Constructing Strategies and Applications of Nitrogen-Rich Energetic Metal–Organic Framework Materials
Abstract
:1. Introduction
2. The Family of EMOF Materials
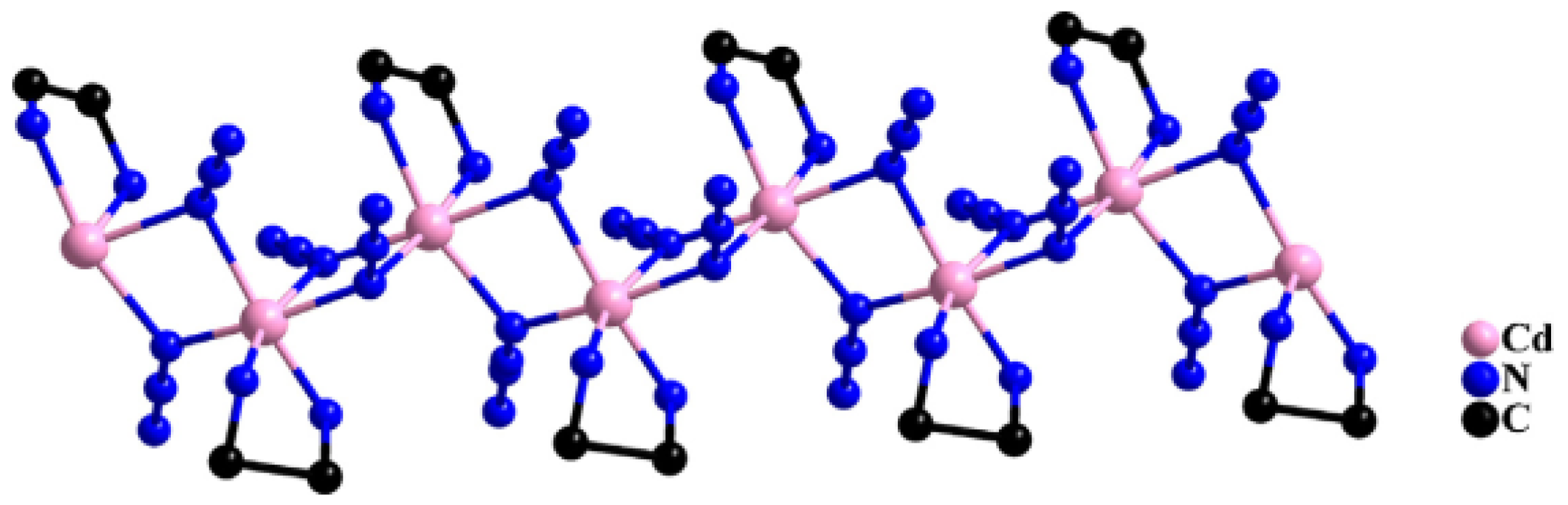
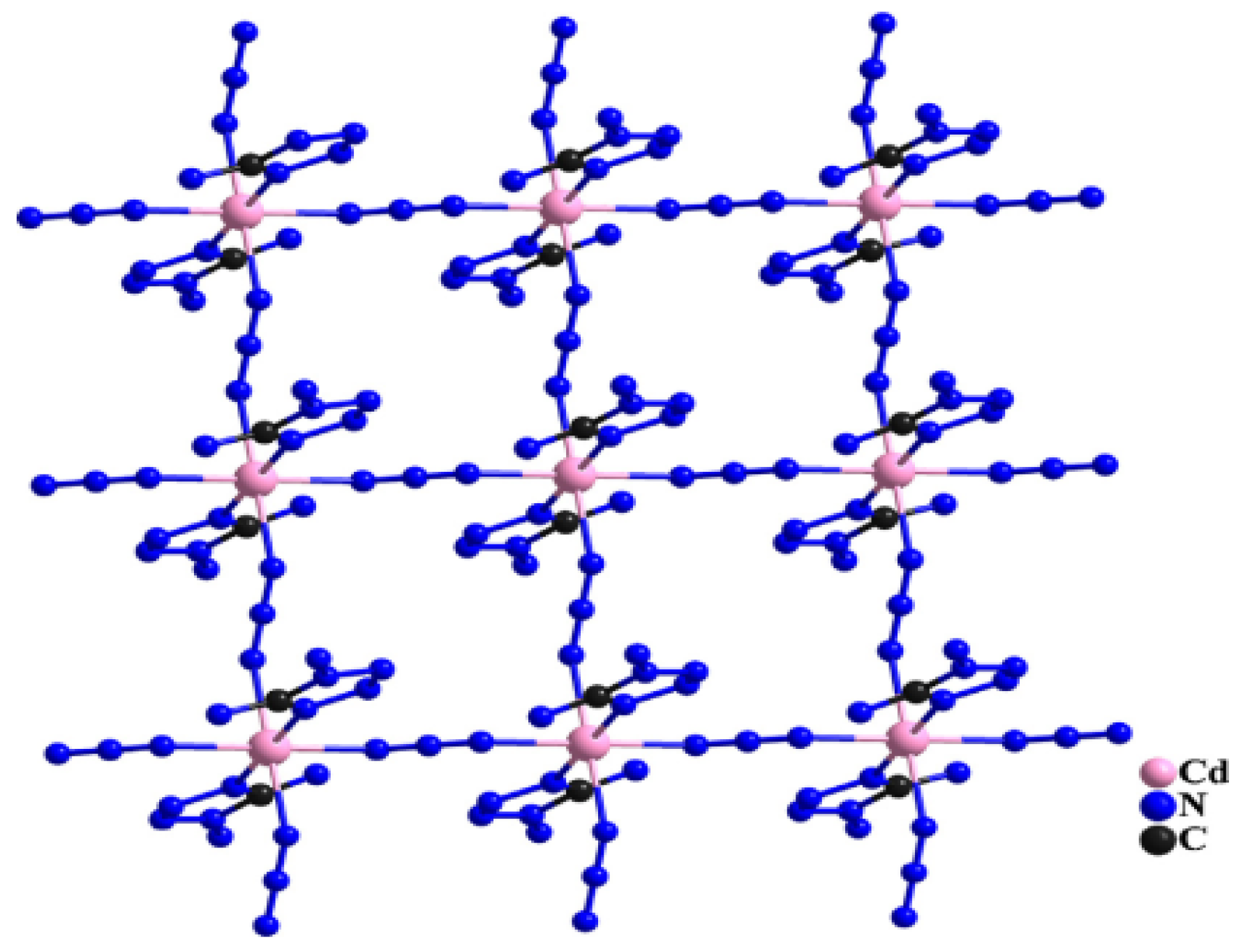
2.1. Materials of 1D, 2D and 3D EMOFs
2.2. Neutral, Cationic and Anion EMOFs
3. Constructing Strategies of N-Rich EMOFs
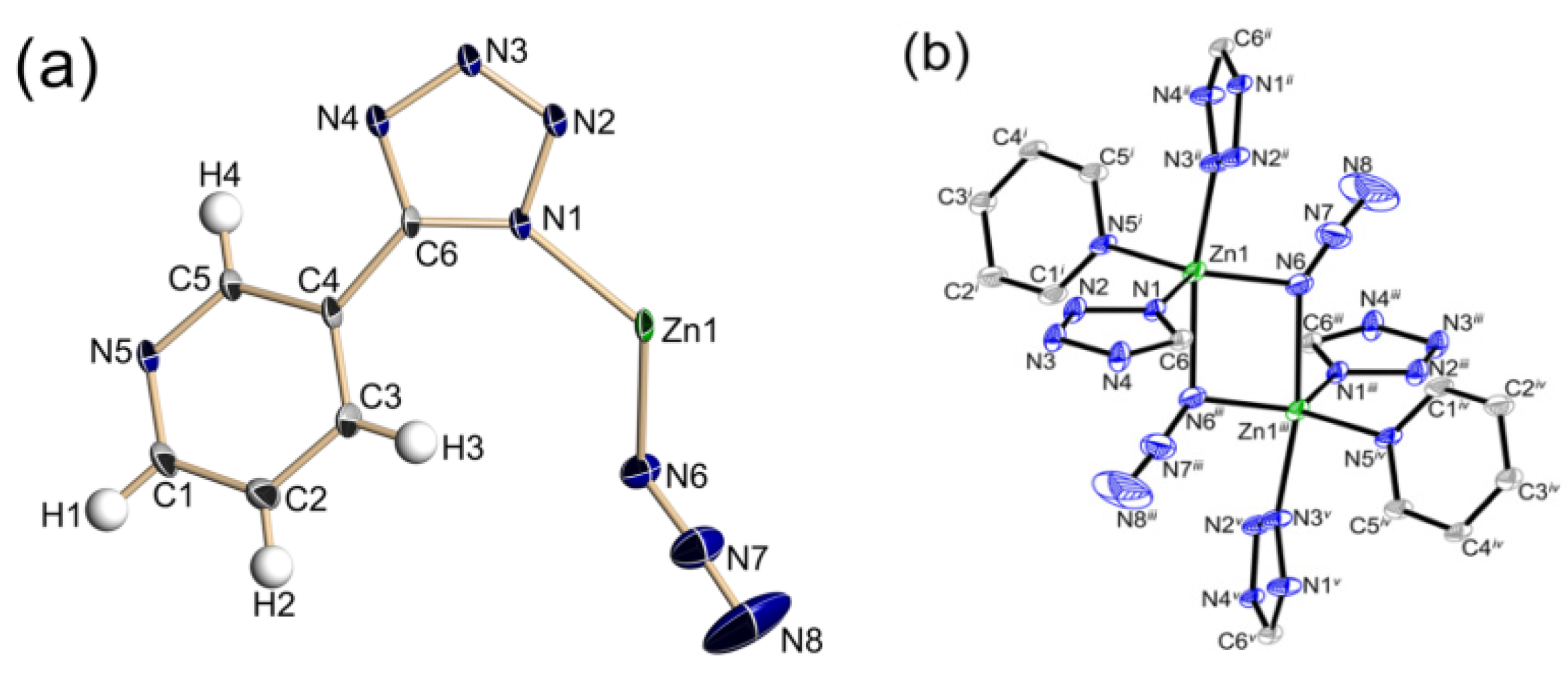
3.1. Energetic Ligands
3.2. Ion-Exchange Modification
3.3. GO-Cu(II) Modification
4. Applications of Nitrogen-Rich EMOFs
4.1. Green Primary Explosives
4.2. Heat-Resistant Explosives
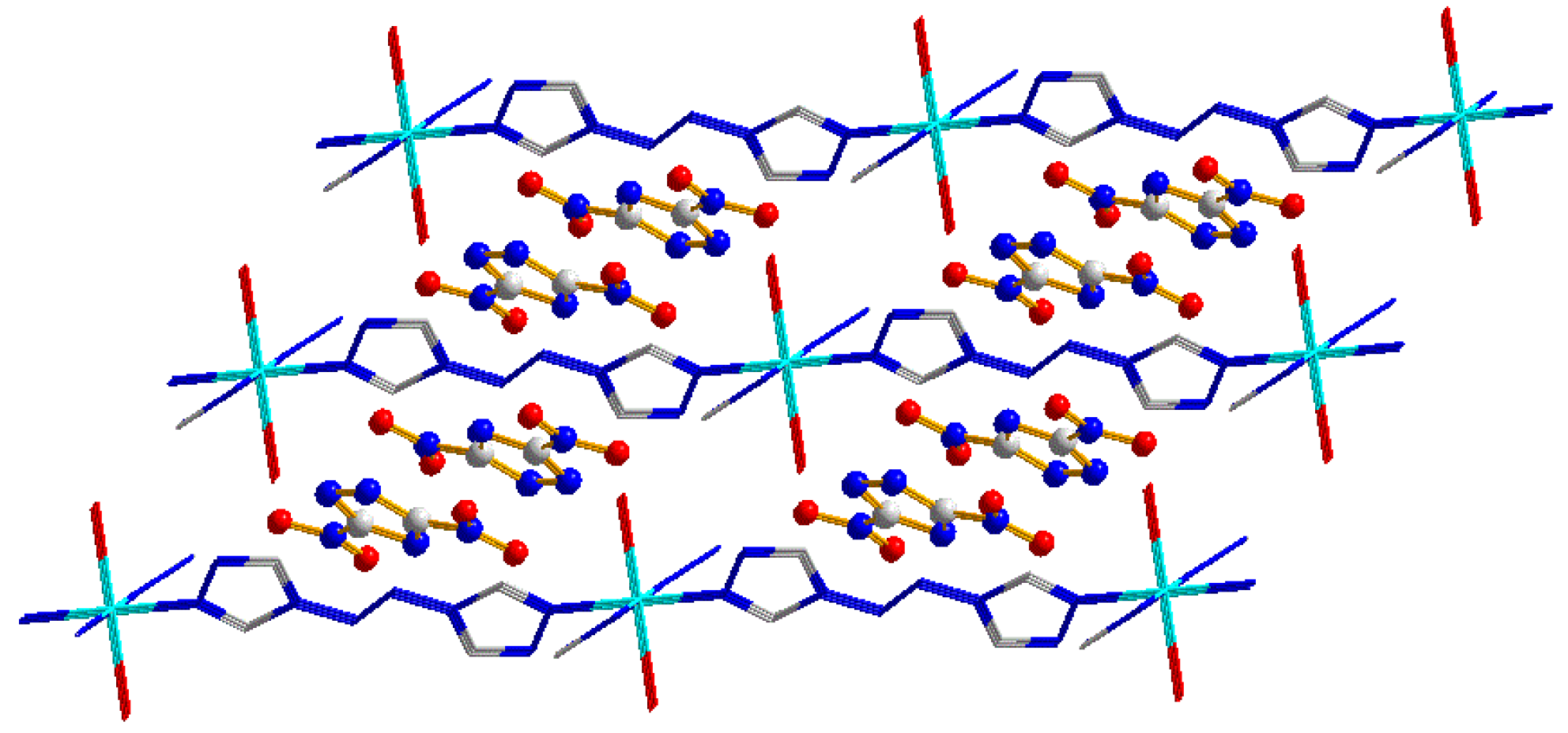
4.3. EMOFs-Based Metastable Composites
4.4. Catalysts
5. Conclusions and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yaghi, O.M.; Li, H.L.; Davis, C.; Richardson, D.; Groy, T.L. Synthetic strategies, structure patterns, and emerging properties in the chemistry of modular porous solids. Acc. Chem. Res. 1998, 31, 474–484. [Google Scholar] [CrossRef]
- Wang, B.; Cote, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Colossal cages in zeoliticimidazolate frameworks as selective carbon dioxide reservoirs. Nature 2008, 453, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bu, X.H.; Li, N.; Xu, J.; Hu, T.L.; Bu, X.H. Governing metal-organic frameworks towards high stability. Chem. Commun. 2016, 52, 8501–8513. [Google Scholar]
- Chen, Q.; Chang, Z.; Song, W.C.; Song, H.; Song, H.B.; Hu, T.L.; Bu, X.H. A controllable gate effect in cobalt(II) organic frameworks by reversible sturcture transformations. Angew. Chem. Int. Ed. Engl. 2013, 52, 11550–11553. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Tao, Y.; Yu, Q.; Bu, X.H.; Sakamoto, H.; Kitagawa, S. Selective gas adsorption and unique structural topology of a highly stable guest-free zeolite-type MOF material with N-riched chiral open channels. Chem. Eur. J. 2008, 14, 2771–2776. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.Y.; Leblanc, R.J.; Xie, S.Y.; Yue, Y.F. Recent progress in the syntheses of mesoporous metal-organic framework materials. Coordin. Chem. Rev. 2018, 369, 76–90. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.Y.; Feng, X.; Wang, B. Explosives in the cage: Metal-organic frameworks for high-Energy materials sensing and desensitization. Adv. Mater. 2017, 29, 1701898. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhu, Q.L.; Zou, R.Q.; Xu, Q. Metal-organic frameworks for energy applications. Chem 2017, 2, 52–80. [Google Scholar] [CrossRef] [Green Version]
- He, Y.B.; Chen, F.L.; Li, B.; Qian, G.D.; Zhou, W.; Chen, B.L. Porous metal-organic frameworks for fuel storage. Coordin. Chem. Rev. 2018, 373, 167–198. [Google Scholar] [CrossRef]
- Junghans, U.; Kobalz, M.; Erhart, O.; Preißler, H.; Lincke, J.; Möllmer, J.; Krautscheid, H.; Gläser, R. A series of robust copper-based triazolylisophthalateMOFs: Impact of linker functionalization on gas sorption and catalytic activity. Materials 2017, 10, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.X.; Doonan, C.J.; Furukawa, H.; Ferreira, R.B.; Towne, J.; Knobler, C.B.; Wang, B.; Yaghi, O.M. Multiple functional groups of varying ratios in metal-organic frameworks. Science 2010, 327, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.C.; Saines, P.J.; Bithell, E.G.; Cheetham, A.K. Hybrid nanosheets of an inorganic-organic framework material: Facile synthesis, structure, and elastic properties. ACS Nano 2012, 6, 615–621. [Google Scholar] [CrossRef]
- Xiang, Z.H.; Leng, S.H.; Cao, D.P. Functional group modification of metal-organic frameworks for CO2 capture. J. Phys. Chem. C 2012, 116, 10573–10579. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal engineering: From molecules to materials. J. Mol. Struct. 2003, 67, 5–15. [Google Scholar] [CrossRef]
- Sun, T.J.; Ren, X.Y.; Hu, J.L.; Wang, S.D. Expanding pore size of Al-BDC metal-organic frameworks as a way to achieve high adsorptionselectivity for CO2/CH4 separation. J. Phys. Chem. C 2014, 118, 15630–15639. [Google Scholar] [CrossRef]
- Yang, L.Z.; Xu, C.L.; Ye, W.C.; Liu, W.S. An electrochemical sensor for H2O2 based on a new Co-metal-organic framework modified electrode. Sens. Actuators B Chem. 2015, 215, 489–496. [Google Scholar] [CrossRef]
- Singha, D.K.; Mahata, P. Highly selective and sensitive luminescence turn-on-based sensing of Al3+ ions in aqueous medium using a MOF with free functional sites. Inorg. Chem. 2015, 54, 6373–6379. [Google Scholar] [CrossRef]
- Shi, L.B.; Zhu, X.; Liu, T.T.; Zhao, H.L.; Lan, M.B. Encapsulating Cu nanoparticles into metal-organic frameworks for nonenzymatic glucose sensing. Sens. Actuators B Chem. 2016, 227, 583–590. [Google Scholar] [CrossRef]
- Yadav, M.; Bhunia, A.; Roesky, P.W.; Roesky, P.W. Manganese-and lanthanide-based 1D chiral coordination polymers as an enantioselective catalyst for sulfoxidation. Inorg. Chem. 2016, 55, 2701–2708. [Google Scholar] [CrossRef]
- Kim, B.R.; Oh, J.S.; Kim, J.; Lee, C.Y. Robust aerobic alcohol oxidation catalyst derived from metal-organic frameworks. Catal. Lett. 2016, 146, 734–743. [Google Scholar] [CrossRef]
- Xu, S.; Meng, W.; Dai, L.; He, Z.X.; Wang, L. Application of porous materials with metal-organic framework. Chin. J. North China Univ. Sci. Techno. (Nat. Sci. Ed.) 2016, 38, 45–56. [Google Scholar]
- Zhang, H.B.; Zhang, M.J.; Lin, P.; Malgras, V.; Tang, J.; Alshehri, S.M.; Yamauchi, Y.; Du, S.W.; Zhang, J. A highly energetic N-rich metal-organic framework as a new high energy density material. Chem. Eur. J. 2016, 22, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Shreeve, J.M. 3D Nitrogen-rich metal–organic frameworksopportunities for safer energetics. Dalton. Trans. 2016, 45, 2363–2368. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Jin, B.; Zhang, Q.C.; Shang, Y. Nitrogen-rich energetic metal-organic framework: Synthesis, structure, properties, and thermal behaviors of Pb(II) complex based on N,N-bis(1H-tetraxole-5-yl)-amine. Materials 2016, 9, 681. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Lu, J.Y. A new tri-nuclear Ag(I) MOF as potential energetic materials constructed using (1e)-N’-hydroxy-2-(1H-1,2,4-triazol-1-yl)ethanimidamide. Inorg. Chem. Commun. 2017, 84, 33–35. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, S.; Zhang, W.D.; Yang, Q.; Wei, Q.; Xie, G.; Chen, S.P.; Gao, S.L.; Lu, J.Y. 3D solvent-free energetic metal-organic framework achieved by removing inclusion molecules from a new coordination polymer. CrystEngComm 2019, 21, 583–588. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, S.; Sun, L.; Yang, Q.; Han, J.; Wei, Q.; Xie, G.; Chen, S.P.; Gao, S.L. Solvent-free dense energetic metal-organic framework (EMOF): To improve stability and energetic performance via in situ microcalorimetry. Chem. Commun. 2017, 53, 3034–3037. [Google Scholar] [CrossRef]
- Friedrich, M.; Gálvez-Ruiz, J.C.; Klapötke, T.M.; Mayer, P.; Weber, B.; Weigand, J.J. BTA copper complexes. Inorg. Chem. 2005, 44, 8044–8052. [Google Scholar] [CrossRef]
- Joo, Y.H.; Shreeve, J.M. High-density energetic mono-or bis(oxy)-5-nitroiminotetrazoles. Angew. Chem. Int. Ed. 2010, 49, 7320–7323. [Google Scholar] [CrossRef]
- Cudzilo, S.; Nita, M. Synthesis and explosive properties of copper(II) chlorate(VII) coordination polymer with 4-amino-1,2,4-triazole bridging ligand, stanisławcudziło, marcinnita. J. Hazard. Mater. 2010, 177, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Y.; Liu, X.Y.; Duan, L.Q.; Yang, Q.; Wei, Q.; Xie, G.; Chen, S.P.; Yang, X.W.; Gao, S.L. In situ synthesized 3D heterometallic metal-organic framework(MOF)as a high-energy-density material shows high heat of detonation, good thermostability and insensitivity. Dalton. Trans. 2015, 44, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.D.; Li, Y.H.; Xu, J.G.; Zheng, F.K.; Guo, G.C.; Lv, R.X.; He, W.C.; Huang, Z.Z.; Liu, J.F. Stabilizing volatile axido in a 3D nitrogen-rich energetic metal-organic framework with excellent energetic performance. J. Solid State Chem. 2018, 265, 42–49. [Google Scholar] [CrossRef]
- Liu, X.; Qu, X.; Zhang, S.; Ke, H.S.; Yang, Q.; Shi, Q.; Wei, Q.; Xie, G.; Chen, S.P. High-performance energetic characteristics and magnetic properties of a three-dimensional Cobalt(Ⅱ) metal-organic framework assembled with azido and triazole. Inorg. Chem. 2015, 54, 11520–11525. [Google Scholar] [CrossRef]
- Guo, D.Z.; An, Q. Thermal stability and detonation properties of potassium 4,4′-bis(dinitromethyl)-3,3′-azofurazanate, an environmentally friendly energetic three-dimensional metal-organic framework. ACS Appl. Mater. Interfaces 2019, 11, 1512–1519. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; Brown, P.; Maiti, A.; Gee, R.H.; Peterson, G.R.; Weeks, B.L.; Hope-Weeks, L.J. Ionic polymers as a new structural motif for high-energy-density materials. J. Am. Chem. Soc. 2012, 134, 1422–1425. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Liu, X.Y.; Qu, X.N.; Wei, Q.; Xie, G.; Chen, S.P.; Gao, S.L. High-energy metal-organic framework(HE-MOFs): Synthesis, structure and energetic performance. Chem. Soc. Rev. 2016, 307, 292–312. [Google Scholar] [CrossRef]
- Zhang, J.C. Synthesis and Properties Study of Energetic Metal-Organic-Frameworks Materials. Ph.D. Thesis, Beijing Institute of Technology, Beijing, China, 2016. [Google Scholar]
- Yang, L.; Wu, B.D.; Zhang, T.L. Preparation, crystal structure, thermal decomposition and explosive properties of [Cd(en) (N3)2]n. Propell. Explos. Pyrot. 2010, 35, 521–528. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, J.G.; Liu, Z.H.; Zhang, T.L.; Yang, L.; Qiao, X.J. Synthesis, structural characterization and thermal analysis of a high nitrogen-contented cadmium(II) coordination polymer based on 1,5-diaminotetrazole. J. Mol. Struct. 2011, 1004, 8–12. [Google Scholar] [CrossRef]
- Ignacio, C.D.; Javier, E.; Carolina, M.; Fritz, R.A.; Vega, A.; Serafini, D.; Herrera., F.; Singh, D.P. Azide-based high-energy metal-oganic frameworks with enhanced thermal stability. ACS Omega 2019, 4, 14398–14403. [Google Scholar]
- Seth, S.; Matzger, A.J. Coordination polymerization of 5,5′-Dinitro-2 H, 2 H′-3,3′-bi-1,2,4-triazole leads to a dense explosive with high thermal stability. Inorg. Chem. 2017, 56, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Bushuyev, O.S.; Peterson, G.R.; Brown, P.; Maiti, A.; Gee, R.H.; Weeks, B.L.; Hope-Weeks, L.J. Metal-organicframeworks (MOFs) as safer, structurally reinforced energetic. Chem. Eur. J. 2013, 19, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Gao, W.J.; Sun, P.P.; Su, Z.Y.; Chen, S.P.; Wei, Q.; Xie, G.; Gao, S.L. Environmentally friendly high-energy MOFs: Crystal structures, thermostability, insensitivity and remarkable detonation performances. Green Chem. 2015, 17, 831–836. [Google Scholar] [CrossRef]
- Li, S.H.; Wang, Y.; Qi, C.; Zhao, J.Z.; Zhang, J.C.; Pang, S.P. 3D energetic metal-organic frameworks: Synthesis and properties of high energy materials. Ang. Chem. Int. Ed. 2013, 52, 14031–14035. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.N.; Zhang, S.; Wang, B.Z.; Yang, Q.; Han, J.; Wei, Q.; Xie, G.; Chen, S.P. Ag(I)Energetic metal-organic framework assembled with the energetic combination of furazan and tetrazole: Synthesis, structure and energetic performance. Dalton. Trans. 2016, 45, 6968–6973. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.X.; He, C.L.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.M. Potassium 4,4-bis(dinitromethyl)-3,3-azofurazanate:a highly energetic 3D metal-organic framework as a promising primary explosive. Ang. Chem. Int. Ed. 2016, 55, 5565–5567. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, C.; Tǎbǎcaru, A.; Galli, S. Coordination polymers and metal-organic frameworks based on poly(pyrazole)-containing ligands. Coordin. Chem. Rev. 2016, 307, 1–31. [Google Scholar] [CrossRef]
- Feng, Y.G.; Bi, Y.G.; Zhao, W.Y.; Zhang, T.L. Anionic metal-organic frameworks lead the way to eco-friendly high-energy-density materials. J. Mater. Chem. A 2016, 4, 7596–7600. [Google Scholar] [CrossRef]
- Gao, W.J.; Liu, X.Y.; Su, Z.Y.; Zhang, S.; Yang, Q.; Wei, Q.; Chen, S.P.; Xie, G.; Yang, X.W.; Gao, S.L. High-energy-density materials with remarkable thermostability and insensitivity: Syntheses, structures and physicochemical properties of Pb(II) compounds with 3-(tetrazol-5-yl) triazole. J. Mater. Chem. A 2014, 2, 11958–11965. [Google Scholar] [CrossRef]
- Qiao, M.; Niu, J.R.; Zhong, W.Z.; Hou, Y.Q.; Li, Y.H.; Li, Z.X.; Zhou, B. Preparation and application of metal-organic frameworks. Chin. Hebei J. Indust. Sci. Technol. 2018, 35, 72–76. [Google Scholar]
- Karmakar, A.; Desai, A.V.; Ghosh, S. KIonic metal-organic frameworks (iMOFs): Design principles and applications. Coordin. Chem. Rev. 2016, 307, 313–341. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, W.; Li, C.; Gao, H.Q.; Tang, L.W.; Zhou, Z.M. (E)-1,2-bis(3,5-dinitro-1H-pyrazol-4-yl)diazene:Its 3D potassium metal organic framework and organic salts with super-heat -resistant properties. Eur. J. Inorgan. Chem. 2017, 2017, 2883–2891. [Google Scholar] [CrossRef]
- Badgujar, D.M.; Talawar, M.B.; Asthana, S.N.; Mahulikar, P.P. Advances in science and technology of modern energetic materials: An overview. J. Hazard. Mater. 2008, 151, 289–305. [Google Scholar] [CrossRef]
- Zhang, J.P.; Zhou, H.L.; Zhou, D.D.; Liao, P.Q.; Chen, X.M. Controlling flexibility of metal-organic frameworks. Natl. Sci. Rev. 2018, 5, 907–919. [Google Scholar] [CrossRef]
- Weinrauch, I.; Savchenko, I.; Denysenko, D.; Souliou, S.M.; Kim, H.H.; Tacon, M.L.; Daemen, L.L.; Cheng, Y.; Mavrandonakis, A.; Ramirez-Cuesta, A.J.; et al. Capture of heavy hydrogen isotopes in a metal-organic framework with active Cu(I) sites. Nat. Commun. 2017, 8, 14496. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.H.; Xu, H.Y.; Guo, Y.; Sa, R.J.; Shreeve, J.M. Bis[3 -(5-nitroimino-1,2,4-triazolate)]-based energetic salts: Synthesis and promising properties of a new family of high-density insensitive materials. J. Am. Chem. Soc. 2010, 132, 11904–11905. [Google Scholar] [CrossRef]
- Fischer, D.; Klapötke, T.M.; Stierstorfer, J. Potassium 1,1′-dinitramino-5,5′-bistetrazolate: A primary explosive with fast detonation and high initiation power. Ang. Chem. Int. Ed. 2014, 53, 8172–8175. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.X.; Shreeve, J.M. Azole-based energetic salts. Chem. Rev. 2011, 111, 7377–7436. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Martin, F.A.; Stierstorfer, J. C2N14: An energetic and highly sensitive binary azidotetrazole. Ang. Chem. Int. Ed. 2011, 50, 4227–4229. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, J.; He, C.; Parrish, D.A.; Shreeve, J.M. Polynitro-substituted pyrazoles and triazoles as potential energetic materials and oxidizers. J. Mater. Chem. A 2014, 2, 3200–3209. [Google Scholar] [CrossRef]
- Martinez, M.R.; Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routs towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Qi, C.; Li, S.H.; Li, Y.C.; Qi, C.; Li, S.H.; Zhang, H.J.; Sun, C.H.; Yu, Y.Z.; Pang, S.P. 1,1’-Azobis-1,2,3-triazole: A high-nitrogen compound with stable N8 structure and photochromism. J. Am. Chem. Soc. 2010, 132, 12172–12173. [Google Scholar] [CrossRef] [PubMed]
- Deblitz, R.; Hrib, C.G.; Blaurock, S.; Jones, P.G.; Plenikowski, G.; Edelmann, F.T. Explosive werner-type cobalt(iii) complexes. Inorg. Chem. Front. 2014, 1, 621–640. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.D.; Garai, B.; Reinsch, H.; Li, W.J.; Dissegna, S.; Bon, V.; Senkovska, I.; Fisher, R.A.; Kaskel, S.; Janiak, C.; et al. Metal-organic frameworks in germany: From synthesis to function. Coordin. Chem. Rev. 2019, 380, 378–418. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.X.; Huang, H.F.; Wang, K.; Bian, C.M. Oxy-bridged bis(1H-tetrazol-5-yl)furazan and its energetic salts paired with nitrogen-rich cations: Highly thermally stable energetic materials with low sensitivity. J. Mater. Chem. 2012, 22, 21954–21964. [Google Scholar] [CrossRef]
- Liang, L.; Wang, K.; Bian, C.; Ling, L.M.; Zhou, Z.M. 4-Nitro-3-(5-tetrazole)furoxan and its salts: Synthesis, characterization, and energetic properties. Chem. Eur. J. 2013, 19, 14902–14910. [Google Scholar] [CrossRef]
- Zhang, J.G.; Li, J.Y.; Zang, Y.; Zhang, T.L.; Yang, L.; Power, P.P. Synthesis and characterization of a novel energetic complex [Cd(DAT)6](NO3)2 (DAT=1,5-diamino-tetrazole) with high nitrogen content. Z. Anorg. Allg. Chem. 2010, 636, 1147–1151. [Google Scholar] [CrossRef]
- Tong, W.C.; Liu, J.C.; Wang, Q.Y.; Yang, L.; Zhang, T.L. Eco-friendly trifoliate stable energetic zinc nitrate coordination compounds: Synthesis, structures, thermal and explosive properties. Z. Anorg. Allg. Chem. 2014, 640, 2991–2997. [Google Scholar] [CrossRef]
- Zhang, T.L.; Wu, B.D.; Yang, L.; Zhou, Z.N.; Zhang, J.G. Recent research progresses in energetic coordination compounds. Chin. J. Energ. Mater. 2013, 21, 137–151. [Google Scholar]
- Bi, F.Q.; Fan, X.Z.; Xu, C.; Wang, B.Z.; Zheng, Y.F.; Ge, Z.X.; Liu, Q. Review on insensitive non-metallic energetic ionic compounds of tetrazolateanions. Chin. J. Energ. Mater. 2012, 20, 805–811. [Google Scholar]
- Zhang, X.J.; Li, Y.C.; Liu, W.; Yang, Y.Z.; Peng, L. Review on triazines energetic compounds. Chin. J. Energ. Mater. 2012, 20, 491–500. [Google Scholar]
- Ju, R.H.; Fan, X.Z.; Wei, H.J.; Bi, F.Q.; Li, J.Z.; Wang, H.; Fu, X.L. Research progress in application of tetrazine-based energetic compounds. Chem. Propell. Polymeric Mater. 2013, 11, 23–28. [Google Scholar]
- Xue, J.Q.; Shang, B.K.; Wang, W.; Liu, F.; Wang, L.X. Research advances in tetrazine-based high-nitrogen molecular and ionic energetic compounds. Chem. Propell. Polymeric Mater. 2011, 9, 1–8. [Google Scholar]
- Zhang, S.Z.; Feng, X.J.; Zhu, T.B.; Miao, C.C.; Ma, H.Q. Research progress in novel energetic materials-furazan-based compounds. Chem. Propell. Polymeric Mater. 2013, 11, 1–5. [Google Scholar]
- Wang, Z.; Wang, Y.; Wang, K.C.; Zhang, Q.H. Research progress in energetic metal-organic frameworks. Chin. J. Energ. Mater. 2017, 25, 442–450. [Google Scholar]
- Qu, X.N.; Zhai, L.J.; Wang, B.Z.; Wei, Q.; Xie, G.; Chen, S.P.; Gao, S.L. Copper-based energetic MOFs with 3-nitro-1H-1,2,4-triazole:solvent-dependent syntheses, structures and energetic performances. Dalton. Trans. 2016, 45, 17304–17311. [Google Scholar] [CrossRef]
- Wu, B.D.; Li, X.Y.; Liang, J.; Geng, X.H.; Huang, H.S.; Huang, H.; Wang., J.Y. 1D energetic metal organic frameworks assembled with energetic combination of furazan and tetrazole. Polyhedron 2018, 39, 148–154. [Google Scholar] [CrossRef]
- Wu, B.D.; Bin, Y.G.; Zhou, M.R.; Zhang, T.L.; Yang, L.; Zhou, Z.N.; Zhang, J.G. Stale high-nitrogen energetictrinuclear compounds based on 4-amino-3,5dimethyl-1,2,4- triazole: Synthesis, structures, thermal and explosive properties. Z. Anorg. Allg. Chem. 2014, 640, 1467–1473. [Google Scholar] [CrossRef]
- Zhang, J.; Du, Y.; Dong, K.; Su, H.; Zhang, S.W.; Li, S.H.; Pang, S.P. Tamingdinitramide anions within an energetic metal-organic framework: A new strategy for synthesis and tunable properties of high energy materials. Chem. Mater. 2016, 28, 1472–1480. [Google Scholar] [CrossRef]
- Calogero, G.P.; Angelos, P.; Maud, S.; Dusan, B.; Stefan, L. Synthesis of metal-organic frameworks for energetic applications. In Proceedings of the 49th International AnnualConference of the Fraunhofer ICT, Convention Center, Gartenhalle, Karlsruhe, Germany, 26–29 June 2018; p. 102. [Google Scholar]
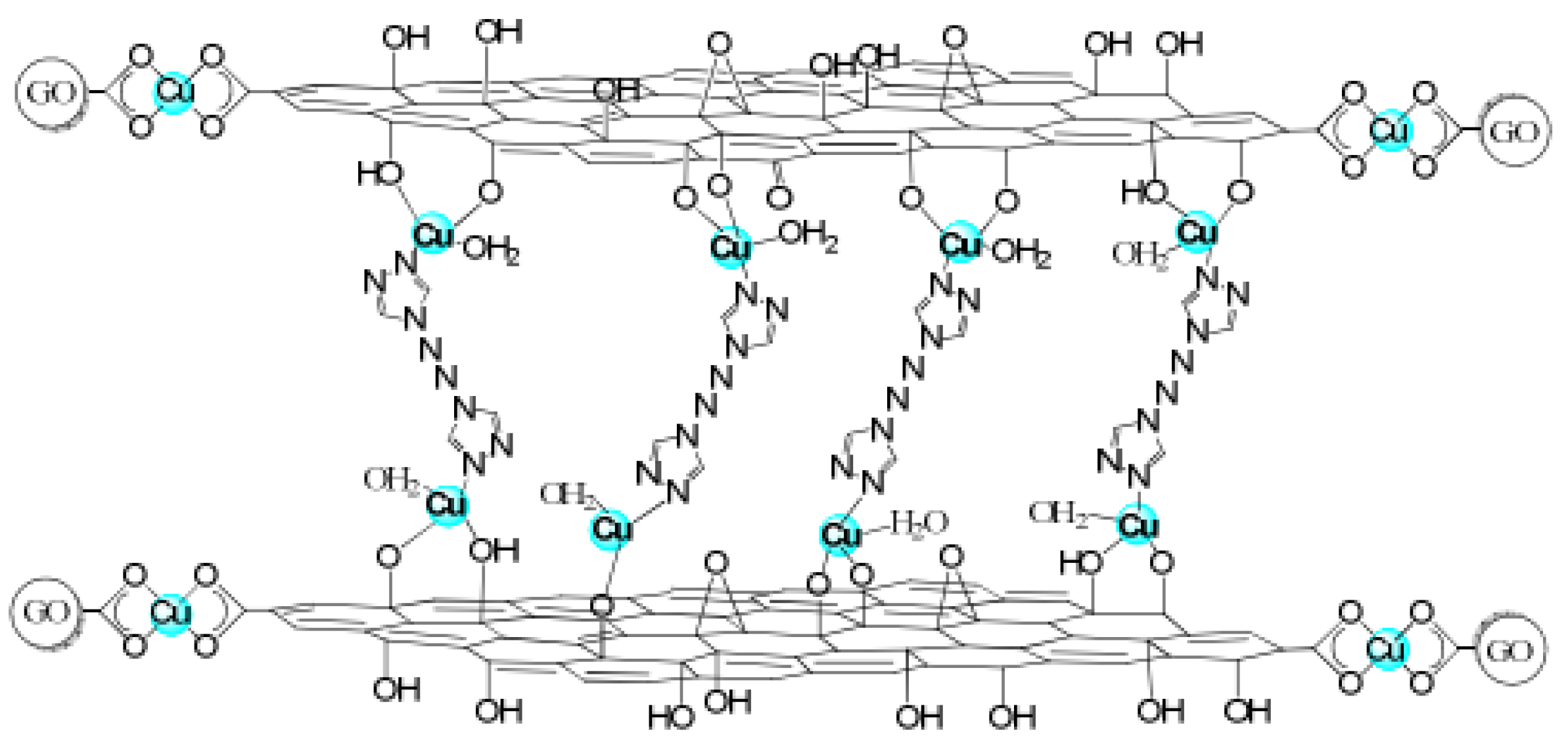
- Xu, M.; Chai, J.; Hu, N.; Huang, D.; Wang, Y.X.; Huang, X.L.; Wei, H.; Yang, Z.; Zhang, Y.F. Facile synthesis of soluble functional grapheme by reduction of graphene oxide via acetylacetone and its adsorption of heavy metal ions. Nanotechnology 2014, 25, 395602. [Google Scholar] [CrossRef]
- Cohen, A.; Yang, Y.Z.; Yan, Q.L.; Shlomovich, A.; Petrutik, N.; Burstein, L.; Pang, S.P.; Gozin, M. Highly thermostable and insensitive energetic hybrid coordination polymers based on grapheneoxide-Cu(Ⅱ)complex. Chem. Mater. 2016, 28, 6118–6126. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, D.; Jing, D.; Fan, G.J.; He, L.; Li, H.Z.; Wang, W.T.; Nie, F. Access to green primary explosives via constructing coordination polymers based on bis-tetrazole oxide and non-lead metals. Green Chem. 2019, 21, 1947–1955. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, B.J.; Zhang, M.; Li, C.; Gao, H.Q.; Zhang, J.; Zhou, Z.M. 3,4-Dinitro-1-(1H-tetrazol-5-yl)-1H-pyrazol-5-amine (HANTP) and its salts primary and secondary explosives. J. Mater. Chem. A 2017, 5, 5044–5054. [Google Scholar] [CrossRef]
- Shen, C.; Liu, Y.; Zhu, Z.Q.; Xu, Y.Q.; Lu, M. Self-assembly of silver(I)-based high-energy metal-organic frameworks(HE-MOFs) at ambient temperature and pressure: Synthesis, structure and superior explosive performance. Chem. Commun. 2017, 53, 7489–7492. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Song, X.X.; Yang, G.L.; Xie, G.; Chen, S.P.; Gao, S.L.l. The Invention Relates to a Heat Resistant High Density Energy-Containing Metal-Organic Skeleton Material and a Preparation Method. China Patent CN106977744A, 25 July 2017. [Google Scholar]
- Li, C.; Zhang, M.; Chen, Q.S.; Li, Y.Y.; Gao, H.Q.; Fu, W.; Zhou, Z.M. Three-dimensional metal-organic framework as super heat-resistant explosive: Potassium 4-(5-amino-3 -nitro-1H-1,2,4-triazol-1-yl)-3,5-dinitropyrazole. Chem. Eur. J. 2017, 23, 1490–1493. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Wang, S.; Feng, X.; Wu, L.; Zhang, G.Y.; Zhou, M.R.; Wang, B.; Li, Y. A heat-resistant and energetic metal-organic framework assembled by chelating ligand. Appl. Mater. Inter. 2017, 9, 37542–37547. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zhao, F.Q.; Yi, J.H.; Xuan, C.L.; Luo, Y. Research progress of MOFs as combustion catalysts and high energy additives for solid propellants. Chin. J. Energ. Mater. 2016, 24, 1225–1231. [Google Scholar]
- Su, H.; Zhang, J.C.; Du, Y.; Zhang, P.C.; Li, S.H.; Fang, T.; Pang, S.P. New roles of metal-organic frameworks: Fuels for aluminum-free energetic thermites with low ignition temperatures, high peak pressures and high activity. Combust. Flame 2018, 191, 32–38. [Google Scholar] [CrossRef]
- Jian, G.Q.; Feng, J.Y.; Jacob, R.J.; Egan, G.; Zachariah, M. Super-reactive nanoenergetic gas generators based on periodate salts. Angew. Chem. Int. Ed. 2013, 52, 9743–9746. [Google Scholar] [CrossRef]
- Slocik, J.M.; Crouse, C.A.; Spowart, J.E.; Naik, R.R. Biologically tunable reactivity of energetic nanomaterials using protein cages. Nano Lett. 2013, 13, 2535–2540. [Google Scholar] [CrossRef]
- Comet, M.; Vidick, G.; Schnell, F.; Suma, Y.; Baps, B.; Spitzer, D. Sulfates-basednanothermites: An expanding horizon for metastable interstitial composites. Angew. Chem. Int. Ed. 2015, 54, 4458–4462. [Google Scholar] [CrossRef] [PubMed]
- Petrantoni, M.; Rossi, C.; Salvagnac, L.; Conédéra, V.; Estève, A.; Tenailleau, C.; Alphonse, P.; Chabal, Y.J. Multilayered Al/CuO thermite formation by reactive magnetron sputtering: Nano versus micro. J. Appl. Phys. 2010, 108, 084323. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.C.; Yin, B.Q.; Shen, R.Q.; Ye, J.H. Significantly enhanced energy output from 3D ordered macroporous structured Fe2O3/Al nanothermite film. ACS Appl. Mater. Interfaces 2013, 5, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.G.; Yang, Y.; Cheng, H.; Li, Y.Y.; Zhang, K.L. Integration of nano-Al with Co3O4nanorods to realize high-exothermic core–shell nanoenergetic materials on a silicon substrate. Combust. Flame 2012, 159, 2202–2209. [Google Scholar] [CrossRef]
- Li, D.Y. The Preparation of Core-Shell Structure Nano Energetic Materials and Research on Their Performance. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2016. [Google Scholar]
- He, W.; Ao, W.; Yang, G.C.; Yang, Z.J.; Guo, Z.Q.; Liu, P.J.; Yan, Q.L. Metastable energetic nanocomposites of MOF-activated aluminum featured with multi-level energy releases. Chem. Eng. J. 2020, 381, 122623. [Google Scholar] [CrossRef]
- He, W.; Li, Z.H.; Chen, S.W.; Yang, G.C.; Yang, Z.J.; Liu, P.J.; Yan, Q.L. Energetic metastable n-Al@PVDF/EMOF composite nanofibers with improved combustion performances. Chem. Eng. J. 2020, 383, 123146. [Google Scholar] [CrossRef]
- Singh, R.P.; Verma, R.D.; Meshri, D.T.; Shreeve, J. Energetic nitrogen-rich salts and ionic liquids. Ang. Chem. Int. Ed. 2006, 45, 3584–3601. [Google Scholar] [CrossRef]
- Xia, Z.Q.; Chen, S.P.; Wei, Q.; Qiao, C.F. Syntheses and characterization of energetic compounds constructed from alkaline earth metal cations (Sr and Ba) and 1,2-bis(tetrazol-5-yl)ethane. J. Solid State Chem. 2011, 184, 1777–1783. [Google Scholar] [CrossRef]
- Ma, X.H.; Cai, C.; Sun, W.J.; Song, W.M.; Ma, Y.L.; Liu, X.Y.; Xie, G.; Chen, S.P.; Gao, S.L. Enhancing energetic performance of multinuclear Ag(I)-cluster MOF-based high-energy-density materials by thermal dehydration. Appl. Mater. Int. 2019, 11, 9233–9238. [Google Scholar] [CrossRef]

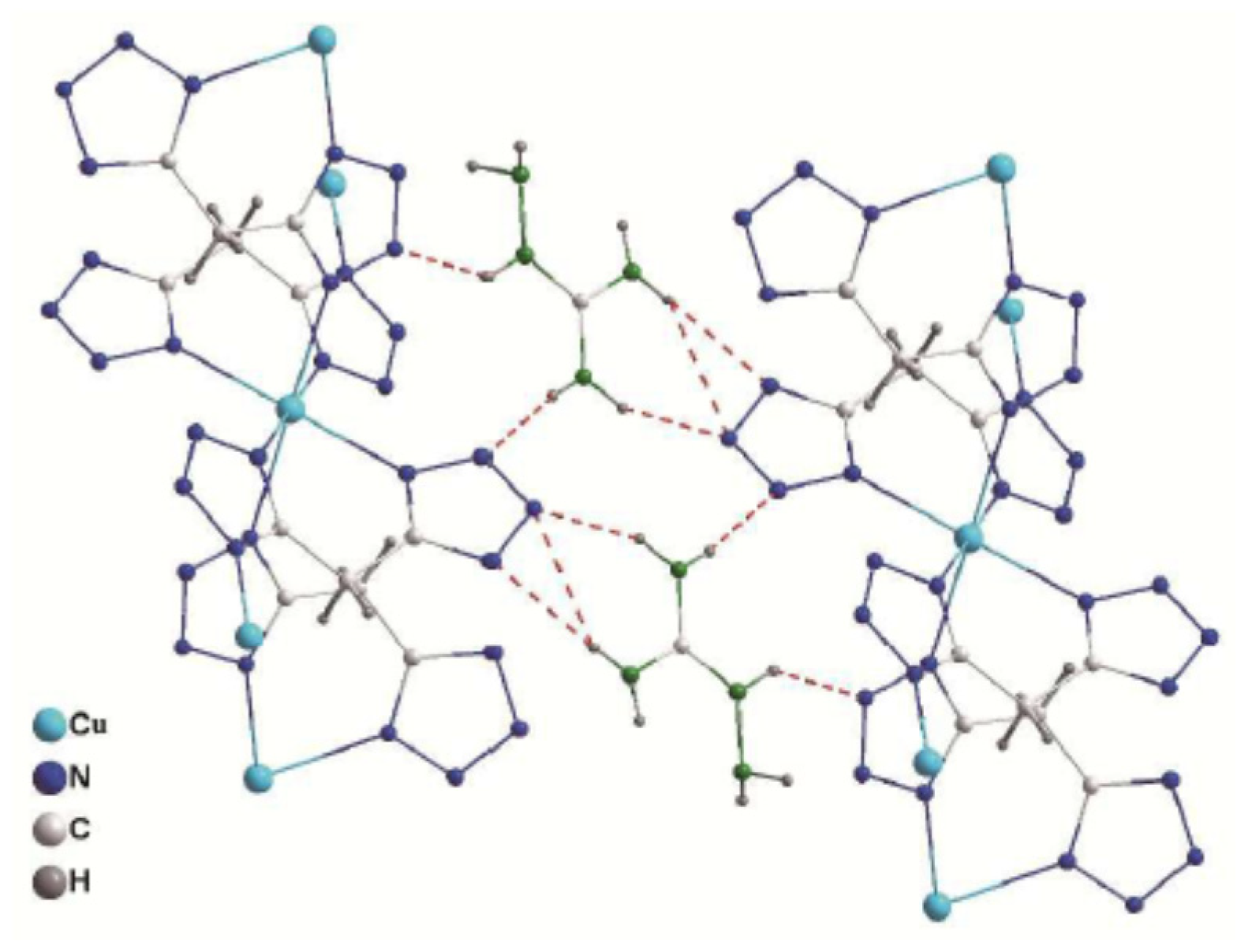



| Compound | ρ/g·cm−3 | N/% | Td./°C | ΔHdet/kJ··g−1 | P/GPa | D/km·s−1 | IS (J),FS (N),ES (J) |
|---|---|---|---|---|---|---|---|
| Pb(N3)2 [47] | 4.800 | 28.9 | 315 | - | 33.4 | 5.877 | 2.5–4,0.1–1,- |
| {[Cu(ATZ)](ClO4)2}n [31] | 1.400 | 32.66 | >250 | - | - | 6.5 | 1,10,- |
| {[Zn(ATZ)3](PA)2·2.5H2O}n [31] | 1.757 | 30.89 | 276.3 | - | - | - | 27.8,-,- |
| [Mn2(HATr)4(NO3)4·2H2O]n [37] [Cd2(HATr)4(NO3)4·H2O]n [37] | 1.864 2.021 | 46.12 41.4 | 260 295 | - - | - - | - - | -,-,- -,-,- |
| CHHP [43] ZnHHP [43] | 2.000 2.117 | 23.58 23.61 | 231 293 | 6.277 6.202 | 17.96 23.58 | 6.205 7.016 | 0.8,-,- -,-,- |
| [Cu(atrz)3(NO3)2]n [45] [Ag(atrz)1.5(NO3)]n [45] | 1.680 2.160 | 53.35 43.76 | 243 257 | 4.562 5.802 | 35.68 29.7 | 9.16 7.773 | 22.5,112,- 30,-,- |
| {[Cu(tztr)]·H2O}n [44] [Cu(tztr)]n [44] | 2.316 2.216 | - - | 80/325 360 | 5.524 14.229 | 31.99 40.02 | 7.920 8.429 | >40,>360,>24.7,>32, >360,>24.75 |
| K2DNMAF [47] | 2.039 | 31.1 | 229 | - | 30.1 | 8.137 | 2,20,- |
| [Ag2(DNMAF)·(H2O)2]n [86] [Ag2(DNMAF)]n [86] | 2.545 2.796 | - - | 230 212 | 8.160 9.165 | 50.01 58.30 | 9.673 10.242 | 10,160,- 8,120,- |
| [Pb(bta)(H2O)]n [87] | 3.412 | 33.50 | 314 | - | - | - | - |
| TNT | 1.654 | 18.5 | 295 | 4.144 | 19.53 | 6.881 | 15,350,0.57 |
| HMX | 1.950 | 37.84 | 287 | 5.525 | 38.39 | 8.900 | 7.4,-,0.2 |
| RDX | 1.800 | 34.31 | 210 | 5.710 | 34.1 | 8.906 | 7.9,120,0.15 |
| CL-20 | 2.035 | 38.36 | 245 | 6.168 | 44.9 | 9.385 | 4,54,0.13 |
| PETN | 1.778 | - | 202 | 6.404 | 32.1 | 8.665 | 3,60,- |
| FOX-7 | 1.885 | - | 240 | - | 36.6 | 9.09 | -,-,- |
| TATB | 1.930 | - | 330 | 4.445 | 31.2 | 8.114 | 50,-,- |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Qiao, J.; Xu, S.; Zhao, X.; Gong, W.; Huang, T. Constructing Strategies and Applications of Nitrogen-Rich Energetic Metal–Organic Framework Materials. Catalysts 2020, 10, 690. https://doi.org/10.3390/catal10060690
Xu L, Qiao J, Xu S, Zhao X, Gong W, Huang T. Constructing Strategies and Applications of Nitrogen-Rich Energetic Metal–Organic Framework Materials. Catalysts. 2020; 10(6):690. https://doi.org/10.3390/catal10060690
Chicago/Turabian StyleXu, Luping, Juan Qiao, Siyu Xu, Xiaoyu Zhao, Wanjun Gong, and Taizhong Huang. 2020. "Constructing Strategies and Applications of Nitrogen-Rich Energetic Metal–Organic Framework Materials" Catalysts 10, no. 6: 690. https://doi.org/10.3390/catal10060690






