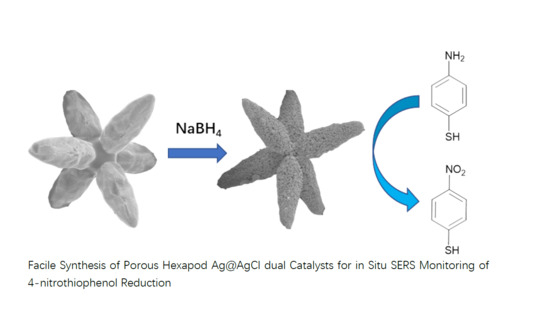
Facile Synthesis of Porous Hexapod Ag@AgCl Dual Catalysts for In Situ SERS Monitoring of 4-Nitrothiophenol Reduction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Hexapod Ag@AgCl Microstructures and Their Characterization
2.2. In Situ Monitoring of Catalytic Reactions by UV–Vis and Catalytic Performance
2.3. In Situ Monitoring of Catalytic Reactions with SERS
3. Experimental
3.1. Materials and Chemicals
3.2. Characterization and Instruments
3.3. Preparation of Hexapod Ag@AgCl Microstructures
3.4. In Situ Detection of Catalytic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Duan, H.H.; Yan, N.; Li, Y.D. Ultrathin rhodium nanosheets. Nat. Commun. 2014, 5, 3093–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, T.S.; Lee, Y.C.; Wang, D.Y. Plasmon-Enhanced Hydrogen Evolution on Specific Facet of Silver. Chem. Mater. 2019, 31, 3722–3728. [Google Scholar] [CrossRef]
- Liu, D.L.; Fang, L.L.; Li, Y. Ultrasensitive and Stable Au Dimer-Based Colorimetric Sensors Using the Dynamically Tunable Gap-Dependent Plasmonic Coupling Optical Properties. Adv. Funct. Mater. 2018, 28, 1707392–1707402. [Google Scholar] [CrossRef]
- Ma, Y.; Promthaveepong, K.; Li, N. Chemical Sensing on a Single SERS Particle. ACS Sens. 2017, 2, 135–139. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Ma, D. Construction of Stable Chainlike Au Nanostructures via Silica Coating and Exploration for Potential Photothermal Therapy. Small 2014, 10, 3619–3624. [Google Scholar] [CrossRef]
- Paterson, S.; Thompson, S.A.; de la Rica, R. Self-assembly of gold supraparticles with crystallographically aligned and strongly coupled nanoparticle building blocks for SERS and photothermal therapy. Chem. Sci. 2016, 7, 6232–6237. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.Q.; Tang, S.H.; Zheng, N.F. Etching Growth under Surface Confinement: An Effective Strategy to Prepare Mesocrystalline Pd Nanocorolla. J. Am. Chem. Soc. 2011, 133, 15946–15949. [Google Scholar] [CrossRef]
- Scarabelli, L.; Coronado-Puchau, M.; Luis, M. Monodisperse Gold Nanotriangles: Size Control, Large-Scale Self-Assembly, and Performance in Surface-Enhanced Raman Scattering. ACS Nano 2014, 8, 5833–5842. [Google Scholar] [CrossRef]
- Yu, B.R.; Li, P.; Zhou, B.B.; Tang, X.H.; Li, S.F.; Yang, L.B. Sodium Chloride Crystal-Induced SERS Platform for Controlled Highly Sensitive Detection of Illicit Drugs. Chem. Eur. J. 2018, 24, 4800–4804. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Xia, X.H.; Zeng, J.; McDearmon, B.; Zheng, Y.Q.; Li, Q.G.; Xia, Y.N. Silver Nanocrystals with Concave Surfaces and Their Optical and Surface-Enhanced Raman Scattering Properties. Angew. Chem. Int. Ed. 2011, 50, 12542–12546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, Y.; Dai, X.; Fang, G.; Wu, R.F.; Zhao, L.; Ma, X.C.; Tian, X.; Lee, S.Y.; Zhang, C.; Chen, C.Y.; et al. Palladium concave nanocrystals with high-index facets accelerate ascorbate oxidation in cancer treatment. Nat. Commun. 2018, 9, 4861–4871. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.G.; Xia, Y.N. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.H.; Zeng, J.; Oetjen, L.K.; Li, Q.G.; Xia, Y.N. Quantitative analysis of the role played by poly(vinylpyrrolidone) in seed-mediated growth of Ag nanocrystals. J. Am. Chem. Soc. 2012, 134, 1793–1801. [Google Scholar] [CrossRef] [Green Version]
- Kim, F.; Connor, S.; Song, H.; Kuykendall, T.; Yang, P.D. Platonic Gold Nanocrystals. Angew. Chem. 2004, 116, 3759–3763. [Google Scholar] [CrossRef]
- Huo, D.; Cao, Z.M.; Li, J.; Xie, M.H.; Tao, J.; Xia, Y.N. Seed-Mediated Growth of Au Nanospheres into Hexagonal Stars and the Emergence of a Hexagonal Close-Packed Phase. Nano Lett. 2019, 19, 3115–3121. [Google Scholar] [CrossRef]
- Garcia-Leis, A.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Silver Nanostars with High SERS Performance. J. Phys. Chem. C 2013, 117, 7791–7795. [Google Scholar] [CrossRef]
- Walsh Michael, J.; Tong, W.M.; Funston Alison, M. A Mechanism for Symmetry Breaking and Shape Control in Single-Crystal Gold Nanorods. Acc. Chem. Res. 2017, 50, 2925–2935. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Zhang, D.C.; Li, H.X.; Liu, G.H. Magnetically recoverable nanoparticles as efficient catalysts for organic transformations in aqueous medium. Green Chem. 2014, 16, 3401–3427. [Google Scholar] [CrossRef]
- Saran, S.; Manjari, G.; Devipriya, S.P. Synergistic eminently active catalytic and recyclable Ag, Cu and Ag-Cu alloy nanoparticles supported on TiO2 for sustainable and cleaner environmental applications: A phytogenic mediated synthesis. J. Clean. Prod. 2018, 177, 134–143. [Google Scholar] [CrossRef]
- Arora, N.; Mehta, A.; Mishra, A.; Basu, S. 4-Nitrophenol reduction catalysed by Au-Ag bimetallic nanoparticles supported on LDH: Homogeneous vs. heterogeneous catalysis. Appl. Clay Sci. 2018, 151, 1–9. [Google Scholar] [CrossRef]
- Xie, W.; Herrmann, C.; Kömpe, K.; Haase, M.; Schlücke, S. Synthesis of Bifunctional Au/Pt/Au Core/Shell Nanoraspberries for in Situ SERS Monitoring of Platinum-Catalyzed Reactions. J. Am. Chem. Soc. 2011, 133, 19302–19305. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.L.; Xu, P.; Zhang, B.; Tsai, H.H.; Han, C.J.; Wang, H.L. Laser wavelength- and power-dependent plasmon-driven chemical reactions monitored using single particle surface enhanced Raman spectroscopy. Chem. Commun. 2013, 49, 3389–3391. [Google Scholar] [CrossRef] [PubMed]
- Fenger, R.; Fertitta, E.; Kirmse, H.; Thünemann, A.F.; Rademann, K. Size dependent catalysis with CTAB-stabilized gold nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9343–9349. [Google Scholar] [CrossRef]
- Mudassir, M.A.; Hussain, S.Z.; Rehman, A.; Zaheer, W.; Asma, S.T.; Jilani, A.; Aslam, M.; Zhang, H.F.; Ansari, T.M.; Hussain, I. Development of silver-nanoparticle-decorated emulsion-templated hierarchically porous poly(1-vinylimidazole) beads for water treatment. ACS Appl. Mater. Interfaces 2017, 9, 24190–24197. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.X.; Astruc, D. Anisotropic gold nanoparticles: Synthesis, properties, applications, and toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef]
- Cheng, Z.Q.; Li, Z.W.; Xu, J.H.; Yao, R.; Li, Z.L.; Liang, S.; Cheng, G.L.; Zhou, Y.H.; Luo, X.; Zhong, J. Morphology-Controlled Fabrication of Large-Scale Dendritic Silver Nanostructures for Catalysis and SERS Applications. Nanoscale Res. Lett. 2019, 14, 89–95. [Google Scholar] [CrossRef]
- Rashid, M.H.; Mandal, T.K. Synthesis and catalytic application of nanostructured silver dendrites. J. Phys. Chem. C 2007, 111, 16750–16760. [Google Scholar] [CrossRef]
- Xu, M.; Sui, Y.M.; Wang, C.; Zhou, B.; Wei, Y.J.; Zou, B. Design of porous Ag platelet structures with tunable porosity and high catalytic activity. J. Mater. Chem. A 2015, 3, 22339–22346. [Google Scholar] [CrossRef]
- Hong, X.; Wang, G.-Z.; Wang, Y. Controllable electrochemical synthesis of silver dendritic nanostructures and their SERS properties. Chin. J. Chem. Phys. 2010, 23, 596–602. [Google Scholar] [CrossRef]
- Gatemala, H.; Thammacharoen, C.; Ekgasit, S. 3D AgCl microstructures selectively fabricated via Cl−-induced precipitation from [Ag(NH3)2]+. CrystEngComm. 2014, 16, 6688–6696. [Google Scholar] [CrossRef]
- Lu, Y.X.; Qin, Y.Z.; Zhou, J.G. Stepwise Evolution of AgCl Microcrystals from Octahedron into Hexapod with Mace Pods and their Visible Light Photocatalytic Activity. Crystals 2019, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Zhang, Y.J.; Yang, W.M.; Dong, J.C.; Fan, F.R.; Zhao, Y.; Zhang, H.; Bodappa, Y.; Tian, X.D.; Yang, Z.L.; et al. Size and dimension dependent surface-enhanced Raman scattering properties of well-defined Ag nanocubes. Appl. Mater. Today 2019, 14, 224–232. [Google Scholar] [CrossRef]
- Liao, G.F.; Gong, Y.; Zhong, L.; Fang, J.S.; Zhang, L.; Xu, Z.S.; Gao, H.Y.; Fang, B.Z. Unlocking the door to highly efficient Ag-based nanoparticles catalysts for NaBH4-assisted nitrophenol reduction. Nano Res. 2019, 12, 2407–2436. [Google Scholar] [CrossRef]
- Chen, X.; Cai, Z.; Chen, X.; Oyama, M. AuPd bimetallic nanoparticles decorated on graphene nanosheets: Their green synthesis, growth mechanism and high catalytic ability in 4-nitrophenol reduction. J. Mater. Chem. A 2014, 2, 5668–5674. [Google Scholar] [CrossRef]
- Zhang, J.W.; Winget, S.A.; Wu, Y.R.; Su, D.; Su, X.J.; Xie, Z.X.; Qin, D. Ag@Au Concave Cuboctahedra: A Unique Probe for Monitoring Au-Catalyzed Reduction and Oxidation Reactions by Surface-Enhanced Raman Spectroscopy. ACS Nano 2016, 10, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Wu, D.Y.; Zhu, H.P.; Zhao, L.B.; Liu, G.K.; Ren, B.; Tian, Z.Q. Surface-enhanced Raman spectroscopic study of p-aminothiophenol. Phys. Chem. Chem. Phys. 2012, 14, 8485–8497. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wunder, S.; Lu, Y.; Ballauff, M. Kinetic Analysis of the Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles. J. Phys. Chem. C 2014, 118, 18618–18625. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Mao, J.; Wang, Z.; Qin, Y.; Zhou, J. Facile Synthesis of Porous Hexapod Ag@AgCl Dual Catalysts for In Situ SERS Monitoring of 4-Nitrothiophenol Reduction. Catalysts 2020, 10, 746. https://doi.org/10.3390/catal10070746
Lu Y, Mao J, Wang Z, Qin Y, Zhou J. Facile Synthesis of Porous Hexapod Ag@AgCl Dual Catalysts for In Situ SERS Monitoring of 4-Nitrothiophenol Reduction. Catalysts. 2020; 10(7):746. https://doi.org/10.3390/catal10070746
Chicago/Turabian StyleLu, Yuxiang, Jikai Mao, Zelin Wang, Yazhou Qin, and Jianguang Zhou. 2020. "Facile Synthesis of Porous Hexapod Ag@AgCl Dual Catalysts for In Situ SERS Monitoring of 4-Nitrothiophenol Reduction" Catalysts 10, no. 7: 746. https://doi.org/10.3390/catal10070746