, E.; Pereira, T.C.; Stradiotto, N.R. Electrochemical sensors based on molecularly imprinted polymer on nanostructured carbon materials: A review. J. Electroanal. Chem. 2019, 840, 343–366. [Google Scholar] [CrossRef]
- Purushothama, H.; Nayaka, Y.A.; Vinay, M.; Manjunatha, P.; Yathisha, R.; Basavarajappa, K. Pencil graphite electrode as an electrochemical sensor for the voltammetric determination of chlorpromazine. J. Sci. Adv. Mater. Devices 2018, 3, 161–166. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.; Lai, G.; Lin, C.-T.; Yu, J.; Yu, A.; Liu, Z.; Xie, K.; Su, W.T. Correction to: A glassy carbon electrode modified with N-doped carbon dots for improved detection of hydrogen peroxide and paracetamol. Microchim. Acta 2019, 186, 413. [Google Scholar] [CrossRef]
- Asadian, E.; Ghalkhani, M.; Shahrokhian, S. Electrochemical sensing based on carbon nanoparticles: A review. Sens. Actuators B Chem. 2019, 293, 183–209. [Google Scholar] [CrossRef]
- Szot-Karpińska, K.; Leśniewski, A.; Jönsson-Niedziółka, M.; Marken, F.; Niedziółka-Jönsson, J. Electrodes modified with bacteriophages and carbon nanofibres for cysteine detection. Sens. Actuators B Chem. 2019, 287, 78–85. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, R.; Wang, F. Facile synthesis of CoxP decorated porous carbon microspheres for ultrasensitive detection of 4-nitrophenol. Talanta 2018, 179, 448–455. [Google Scholar] [CrossRef]
- Sinha, A.; Dhanjai Jain, R.; Zhao, H.; Karolia, P.; Jadon, N. Voltammetric sensing based on the use of advanced carbonaceous nanomaterials: A review. Microchim. Acta 2018, 185, 89. [Google Scholar] [CrossRef] [PubMed]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review—Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Bezzon, V.D.; Montanheiro, T.L.D.A.; De Menezes, B.R.C.; Ribas, R.G.; Righetti, V.A.N.; Rodrigues, K.F.; Thim, G.P. Carbon Nanostructure-based Sensors: A Brief Review on Recent Advances. Adv. Mater. Sci. Eng. 2019, 2019, 1–21. [Google Scholar] [CrossRef]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Jiang, H.; Wang, X. Review—Intracellular Sensors Based on Carbonaceous Nanomaterials: A Review. J. Electrochem. Soc. 2020, 167, 037540. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M.; Saleh, T.A. Recent trends in nanomaterial-modified electrodes for electroanalytical applications. TrAC Trends Anal. Chem. 2019, 111, 47–61. [Google Scholar] [CrossRef]
- Commission Recommendation of 18 October 2011 on the Definition of Nanomaterial. Available online: https://ec.europa.eu/research/industrial_technologies/pdf/policy/commission-recommendation-on-the-definition-of-nanomater-18102011_en.pdf (accessed on 5 November 2019).
- Mehra, N.K.; Jain, A.K.; Nahar, M. Carbon nanomaterials in oncology: An expanding horizon. Drug Discov. Today 2018, 23, 1016–1025. [Google Scholar] [CrossRef]
- George, J.M.; Antony, A.; Mathew, B. Metal oxide nanoparticles in electrochemical sensing and biosensing: A review. Microchim. Acta 2018, 185, 358. [Google Scholar] [CrossRef]
- Zhong, C.; Yang, B.; Jiang, X.; Li, J. Current Progress of Nanomaterials in Molecularly Imprinted Electrochemical Sensing. Crit. Rev. Anal. Chem. 2017, 48, 15–32. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, M.; Wang, T.; Liu, B.; Meng, J. Application Technology of Electrochemical Sensors Based on New Nanomaterials. Acta Microsc. 2019, 28, 2019. [Google Scholar]
- Thakur, S.; Thakur, S.; Kumar, R. Bio-Nanotechnology and its Role in Agriculture and Food Industry. J. Mol. Genet. Med. 2018, 12, 2018. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; Alothman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ.-Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Lim, J.Y.; Mubarak, N.; Abdullah, E.C.; Nizamuddin, S.; Khalid, M. Inamuddin. Recent trends in the synthesis of graphene and graphene oxide based nanomaterials for removal of heavy metals—A review. J. Ind. Eng. Chem. 2018, 66, 29–44. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties; applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Hunyadi Murph, S.E.; Larsen, G.K.; Coopersmith, K.J. (Eds.) Anisotropic and Shape-Selective Nanomaterials: Structure-Property Relationships; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Study of Different Carbonaceous Materials as Modifiers of Screen-Printed Electrodes for Detection of Catecholamines. IEEE Sensors J. 2014, 15, 3094–3101. [Google Scholar] [CrossRef]
- Thangamuthu, M.; Gabriel, W.E.; Santschi, C.; Martin, O. Electrochemical Sensor for Bilirubin Detection Using Screen Printed Electrodes Functionalized with Carbon Nanotubes and Graphene. Sensors 2018, 18, 800. [Google Scholar] [CrossRef] [PubMed]
- Alim, S.; Vejayan, J.; Yusoff, M.; Kafi, A. Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: A review. Biosens. Bioelectron. 2018, 121, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Saadati, A.; Hassanpour, S.; De La Guardia, M.; Mosafer, J.; Hashemzaei, M.; Mokhtarzadeh, A.; Baradaran, B. Recent advances on application of peptide nucleic acids as a bioreceptor in biosensors development. TrAC Trends Anal. Chem. 2019, 114, 56–68. [Google Scholar] [CrossRef]
- Diao, W.; Tang, M.; Ding, S.-J.; Li, X.; Cheng, W.; Mo, F.; Yan, X.; Ma, H.; Yan, Y. Highly sensitive surface plasmon resonance biosensor for the detection of HIV-related DNA based on dynamic and structural DNA nanodevices. Biosens. Bioelectron. 2018, 100, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Emran, M.Y.; Mekawy, M.; Akhtar, N.; Shenashen, M.A.; El-Sewify, I.M.; Faheem, A.; El-Safty, S.A. Broccoli-shaped biosensor hierarchy for electrochemical screening of noradrenaline in living cells. Biosens. Bioelectron. 2018, 100, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Deshours, F.; Alquie, G.; Kokabi, H.; Rachedi, K.; Tlili, M.; Hardinata, S.; Koskas, F. Improved microwave biosensor for non-invasive dielectric characterization of biological tissues. Microelectron. J. 2019, 88, 137–144. [Google Scholar] [CrossRef]
- Liu, G.; Linb, Y. Nanomaterial labels in electrochemical immunosensors and immunoassays. Talanta 2007, 74, 308–317. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Nanostructures for nonlabeled and labeled electrochemical immunosensors: Simultaneous electrochemical detection of cancer markers: A review. Talanta 2019, 205, 120153. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Gu, X.; Trujillo, M.J.; Olson, J.E.; Camden, J. SERS Sensors: Recent Developments and a Generalized Classification Scheme Based on the Signal Origin. Annu. Rev. Anal. Chem. 2018, 11, 147–169. [Google Scholar] [CrossRef]
- Nag, A.; Afsarimanesh, N.; Feng, S.C. Mukhopadhyay. Strain induced graphite/PDMS sensors for biomedical applications. Sens. Actuators A Phys. 2018, 271, 257–269. [Google Scholar] [CrossRef]
- T Tran, T.S.; Dutta, N.K.; Choudhury, N.R. Graphene inks for printed flexible electronics: Graphene dispersions, ink formulations, printing techniques and applications. Adv. Colloid Interface Sci. 2018, 261, 41–61. [Google Scholar] [CrossRef]
- Woltornist, S.; Carrillo, J.-M.Y.; Xu, T.O.; Dobrynin, A.V.; Adamson, D.H. Polymer/Pristine Graphene Based Composites: From Emulsions to Strong; Electrically Conducting Foams. Macromolecules 2015, 48, 687–693. [Google Scholar] [CrossRef]
- Hendawy, H.A.M.; Salem, W.M.; Abd-Elmonem, M.S.; Khaled, E. Nanomaterial-Based Carbon Paste Electrodes for Voltammetric Determination of Naproxen in Presence of Its Degradation Products. J. Anal. Methods Chem. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Muñoz, J.; Baeza, M. Customized Bio-functionalization of Nanocomposite Carbon Paste Electrodes for Electrochemical Sensing: A Mini Review. Electroanalysis 2017, 29, 1660–1669. [Google Scholar] [CrossRef]
- Chu, Z.; Peng, J.; Jin, W. Advanced nanomaterial inks for screen-printed chemical sensors. Sens. Actuators B Chem. 2017, 243, 919–926. [Google Scholar] [CrossRef]
- da Silva Neves, M.M.P.; González-Garcia, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Future trends in the market for electrochemical biosensing. Curr. Opin. Electrochem. 2018, 10, 107–111. [Google Scholar] [CrossRef]
- Scordo, G.; Moscone, D.; Palleschi, G.; Arduini, F. A reagent-free paper-based sensor embedded in a 3D printing device for cholinesterase activity measurement in serum. Sens. Actuators B Chem. 2018, 258, 1015–1021. [Google Scholar] [CrossRef]
- Dias, D.; Cunha, J.P.S. Wearable Health Devices—Vital Sign Monitoring; Systems and Technologies. Sensors 2018, 18, 2414. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.W.; Kadara, R.O.; Banks, C.E. Fabricating Screen-Printed Electrochemical Architectures: Successful Design and Fabrication. In Screen-Printing Electrochemical Architectures; Banks, C.E., Foster, C.W., Kadara, R.O., Eds.; Springer International Publishing: New York, NY, USA, 2016; pp. 25–33. [Google Scholar]
- Li, Q.; Zhang, J.; Li, Q.; Li, G.; Tian, X.; Luo, Z.; Qiao, F.; Wu, X.; Zhang, J. Review of printed electrodes for flexible devices. Front. Mater. 2019, 5, 77. [Google Scholar] [CrossRef]
- Gwent Group, Leaders in Paste Manufacturing, Sensor/Biosensor Development and Instrumentation. Available online: http://www.gwent.org/gem_screen_printing.html (accessed on 25 February 2020).
- Couto, R.A.S.; Lima, J.L.F.C.; Quinaz, M.B. Recent developments; characteristics and potential applications of screen-printed electrodes in pharmaceutical and biological analysis. Talanta 2016, 146, 801–814. [Google Scholar] [CrossRef]
- Faria, R.A.D.; Messaddeq, Y.; Heneine, G.D.; Matencio, T. Application of screen-printed carbon electrode as an electrochemical transducer in biosensors. Int. J. Biosens. Bioelectron. 2019, 5, 2019. [Google Scholar] [CrossRef]
- Cordeiro, T.A.; Gonçalves, M.V.; Franco, D.L.; Reis, A.B.; Martins, H.R.; Ferreira, L.F. Label-free electrochemical impedance immunosensor based on modified screen-printed gold electrodes for the diagnosis of canine visceral leishmaniasis. Talanta 2019, 195, 327–332. [Google Scholar] [CrossRef]
- De Oliveira Silva, R.; da Silva, É.A.; Fiorucci, A.R.; Ferreira, V.S. Electrochemically activated multi-walled carbon nanotubes modified screen-printed electrode for voltammetric determination of sulfentrazone. J. Electroanal. Chem. 2019, 835, 220–226. [Google Scholar] [CrossRef]
- Rosal, M.; Ceto, X.; Serrano, N.; Ariño, C.; Esteban, M.; Díaz-Cruz, J.M. Dimethylglyoxime modified screen-printed electrodes for nickel determination. J. Electroanal. Chem. 2019, 839, 83–89. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, W.; Yan, T.; Gao, J.; Zhanga, J. Fuel cell-based self-powered electrochemical sensors for biochemical detection. Nano Energy 2019, 2019. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, R.; Luo, F.; Wang, P.; Lin, Z. Miniaturized electrochemical sensors and their point-of-care applications. Chin. Chem. Lett. 2020, 31, 589–600. [Google Scholar] [CrossRef]
- Colmati, F.; Sgobbi, L.F.; Teixeira, G.F.; Vilela, R.S.; Martins, T.D.; Figueiredo, G.O. Electrochemical biosensors containing pure enzymes or crude extracts as enzyme sources for pesticides and phenolic compounds with pharmacological property detection and quantification. In Environmental Biosensors; IntechOpen: London, UK, 2019. [Google Scholar]
- Liu, X.; Yao, Y.; Ying, Y.; Ping, J. Recent advances in nanomaterial-enabled screen-printed electrochemical sensors for heavy metal detection. TrAC Trends Anal. Chem. 2019, 115, 187–202. [Google Scholar] [CrossRef]
- Cinti, S.; Santella, F.; Moscone, D.; Arduini, F. Hg2+ detection using a disposable and miniaturized screen-printed electrode modified with nanocomposite carbon black and gold nanoparticles. Environ. Sci. Pollut. Res. 2016, 23, 8192–8199. [Google Scholar] [CrossRef]
- Ali, S.M.U.; Nur, O.; Willander, M.; Danielsson, B. A fast and sensitive potentiometric glucose microsensor based on glucose oxidase coated ZnO nanowires grown on a thin silver wire. Sens. Actuators B Chem. 2010, 145, 869–874. [Google Scholar] [CrossRef]
- Hayat, A.; Marty, J.L. Disposable screen printed electrochemical sensors: Tools for environmental monitoring. Sensors (Basel) 2014, 14, 10432–10453. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized enzymes in biosensor applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef]
- Svalova, T.S.; Medvedeva, M.V.; Saigushkina, A.A.; Kozitsin, I.V.; Malysheva, N.N.; Zhdanovskikh, V.O.; Okhokhonin, A.V.; Kozitsina, A.N. A label-free impedimetric immunosensor based on covalent immobilization of anti-E. Coli antibody via a copper-catalyzed azide-alkyne cycloaddition reaction. Anal. Bioanal. Chem. 2020, 1–11. [Google Scholar] [CrossRef]
- Fortunati, S.; Rozzi, A.; Curti, F.; Giannetto, M.; Corradini, R.; Careri, M. Novel amperometric genosensor based on peptide nucleic acid (PNA) probes immobilized on carbon nanotubes-screen printed electrodes for the determination of trace levels of non-amplified DNA in genetically modified (GM) soy. Biosens. Bioelectron. 2019, 129, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.; Ogawa, Y.; Kato, Y.; Kai, H.; Nishizawa, M. Printing of Enzyme Electrodes on Nonwoven Fabric for Flexible Biofuel Cells. ECS Meet. Abstr. 2016, MA2016-02, 3211. [Google Scholar] [CrossRef]
- Bragança, I.; Lemos, P.C.; Delerue-Matos, C.; Domingues, V.F. Pyrethroid pesticide metabolite, 3-PBA, in soils: Method development and application to real agricultural soils. Environ. Sci. Pollut. Res. 2018, 26, 2987–2997. [Google Scholar] [CrossRef]
- Fu, L.; Xu, Y.; Du, J.; Cao, D.; Liu, Q. Electroanalytical Methods for Fish Drug Determination and Control: A Review and Outlook. Int. J. Electrochem. Sci. 2019, 14, 4383. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical biosensors for autoantibodies in autoimmune and cancer diseases. Anal. Methods 2019, 11, 871–887. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M. Advances in celiac disease testing. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–29. [Google Scholar]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosens. Bioelectron. 2019, 141, 111417. [Google Scholar] [CrossRef]
- Ahn, M.-S.; Ahmad, R.; Bhat, K.S.; Yoo, J.-Y.; Mahmoudi, T.; Hahn, Y.-B. Fabrication of a solution-gated transistor based on valinomycin modified iron oxide nanoparticles decorated zinc oxide nanorods for potassium detection. J. Colloid Interface Sci. 2018, 518, 277–283. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Ahn, M.-S.; Yoo, J.-Y.; Hahn, Y.-B. Preparation of a Highly Conductive Seed Layer for Calcium Sensor Fabrication with Enhanced Sensing Performance. ACS Sens. 2018, 3, 772–778. [Google Scholar] [CrossRef]
- Hayat, A.; Rhouati, A.; Mishra, R.; A Alonso-Silverio, G.; Nasir, M.; Istamboulie, G.; Marty, J.L. An electrochemical sensor based on TiO2/activated carbon nanocomposite modified screen printed electrode and its performance for phenolic compounds detection in water samples. Int. J. Environ. Anal. Chem. 2016, 96, 1–10. [Google Scholar] [CrossRef]
- De Oliveira, T.R.; Fonseca, W.T.; Setti, G.D.O.; Faria, R.C. Fast and flexible strategy to produce electrochemical paper-based analytical devices using a craft cutter printer to create wax barrier and screen-printed electrodes. Talanta 2019, 195, 480–489. [Google Scholar] [CrossRef]
- Maleki, B.; Baghayeri, M.; Ghanei-Motlagh, M.; Zonoz, F.M.; Amiri, A.; Hajizadeh, F.; Hosseinifar, A.; Esmaeilnezhad, E. Polyamidoamine dendrimer functionalized iron oxide nanoparticles for simultaneous electrochemical detection of Pb2+ and Cd2+ ions in environmental waters. Measurement 2019, 140, 81–88. [Google Scholar] [CrossRef]
- Bernalte, E.; Arévalo, S.; Taborda, J.A.P.; Wenkab, J.; Estrelabce, P.; Avila, A.; Di Lorenzo, M. Rapid and on-site simultaneous electrochemical detection of copper; lead and mercury in the Amazon river. Sens. Actuators B Chem. 2020, 307, 127620. [Google Scholar] [CrossRef]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gil, E. Fast and direct amperometric analysis of polyphenols in beers using tyrosinase-modified screen-printed gold nanoparticles biosensors. Talanta 2018, 193, 93–99. [Google Scholar] [CrossRef] [PubMed]
- De Araujo Andreotti, I.A.; Orzari, L.O.; Camargo, J.R.; Faria, R.C.; Marcolino-Junior, L.H.; Bergamini, M.F.; Gatti, A.; Janegitz, B.C. Disposable and flexible electrochemical sensor made by recyclable material and low cost conductive ink. J. Electroanal. Chem. 2019, 840, 109–116. [Google Scholar] [CrossRef]
- Liu, S.; Leng, J.; Aquino, T.C. Development of Disposable Single-Use Biosensor for Fructosyl Valine and Glycated Hemoglobin A1c. J. Sens. Technol. 2019, 9, 45–53. [Google Scholar] [CrossRef]
- Rocha, R.G.; Stefano, J.S.; Arantes, I.V.S.; Ribeiro, M.M.A.C.; Santana, M.H.P.; Richter, E.M.; Munoz, R.A.A. Simple Strategy for Selective Determination of Levamisole in Seized Cocaine and Pharmaceutical Samples Using Disposable Screen-printed Electrodes. Electroanalysis 2019, 31, 153–159. [Google Scholar] [CrossRef]
- Trevino, N.; Montes, W.; Ganta, D. Disposable chronoamperometric sensor for detecting ciprofloxacin. Eng. Res. Express 2019, 1, 015031. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Hossain, M.M.; Safavieh, M.; Wong, Y.L.; Rahman, I.A.; Zourob, M.; Tamiya, E. Toward the development of smart and low cost point-of-care biosensors based on screen printed electrodes. Crit. Rev. Biotechnol. 2015, 36, 495–505. [Google Scholar] [CrossRef]
- Montes, C.; Contento, A.M.; Villaseñor, M.J.; Ríos, Á. A screen-printed electrode modified with silver nanoparticles and carbon nanofibers in a nafion matrix for ionic liquid-based dispersive liquid-liquid microextraction and voltammetric assay of heterocyclic amine 8-MeIQx in food. Microchim. Acta 2020, 187, 1–11. [Google Scholar] [CrossRef]
- Šišoláková, I.; Hovancová, J.; Oriňaková, R.; Oriňak, A.; Trnková, L.; Třísková, I.; Farka, Z.; Pastucha, M.; Radoňák, J. Electrochemical determination of insulin at CuNPs/chitosan-MWCNTs and CoNPs/chitosan-MWCNTs modified screen printed carbon electrodes. J. Electroanal. Chem. 2020, 860, 113881. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, C.; Mediavilla, M.; Guerrero-Esteban, T.; Revenga-Parra, M.; Pariente, F.; Lorenzo, E. Direct covalent immobilization of new nitrogen-doped carbon nanodots by electrografting for sensing applications. Carbon 2020, 159, 303–310. [Google Scholar] [CrossRef]
- Llaver, M.; Martinis, E.M.; Sotolongo, A.C.; Wuilloud, R.G. Wuilloud. Modern Analytical Nanotechnologies for Beverages Quality Control. In Nanoengineering in the Beverage Industry 2020; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–103. [Google Scholar] [CrossRef]
- Jeon, J.-Y.; Kang, B.-C.; Ha, T.-J. Flexible pH sensors based on printed nanocomposites of single-wall carbon nanotubes and Nafion. Appl. Surf. Sci. 2020, 145956. [Google Scholar] [CrossRef]
- Jahromi, Z.; Mirzaei, E.; Savardashtaki, A.; Afzali, M.; Afzali, Z. A rapid and selective electrochemical sensor based on electrospun carbon nanofibers for tramadol detection. Microchem. J. 2020, 157, 104942. [Google Scholar] [CrossRef]
- Abazar, F.; Noorbakhsh, A. Chitosan-carbon quantum dots as a new platform for highly sensitive insulin impedimetric aptasensor. Sens. Actuators B Chem. 2020, 304, 127281. [Google Scholar] [CrossRef]
- Thunkhamrak, C.; Chuntib, P.; Ounnunkad, K.; Banet, P.; Aubert, P.-H.; Saianand, G.; Gopalan, A.-I.; Jakmunee, J. Highly sensitive voltammetric immunosensor for the detection of prostate specific antigen based on silver nanoprobe assisted graphene oxide modified screen printed carbon electrode. Talanta 2020, 208, 120389. [Google Scholar] [CrossRef]
- Eissa, S.; AlShehri, N.; Rahamn, A.A.; Dasouki, M.; Abu-Salah, K.M.; Zourob, M. Electrochemical immunosensors for the detection of survival motor neuron (SMN) protein using different carbon nanomaterials-modified electrodes. Biosens. Bioelectron. 2018, 101, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Kim, K.-H. Recent advances in nanomaterial-based electrochemical detection of antibiotics: Challenges and future perspectives. Biosens. Bioelectron. 2020, 153, 112046. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.V.F.; Silva, M.N.; Tormin, T.F.; Santana, M.H.; Nossol, E.; Richter, E.M.; Munoz, R.A.A. Highly-sensitive voltammetric detection of trinitrotoluene on reduced graphene oxide/carbon nanotube nanocomposite sensor. Anal. Chim. Acta 2018, 1035, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.; Morais, S. New Generation of Electrochemical Sensors Based on Multi-Walled Carbon Nanotubes. Appl. Sci. 2018, 8, 1925. [Google Scholar] [CrossRef]
- Deroco, B.; Melo, I.G.; Silva, L.S.; Eguiluz, K.I.B.; Salazar-Banda, G.R.; Fatibello-Filho, O. Carbon black supported Au–Pd core-shell nanoparticles within a dihexadecylphosphate film for the development of hydrazine electrochemical sensor. Sens. Actuators B Chem. 2018, 256, 535–542. [Google Scholar] [CrossRef]
- Baig, N.; Saleh, T.A. Electrodes modified with 3D graphene composites: A review on methods for preparation, properties and sensing applications. Microchim. Acta 2018, 185, 283. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, Y.; Zhang, M.; Cui, B.; Hu, Q.; Wang, L. A novel electrochemical strategy based on porous 3D graphene-starch architecture and silver deposition for ultrasensitive detection of neuron-specific enolase. Analyst 2019, 144, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress; Applications; and Future Perspective. Chem. Rev. 2018, 119, 120–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Speranza, G. Functionalization of Carbon Nanomaterials for Biomedical Applications. C—J. Carbon Res. 2019, 5, 72. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Abdullah, E.C. Functionalized Carbon Nanomaterial for Artificial Bone Replacement as Filler Material. In Sustainable Polymer Composites and Nanocomposites 2019; Inamuddin Thomas, S., Kumar Mishra, R., Asiri, A.M., Eds.; Springer International Publishing: Cham, Switzeland, 2019; pp. 783–804. [Google Scholar] [CrossRef]
- Wang, C.; Yin, J.; Han, S.; Jiao, T.; Bai, Z.; Zhou, J.; Zhang, L.; Peng, Q. Preparation of Palladium Nanoparticles Decorated Polyethyleneimine/Polycaprolactone Composite Fibers Constructed by Electrospinning with Highly Efficient and Recyclable Catalytic Performances. Catalysts 2019, 9, 559. [Google Scholar] [CrossRef]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Skákalová, V.; Kotrusz, P.; Jergel, M.; Susi, T.; Mittelberger, A.; Vretenár, V.; Siffalovic, P.; Kotakoski, J.; Meyer, J.C.; Hulman, M. Chemical Oxidation of Graphite: Evolution of the Structure and Properties. J. Phys. Chem. C 2017, 122, 929–935. [Google Scholar] [CrossRef]
- Chen, K.; Shi, L.; Zhang, Y.; Liu, Z. Scalable chemical-vapour-deposition growth of three-dimensional graphene materials towards energy-related applications. Chem. Soc. Rev. 2018, 47, 3018–3036. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, B.; Madjet, M.E.; Shahrokhi, M.; Ahzi, S.; Zhuang, X.; Rabczuk, T. Nanoporous graphene: A 2D semiconductor with anisotropic mechanical, optical and thermal conduction properties. Carbon 2019, 147, 377–384. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, P.; Su, Z. Fabrication technologies and sensing applications of graphene-based composite films: Advances and challenges. Biosens. Bioelectron. 2017, 89, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, K. Recent progress on graphene-analogous 2D nanomaterials: Properties, modeling and applications. Prog. Mater. Sci. 2019, 100, 99–169. [Google Scholar] [CrossRef]
- Li, X.; Tao, L.; Chen, Z.; Fang, H.; Li, X.; Wang, X.; Xu, J.; Zhu, H. Graphene and related two-dimensional materials: Structure-property relationships for electronics and optoelectronics. Appl. Phys. Rev. 2017, 4, 021306. [Google Scholar] [CrossRef]
- Abdelkader, A.M. Electrochemical synthesis of highly corrugated graphene sheets for high performance supercapacitors. J. Mater. Chem. A 2015, 3, 8519–8525. [Google Scholar] [CrossRef]
- Jiang, L.; Fan, Z. Design of advanced porous graphene materials: From graphene nanomesh to 3D architectures. Nanoscale 2014, 6, 1922–1945. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Jung, G.; Kang, M.J.; Buehler, M.J. The mechanics and design of a lightweight three-dimensional graphene assembly. Sci. Adv. 2017, 3, e1601536. [Google Scholar] [CrossRef]
- Al Dream, J.; Zequine, C.; Siam, K.; Kahol, P.K.; Mishra, S.; Gupta, R.K. Electrochemical Properties of Graphene Oxide Nanoribbons/Polypyrrole Nanocomposites. C—J. Carbon Res. 2019, 5, 18. [Google Scholar] [CrossRef]
- Sang, M.; Shin, J.; Kim, K.; Yu, K. Electronic and Thermal Properties of Graphene and Recent Advances in Graphene Based Electronics Applications. Nanomaterials 2019, 9, 374. [Google Scholar] [CrossRef]
- Taniselass, S.; Arshad, M.M.; Gopinath, S.C. Graphene-based electrochemical biosensors for monitoring noncommunicable disease biomarkers. Biosens. Bioelectron. 2019, 130, 276–292. [Google Scholar] [CrossRef]
- Imran, H.; Manikandan, P.N.; Prabhu, D.; Dharuman, V.; Jeyakanthan, J.; Hahn, J.H.; Mainkandan, P.N. Ultra selective label free electrochemical detection of cancer prognostic p53-antibody at DNA functionalized graphene. Sens. Bio-Sens. Res. 2019, 23, 100261. [Google Scholar] [CrossRef]
- Kovalska, E.; Lesongeur, P.; Hogan, B.; Baldycheva, A. Multi-layer graphene as a selective detector for future lung cancer biosensing platforms. Nanoscale 2019, 11, 2476–2483. [Google Scholar] [CrossRef]
- Yang, W.; Ratinac, K.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon Nanomaterials in Biosensors: Should You Use Nanotubes or Graphene? Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Hossain, B.; Rana, M.; Abdulrazak, L.F.; Mitra, S.; Rahman, M. Design and analysis of graphene–MoS2 hybrid layer based SPR biosensor with TiO2–SiO2 nano film for formalin detection: Numerical approach. Opt. Quantum Electron. 2019, 51, 195. [Google Scholar] [CrossRef]
- Afkhami, A.; Shirzadmehr, A.; Madrakian, T.; Bagheri, H. New nano-composite potentiometric sensor composed of graphene nanosheets/thionine/molecular wire for nanomolar detection of silver ion in various real samples. Talanta 2015, 131, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.; Mazumder, B.; Chattopadhyay, P.; Bora, N.S.; Goyary, D.; Karmakar, S. Graphene Family of Nanomaterials: Reviewing Advanced Applications in Drug delivery and Medicine. Curr. Drug Deliv. 2019, 16, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Tahernejad-Javazmi, F.; Shabani-Nooshabadi, M.; Karimi-Maleh, H. 3D reduced graphene oxide/FeNi3-ionic liquid nanocomposite modified sensor; an electrical synergic effect for development of tert-butylhydroquinone and folic acid sensor. Compos. Part B Eng. 2019, 172, 666–670. [Google Scholar] [CrossRef]
- Khaliji, K.; Biswas, S.R.; Hu, H.; Yang, X.; Dai, Q.; Oh, S.-H.; Avouris, P.; Low, T. Plasmonic Gas Sensing with Graphene Nanoribbons. Phys. Rev. Appl. 2020, 13, 011002. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Li, H.; Yan, F.; Pang, P.; Wang, H.; Wu, Z.; Yang, W. A molybdenum disulfide/gold nanorod composite-based electrochemical immunosensor for sensitive and quantitative detection of microcystin-LR in environmental samples. Sens. Actuators B Chem. 2017, 244, 606–615. [Google Scholar] [CrossRef]
- De Melo-Diogo, D.; Sousa, A.R.L.; Alves, C.G.; Costa, E.; Louro, R.O.; Correia, I.J. Functionalization of graphene family nanomaterials for application in cancer therapy. Colloids Surf. B Biointerfaces 2018, 171, 260–275. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Yu, J.; He, J.; Yang, H.; Ye, Y.; Song, Y. A green and simple strategy to prepare graphene foam-like three-dimensional porous carbon/Ni nanoparticles for glucose sensing. Sens. Actuators B Chem. 2017, 239, 172–179. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Manickam, S.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef] [PubMed]
- Shim, G.; Kim, M.-G.; Park, J.Y.; Oh, Y.-K. Graphene-based nanosheets for delivery of chemotherapeutics and biological drugs. Adv. Drug Deliv. Rev. 2016, 105, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.-R.; Govindhan, M.; Chen, A. Sensitive detection of acetaminophen with graphene-based electrochemical sensor. Electrochim. Acta 2015, 162, 198–204. [Google Scholar] [CrossRef]
- Govindhan, M.; Amiri, M.; Chen, A. Au nanoparticle/graphene nanocomposite as a platform for the sensitive detection of NADH in human urine. Biosens. Bioelectron. 2015, 66, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, X.; Huang, L.; Zhang, Z.; Du, Y. One-Pot Synthesis of Fe3O4 Nanoparticle Loaded 3D Porous Graphene Nanocomposites with Enhanced Nanozyme Activity for Glucose Detection. ACS Appl. Mater. Interfaces 2017, 9, 7465–7471. [Google Scholar] [CrossRef]
- Salahandish, R.; Ghaffarinejad, A.; Naghib, S.M.; Niyazi, A.; Majidzadeh, K.; Janmaleki, M.; Nezhad, A.S. Sandwich-structured nanoparticles-grafted functionalized graphene based 3D nanocomposites for high-performance biosensors to detect ascorbic acid biomolecule. Sci. Rep. 2019, 1226. [Google Scholar] [CrossRef] [PubMed]
- Altun, M.; Kamaç, M.B.; Bilgi, A.; Yılmaz, M. Dopamine biosensor based on screen-printed electrode modified with reduced graphene oxide, polyneutral red and gold nanoparticle. Int. J. Environ. Anal. Chem. 2020, 100, 451–467. [Google Scholar] [CrossRef]
- Ji, D.; Shi, Z.; Liu, Z.; Low, S.S.; Zhu, J.; Zhang, T.; Chen, Z.; Yu, X.; Lu, Y.; Lu, D.; et al. Smartphone-based square wave voltammetry system with screen-printed graphene electrodes for norepinephrine detection. Smart Mater. Med. 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Mathur, R.B.; Singh, B.P.; Pande, S. Carbon Nanomaterials: Synthesis, Structure, Properties and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Mishra, R.; Militky, J. Nanotechnology in Textiles: Theory and Application, 1st ed.; Woodhead Publishing: Duxford, UK, 2018; pp. 163–179. [Google Scholar] [CrossRef]
- O’Connell, M.J. Carbon Nanotubes: Properties and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; p. 18. [Google Scholar] [CrossRef]
- Sutradhar, S.; Patnaik, A. A new fullerene-C60—Nanogold composite for non-enzymatic glucose sensing. Sens. Actuators B Chem. 2017, 241, 681–689. [Google Scholar] [CrossRef]
- Nimibofa, A.; Newton, E.A.; Cyprain, A.Y.; Donbebe, W. Fullerenes: Synthesis and Applications. JMSR 2018, 7, 22. [Google Scholar] [CrossRef]
- Valentini, F.; Ciambella, E.; Cataldo, F.; Calcaterra, A.; Menegatti, L.; Talamo, M. Fullerene Black Modified Screen Printed Electrodes for the Quantification of Acetaminophen and Guanine. Electroanalysis 2017, 29, 2863–2872. [Google Scholar] [CrossRef]
- Palanisamy, S.; Thirumalraj, B.; Chen, S.-M.; Ali, M.A.; Al Hemaid, F.M. Palladium nanoparticles decorated on activated fullerene modified screen printed carbon electrode for enhanced electrochemical sensing of dopamine. J. Colloid Interface Sci. 2015, 448, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure; Properties; Functionalization; and Applications of Carbon Nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Sun, H.; Zou, B.; Liu, Z.; Sun, N.; Yi, Y.; Wong, K.-Y. Electrochemical sensing of 4-nitrochlorobenzene based on carbon nanohorns/graphene oxide nanohybrids. Biosens. Bioelectron. 2018, 106, 136–141. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Chen, Z. A highly sensitive electrochemical sensor for bisphenol A using cetyltrimethylammonium bromide functionalized carbon nanohorn modified electrode. Ionics 2018, 24, 2123–2134. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, H.; Ping, J. Simultaneous determination of Cd(II) and Pb(II) ions in honey and milk samples using a single-walled carbon nanohorns modified screen-printed electrochemical sensor. Food Chem. 2019, 274, 8–15. [Google Scholar] [CrossRef]
- Carli, S.; Lambertini, L.; Zucchini, E.; Ciarpella, F.; Scarpellini, A.; Prato, M.; Castagnola, E.; Fadiga, L.; Ricci, D. Single walled carbon nanohorns composite for neural sensing and stimulation. Sens. Actuators B Chem. 2018, 271, 280–288. [Google Scholar] [CrossRef]
- Zhu, G.; Fiston, M.N.; Qian, J.; Kingsford, O.J. Highly sensitive electrochemical sensing of para-chloronitrobenzene using a carbon nanohorn–nanotube hybrid modified electrode. Anal. Methods 2019, 11, 1125–1130. [Google Scholar] [CrossRef]
- Hasani, A. Approaches to Graphene; Carbon Nanotube and Carbon nanohorn; Synthesis; Properties and Applications. Nanosci. Nanotechnol.-Asia 2020, 10, 4–11. [Google Scholar] [CrossRef]
- Kausar, A. Prevailing Research Trends in Carbon Nanohorn and Polymer-based Hybrids. Polym.-Plast. Technol. Eng. 2018, 57, 118–132. [Google Scholar] [CrossRef]
- Chen, X.; Ma, Y.; Chen, D.; Ma, M.; Li, C. Electrochemical fabrication of polymerized imidazole-based ionic liquid bearing pyrrole moiety for sensitive determination of hexestrol in chicken meat. Food Chem. 2015, 180, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, P.; Feng, W.; Ding, S.; Chen, J.; Li, L.; He, H.; Zhang, S.; Dong, F. Carbon nanohorns/poly(glycine) modified glassy carbon electrode: Preparation, characterization and simultaneous electrochemical determination of uric acid, dopamine and ascorbic acid. J. Electroanal. Chem. 2016, 760, 24–31. [Google Scholar] [CrossRef]
- Wang, H.; Pan, L.; Liu, Y.; Ye, Y.; Yao, S. Electrochemical sensing of nitenpyram based on the binary nanohybrid of hydroxylated multiwall carbon nanotubes/single-wall carbon nanohorns. J. Electroanal. Chem. 2020, 862, 113955. [Google Scholar] [CrossRef]
- Cabello, C.; Rincón, S.; Bartolo, P.; Ruiz-Espinoza, J.; Zepeda, A. Incorporation of organic groups on the surface of multi-walled carbon nanotubes using an ultrasonic tip. Full-Nanotub. Carbon Nanostruct. 2018, 26, 502–509. [Google Scholar] [CrossRef]
- Kim, H.; Wang, M.; Lee, S.K.; Kang, J.; Nam, J.-D.; Ci, L.; Suhr, J. Tensile properties of millimeter-long multi-walled carbon nanotubes. Sci. Rep. 2017, 7, 9512. [Google Scholar] [CrossRef]
- Weng, G.-M.; Li, J.; Alhabeb, M.; Karpovich, C.; Wang, H.; Lipton, J.; Maleski, K.; Kong, J.; Shaulsky, E.; Elimelech, M. Layer-by-Layer Assembly of Cross-Functional Semi-transparent MXene-Carbon Nanotubes Composite Films for Next-Generation Electromagnetic Interference Shielding. Adv. Funct. Mater. 2018, 28, 1803360. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zbořil, R. Broad Family of Carbon Nanoallotropes: Classification; Chemistry; and Applications of Fullerenes; Carbon Dots; Nanotubes; Graphene; Nanodiamonds; and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Kamalian, S.; Shayeghi, M.; Yousefi, M.; Heidarinejad, Z.; Agarwal, S.; Gupta, V.K. High-performance removal of diazinon pesticide from water using multi-walled carbon nanotubes. Microchem. J. 2019, 145, 486–491. [Google Scholar] [CrossRef]
- Yadav, M.D.; Dasgupta, K.; Patwardhan, A.W.; Joshi, J.B. Controlling the carbon nanotube type with processing parameters synthesized by floating catalyst chemical vapour deposition. Mater. Today Proc. 2019, 18, 1039–1043. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, T.-K.; Peng, X.; Hu, J.; Yang, W. Catalyst effect on the preparation of single-walled carbon nanotubes by a modified arc discharge. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 52–57. [Google Scholar] [CrossRef]
- Ismail, R.A.; Mohsin, M.H.; Ali, A.K.; Hassoon, K.I.; Erten-Ela, S. Preparation and characterization of carbon nanotubes by pulsed laser ablation in water for optoelectronic application. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 119, 113997. [Google Scholar] [CrossRef]
- García-Carmona, L.; Moreno-Guzmán, M.; Sierra, T.; González, M.C.; Escarpa, A. Filtered carbon nanotubes-based electrodes for rapid sensing and monitoring of L-tyrosine in plasma and whole blood samples. Sens. Actuators B Chem. 2018, 259, 762–767. [Google Scholar] [CrossRef]
- Alagappan, M.; Immanuel, S.; Sivasubramanian, R.; Kandaswamy, A. Development of cholesterol biosensor using Au nanoparticles decorated f-MWCNT covered with polypyrrole network. Arab. J. Chem. 2020, 13, 2001–2010. [Google Scholar] [CrossRef]
- Viet, N.X.; Hoan, N.X.; Takamura, Y. Development of highly sensitive electrochemical immunosensor based on single-walled carbon nanotube modified screen-printed carbon electrode. Mater. Chem. Phys. 2019, 227, 123–129. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Jan, M.R. Ultrasensitive Electrical Biosensing of Proteins and DNA: Carbon-Nanotube Derived Amplification of the Recognition and Transduction Events. J. Am. Chem. Soc. 2004, 126, 3010–3011. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Dhanjai Mugo, S.M.; Zhao, H.; Chen, J.; Jain, R. Electrochemical Immunosensors for Rapid Detection of Breast Cancer Biomarkers. In Advanced Biosensors for Health Care Applications 2019; Elsevier: Amsterdam, The Netherlands, 2019; pp. 147–169. [Google Scholar] [CrossRef]
- Nie, Q.; Zhang, W.; Wang, L.; Guo, Z.; Li, C.; Yao, J.; Li, M.; Wu, D.; Zhou, L. Sensitivity enhanced, stability improved ethanol gas sensor based on multi-wall carbon nanotubes functionalized with Pt-Pd nanoparticles. Sens. Actuators B Chem. 2018, 270, 140–148. [Google Scholar] [CrossRef]
- Ma, X.; Tu, X.; Gao, F.; Xie, Y.; Huang, X.; Fernandez, C.; Qu, F.; Liu, G.; Lu, L.; Yu, Y. Hierarchical porous MXene/amino carbon nanotubes-based molecular imprinting sensor for highly sensitive and selective sensing of fisetin. Sens. Actuators B Chem. 2020, 309, 127815. [Google Scholar] [CrossRef]
- D’Souza, O.J.; Mascarenhas, R.J.; Satpati, A.K.; Aiman, L.V.; Mekhalif, Z. Electrocatalytic oxidation of l-tyrosine at carboxylic acid functionalized multi-walled carbon nanotubes modified carbon paste electrode. Ionics 2015, 22, 405–414. [Google Scholar] [CrossRef]
- Dyachkova, T.; Rukhov, A.; Tkachev, A.; Tugolukov, E.N. Functionalization of Carbon Nanotubes: Methods; Mechanisms and Technological Realization. Adv. Mater. Technol. 2018, 2, 018–041. [Google Scholar] [CrossRef]
- Hirsch, A. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed. Engl. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Sezer, N.; Koç, M. Oxidative acid treatment of carbon nanotubes. Surf. Interfaces 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Mallakpour, S.; Soltanian, S. Surface functionalization of carbon nanotubes: Fabrication and applications. RSC Adv. 2016, 6, 109916–109935. [Google Scholar] [CrossRef]
- Liu, J.; Ye, Y.; Xue, Y.; Xie, X.; Mai, Y.-W. Recent advances in covalent functionalization of carbon nanomaterials with polymers: Strategies and perspectives. J. Polym. Sci. Part A Polym. Chem. 2016, 55, 622–631. [Google Scholar] [CrossRef]
- Bai, L.; Li, Z.; Zhao, S.; Zheng, J. Covalent functionalization of carbon nanotubes with hydroxyl-terminated polydimethylsiloxane to enhance filler dispersion, interfacial adhesion and performance of poly(methylphenylsiloxane) composites. Compos. Sci. Technol. 2018, 165, 274–281. [Google Scholar] [CrossRef]
- Topcu, C.; Caglar, B.; Güner, E.K.; Coldur, F.; Caglar, S.; Yıldırım, Ö.; Özdokur, K.V.; Cubuk, O. Novel Copper(II)-Selective Potentiometric Sensor Based on a Folic Acid-Functionalized Carbon Nanotube Material. Anal. Lett. 2019, 52, 2524–2545. [Google Scholar] [CrossRef]
- Rashid, M.H.-O.; Ralph, S.F. Carbon Nanotube Membranes: Synthesis; Properties; and Future Filtration Applications. Nanomaterials 2017, 7, 99. [Google Scholar] [CrossRef]
- Ata, M.S.; Poon, R.; Syed, A.M.; Milne, J.; Zhitomirsky, I. New developments in non-covalent surface modification, dispersion and electrophoretic deposition of carbon nanotubes. Carbon 2018, 130, 584–598. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef] [PubMed]
- Erol, O.; Uyan, I.; Hatip, M.; Yılmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Boil. Med. 2017, 14, 2433–2454. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Patharkar, A.; Kuche, K.; Maheshwari‡, R.; Deb, P.K.; Kalia, K.; Tekade, R.K. Functionalized carbon nanotubes as emerging delivery system for the treatment of cancer. Int. J. Pharm. 2018, 548, 540–558. [Google Scholar] [CrossRef] [PubMed]
- Ménard-Moyon, C. Applications of Carbon Nanotubes in the Biomedical Field. In Smart Nanoparticles for Biomedicine 2018; Elsevier: Amsterdam, The Netherlands, 2018; pp. 83–101. [Google Scholar] [CrossRef]
- Sajid, M.I.; Jamshaid, U.; Jamshaid, T.; Zafar, N.; Fessi, H.; Elaissari, A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 2016, 501, 278–299. [Google Scholar] [CrossRef] [PubMed]
- Ajori, S.; Haghighi, S.; Ansari, R. A molecular dynamics study on the thermal conductivity of endohedrally functionalized single-walled carbon nanotubes with gold nanowires. Eur. Phys. J. D 2018, 72, 24. [Google Scholar] [CrossRef]
- Iglesias, D.; Melchionna, M. Enter the Tubes: Carbon Nanotube Endohedral Catalysis. Catalysts 2019, 9, 128. [Google Scholar] [CrossRef]
- Pagán, M.; Suazo-Dávila, D.; Del Toro, N.; Griebenow, K. A comparative study of different protein immobilization methods for the construction of an efficient nano-structured lactate oxidase-SWCNT-biosensor. Biosens. Bioelectron. 2015, 64, 138–146. [Google Scholar] [CrossRef]
- Nayak, L.; Rahaman, M.; Giri, R. Surface Modification/Functionalization of Carbon Materials by Different Techniques: An Overview. In Carbon-Containing Polymer Composites; Rahaman, M., Khastgir, D., Aldalbahi, A.K., Eds.; Springer: Singapore, 2019; pp. 65–98. [Google Scholar]
- Antonucci, A.; Kupis-Rozmysłowicz, J.; Boghossian, A.A. Noncovalent Protein and Peptide Functionalization of Single-Walled Carbon Nanotubes for Biodelivery and Optical Sensing Applications. ACS Appl. Mater. Interfaces 2017, 9, 11321–11331. [Google Scholar] [CrossRef]
- Sudha, V.; Kumar, S.M.S.; Thangamuthu, R. Simultaneous electrochemical sensing of sulphite and nitrite on acid-functionalized multi-walled carbon nanotubes modified electrodes. J. Alloy. Compd. 2018, 749, 990–999. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, F.; Huang, L.; Wang, L. Electrochemical nonenzymatic sensor based on cetyltrimethylammonium bromide and chitosan functionalized carbon nanotube modified glassy carbon electrode for the determination of hydroxymethanesulfinate in the presence of sulfite in foods. Food Chem. 2018, 259, 213–218. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Khoshroo, A.; Hosseinzadeh, L. Simultaneous determination of hydrazine and hydroxylamine based on fullerene-functionalized carbon nanotubes/ionic liquid nanocomposite. Sens. Actuators B Chem. 2015, 214, 132–137. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Khoshroo, A.; Mazloum-Ardakani, M. Electrochemical determination of diazepam in real samples based on fullerene-functionalized carbon nanotubes/ionic liquid nanocomposite. Sens. Actuators B Chem. 2017, 240, 125–131. [Google Scholar] [CrossRef]
- Devi, R.K.; Muthusankar, G.; Gopu, G.; Berchmans, L.J. A simple self-assembly fabrication of tin oxide nanoplates on multiwall carbon nanotubes for selective and sensitive electrochemical determination of antipyretic drug. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124825. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Y.; Leblanc, R.M. Recent development of carbon quantum dots regarding their optical properties, photoluminescence mechanism, and core structure. Nanoscale 2019, 11, 4634–4652. [Google Scholar] [CrossRef]
- Zhao, D.L.; Chung, T.-S. Applications of carbon quantum dots (CQDs) in membrane technologies: A review. Water Res. 2018, 147, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Kim, J.; Malgras, V.; Reddy, K.R.; Ward, A.C.; You, J.; Bando, Y.; Hossain, S.A.; Yamauchi, Y. Recent Advances in Graphene Quantum Dots: Synthesis; Properties; and Applications. Small Methods 2018, 2, 1800050. [Google Scholar] [CrossRef]
- Chen, F.; Gao, W.; Qiu, X.; Zhang, H.; Liu, L.; Liao, P.; Fu, W.; Luo, Y. Graphene quantum dots in biomedical applications: Recent advances and future challenges. Front. Lab. Med. 2017, 1, 192–199. [Google Scholar] [CrossRef]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Xu, T.; Liao, H.; Yao, C.; Liu, Y.; Li, Z.; Chen, Z.; Pan, D.; Sun, L.; et al. Gram-scale synthesis of single-crystalline graphene quantum dots with superior optical properties. Nat. Commun. 2014, 5, 5357. [Google Scholar] [CrossRef]
- Bak, S.; Kim, D.; Lee, H. Graphene quantum dots and their possible energy applications: A review. Curr. Appl. Phys. 2016, 16, 1192–1201. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Liang, R.; Tjandra, R.; Yu, A. Graphene quantum dot induced tunable growth of nanostructured MnCo2O4.5 composites for high-performance supercapacitors. Sustain. Energy Fuels 2019, 3, 2499–2508. [Google Scholar] [CrossRef]
- Chao, D.; Zhu, C.; Xia, X.; Liu, J.; Zhang, X.; Wang, J.; Liang, P.; Lin, J.; Zhang, H.; Shen, Z.X.; et al. Graphene quantum dots coated VO2 arrays for highly durable electrodes for Li and Na ion batteries. Nano Lett. 2014, 15, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Yang, L.; Li, R.S.; Chen, B.B.; Liu, H.; Huang, C.Z. Large-scale simultaneous synthesis of highly photoluminescent green amorphous carbon nanodots and yellow crystalline graphene quantum dots at room temperature. Green Chem. 2017, 19, 3611–3617. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing Graphene Quantum Dots and Carbon Dots: Properties; Syntheses; and Biological Applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Wu, N. Fluorescence and Sensing Applications of Graphene Oxide and Graphene Quantum Dots: A Review. Chem. Asian J. 2017, 12, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-Doped Graphene Quantum Dots with Oxygen-Rich Functional Groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef]
- Xu, Q.; Mi, H.-Y.; Liu, Y.; Cai, L.; Peng, X.; Sreeprasad, T.S.; Zhao, P.; Yu, Z.; Li, N. Heteroatom-doped carbon dots: Synthesis; characterization; properties; photoluminescence mechanism and biological applications. J. Mater. Chem. B 2016, 4, 7204–7219. [Google Scholar] [CrossRef]
- Tan, X.; Li, Y.; Zhou, S.; Fan, L.; Yang, S. Electrochemical synthesis of small-sized red fluorescent graphene quantum dots as a bioimaging platform. Chem. Commun. 2015, 51, 2544–2546. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Pang, H.; Bin Yang, H.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef]
- Li, X.; Rui, M.; Song, J.; Shen, Z.; Zeng, H. Carbon and Graphene Quantum Dots for Optoelectronic and Energy Devices: A Review. Adv. Funct. Mater. 2015, 25, 4929–4947. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of graphene and related materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef]
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent Advances on Graphene Quantum Dots: From Chemistry and Physics to Applications. Adv. Mater. 2019, 31, 1808283. [Google Scholar] [CrossRef] [PubMed]
- Shinde, D.B.; Pillai, V.K. Electrochemical Resolution of Multiple Redox Events for Graphene Quantum Dots. Angew. Chem. Int. Ed. 2013, 52, 2482–2485. [Google Scholar] [CrossRef] [PubMed]
- Kunpatee, K.; Traipop, S.; Chailapakul, O.; Chuanuwatanakul, S. Simultaneous determination of ascorbic acid; dopamine; and uric acid using graphene quantum dots/ionic liquid modified screen-printed carbon electrode. Sens. Actuators B Chem. 2020, 314, 128059. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Zheng, J. Electrochemical behavior of modified electrodes with carbon nanotubes and nanofibers: Application to the sensitive measurement of uric acid in the presence of ascorbic acid. Measurement. 2015, 59, 177–183. [Google Scholar] [CrossRef]
- Rezaei, B.; Ghani, M.; Shoushtari, A.M.; Rabiee, M. Electrochemical biosensors based on nanofibres for cardiac biomarker detection: A comprehensive review. Biosens. Bioelectron. 2016, 78, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Hyun, Y. Synthesis and Characteristics of Carbon Nanofibers/Silicon Composites and Application to Anode Materials of Li Secondary Batteries. Nanofiber Res. Reach. New Heights 2016. [Google Scholar] [CrossRef]
- Matlock-Colangelo, L.; Baeumner, A.J. Recent progress in the design of nanofiber-based biosensing devices. Lab Chip 2012, 12, 2612. [Google Scholar] [CrossRef]
- Macagnano, A.; Zampetti, E.; Kny, E. (Eds.) Electrospinning for High Performance Sensors, 2015th ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Sadir, S.; Prabhakaran, M.P.; Wicaksono, D.H.B.; Ramakrishna, S. Fiber based enzyme-linked immunosorbent assay for C-reactive protein. Sens. Actuators B Chem. 2014, 205, 50–60. [Google Scholar] [CrossRef]
- Kimuam, K.; Rodthongkum, N.; Ngamrojanavanich, N.; Chailapakul, O.; Ruecha, N. Single step preparation of platinum nanoflowers/reduced graphene oxide electrode as a novel platform for diclofenac sensor. Microchem. J. 2020, 155, 104744. [Google Scholar] [CrossRef]
- Apetrei, I.; Apetrei, C. A modified nanostructured graphene-gold nanoparticle carbon screen-printed electrode for the sensitive voltammetric detection of rutin. Measurement 2018, 114, 37–43. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Voltammetric determination of melatonin using a graphene-based sensor in pharmaceutical products. Int. J. Nanomed. 2016, 11, 1859–1866. [Google Scholar] [CrossRef]
- Palisoc, S.; Sow, V.A.; Natividad, M.T. Fabrication of a bismuth nanoparticle/Nafion modified screen-printed graphene electrode for in situ environmental monitoring. Anal. Methods 2019, 11, 1591–1603. [Google Scholar] [CrossRef]
- Charoenkitamorn, K.; Chaiyo, S.; Chailapakul, O.; Siangproh, W. Low-cost and disposable sensors for the simultaneous determination of coenzyme Q10 and α-lipoic acid using manganese (IV) oxide-modified screen-printed graphene electrodes. Anal. Chim. Acta 2018, 1004, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Dechtrirat, D.; Sookcharoenpinyo, B.; Prajongtat, P.; Sriprachuabwong, C.; Sanguankiat, A.; Tuantranont, A.; Hannongbua, S. An electrochemical MIP sensor for selective detection of salbutamol based on a graphene/PEDOT:PSS modified screen printed carbon electrode. RSC Adv. 2018, 8, 206–212. [Google Scholar] [CrossRef]
- Chen, S.-M.; Manavalan, S.; Rajaji, U.; Govindasamy, M.; Chen, T.-W.; Ali, M.A.; Alnakhli, A.K.; Al-Hemaid, F.M.A.; Elshikh, M.S. Determination of the antioxidant propyl gallate in meat by using a screen-printed electrode modified with CoSe2 nanoparticles and reduced graphene oxide. Microchim. Acta 2018, 185, 520. [Google Scholar] [CrossRef] [PubMed]
- Rowley-Neale, S.J.; Brownson, D.A.C.; Smith, G.; Banks, C.E. Graphene Oxide Bulk-Modified Screen-Printed Electrodes Provide Beneficial Electroanalytical Sensing Capabilities. Biosensors 2020, 10, 27. [Google Scholar] [CrossRef]
- Chaiyo, S.; Jampasa, S.; Thongchue, N.; Mehmeti, E.; Siangproh, W.; Chailapakul, O.; Kalcher, K. Wide electrochemical window of screen-printed electrode for determination of rapamycin using ionic liquid/graphene composites. Microchim. Acta 2020, 187, 245. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Khajesharifi, H.; Thompson, M. Simultaneous Determination of Streptomycin and Oxytetracycline Using a Oracet-Blue/ Silver-Nanoparticle/ Graphene-Oxide/ Modified Screen-Printed Electrode. Biosensors 2020, 10, 23. [Google Scholar] [CrossRef]
- Muthumariappan, A.; Govindasamy, M.; Chen, S.-M.; Sakthivel, K.; Mani, V. Screen-printed electrode modified with a composite prepared from graphene oxide nanosheets and Mn3O4 microcubes for ultrasensitive determination of nitrite. Microchim. Acta 2017, 184, 3625–3634. [Google Scholar] [CrossRef]
- Govindasamy, M.; Mani, V.; Chen, S.-M.; Chen, T.-W.; Sundramoorthy, A.K. Methyl parathion detection in vegetables and fruits using silver@graphene nanoribbons nanocomposite modified screen printed electrode. Sci. Rep. 2017, 7, 46471. [Google Scholar] [CrossRef]
- Redin, G.G.I.; Wilson, D.; Gonçalves, D.; Oliveira, O. Low-cost screen-printed electrodes based on electrochemically reduced graphene oxide-carbon black nanocomposites for dopamine, epinephrine and paracetamol detection. J. Colloid Interface Sci. 2018, 515, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Seifati, S.M.; Nasirizadeh, N.; Azimzadeh, M. Nano-biosensor based on reduced graphene oxide and gold nanoparticles, for detection of phenylketonuria-associated DNA mutation. IET Nanobiotechnol. 2018, 12, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Dinu, A.; Apetrei, C. A Review on Electrochemical Sensors and Biosensors Used in Phenylalanine Electroanalysis. Sensors 2020, 20, 2496. [Google Scholar] [CrossRef] [PubMed]
- Uygun, H.D.E.; Uygun, Z.O.; Canbay, E.; Sağın, F.G.; Sezer, E.D.; Ertuğrul, H.D.; Sağin, F.G. Non-invasive cortisol detection in saliva by using molecularly cortisol imprinted fullerene-acrylamide modified screen printed electrodes. Talanta 2020, 206, 120225. [Google Scholar] [CrossRef]
- Palanisamy, S.; Thirumalraj, B.; Chen, S.-M. Electrochemical fabrication of gold nanoparticles decorated on activated fullerene C60: An enhanced sensing platform for trace level detection of toxic hydrazine in water samples. RSC Adv. 2015, 5, 94591–94598. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Hosseinzadeh, L.; Khoshroo, A. Label-free electrochemical immunosensor for detection of tumor necrosis factor α based on fullerene-functionalized carbon nanotubes/ionic liquid. J. Electroanal. Chem. 2015, 757, 58–64. [Google Scholar] [CrossRef]
- Yi, Y.; Kingsford, O.J.; Fiston, M.N.; Qian, J.; Liu, Z.; Liu, L.; Zhu, G. Perylenetetracarboxylic acid noncovalently functionalizes carbon nanohorn nanohybrids for electrochemical sensing of 4;4′-diaminobiphenyl. J. Electroanal. Chem. 2017, 801, 38–42. [Google Scholar] [CrossRef]
- Sánchez-Tirado, E.; González-Cortés, A.; Yudasaka, M.; Iijima, S.; Langa, F.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical immunosensor for the determination of 8-isoprostane aging biomarker using carbon nanohorns-modified disposable electrodes. J. Electroanal. Chem. 2017, 793, 197–202. [Google Scholar] [CrossRef]
- Valentini, F.; Ciambella, E.; Boaretto, A.; Rizzitelli, G.; Carbone, M.; Conte, V.; Cataldo, F.; Russo, V.; Casari, C.S.; Martino, D.F.C.; et al. Sensor Properties of Pristine and Functionalized Carbon Nanohorns. Electroanalysis 2016, 28, 2489–2499. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, L.; Wang, T.; Han, Q.; Xu, S. A high-performance hydrazine electrochemical sensor based on gold nanoparticles/single-walled carbon nanohorns composite film. Appl. Surf. Sci. 2016, 369, 36–42. [Google Scholar] [CrossRef]
- Phonklam, K.; Wannapob, R.; Sriwimol, W.; Thavarungkul, P.; Phairatana, T. A novel molecularly imprinted polymer PMB/MWCNTs sensor for highly-sensitive cardiac troponin T detection. Sens. Actuators B Chem. 2020, 308, 127630. [Google Scholar] [CrossRef]
- Pasakon, P.; Mensing, J.P.; Phokaratkul, D.; Karuwan, C.; Lomas, T.; Wisitsoraat, A.; Tuantranont, A. A high-performance, disposable screen-printed carbon electrode modified with multi-walled carbon nanotubes/graphene for ultratrace level electrochemical sensors. J. Appl. Electrochem. 2018, 49, 217–227. [Google Scholar] [CrossRef]
- Jeromiyas, N.; Elaiyappillai, E.; Kumar, A.S.; Huang, S.-T.; Mani, V. Bismuth nanoparticles decorated graphenated carbon nanotubes modified screen-printed electrode for mercury detection. J. Taiwan Inst. Chem. Eng. 2019, 95, 466–474. [Google Scholar] [CrossRef]
- Muhammad, A.; Hajian, R.; Yusof, N.; Shams, N.; Abdullah, J.; Woi, P.M.; Garmestani, H. A screen printed carbon electrode modified with carbon nanotubes and gold nanoparticles as a sensitive electrochemical sensor for determination of thiamphenicol residue in milk. RSC Adv. 2018, 8, 2714–2722. [Google Scholar] [CrossRef]
- Kunene, K.; Sabela, M.; Kanchi, S.; Bisetty, K. High Performance Electrochemical Biosensor for Bisphenol A Using Screen Printed Electrodes Modified with Multiwalled Carbon Nanotubes Functionalized with Silver-Doped Zinc Oxide. Waste Biomass Valorization 2018, 11, 1085–1096. [Google Scholar] [CrossRef]
- Hadi, M.; Ahmadvand, E.; Ehsani, A. Electroanalytical Sensing of Piperazine at Carbon Nanotubes/Nafion Composite-modified Glassy Carbon and Screen-printed Carbon Electrodes in Human Plasma. J. Anal. Chem. 2020, 75, 238–245. [Google Scholar] [CrossRef]
- Ji, D.; Xu, N.; Liu, Z.; Shi, Z.; Low, S.S.; Liu, J.; Cheng, C.; Zhu, J.; Zhang, T.; Xu, H.; et al. Smartphone-based differential pulse amperometry system for real-time monitoring of levodopa with carbon nanotubes and gold nanoparticles modified screen-printing electrodes. Biosens. Bioelectron. 2019, 129, 216–223. [Google Scholar] [CrossRef]
- Dos Santos, W.T.; Compton, R.G. A simple method to detect the stimulant modafinil in authentic saliva using a carbon-nanotube screen-printed electrode with adsorptive stripping voltammetry. Sens. Actuators B Chem. 2019, 285, 137–144. [Google Scholar] [CrossRef]
- Hassan, S.S.; Kamel, A.H.; Amr, A.E.-G.E.; Hashem, H.M.; Bary, E.M.A. Imprinted Polymeric Beads-Based Screen-Printed Potentiometric Platforms Modified with Multi-Walled Carbon Nanotubes (MWCNTs) for Selective Recognition of Fluoxetine. Nanomaterials 2020, 10, 572. [Google Scholar] [CrossRef]
- Sasal, A.; Tyszczuk-Rotko, K.; Wójciak, M.; Sowa, I. First Electrochemical Sensor (Screen-Printed Carbon Electrode Modified with Carboxyl Functionalized Multiwalled Carbon Nanotubes) for Ultratrace Determination of Diclofenac. Materials 2020, 13, 781. [Google Scholar] [CrossRef]
- Serrano, N.; Castilla, Ò.; Ariño, C.; Díaz-Cruz, M.S.; Díaz-Cruz, J. Commercial Screen-Printed Electrodes Based on Carbon Nanomaterials for a Fast and Cost-Effective Voltammetric Determination of Paracetamol; Ibuprofen and Caffeine in Water Samples. Sensors 2019, 19, 4039. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, M.R.; Dourandish, Z.; Beitollahi, H.; Tajik, S.; Hajiaghababaei, L.; Larijani, B. Highly Sensitive Determination of Theophylline Based on Graphene Quantum Dots Modified Electrode. Int. J. Electrochem. Sci. 2018, 2448–2461. [Google Scholar] [CrossRef]
- Beitollahi, H.; Dourandish, Z.; Ganjali, M.; Shakeri, S. Voltammetric determination of dopamine in the presence of tyrosine using graphite screen-printed electrode modified with graphene quantum dots. Ionics 2018, 24, 4023–4031. [Google Scholar] [CrossRef]
- Gevaerd, A.; Banks, C.E.; Bergamini, M.F.; Marcolino-Junior, L.H. Nanomodified Screen-Printed Electrode for direct determination of Aflatoxin B1 in malted barley samples. Sens. Actuators B Chem. 2020, 307, 127547. [Google Scholar] [CrossRef]
- Gevaerd, A.; Banks, C.E.; Bergamini, M.F.; Marcolino-Junior, L.H. Graphene Quantum Dots Modified Screen-printed Electrodes as Electroanalytical Sensing Platform for Diethylstilbestrol. Electroanalysis 2019, 31, 838–843. [Google Scholar] [CrossRef]
- Roushani, M.; Jalilian, Z.; Nezhadali, A. Screen printed carbon electrode sensor with thiol graphene quantum dots and gold nanoparticles for voltammetric determination of solatol. Heliyon 2019, 5, e01984. [Google Scholar] [CrossRef]
- Mollarasouliab, F.; Serafín, V.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M.; Asadpour-Zeynali, K. Ultrasensitive determination of receptor tyrosine kinase with a label-free electrochemical immunosensor using graphene quantum dots-modified screen-printed electrodes. Anal. Chim. Acta 2018, 1011, 28–34. [Google Scholar] [CrossRef]
- Dourandish, Z.; Beitollahi, H. Electrochemical sensing of isoproterenol using graphite screen-printed electrode modified with graphene quantum dots. Anal. Bioanal. Chem. 2018, 10, 192–202. [Google Scholar]
- Pathak, P.K.; Kumar, A.; Prasad, B.B. Functionalized nitrogen doped graphene quantum dots and bimetallic Au/Ag core-shell decorated imprinted polymer for electrochemical sensing of anticancerous hydroxyurea. Biosens. Bioelectron. 2019, 127, 10–18. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Doong, R.-A. Functionalized N-doped graphene quantum dots for electrochemical determination of cholesterol through host-guest inclusion. Microchim. Acta 2018, 185, 526. [Google Scholar] [CrossRef]
- Punrat, E.; Maksuk, C.; Chuanuwatanakul, S.; Wonsawat, W.; Chailapakul, O. Polyaniline/graphene quantum dot-modified screen-printed carbon electrode for the rapid determination of Cr(VI) using stopped-flow analysis coupled with voltammetric technique. Talanta 2016, 150, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, I.M.; Bejinaru, A.A.; Boev, M.; Apetrei, C.; Buzia, O.D. Determination of ibuprofen based on screen-printed electrodes modified with carbon nanofibers. Farmacia 2017, 65, 5. [Google Scholar]
- Apetrei, I.M.; Apetrei, C. Highly sensitive voltamperometric determination of pyritinol using carbon nanofiber/gold nanoparticle composite screen-printed carbon electrode. IJN 2017, 12, 5177–5188. [Google Scholar] [CrossRef]
- Sasal, A.; Tyszczuk-Rotko, K.; Chojecki, M.; Korona, T.; Nosal-Wiercińska, A. Direct Determination of Paracetamol in Environmental Samples Using Screen-printed Carbon/Carbon Nanofibers Sensor- Experimental and Theoretical Studies. Electroanalysis 2020. [Google Scholar] [CrossRef]
- Tyszczuk-Rotko, K.; Pietrzak, K.; Sasal, A. Adsorptive stripping voltammetric method for the determination of caffeine at integrated three-electrode screen-printed sensor with carbon/carbon nanofibers working electrode. Adsorption 2019, 25, 913–921. [Google Scholar] [CrossRef]
- Nellaiappan, S.; Kumar, A.S. Electrocatalytic oxidation and flow injection analysis of isoniazid drug using a gold nanoparticles decorated carbon nanofibers-chitosan modified carbon screen printed electrode in neutral pH. J. Electroanal. Chem. 2017, 801, 171–178. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Sun, X.; Guo, Y. Multiplex electrochemical aptasensor for detecting multiple antibiotics residues based on carbon fiber and mesoporous carbon-gold nanoparticles. Sens. Actuators B Chem. 2018, 265, 217–226. [Google Scholar] [CrossRef]
- Muschietti, A.; Serrano, N.; Ariño, C.; Díaz-Cruz, M.S.; Díaz-Cruz, J.M. Screen-Printed Electrodes for the Voltammetric Sensing of Benzotriazoles in Water. Sensors 2020, 20, 1839. [Google Scholar] [CrossRef]
- Nellaiappan, S.; Pillai, K.C.; Kumar, A.S.; Subramanain, N.; Pillai, P.C. Flow-injection analysis coupled with electrochemical detection of poisonous inorganic arsenic(iii) species using a gold nanoparticle/carbon nanofiber/chitosan chemically modified carbon screen printed electrode in neutral pH solution. Anal. Methods 2018, 10, 799–808. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).